Abstract
Background
Data on the protection conferred by COVID-19 vaccination and previous SARS-CoV-2 infection against omicron (B.1.1.529) infection in young children are scarce. We aimed to estimate the time-varying effects of primary and booster COVID-19 vaccination and previous SARS-CoV-2 infection on subsequent omicron infection and severe illness (hospital admission or death) in children younger than 12 years of age.
Methods
In this observational cohort study, we obtained individual-level records on vaccination with the BNT162b2 and mRNA-1273 vaccines and clinical outcomes from the North Carolina COVID-19 Surveillance System and the COVID-19 Vaccine Management System for 1 368 721 North Carolina residents aged 11 years or younger from Oct 29, 2021 (Oct 29, 2021 for children aged 5–11 years and June 17, 2022 for children aged 0–4 years), to Jan 6, 2023. We used Cox regression to estimate the time-varying effects of primary and booster vaccination and previous infection on the risks of omicron infection, hospital admission, and death.
Findings
For children 5–11 years of age, the effectiveness of primary vaccination against infection, compared with being unvaccinated, was 59·9% (95% CI 58·5–61·2) at 1 month, 33·7% (32·6–34·8) at 4 months, and 14·9% (95% CI 12·3–17·5) at 10 months after the first dose. Compared with primary vaccination only, the effectiveness of a monovalent booster dose after 1 month was 24·4% (14·4–33·2) and that of a bivalent booster dose was 76·7% (45·7–90·0). The effectiveness of omicron infection against reinfection was 79·9% (78·8–80·9) after 3 months and 53·9% (52·3–55·5) after 6 months. For children 0–4 years of age, the effectiveness of primary vaccination against infection, compared with being unvaccinated, was 63·8% (57·0–69·5) at 2 months and 58·1% (48·3–66·1) at 5 months after the first dose, and the effectiveness of omicron infection against reinfection was 77·3% (75·9–78·6) after 3 months and 64·7% (63·3–66·1) after 6 months. For both age groups, vaccination and previous infection had better effectiveness against severe illness as measured by hospital admission or death as a composite endpoint than against infection.
Interpretation
The BNT162b2 and mRNA-1273 vaccines were effective against omicron infection and severe outcomes in children younger than 12 years, although the effectiveness decreased over time. Bivalent boosters were more effective than monovalent boosters. Immunity acquired via omicron infection was high and waned gradually over time. These findings can be used to develop effective prevention strategies against COVID-19 in children younger than 12 years.
Funding
US National Institutes of Health.
Introduction
Omicron (B.1.1.529) is highly transmissible and can cause severe illness, especially in individuals who are immunocompromised. COVID-19 vaccination and previous SARS-CoV-2 infection have been shown to reduce the risk of omicron infection and severe outcomes in adults and adolescents.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 However, data on the effectiveness of COVID-19 vaccination in children younger than 12 years of age are scarce, and the effects of previous SARS-CoV-2 infection on omicron infection in these children are not well understood.3, 4, 12, 13, 14, 15, 16, 18, 19, 20, 21, 22, 23 Using surveillance data on COVID-19 vaccination and disease incidence from the state of North Carolina, USA, as of June 3, 2022, we previously estimated the effects of the BNT162b2 (Pfizer–BioNTech) vaccine and previous SARS-CoV-2 infection on infection with omicron's BA.1 and BA.2 lineages among children aged 5–11 years.24 Because of short follow-up, the long-term effects of vaccination and previous infection were unclear.
This Article presents the North Carolina surveillance data on COVID-19 vaccination and clinical outcomes in children younger than 12 years up to Jan 6, 2023. The expanded dataset allowed characterisation of the long-term effects of primary vaccination with the BNT162b2 or mRNA-1273 (Moderna) vaccine and previous SARS-CoV-2 infection on omicron infection and severe outcomes among children aged 5–11 years. The dataset also allowed comparison of the effectiveness of monovalent and bivalent boosters in this age group. Finally, it allowed estimation of the time-varying effects of primary vaccination with the BNT162b2 or mRNA-1273 vaccine and previous SARS-CoV-2 infection on omicron infection and severe outcomes among children younger than 5 years. This study covered all lineages of the omicron variant, including BA.1, BA.2, BA.4, BA.5, BQ.1-BQ.1.1, and XBB-XBB.1.5.
Research in context.
Evidence before this study
We searched PubMed from inception for reports up to Feb 12, 2023, without any language restrictions using the keywords (“COVID-19” OR “omicron”) AND (“vaccine*” OR “vaccination” OR “BNT162b2” OR “mRNA-1273”) AND (“protection” OR “effect*” OR “effectiveness” OR “evaluation” OR “VE”) AND “children”. We identified 13 studies on the efficacy or effectiveness of COVID-19 vaccination in children younger than 12 years of age, of which two were clinical trials and 11 were observational studies in real-world settings. Of the two clinical trials, one evaluated the safety, immunogenicity, and efficacy of mRNA-1273 vaccine in children 6–11 years of age at a time when B.1.617.2 (delta) was the dominant circulating variant, and one evaluated the safety, immunogenicity, and efficacy of mRNA-1273 vaccine in children 6 months to 5 years of age at a time when B.1.1.529 (omicron) was the dominant circulating variant. The 11 observational studies evaluated the effectiveness of the BNT162b2 or mRNA-1273 vaccine in children 5–11 years of age when B.1.617.2, B.1.1.529, BA.2/BA.2.12.1, or BA.4-BA.5 were the dominant circulating variants. The follow-up was shorter than 3 months for most studies. None of the studies evaluated the protection conferred by previous infection or bivalent vaccines in children 5–11 years of age, or the protection conferred by vaccination or previous infection in children younger than 5 years of age.
