Abstract
Objectives:
To examine temporal trends in all-cause and cause-specific mortality in patients with rheumatoid arthritis (RA) in the Veteran’s Health Administration (VHA).
Methods:
We conducted a matched cohort study in the VHA from 1/1/2000-12/31/2017. Incident RA patients were matched up to 1:10 on age, sex, and VHA enrollment year to non-RA patients, then followed until death or end of study period. Cause of death was obtained from the National Death Index. Multivariable Cox regression models stratified by RA diagnosis years were used to examine trends in RA-related risk of all-cause and cause-specific mortality.
Results:
Among 29,779 incident RA patients (matched to 245,226 non-RA patients), 9,565 deaths occurred. RA patients were at increased risk of all-cause (aHR 1.23, 95% CI 1.20-1.26), cardiovascular (aHR 1.19, 1.14-1.23), cancer (aHR 1.19, 1.14-1.24), respiratory (aHR 1.46, 1.38-1.55), and infection-related mortality (aHR 1.59, 1.41-1.80). Interstitial lung disease (ILD) was the cause of death most strongly associated with RA (aHR 3.39, 2.88-3.99). Nearly 70% of excess deaths in RA were attributable to cardiopulmonary disease. All-cause mortality risk related to RA was lower among those diagnosed during 2012-2017 (aHR 1.10, 1.05-1.15) compared to 2000-2005 (aHR 1.31, 1.26-1.36), but still higher than non-RA controls (p<0.001). Cause-specific mortality trends were similar.
Conclusion:
Excess RA-related mortality was driven by cardiovascular, cancer, respiratory, and infectious causes, particularly cardiopulmonary diseases. Though our findings support that RA-related mortality risk is decreasing over time, a mortality gap remains for all-cause and cause-specific mortality in RA.
The potential for rheumatoid arthritis (RA) to shorten patients’ lifespans has been well described for several decades (1). It has been estimated that RA increases the mortality risk by approximately 50% and prematurely abbreviates longevity by up to 10 years (2–4). Several advances in RA management have occurred over recent decades including earlier diagnosis and treatment initiation, the marked expansion of disease modifying anti-rheumatic drug (DMARD) options, and the use of treat-to-target management strategies, collectively leading to improved patient outcomes in the domains of joint damage, physical function, and quality of life (5–8). However, there is conflicting evidence on whether these advances have yielded survival benefit for patients with RA (9, 10). Understanding if a mortality gap persists is crucial for determining if more holistic management strategies are needed to preserve patients’ longevity.
Prior mortality studies have demonstrated that RA differentially impacts the risk of different causes of death. While cardiovascular disease (CVD), cancer, and respiratory diseases are the most frequent causes of death in both RA and the general population, respiratory-related mortality has been shown to be the most overrepresented cause of death in RA (1, 11). Though not among the most frequent causes of death, infectious-related complications are of particular concern in patients with RA given their predisposition related to immunosuppressive therapy use, especially with more widespread availability and implementation of biologic and targeted-synthetic DMARD therapy over the last two decades, and further underscored by the ongoing COVID-19 pandemic (12). Due to the large sample sizes needed for detailed investigations, most prior studies of cause-specific mortality in RA have examined broad categorical causes of death (e.g. CVD-related death). Few studies, outside of those investigating RA-associated interstitial lung disease (RA-ILD), have directly compared the risk of specific causes of CVD, cancer, and respiratory related mortality in RA (9, 11, 13). Additionally, it remains poorly understood how temporal trends in excess mortality from these causes of death may explain the broader RA “mortality gap”.
We aimed to comprehensively evaluate all-cause and cause-specific mortality in patients with RA, including an examination of temporal trends from 2000-2017 in all-cause, CVD-, cancer-, respiratory-, and infectious-related deaths. We conducted this matched cohort study within the Veteran’s Health Administration (VHA), the largest integrated health system in the U.S. and one that possesses robust administrative and electronic heath record data as well as linkages with external data sources.
Patients and Methods
Study Population and Design
We conducted a retrospective, matched cohort study, constructing an inception RA cohort using national VHA data within the VA Corporate Data Warehouse (CDW) data between January 1, 2000 and December 31, 2017. Data prior to this time-period is limited due to the timing of when the electronic medical record was established in the VHA. Patients with RA were identified using validated administrative algorithms requiring the presence of ≥2 diagnostic codes for RA, a rheumatologist diagnosis of RA, and either a positive RA autoantibody (rheumatoid factor [RF] or anti-cyclic citrullinated protein [anti-CCP] antibody) or a fill of a DMARD (14). Patients with RA were matched up to 1:10 on exact calendar year of birth, sex, and year of VHA enrollment to patients with no RA diagnostic codes during the study period. To reduce the risk of survival bias, we subsequently selected patients with incident RA following an administrative-based algorithm proposed by Curtis et al with a PPV of 70-80% (15). This algorithm required that patients received care in the VHA for ≥365 days without an RA diagnostic code or receipt of a DMARD prescription fill at any point prior to incident RA date. Patients were followed from the time the RA algorithm was fulfilled (index date; corresponding calendar date for matched non-RA patients) until the earliest of death or December 31, 2019. This study received institutional review board approval.
Vital Status and Cause of Death
Vital status was assessed through linkage with the National Death Index (NDI, National Center for Health Statistics). Broad, categorical causes of death were first defined according to International Classification of Diseases, 10th Edition (ICD-10) codes assigned to the immediate underlying cause of death and grouped according to ICD-10 chapters including infection, cancer, non-malignant blood disorders, endocrine, mental health, nervous system, cardiovascular, respiratory, gastrointestinal, skin, genitourinary, and external causes of death. We further classified more specific causes of CVD, cancer, and respiratory-related mortality using the Clinical Classifications Software Refined (CCSR), a Healthcare Cost and Utilization Project (HCUP) tool that assigns ICD-10 diagnostic codes to a clinical disease category (Supplementary Table 1). Because RA-ILD represents a key cause of mortality in patients with RA and is poorly defined by current CCSR categories, we leveraged previously validated diagnostic codes to identify ILD-related deaths in this study (16).
Covariates
Covariates were selected a priori, obtained from the CDW, and included race (Black, White, Other, Unknown), ethnicity (Hispanic, Non-Hispanic, Unknown), smoking status (current, former, or never), body mass index (BMI) category, and comorbidity burden. Demographics were collected from VHA enrollment records. Smoking status was defined based on “health factors”, which represent retrievable clinical data in the electronic health record collected for various conditions (17). BMI was calculated from weight at nearest visit preceding the index date and the modal height using vital signs from VHA encounters (18, 19). Comorbidity burden was assessed using the Rheumatic Disease Comorbidity Index (RDCI), a validated comorbidity index that includes lung disease, CVD, diabetes, cancer, depression, fracture, and gastric disease (20). The presence of these comorbid conditions was defined by ≥2 diagnostic codes on separate days prior to the index date.
