Abstract
A national nicotine reduction policy has the potential to reduce cigarette smoking and associated adverse health impacts among vulnerable populations. However, possible unanticipated adverse effects of reducing nicotine content in cigarettes, such as increasing the use of alcohol or other abused substances, must be examined. The purpose of this study was to evaluate the effects of exposure to varying doses of nicotine in cigarettes on use of other substances. This was a secondary analysis (n = 753) of three simultaneous, multisite, double-blind, randomized-controlled trials examining 12 weeks of exposure to study cigarettes varying in nicotine content (0.4, 2.4, 15.8 mg nicotine/g tobacco) among daily smokers from three vulnerable populations: individuals with affective disorders (n = 251), individuals with opioid use disorder (n = 256), and socioeconomically-disadvantaged women of reproductive age (n = 246). Effect of study cigarette assignment on urine toxicology screens (performed weekly) and responses to drug and alcohol use questionnaires (completed at study weeks 6 and 12) were examined using negative binomial regression, logistic regression, or repeated measures analysis of variance, controlling for sex, age, and menthol status. The most common substances identified using urine toxicology included tetrahydrocannabinol (THC; 44.8%), cocaine (9.2%), benzodiazepine (8.6%), and amphetamines (8.0%), with 57.2% of participants testing positive at least once for substance use (27.3% if excluding THC). No significant main effects of nicotine dose were found on any of the examined outcomes. These results suggest that reducing nicotine content does not systematically increase use of other substances, even among individuals at increased risk of substance use.
Keywords: Nicotine reduction, Substance use, Vulnerable populations
Tobacco-related morbidity and mortality remain a significant public health burden in the U.S., driven primarily by use of cigarettes and other combustible products (United States Department of Health and Human Services, 2014; Wang et al., 2017). Nicotine is the addictive tobacco constituent that promotes repeated use of cigarettes upon smoking initiation, and reduction of nicotine content in cigarettes should reduce use and dependence risk (Benowitz, 1988, 2010). The 2009 Family Smoking Prevention and Tobacco Control Act (Tobacco Control Act) gave the U.S. Food and Drug Administration (FDA) authority to regulate tobacco products in the interest of public health (Family Smoking Prevention and Tobacco Control Act, 2009) including broad regulatory authority over the manufacturing, sale, and distribution of tobacco products (U.S. Food and Drug Administration, 2020). While the Tobacco Control Act explicitly denies FDA regulatory authority to require that nicotine content in tobacco products be reduced to zero (U.S. Food and Drug Administration, 2020), it gives FDA the authority to enact product standards including reducing the nicotine content of cigarettes to levels unlikely to create or maintain dependence.
An important research question is whether such a nicotine-reduction policy may have unanticipated adverse consequences. Previous investigations have assessed the effects of reducing the nicotine content of combustible cigarettes on various aspects of smoking. Investigations in general populations have shown that those who are randomized to receive reduced nicotine content cigarettes generally show reductions in total cigarette consumption, toxin exposure, and dependence severity, without evidence of potential negative consequences, such as compensatory smoking (i.e., increasing smoking rate or altering smoking topography to sustain desired nicotine blood levels) or increases in depressive symptoms (Donny et al., 2015; Hatsukami et al., 2018).
However, effects of such a policy must be carefully considered in vulnerable populations. That is, in populations in which smoking is overrepresented and who are at increased risk of the adverse health impacts of smoking. Some examples of vulnerable populations include those who are socioeconomically-disadvantaged or have comorbid psychiatric disorders (National Cancer Institute, 2017; United States Department of Health and Human Services, 2014). As these populations make up the majority of adults who smoke currently (United States Department of Health and Human Services, 2014), it is imperative to look at outcomes of a reduced-nicotine standard in these groups.
Research conducted thus far in vulnerable groups is consistent with research in the general population of those who smoke. Participants from vulnerable populations who are randomly assigned to reduced nicotine content cigarettes also show reductions in cigarette consumption and dependence severity, without evidence of compensatory smoking or increases or exacerbation in co-morbid conditions (e.g., depressive symptoms) (Gaalema et al., 2019; Higgins et al., 2017, 2020; Tidey et al., 2016, 2019). Despite these promising findings, there is need for continued vigilance regarding whether a reduced nicotine policy might result in unintended adverse consequences (Gottlieb and Zeller, 2017).
One potential consequence that should be examined is the use of other substances. Vulnerable populations, such as adults with affective or opioid use disorders, may be more likely than the general population to be susceptible to co-use of tobacco, alcohol, and/or cannabis (Degenhardt and Hall, 2001; Parker and Villanti, 2021), underscoring the need to evaluate whether tobacco users who use other substances exhibit a cross substance coping response (i.e. nicotine reduction could increase urges to drink alcohol) or cross substance cue reactivity (i.e. reduction in urges to smoke due to reduced nicotine exposure may also reduce urges to use alcohol if both substances are conditioned stimuli) (Cooney et al., 2007). There is mixed evidence supporting each hypothesis (Cooney et al., 2007) and the extent to which nicotine reduction may influence other substance use depends on whether they serve as economic substitutes or complements for one another. If substances are functioning as economic substitutes (i.e. reduction in the use of one substance results in an increase in use of another) then a decrease in nicotine content of cigarettes and associated reductions in smoking rate could lead to an increase in use of other substances (e.g. alcohol or other drugs). Among studies assessing the substitutability of nicotine and other drugs, the literature suggests that smoking and THC may in fact serve as economic complements, where a reduction in use of one leads to a reduction in use of the other (e.g. Agrawal et al., 2012) and a similar pattern can be seen with smoking and alcohol use (Tauchmann et al., 2013). However, the substitutability of nicotine and other drugs remains understudied, especially among populations most vulnerable to dependence on nicotine and other drugs.