Added value of this study
We did an observational cohort study of more than 1 million children aged 11 years or younger from Oct 29, 2021, to Jan 6, 2023. We characterised the long-term effects of vaccination and previous infection on Omicron infection and severe outcomes in children 5–11 years of age. In addition, we compared the effectiveness of monovalent and bivalent boosters in this age group. Finally, we estimated the time-varying effects of vaccination and previous infection on Omicron infection and severe outcomes in children 0–4 years of age. Our study covered all lineages of the Omicron variant, including BA.1, BA.2, BA.4, BA.5, BQ.1-BQ.1.1, and XBB-XBB.1.5. We found that vaccination was effective against Omicron infection and severe outcomes, although the effectiveness decreased over time, bivalent boosters were more effective than monovalent boosters, and Immunity acquired via Omicron infection was very high and waned gradually over time.
Implications of all the available evidence
The findings from our study and previous studies can be leveraged to devise effective vaccination programmes for children younger than 12 years. Further research would be required to evaluate the effectiveness of bivalent vaccines against current and future circulating variants.
Methods
Study design and participants
In this observational cohort study in the USA, we considered children in two age groups, those aged 5–11 years and those aged 0–4 years. The US Food and Drug Administration (FDA) authorised the two-dose primary series of the BNT162b2 vaccine (10 μg per dose) for emergency use in children aged 5–11 years on Oct 29, 2021, and expanded eligibility for the BNT162b2 vaccine booster dose (10 μg per dose) to this age group on May 17, 2022.25, 26 On June 17, 2022, the FDA authorised the two-dose primary series of the mRNA-1273 vaccine for children as young as 6 months of age (25 μg per dose for children aged 6 months to 5 years, and 50 μg per dose for children aged 6–11 years) and the three-dose primary series of the BNT162b2 vaccine (3 μg per dose) for children aged 6 months to 4 years.27 The FDA authorisations were followed soon with Centers for Disease Control and Prevention recommendations, after which vaccines became widely available.
Data sources
The North Carolina COVID-19 Surveillance System (NC COVID) is a web-based central repository of person-level laboratory and communicable-disease investigation data. COVID-19 cases are populated according to lab reports from clinical laboratories that are mandated to report results. These cases include those identified by both molecular and antigen detection assays, but in general, only tests done under a Clinical Laboratory Improvement Amendments certificate or certificate of waiver are reported. At-home tests results are not included. The database contains positive SARS-CoV-2 test results for all cases and index reinfections using a unique person identifier and person-event infection variables. COVID-19-related hospital admission and deaths are documented through local health-department case investigation. For COVID-19 cases reported on Jan 1, 2022 onwards, vital records have been used to improve the completeness and accuracy of the COVID-19 death reporting.
The COVID-19 Vaccine Management System (CVMS) is a secure, cloud-based system that tracks information about provider enrolment and vaccine products administered and allows the state to manage vaccine supply. CVMS records are transferred daily to the North Carolina Department of Health and Human Services Business Intelligence Data Platform, where the data are processed to create a recipient-based view of COVID-19 vaccination history.
Procedures
We extracted individual-level data on vaccination histories from the authorisation date of Oct 29, 2021 to the study end date of Jan 6, 2023 for children aged 5–11 years and from the authorisation date of June 17, 2022 to Jan 6, 2023 for children 0–4 years of age, and we also collected individual-level data on SARS-CoV-2 infection, hospital admission, and death from March 11, 2020, to Jan 6, 2023 for children 0–11 years of age, by linking the NC COVID and CVMS databases through a Master Patient Index. We included both healthy children and children with underlying conditions, including those who were immunocompromised. Of note, infection was both an exposure and an outcome, in that we studied the effects of previous infection on future infection and severe outcomes. Although we were primarily interested in the omicron variant, we considered all infections that had been recorded, because any previous infections might affect the risk of omicron infection and might also change vaccine effectiveness against omicron.
The NC COVID and CVMS databases covered all residents who had been vaccinated or had positive lab results reported to health departments. Residents who had been neither vaccinated nor infected were not included. To establish the number of those residents and their demographic characteristics, we used the 2020 Bridged-Race Population estimates produced by the US Census Bureau to determine the total number of residents with each combination of demographic variables (ie, age, sex, race and ethnicity, geographical region such as coastal plains, Piedmont, or mountains, and county-level vaccination rates).
We used the population-level variant prevalence data specific to the state of North Carolina, available from CoVariants.org. The delta (B.1.617.2) variant was predominant in November and early December, 2021, whereas the omicron variant became predominant by late December, 2021. Thus, we studied vaccine effectiveness from the tail of the delta period through the entire omicron period for children aged 5–11 years and only during the omicron period for children aged 0–4 years.
This study was approved by the University of North Carolina at Chapel Hill Institutional Review Board. The study was determined to be public health surveillance as defined in the US Department of Health and Human Services, Title 45 Code of Federal Regulations, §46 Protection of Human Subjects (45 CFR §46.102[l]), and thus informed consent was not required.