Statistical Analysis
Baseline characteristics of RA and matched non-RA patients were compared using descriptive statistics. Categorical causes of death were tabulated, and crude mortality rates were calculated, stratified by RA status. Premature deaths in RA were quantified as the proportion of excess deaths on an absolute scale using non-RA mortality rates. We used Cox regression models to assess all-cause and cause-specific mortality risk amongst patients with RA compared to non-RA patients, clustering by matched patient groups to ensure the standard error accounts for the correlated nature of the matched set. Multivariable models were adjusted for baseline age, sex, race, ethnicity, smoking status, BMI, RDCI, and index year. Time-dependent changes in all-cause and cause-specific mortality were then examined using similar Cox regression models, testing the interaction of RA status and sequential time periods in a linear manner. Models were then stratified to include matched pairs with index dates falling within sequential 5-year time periods (2000-2005, 2006-2011, and 2012-2017) chosen to evenly divide the available study period. Trends in cause-specific mortality focused on pre-defined causes of death felt to be most clinically relevant in RA: CVD-, cancer-, respiratory-, and infectious-related mortality. Sensitivity analyses were performed to homogenize duration of follow-up to a maximum of 5 years amongst patients in different time periods. As changes in RA diagnostic patterns over time may influence mortality risk estimates, we additionally examined mortality trends after restricting to matched pairs containing seropositive (RF and/or anti-CCP) RA patients. In secondary analyses, all-cause and cause-specific mortality risks related to RA were estimated in models stratified by sex, seropositivity, and restricting to patients <65 years of age. Missing covariate data was addressed using multiple imputation with 10 imputations. All analyses were completed using Stata MP Version 16 (StataCorp) within the VA Informatics and Computing Infrastructure (VINCI) (21).
Results
Baseline Characteristics and Causes of Death
We identified 29,779 patients with incident RA. These patients were matched to 245,226 non-RA patients. Reflecting the VHA population, RA and non-RA patients were predominantly male (85.5% in RA, 87.1% in non-RA; Table 1). The mean age of RA patients in this study was 64.0 (62.4 in non-RA). White race was more common in RA (73%) than non-RA patients (62%), reflecting a higher proportion of missingness in non-RA patients (17.8% vs 7.8%). Patients with RA were more likely to have ever smoked (85% vs. 70%) and had a higher comorbidity burden (mean RDCI 2.3 vs. 1.6). Among RA patients with autoantibody testing results, 67.1% were seropositive for RF and/or anti-CCP. At baseline, the majority of RA patients had received a conventional DMARD (77.4%) and/or glucocorticoids (57.3%), whereas 16.4% of patients had received a biologic or targeted-synthetic DMARD (16.4%).
Table 1.
Baseline Characteristics of Patients with and without RA
| Characteristic | RA (N = 29,779) | Non-RA (N = 245,226) |
|---|---|---|
| Male sex (%) | 85.5 | 87.1 |
| Age, years (mean, SD) | 64.0 (11.6) | 62.4 (11.3) |
| Index year (%) | ||
| 2000-05 | 18.3 | 19.6 |
| 2006-11 | 34.1 | 34.1 |
| 2012-17 | 47.6 | 46.3 |
| Race (%) | ||
| Black | 16.3 | 17.0 |
| White | 72.9 | 62.4 |
| Other | 3.0 | 2.8 |
| Unknown | 7.8 | 17.8 |
| Ethnicity (%) | ||
| Hispanic | 5.2 | 3.8 |
| Non-Hispanic | 89.1 | 81.3 |
| Unknown | 5.7 | 14.9 |
| Smoking status (%) | ||
| Current | 53.6 | 43.7 |
| Former | 31.4 | 26.2 |
| Never | 12.8 | 18.4 |
| Unknown | 2.2 | 11.7 |
| BMI categories (%) | ||
| <20 kg/m2 | 1.4 | 1.4 |
| 20-24.9 kg/m2 | 9.0 | 10.0 |
| 25-29.9 kg/m2 | 29.4 | 29.3 |
| 30-34.9 kg/m2 | 29.8 | 27.6 |
| 35-39.9 kg/m2 | 16.8 | 14.3 |
| ≥40 kg/m2 | 13.4 | 10.4 |
| Unknown | 0.2 | 6.9 |
| RDCI score (mean, SD) | 2.3 (1.6) | 1.6 (1.6) |
| Seropositivity (RF and/or anti-CCP, %) | 67.1 | - |
| DMARD exposure prior to index (%) | ||
| csDMARD | 77.4 | - |
| bDMARD | 16.4 | - |
| Glucocorticoids | 57.3 | 7.4 |
Abbreviations: BMI, body mass index; CCP, cyclic citrullinated peptide; DMARD, disease modifying antirheumatic drug; RA, rheumatoid arthritis; RDCI, rheumatic disease comorbidity index; RF, rheumatoid factor; SD, standard deviation
Our study population was followed for a total of 2,053,993 patient-years (PY). Mean follow-up time was 12.6 years in patients with RA (median 11.5 years) and 14.2 years (median 14.3 years) in non-RA patients. We observed 72,461 deaths, of which 9,565 occurred in patients with RA. The most frequent causes of death in patients with RA were CVD (N=2,988), cancer (N=2,305), and respiratory-related (N=1,531) (Table 2). CVD-related death was most commonly due to coronary artery disease and heart failure, whereas cancer-related death was most frequently due to lung and gastrointestinal malignancy. Chronic obstructive pulmonary disease (COPD) and ILD were the most common causes of respiratory-related mortality. With the exception of ILD-related mortality, the most frequent causes of death (CVD-, cancer-, and respiratory) in patients with RA were also the most frequent among controls.
Table 2.
