Abstract
Youth antisocial behavior (AB) is associated with deficits in socioemotional processing, reward and threat processing and executive functioning. These deficits are thought to emerge from differences in neural structure, functioning and connectivity, particularly within the default, salience and frontoparietal networks. However, the relationship between AB and the organization of these networks remains unclear. To address this gap, the current study applied unweighted, undirected graph analyses to resting-state functional magnetic resonance imaging data in a cohort of 161 adolescents (95 female) enriched for exposure to poverty, a risk factor for AB. As prior work indicates that callous-unemotional (CU) traits may moderate the neurocognitive profile of youth AB, we examined CU traits as a moderator. Using multi-informant latent factors, AB was found to be associated with less efficient frontoparietal network topology, a network associated with executive functioning. However, this effect was limited to youth at low or mean levels of CU traits, indicating that these neural differences were specific to those high on AB but not CU traits. Neither AB, CU traits nor their interaction was significantly related to default or salience network topologies. Results suggest that AB, specifically, may be linked with shifts in the architecture of the frontoparietal network.
Keywords: youth antisocial behavior, callous-unemotional traits, frontoparietal network, resting-state fMRI, graph analysis
Youth antisocial behavior (AB) encompasses a broad spectrum of behaviors (e.g. aggression and violence) and imposes significant economic, emotional and health burdens to victims and their families, as well as to those engaging in these behaviors, their families and society at large (Rivenbark et al., 2018; Roberts et al., 2018). Moreover, youth AB is a major risk factor for substance use disorders, major depressive episodes, poor physical health outcomes and continuing engagement in AB into adulthood (Laub and Vaillant, 2000; Simonoff et al., 2004; McGue and Iacono, 2005; Colman et al., 2009). Unfortunately, AB during adolescence is quite common (Moffitt, 1993), underscoring the public health priority to understanding the etiology of AB.
Youth AB and neurocognitive functioning
Youth AB has been consistently associated with deficits across several domains of neurocognitive functioning, including socioemotional processing, reward processing and executive functioning (see Blair et al., 2014 for review). For example, youth with high rates of AB have difficulties identifying others’ emotions (see Hawes and Dadds, 2012; Tillem et al., 2020; Chang et al., 2021 for reviews), may view ambiguous social cues as threatening (see Martinelli et al., 2018 for review) and show differences in neural activity in the medial prefrontal cortex, precuneus and amygdala during socioemotional processing tasks (Dalwani et al., 2014; Zhou et al., 2016; Dotterer et al., 2017). These youth also perseverate on previously rewarding behavioral patterns (see Estrada et al., 2018 for review) and show blunted neural responses in the anterior insula, anterior cingulate cortex and caudate during reward processing tasks (White et al., 2013, 2014). Finally, youth AB is related to differences in decision-making, sustained attention and response inhibition, particularly when under stress (Fairchild et al., 2009; Hobson et al., 2011; Schoorl et al., 2018), with reduced neural responses in the dorsolateral prefrontal cortex during executive functioning tasks (Rubia et al., 2009; Crowley et al., 2010). Moreover, AB (including symptoms of conduct disorder and oppositional defiant disorder (ODD), as well as lower-level rule breaking and aggression) is part of the externalizing meta-factor, which is marked by inhibitory control deficits (Iacono et al., 2008).
Though prior research has identified structural and functional differences in several discrete brain regions related to these behavioral differences, work in basic neuroscience has highlighted that complex behaviors are supported by the coordination of large-scale neural networks, rather than activity in single regions of interest (ROIs) (Meehan and Bressler, 2012). Specifically, the default, salience and frontoparietal networks are thought to be critical to understanding neurocognitive functioning and complex behaviors across mental health diagnoses, generally (Menon, 2011), and youth AB, specifically (Cohn et al., 2015). Furthermore, these networks may be of particular importance to youth AB as they are believed to primarily support socioemotional functioning (e.g. interpreting other agents’ affective cues; Spreng and Grady, 2010; Jack et al., 2013), salience processing (e.g. fixating on potentially rewarding and/or threatening stimuli; Uddin, 2016) and executive functioning (e.g. response inhibition; Marek and Dosenbach, 2018). Not surprisingly, these three networks contain many of the neural structures previously associated with youth AB, including the medial prefrontal cortex and precuneus (Dalwani et al., 2014; Zhou et al., 2016), the anterior insula and anterior cingulate cortex (White et al., 2013, 2014) and the dorsolateral prefrontal cortex (Rubia et al., 2009; Crowley et al., 2010). However, to properly capture these complex neural networks, it is important to understand the overall organization and functioning of these networks as a whole, rather than simply examining specific structures or subsets of connections within these networks (Reijneveld et al., 2007; Stam and Reijneveld, 2007; Bullmore and Sporns, 2009).
Graph analytic techniques provide a way to examine the higher-level organization of distributed neural networks. By computing the overall organization, or topology, of neural networks, graph analysis can provide quantifiable metrics for the ‘optimality’ (e.g. efficiency and robustness) of neural information processing throughout a neural network or the brain as a whole. For example, graph analysis can calculate the global efficiency of a network, delineating the resources necessary for information to be communicated and integrated throughout a network. Similarly, graph analysis can provide a clustering metric, which taps the degree to which the functioning of a neural network may be robust to disruptions (e.g. damage or overload; Reijneveld et al., 2007; Stam and Reijneveld, 2007; Bullmore and Sporns, 2009). These neuro-topological features play critical roles in neurocognitive functioning, with more efficient and/or robust neural network topologies supporting positive cognitive outcomes (e.g. higher IQ; Langer et al., 2012; Suprano et al., 2019). Similarly, less efficient or robust network organization has been linked to psychopathology (e.g. schizophrenia, autism; Itahashi et al., 2014; Yang et al., 2020). However, prior research applying graph analytic methods in youth AB has been limited.
Youth AB and neural topology
Thus far, the few existing studies using graph analysis to explore functional neural topology in youth AB have yielded conflicting results. Two case–control studies of conduct disorder, a developmental disorder characterized by persistent engagement in AB during childhood or adolescence, found that conduct disorder was linked with less efficiently organized neural communication throughout the entire brain, but found no differences in global clustering (an indication of functional segregation and robustness; Jiang et al., 2016, 2021). In contrast, a recent study found that, in a large representative cohort, conduct disorder symptomatology was related to enhanced global clustering but was not related to differences in global efficiency (Tillem et al., 2021). Finally, a third case–control study found no relationship between conduct disorder and differences in either global efficiency or global clustering (Lu et al., 2017).