Statistical analysis
We considered two types of outcomes, SARS-CoV-2 infection and composite endpoint of hospital admission and death. The composite endpoint was defined as time to severe SARS-CoV-2 infection that results in hospital admission or death. We treated SARS-CoV-2 infection as a recurrent event and related the rate of infection to immunity-conferring events (ie, vaccination and previous infection) through the proportional rates model.28 In addition, we related the hazard of severe SARS-CoV-2 infection to immunity-conferring events through the proportional hazards model.29
The effect of each exposure (ie, vaccination or previous infection) on the risk of SARS-CoV-2 infection was characterised by a time-varying rate ratio (RR), and its effect on severe SARS-CoV-2 infection was characterised by a time-varying hazard ratio (HR), as a function of the time elapsed since the exposure. Stratification by the date of exposure and interactions between the two exposures were allowed.8, 24 We approximated the log RR or HR for the vaccine effect by a piecewise linear function with change points at 1 month and 2 months after the first dose, and we approximated the log RR or HR for the effect of previous infection by a linear function starting 2 weeks after the initial diagnosis. We measured each event time from the start of the study to control for time-varying confounders (eg, circulating strains, use of masks, and school opening) by comparing disease incidence between vaccinated and unvaccinated children, and between children previously infected and those uninfected, on the same date.8, 24 We included demographic variables (age, sex, race and ethnicity, geographical region, and county-level vaccination rate) as covariates to adjust for potential confounding by individual characteristics and geographical location.
In the first set of analyses, we estimated the effects of primary vaccination (two doses) and previous SARS-CoV-2 infection on the two types of outcomes in children 5–11 years of age. We estimated the overall effects of these two exposures and the effects of vaccination on infection by date of first dose and by previous infection status, and the effects of previous infection on future infection by virus strain and by vaccination status. Thus, we estimated the effects of each exposure alone, rather than in combination, but accommodated interactions (ie, allowing vaccine effectiveness to be modified by previous infection status and allowing the effect of previous infection to be modified by vaccination status). We included participants with only one dose in the model but did not plan to report the effects of one dose.
In the second set of analyses, we estimated the effectiveness of booster vaccination (third dose), relative to only primary vaccination, and compared monovalent and bivalent boosters in children 5–11 years of age. We included the date of primary series as an additional covariate in the model.
The third set of analyses was similar to the first, but for children 0–4 years of age. For this age group, the third dose of the BNT162b2 vaccine was considered part of the primary series, whereas the third dose of the mRNA-1273 vaccine was considered a booster. In both cases, the third dose was recommended to be administered at least 8 weeks after the second dose, such that not many children had taken a third dose. Thus, we estimated only the effects of the first two doses for both the mRNA-1273 and BNT162b2 vaccines.
The effectiveness of vaccination and previous infection was defined by one minus the RR or HR, multiplied by 100%. The parameters in each model were estimated by maximising the partial likelihood with potentially censored observations.8 Corresponding 95% CIs were constructed.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The demographic characteristics, vaccine uptakes, and clinical outcomes of the 1 368 721 study participants have been summarised (table ). As of Jan 6, 2023, 39 261 children 5–11 years of age had only received one dose of an mRNA vaccine, 216 330 had received only two doses, and 46 895 had received three doses; 11 235 children 0–4 years of age had received only one dose of an mRNA vaccine, 28 066 has received only two doses, and 11 529 had received three doses.
Table.
Demographic and clinical characteristics of study participants
|
0–4 years of age |
5–11 years of age |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of children | Infections | Hospitalisations | Deaths | Number of children | Infections | Hospitalisations | Deaths | ||
| Total | 481 528 | 127 211 | 691 | 5 | 887 193 | 257 592 | 426 | 10 | |
| Unvaccinated | 430 698 | 584 707 | |||||||
| Before authorisation | 90 221 | 509 | 4 | 76 619 | 176 | 4 | |||
| After authorisation | 27 449 | 161 | 1 | 116 108 | 141 | 5 | |||
| Vaccinated | 50 830 | 302 486 | |||||||
| Before authorisation | 7447 | 15 | 0 | 22 381 | 57 | 0 | |||
| After authorisation | 2094 | 6 | 0 | 42 484 | 52 | 1 | |||
| One dose only | 11 235 | 39 261 | |||||||
| Before first dose | 2083 | 6 | 0 | 5372 | 9 | 0 | |||
| After first dose | 159 | 0 | 0 | 3319 | 3 | 0 | |||
| Two doses only | 28 066 | 216 330 | |||||||
| Before second dose | 4913 | 8 | 0 | 28 550 | 56 | 0 | |||
| After second dose | 341 | 1 | 0 | 18 132 | 26 | 1 | |||
| Three doses | 11 529 | 46 895 | |||||||
| Before third dose | 1966 | 6 | 0 | 8584 | 14 | 0 | |||
| After third dose | 79 | 0 | 0 | 908 | 1 | 0 | |||
| Sex | |||||||||
| Female | 235 838 | 60 859 | 308 | 4 | 434 917 | 125 552 | 199 | 4 | |
| Male | 245 690 | 66 352 | 383 | 1 | 452 276 | 132 040 | 227 | 6 | |
| Race and ethnicity | |||||||||
| Black or Hispanic | 206 120 | 49 191 | 333 | 1 | 369 667 | 98 221 | 191 | 4 | |
| Other | 275 408 | 78 020 | 358 | 4 | 517 526 | 159 371 | 235 | 6 | |
| Geographical region | |||||||||
| Coastal plain | 139 930 | 34 034 | 205 | 0 | 250 132 | 69 609 | 117 | 1 | |
| Piedmont | 299 124 | 83 002 | 441 | 5 | 557 837 | 165 417 | 264 | 8 | |
| Mountain | 42 474 | 10 175 | 45 | 0 | 79 224 | 22 566 | 45 | 1 | |
| County vaccination | |||||||||
| <62% | 165 702 | 41 659 | 270 | 1 | 299 029 | 83 985 | 152 | 1 | |
| 62–75% | 143 831 | 37 474 | 234 | 1 | 274 978 | 78 769 | 149 | 5 | |
| >75% | 171 995 | 48 078 | 187 | 3 | 313 186 | 94 838 | 125 | 4 | |
Between the vaccination authorisation date of Oct 29, 2021 and the study end date of Jan 6, 2023, the 584 707 children 5–11 years of age who were unvaccinated had 116 108 SARS-CoV-2 infections, 141 of which were known to result in hospital admission and five of which were known to result in death, whereas the 302 486 children 5–11 years of age who were vaccinated had 42 484 SARS-CoV-2 infections, 52 of which were known to result in hospital admission and only one of which was known to result in death. Between the vaccine authorisation date of June 17, 2022 and Jan 6, 2023, the 430 698 children 0–4 years of age who were unvaccinated had 27 449 SARS-CoV-2 infections, 161 of which were known to result in hospital admission and one was known to result in death, whereas the 50 830 children 0–4 years of age who were vaccinated had 2094 SARS-CoV-2 infections, six of which were known to result in hospital admission and none of which were known to result in death.