Causes of death and crude mortality rates in patients with and without RA
| RA (N=29,779) | Non-RA (N=245,226) | Estimated Excess Deaths‡ | |||
|---|---|---|---|---|---|
| Event Type | Deaths | Mortality Rate* | Deaths | Mortality Rate* | |
| All Cause | 9,565 | 46.0 | 62,896 | 33.8 | 2,535 |
| Cardiovascular | 2,988 | 14.4 | 20,335 | 10.9 | 721 |
| CAD | 1,480 | 7.1 | 10,059 | 5.4 | 357 |
| Heart failure | 441 | 0.2 | 2,673 | 0.1 | 420 |
| Hypertension | 224 | 1.1 | 1,706 | 0.9 | 37 |
| Arrhythmia | 171 | 0.8 | 1,210 | 0.7 | 25 |
| Stroke | 148 | 0.7 | 1,250 | 0.7 | 2 |
| Valvular disease | 78 | 0.4 | 371 | 0.2 | 36 |
| PVD | 44 | 0.2 | 336 | 0.2 | 2 |
| VTE | 38 | 0.2 | 199 | 0.1 | 17 |
| Cancer | 2,305 | 11.1 | 15,784 | 8.6 | 516 |
| Lung | 951 | 4.6 | 5,009 | 2.7 | 389 |
| GI | 304 | 1.5 | 2,747 | 1.5 | −8 |
| Hematologic | 288 | 1.4 | 1,609 | 0.9 | 101 |
| Renal | 140 | 0.7 | 961 | 0.5 | 36 |
| Male GU | 134 | 0.6 | 1,417 | 0.8 | −32 |
| Endocrine | 116 | 0.6 | 958 | 0.5 | 12 |
| Head/neck | 80 | 0.4 | 623 | 0.3 | 18 |
| Skin | 55 | 0.3 | 345 | 0.2 | 13 |
| Brain | 20 | 0.1 | 292 | 0.2 | −22 |
| Respiratory | 1,531 | 7.3 | 7,963 | 4.3 | 637 |
| COPD | 860 | 4.1 | 5,037 | 2.7 | 298 |
| ILD | 223 | 1.1 | 519 | 0.3 | 161 |
| Infectious | 126 | 0.6 | 665 | 0.4 | 43 |
| Endocrine | 389 | 1.9 | 3,084 | 1.7 | 35 |
| Nervous system | 346 | 1.7 | 3,327 | 1.8 | −28 |
| Infection | 338 | 1.6 | 1,665 | 0.9 | 151 |
| Gastrointestinal | 328 | 1.6 | 2,495 | 1.4 | 37 |
| External causes | 318 | 1.9 | 2,399 | 1.6 | −15 |
| Mental health | 252 | 1.2 | 2,305 | 1.2 | 2 |
| Genitourinary | 239 | 1.1 | 1,678 | 0.9 | 52 |
| Blood disorders | 54 | 0.3 | 202 | 0.1 | 33 |
| Skin | 23 | 0.1 | 96 | 0.05 | 13 |
Abbreviations: CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; GU, genitourinary; ILD, interstitial lung disease; PVD, peripheral vascular disease; PY, person years; RA, rheumatoid arthritis; VTE, venous thromboembolism.
Per 1000 person-years
Estimated excess deaths in RA = Observed deaths – (Non-RA mortality rate x RA person-years of follow-up)
All-Cause Mortality and Broad Causes of Death
Unadjusted incidence rates for all-cause and cause-specific mortality in RA and matched non-RA patients are shown in Table 2. All-cause mortality rates were higher in patients with RA (46.0 deaths per 1000 PY, 95% CI 45.1-46.9) relative to non-RA patients (34.1 deaths per 1000 PY, 95% CI 33.8–34.3), as were rates of CVD-, cancer-, respiratory-, and infectious-related mortality rates. In total, 2,535 excess deaths occurred amongst patients with RA, of which 68.9% were attributable to cardiopulmonary disease (CVD, respiratory disease, and lung cancer). Kaplan-Meier survival curves for all-cause mortality and the most frequent categorical causes of death (CVD, cancer, and respiratory death) are shown in Supplementary Figure 1.
After matching on age, sex, and enrollment year and adjusting for pre-defined covariates, patients with RA were at a 23% increased risk of all-cause mortality (adjusted hazard ratio [aHR] 1.23, 95% CI 1.20-1.26) (Figure 1). RA was associated with a heightened risk of broad categories of deaths including CVD (aHR 1.19, 95% CI 1.14-1.23), cancer (aHR 1.19, 95% CI 1.14-1.24), and respiratory-related deaths (aHR 1.46, 95% CI 1.38-1.55), as well as a higher risk of death from infection (aHR 1.59, 95% CI 1.41-1.80). RA was also associated with a higher risk of external causes of death (aHR 1.11, 95% CI 1.00-1.24), skin disorders (aHR 2.73, 95% CI 2.11-3.54), and non-malignant blood disorders (primarily attributable to aplastic anemia, immunodeficiency, and hemorrhagic/coagulopathic etiologies; aHR 2.55, 95% CI 2.15-3.03), though these were infrequent. RA was not associated with deaths from endocrine, neurologic, gastrointestinal, mental health, or genitourinary causes.
Figure 1:
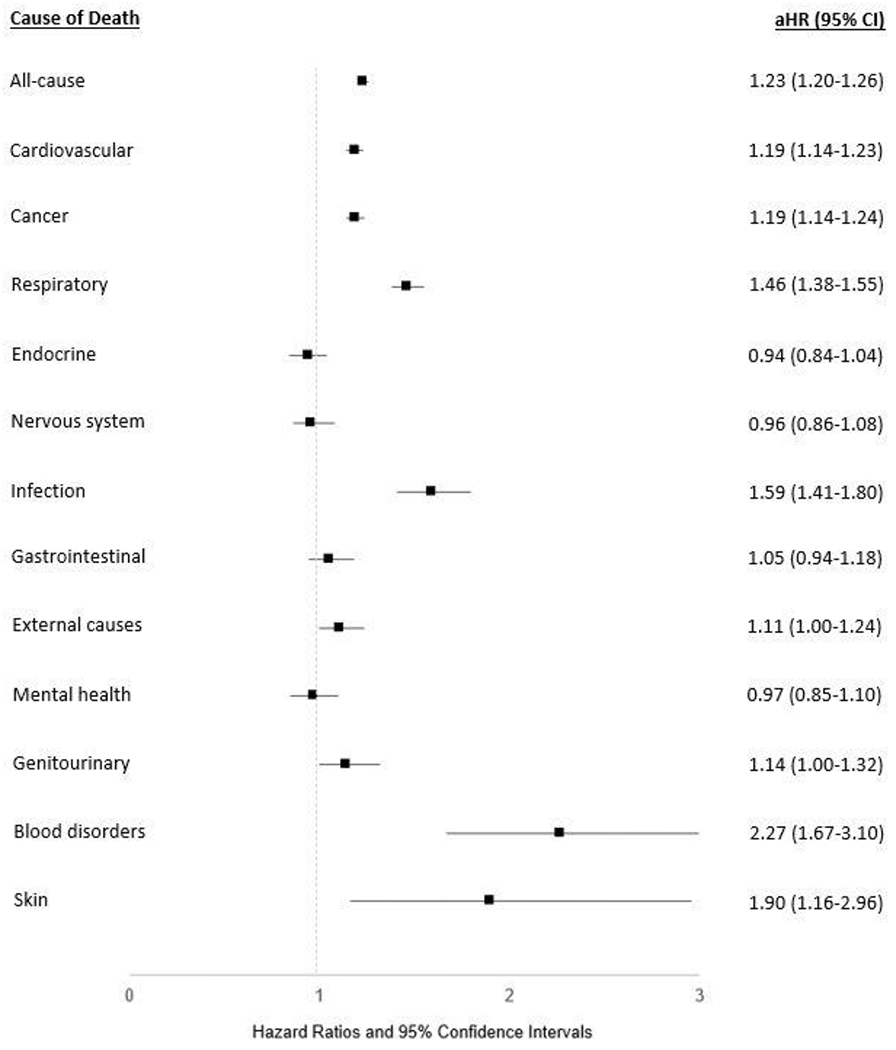
Risk of all-cause and categorical cause-specific mortality in U.S. Veterans with rheumatoid arthritis (RA). Forest plot illustrates the adjusted hazard ratios (aHR) and 95% confidence intervals (CI) for all-cause mortality and categorical causes of death in patients with RA vs. matched non-RA controls. Assessed using multivariable Cox regression models adjusting for age, sex, race, ethnicity, BMI, smoking status, comorbidity burden, and index year. Causes of death listed in order of decreasing frequency.