Three studies have examined effects of reduced nicotine-content cigarettes on substance use, although these studies were in the general population of people who smoke. Two secondary analyses examined substance use outcomes in a large clinical trial of adults, who smoked daily, who were randomized to receive cigarettes varying in nicotine content for 6 weeks (Donny et al., 2015). Nicotine content had no effect on prevalence or frequency of using cannabis (Pacek et al., 2016) or alcohol (Dermody et al., 2016). Likewise, in a secondary analysis of a trial that compared the effects of immediate versus gradual reductions in cigarette nicotine content in adults who smoked daily (Hatsukami et al., 2018), there were no negative effects of nicotine reduction. Instead, among those with a history of alcohol use, assignment to reduced nicotine content cigarettes was associated with reductions in alcohol use and binge drinking (Dermody et al., 2021).
While these initial results in the general population are promising, it is important that these outcomes also be examined in vulnerable populations and across a wider range of substances. Vulnerable populations are more likely to use other substances and engage in problematic drinking (Conway et al., 2017; Hiscock et al., 2012; Substance Abuse and Mental Health Services Administration;, 2019) so examining other substance use as a potential consequence of a nicotine reduction policy in these vulnerable populations is crucial. As such, the purpose of the present study is to examine the potential consequences of a nicotine reduction policy on use of substances, other than tobacco, in samples drawn from vulnerable populations.
1. Methods
1.1. Study sample
Data for this secondary analysis are from three multi-site clinical trials conducted from October 2016 to September 2019. Details of the parent trials have been reported previously (Higgins et al., 2020). Briefly, the trial consisted of three multisite, double-blind, randomized controlled trials examining 12 weeks of exposure to study cigarettes varying in nicotine content (0.4, 2.4, or 15.8 mg nicotine/g tobacco) among adults who smoke daily from three vulnerable populations (individuals with a current affective disorders (n = 258), individuals enrolled in medication treatment for opioid use disorder (OUD) (n = 260) and socioeconomically-disadvantaged women of reproductive age (n = 257). Eligibility was determined at a screening visit conducted prior to any study procedures. The study was inclusive of adults who smoked at least 5 cigarettes per day, used primarily combustible cigarettes within the past 30 days (fewer than 9 days of other tobacco product use), and who had experienced no significant negative health changes in the previous 90 days. Exclusion criteria included plans to quit smoking within the next 30 days, and 3 or more days of smoking abstinence in the past 30 days. Individuals with a positive urine test for illicit drugs, excluding THC were considered ineligible, although they were allowed to rescreen at a later date. Of particular relevance to these analyses, those in the OUD sample had to be on a stable dose of either methadone or buprenorphine and have multiple recent drug screens negative for illicit substances prior to study enrollment. We required a negative urine screen prior to enrollment as an effort to recruit a fairly stable sample, that could be expected to be retained over a 12-week study. All participants completed informed consent as part of these trials.
Participants completed questionnaires during screening and baseline sessions concerning their tobacco use, nicotine dependence, and sociodemographic and household characteristics. During the baseline visits, nicotine dependence and tobacco use were assessed using the Fagerstrom Test of Nicotine Dependence (FTND) (Sato et al., 2012), and the Heaviness of Smoking Index (HSI) (Borland et al., 2010). Measures associated with dependence and tobacco use were also assessed at baseline, including nicotine metabolite ratio (NMR), cotinine, and expired breath carbon monoxide (CO).
Objective substance-use outcomes were based on the results of weekly urine toxicology testing for recent drug use using the Instant Drug Test Cup/Card II (IDTC II, Advin Biotech Inc). These test cards are sensitive to recent use of 12 substances: amphetamines, barbiturates, buprenorphine, benzodiazepines, cocaine, methylenedioxymethamphetamine (MDMA), methamphetamine, methadone, opiates, oxycodone, phencyclidine (PCP), and tetrahydrocannabinol (THC). Following the urine test done at screening, these tests were also conducted at the two baseline visits, prior to participants being given the cigarettes varying in nicotine content, as well as at the weekly study visits. Participants were aware of the results of these tests and cautioned that repeated illicit use could jeopardize their continued participation in the study. However, no participant was withdrawn based on positive tests. These weekly urine screens were conducted to characterize substance use, as several of the substances tested for have known interactive effects with nicotine and we were seeking to measure, and if needed, control for, those potential effects.
Self-reported drug use was assessed twice during the study (Weeks 6 and 12) using questionnaires that asked participants whether they had used each of 16 substances in the past 30 days and, if so, on how many days. Substances included in the questionnaire were amphetamines, benzodiazepines, barbiturates, club drugs, cocaine, hallucinogens, heroin, inhalants, marijuana, methamphetamines, other opiates, other tranquilizers, PCP, synthetic cannabinoids, synthetic stimulants, and other drugs.
Self-reported alcohol-use was also assessed at Weeks 6 and 12; the alcohol-use questionnaire asked participants to indicate how long it had been since they used alcohol (if ever) and how frequently they consumed alcohol within the past 30 days, how many drinks they consumed on a typical day, and how frequently they engaged in binge drinking (i.e., ≥5 drinks for males, ≥4 drinks for females within a two-hour period).
