Abstract
Social isolation and lack of social support are risk factors for cardiovascular and cerebrovascular disease (CVD). This study explored the relationship between measures of social support and subclinical measures of CVD risk. 58 healthy adults ages 18 to 85 years participated in this study. The Berkman-Syme Social Network Inventory (SNI) was used to assess social isolation, with higher scores signifying less isolation. Social support was defined using the 12-Item Interpersonal Support Evaluation List (ISEL-12) with a higher score signifying higher social support. Subclinical CVD measures included carotid-femoral pulse wave velocity (cfPWV), carotid beta-stiffness index, and middle cerebral artery (MCA) pulsatility index. Path analysis models for both the SNI and ISEL appraisal domain predicting cfPWV and cerebrovascular pulsatility fit the data well. Path analyses showed significant direct paths from the SNI (β =−.363, t=−2.91) and ISEL appraisal domain (β=−.264, t=−2.05) to cfPWV. From cfPWV, both models revealed significant direct paths to carotid stiffness (β =.488, t=4.18) to carotid pulse pressure (β =.311, t=2.45) to MCA pulsatility (β =.527, t=4.64). Social isolation and appraisal of social support are related to unfavorably higher aortic stiffness, with subsequent detrimental effects on cerebrovascular hemodynamic pulsatility.
Keywords: isolation, social support, appraisal, arterial stiffness, cerebrovascular pulsatility
Introduction
Cardiovascular and cerebrovascular diseases (CVDs) remain among the most prevalent killers in the United States in men and women alike, despite improvements in detection and treatment (Go et al., 2014a, 2014b; Lakatta & Levy, 2003b, 2003a). Research examining how psychosocial factors influence CVD risk have shown that low social support is as potent a risk factor for CVD as hypertension, smoking, obesity, hyperlipidemia and diabetes (Brinkhues et al., 2017; Holt-Lunstad et al., 2010; House et al., 1988; Pantell et al., 2013). The exact mechanism through which social relationships influence CVD risk has yet to be elucidated. In the current work, we examine how social relationships associate with arterial stiffness and cerebrovascular hemodynamic pulsatility, testing the hypothesis that vascular aging is the mechanism through which social relationships ultimately impact CVD risk.
Arterial Stiffness, Hemodynamic Pulsatility & CVD Risk
Research aimed at understanding prodromal risk of CVD has focused on the biological and physiological mechanisms with which the large arteries such as the aorta and carotid artery become stiffer (Lakatta & Levy, 2003a), as this vascular senescence is known to contribute to increased CVD morbidity and mortality (Ben-Shlomo et al., 2014; Giannarelli et al., 2012; Vlachopoulos et al., 2010). Increases in arterial stiffness with age (i.e., vascular aging) precede the development of hypertension (Kaess et al., 2012) and increase risk for related vascular events such as myocardial infarction and stroke (Mitchell et al., 2010; Sutton-Tyrrell et al., 2005). Functionally, the elastic properties of the large central arteries function to dampen the amplitude of fluctuations in pressure and flow produced during a typical cardiac cycle, thereby preventing transmission of excess pulsatile energy into target organs (Mitchell, 2008). Increased arterial stiffness and its associated sequela (i.e. increased hemodynamic pulsatility) mechanistically contribute to target organ damage such as left ventricular hypertrophy (Roman et al., 2000), renal dysfunction (Sedaghat et al., 2014) and brain/cerebral structural damage (Mitchell, 2008) and has been linked to related pathologies of aging such as heart failure (Chirinos et al., 2014), renal disease (Madero et al., 2013), and cogntive dysfunction (Mitchell et al., 2011), respectively. As such, understanding factors that contribute to arterial stiffness as a reflection of vascular aging and related hemodynamic pulsatility has important implications for the prevention of age-associated target organ damage, and CVD morbidity and mortality. Although numerous biological and physiological mechanisms have been investigated, the potential impact of psychosocial factors on arterial stiffness remains largely unexplored.
Psychosocial Determinants of CVD Risk
A new position statement by the American Heart Association acknowledges a strong and clinically meaningful relationship between psychological well-being and physical health referred to as the “mind-heart-body connection.” Social isolation is considered an important psychosocial stressor that may increase CVD risk upwards of 50% (Levine et al., 2021). This link between psychosocial factors and CVD risk is well established (Rosengren et al., 2004; Rozanski et al., 1999) with numerous studies observing associations between a lack of positive social relationships and future CVD morbidity and mortality (Eng et al., 2002; Horsten et al., 2000; Kawachi et al., 1996; Steptoe et al., 2013; Valtorta et al., 2018). Low social support predicts the development of hypertension (Yang et al., 2015) and cognitive decline (Cacioppo & Cacioppo, 2014; Shankar et al., 2013) and is associated with numerous subclinical measures of CVD risk such as systemic inflammation (Yang et al., 2013), absence of nocturnal blood pressure dipping measured from 24-hour ambulatory blood pressure monitoring (Steptoe, 2000), left ventricular hypertrophy measured via transthoracic echocardiograms (Rodriguez et al., 2011), and coronary artery calcium determined from electron beam tomography (Kop et al., 2005).
To date, most studies examining the association between social relationships and CVD risk, including those acknowledged above, have explored global indices of social relationships. Moreover, these studies focus on social relationship quantity (i.e., the number of social contacts) and not the functional nature of the social relationships. Social support can be stratified according to distinct functional dimensions: appraisal (informational), tangible (instrumental) and belonging (Cohen & Wills, 1985). Appraisal support encompasses the provision of information, guidance, suggestions or advice that holds the potential to help others solve problems. With said information and coping strategies, individuals may re-appraise certain situations as less stressful or threatening. Tangible support describes the provision of material aid or services equating to direct assistance of a problem or stressful situation. Belonging support involves inclusion of social activities such that an individual identifies a social connection or companionship. Examination of these specific dimensions with measures of arterial stiffness may provide greater insight into potential vascular pathways through which different types of social support may affect vascular aging and CVD risk.
The primary purpose of this study was to examine the association between social support and aortic stiffness in apparently healthy men and women. We hypothesized that low social support would be a predictor of increased aortic stiffness. A secondary purpose of this study was to examine the cerebrovascular consequences of increased aortic stiffness in this cohort. We hypothesized that increased aortic stiffness would be further associated with increased carotid artery stiffness, and ultimately hemodynamic pulsatility in the carotid and cerebral arteries.
Method
Fifty-eight apparently healthy adults (N = 26 women) between the ages of 18 and 85 participated in this study (47 ± 17 years). Participants were recruited from the broader Syracuse, and Central New York community via several methods including: targeted radio commercials on a local public access radio station, flyers, local newspaper advertisements and social media postings. Exclusion criteria were history of smoking, stroke, diabetes mellitus, pulmonary disease, renal disease, neurological disease, severe arrhythmia, and peripheral artery disease. This study was approved by the Institutional Review Board of Syracuse University. All participants provided written informed consent.