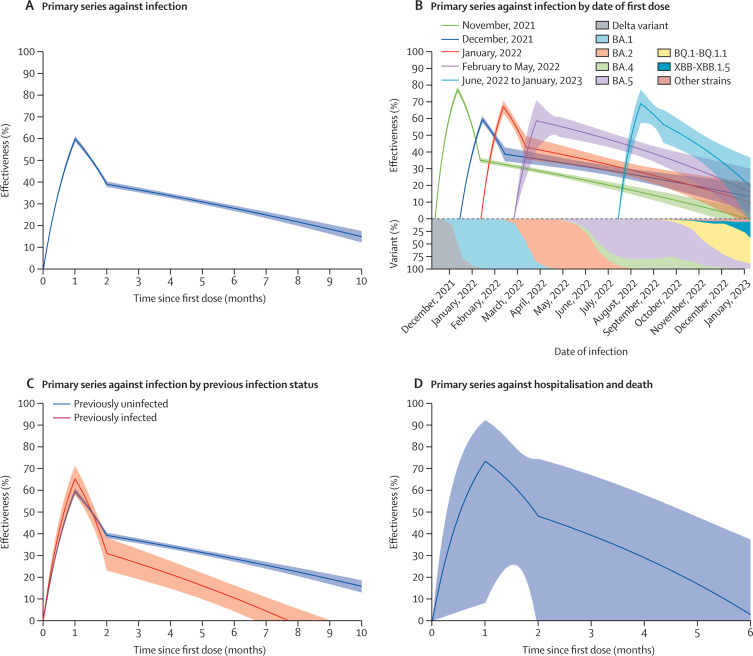
We estimated the effectiveness of the primary vaccine series over time, as compared with being unvaccinated, in children 5–11 years of age (figure 1 ). The effectiveness against infection reached 59·9% (95% CI 58·5–61·2) at 1 month after the first dose, and it decreased to 33·7% (32·6–34·8) at 4 months and to 14·9% (12·3–17·5) at 10 months (figure 1A; appendix p 1). The effectiveness was lower in Black and Hispanic children than in children of other ethnicities (appendix p 16). The effectiveness patterns varied among children who were vaccinated on different dates, and the emergence of the BQ.1-BQ.1.1 and XBB-XBB.1.5 strains seemed to reduce vaccine effectiveness (figure 1B; appendix p 2).
Figure 1.
Effectiveness of two doses of an mRNA vaccine, compared with no vaccination, against omicron infection and severe outcomes in children 5–11 years of age
(A) Vaccine effectiveness against infection. (B) Vaccine effectiveness against infection by date of first dose. Each curve starts at the median date of the first dose for the children in that cohort. (C) Vaccine effectiveness against infection by previous infection status. Effectiveness was calculated for vaccination alone given the status of previous infection. Estimates of effectiveness are shown by solid curves, and 95% CIs are shown by shaded bands. (D) Vaccine effectiveness against severe infection resulting in hospitalisation or death.
Among children with a previous infection, the effectiveness of primary vaccination (compared with being unvaccinated) against infection reached 65·3% (95% CI 58·1–71·2) at 1 month and declined to 26·4% (19·2–33·0) at 3 months and to 10·6% (4·4–16·4) at 6 months; among children previously uninfected, the effectiveness of primary vaccination reached a level of 59·7% (58·3–61·0) at 1 month and declined to 36·8% (35·7–38·0) at 3 months and to 28·6% (27·3–29·9) at 6 months (figure 1C; appendix p 3).
The effectiveness of primary vaccination (compared with being unvaccinated) against severe infection resulting in hospital admission or death reached a level of 73·3% (8·3–92·3) at 1 month and waned afterwards (figure 1D; appendix p 4). The estimates were highly variable because of the small number of events.