Participants with no urine toxicology results during the course of the study (n = 22) were excluded from the analyses resulting in a final sample of 753 participants, including 251 with affective disorders, 256 with opioid-use disorder, and 246 socioeconomically disadvantaged women of reproductive age. Given the differences between self-report and urine measurements in: the frequency of collection, the time frame covered (subjective was past 30 days, objective measures are dependent on the half-life of metabolites of the substances), and the substances queried, results from objective and subjective measures were examined separately.
1.2. Statistical analyses
Drug-use outcomes included 1) the proportion of participants ever testing positive on a weekly urine screen and 2) the number of positive urine tox screens for 1 or more substances. Effect of nicotine dose assignment on urine toxicology screens (performed weekly) and responses to drug use questionnaires (completed at study weeks 6 and 12) were examined using negative binomial regression or logistic regression. Negative binomial regression models estimating the number of weeks an individual tested positive (range: 0–12) included covariates representing whether a participant tested positive for one or more substances on at least one of the two baseline urine toxicology screens (which occur prior to participants being given the cigarettes varying in nicotine levels, coded as testing positive on at least one versus no positive tests), menthol status, sex, age, and vulnerable population. Logistic regression models estimating the proportion of participants ever testing positive (“Yes” for any positive test versus “No” for no positive tests) were adjusted for the same covariates described above. Similar logistic regression models examined participant-reported drug use at study weeks 6 or 12. Methadone and buprenorphine were not included as outcomes for those with OUD, as they were required to be taking one of these medications. Accordingly, for these participants results for buprenorphine and methadone are not reported in the tables, nor used in the summary measure of any substance use, nor are they used in the nicotine dose analyses. Methadone and buprenorphine are included in analyses for the other two groups (those with affective disorders and lower-SES women). Tests results were not differentiated by source (e.g. if a participant reported/did not report a prescription for a substance). An insufficient number of participants tested positive for some of the compounds included in the toxicology screens to allow for analysis (i.e., MDMA, barbiturates).
Alcohol-use was examined in those who reported using alcohol in the last 12 months at study intake. Alcohol-use outcomes included 1) mean number of drinks per month at Week 6 and Week 12 assessments, and 2) the proportion of participants reporting one or more episodes of binge drinking in the past 30 days at the Week 6 and Week 12 assessments. We used negative binomial regression to model the probability of reporting binge drinking (Never, at one time point, at both time points), adjusting for covariates including self-reported binge drinking at screening, menthol status, sex, and age. We also modelled variation in mean total number of drinks per month (using a square root transformation to improve normality) at Weeks 6 and 12, using repeated measures analysis of variance. Among the 529 participants who indicated using alcohol at least once in the previous 12 months, 23 (4.4%) provided responses at either Weeks 6 or 12 that were discordant (e.g. reporting a number of drinks consumed on a typical day that was higher than the number reported on their highest drinking day) and were subsequently removed from alcohol use analyses.
All analyses included a vulnerable population-by-dose interaction or vulnerable population-by-dose-by-time interaction term; however, the interaction terms were dropped from models in which they were not statistically significant (alpha≥0.05). We tested the sensitivity of our results to the number of weeks of urine testing data available per participant as well as to the extent the participant reported use of non-study cigarettes. Neither of these sets of analyses changed the results. All analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC).
2. Results
Participant characteristics can be seen in Table 1 broken down by vulnerable population. There were no characteristics that differed by nicotine dose condition in the parent trials, except menthol status, which was included as a covariate in all analyses. Study completion also did not differ by nicotine dose condition. The majority of study participants were female (due to one of the three trials including only women) (71.2%), identified race and ethnicity as Non-Latinx white (81.8%), were never married (59.0%), and indicated that they had achieved a high school degree or some college (73.4%). A majority (70.8%) reported having consumed alcohol in the prior year. The majority of participants preferred non-menthol cigarettes (54.6%), smoked an average of 18 cigarettes per day (CPD), and started smoking regularly in adolescence (16.2 years of age). Across vulnerable populations, adults with OUD exhibited higher levels of daily smoking than the disadvantaged women and those with affective disorders (22.5 versus 15.3 and 15.9 CPD), as well as higher breath CO (19.5 versus 16.2 and 18.6 ppm), and higher FTND (6.6 versus 4.9 and 5.3) and HSI scores (4.2 versus 3.1 and 3.2).
Table 1.
Participant characteristics.