Procedure
Participants reported to the Human Performance Laboratory on two separate occasions. The initial screening visit was carried out in the early morning (0600–0900) following an overnight fast. Following consent, participants completed a health history questionnaire, body composition assessment, urinalysis, fasting glucose and lipid assessment via finger stick, depressive symptomology appraisal via the Center for Epidemiologic Studies Depression Scale (CES-D), social support questionnaires, global cognitive appraisal via the Montreal Cognitive Assessment (MOCA), and cognitive function evaluation using the Trail Making Test (TMT).
Participants reported back to the Human Performance Laboratory on a second day for vascular and hemodynamic data acquisition. Vascular-hemodynamic testing was conducted in a quiet, dimly lit, temperature-controlled laboratory. Participants were instructed to fast for ≥ 4 hours and avoid vigorous exercise and consumption of caffeine or alcohol ≥ 12 hours before testing. Participants were familiarized with all instrumentation. Following familiarization, a blood pressure cuff and ECG electrodes were applied. Participants were then allowed to relax in the supine position on a cushioned exam bed for a period of 10–15 minutes. Resting measures of pulsatile blood pressure (brachial and carotid), arterial stiffness (carotid and aortic) and pulsatile blood flow (cerebral) were then simultaneously obtained.
Measures
Social Support Questionnaires.
The Interpersonal Support Evaluation List (ISEL) was used to assess perceived social support. Rather than use the full 40 items, we used the psychometrically-sound 12 item version of the ISEL (Payne et al., 2012). This scale assesses three dimensions of social support: appraisal, tangible, and belonging. To assess appraisal, items such as “There is someone I can turn to for advice about handling problems with my family,” are included. Tangible support is assessed with items such as “If I were sick, I could easily find someone to help me with my daily chores.” Belonging is assessed with items such as “If I decide one afternoon that I would like to go to a movie that evening, I could easily find someone to go with me.” All items were rated on a scale from 0 (definitely false) to 3 (definitely true), and each subscale evidenced acceptable reliability (appraisal α = .77; tangible α = .76; belonging α = .78). Higher scores are indicative of higher social support.
Additionally, we administered the Berkman-Syme Social Network Index (SNI), which is a self-report questionnaire that evaluates social network size and frequency of social contact, as an index of overall social isolation (Berkman & Breslow, 1983). The SNI includes 4 types of social connections in the measure of social support: (1) marital status, (2) number of close friends, (3) regular attendance at religious services, and (4) participation in community or volunteer groups or other organizations such as church-connected groups, self-help groups, charity, and public service. Scores were summed and a higher score is indicative of higher social support. Depressive symptomology was appraised via the CES-D as a potential covariate for any identified relationships between psychosocial function and aortic stiffness.
Anthropometrics.
Height and weight were assessed via wall-mounted ruler and electronic scale, respectively. Body composition was measured via air displacement plethysmography (BodPod; COSMED, Concord, CA).
Fasting Lipids and Glucose.
Total cholesterol, high-density lipoprotein cholesterol and fasting glucose levels were measured using an automated point-of-care blood lipid analysis device (Cholestech, Alere). Small samples of blood were obtained via finger lancet on the non-dominant hand of the participant. After immediate blood collection in small heparin lined capillary tubes, the blood was transferred to Cholestech cartridges and loaded in the device to be analyzed. This technique has previously been established to be valid and reliable (Carey et al., 2006).
Brachial and Central Blood Pressure.
Systolic blood pressure (SBP) and diastolic brachial blood pressure (DBP) was measured on the left arm using a validated, automated oscillometric cuff (EW3109, Panasonic Electric Works, Secaucus NJ) (Bonso et al., 2010). Mean arterial pressure (MAP) was calculated as: . Carotid pressure waveforms were obtained in the right carotid artery using applanation tonometry from a 10 s epoch (SphygmoCor, AtCor Medical, Sydney, Australia). The validity of this approach has been previously established; carotid pressure waveforms registered non-invasively via applanation tonometry are comparable to waveforms obtained invasively via a micromanometer-tipped catheter (Chen et al., 1996). Carotid pressure waveforms were calibrated to brachial MAP and DBP. Pulse pressure (PP) was calculated as SBP minus DBP.
Aortic Stiffness.
A single high-fidelity pressure transducer (SphygmoCor, AtCor Medical, Sydney, Australia) was used to consecutively measure pressure waveforms between the right common carotid artery (CCA) and the right femoral artery. Surface distances between the carotid pulse and suprasternal notch and the suprasternal notch and femoral artery were measured using a tape measure. Carotid-femoral wave velocity (cfPWV) was calculated from the distances between measurement points, after subtraction of the distance from the carotid artery to the suprasternal notch to account for the bi-directional nature of pressure propagation, and the measured time delay (Δt) between proximal and distal waveforms determined from simultaneous ECG R-wave gating. Validity of this approach has been established and measurements were carried out following guidelines of the American Heart Association (Townsend et al., 2015).
Carotid Artery Stiffness.
Images of the left CCA were obtained using Doppler ultrasound (ProSound α7, Aloka, Tokyo, Japan) and 7.5–10.0 MHz linear-array probe. Images were acquired 5–10 mm below the carotid bulb. The distance from the near wall to far wall lumen-intima interface was continuously traced using eTracking software and used to determine CCA diameters during systole and diastole (determined from ECG gating from a single lead modified CM5 configuration). The echo-tracking system measures diameter changes within 1/16th of an ultrasound wavelength (0.013 mm) creating a distension waveform almost identical to a pressure waveform (Van Bortel et al., 2001). Carotid stiffness was measured using the beta stiffness index () calculated as:
where P and D correspond to pressure and diameter respectively, and Max and Min refer to maximum (systolic) and minimum (diastolic) values during the cardiac cycle. These particular parameters were selected as they are more reflective of intrinsic vessel wall elastic properties and are less sensitive to changes in pressure.
Cerebral Blood Flow Velocity Pulsatility.
Middle cerebral artery (MCA) blood velocity was assessed using a 2-MHz transcranial Doppler (TCD) ultrasound probe (DWL Doppler Box-X, Compumedics, Germany) applied to the left temporal window (Purkayastha & Sorond, 2012). Cerebral blood flow velocity was measured at insonation depths of 45–60mm. The envelope of the velocity spectrum and mean velocity were calculated by a standard algorithm implemented on the instrument with use of a fast Fourier transform. MCA pulsatility index (PI) was calculated via an automated waveform tracking function using the following equation:, where is the peak systolic velocity, diastolic velocity and the mean velocity.
Cognitive Outcomes.