Though these studies suggest that AB, at least as measured narrowly by conduct disorder symptoms, may be related to differences in global neural topology, they are limited in several ways. First, these studies targeted neural topology globally, that is, across all the networks in the brain, even though prior empirical and theoretical work suggest only certain networks are likely to be affected in youth AB. That is, no prior studies have examined the neural topology of the default, salience or frontoparietal networks despite their theoretical and empirical relevance to youth AB (Dalwani et al., 2011, 2014; Cohn et al., 2015; Zhou et al., 2016; Sethi et al., 2018; Waller et al., 2020).
Second, with one exception (Tillem et al., 2021), prior research has exclusively used case–control studies with small samples with conduct disorder. However, evidence continues to accumulate that AB occurs on a continuum ranging from relatively normative levels of rule breaking and defiance to more extreme behaviors such as violence and aggression (Patrick et al., 2002; Krueger et al., 2007). Accordingly, research using dimensional methods that capture the entire spectrum of youth AB is needed, particularly in well-sampled cohorts that have enrichment for risk for AB. These types of samples provide greater generalizability but still contain youth exhibiting a wide range of AB, including some who meet diagnostic criteria for conduct disorder or other AB diagnoses (e.g. ODD).
Finally, there is growing evidence of the importance of callous-unemotional (CU) traits in understanding the etiology of youth AB. CU traits are defined by low empathy and guilt, as well as low or manipulative interpersonal emotions (Frick et al., 2014) and are a specifier for the diagnosis of conduct disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013). Youth high on AB and CU traits engage in significantly more varied and violent AB (Enebrink et al., 2005; Pardini and Fite, 2010), are more likely to continue engaging in AB into adulthood (McMahon et al., 2010; Kahn et al., 2013) and are likely at increased risk to develop psychopathy in adulthood (Frick et al., 2014; Frogner et al., 2016; Viding and McCrory, 2018). Critically, CU traits appear to moderate some neurocognitive correlates of youth AB, including the associations among AB, emotion regulation and amygdala reactivity during socioemotional processing (Viding et al., 2012a, 2012b; Dotterer et al., 2020b). In fact, one of the few studies linking youth conduct disorder to global neural topology found that associations were specific to youth with conduct disorder and CU traits (Jiang et al., 2021). Thus, it is important to examine whether any AB-related neural effects are unique to youth AB, CU traits and/or their interaction.
Current study
To address these gaps in the literature, we examined whether dimensional measures of AB, CU traits or their interaction significantly related to differences in the topology of neural communication within the default, salience and/or frontoparietal networks by completing three unweighted, undirected graph analyses, using a proportional thresholding approach. We examined these questions using resting-state functional magnetic resonance imaging (rs-fMRI) data collected from a birth cohort that was over-sampled for low-income, urban families with nonmarital births. This sampling frame increased risk for poverty, which is, unfortunately, a robust risk factor for the development of youth AB (see Bradley and Corwyn, 2002 for review).
Given differences in socioemotional processing, reward and threat processing and executive functioning associated with youth AB, we expected youth AB to be associated with less optimal network topologies for each of the three networks. Specifically, given prior research linking differences in global efficiency and global clustering to neurocognitive functioning, in general (Langer et al., 2012; Suprano et al., 2019), and to youth AB, in particular (Jiang et al., 2016, 2021; Tillem et al., 2021), we hypothesized that youth AB would be associated with lower global efficiency and clustering in all three networks. Though our central focus of this study was on neural correlates of AB, given that CU traits have been associated with abnormal connectivity within the default network (Cohn et al., 2015), we hypothesized that CU traits also would be associated with lower global efficiency and clustering in the default network.
Finally, given the evidence suggesting that the presence of CU traits moderates the impact of youth AB on socioemotional processing and threat detection (Blair et al., 2014), we hypothesized that CU traits would moderate the association between AB and differences in default and salience network topologies. In contrast, since deficits in response inhibition and decision-making may be associated with AB more generally, independent of levels of CU traits (Iacono et al., 2008; Blair et al., 2014), we hypothesized that CU traits would not moderate the impact of youth AB on frontoparietal network topology.
Methods
Participants
The study sample was drawn from 183 adolescents from Detroit, Toledo or Chicago who were part of the Study of Adolescent Neural Development (SAND; Hein et al., 2018; Goetschius et al., 2019; Dotterer et al., 2020b; Goetschius et al., 2020), a substudy of the Future of Families and Child Wellbeing Study (FFCWS; Reichman et al., 2001), which contains multiple measures of context, psychopathology, brain function and biology. The FFCWS is a longitudinal cohort of 4898 (52.4% boys) children sampled from births in 20 large US cities from 1998 to 2000 (Reichman et al., 2001) with an over-sample for nonmarital births (∼3:1). Families living in Detroit, Toledo and Chicago were invited to take part in additional data collection at the University of Michigan as part of the SAND when the focused child was 15 years old. The complete list of measures and data for this project is publicly available from the National Institute of Mental Health data archive (https://nda.nih.gov/). The University of Michigan Medical School Institutional Review Board approved this study (UM IRBMED: HUM00074392). All adolescent participants provided written informed assent, and their primary caregivers provided written consent for both themselves and their adolescent children. Of the 183 adolescents in the study sample, 22 participants were excluded due to issues in MRI data quality and/or missing behavioral or demographic data (see Supplementary Table S1), resulting in a final sample of 161 adolescents with complete, available, and high-quaility rs-fMRI data. Of the 161 adolescents included in the final sample, 59% were female, 75.2% were identified as Black/African American, 11.2% were identified as White/European American; 46.6% of families reported annual income below $25 000 (see Supplementary Table S2 for additional demographics).