| Participant groups | ||||
|---|---|---|---|---|
|
|
|
|
||
| All (n = 753) | Lower-SES women (n = 246) | Opioid-use disorder (n = 256) | Affective disorders (n = 251) | |
|
| ||||
| Age (M ± SD) | 35.8 (11.1) | 31.1 (7.0) | 38.6 (10.4) | 37.6 (13.3) |
| Gender (% women) | 536 (71.2%) | 246 (100.0%) | 142 (55.5%) | 148 (59.0%) |
| Race | ||||
| Non-Latinx black | 68 (9.1%) | 33 (13.5%) | 22 (8.7%) | 13 (5.2%) |
| Non-Latinx white | 610 (81.8%) | 193 (79.1%) | 208 (82.5%) | 209 (83.6%) |
| Latinx | 22 (3.0%) | 3 (1.2%) | 6 (2.4%) | 13 (5.2%) |
| Non-Latinx other | 46 (6.2%) | 15 (6.2%) | 16 (6.4%) | 15 (6.0%) |
| Education | ||||
| Some high school | 101 (13.4%) | 36 (14.6%) | 48 (18.8%) | 17 (0.1%) |
| High school grad, some college | 553 (73.4%) | 209 (85.0%) | 185 (72.3% | 159 (63.4%) |
| Associate’s degree | 38 (5.1%) | 1 (0.4%) | 12 (4.7%) | 25 (10.0%) |
| College graduate or more | 61 (8.1%) | 0 (0.0%) | 11 (4.3%) | 50 (19.9%) |
| Marital status | ||||
| Never married | 444 (59.0%) | 139 (56.5%) | 159 (62.1%) | 146 58.2%) |
| Married | 111 (14.7%) | 51 (20.7%) | 27 (10.6%) | 33 (13.2%) |
| Divorced, separated | 198 (26.3%) | 56 (22.8%) | 70 (27.3%) | 72 (28.7%) |
| Menthol status | ||||
| Menthol | 342 (45.4%) | 115 (46.8%) | 116 (45.3%) | 111 (44.2%) |
| Non-menthol | 411 (54.6%) | 131 (53.3%) | 140 (54.7%) | 140 (55.8%) |
| CPD (M ± SD) | 18.0 (9.2) | 15.3 (7.5) | 22.5 (9.8) | 15.9 (8.4) |
| CO (M ± SD) | 18.1 (9.9) | 16.2 (8.2) | 19.5 (9.8) | 18.6 (11.2) |
| Cotinine (M ± SD) | 4925.4 (3747.8) | 5059.8 (3592.0) | 5341.3 (3744.9) | 4381.1 (3834.2) |
| Age started smoking reg (M ± SD) | 16.2 (4.0) | 16.1 (3.0) | 15.7 (4.1) | 16.8 (4.7) |
| FTND (M ± SD) | 5.6 (2.3) | 4.9 (2.2) | 6.6 (2.0) | 5.3 (2.4) |
| HSI (M ± SD) | 3.5 (1.5) | 3.1 (1.4) | 4.2 (1.3) | 3.2 (1.6) |
| % reporting alcohol use in last year | 529 (70.3%) | 215 (87.4%) | 116 (45.3%) | 198 (78.9%) |
Abbreviations: CPD - cigarettes per day, CO - carbon monoxide, FTND - Fagerstrom Test for Nicotine Dependence, HSI - Heaviness of Smoking Index.
2.1. Any use of substances
Summary of urine testing results can be seen in Table 2. Over half (57.2%) of participants tested positive at least once for any substance (27.3% if THC is excluded). The most commonly identified substances included THC (44.8%), cocaine (9.2%), benzodiazepine (8.6%), and amphetamines (8.0%). Within the 70.3% reporting past-year alcohol use, 41.4% reported binge drinking at least once during the study (Supplemental Table 1).
Table 2.
Distribution of positive urine screens for other substance use.
| Participant groups | |||||
|---|---|---|---|---|---|
|
|
|
|
|
||
| All (n = 753) | Lower-SES women (n = 246) | Opioid-use disorder (n = 256) | Affective disorders (n = 251) | p | |
|
| |||||
| Urine screens n (%) | |||||
| Amphetamines | 60 (8.0%) | 15 (6.1%) | 23 (9.0%) | 22 (8.8%) | 0.30 |
| Barbiturates | 3 (0.4%) | 0 (0.0%) | 2 (0.8%) | 1 (0.4%) | 0.99 |
| Buprenorphine | 8 (1.6%) | 4 (1.6%) | - | 4 (1.6%) | 0.50 |
| Benzodiazepines | 65 (8.6%) | 13 (5.3%)a | 16 (6.3%)ab | 36 (14.3%)b | 0.03 |
| Cocaine | 69 (9.2%) | 10 (4.1%)a | 35 (13.7%)b | 24 (9.6%)ab | 0.01 |
| MDMA* | 5 (0.7%) | 3 (1.2%) | 0 (0.0%) | 2 (0.8%) | 0.17 |
| Methamphetamine | 16 (2.1%) | 3 (1.2%) | 6 (2.3%) | 7 (2.8%) | 0.39 |
| Methadone | 2 (0.4%) | 1 (0.4%) | - | 1 (0.4%) | 0.99 |
| Opiates | 35 (4.6%) | 11 (4.5%) | 10 (3.9%) | 14 (5.6%) | 0.72 |
| Oxycodone | 28 (3.7%) | 14 (5.7%) | 6 (2.3%) | 8 (3.2%) | 0.12 |
| Phencyclidine** | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) | - |
| THC | 337 (44.8%) | 103 (41.9%) | 108 (42.2%) | 126 (50.2%) | 0.73 |
| Any substance*** | 433 (57.2%) | 127 (51.6%) | 142 (55.5%) | 164 (65.3%) | 0.65 |
| Any substance*** | 207 | 51 | 72 | 84 | 0.04 |
| w/o THC | (27.3%) | (20.7%)a | (28.1%)ab | (33.5%)b | |
Notes: Results reported for urine screens represent the % ever positive during the 12-week study period. Groups that are not equivalent in post-hoc comparisons are denoted by different superscript letters.
Number of positive test results was too small to conduct the logistic regression, P-value is computed from a Fisher’s Exact Test.