Neurophysiological/cognitive function was assessed using the Trail Making Test (TMT). Elevations in MCA PI may be related to deterioration in multiple domains of cognitive function (Altmann et al., 2016; Mitchell et al., 2011). The TMT is associated with several components of neurophysiological function including visual scanning, working memory, graphomotor and visuomotor processing (Llinas-Regla et al., 2017; Salthouse, 2011) and therefore provides a comprehensive measure of global cognitive function that may be impacted by cerebral pulsatility. The TMT was administered in 2 parts, Trail A and Trail B. For Trail A, participants were tasked with connecting 25 circles numbered 1–25 in numerical order as quickly as possible while maintaining full accuracy. For Trail B, participants were asked to connect 13 circles numbered 1–13 and 13 circles labeled A-L in alternating numerical/alphabetical order (i.e. 1-A-2-B-3-C etc.) (Vazzana et al., 2010). If an error was made at any point, participants were instructed to return to the circle where the error originated and continue the sequence from that point. The total time to complete each trail was recorded and served as the participants’ score. These 2 trails are considered to measure cognitive domains of processing speed, sequencing, mental flexibility and visual motor skills (Vazzana et al., 2010). TMT B-A score was calculated as the difference between times to complete Trail A and Trail B. This value was considered as a measure of cognitive flexibility independent of manual dexterity (Vazzana et al., 2010).
Results
Participant descriptive characteristics are presented in Table 1 (mean ± SD). In addition to reported characteristics in Table 1, no abnormal urinary levels of glucose, protein, enzymes, blood cells or specific gravity were detected in our sample.
Table 1.
Participant Characteristics
| Mean±SD | |
|---|---|
|
| |
| Age (years) | 47±17 |
| BMI (kg/m2) | 25.4±4.0 |
| Body Fat (%) | 24.0±9.9 |
| SBP (mmHg) | 120±10 |
| DBP (mmHg) | 76±6 |
| Fasting Glucose (mg/dL) | 91.4±10.8 |
| Total Cholesterol (mg/dL) | 183.7±34.9 |
| HDL-Cholesterol (mg/dL) | 63.9±21.8 |
| Berkman-Syme Index Score | 8±3 |
| 12-Item ISEL (max score 36) | 30±6 |
| CES-D (max score 60) | 7±5 |
| MOCA (max score 30) | 28±2 |
| Trails B-A (sec) | 22.8±15.1 |
| cfPWV (m/s) | 6.5±1.9 |
| Carotid Pulse Pressure (mmHg) | 37±8 |
| Carotid Beta Stiffness (aU) | 5.1±1.8 |
| MCA Pulsatility Index (aU) | 0.79±0.15 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HDL, high-density lipoprotein; ISEL, interpersonal support evaluation list; MOCA, Montreal Cognitive Assessment; CES-D, Center for Epidemiologic Studies Depression Scale; cfPWV, carotid-femoral pulse wave velocity; PP, pulse pressure; MCA PI, middle cerebral artery pulsatility index.
Analytic Plan
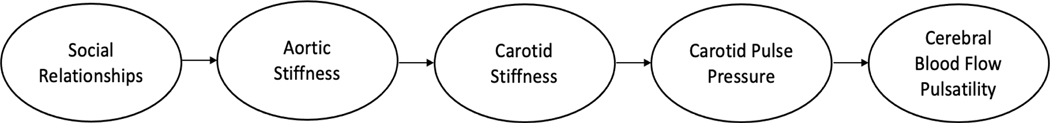
Analyses were carried out in two steps. First, we explored bivariate associations between parameters using Pearson Correlations. We then additionally assessed the association between the different measures of social relationships (i.e., dimensions of social support and social isolation) and cfPWV (a measure of aortic stiffness) using multiple linear regression to determine which, if any, dimensions of social relationships predict aortic stiffness and whether their contribution to cfPWV (i.e., the measure of aortic stiffness employed) is moderated by any known risk factors for arterial stiffness. To test for moderation in this setting, we created interaction terms between independent variables (age, sex, body fat, blood pressure appraised as mean arterial pressure, depressive symptoms appraised as CES-D and glucose) and moderator variables (isolation and appraisal) and entered terms into regression models. Primary analyses were conducted using both SAS and IBM Statistical Package for Social Sciences (SPSS, version 27, IBM, Chicago IL). Next, we used path analysis to examine whether social relationships begin a series of cerebrovascular consequences leading to increased blood flow pulsatility in the MCA. Specifically, we examined whether social relationship qualities predict aortic stiffness, which in turn predicts carotid artery stiffness, then carotid pulse pressure, and then cerebral blood flow pulsatility. See Figure 1 for the theoretical model.
Figure 1: Theoretical Model.
Theoretical model linking social relationships to arterial stiffness and hemodynamic pulsatility as a potential mechanism explaining social isolation as a risk factor for cardiovascular and cerebrovascular diseases.
Social Support and Pulse Wave Velocity
Prior to examining our model of interest, we sought to determine which dimensions of social relationships were relevant to aortic stiffness. In bivariate tests, SNI was significantly associated with cfPWV. Among the social support measures derived from the ISEL, only appraisal was significantly correlated with cfPWV. See Table 2 for the bivariate associations among primary study variables. Multiple linear regression then revealed that of our dimensions of social relationships, SNI and appraisal were the only parameters to predict cfPWV. See Table 3 for multiple linear regression results between dimensions of social support and cfPWV. Results are presented as unstandardized betas and associated 95% confidence intervals.
Table 2.
Bivariate Correlations Among Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Social Support | ||||||||||
| 1. Appraisal (ISEL) | . | |||||||||
| 2. Tangible (ISEL) | .54*** | . | ||||||||
| 3. Belonging (ISEL) | .65*** | .60*** | . | |||||||
| 4. Isolation (SNI) | .36** | .23 | .21 | . | ||||||
|
| ||||||||||
| Vascular Outcomes | ||||||||||
| 5. Pulse Wave Velocity | −.26* | −.18 | −.19 | −.36** | . | |||||
| 6. Carotid Beta Stiffness | −.079 | −.050 | −.14 | −.22 | .49*** | . | ||||
| 7. Carotid Pulse Pressure | −.31* | .042 | −.21 | −.054 | .24 | .31* | . | |||
| 8. MCA Blood Flow Pulsatility | −.27* | −.16 | −.31* | −.10 | .27* | .30 | .53*** | . | ||
|
| ||||||||||
| Cognitive Outcomes | ||||||||||
| 9. Trail A | −.26 | −.18 | −.16 | −.14 | .38** | .24 | .14 | .37*** | . | |
| 10. Trail B | −.18 | −.28* | −.27* | −.073 | .33* | .17 | −.023 | .24 | .66*** | . |
Note.
p < .001
p < .01
p < .05.
Table 3.
Social Support Dimensions as Predictors of cfPWV
| Social Support Dimension | Unstandardized Beta (95% Confidence Interval) | p value |
|---|---|---|
|
| ||
| Appraisal (ISEL) | −0.26 (−0.51 to −0.01) | .045* |
| Tangible (ISEL) | −0.17 (−0.43 to 0.08) | .168 |
| Belonging (ISEL) | −0.26 (−0.37 to 0.06) | .148 |
| Isolation (SNI) | −0.22 (−0.36 to −0.07) | .005** |
Note.
p < .001
p < .01
p < .05.