AB and CU traits
Latent factors for both AB and CU traits were previously generated for the SAND sample using a multi-informant, multimethod approach, allowing us to mitigate reporter-specific and/or method-specific sources of error (e.g. informant bias; see Dotterer et al., 2020b for details). For AB, the latent factor was generated combining indicators from the following measures: (a) parent-reported rule breaking and (b) aggression from the Child Behavior Checklist (Achenbach, 1994), (c) the total score (excluding substance use items) of the youth-reported Self-Report of Delinquency (Elliott et al., 1985) and (d) combined lifetime symptom count (i.e. past/lifetime and present subclinical and clinical threshold symptoms) of the DSM-5 for conduct disorder and ODD (American Psychiatric Association, 2013) on the basis of clinician ratings assessed via a modified version of the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 1997). Thus, the latent factor captured AB as a dimension including broad behaviors from minor rule breaking and defiance to more serious aggression to full clinical symptoms of conduct disorder and ODD. For CU traits, the latent factor was generated combining the following measures: total scores for (a) parent-reported and (b) youth-reported Inventory of Callous-Unemotional Traits (ICU) (Frick et al., 2000; consistent with prior studies, two items were excluded from the total score based on an examination of polychoric inter-item correlations; Waller et al., 2015) and (c) clinician ratings of total lifetime symptom counts (i.e. past/lifetime and present subclinical and clinical threshold symptoms) using the Michigan Addendum to the K-SADS (Walker et al., 2021), which consists of items that are meant to overlap with the recently developed DSM-5 ‘limited prosocial emotions’ specifier (American Psychiatric Association, 2013) derived from the Clinical Assessment of Prosocial Emotions (Frick, 2013) and embedded into the K-SADS interview. Both latent factors were calculated using Confirmatory Factor Analysis in Mplus (Version 7.3; Muthén and Muthén, 2015), with maximum likelihood estimation with robust standard errors (to account for skew and zero-inflation). See Tables 1 and 2 for factor loadings and model fit statistics.
Table 1.
Antisocial behavior factor loadings and model fit
| Variable | Loadings |
|---|---|
| Factor loadings | |
| CBCL aggression | 0.88 |
| CBCL rule breaking | 0.93 |
| SRD total score | 0.39 |
| K-SADS ODD/CD symptoms | 0.69 |
| Model fit statistics | |
| Chi-square test of model fit | 2.67, df = 1, P = 0.10 |
| CFI | 0.99 |
| TLI | 0.96 |
| RMSEA | 0.08 |
| SRMR | 0.01 |
Factor loadings and model fit statistics for the antisocial behavior factor previously generated by Dotterer et al. (2020b) using a confirmatory factor analysis with a maximum likelihood estimation approach and robust standard errors. CBCL = Child Behavior Checklist; SRD = Self-Report of Delinquency; K-SADS = Kiddie Schedule for Affective Disorders and Schizophrenia; ODD = oppositional defiant disorder; CD = conduct disorder; df = degrees of freedom; TLI = Tucker–Lewis index; CFI = comparative fit index; RMSEA = root mean square error of approximation; SRMR = standardized root mean residual.
Table 2.
Callous-unemotional traits factor loadings and model fit
| Variable | Loadings |
|---|---|
| Factor loadings | |
| ICU parent-report total score | 0.40 |
| ICU self-report total score | 0.33 |
| CAPE/K-SADS limited prosocial emotions symptoms | 0.87 |
| Model fit statistics | |
| Chi-square test of model fit | 0, df = 0, P < 0.001 |
| CFI | 1.00 |
| TLI | 1.00 |
| RMSEA | 0.00 |
| SRMR | 0.00 |
Factor loadings for the callous-unemotional traits factor previously generated by Dotterer et al. (2020b) using a confirmatory factor analysis with a maximum likelihood estimation approach and robust standard errors. The model is saturated. ICU = Inventory of Callous-Unemotional Traits; K-SADS = Kiddie Schedule for Affective Disorders and Schizophrenia; CAPE = Clinical Assessment of Prosocial Emotions; df = degrees of freedom; TLI = Tucker–Lewis index; CFI = comparative fit index; RMSEA = root mean square error of approximation; SRMR = standardized root mean residual.
While the current study was designed to examine AB and CU traits dimensionally across a broad continuum of behaviors, it is important to note that participants reported a range of AB and CU traits scores from normative to clinical level. For AB, several participants met diagnostic criteria for conduct disorder (past diagnosis: n = 13, 8.1%; current diagnosis: n = 5, 3.1%; any CD diagnosis: n = 13, 8.1%) and ODD [past diagnosis: n = 12, 7.5%; current diagnosis: n = 8, 5.1%; any ODD diagnosis: n = 13, 8.1% (n = 8 participants who met criteria for ODD also met criteria for past or present CD)]. For CU traits, some participants did meet diagnostic criteria for the ‘with limited prosocial emotion’ specifier (past diagnosis: n = 6, 3.7%; current diagnosis: n = 5, 3.1%; any diagnosis: n = 6, 3.7%). Similarly, ICU total scores for several participants fell within the ‘clinical’ (n = 9, 5.6%) and ‘at-risk’ (n = 10, 6.2%) score ranges for community samples (https://faculty.lsu.edu/pfricklab/icu.php).
Imaging procedures and processing
MRI acquisition and preprocessing
MRI image data for the SAND were acquired on a GE Discovery MR750 3 T MRI scanner with an 8-channel head coil. Data acquisition included a T1-weighted structural scan and an 8 min rs-fMRI scan obtained using functional T2*-weighted BOLD images with a gradient echo spiral sequence (TR = 2000 ms, TE = 30 ms, 40 contiguous 3 mm axial slices, flip angle = 90°, FOV = 22 cm, voxel size = 3.44 mm × 3.44 mm × 3 mm) aligned with the AC-PC plane. Resting-state functional images were collected while participants were awake, passively viewing a fixation cross at the end of the scanning session. Slices were acquired contiguously. Images were reconstructed offline using processing steps to remove distortions caused by magnetic field inhomogeneity and other sources of misalignment to the structural data. Standard preprocessing, slice timing, realignment and coregistration to the structural scans, and normalization to MNI 152 space, and a spatial smoothing using a Gaussian kernel (6 mm) was completed in SPM12 using defaults. The top five white matter components were regressed out as well. All brain activity was filtered through a bandpass filter between 0.01 and 0.1 Hz.
Motion correction and denoising
A conservative, multistep procedure was used to correct for motion artifacts combining multiple correction strategies (Parkes et al., 2018). First, 8 min scans were motion scrubbed to identify and remove motion artifacts from the fMRI time series, using a mean frame displacement cutoff value of 0.5 mm (Power et al., 2012). Second, independent component analysis-based Automatic Removal of Motion Artifacts was applied to data at the subject level to remove motion-related artifacts (Pruim et al., 2015a, 2015b).
Brain connectome generation
To produce a whole-brain resting functional connectome, we placed 264 ROIs following the Power et al. (2011) atlas. Each ROI consisted of a 3.2 voxel center-to-voxel center radius pseudosphere. Connection strength, measured as the strength of BOLD signal correlation between each of the ROIs, was then calculated for connectome generation. Following the connectome generation, connectivity matrices for each network of interest (i.e. default, salience and frontoparietal networks) were extracted from the whole-brain connectome.