Number of positive test results was too small to conduct analysis.
Excluding buprenorphine and methadone for the opioid-use disorder group. THC = Tetrahydrocannabinol; MDMA = Methylenedioxymethamphetamine.
2.2. Differences by vulnerable population
Differences in urine toxicology by vulnerable population can be seen in Tables 2 and 3. When looking at ever use during the study (Table 2), fewer positives were seen in the lower-SES women than the other two populations for benzodiazepines, cocaine, and the summary measure of the use of any substance (excluding THC). A similar pattern was seen for benzodiazepines and cocaine when looking at the mean number of weeks testing positive (Table 3). When looking at self-reported alcohol use (Supplemental Table 1), participants with OUD reported lower levels of alcohol consumption, both as measured by mean number of drinks per month and binge drinking.
Table 3.
Mean number of weeks with positive urine screen.
| Participant groups | |||||
|---|---|---|---|---|---|
|
|
|
|
|
||
| All (n = 753) | Lower-SES women (n = 246) | Opioid-use disorder (n = 256) | Affective disorders (n = 251) | p | |
|
| |||||
| Urine screens (M ± SD) | |||||
| Amphetamines | 0.39 (1.72) | 0.37 (1.81) | 0.33 (1.33) | 0.47 (1.97) | 0.23 |
| Barbiturates* | 0.02 (0.38) | 0.00 (0.00) | 0.02 (0.20) | 0.04 (0.63) | 0.38 |
| Buprenorphine* | 0.04 (0.47) | 0.03 (0.34) | - | 0.05 (0.58) | 0.98 |
| Benzodiazepines | 0.39 (1.75) | 0.15 (0.94)a | 0.18 (1.05)ab | 0.86 (2.63)b | 0.005 |
| Cocaine | 0.17 (0.69) | 0.05 (0.27)a | 0.29 (0.90)b | 0.17 (0.71)b | <0.001 |
| MDMA* | 0.01 (0.10) | 0.02 (0.16) | 0.00 (0.00) | 0.01 (0.09) | 0.23 |
| Methamphetamine | 0.04 (0.45) | 0.02 (0.16) | 0.03 (0.19) | 0.08 (0.75) | 0.37 |
| Methadone* | 0.01 (0.10) | 0.01 (0.13) | - | 0.00 (0.06) | 0.99 |
| Opiates | 0.08 (0.47) | 0.07 (0.36) | 0.08 (0.50) | 0.10 (0.54) | 0.65 |
| Oxycodone | 0.08 (0.55) | 0.15 (0.85) | 0.04 (0.30) | 0.05 (0.32) | 0.09 |
| Phencyclidine | 0.00 (0.04) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.06) | 0.37 |
| THC | 3.08 (4.42) | 2.91 (4.39) | 2.76 (4.27) | 3.57 (4.57) | 0.54 |
| Any substance** | 3.73 (4.53) | 3.23 (4.41) | 3.36 (4.31) | 4.58 (4.75) | 0.50 |
| Any substance** | 1.07 | 0.74 | 0.82 | 1.64 | 0.75 |
| w/o THC | (2.54) | (2.16) | (1.89) | (3.27) | |
Notes: Results reported for urine screens represent the mean number of weeks testing positive during the 12-week study period. Groups that are not equivalent in post-hoc comparisons are denoted by different superscript letters.
Number of positive test results was too small to conduct the negative binomial regression, P- value is computed from a Wilcoxon Sign Rank Test.
Excluding buprenorphine and methadone for the opioid-use disorder group. THC = Tetrahydrocannabinol; MDMA = Methylenedioxymethamphetamine.
2.3. Effects of nicotine dose
There was no main effect of nicotine dose on use of any of the substances examined through urine testing, either in terms of the percent who ever tested positive or the mean number of weeks with a positive test (Fig. 1). There was also no main effect of nicotine dose for any of the self-reported substance use measures (Supplemental Tables 2 and 3).
Fig. 1.