Next, we examined whether any known predictors of aortic stiffness moderated the associations between SNI and appraisal with cfPWV. The majority of the predictors of aortic stiffness that we examined failed to significantly interact with either isolation or appraisal, including age (isolation: t(54) = −0.54, p = .59; appraisal: t(54) = 0.25, p = .80), sex (isolation: t(54) = 0.64, p = .53; appraisal: t(54) = −0.16, p = .87), body fat (isolation: t(54) = 0.51, p = .48; appraisal: t(54) = −0.33, p = .74), CES-D score (isolation: t(54) = 1.62, p = .11; appraisal: t(54) = −0.15, p = .88), or MAP (isolation: t(54) = −0.65, p = .52; appraisal: t(54) = −0.51, p = .61). Glucose, however, did interact with both isolation (t(54) = −3.16, p < .01) and appraisal (t(54) = −2.39, p < .05), such that for individuals with low glucose (−1SD), the association between social relationships and cfPWV was non-significant (isolation: t = −0.87, p =.39; appraisal: t = 0.15, p = .88), whereas for those with high glucose (+1SD) the association was significantly negative (isolation: t = −5.14, p < .001; appraisal: t = −3.17, p < .01). Importantly, however, after controlling for the effect of glucose, both the association between isolation and cfPWV (t(55) = −3.96, p < .001) and between appraisal and cfPWV were significant (t(55) = −1.97, p = .054). Thus, we moved to model testing, confident that both social isolation (SNI) and the appraisal dimension of social support (from ISEL) are unique predictors of cfPWV, not impacted by known risk factors for aortic stiffness.
Model Testing
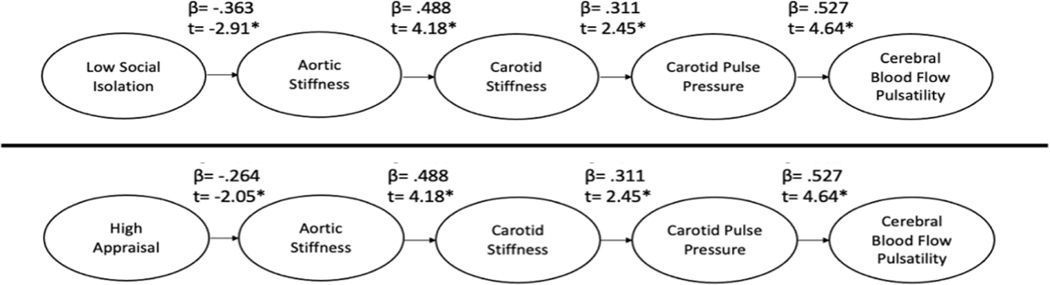
To examine the downstream consequences of social relationships, we tested the path model depicted in Figure 1 using PROC CALIS in SAS 9.4 and verified with SPSS AMOS. First, we examined how social isolation (SNI) influenced the subsequent associations. Complete results are shown in the top half of Figure 2. Results here are presented as standardized betas with associated t-statistics. Overall, the model fit the data well [Χ2(6) = 5.24, GFI = .96, CFI = 1.00, RMSEA = .00]. Next, we examined the same model, but replaced social isolation (from SNI) with appraisal support (from ISEL). Complete results can be seen in the bottom half of Figure 2. Again, the model fit the data well [Χ2(6) = 7.29, GFI = .95, CFI = .97, RMSEA = .06] suggesting that social isolation and appraisal support impact aortic stiffness, in turn influencing carotid stiffness and pulse pressure, and finally cerebral blood flow pulsatility.
Figure 2: Empirical Model.
Path analysis of downstream consequences of low social isolation and high appraisal support based on theoretical model. Note. *significant path associations
Discussion
This study primarily sought to assess the association between social support and aortic stiffness in apparently healthy adults. We then aimed to elucidate a potential direct pathway that connects psychosocial function (i.e., social isolation and appraisal support) to cardiovascular and cerebrovascular health based on associations observed within our data. The findings of the current study can be summarized as follows: 1) Measures of psychosocial function (social isolation and low appraisal support) were associated with higher aortic stiffness, measured as cfPWV. 2) There was a direct path from social isolation and low appraisal support to arterial stiffness (aortic then carotid artery), followed by hemodynamic pulsatility in the carotid artery and middle cerebral artery, respectively. Collectively, our findings suggest that psychosocial factors may elevate CVD risk through effects on central cardiovascular and cerebrovascular hemodynamics.
Types of Social Support
The psychological systems in place to assess social interaction and identify social pain (“threat-detection systems”) are contributors to both cardiovascular health and cerebrovascular function, suggesting a physiological interplay between the 3 elements (White et al., 2015). Of particular importance to understanding these relationships may be to examine whether social relationships provide support, indicating the capacity to reduce stress and are sufficiently present as to not cause social pain (feelings of interpersonal rejection or loss) (MacDonald & Jensen-Campbell, 2011). This concept is reiterated in a recent review, referring to the impact of social relationships as a “double-edged sword”- contributing both beneficial and deleterious effects to mental and physical health (Song et al., 2021).
Research suggests appraisal support may have a more positive influence on cardiovascular health compared to other forms of support (Bowen et al., 2013). For example, individuals can always benefit from useful information that may help with coping of habitual stressful life situations. Conversely, individuals may only benefit from tangible support during an acute stressful situation that requires that particular form of support (i.e., material aid to change a flat tire). We found evidence corroborating this notion in the current study, in which appraisal predicted aortic stiffness whilst other forms of support did not. Similarly, higher appraisal (informational) support was previously identified as a significant predictor of lower ambulatory systolic blood pressure only (Bowen et al., 2013) relating directly to the arterial measures in the current study. Likewise, informational support was reported as the most consistent “stress-buffering predictor” of ambulatory blood pressure, corresponding with both ambulatory systolic and diastolic blood pressure measurement in another study (Bowen et al., 2014). Furthermore, appraisal may be the most accessible form of social support, and therefore the most important to consider.
Mechanisms
Despite mounting evidence reinforcing the absence of positive social relationships as a CVD risk factor (Alcaraz et al., 2019; Valtorta et al., 2016), psychosocial factors are not routinely considered in clinical practice when assessing CVD risk in asymptomatic patients, in accordance with current American Heart Association guidelines (Manzoni et al., 2011). As previously discussed, it may be more important to understand the quality of social support (i.e., the ability for one’s social support to mitigate stress) rather than the quantity of social contacts when considering effects on CVD risk. One with low social support conceivably does not have the ability to moderate stressful situations, resulting in a chronic state of arousal and stress. The sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis are responsible for the physiological changes associated with the stress response. SNS activation elicits a well-known increase in hemodynamic load, while the HPA axis primarily functions to release glucocorticoids such as cortisol (and further activation of the SNS) (Xia & Li, 2018). Overactivation of the HPA axis during prolonged stress therefore results in autonomic imbalances in favor of sympathetic tone and elevated heart rate, ultimately exacerbating vasoconstriction and subsequent increases in blood pressure. Indeed individuals with low social support have been shown to have exaggerated pressor responses to psychosocial stressors with a delay in recovery owing to augmented sympatho-excitation (Glynn et al., 1999; Grant et al., 2009; Nausheen et al., 2007; O’Donovan & Hughes, 2007). Increases in blood pressure paired with increased peripheral resistance intensify hemodynamic pulsatility and over time results in vascular fatigue-fracture, contributing to a detrimental remodeling response by the vessel via arterial stiffening (Spartano et al., 2014). Moreover, these hemodynamic alterations contribute to a chronically altered redox balance in favor of a pro-inflammatory state and further activation of the SNS/HPA axis (Palombo & Kozakova, 2016; White et al., 2015). Social relationships may provide vascular benefit via their roles as stress-buffers (Cohen & Wills, 1985; Thoits, 2011). Individuals with adequate social support may perceive taxing life events as less stressful, reducing physiological arousal and overall allostatic load.