Graph analysis
All graph analyses were completed in Matlab (version 2018b) using a combination of the Brain Connectivity Toolbox (Rubinov and Sporns, 2010) and the MIT graph toolbox (http://strategic.mit.edu/downloads.php?page=matlab_networks. To ensure all graphs were fully connected, a minimum spanning tree analysis using the Kruskal algorithm (Kruskal, 1956) was implemented to generate an initial fully connected subgraph for each network, for each participant. These subgraphs acted as an initial skeletal structure for the main, proportional thresholded graph analysis.
Following this initial subgraph generation, connections were added to each subgraph at proportional thresholds of 0.01 to 0.35 at 0.01 step intervals to generate 35 unweighted, undirected graphs of differing levels of sparsity per network, per participant. Our two graph metrics of interest, efficiency and clustering, were then extracted from each of these thresholded graphs for each participant. To help ensure that our graph metrics accurately reflected neural organization across different levels of sparsity, the area under the curve (AUC) was calculated for each graph metric across sparsity levels (Ginestet et al., 2011; Hosseini et al., 2012), producing one AUC value, per metric, per network, per participant. All AUC graph metrics were winsorized to limit the leverage of outliers on the subsequent regression analyses.
Graph metrics
Efficiency
Efficiency was calculated as the inverse average shortest path length across the graphs. Accordingly, within graphs with higher efficiency, information theoretically travels through fewer connections to get from any node to any other node in the network, allowing for more efficient neural communication and information integration (i.e. communication/integration requiring less time or neural resources; Bullmore and Sporns, 2009).
Clustering
Clustering was calculated as the global fraction of nodes in a graph, which form triangular connections (i.e. the fraction of nodes in a graph whose neighbors are also interconnected with each other). Graphs with higher clustering tend to exhibit higher degrees of functional segregation and may be more robust to disruptions or damage (Bullmore and Sporns, 2009).
Data analysis
Separate linear regression models were run for each of the graph metrics of interest in each of the three network analyses. In each of these regression models, the latent AB factor, the latent CU traits factor and the AB × CU interaction were entered as simultaneous predictors of interest. Additionally, consistent with prior studies examining these factors in this sample (see also Dotterer et al., 2020b), self-reported gender (dichotomously coded, female vs male), self-reported race (a social construct included to control for differences in exposure to discrimination and structural racism and inequality in opportunity; two simultaneous, dichotomously coded variables: Black vs Non-Black and White vs Non-White), familial income (z-scored) and pubertal development score (z-scored; as measured by the Pubertal Development Scale; Petersen et al., 1988) were included as nuisance regressors in the models (see Supplementary Table S3 for zero-order correlations).
All β-values were Bonferroni corrected within each network analysis separately to control for multiple comparisons. P-values reported in the results section reflect the Bonferroni-corrected P-values. Any significant moderation effects were decomposed and graphed using the online utility by Preacher et al. (2006) to assess simple slopes and regions of significance. While all results were assessed via Bonferroni-corrected P-values, we also generated supplementary Bayes Factors (BF) for any null findings to provide additional information on the strength and confidence of any null results (see Supplementary Materials).
Supplementary analyses
Since prior work in this field has examined the relationship between youth AB at different levels of analysis (e.g. whole-brain graph theory metrics; Jiang et al., 2016, 2021; Tillem et al., 2021), we also ran supplementary, exploratory analyses at the whole-brain and node levels. These analyses can be found in the Supplementary Materials to aide future research and were not part of our focal analyses.
Results
AB was significantly related to lower efficiency in the frontoparietal network (β = −0.25, P = 0.024; Table 3). However, the effect of AB was qualified by a significant AB × CU traits interaction (β = 0.28, P = 0.028). A region of significance analysis for this moderation effect revealed that the association between lower frontoparietal efficiency and AB was only significant at low or average levels of CU traits (CU traits ≤0.23 s.d. above the mean; see Figure 1 for simple slopes). In contrast, at extremely high levels of CU traits (CU traits ≥3.42 s.d. above the mean), AB was associated with higher efficiency in the frontoparietal network. However, this frontoparietal hyperefficiency should be interpreted with caution as, in the current sample, only three participants had CU trait scores within this region of significance (i.e. CU trait scores ≥3.42 SD above the mean).
Table 3.
Regression results
| β | t | P | P corrected | |
|---|---|---|---|---|
| Frontoparietal network | ||||
| Efficiency | ||||
| AB | −0.25* | −2.53 | 0.012 | 0.024 |
| CU traits | −0.08 | −0.71 | 0.482 | 0.964 |
| AB × CU traits | 0.28* | 2.49 | 0.014 | 0.028 |
| Clustering | ||||
| AB | 0.15 | 1.60 | 0.111 | 0.222 |
| CU traits | 0.02 | 0.20 | 0.840 | 1.000 |
| AB × CU traits | −0.19 | −1.69 | 0.093 | 0.186 |
| Default network | ||||
| Efficiency | ||||
| AB | 0.04 | 0.39 | 0.701 | 1.000 |
| CU traits | −0.06 | −0.54 | 0.587 | 1.000 |
| AB × CU traits | −0.03 | −0.22 | 0.826 | 1.000 |
| Clustering | ||||
| AB | −0.09 | −0.97 | 0.331 | 0.662 |
| CU traits | 0.04 | 0.40 | 0.689 | 1.000 |
| AB × CU traits | 0.13 | 1.17 | 0.244 | 0.488 |
| Salience network | ||||
| Efficiency | ||||
| AB | −0.07 | 0.68 | 0.499 | 0.998 |
| CU traits | 0.08 | 0.67 | 0.503 | 1.000 |
| AB × CU traits | −0.08 | −0.70 | 0.485 | 0.970 |
| Clustering | ||||
| AB | −0.01 | −0.06 | 0.954 | 1.000 |
| CU traits | −0.05 | −0.41 | 0.684 | 1.000 |
| AB × CU traits | 0.11 | 0.97 | 0.336 | 0.672 |
Results from six linear regression models examining the relationship between network-specific graph analysis metrics (i.e. global efficiency and global clustering) and youth AB, CU traits and the AB × CU traits interaction across three networks (the frontoparietal network, the default network and the salience network). Models controlled for self-reported gender (dichotomously coded, female vs male), self-reported race (two dichotomously coded variables: Black vs Non-Black and White vs Non-White), familial income (z-scored) and pubertal development score (z-scored). Given the correlations among youth AB, CU traits and pubertal status, all models were rerun excluding CU trait and puberty scores from the models to ensure that the current AB findings were not due to suppression effects. Excluding these variables from the models did not meaningfully change any findings..