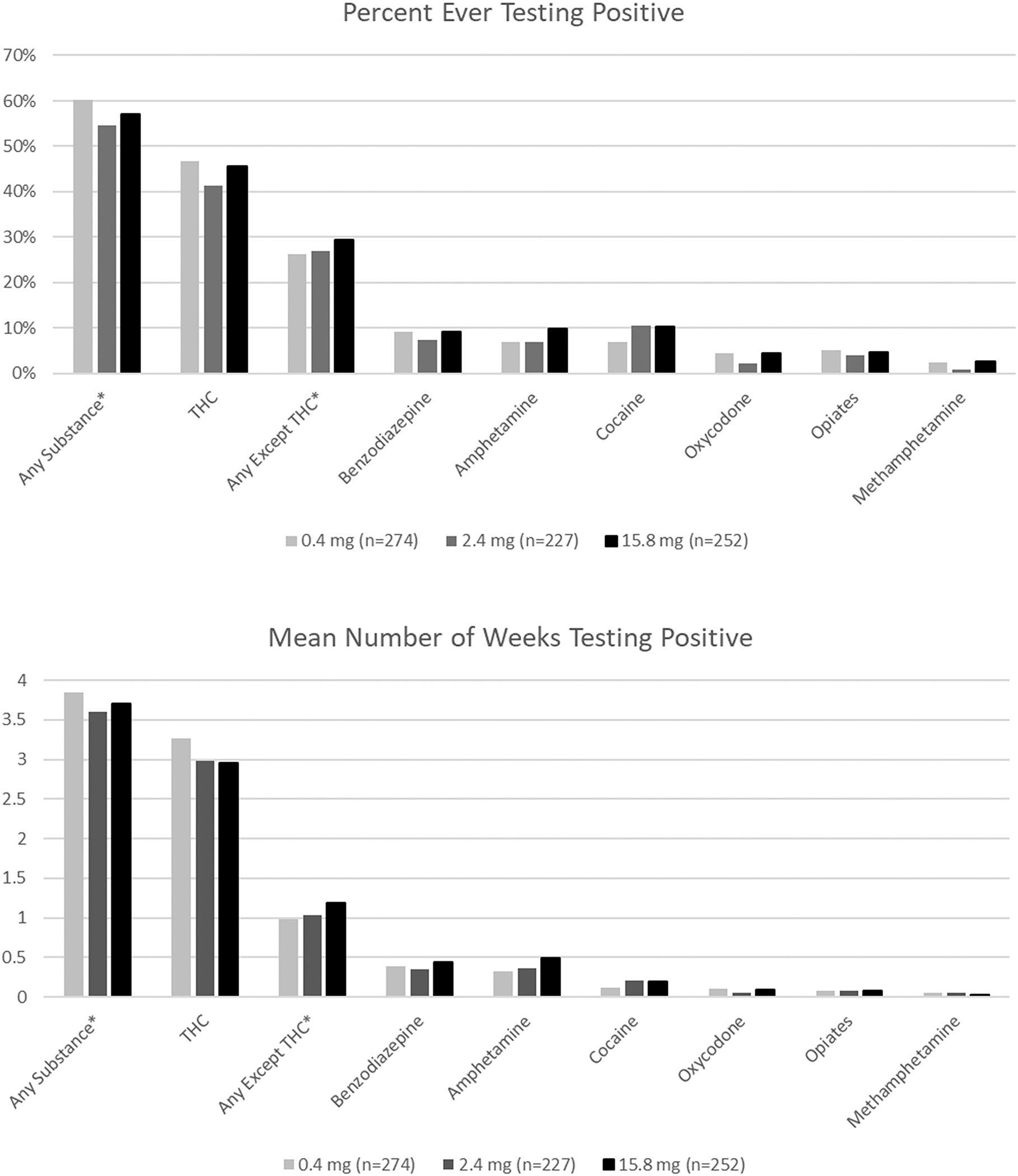
Urine test results by assigned cigarette nicotine content for selected substances.
*Any substance includes testing positive for any of the substances listed in Table 2. For the opioid-use disorder group methadone and buprenorphine are not included in these analyses. Results are presented for the most common substance combinations and individual substances.
2.4. Interactions
We saw no significant dose-by-population interactions on urine screens or on self-report measures. There was one significant population-dose-time interaction regarding self-reported alcohol use. Examination of the comparisons of interest suggested that the alcohol intake for those with OUD was significantly lower than that reported among those individuals with affective disorders at 6 weeks (differences observed among those assigned to the 2.4 mg and 15.8 mg dose, but not the 0.4 mg dose) and at 12 weeks (differences observed among those assigned to the 0.4 mg and 2.4 mg doses but not the 15.8 mg dose). While the differences in alcohol intake between these two groups in the 0.4 mg dose at 6 weeks were similar to the differences seen at 12 weeks, and the differences at 12 weeks in the 15.8 mg dose were similar to those seen at 6 weeks, they were not statistically significant. As the direction of effects of vulnerable population, nicotine dose, or time point within the interaction were largely in a single direction and differed only with respect to minor variations in magnitude, this interaction and all non-significant two-way interactions were removed from the model and alcohol results are reported collapsed across dose and time point.
3. Discussion
The results of the current study suggest that reducing the nicotine content in cigarettes during double-blind testing does not increase use of other substances in a sample drawn from vulnerable populations. These findings are encouraging in the context of a nicotine reduction policy as they suggest that reducing nicotine content in cigarettes should not lead to increases in use of other substances, even among vulnerable populations, which are at increased risk for substance use. However, it should be noted that these participants had to screen negative for illicit substances at least once to enter the study.
The lack of a nicotine dose effect on substance use in this vulnerable sample is consistent with the extant literature within the general population of those who smoke that was described above. Three studies examining effects of nicotine content on substance use within a general population found nicotine dose to have no systematic negative effect on prevalence or frequency of using cannabis (Pacek et al., 2016) or alcohol (Dermody et al., 2016; Dermody et al., 2021). Results from the current study extend these findings to vulnerable populations and to use of a broader range of substances.
This lack of a negative effect of reducing the nicotine content in cigarettes is also consistent with studies examining the effects of quitting smoking. Results from smoking cessation studies and naturalistic observations of the relationships between quitting smoking and substance use have generally either found no increase, or a decrease, in use of other substances following smoking cessation (Dunn et al., 2009; Baca and Yahne, 2009; Gaalema et al., 2013; Kohn et al., 2003; McKelvey et al., 2017).