Our findings indicate that SNI as a measure of social isolation and low appraisal support were significantly associated with aortic stiffness independent of other potential confounders such as age, sex, body fat, and blood pressure. The sole predictor of aortic stiffness that remained in our analysis was blood glucose, such that low blood glucose deemed the associations between social isolation/appraisal support and cfPWV nonsignificant, and high blood glucose strengthened said significant association. This is foreseeable as glucose plays a notable role in the glycation process that occurs during vascular remodeling and arterial stiffening (Palombo & Kozakova, 2016), and therefore has a direct influence on this development. Additionally, overactivation of the HPA axis results in excessive glucocorticoid release, which has been reported to be linked to increased insulin resistance and a chronic inflammatory state, both contributing to the vascular remodeling process (Palombo & Kozakova, 2016; Xia & Li, 2018).
A risk factor of note that failed to interact with either association observed was depressive symptomology. Depression is closely related to psychosocial measures such as social isolation and loneliness, posing as risk factors for both cardiovascular and cerebrovascular events (Bhatti & Haq, 2017; Xia & Li, 2018). While closely related, depressive symptomology is a common symptom of both social isolation and loneliness, but one does not necessarily indicate the other (Matthews et al., 2016). Individuals can be socially isolated and depressed but not lonely, and vice versa. As we know that depressive symptomology interacts closely with many of the psychosocial and physiological factors discussed above, it is important to note the distinction that is being implicated by our findings. Despite its integral role in psychosocial and physiological function, depressive symptomology may play only an episodic role rather than a chronic one. A previous study examining depression and coronary artery disease severity showed that “chronic” factors such as low social support may be more important than unsustained “episodic” factors such as depressive symptomology when discussing cardiovascular health (Kop et al., 2005). These findings may be echoed in the current study, as depressive symptomology was assessed using the CES-D, as a measure of current (episodic) symptomology rather than chronic depression and failed to interact with aortic stiffness.
It is important to underscore that this study did not assess the construct of loneliness, and this is a notable limitation. Social support, social isolation and loneliness are all interconnected and partly overlapping phenomena. While these measures are closely related, they are conceptually different. Social isolation is an objective measure of lack of social connection or relationships (Xia & Li, 2018). In contrast, loneliness is defined as a discrepancy between desired and actual social relationships (Xia & Li, 2018) and acts as a psychological manifestation of social isolation (Yanguas et al., 2018). Both of these factors play an important role in cardiovascular health, as they are common sources of chronic stress in adults (Li & Xia, 2020; Steptoe & Kivimäki, 2013; Xia & Li, 2018). While both social isolation and loneliness have displayed strong relationships with CVD outcomes (Brown et al., 2019; White et al., 2015), a recent review suggests that loneliness is more researched as a potential risk factor for CVD compared to social isolation (Courtin & Knapp, 2017). As such, we chose to focus our investigation on social isolation. More research regarding social isolation and loneliness is necessary to truly compare the impact of each psychosocial factor on CVD risk.
Cerebrovascular Impact
Based on our path analysis, social isolation and low social support may trigger a series of cerebrovascular consequences, beginning with aortic stiffness and ending with cerebral blood flow pulsatility, and this may have implications for cognitive health. More specifically, we observed a direct path from social isolation/appraisal support to arterial stiffness (aortic then carotid), and subsequently to cerebral blood flow pulsatility. This is important to note as large-artery stiffness mediated increases in MCA blood flow pulsatility are associated with detrimental cerebrovascular remodeling and reduced cognitive function (Heffernan et al., 2018). Indeed, in our study we noted associations between various measures of arterial stiffness and cerebrovascular pulsatility and cognitive function measured using the Trails A/B tests (Table 2). From a mechanistic standpoint, it appears that the increase in hemodynamic pulsatility associated with SNS and HPA axis activation, chronic inflammation and subsequent vascular remodeling that occurs, ultimately may result in negative alterations to blood flow delivery, increased ischemia susceptibility, and target organ damage at the brain (Palombo & Kozakova, 2016). This upholds previous findings where loneliness was associated with cognitive deterioration, degree of impairment, and progression of Alzheimer’s (Raz et al., 2016; Wilson et al., 2007; Yanguas et al., 2018) akin to findings with social isolation and similar negative cognitive outcomes (White et al., 2015).
The findings of the current study and previous investigations collectively suggest that lack of social support may increase cerebrovascular risk and incidence of poor cognitive outcomes. An implication of this observation is that social support may be a novel therapeutic strategy to consider for both prevention and rehabilitation of CVDs. Higher social support has been shown to be related to decelerated cognitive decline and lowering risk for adverse cognitive events (Grothe et al., 2021; Read et al., 2020; Salinas et al., 2021; Seeman et al., 2001), potentially explicated by the psychosocial-vascular pathway elucidated in the current study. Improving social support may be a viable therapeutic strategy for abetting healthy vascular aging and preserving cognitive function. Similarly, psychosocial interventions may be successful in improving vascular risk and cognitive function in rehabilitation settings. Those with higher social support have demonstrated better recovery responses following cognitive decline/injury (Elloker & Rhoda, 2018), suggesting psychosocial factors could be considered in clinical settings. Furthermore, family-supported interventions have been shown to be significantly more successful than clinician-derived regimens, suggesting perception of social support (i.e., appraisal) may be an important tool for improving recovery in individuals with a history cognitive injury (Braga et al., 2005).
Limitations/Implications
We do acknowledge that there are additional limitations and considerations to the current study. Our secondary path analyses assume a direct, unidirectional interaction between our study variables and did not account for potential multidirectional interplay between these variables. For example, cerebrovascular damage caused by arterial stiffness may contribute to mental health status (notably depression (Van Sloten et al., 2016)), which may affect appraisal of social support. Nonetheless, we did control for depressive symptomology in our study and are confident in the hypothesized direction of our path. Likewise, there is evidence to suggest that apathy and related disengagement from social relationships is associated with cerebrovascular damage as per the vascular apathy hypothesis, however the true interplay and direction of this pathway requires further investigation (Wouts et al., 2020). Furthermore, as vascular remodeling occurs with aging, our large age range (18–85 years) may influence some of our vascular/hemodynamic outcomes. We did control for age in our analysis and with the noted failed interaction, posit that age did not significantly affect the found associations between psychosocial function and arterial stiffening. Social support may be important for CVD protection across the lifespan irrespective of age. Finally, it is important to note that this study is observational in design, therefore we cannot claim causality of our relationships. More research will be needed to understand how changes in social relationships over time affect the dynamic vascular aging process.