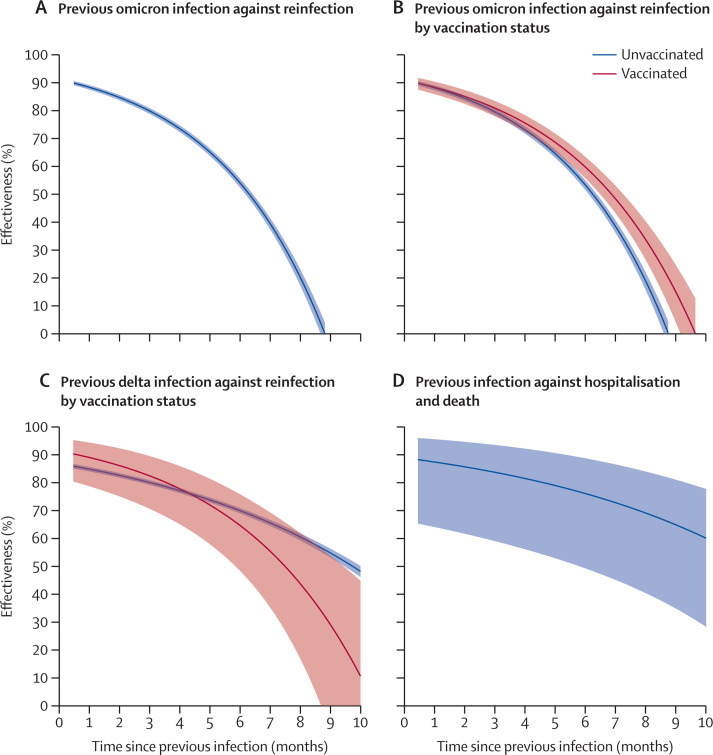
The effectiveness of previous SARS-CoV-2 infection over time, as compared with no previous infection, in children 5–11 years of age was calculated (figure 2 ). The effectiveness of previous infection with the omicron variant against reinfection with the omicron variant was 79·9% (78·8–80·9) at 3 months and 53·9% (52·3–55·5) at 6 months (figure 2A; appendix p 5). The long-term effectiveness of previous infection (compared with no previous infection) was higher among children who were vaccinated than those who were unvaccinated (figure 2B; appendix p 6). The effectiveness of previous infection with the delta variant (compared with no previous infection) against future infection was similar between vaccinated and unvaccinated children (figure 2C; appendix p 7). The effectiveness of previous infection (compared with no previous infection) against severe reinfection resulting in hospital admission or death was 83·8% (59·1–93·6) at 3 months, 76·2% (49·4–88·8) at 6 months, and 64·9% (34·9–81·1) at 9 months (figure 2D; appendix p 8).
Figure 2.
Effectiveness of previous SARS-CoV-2 infection, compared with no previous infection, against future infection and severe outcomes in children 5–11 years of age
(A) Effectiveness of previous omicron infection against Omicron reinfection. (B) Effectiveness of previous omicron infection against omicron reinfection by vaccination status. (C) Effectiveness of previous infection with the delta variant against reinfection by vaccination status. (D) Effectiveness of previous infection against severe reinfection resulting in hospitalisation or death. Effectiveness was calculated for previous infection alone given the vaccination status. Estimates of effectiveness are shown by solid curves, and 95% CIs are shown by shaded bands (B, C).
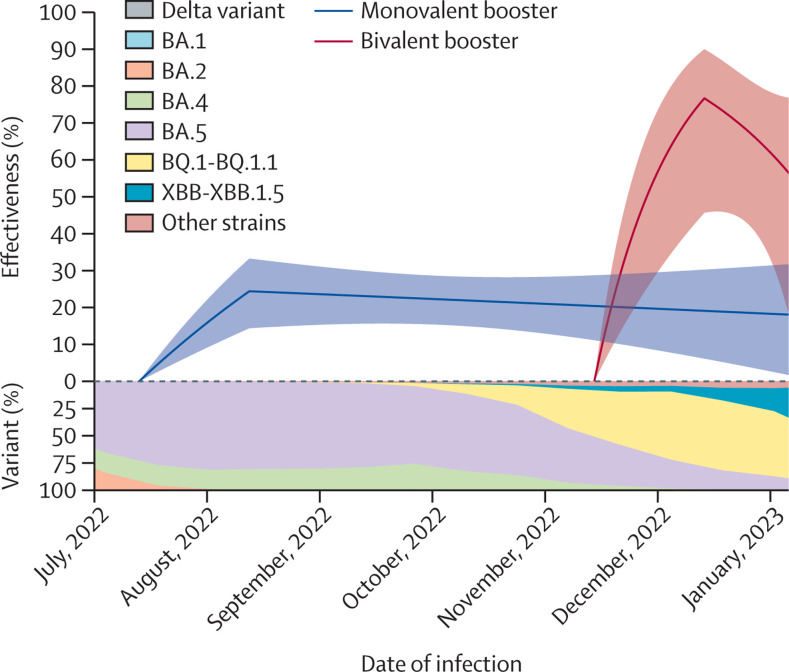
The effectiveness of a booster (third) dose, compared with two doses, against SARS-CoV-2 infection in children 5–11 years of age was calculated (figure 3 ). 31 319 children received monovalent boosters, and 15 576 received bivalent boosters. The effectiveness of a monovalent booster dose was 24·4% (14·4 to 33·2) after 1 month and 23·1% (15·6 to 30·0) after 2 months, whereas that of a bivalent booster dose was 76·7% (45·7 to 90·0) after 1 month and 47·3% (–17·9 to 76·4) after 2 months (appendix p 9). We were unable to obtain an accurate estimate for the booster effectiveness against hospital admission or death because there was only 1 hospital admission and no deaths after receipt of a booster dose (table).