Our findings are also consistent with existing research that suggests a nicotine-reduction policy would have minimal adverse consequences more broadly. Cigarettes with lower nicotine content have been shown to reduce smoking rate, toxin exposure, and nicotine dependence severity, without evidence of many concerning adverse consequences such as compensatory smoking in a general population (Donny et al., 2015; Hatsukami et al., 2018). However, unexpected consequences should continue to be examined as, for example, weight gain has been demonstrated as an outcome of use of reduced nicotine content cigarettes (Rupprecht et al., 2017). Overall, these benefits, and general lack of adverse consequences, have been shown to extend to vulnerable populations such as those with psychiatric or substance use disorders or those of lower-socioeconomic status (Higgins et al., 2017, 2020; Tidey et al., 2016, 2019; Krebs et al., 2021). The current results suggest that the consistent findings between general and vulnerable populations in lack of most negative consequences extends to substance use.
Several limitations of this study should be noted. This was a secondary analysis, meaning that the parent trials were not designed specifically to test effects of reductions in nicotine content in cigarettes on use of other substances. Thus, we may be underpowered to detect statistically-significant effects of nicotine dose on other substance use. People with regular drug use were screened out in the parent trials, as all participants had to have at least one negative urine screen for substances other than THC prior to enrollment. This may limit the extent to which our results may generalize to smokers from these vulnerable populations. There were also instances of non-adherence with use of the reduced nicotine content cigarettes, which could have affected nicotine intake and possibly mitigated the effects of nicotine reduction on substance use. Additionally, the analyses did not differentiate between prescribed and illicit use of substances, results from objective and subjective measurements of substance use could not be combined, and participants may have been less likely to use substances given weekly urine screens. However, the strengths of this study include random assignment to nicotine dose, double-blind nicotine dosing, objective measures of substance use, and the inclusion of a large sample of participants from three vulnerable populations. Overall, findings from the current examination, as well as existing literature, provide no evidence that a nicotine reduction policy would lead to an increase in use of other substances, even among vulnerable populations. Further research of this important topic especially among those with recent histories of problematic drug use would be helpful.
Supplementary Material
Funding
This research was supported by the Tobacco Centers of Regulatory Science award U54DA036114 from the National Institute on Drug Abuse (DCL, DEG, JRH, JWT, SCS, SHH, STH). Manuscript preparation was also supported by NIH awards U54DA031659 (JWT), P20GM103644 (DEG, SCS, STH), and P20GM130414 (JWT).
Footnotes
Declaration of Competing Interest
Dr. Hughes has received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction and from several non-profit organizations that promote tobacco control. He has recently received consulting fees from Swedish Match, Altria and Philip Morris International to assist their efforts to develop less risky tobacco products. All other authors have no disclosures to declare.
CRediT authorship contribution statement
Diann E. Gaalema: Conceptualization, Writing – original draft. L. Morgan Snell: Writing – original draft. Jennifer W. Tidey: Supervision, Writing – review & editing. Stacey C. Sigmon: Supervision, Writing – review & editing. Sarah H. Heil: Supervision, Writing – review & editing. Dustin C. Lee: Supervision, Writing – review & editing. Janice Y. Bunn: Formal analysis. Claire Park: Investigation, Writing – review & editing. John R. Hughes: Supervision, Writing – review & editing. Stephen T. Higgins: Writing – review & editing, Conceptualization, Supervision.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2022.107290.
ClinicalTrials.gov Identifiers: NCT02232737, NCT2250664, NCT2250534.
Data availability
Data from the parent trial can be requested with an appropriate data sharing agreement
References
- Agrawal A, Budney AJ, Lynskey MT, 2012. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction 107 (7), 1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baca CT, Yahne CE, 2009. Smoking cessation during substance abuse treatment: what you need to know. J. Subst. Abus. Treat. 36 (2), 205–219. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, 1988. Pharmacologic aspects of cigarette smoking and nicotine addiction. N. Engl. J. Med. 319 (20), 1318–1330. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, 2010. Nicotine addiction. N. Engl. J. Med. 362 (24), 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland R, Yong H-H, O’Connor RJ, Hyland A, Thompson ME, 2010. The reliability and predictive validity of the heaviness of smoking index and its two components: findings from the international tobacco control four country study. Nicotine Tob. Res. 12 (Supplement 1), S45–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Green VR, Kasza KA, Silveira ML, Borek N, Kimmel HL, Sargent JD, Stanton CA, Lambert E, Hilmi N, Reissig CJ, Jackson KJ, Tanski SE, Maklan D, Hyland AJ, Compton WM, 2017. Co-occurrence of tobacco product use, substance use, and mental health problems among adults: findings from wave 1 (2013 & 2014) of the population assessment of tobacco and health (PATH) study. Drug Alcohol Depend. 177 (February 2017), 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinberg HR, Oncken CA, 2007. Alcohol and tobacco cessation in alcohol-dependent smokers: analysis of real-time reports. Psychol. Addict. Behav. 21 (3), 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall AW, 2001. The relationship between tobacco use, substance-use disorders and mental health: results from the National Survey of mental health and well-being. Nicotine Tob. Res. 3 (3), 225–234. [DOI] [PubMed] [Google Scholar]
- Dermody SS, Tidey JW, Denlinger RL, Pacek LR, Al’absi M, Drobes DJ, Donny EC, 2016. The impact of smoking very low nicotine content cigarettes on alcohol use. Alcohol. Clin. Exp. Res. 40 (3), 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody SS, Tessier KM, Meier E, Al’absi M, Denlinger-Apte RL, Drobes DJ, Hatsukami D, 2021. An evaluation of potential unintended consequences of a nicotine product standard: a focus on drinking history and outcomes. Nicotine Tob. Res. 23 (7), 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny, et al. , 2015. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med. 373 (14), 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Reimann E, Heil SH, Higgins ST, 2009. Effects of smoking cessation on illicit drug use among opioid maintenance patients: a pilot study. J. Drug Issues 39 (2), 313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Family Smoking Prevention and Tobacco Control Act, 2009. https://www.govinfo.gov/content/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf.