Mortality Risk for Specific Causes of Death
We then investigated the risk of specific causes of CVD-, cancer-, and respiratory-related mortality. Given their high frequency, the absolute risk difference in patients with RA was largest for death related to heart failure, lung cancer, coronary artery disease (CAD), and COPD (Table 2). After matching and multivariable adjustment, ILD was the most over-represented cause of cardiopulmonary or cancer-related death in patients with RA (aHR 3.39, 95% CI 2.88-3.99) (Figure 2). RA was also associated with a higher risk of respiratory-related infections (aHR 1.70, 95% CI 1.40-2.08) and COPD (aHR 1.24, 95% CI 1.15-1.33).
Figure 2:
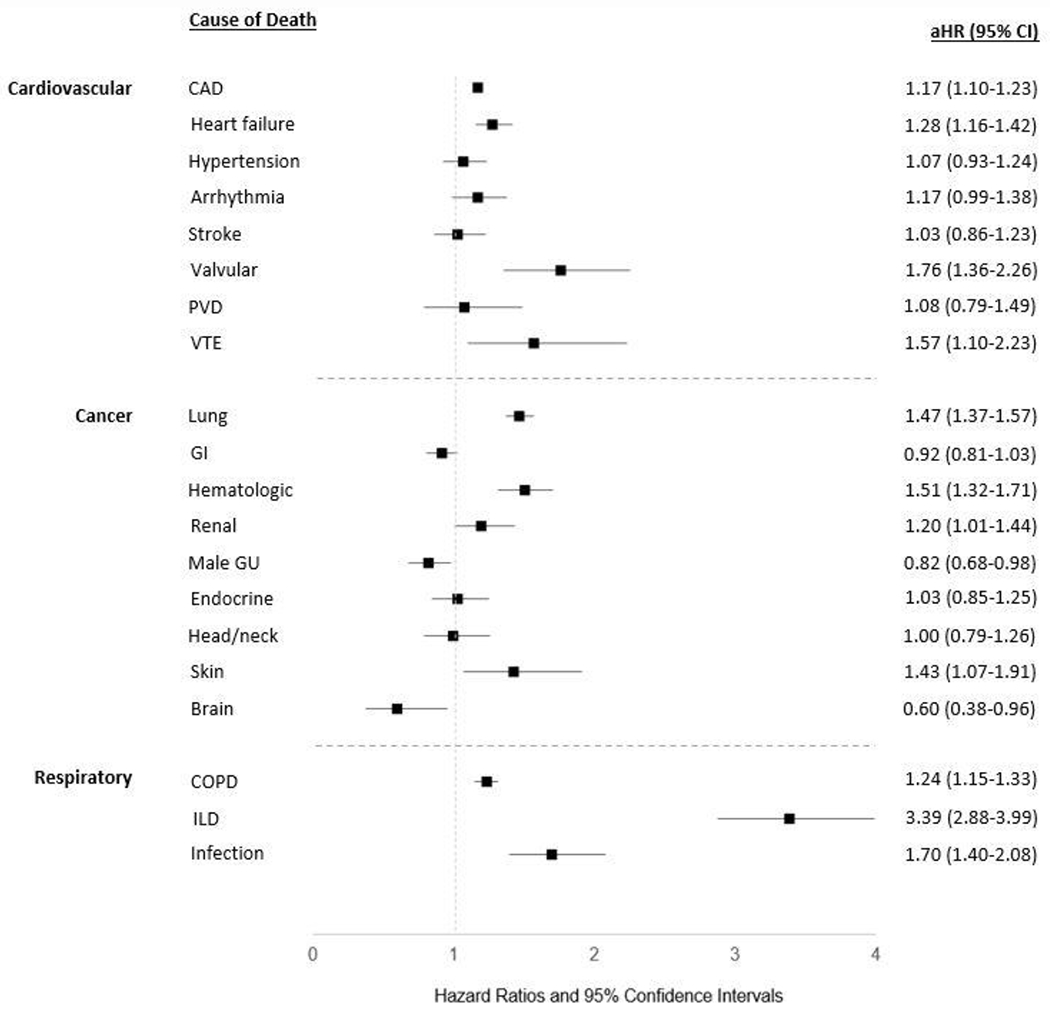
Risk of specific causes of cardiovascular-, cancer-, and respiratory-related mortality in U.S. Veterans with rheumatoid arthritis (RA). Forest plot illustrates the adjusted hazard ratio (aHR) and 95% confidence intervals (CI) for specific causes of death in patients with RA vs. matched non-RA controls. Assessed using multivariable Cox regression models adjusting for age, sex, race, ethnicity, BMI, smoking status, comorbidity burden, and index year.
Several causes of CVD-related death were overrepresented in patients with RA. The leading causes of CVD-related death, CAD (aHR 1.17, 95% CI 1.10-1.23) and heart failure (HF; aHR 1.28, 95% CI 1.16-1.42), were both more frequent in patients with RA. Higher risks of death from valvular heart disease (aHR 1.76, 95% CI 1.36-2.26) and venous thromboembolism (VTE; aHR 1.57, 95% CI 1.10-2.51) were also observed in patients with RA. RA was not associated with death from fatal stroke, peripheral vascular disease, arrhythmias, or hypertensive events. Within cancer-related causes of death, RA was associated with a higher risk of hematologic (aHR 1.51, 95% CI 1.32-1.71), lung (aHR 1.47, 95% CI 1.37-1.57), skin (aHR 1.43, 95% CI 1.07-1.91), and renal cancers (aHR 1.20, 95% CI 1.01-1.44). Patients with RA were not at increased risk of death from endocrine, head and neck, or gastrointestinal cancers, and were at a reduced risk of death from brain (aHR 0.60, 95% CI 0.38-0.96) and male genitourinary malignancies (aHR 0.82, 95% CI 0.68-0.98).