Figure 3.
Effectiveness of a monovalent or bivalent booster dose, compared with two monovalent doses, against omicron infection in children 5–11 years of age
Estimates of effectiveness are shown by solid curves, and 95% CIs are shown by shaded bands.
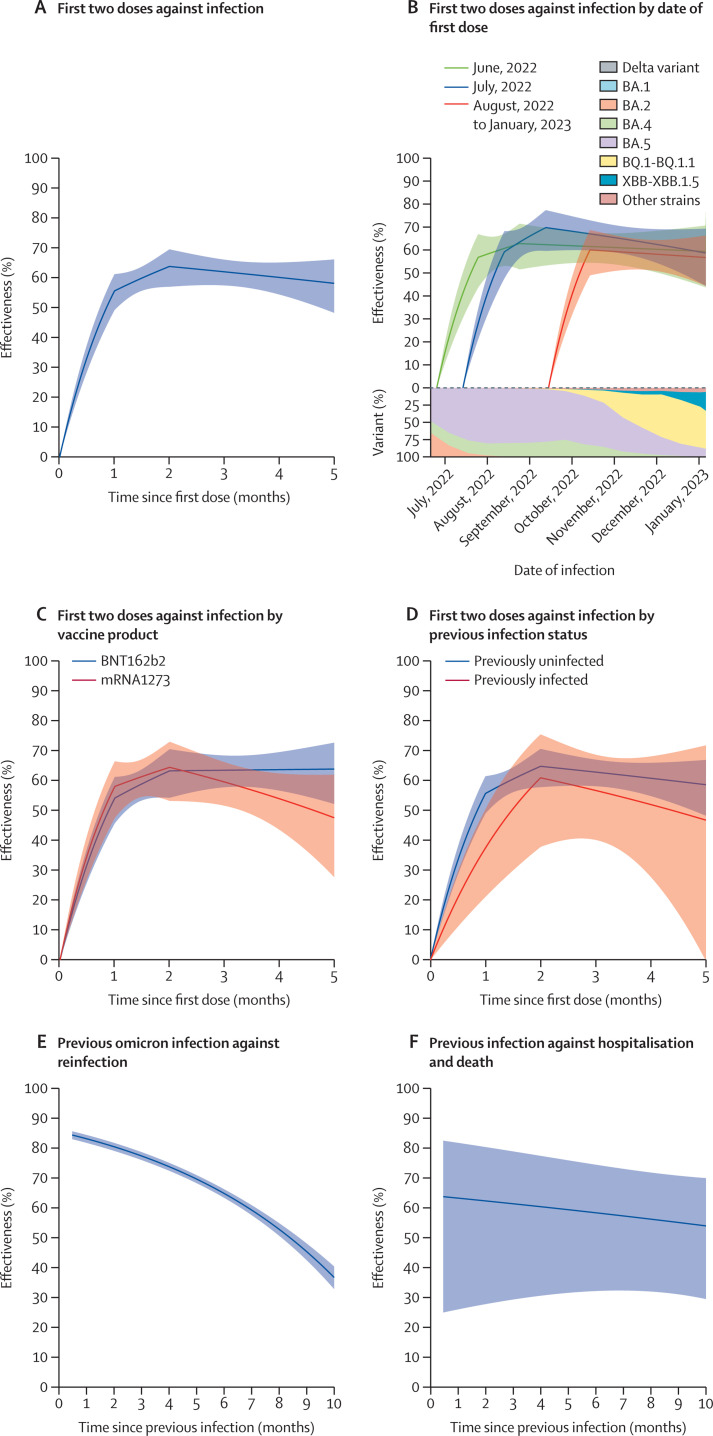
The effectiveness of the two doses of a primary vaccine series, compared with being unvaccinated, against SARS-CoV-2 infection over time in children 0–4 years of age was calculated (figure 4A–D ). The effectiveness reached a level of 63·8% (57·0–69·5) at 2 months after the first dose and decreased to 58·1% (48·3–66·1) at 5 months (figure 4A; appendix p 10). The effectivenss patterns varied slightly among children who were vaccinated on different dates (figure 4B; appendix p 11). Both the mRNA-1273 and BNT162b2 vaccines were effective (figure 4C; appendix p 12). Vaccination was effective for children previously infected and previously uninfected (figure 4D; appendix p 13).
Figure 4.
Effectiveness of two doses of an mRNA vaccine, compared with no vaccination, and effectiveness of previous SARS-CoV-2 infection, compared with no previous infection, against subsequent infection and severe outcomes in children 0–4 years of age
(A) Vaccine effectiveness against infection. (B) Vaccine effectiveness against infection by date of first dose. Each curve starts at the median date of the first dose for the children in that cohort. (C) Vaccine effectiveness against infection by vaccine product. (D) Vaccine effectiveness against infection by previous infection status. Effectiveness was calculated for vaccination alone given the status of previous infection. (E) Effectiveness of omicron infection against reinfection with Omicron. (F) Effectiveness of previous infection against severe reinfection resulting in hospitalisation or death. Estimates of effectiveness are shown by solid curves, and 95% CIs are shown by shaded bands.