- Gaalema DE, Higgins ST, Pepin CS, Heil SH, Bernstein IM, 2013. Illicit drug use among pregnant women enrolled in treatment for cigarette smoking cessation. Nicotine Tob. Res. 15 (5), 987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaalema DE, Tidey JW, Davis DR, Sigmon SC, Heil SH, Stitzer ML, Desarno MJ, Diaz V, Hughes JR, Higgins ST, 2019. Potential moderating effects of psychiatric diagnosis and symptom severity on subjective and behavioral responses to reduced nicotine content cigarettes. Nicotine Tob. Res. 21 (Supplement_1), S29–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Zeller M, 2017. A nicotine-focused framework for public health. N. Engl. J. Med. 377 (12), 1111–1114. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Luo X, Jensen JA, Al’Absi M, Allen SS, Carmella SG, Donny EC, 2018. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA 320 (9), 880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Sigmon SC, Tidey JW, Gaalema DE, Hughes JR, Tursi L, 2017. Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry 74 (10), 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Tidey JW, Sigmon SC, Heil SH, Gaalema DE, Lee D, Harfmann RF, 2020. Changes in cigarette consumption with reduced nicotine content cigarettes among smokers with psychiatric conditions or socioeconomic disadvantage: 3 randomized clinical trials. JAMA Netw. Open 3 (10), e2019311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M, 2012. Socioeconomic status and smoking: a review. Ann. N. Y. Acad. Sci. 1248 (1), 107–123. [DOI] [PubMed] [Google Scholar]
- Kohn CS, Tsoh JY, Weisner CM, 2003. Changes in smoking status among substance abusers: baseline characteristics and abstinence from alcohol and drugs at 12-month follow-up. Drug Alcohol Depend. 69 (1), 61–71. [DOI] [PubMed] [Google Scholar]
- Krebs NM, Zhu J, Wasserman E, Kuprewicz R, Martinez DJ, Veldheer S, Muscat JE, 2021. Switching to progressively reduced nicotine content cigarettes in smokers with low socioeconomic status: a double-blind randomized clinical trial. Nicotine Tob. Res. 23 (6), 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey K, Thrul J, Ramo D, 2017. Impact of quitting smoking and smoking cessation treatment on substance use outcomes: an updated and narrative review. Addict. Behav. 65, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute, 2017. NCI Tobacco Control Monograph 22: A Socioecological Approach to Addressing Tobacco-Related Health Disparities. https://cancercontrol.cancer.gov/brp/tcrb/monographs/22/docs/m22_complete.pdf.
- Pacek LR, Vandrey R, Dermody SS, Denlinger-Apte RL, Lemieux A, Tidey JW, Strasser AA, 2016. Evaluation of a reduced nicotine product standard: moderating effects of and impact on cannabis use. Drug Alcohol Depend. 167, 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Villanti AC, 2021. Relationship between comorbid drug use disorders, affective disorders, and current smoking. Subst. Use Misuse 56, 93–100. 10.1080/10826084.2020.1840591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht LE, Koopmeiners JS, Dermody SS, Oliver JA, Al’Absi M, Benowitz NL, Donny EC, 2017. Reducing nicotine exposure results in weight gain in smokers randomised to very low nicotine content cigarettes. Tob. Control. 26 (e1), e43–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Sato T, Nozawa A, 2012. Assessment scales for nicotine addiction. J. Addict Res. Ther. 01 (S1), 1–5. 10.4172/2155-6105.S1-008. [DOI] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2019. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf.
- Tauchmann H, Lenz S, Requate T, Schmidt CM, 2013. Tobacco and alcohol: complements or substitutes? Empir. Econ. 45 (1), 539–566. [Google Scholar]
- Tidey JW, Pacek LR, Koopmeiners JS, Vandrey R, Nardone N, Drobes DJ, Donny EC, 2016. Effects of 6-week use of reduced-nicotine content cigarettes in smokers with and without elevated depressive symptoms. Nicotine Tob. Res. 19 (1), 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Colby SM, Denlinger-Apte RL, Goodwin C, Cioe PA, Cassidy RN, Hecht SS, 2019. Effects of 6-week use of very low nicotine content cigarettes in smokers with serious mental illness. Nicotine Tob. Res. 21 (Supplement_1), S38–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, 2020. Family Smoking Prevention and Tobacco Control Act - An Overview. https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/family-smoking-prevention-and-tobacco-control-act-overview.
- United States Department of Health and Human Services, 2014. The Health Consequences of Smoking—50 Years of Progress A Report of the Surgeon General. A Report of the Surgeon General, 1081. [Google Scholar]
- Wang T, Asman K, Gentzke A, Cullen KA, Holder-Hayes E, Reyes-Guzman C, Jamal A, Neff LJ, King BA, 2017. Tobacco product use among adults — United States, 2017. MMWR Morb. Mortal. Wkly Rep. 67 (44). https://www.cdc.gov/mmwr/volumes/66/wr/mm6644a2.htm?s_cid=mm6644a2_w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the parent trial can be requested with an appropriate data sharing agreement