Sub-Analyses of Mortality Risk based on Sex, Seropositivity, and Age
RA associations with all-cause (aHR 1.23, 95% CI 1.21-1.26 in males; aHR 1.15, 95% CI 1.04-1.27 in females) and CVD-related mortality (aHR 1.19, 95% CI 1.14-1.23 in males; aHR 1.19, 95% CI 0.98-1.45 in females) were similar among males and females (Supplementary Figure 2). The higher risk of respiratory (aHR 1.47, 95% CI 1.39-1.56 in males; aHR 1.24, 95% CI 0.92-1.67 in females) and cancer-related (aHR 1.20, 95% CI 1.14-1.25 in males; aHR 1.02, 95% CI 0.82-1.27 in females) mortality observed in RA was accentuated among males. Patients with RA who were seropositive for RF and/or anti-CCP were at higher risk of all-cause (aHR 1.31, 95% CI 1.27-1.34), CVD (aHR 1.24, 95% CI 1.18-1.30), respiratory (aHR 1.67, 95% CI 1.56-1.79), and cancer-related (aHR 1.25, 95% CI 1.18-1.32) mortality. This was in contrast to seronegative RA patients in this cohort, who did not experience heightened risks of all-cause or cause-specific mortality (aHR range 0.94-1.02) (Supplementary Figure 3). Compared to the overall cohort, patients under 65 years of age experienced slightly attenuated risk of all-cause (aHR 1.16, 95% CI 1.11-1.20), CVD (aHR 1.16, 95% CI 1.08-1.25), cancer (aHR 1.10, 95% CI 1.02-1.19), and respiratory-related mortality (aHR 1.43, 95% CI 1.28-1.59).
Temporal Trends in Mortality
The excess all-cause mortality observed in RA significantly improved over sequential time periods (2000-2005 aHR 1.31, 95% CI 1.26-1.36; 2006-2011 aHR 1.23, 95% CI 1.19-1.27; 2012-2017 aHR 1.10, 95% CI 1.05-1.15; p = 0.01 for RA and time-period interaction) (Figure 3). RA-related risk of CVD, cancer, and respiratory mortality was numerically lowest in the most recent cohort, whereas the risk of infection related death in the 2006-2011 and 2012-2017 time periods were similar. While CVD and cancer-related mortality risk tended to improve over our study period, patterns of improvement were less consistent over time for respiratory and infectious-related mortality. Linear interactions between RA status and time period on the risk of these select causes of death were not significant. During the most recent time period (2012-2017), RA patients continued to have a 10% increased risk of all-cause mortality, 55% increased risk of infectious-related death, 33% increased risk of respiratory-related mortality, and 11% increased risk of death from CVD. Patients with RA also continued to experience a modestly higher risk of cancer related causes of death in the 2012-2017 time period (9%), but this did not reach statistical significance.
Figure 3:
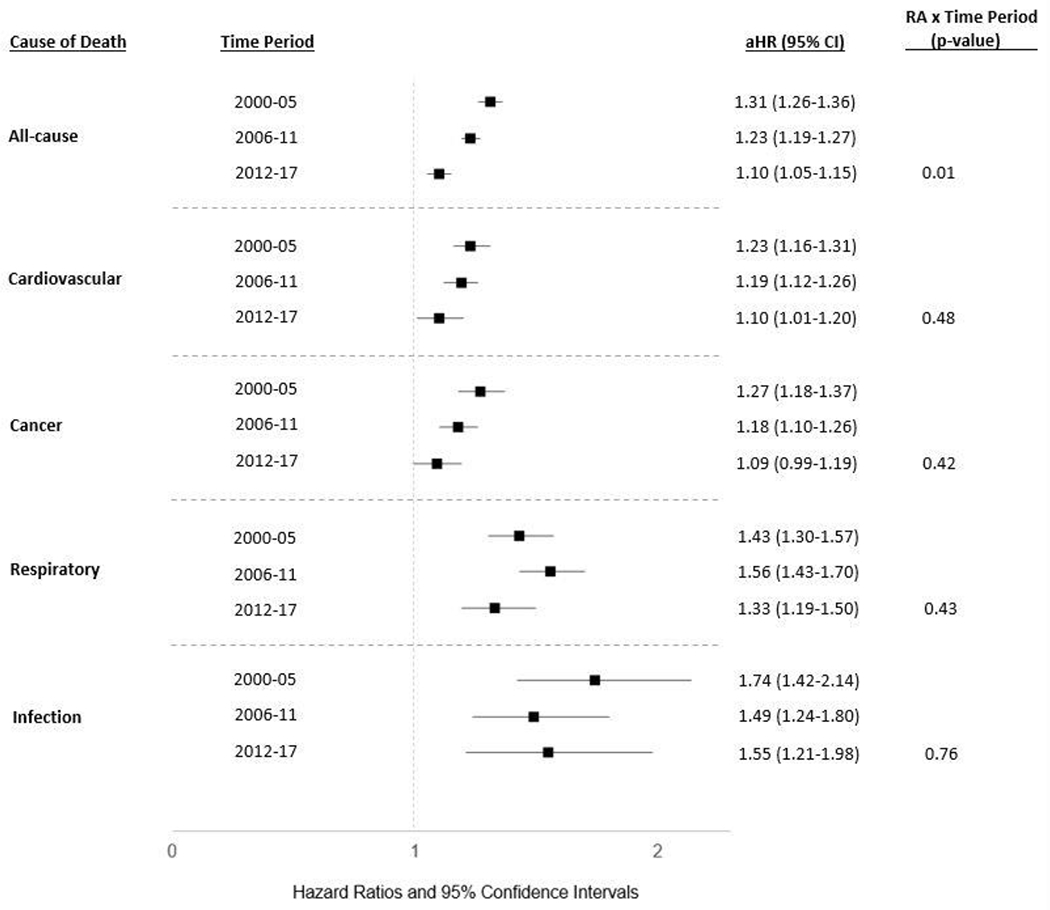
Temporal trends in all-cause and cause-specific mortality in U.S. Veterans with rheumatoid arthritis (RA). Forest plot illustrates the adjusted hazard ratios (aHR) and 95% confidence intervals (CI) for specific causes of death in multivariable Cox regression models stratified by time periods: 2000-2005, 2006-2011, and 2012-2017. A linear interaction (RA x sequential time periods) was used to examine time dependent changes in the RA-related risk of cause-specific mortality. Models adjusted for age, sex, race, ethnicity, BMI, smoking status, comorbidity burden, and index year.