Only one of the 50 830 vaccinated children was known to be hospitalised after vaccination and none were known to die (table 1).
The effectiveness of previous SARS-CoV-2 infection over time, compared with no previous infection, in children 0–4 years of age was calculated (figure 4E–F). The effectiveness of previous infection with the omicron variant against reinfection with the omicron variant was 77·3% (75·9–78·6) at 3 months, 64·7% (63·3–66·1) at 6 months, and 45·2% (42·4–48·0) at 9 months (figure 4E; appendix p 14). The effectiveness of previous infection against severe reinfection resulting in hospital admission or death was 61·4% (29·4–78·9) at 3 months, 58·4% (32·2–74·5) at 6 months, and 55·1% (31·1–70·8) at 9 months (figure 4F; appendix p 15).
Discussion
This study yielded important findings about COVID-19 vaccination and previous SARS-CoV-2 infection in children younger than 12 years. First, vaccination was effective against omicron infection and severe illness (hospital admission or death as composite endpoint), although the effectiveness waned over time. Second, both the mRNA-1273 and BNT162b2 vaccines were effective. Third, bivalent boosters were more effective than monovalent boosters. Fourth, previous SARS-CoV-2 infection induced strong immunity against future infection, although the immunity waned gradually over time. Fifth, vaccination provided additional protection for previously infected children, and omicron infection induced strong immunity in both vaccinated and unvaccinated children. This finding is consistent with previous knowledge that vaccination provides a better cross-variant protective immune response than infection alone.30 Finally, the effectiveness of both vaccination and previous infection was higher for children younger than 5 years than for children 5–11 years of age.
The number of deaths was too small to permit separate estimation of the vaccine effectiveness on death. Some children died before being hospitalised, so it would be difficult to consider hospital admission in isolation. Because most deaths occurred after hospital admissions and there were many more hospital admissions than deaths, the composite endpoint of hospitalisation and death is dominated by hospitalisation, such that the vaccine effectiveness on the composite endpoint represents primarily the vaccine effectiveness on hospital admission.
Our estimates for the effectiveness of vaccination in children younger than 12 years were lower than those of adults and adolescents previously reported.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 31 However, vaccines were administered in children younger than 12 years later than in adults and adolescents, and the emergence of recent variants and the high prevalence of natural immunity reduced vaccine effectiveness. We note also that the dosages for children younger than 12 years were lower than those of older individuals. For individuals 12 years and older, the BNT162b2 vaccine was administered at 30 μg per dose, and the mRNA-1273 vaccine was administered at 100 μg per dose in the primary series and 50 μg per dose as a booster.
Our observation that bivalent boosters were more effective than monovalent boosters in children 5–11 years of age was consistent with findings from a recent study in adults and adolescents.17 However, bivalent boosters were not authorised in children 5–11 years of age until Oct 12, 2022. Thus, the number of children receiving bivalent boosters was small, and the follow-up was short.
Immunity acquired via omicron infection against omicron reinfection appeared similar between children younger than 12 years and older individuals.8 However, the protection of omicron infection against reinfection in adults and adolescents was studied over a relatively short time period.8
Our study had some limitations. Vaccination rates were much lower in children younger than 12 years than in adults and adolescents, such that there was a great potential for selection bias in our study. Children who were immunocompromised were more likely to be vaccinated than those who were immunocompetent, which would dilute vaccine effectiveness estimates. Indeed, the third dose was part of the primary series for children who were immunocompromised rather than a booster dose. We did not have the information to separate additional doses for children who were immunocompromised from booster doses, such that our estimates of booster effectiveness pertained to third doses, which might not be booster doses.
Our records on admissions to intensive care units were incomplete, and we did not collect data on non-invasive ventilation. In addition, we did not collect data on myocarditis or other side-effects.
Lastly, at-home SARS-CoV-2 tests became more common since January, 2022. Our database did not include home-based testing results and thus under-represented the cases of SARS-CoV-2 infection. In addition, some COVID-19 cases were not investigated by local health departments or families could not be contacted, such that their hospital admission and survival status was unknown. Starting on Jan 1, 2022, vital records were used as an additional step to identify COVID-19 deaths. Thus, it was unlikely for COVID-19 deaths to be unreported, given the rarity of these events in young children and the higher degree of scrutiny. Our estimates of vaccine effectiveness would be biased if there were differential ascertainments of COVID-19 cases or clinical outcomes between children who were vaccinated and children who were unvaccinated.
Despite their limitations, surveillance data are crucially important to our understanding of the protection conferred by vaccination and previous infection in young children. Compared with the test-negative design, the cohort design (with the calendar date as the time index) provides better control of time-varying confounders and allows more precise characterisation of the waning immunity from vaccination and previous infection.
Data sharing
De-identified individual-level participant data and data dictionary will be available for sharing after approval of a proposal by the North Carolina Department of Public Health and following a signed data access agreement. Requests for data sharing can be directed to D-YL.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This study was partly supported by a Dennis Gillings Distinguished Professorship (to D-YL) and National Institutes of Health R01 grants (D-YL and DZ).
Contributors
D-YL designed the study, supervised the data analysis, and prepared the manuscript. YX and YG did the data analysis and prepared the figures and tables. DZ supervised the data analysis. BW, HY, ZM, and SKS collected data and contributed to data interpretation. SKS, YX, and YG directly accessed and verified the data. D-YL decided to submit the manuscript for publication. All authors reviewed and approved the final text.