P < 0.05.
Fig. 1.
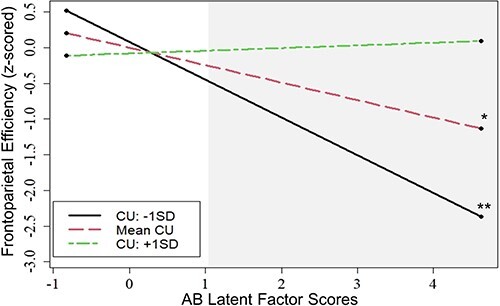
Antisocial behavior × callous-unemotional traits interaction for frontoparietal efficiency. Simple slopes for the significant AB × CU traits interaction effect on frontoparietal efficiency, controlling for self-reported gender (dichotomously coded, female vs male), self-reported race (two dichotomously coded variables: Black vs Non-Black and White vs Non-White), familial income (z-scored) and pubertal development score (z-scored). The significance of the simple slopes was evaluated via a region of significance analysis. A secondary region of significance analysis was run to evaluate at what levels of AB the main effect of the moderator, CU traits, was significant. The secondary analysis revealed that the main effect of CU traits was significant in youth with AB scores ≥1.05 s.d. above the mean. The shaded region of the figure represents this region of significance. *P < 0.05, **P < 0.01.
In contrast, the main effect of CU traits on efficiency within the frontoparietal network was not significant (β = −0.08, P = 0.964). Similarly, neither the main effect of AB (β = 0.15, P = 0.222), the main effect of CU traits (β = 0.02, P = 1.000), nor the AB × CU interaction (β = −0.19, P = 0.186) was significant for clustering in this network.
For both the default and salience networks, there were no significant associations between AB, CU traits, nor their interaction and measures of efficiency or clustering (see Table 3 for full regression results). Moreover, based on the BF findings, the current study provided ‘strong’ evidence in favor of the null hypothesis for our model examining efficiency in the default network (BF = 0.07) and ‘substantial’ evidence for the null hypothesis for the remaining models examining the default and salience networks (BFs range from 0.012 to 0.20).
Supplementary results
Full results from the supplementary, exploratory analyses can be found in the Supplementary Materials; however, briefly, neither AB, CU nor their interaction were significantly related to differences in whole-brain efficiency, whole-brain clustering or inter-network communication. The exploratory hubness analysis, however, revealed that, within the default network, CU traits were related to increased hubness in the right-temporal pole (β = 0.491, P < 0.001). No other hubness effects were significant.
Discussion
By applying graph analytic techniques to rs-fMRI data collected from a well-sampled community cohort with increased exposure to poverty and thus risk for AB, we found that youth AB was associated with a less efficiently organized frontoparietal network at rest, but this was only true for youth with average or low levels of CU traits. In contrast, neither youth AB nor CU traits were significantly related to differences in the topology of the default or salience networks. Collectively, these findings highlight the specificity of neural network topology alterations to the frontoparietal network and to youth with AB but not high CU traits.
Youth AB and the frontoparietal network
In line with our hypotheses, youth AB was associated with less efficient frontoparietal topology. These network findings are consistent with a prior resting-state connectivity study that linked youth AB to blunted connectivity in frontoparietal regions (Cohn et al., 2015). Based on prior graph theory work in neurotypical populations, lower efficiency in a neural network may slow neural communication and information integration within the network, impeding rapid and cost-effective information processing and impairing associated neurocognitive functions (Reijneveld et al., 2007; Stam and Reijneveld, 2007; Bullmore and Sporns, 2009). The frontoparietal network supports executive functioning, including response inhibition, sustained attention and decision-making (Marek and Dosenbach, 2018). Thus, these findings may help explain why youth who engage in AB have difficulties with inhibitory control and other executive functioning, particularly in time-limited and/or stressful contexts in which resources may be limited or rapid information processing may be critical (Fairchild et al., 2009; Hobson et al., 2011; Schoorl et al., 2018).
Divergent neurocognitive profiles associated with youth AB at differing levels of CU traits
Contrary to our a priori hypotheses, the association between youth AB and lower frontoparietal efficiency was only present ‘at low and average’ (and not high) levels of CU traits, indicating that this neural profile is specific to youth engaged in AB who do not show relatively higher levels of CU traits. Moreover, in youth with extremely elevated levels of CU traits, AB actually was associated with greater efficiency in the frontoparietal network. Though this greater efficiency of the frontoparietal network should be interpreted with caution given the few participants in our sample at this level of CU traits, this finding, combined with the specificity of lower efficiency in the frontoparietal network for those with low or average levels of CU traits, is inconsistent with the idea that deficits in executive functioning, and the neural processes supporting them, are related to AB generally (i.e. regardless of the CU trait levels; Iacono et al., 2008; Blair et al., 2014).
This pattern of results, however, may not be overly surprising. Recent studies have reported similar interactions between youth AB, CU traits and executive functioning. For example, consistent with the current findings, Dotterer et al. (2021) reported that, at low levels of CU traits, youth AB was associated with sustained attention deficits, whereas at higher levels of CU traits, youth AB was associated with improved sustained attention. Similarly, Graziano et al. (2019) found that youth with elevated AB and CU traits performed better on standardized measures of executive functioning than youth with AB without CU traits. Although not conclusive, these prior studies, combined with the current findings, suggest that the presence of CU traits may moderate both behavioral and neural executive functioning deficits in youth AB.
Youth AB, the default network and the salience network
Counter to our a priori hypotheses, we did not find any evidence that youth AB was related to altered network topology in either the default or salience networks. Though some studies have linked youth AB to differences in functioning or connectivity in regions within these networks (Dalwani et al., 2014; Zhou et al., 2016; Sethi et al., 2018), it may be that AB-related differences are related to activation in or connectivity between very specific regions, and not to the broader pattern of connectivity within these networks. Alternatively, the current null findings may be due to the study’s sample size; however, the BF findings suggest that we have relatively substantial evidence supporting our null results. Similarly, it is possible that the specific level of analysis we examined (i.e. the network-level) simply may not capture the types of disruptions that are present in these networks (e.g. differences in node-level hubness, Jiang et al., 2016; or inter-network communication, Dotterer et al., 2020a); however, our exploratory node-level and whole-brain analyses found no evidence that youth AB was linked with differences at these different levels of analysis (see Supplementary Materials).