To evaluate whether observed temporal trends were influenced by longer follow-up in earlier RA cohorts, sensitivity analyses were performed truncating follow-up for all time-periods to a maximum of 5 years. Compared to a near 1.5-fold increased risk of death in the 2000-2005 cohort (aHR 1.47, 95% CI 1.38-1.57), all-cause mortality significantly improved over time in the 2006-2011 (aHR 1.15, 95% CI 1.10-1.21) and 2012-2017 time periods (aHR 1.11, 95% CI 1.06-1.17; p=0.04 for RA and time period interaction). We again observed that the RA-related risk of death due to CVD, cancer, respiratory, and infectious diseases improved in the most contemporary cohorts but remained significantly elevated across all categories (Supplementary Figure 4). Lastly, we examined whether changes in RA diagnostic patterns impacted observed temporal trends by evaluating only matched pairs with RA patients positive for RF and/or anti-CCP. Restricting to seropositive RA patients resulted in modestly higher mortality risk estimates compared to the overall cohort (increase in aHR of 0.04-0.24), though temporal trends demonstrated similar patterns of numerical improvement in RA-related mortality risk in more recent time periods (Supplementary Figure 5). The linear trend interaction between RA status and time period did not reach statistical significance for all-cause or cause-specific mortality when restricting to seropositive patients.
Discussion
In this national, matched cohort study within the VHA, patients with RA were at a heightened risk of all-cause mortality, as well as death related to CVD, cancer, respiratory disease, and infection. Over an 18-year study period in the recent treatment era, improvements were observed in RA-associated risks of all-cause and cause-specific mortality. However, over 25% of deaths among patients with RA were premature, and a significant mortality gap persisted in RA during the most recent time period extending through 2017, emphasizing that continued efforts are needed to improve longevity in this high-risk population. Our results suggest that these efforts should be directed at cardiopulmonary diseases that included CVD, respiratory disease, and lung cancer, which together explained nearly 70% of the >2,500 excess deaths that occurred among patients with RA. This is the first study, to our knowledge, to examine risk and temporal trends in cause-specific mortality in RA using national-level data in the VHA, the largest integrated healthcare system in the U.S.
We found that RA-associated all-cause mortality risk improved significantly over our study time period. This is consistent with other studies that have shown the mortality gap between RA and the general population is narrowing (9, 10, 22). Despite this improvement, a mortality gap persisted in the most contemporary RA cohort, a finding shared by a population-based cohort study in the UK (10). In contrast, a matched cohort study of incident RA in British Columbia found that, following temporal improvements, patients with RA were no longer at an elevated risk of all-cause mortality (9). These differences may be related to variability in study time periods, age of study populations, and duration of follow-up as excess RA mortality is most evident after 10 years of disease (23). However, in sensitivity analyses restricting follow-up to a maximum of 5 years, all-cause mortality risk and trends did not significantly change in our study. Our study is additionally among the first to compare temporal trends in major causes of death amongst patients with RA (13, 22). Paralleling significant improvements in all-cause mortality, we found favorable trends in CVD-, cancer-, respiratory-, and infectious-related deaths. However, persistently increased risk of respiratory (33%), CVD (10%), and infectious-related death (55%), as well as a near-significant increase in cancer-related mortality (9%) among patients diagnosed with RA between 2012-2017 illustrates the need for continued efforts targeting the management and prevention of these conditions to improve longevity in patients with RA.
Chronic lung diseases are critical extra-articular manifestations in RA and account for a significant degree of morbidity and mortality in patients with RA (24). In this study, RA patients suffered from a near 50% increased risk of respiratory-related deaths. Leveraging validated administrative algorithms developed by our group to accurately capture ILD (16), we found that patients with RA were at an over three-fold increased risk of death related to ILD (HR 3.39, 95% CI 2.88-3.99), making this the most over-represented cause of death studied amongst patients with RA. The most frequent cause of respiratory-related death was COPD, corroborating previous findings in male and female RA cohorts (1, 11). Our findings reflect the increased incidence of both ILD and COPD in RA, even after accounting for cigarette smoking history, and the impact of these conditions on survival (24-26). Despite recognition of these epidemiologic findings, there are no standard approaches for the identification or management of RA-associated lung diseases. This study emphasizes the need for developing and evaluating such approaches to eliminate the persistent mortality burden posed by these conditions in patients with RA.
Consistent with prior literature (1, 11), CVD was the most frequent cause of death in patients with RA. CAD and HF remain the primary drivers of CVD mortality, but our study has highlighted additional, less frequent causes of CVD mortality afflicting RA patients. RA patients were at a 1.7-fold higher risk of valvular CVD mortality and a 1.5-fold higher risk of VTE-related mortality compared to matched non-RA patients. While there has been increasing interest in VTE risk with DMARDs, in particular Janus kinase inhibitors (JAKi), VTE risk related to RA itself should be recognized. Recent studies in RA cohorts have reported up to a nearly 2-fold increased risk of incident VTE independent of VTE risk factors and most pronounced in early and more active RA (27, 28). Given our study time periods and prior findings that JAKi use is infrequent in the VHA (12), we believe RA, rather than DMARDs, was the driver of VTE-mortality risk in our study. In contrast to VTE, there is a paucity of literature evaluating the risk of valvular heart disease in patients with RA. Small studies have demonstrated that valvular pathology assessed by echocardiography is more common in patients with RA, including valvular nodules and calcifications (29-31). Though acute phase responses were associated with more rapid progression of aortic stenosis in a RA cohort, rates of progression were no greater than those observed amongst patients in previous non-RA cohorts (32). Finally, patients with RA may have higher mortality rates following surgical aortic valve replacement (33). These novel findings of increased mortality related to valvular heart disease in RA call for clinical and epidemiologic investigations to better characterize valvular disorders in RA.
The immune system serves a key role in cancer prevention, and RA patients are recognized to have an increased risk of several cancers such as lymphoproliferative, lung, and skin cancers (34). In line with cancer incidence studies (1, 34, 35), we observed a 19% increased risk of cancer mortality, driven by increases in lung cancer (HR 1.47, 95% CI 1.37-1.57) and hematologic malignancies (HR 1.51, 95% CI 1.32-1.71). Trends towards lower gastrointestinal cancer mortality in RA patients in this study (HR 0.92, 95% CI 0.81-1.03) parallel the decreased incidence in colorectal cancers seen in RA cohorts previously and hypothesized to be related to nonsteroidal use (34, 36). We also observed a reduced risk of genitourinary and brain cancers amongst patients with RA in this cohort. A possible biological mechanism for these findings is unknown and we speculate this may be due to the competing risk of death from other causes. Encouragingly, cancer-related mortality risk numerically improved during our study and was no longer significant in the most recent time period, suggesting that despite more widespread use of biologic DMARDs, this has not negatively impacted cancer-related outcomes.