Supplementary Material
References
- 1.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386:1603–1614. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price AM, Olson SM, Newhams MM, et al. BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med. 2022;386:1899–1909. doi: 10.1056/NEJMoa2202826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming-Dutra KE, Britton A, Shang N, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA. 2022;327:2210–2219. doi: 10.1001/jama.2022.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. 2022;386:1712–1720. doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin D-Y, Gu Y, Xu Y, et al. Association of primary and booster vaccination and prior infection with SARS-CoV-2 infection and severe COVID-19 outcomes. JAMA. 2022;328:1415–1426. doi: 10.1001/jama.2022.17876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Protective effect of previous SARS-CoV-2 infection against omicron BA.4 and BA.5 subvariants. N Engl J Med. 2022;387:1620–1622. doi: 10.1056/NEJMc2209306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal U, Bedston S, McCowan C, et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet. 2022;400:1305–1320. doi: 10.1016/S0140-6736(22)01656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin ET, Leidner D, Lamson L, et al. Protection against omicron from vaccination and previous infection in a prison system. N Engl J Med. 2022;387:1770–1782. doi: 10.1056/NEJMoa2207082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chemaitelly H, AlMukdad S, Ayoub HH, et al. Covid-19 vaccine protection among children and adolescents in Qatar. N Engl J Med. 2022;387:1865–1876. doi: 10.1056/NEJMoa2210058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amir O, Goldberg Y, Mandel M, et al. Initial protection against SARS-CoV-2 omicron lineage infection in children and adolescents by BNT162b2 in Israel: an observational study. Lancet Infect Dis. 2023;23:67–73. doi: 10.1016/S1473-3099(22)00527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5–17 years. VISION network, 10 states, April 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:352–358. doi: 10.15585/mmwr.mm7109e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowlkes AL, Yoon SK, Lutrick K, et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in preventing SARS-CoV-2 infection among children aged 5–11 years and adolescents aged 12–15 years. PROTECT cohort, July 2021–February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:422–428. doi: 10.15585/mmwr.mm7111e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castelli JM, Rearte A, Olszevicki S, et al. Effectiveness of mRNA-1273, BNT162b2, and BBIBP-CorV vaccines against infection and mortality in children in Argentina, during predominance of delta and omicron covid-19 variants: test negative, case-control study. BMJ. 2022;379:e073070. doi: 10.1136/bmj-2022-073070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin D-Y, Xu Y, Gu Y, et al. Effectiveness of bivalent boosters against severe omicron infection. N Engl J Med. 2023;388:764–766. doi: 10.1056/NEJMc2215471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen-Stavi CJ, Magen O, Barda N, et al. BNT162b2 vaccine effectiveness against omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:227–236. doi: 10.1056/NEJMoa2205011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan SHX, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 vaccine against omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:525–532. doi: 10.1056/NEJMoa2203209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creech CB, Anderson E, Berthaud V, et al. Evaluation of mRNA-1273 Covid-19 vaccine in children 6 to 11 years of age. N Engl J Med. 2022;386:2011–2023. doi: 10.1056/NEJMoa2203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson EJ, Creech CB, Berthaud V, et al. Evaluation of mRNA-1273 vaccine in children 6 months to 5 years of age. N Engl J Med. 2022;387:1673–1687. doi: 10.1056/NEJMoa2209367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacco C, Del Manso M, Mateo-Urdiales A, et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5–11 years in Italy: a retrospective analysis of January–April, 2022. Lancet. 2022;400:97–103. doi: 10.1016/S0140-6736(22)01185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan FL, Nguyen JL, Singh TG, et al. Estimated BNT162b2 vaccine effectiveness against infection with delta and omicron variants among US children 5 to 11 years of age. JAMA Netw Open. 2022;5:e2246915. doi: 10.1001/jamanetworkopen.2022.46915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin D-Y, Gu Y, Xu Y, et al. Effects of vaccination and previous infection on omicron infections in children. N Engl J Med. 2022;387:1141–1143. doi: 10.1056/NEJMc2209371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Food and Drug Administration FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in children 5 through 11 years of age. Oct 29, 2021. www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age
- 26.US Food and Drug Administration Coronavirus (COVID-19) update: FDA expands eligibility for Pfizer-BioNTech COVID-19 vaccine booster dose to children 5 through 11 years. May 17, 2022. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-pfizer-biontech-covid-19-vaccine-booster-dose
- 27.US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes Moderna and Pfizer-BioNTech COVID-19 vaccines for children down to 6 months of age. June 17, 2022. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-covid-19-vaccines-children
- 28.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Series B Stat Methodol. 2000;62:711–730. [Google Scholar]
- 29.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 30.Buonsenso D, Cusenza F, Passadore L, Bonanno F, De Guido C, Esposito S. Duration of immunity to SARS-CoV-2 in children after natural infection or vaccination in the omicron and pre-omicron era: a systematic review of clinical and immunological studies. Front Immunol. 2023;13:1024924. doi: 10.3389/fimmu.2022.1024924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin D-Y, Gu Y, Wheeler B, et al. Effectiveness of Covid-19 vaccines over a 9-month period in North Carolina. N Engl J Med. 2022;386:933–941. doi: 10.1056/NEJMoa2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual-level participant data and data dictionary will be available for sharing after approval of a proposal by the North Carolina Department of Public Health and following a signed data access agreement. Requests for data sharing can be directed to D-YL.