Limitations
While the current findings provide evidence that youth AB is associated with altered frontoparietal topology dimensionally in a unique, enriched community sample, they must be considered in light of limitations. First, the current study was limited to examining resting-state data and AB; therefore, any theorized links between frontoparietal efficiency and executive functioning remain speculative. Although there is increasing evidence that neural topology at rest relates to various aspects of psychopathology, neurocognitive functioning and behavior (Kong et al., 2018; Tillem et al., 2021; Chan et al., 2022), additional research is needed to link frontoparietal network topology at rest to behavioral measures of executive functioning in youth AB directly. Second, while the cohort who engaged in this study were at higher risk for AB based on their families SES at birth and associated increased risk for exposure to adversity, and we did have several cases that met diagnosable levels of AB (n = 14) and CU traits (n = 6), most of the sample did not meet diagnostic criteria, making it impossible to do a case–control comparison. Thus, our findings may not generalize to the more extreme levels of AB and CU traits found in clinical or forensic samples. Third, while the sample size of the current study is larger than previous case–control studies examining youth AB and neural topology, it is currently underpowered to explore more complex models with additional moderators (e.g. examining three-way interactions between AB × CU traits × gender). Fourth, the current study is cross-sectional, limiting our ability to examine whether these neural correlates are causes or consequences of AB. Finally, future directions in this dataset and others are needed to identify the complex etiology of individual differences in network topology that may give rise to AB (e.g. examining experiences that may impact these circuits during development).
Conclusions
Youth AB was associated with alterations in the topology of the frontoparietal network; however, those alterations are dependent upon an individual’s level of CU traits. Specifically, in youth with low or average levels of CU traits, youth AB was associated with reduced efficiency in the topology of the frontoparietal, but not the salience or default, network at rest. The specificity of these findings (1) suggest that differences in the frontoparietal network are related to AB but not CU traits, (2) indicate that network topology differences in youth AB may be specific to the frontoparietal network, which supports executive functioning and (3) add to a growing body of literature showing that the presence of CU traits may moderate executive functioning deficits in youth AB.
Supplementary Material
Acknowledgements
We acknowledge the past work of the Future Families and Child Wellbeing Study, the families for sharing their experiences with us and the project staff for making the study possible.
Contributor Information
Scott Tillem, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, USA.
Hailey L Dotterer, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, USA.
Leigh G Goetschius, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, USA.
Nestor Lopez-Duran, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, USA.
Colter Mitchell, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, USA.
Christopher S Monk, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, USA.
Luke W Hyde, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, USA.
Supplementary data
Supplementary data is available at SCAN online.
Data availability
Data from the Study of Adolescent Neurodevelopment (https://nda.nih.gov/edit_collection.html?id=2106) and the Fragile Families and Child Wellbeing Study (https://opr.princeton.edu/archive/) are publicly available. The fMRI data are not publicly posted as this was not supported by the National Institutes of Health at the time of data collection.
Funding
This work was supported by the National Institutes of Health Grant R01-MH103761 (to C.S.M). L.W.H was supported by a NARSAD Young Investigator Award from the Brain and Behavior Foundation. H.L.D was supported by a National Science Foundation Graduate Research Fellowship. S.T. was supported by the National Institutes of Health Grant T32-HD007109 (Monk and Gelman).
Conflict of interest
The authors declared that they had no conflict of interest with respect to their authorship or the publication of this article.
References
- Achenbach T.M. (1994). Integrative Guide for the 1991 CBCL/4-18, YSR, and TRF Profiles. Burlington, Vermont: University of Vermont. [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5, Vol. 5. Washington, DC: American Psychiatric Association. [Google Scholar]
- Blair J., Leibenluft E., Pine D.S. (2014). Conduct disorder and callous–unemotional traits in youth. New England Journal of Medicine, 371(23), 2207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F. (2002). Socioeconomic status and child development. Annual Review of Psychology, 53(1), 371–99. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10(3), 186–98. [DOI] [PubMed] [Google Scholar]
- Chang S.A., Tillem S., Benson-Williams C., Baskin-Sommers A.R. (2021). Cognitive empathy in subtypes of antisocial individuals. Frontiers in Psychiatry, 12, 677975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L., Simmons C., Tillem S., Conley M.I., Brazil I.A., Baskin-Sommers A.R. (2022). Classifying conduct disorder using a biopsychosocial model and machine learning method. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M.D., Pape L.E., Schmaal L., et al. (2015). Differential relations between juvenile psychopathic traits and resting state network connectivity. Human Brain Mapping, 36(6), 2396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman I., Murray J., Abbott R.A., et al. (2009). Outcomes of conduct problems in adolescence: 40 year follow-up of national cohort. BMJ, 338, a2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T.J., Dalwani M.S., Mikulich-Gilbertson S.K., et al. (2010). Risky decisions and their consequences: neural processing by boys with antisocial substance disorder. PLoS One, 5(9), e12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwani M.S., Sakai J.T., Mikulich-Gilbertson S.K., et al. (2011). Reduced cortical gray matter volume in male adolescents with substance and conduct problems. Drug and Alcohol Dependence, 118(2–3), 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwani M.S., Tregellas J.R., Andrews-Hanna J.R., et al. (2014). Default mode network activity in male adolescents with conduct and substance use disorder. Drug and Alcohol Dependence, 134, 242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterer H.L., Hyde L.W., Shaw D.S., Rodgers E.L., Forbes E.E., Beltz A.M. (2020a). Connections that characterize callousness: affective features of psychopathy are associated with personalized patterns of resting-state network connectivity. NeuroImage: Clinical, 28, 102402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterer H.L., Hyde L.W., Swartz J.R., Hariri A.R., Williamson D.E. (2017). Amygdala reactivity predicts adolescent antisocial behavior but not callous-unemotional traits. Developmental Cognitive Neuroscience, 24, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterer H.L., Tomlinson R.C., Burt S.A., Weigard A.S., Klump K.L., Hyde L.W. (2021). Neurocognitive abilities associated with antisocial behavior with and without callous-unemotional traits in a community sample. Neuropsychology, 35(4), 374–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterer H.L., Waller R., Hein T.C., et al. (2020b). Clarifying the link between amygdala functioning during emotion processing and antisocial behaviors versus callous-unemotional traits within a population-based community sample. Clinical Psychological Science, 8, 918–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D.S., Huizinga D., Ageton S.S. (1985). Explaining Delinquency and Drug Use. Beverly Hills, CA: Sage Publications. [Google Scholar]