Death from another possible complication of both RA and DMARD therapy, infection (37), was also strongly associated with RA in this study (HR 1.59, 95% CI 1.41-1.80). Temporal trends of infectious-related mortality in RA during the biologic era are unknown. Infectious-related mortality in RA improved numerically in our study relative to the earliest time period. However, patients with RA continued to exhibit a 55% higher risk of infectious-related death in the 2012-2017 time period, the most over-represented categorical cause of death studied in our most contemporary RA cohort. These findings illustrate the need for provider vigilance toward infectious disease prevention, including counseling on recommended vaccinations, minimizing glucocorticoid use and dose (38), and carefully considering risks when initiating biologic and targeted synthetic DMARDs.
Consistent with the VHA population, our cohorts were male-predominant which may limit the generalizability of our findings. However, our sample size permitted stratified analyses by sex for major causes of death, finding similar risks of all-cause and CVD mortality in males and females with RA. The observed higher risk of respiratory and cancer-related mortality amongst males with RA is consistent with prior epidemiologic studies of respiratory disease and cancer incidence (39-41). RA and incident RA status were based on validated administrative algorithms and misclassification of RA status is possible, particularly amongst seronegative RA patients, which would bias our results to the null. Notably, seronegative patients did not experience elevated mortality risk in this study, findings consistent with other observational cohorts (11, 42, 43). As with any observational study, there is a risk for residual and/or unmeasured confounding since risk factors that may be associated with RA development and severity may also be associated with other health risks (e.g. diet, smoking, socioeconomic status). Future pharmacoepidemiologic studies are needed to evaluate whether temporal trends in RA medications, including glucocorticoids, or disease control may mediate temporal differences in mortality risk. Only baseline smoking status was available for adjustment, which may not fully capture the impact of smoking intensity and duration, or differential smoking cessation rates, on mortality risk. Small differences in age due to matching on calendar year of birth and variable matching frequency may overestimate RA-related mortality risk. There may also be misclassification of causes of death related to the use of NDI data, though such misclassification would be anticipated to be non-differential. Algorithms implemented to identify ILD in this cohort were previously validated in a RA cohort and may perform differently in non-RA patients. Lastly, temporal trends were assessed with linear-trend interactions, which may be underpowered to detect statistically significant improvements in cause-specific mortality.
In conclusion, we found that patients with RA experience premature all-cause, CVD, respiratory, cancer, and infectious mortality. Though favorable mortality trends were observed amongst patients diagnosed with RA over an 18-year period, there remains a persistent mortality gap between RA and non-RA patients. Over 2,500 excess deaths were observed amongst patients with RA, most of which were attributable to cardiopulmonary diseases including CVD, respiratory disease, and lung cancer. These findings illustrate the need for continued efforts to provide holistic care to improve the longevity of patients with RA and further underscore the potential for RA lives saved with improved prevention and management of cardiopulmonary disease.
Supplementary Material
Significance and Innovations.
This is among the first studies to evaluate temporal trends in cause-specific mortality in a national sample of patients with incident rheumatoid arthritis (RA).
Building on prior studies evaluating causes of death in RA with broad categories, we have directly assessed the risk of more specific causes of cardiovascular, cancer, and respiratory related mortality.
Despite improvements in RA management, a mortality gap persists in RA, highlighting a continued opportunity to improve longevity of patients with RA through advances in the management of this chronic, systemic disease.
Nearly 70% of excess deaths in RA are attributable to cardiopulmonary disease, (chronic lung disease, cardiovascular disease, and lung cancer), which should inform the development of prioritized prediction, prevention, and management strategies.
Acknowledgements
Work supported by Center of Excellence for Suicide Prevention, Joint Department of Veterans Affairs and Department of Defense Mortality Data Repository – National Death Index.
Funding:
TMJ is supported by the Rheumatology Research Foundation. BRE is supported by the VA CSR&D (CX002203). TRM is supported by the VA BLRD (BX004660), Department of Defense (PR200793), and National Institutes of General Medical Sciences (U54 GM115458). JFB is supported by the VA CSR&D (CX001703).
Disclosures:
BRE has consulted with Boehringer-Ingelheim. TRM has consulted with Pfizer, Sanofi, Gilead, Horizon Therapeutics and received research funding from Horizon Therapeutics.
References
- 1.England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, et al. Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2016;68(1):36–45. [DOI] [PubMed] [Google Scholar]
- 2.Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379–85. [DOI] [PubMed] [Google Scholar]
- 3.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008;26(5 Suppl 51):S35–61. [PubMed] [Google Scholar]
- 4.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37(4):481–94. [DOI] [PubMed] [Google Scholar]
- 5.Alemao E, Joo S, Kawabata H, Al MJ, Allison PD, Rutten-van Molken MP, et al. Effects of Achieving Target Measures in Rheumatoid Arthritis on Functional Status, Quality of Life, and Resource Utilization: Analysis of Clinical Practice Data. Arthritis Care Res (Hoboken). 2016;68(3):308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gullick NJ, Ibrahim F, Scott IC, Vincent A, Cope AP, Garrood T, et al. Real world long-term impact of intensive treatment on disease activity, disability and health-related quality of life in rheumatoid arthritis. BMC Rheumatol. 2019;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radner H, Alasti F, Smolen JS, Aletaha D. Physical function continues to improve when clinical remission is sustained in rheumatoid arthritis patients. Arthritis Res Ther. 2015;17:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ten Klooster PM, Oude Voshaar MAH, Fakhouri W, de la Torre I, Nicolay C, van de Laar M. Long-term clinical, functional, and cost outcomes for early rheumatoid arthritis patients who did or did not achieve early remission in a real-world treat-to-target strategy. Clin Rheumatol. 2019;38(10):2727–36. [DOI] [PubMed] [Google Scholar]
- 9.Lacaille D, Avina-Zubieta JA, Sayre EC, Abrahamowicz M. Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population-closing the mortality gap. Ann Rheum Dis. 2017;76(6):1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Lu N, Peloquin C, Dubreuil M, Neogi T, Avina-Zubieta JA, et al. Improved survival in rheumatoid arthritis: a general population-based cohort study. Ann Rheum Dis. 2017;76(2):408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparks JA, Chang SC, Liao KP, Lu B, Fine AR, Solomon DH, et al. Rheumatoid Arthritis and Mortality Among Women During 36 Years of Prospective Follow-Up: Results From the Nurses’ Health Study. Arthritis Care Res (Hoboken). 2016;68(6):753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England BR, Roul P, Yang Y, Kalil AC, Michaud K, Thiele GM, et al. Risk of COVID-19 in Rheumatoid Arthritis: A National Veterans Affairs Matched Cohort Study in At-Risk Individuals. Arthritis Rheumatol. 2021;73(12):2179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provan SA, Lillegraven S, Sexton J, Angel K, Austad C, Haavardsholm EA, et al. Trends in all-cause and cardiovascular mortality in patients with incident rheumatoid arthritis: a 20-year follow-up matched case-cohort study. Rheumatology (Oxford). 2020;59(3):505–12. [DOI] [PubMed] [Google Scholar]
- 14.Chung CP, Rohan P, Krishnaswami S, McPheeters ML. A systematic review of validated methods for identifying patients with rheumatoid arthritis using administrative or claims data. Vaccine. 2013;31 Suppl 10:K41–61. [DOI] [PubMed] [Google Scholar]
- 15.Curtis JR, Xie F, Chen L, Greenberg JD, Zhang J. Evaluation of a Methodologic Approach to Define an Inception Cohort of Rheumatoid Arthritis Patients Using Administrative Data. Arthritis care & research. 2018;70(10):1541–5. [DOI] [PubMed] [Google Scholar]
- 16.England BR, Roul P, Mahajan TD, Singh N, Yu F, Sayles H, et al. Performance of Administrative Algorithms to Identify Interstitial Lung Disease in Rheumatoid Arthritis. Arthritis care & research. 2020;72(10):1392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melzer AC, Pinsker EA, Clothier B, Noorbaloochi S, Burgess DJ, Danan ER, et al. Validating the use of veterans affairs tobacco health factors for assessing change in smoking status: accuracy, availability, and approach. BMC Med Res Methodol. 2018;18(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker JF, Billig E, Michaud K, Ibrahim S, Caplan L, Cannon GW, et al. Weight Loss, the Obesity Paradox, and the Risk of Death in Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(7):1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.England BR, Baker JF, Sayles H, Michaud K, Caplan L, Davis LA, et al. Body Mass Index, Weight Loss, and Cause-Specific Mortality in Rheumatoid Arthritis. Arthritis care & research. 2018;70(1):11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.England BR, Sayles H, Mikuls TR, Johnson DS, Michaud K. Validation of the rheumatic disease comorbidity index. Arthritis care & research. 2015;67(6):865–72. [DOI] [PubMed] [Google Scholar]
- 21.VA Office of Research & Development - VA Informatics and Computing Infrastructure [updated March 16, 2022]. Available from: https://www.research.va.gov/programs/vinci/default.cfm.