All data collected herein were completed prior to the onset of the COVID-19 pandemic. It is well established that lockdowns initiated as a countermeasure to halt spread of COVID-19 had profound effects on social relationships and mental health. More specifically, restriction of in-person social contact during COVID-19 has decreased the quality of the already reduced number of social interactions and can result in increased feelings of loneliness (Gronewold & Engels, 2022). Furthermore, prevalence estimates of psychosocial factors such as social isolation and loneliness were significantly higher in individuals three months or more into pandemic living compared to at the start of the pandemic (Su et al., 2022), suggesting extended lockdown durations may further impact presence of detrimental psychosocial outcomes. For individuals recovering from COVID-19, isolated social conditions may compound cerebrovascular and cognitive damage that is inherent to COVID-19 pathophysiology (Pai & Vella, 2022). Albeit speculative, our findings may suggest that social isolation experienced during the pandemic may have had a detrimental effect on vascular aging and in turn cerebrovascular health outcomes.
Conclusion
Social isolation and low appraisal social support are significant predictors of higher aortic stiffness, as denoted by increased cfPWV. Our data suggest social isolation and low appraisal social support may elicit unfavorable changes in cardiovascular and cerebrovascular function through arterial stiffness and hemodynamic pulsatility. These data support a direct path through which indicators of social function may alter vascular aging to influence CVD risk.
Source of Funding
This study was funded by NIH NIA P30 AG0344645 05 (Project Director Heffernan; PI Wolf).
Footnotes
Conflicts of Interest: All authors of the present article declare no conflicts of interest.
References
- Alcaraz KI, Eddens KS, Blase JL, Diver WR, Patel AV, Teras LR, Stevens VL, Jacobs EJ, & Gapstur SM (2019). Social Isolation and Mortality in US Black and White Men and Women. American Journal of Epidemiology. 10.1093/aje/kwy231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann M, Thommessen B, Rønning OM, Benth JŠ, Reichenbach AS, & Fure B. (2016). Middle Cerebral Artery Pulsatility Index is Associated with Cognitive Impairment in Lacunar Stroke. Journal of Neuroimaging. 10.1111/jon.12335 [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, … Wilkinson IB (2014). Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology. 10.1016/j.jacc.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, & Breslow L. (1983). Health and Ways on Living; The Alameda County Study. Oxford University Press. [Google Scholar]
- Bhatti AB, & Haq A. ul. (2017). The Pathophysiology of Perceived Social Isolation: Effects on Health and Mortality. Cureus. 10.7759/cureus.994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonso E, Dorigatti F, & Palatini P. (2010). Validation of Panasonic EW3106 and EW3109 devices for blood pressure measurement according to the International Protocol. Blood Pressure Monitoring. 10.1097/MBP.0b013e32833531b3 [DOI] [PubMed] [Google Scholar]
- Bowen KS, Birmingham W, Uchino BN, Carlisle M, Smith TW, & Light KC (2013). Specific dimensions of perceived support and ambulatory blood pressure: Which support functions appear most beneficial and for whom? International Journal of Psychophysiology. 10.1016/j.ijpsycho.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen KS, Uchino BN, Birmingham W, Carlisle M, Smith TW, & Light KC (2014). The stress-buffering effects of functional social support on ambulatory blood pressure. Health Psychology. 10.1037/hea0000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga LW, Da Paz AC, & Ylvisaker M. (2005). Direct clinician-delivered versus indirect family-supported rehabilitation of children with traumatic brain injury: A randomized controlled trial. Brain Injury. 10.1080/02699050500110165 [DOI] [PubMed] [Google Scholar]
- Brinkhues S, Dukers-Muijrers NHTM, Hoebe CJPA, Van Der Kallen CJH, Dagnelie PC, Koster A, Henry RMA, Sep SJS, Schaper NC, Stehouwer CDA, Bosma H, Savelkoul PHM, & Schram MT (2017). Socially isolated individuals are more prone to have newly diagnosed and prevalent type 2 diabetes mellitus - The Maastricht study - The M. BMC Public Health. 10.1186/s12889-017-4948-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EG, Creaven AM, & Gallagher S. (2019). Loneliness and cardiovascular reactivity to acute stress in younger adults. International Journal of Psychophysiology. 10.1016/j.ijpsycho.2018.07.471 [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, & Cacioppo S. (2014). Older adults reporting social isolation or loneliness show poorer cognitive function 4 years later. In Evidence-Based Nursing. 10.1136/eb-2013-101379 [DOI] [PubMed] [Google Scholar]
- Carey M, Markham C, Gaffney P, Boran G, & Maher V. (2006). Validation of a point of care lipid analyser using a hospital based reference laboratory. Irish Journal of Medical Science. 10.1007/BF03167964 [DOI] [PubMed] [Google Scholar]
- Chen CH, Ting CT, Nussbacher A, Nevo E, Kass DA, Pak P, Wang SP, Chang MS, & Yin FCP (1996). Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension. 10.1161/01.HYP.27.2.168 [DOI] [PubMed] [Google Scholar]
- Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Roy J, Pan Q, Go AS, & Townsend RR (2014). Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circulation: Heart Failure. 10.1161/CIRCHEARTFAILURE.113.001041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, & Wills TA (1985). Stress, Social Support, and the Buffering Hypothesis. In Psychological Bulletin. 10.1037/0033-2909.98.2.310 [DOI] [PubMed] [Google Scholar]
- Courtin E, & Knapp M. (2017). Social isolation, loneliness and health in old age: a scoping review. In Health and Social Care in the Community. 10.1111/hsc.12311 [DOI] [PubMed] [Google Scholar]
- Elloker T, & Rhoda AJ (2018). The relationship between social support and participation in stroke: A systematic review. In African Journal of Disability. 10.4102/ajod.v7i0.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng PM, Rimm EB, Fitzmaurice G, & Kawachi I. (2002). Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. American Journal of Epidemiology. 10.1093/aje/155.8.700 [DOI] [PubMed] [Google Scholar]
- Giannarelli C, Bianchini E, Bruno RM, Magagna A, Landini L, Faita F, Gemignani V, Penno G, Taddei S, & Ghiadoni L. (2012). Local carotid stiffness and intima-media thickness assessment by a novel ultrasound-based system in essential hypertension. Atherosclerosis. 10.1016/j.atherosclerosis.2012.05.027 [DOI] [PubMed] [Google Scholar]
- Glynn LM, Christenfeld N, & Gerin W. (1999). Gender, social support, and cardiovascular responses to stress. Psychosomatic Medicine. 10.1097/00006842-199903000-00016 [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, … Turner MB (2014a). Executive summary: Heart Disease and Stroke Statistics - 2014 Update: A report from the American Heart Association. In Circulation. 10.1161/01.cir.0000442015.53336.12 [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, … Turner MB (2014b). Heart Disease and Stroke Statistics - 2014 Update: A report from the American Heart Association. In Circulation. 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant N, Hamer M, & Steptoe A. (2009). Social isolation and stress-related cardiovascular, lipid, and cortisol responses. Annals of Behavioral Medicine. 10.1007/s12160-009-9081-z [DOI] [PubMed] [Google Scholar]