- Enebrink P., Andershed H., Långström N. (2005). Callous–unemotional traits are associated with clinical severity in referred boys with conduct problems. Nordic Journal of Psychiatry, 59(6), 431–40. [DOI] [PubMed] [Google Scholar]
- Estrada S., Tillem S., Stuppy-Sullivan A., Baskin-Sommers A.R. (2018). Specifying the connection between reward processing and antisocial psychopathology across development: review, integration, and future directions. In: Gruber, J., editor. Oxford Handbook of Positive Emotion and Psychopathology. New York, NY: Oxford University Press, 312–32. [Google Scholar]
- Fairchild G., van Goozen S.H.M., Stollery S.J., et al. (2009). Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biological Psychiatry, 66(2), 162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick P.J. (2013). Clinical assessment of prosocial emotions (CAPE). Unpublished Test Manual. [Google Scholar]
- Frick P.J., Bodin S.D., Barry C.T. (2000). Psychopathic traits and conduct problems in community and clinic-referred samples of children: further development of the psychopathy screening device. Psychological Assessment, 12(4), 382. [PubMed] [Google Scholar]
- Frick P.J., Ray J.V., Thornton L.C., Kahn R.E. (2014). Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological Bulletin, 140(1), 1–57. [DOI] [PubMed] [Google Scholar]
- Frogner L., Gibson C.L., Andershed A.K., Andershed H. (2016). Childhood psychopathic personality and callous–unemotional traits in the prediction of conduct problems. American Journal of Orthopsychiatry, 88(2), 211–25. [DOI] [PubMed] [Google Scholar]
- Ginestet C.E., Nichols T.E., Bullmore T., Simmons A. (2011). Brain network analysis: separating cost from topology using cost-integration. PLoS One, 6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetschius L.G., Hein T.C., Mattson W.I., et al. (2019). Amygdala-prefrontal cortex white matter tracts are widespread, variable and implicated in amygdala modulation in adolescents. NeuroImage, 191, 278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetschius L.G., Hein T.C., McLanahan S.S., et al. (2020). Association of childhood violence exposure with adolescent neural network density. JAMA Network Open, 3(9), e2017850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P.A., Landis T., Maharaj A., Ros-Demarize R., Hart K.C., Garcia A. (2019). Differentiating preschool children with conduct problems and callous-unemotional behaviors through emotion regulation and executive functioning. Journal of Clinical Child & Adolescent Psychology, 51, 170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes D.J., Dadds M.R. (2012). Revisiting the role of empathy in childhood pathways to antisocial behavior. In: Langdon, R., Catriona, M., editors. Emotions, Imagination, and Moral Reasoning. New York: Psychology Press, 45–70. [Google Scholar]
- Hein T.C., Mattson W.I., Dotterer H.L., et al. (2018). Amygdala habituation and uncinate fasciculus connectivity in adolescence: a multi-modal approach. NeuroImage, 183, 617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson C.W., Scott S., Rubia K. (2011). Investigation of cool and hot executive function in ODD/CD independently of ADHD. Journal of Child Psychology and Psychiatry, 52(10), 1035–43. [DOI] [PubMed] [Google Scholar]
- Hosseini S.M.H., Hoeft F., Kesler S.R. (2012). GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One, 7(7), e40709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono W.G., Malone S.M., McGue M. (2008). Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annual Review of Clinical Psychology, 4, 325–48. [DOI] [PubMed] [Google Scholar]
- Itahashi T., Yamada T., Watanabe H., et al. (2014). Altered network topologies and hub organization in adults with autism: a resting-state fMRI study. PLoS One, 9(4), e94115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A.I., Dawson A.J., Begany K.L., et al. (2013). fMRI reveals reciprocal inhibition between social and physical cognitive domains. NeuroImage, 66, 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Gao Y., Dong D., Sun X., Situ W., Yao S. (2021). Impaired global efficiency in boys with conduct disorder and high callous unemotional traits. Journal of Psychiatric Research, 138, 560–8. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Liu W., Ming Q., et al. (2016). Disrupted topological patterns of large-scale network in conduct disorder. Scientific Reports, 6(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R.E., Byrd A.L., Pardini D.A. (2013). Callous-unemotional traits robustly predict future criminal offending in young men. Law and Human Behavior, 37(2), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., et al. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–8. [DOI] [PubMed] [Google Scholar]
- Kong R., Li J., Orban C., et al. (2018). Spatial topography of individual-specific cortical networks predicts human cognition, personality, and emotion. Cerebral Cortex, 29, 2533–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R.F., Markon K.E., Patrick C.J., Benning S.D., Kramer M.D. (2007). Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology, 116(4), 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal J.B. (1956). On the shortest spanning subtree of a graph and the traveling salesman problem. Proceedings of the American Mathematical Society, 7(1), 48–50. [Google Scholar]
- Langer N., Pedroni A., Gianotti L.R.R., Hänggi J., Knoch D., Jäncke L. (2012). Functional brain network efficiency predicts intelligence. Human Brain Mapping, 33(6), 1393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub J.H., Vaillant G.E. (2000). Delinquency and mortality: a 50-year follow-up study of 1,000 delinquent and nondelinquent boys. American Journal of Psychiatry, 157(1), 96–102. [DOI] [PubMed] [Google Scholar]
- Lu F.M., Zhou J.S., Zhang J., Wang X.P., Yuan Z. (2017). Disrupted small-world brain network topology in pure conduct disorder. Oncotarget, 8(39), 65506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Dosenbach N.U.F. (2018). The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues in Clinical Neuroscience, 20(2), 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli A., Ackermann K., Bernhard A., Freitag C.M., Schwenck C. (2018). Hostile attribution bias and aggression in children and adolescents: a systematic literature review on the influence of aggression subtype and gender. Aggression and Violent Behavior, 39, 25–32. [Google Scholar]
- McGue M., Iacono W.G. (2005). The association of early adolescent problem behavior with adult psychopathology. American Journal of Psychiatry, 162(6), 1118–24. [DOI] [PubMed] [Google Scholar]
- McMahon R.J., Witkiewitz K., Kotler J.S. (2010). Predictive validity of callous–unemotional traits measured in early adolescence with respect to multiple antisocial outcomes. Journal of Abnormal Psychology, 119(4), 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan T.P., Bressler S.L. (2012). Neurocognitive networks: findings, models, and theory. Neuroscience and Biobehavioral Reviews, 36(10), 2232–47. [DOI] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E. (1993). Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review, 100(4), 674. [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. (2015). Mplus Version 7.3. Los Angeles, CA. [Google Scholar]