- 22.Myasoedova E, Gabriel SE, Matteson EL, Davis JM 3rd, Therneau TM, Crowson CS. Decreased Cardiovascular Mortality in Patients with Incident Rheumatoid Arthritis (RA) in Recent Years: Dawn of a New Era in Cardiovascular Disease in RA? J Rheumatol. 2017;44(6):732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radovits BJ, Fransen J, Al Shamma S, Eijsbouts AM, van Riel PL, Laan RF. Excess mortality emerges after 10 years in an inception cohort of early rheumatoid arthritis. Arthritis care & research. 2010;62(3):362–70. [DOI] [PubMed] [Google Scholar]
- 24.England BR, Sayles H, Michaud K, Thiele GM, Poole JA, Caplan L, et al. Chronic lung disease in U.S. Veterans with rheumatoid arthritis and the impact on survival. Clin Rheumatol. 2018;37(11):2907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuire K, Avina-Zubieta JA, Esdaile JM, Sadatsafavi M, Sayre EC, Abrahamowicz M, et al. Risk of Incident Chronic Obstructive Pulmonary Disease in Rheumatoid Arthritis: A Population-Based Cohort Study. Arthritis care & research. 2019;71(5):602–10. [DOI] [PubMed] [Google Scholar]
- 26.Sparks JA, Lin TC, Camargo CA Jr., Barbhaiya M, Tedeschi SK, Costenbader KH, et al. Rheumatoid arthritis and risk of chronic obstructive pulmonary disease or asthma among women: A marginal structural model analysis in the Nurses’ Health Study. Semin Arthritis Rheum. 2018;47(5):639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Lu N, Avina-Galindo AM, Zheng Y, Lacaille D, Esdaile JM, et al. The risk and trend of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a general population-based study. Rheumatology (Oxford). 2021;60(1):188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molander V, Bower H, Frisell T, Askling J. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis. 2021;80(2):169–75. [DOI] [PubMed] [Google Scholar]
- 29.Bacon PA, Gibson DG. Cardiac involvement in rheumatoid arthritis. An echocardiographic study. Ann Rheum Dis. 1974;33(1):20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roldan CA, DeLong C, Qualls CR, Crawford MH. Characterization of valvular heart disease in rheumatoid arthritis by transesophageal echocardiography and clinical correlates. Am J Cardiol. 2007;100(3):496–502. [DOI] [PubMed] [Google Scholar]
- 31.Yiu KH, Wang S, Mok MY, Ooi GC, Khong PL, Lau CS, et al. Relationship between cardiac valvular and arterial calcification in patients with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 2011;38(4):621–7. [DOI] [PubMed] [Google Scholar]
- 32.Bois JP, Crowson CS, Khullar T, Achenbach SJ, Krause ML, Mankad R. Progression rate of severity of aortic stenosis in patients with rheumatoid arthritis. Echocardiography. 2017;34(10):1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malmberg M, Palomaki A, Sipila JOT, Rautava P, Gunn J, Kyto V. Long-Term Outcomes of Surgical Aortic Valve Replacement in Patients with Rheumatoid Arthritis. J Clin Med. 2021;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji J, Liu X, Sundquist K, Sundquist J. Survival of cancer in patients with rheumatoid arthritis: a follow-up study in Sweden of patients hospitalized with rheumatoid arthritis 1 year before diagnosis of cancer. Rheumatology (Oxford, England). 2011;50(8):1513–8. [DOI] [PubMed] [Google Scholar]
- 36.Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–82. [DOI] [PubMed] [Google Scholar]
- 37.Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386(9990):258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George MD, Baker JF, Winthrop K, Hsu JY, Wu Q, Chen L, et al. Risk for Serious Infection With Low-Dose Glucocorticoids in Patients With Rheumatoid Arthritis : A Cohort Study. Ann Intern Med. 2020;173(11):870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gridley G, McLaughlin JK, Ekbom A, Klareskog L, Adami HO, Hacker DG, et al. Incidence of cancer among patients with rheumatoid arthritis. J Natl Cancer Inst. 1993;85(4):307–11. [DOI] [PubMed] [Google Scholar]
- 41.Parikh-Patel A, White RH, Allen M, Cress R. Risk of cancer among rheumatoid arthritis patients in California. Cancer Causes Control. 2009;20(6):1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikuls TR, Saag KG, Criswell LA, Merlino LA, Kaslow RA, Shelton BJ, et al. Mortality risk associated with rheumatoid arthritis in a prospective cohort of older women: results from the Iowa Women’s Health Study. Ann Rheum Dis. 2002;61(11):994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Schaardenburg D, Hazes JM, de Boer A, Zwinderman AH, Meijers KA, Breedveld FC. Outcome of rheumatoid arthritis in relation to age and rheumatoid factor at diagnosis. J Rheumatol. 1993;20(1):45–52. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