- Gronewold J, & Engels M. (2022). The Lonely Brain – Associations Between Social Isolation and (Cerebro-) Vascular Disease From the Perspective of Social Neuroscience. Frontiers in Integrative Neuroscience. 10.3389/fnint.2022.729621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe J, Röhr S, Luppa M, Scherer M, Weyerer S, König H-H, Wagner M, & Riedel-Heller S. (2021). Social isolation and incident dementia in the oldest-old – A competing risk analysis. Das Soziale in Medizin Und Gesellschaft – Aktuelle Megatrends Fordern Uns Heraus 56. Jahrestagung Der Deutschen Gesellschaft Für Sozialmedizin Und Prävention (DGSMP). 10.1055/s-0041-1732118 [DOI] [Google Scholar]
- Heffernan KS, Augustine JA, Lefferts WK, Spartano NL, Hughes WE, Jorgensen RS, & Gump BB (2018). Arterial stiffness and cerebral hemodynamic pulsatility during cognitive engagement in younger and older adults. Experimental Gerontology, 101, 54–62. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, & Layton JB (2010). Social relationships and mortality risk: A meta-analytic review. In PLoS Medicine. 10.1371/journal.pmed.1000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsten M, Mittleman MA, Wamala SP, Schenck-Gustafsson K, & Orth-Gomér K. (2000). Depressive symptoms and lack of social integration in relation to prognosis of CHD in middle-aged women. The Stockholm Female Coronary Risk Study. European Heart Journal. 10.1053/euhj.1999.2012 [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, & Umberson D. (1988). Social relationships and health. Science. 10.1126/science.3399889 [DOI] [PubMed] [Google Scholar]
- Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, & Mitchell GF (2012). Aortic stiffness, blood pressure progression, and incident hypertension. JAMA - Journal of the American Medical Association. 10.1001/2012.jama.10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Colditz GA, Ascherio A, Rimm EB, Giovannucci E, Stampfer MJ, & Willett WC (1996). A prospective study of social networks in relation to total mortality and cardiovascular disease in men in the USA. Journal of Epidemiology and Community Health. 10.1136/jech.50.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kop WJ, Berman DS, Gransar H, Wong ND, Miranda-Peats R, White MD, Shin M, Bruce M, Krantz DS, & Rozanski A. (2005). Social network and coronary artery calcification in asymptomatic individuals. Psychosomatic Medicine. 10.1097/01.psy.0000161201.45643.8d [DOI] [PubMed] [Google Scholar]
- Lakatta EG, & Levy D. (2003a). Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. In Circulation. 10.1161/01.CIR.0000048892.83521.58 [DOI] [PubMed] [Google Scholar]
- Lakatta EG, & Levy D. (2003b). Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. In Circulation. 10.1161/01.CIR.0000048893.62841.F7 [DOI] [PubMed] [Google Scholar]
- Levine GN, Cohen BE, Commodore-Mensah Y, Fleury J, Huffman JC, Khalid U, Labarthe DR, Lavretsky H, Michos ED, Spatz ES, & Kubzansky LD (2021). Psychological Health, Well-Being, and the Mind-Heart-Body Connection: A Scientific Statement from the American Heart Association. In Circulation. 10.1161/CIR.0000000000000947 [DOI] [PubMed] [Google Scholar]
- Li H, & Xia N. (2020). The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. In Redox Biology. 10.1016/j.redox.2020.101585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas-Regla J, Vilalta-Franch J, Lopez-Pousa S, Calvo-Perxas L, Torrents Rodas D, & Garre-Olmo J. (2017). The Trail Making Test. Assesment, 24(2), 183–196. [DOI] [PubMed] [Google Scholar]
- MacDonald G, & Jensen-Campbell LA (2011). Social pain: Neuropsychological and health implications of loss and exclusion. [Google Scholar]
- Madero M, Peralta C, Katz R, Canada R, Fried L, Najjar S, Shlipak M, Simonsick E, Lakatta E, Patel K, Rifkin D, Hawkins M, Newman A, & Sarnak M. (2013). Association of arterial rigidity with incident kidney disease and kidney function decline: The health ABC study. Clinical Journal of the American Society of Nephrology. 10.2215/CJN.07900812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni GM, Castelnuovo G, & Proietti R. (2011). Assessment of psychosocial risk factors is missing in the 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults. In Journal of the American College of Cardiology. 10.1016/j.jacc.2010.12.015 [DOI] [PubMed] [Google Scholar]
- Matthews T, Danese A, Wertz J, Odgers CL, Ambler A, Moffitt TE, & Arseneault L. (2016). Social isolation, loneliness and depression in young adulthood: a behavioural genetic analysis. Social Psychiatry and Psychiatric Epidemiology. 10.1007/s00127-016-1178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF (2008). Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. In Journal of Applied Physiology. 10.1152/japplphysiol.90549.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, & Benjamin EJ (2010). Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, & Launer LJ (2011). Arterial stiffness, pressure and flow pulsatility and brain structure and function: The Age, Gene/Environment Susceptibility-Reykjavik Study. Brain. 10.1093/brain/awr253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausheen B, Gidron Y, Gregg A, Tissarchondou HS, & Peveler R. (2007). Loneliness, social support and cardiovascular reactivity to laboratory stress. Stress. 10.1080/10253890601135434 [DOI] [PubMed] [Google Scholar]
- O’Donovan A, & Hughes B. (2007). Social support and loneliness in college students: Effects on pulse pressure reactivity to acute stress. International Journal of Adolescent Medicine and Health. 10.1515/IJAMH.2007.19.4.523 [DOI] [PubMed] [Google Scholar]
- Pai N, & Vella S-L (2022). The physical and mental health consequences of social isolation and loneliness in the context of COVID-19. Current Opinion in Psychiatry, 35(5), 305–310. [DOI] [PubMed] [Google Scholar]
- Palombo C, & Kozakova M. (2016). Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. In Vascular Pharmacology. 10.1016/j.vph.2015.11.083 [DOI] [PubMed] [Google Scholar]
- Pantell M, Rehkopf D, Jutte D, Syme SL, Balmes J, & Adler N. (2013). Social isolation: A predictor of mortality comparable to traditional clinical risk factors. American Journal of Public Health. 10.2105/AJPH.2013.301261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne TJ, Andrew M, Butler KR, Wyatt SB, Dubbert PM, & Mosley TH (2012). Psychometric evaluation of the interpersonal support evaluation list-short form in the ARIC study cohort. SAGE Open. 10.1177/2158244012461923 [DOI] [Google Scholar]
- Purkayastha S, & Sorond F. (2012). Transcranial doppler ultrasound: Technique and application. Seminars in Neurology. 10.1055/s-0032-1331812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz L, Knoefel J, & Bhaskar K. (2016). The neuropathology and cerebrovascular mechanisms of dementia. In Journal of Cerebral Blood Flow and Metabolism. 10.1038/jcbfm.2015.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S, Comas-Herrera A, & Grundy E. (2020). Social Isolation and Memory Decline in Later-life. Journals of Gerontology - Series B Psychological Sciences and Social Sciences. 10.1093/geronb/gbz152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CJ, Elkind MSV, Clemow L, Jin Z, Di Tullio M, Sacco RL, Homma S, & Boden-Albala B. (2011). Association between social isolation and left ventricular mass. American Journal of Medicine. 10.1016/j.amjmed.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, & Devereux RB (2000). Impact of arterial stiffening on left ventricular structure. Hypertension. 10.1161/01.HYP.36.4.489 [DOI] [PubMed] [Google Scholar]