- Pardini D.A., Fite P.J. (2010). Symptoms of conduct disorder, oppositional defiant disorder, attention-deficit/hyperactivity disorder, and callous-unemotional traits as unique predictors of psychosocial maladjustment in boys: advancing an evidence base for DSM-V. Journal of the American Academy of Child and Adolescent Psychiatry, 49(11), 1134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L., Fulcher B., Yücel M., Fornito A. (2018). An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage, 171, 415–36. [DOI] [PubMed] [Google Scholar]
- Patrick C.J., Curtin J.J., Tellegen A. (2002). Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment, 14(2), 150–63. [DOI] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. (1988). A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–33. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., et al. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K.J., Curran P.J., Bauer D.J. (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31(4), 437–48. [Google Scholar]
- Pruim R.H.R., Mennes M., Buitelaar J.K., Beckmann C.F. (2015a). Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage, 112, 278–87. [DOI] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. (2015b). ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage, 112, 267–77. [DOI] [PubMed] [Google Scholar]
- Reichman N.E., Teitler J.O., Garfinkel I., McLanahan S.S. (2001). Fragile families: sample and design. Children and Youth Services Review, 23(4–5), 303–26. [Google Scholar]
- Reijneveld J.C., Ponten S.C., Berendse H.W., Stam C.J. (2007). The application of graph theoretical analysis to complex networks in the brain. Clinical Neurophysiology, 118(11), 2317–31. [DOI] [PubMed] [Google Scholar]
- Rivenbark J.G., Odgers C.L., Caspi A., et al. (2018). The high societal costs of childhood conduct problems: evidence from administrative records up to age 38 in a longitudinal birth cohort. Journal of Child Psychology and Psychiatry, 59(6), 703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R., McCrory E., Joffe H., De Lima N., Viding E. (2018). Living with conduct problem youth: family functioning and parental perceptions of their child. European Child & Adolescent Psychiatry, 27(5), 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Halari R., Smith A.B., Mohammad M., Scott S., Brammer M.J. (2009). Shared and disorder‐specific prefrontal abnormalities in boys with pure attention‐deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. Journal of Child Psychology and Psychiatry, 50(6), 669–78. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. (2010). Complex network measures of brain connectivity: uses and interpretations. NeuroImage, 52(3), 1059–69. [DOI] [PubMed] [Google Scholar]
- Schoorl J., van Rijn S., de Wied M., Van Goozen S.H., Swaab H. (2018). Boys with oppositional defiant disorder/conduct disorder show impaired adaptation during stress: an executive functioning study. Child Psychiatry and Human Development, 49(2), 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A., Sarkar S., Dell’Acqua F., et al. (2018). Anatomy of the dorsal default-mode network in conduct disorder: association with callous-unemotional traits. Developmental Cognitive Neuroscience, 30, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E., Elander J., Holmshaw J., Pickles A., Murray R., Rutter M. (2004). Predictors of antisocial personality: continuities from childhood to adult life. The British Journal of Psychiatry, 184(2), 118–27. [DOI] [PubMed] [Google Scholar]
- Spreng R.N., Grady C.L. (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience, 22(6), 1112–23. [DOI] [PubMed] [Google Scholar]
- Stam C.J., Reijneveld J.C. (2007). Graph theoretical analysis of complex networks in the brain. Nonlinear Biomedical Physics, 1(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suprano I., Delon-Martin C., Kocevar G., et al. (2019). Topological modification of brain networks organization in children with high intelligence quotient: a resting-state fMRI study. Frontiers in Human Neuroscience, 13, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillem S., Chang S.A., Baskin-Sommers A.R. (2020). Comparison of socio-affective processing across subtypes of antisocial psychopathology. In: Focquaert, F., Shaw, E., Waller, B., editors. The Routledge Handbook of the Philosophy and Science of Punishment. Abingdon, UK: Routledge, 288–302. [Google Scholar]
- Tillem S., Conley M.I., Baskin-Sommers A.R. (2021). Conduct disorder symptomatology is associated with an altered functional connectome in a large national youth sample. Development and Psychopathology, 34, 1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q. (2016). Salience Network of the Human Brain. San Diego, CA: Academic Press. [Google Scholar]
- Viding E., Fontaine N.M.G., McCrory E.J. (2012a). Antisocial behaviour in children with and without callous-unemotional traits. Journal of the Royal Society of Medicine, 105(5), 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E., McCrory E. (2018). Understanding the development of psychopathy: progress and challenges. Psychological Medicine, 48(1), 566–77. [DOI] [PubMed] [Google Scholar]
- Viding E., Sebastian C.L., Dadds M.R., et al. (2012b). Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. American Journal of Psychiatry, 169(10), 1109–16. [DOI] [PubMed] [Google Scholar]
- Walker T.M., Frick P.J., Matlasz T.M., et al. (2021). Psychometric properties of a semistructured interview to assess limited prosocial emotions. Assessment, 28(7), 1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R., Hawes S.W., Byrd A.L., et al. (2020). Disruptive behavior problems, callous-unemotional traits, and regional gray matter volume in the Adolescent Brain and Cognitive Development Study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(5), 481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R., Wright A.G.C., Shaw D.S., et al. (2015). Factor structure and construct validity of the parent-reported Inventory of Callous-Unemotional Traits among high-risk 9-year-olds. Assessment, 22(5), 561–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S.F., Fowler K.A., Sinclair S., et al. (2014). Disrupted expected value signaling in youth with disruptive behavior disorders to environmental reinforcers. Journal of the American Academy of Child and Adolescent Psychiatry, 53(5), 579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S.F., Pope K., Sinclair S., et al. (2013). Disrupted expected value and prediction error signaling in youths with disruptive behavior disorders during a passive avoidance task. American Journal of Psychiatry, 170(3), 315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Pu W., Wu G., et al. (2020). Connectomic underpinnings of working memory deficits in schizophrenia: evidence from a replication fMRI study. Schizophrenia Bulletin, 46(4), 916–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Yao N., Fairchild G., et al. (2016). Disrupted default mode network connectivity in male adolescents with conduct disorder. Brain Imaging and Behavior, 10(4), 995–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the Study of Adolescent Neurodevelopment (https://nda.nih.gov/edit_collection.html?id=2106) and the Fragile Families and Child Wellbeing Study (https://opr.princeton.edu/archive/) are publicly available. The fMRI data are not publicly posted as this was not supported by the National Institutes of Health at the time of data collection.