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed W, Blackett KN, Sitthi-Amorn C, Sato H, & Yusuf S. (2004). Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet, 364, 953–962. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, & Kaplan J. (1999). Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 10.1161/01.CIR.99.16.2192 [DOI] [PubMed] [Google Scholar]
- Salinas J, O’Donnell A, Kojis DJ, Pase MP, Decarli C, Rentz DM, Berkman LF, Beiser A, & Seshadri S. (2021). Association of Social Support with Brain Volume and Cognition. JAMA Network Open. 10.1001/jamanetworkopen.2021.21122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2011). What cognitive abilities are involved in trail-making performance? Intelligence. 10.1016/j.intell.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat S, Dawkins Arce FG, Verwoert GC, Hofman A, Ikram MA, Franco OH, Dehghan A, Witteman JCM, & Mattace-Raso F. (2014). Association of renal function with vascular stiffness in older adults: the Rotterdam study. Age and Ageing. 10.1093/ageing/afu111 [DOI] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, & Berkman L. (2001). Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychology. 10.1037/0278-6133.20.4.243 [DOI] [PubMed] [Google Scholar]
- Shankar A, Hamer M, McMunn A, & Steptoe A. (2013). Social isolation and loneliness: Relationships with cognitive function during 4 years of follow-up in the English longitudinal study of ageing. Psychosomatic Medicine. 10.1097/PSY.0b013e31827f09cd [DOI] [PubMed] [Google Scholar]
- Song L, Pettis PJ, Chen Y, & Goodson-Miller M. (2021). Social Cost and Health: The Downside of Social Relationships and Social Networks. Journal of Health and Social Behavior, 1–17. [DOI] [PubMed] [Google Scholar]
- Spartano NL, Augustine JA, Lefferts WK, Gump BB, & Heffernan KS (2014). The relationship between carotid blood pressure reactivity to mental stress and carotid intima-media thickness. Atherosclerosis. 10.1016/j.atherosclerosis.2014.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A. (2000). Stress, social support and cardiovascular activity over the working day. International Journal of Psychophysiology. 10.1016/S0167-8760(00)00109-4 [DOI] [PubMed] [Google Scholar]
- Steptoe A, & Kivimäki M. (2013). Stress and cardiovascular disease: An update on current knowledge. In Annual Review of Public Health. 10.1146/annurev-publhealth-031912-114452 [DOI] [PubMed] [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, & Wardle J. (2013). Social isolation, loneliness, and all-cause mortality in older men and women. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1219686110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Rao W, Muzi L, Caron G, D’Arcy C, & Meng X. (2022). Prevalence of loneliness and social isolation among older adults during the COVID-19 pandemic: A systematic review and meta-analysis. International Psychogeriatrics, 1–13. [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, & Newman A. (2005). Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 10.1161/CIRCULATIONAHA.104.483628 [DOI] [PubMed] [Google Scholar]
- Thoits PA (2011). Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior. 10.1177/0022146510395592 [DOI] [PubMed] [Google Scholar]
- Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, & Weber T. (2015). Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement from the American Heart Association. Hypertension. 10.1161/HYP.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtorta NK, Kanaan M, Gilbody S, & Hanratty B. (2018). Loneliness, social isolation and risk of cardiovascular disease in the English Longitudinal Study of Ageing. European Journal of Preventive Cardiology. 10.1177/2047487318792696 [DOI] [PubMed] [Google Scholar]
- Valtorta NK, Kanaan M, Gilbody S, Ronzi S, & Hanratty B. (2016). Loneliness and social isolation as risk factors for coronary heart disease and stroke: Systematic review and meta-analysis of longitudinal observational studies. In Heart. 10.1136/heartjnl-2015-308790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bortel LM, Balkestein EJ, Van Der Heijden-Spek JJ, Vanmolkot FH, Staessen JA, Kragten JA, Vredeveld JW, Safar ME, Boudier HAS, & Hoeks AP (2001). Non-invasive assessment of local arterial pulse pressure: Comparison of applanation tonometry and echo-tracking. Journal of Hypertension. 10.1097/00004872-200106000-00007 [DOI] [PubMed] [Google Scholar]
- Van Sloten TT, Mitchell GF, Sigurdsson S, Van Buchem MA, Jonsson PV, Garcia ME, Harris TB, Henry RMA, Levey AS, Stehouwer CDA, Gudnason V, & Launer LJ (2016). Associations between arterial stiffness, depressive symptoms and cerebral small vessel disease: cross-sectional findings from the AGES-Reykjavik Study. J Psychiatry Neuroscience, 41(3), 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazzana R, Bandinelli S, Lauretani F, Volpato S, Lauretani F, Di Iorio A, Abate M, Corsi AM, Milaneschi Y, Guralnik JM, & Ferrucci L. (2010). Trail making test predicts physical impairment and mortality in older persons. Journal of the American Geriatrics Society. 10.1111/j.1532-5415.2010.02780.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, & Stefanadis C. (2010). Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. European Heart Journal. 10.1093/eurheartj/ehq024 [DOI] [PubMed] [Google Scholar]
- White CN, VanderDrift LE, & Heffernan KS (2015). Social isolation, cognitive decline, and cardiovascular disease risk. Current Opinion in Psychology, 5, 18–23. [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, Tang Y, & Bennett DA (2007). Loneliness and risk of Alzheimer disease. Archives of General Psychiatry. 10.1001/archpsyc.64.2.234 [DOI] [PubMed] [Google Scholar]
- Wouts L, Van Kessel M, Beekman ATF, Marijinissen RM, & Oude Voshaar RC (2020). Empirical support for the vascular apathy hypothesis: A structured review. International Journal of Geriatric Psychiatry, 35, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia N, & Li H. (2018). Loneliness, Social Isolation, and Cardiovascular Health. In Antioxidants and Redox Signaling. 10.1089/ars.2017.7312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Boen C, & Mullan Harris K. (2015). Social relationships and hypertension in late life: Evidence from a nationally representative longitudinal study of older adults. Journal of Aging and Health. 10.1177/0898264314551172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, McClintock MK, Kozloski M, & Li T. (2013). Social Isolation and Adult Mortality: The Role of Chronic Inflammation and Sex Differences. Journal of Health and Social Behavior. 10.1177/0022146513485244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanguas J, Pinazo-Henandis S, & Tarazona-Santabalbina FJ (2018). The Compleixty of Loneliness. Acta Biomedica, 89, 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]