Abstract
Climate change is considered the greatest threat to global health. Greenhouse gases as well as global surface temperatures have increased causing more frequent and intense heat and cold waves, wildfires, floods, drought, altered rainfall patterns, hurricanes, thunderstorms, air pollution, and windstorms. These extreme weather events have direct and indirect effects on the immune system, leading to allergic disease due to exposure to pollen, molds, and other environmental pollutants. In this review, we will focus on immune mechanisms associated with allergy and asthma-related health risks induced by climate change events. We will review current understanding of the molecular and cellular mechanisms by which the changing environment mediates these effects.
Keywords: Climate change, pollution, pollen, allergy, asthma, wildfire
The environmental exposures that the human immune system encounters is vastly different from that during preindustrial times. The increased use of fossil fuels in conjunction with deforestation, urbanization, loss of biodiversity, and effluents from mining, agriculture, and industry have led to fundamental shifts in the physical and chemical nature of Earth. Here, we review these fundamental shifts in the environment, extreme weather events and their association with increased prevalence of allergic diseases, and the mechanism by which they alter immune responses leading to allergic sensitization and reactions.
Environmental shifts caused by fossil fuel usage: Climate change and air pollution.
Burning of fossil fuels has led to increases in heat-trapping greenhouse gases (eg., carbon dioxide (CO2), methane, nitrous oxides, and fluorinated compounds) resulting in increased surface global temperatures, which are now 1.1°C higher than in 1880.[1] In May 2022, CO2 levels were 421 parts per million, levels last seen four million years ago during the Pliocene Era[2] and 50% higher than pre-industrial levels.[3] The ocean has absorbed enough CO2 since the start of the preindustrial age to lower its pH by 0.1 units, from 8.21 to 8.10, a 30% increase in acidity.[4] These fundamental shifts are altering weather patterns and leading to increases in the intensity, frequency, and duration of extreme weather events, such as thunderstorms, heat waves, and drought.[5–7]
Two extreme weather events that are increasing air pollutants are wildfires and sandstorms. Wildfire smoke is a complex mixture of gases, particulate matter, complex hydrocarbons, trace minerals, and several other toxic and carcinogenic compounds. Particle matter, that are ≤2.5μm in diameter (PM2.5) are called fine particulate matter and are small enough to penetrate deep into the lungs and enter the bloodstream, while larger ones are deposited in the upper respiratory tract. Wildfires also emit greenhouse gases such as CO2, methane, and nitrous oxides, which further exacerbate global warming and increase risk of wildfires creating a vicious circle. Other gaseous particulates emitted by wildfires include carbon monoxide, sulfur dioxide, and biogenic volatile organic compounds. Volatile organic compounds are precursors of O3. Sand and dust storms (SDS) are common in Northern Africa and Asia[8] and concentrations of dust and sand over 6,000 μg/m−3 have been observed during major storms. Dust typically contains a complex mixture of PM2.5 (e.g., silicates, clay, minerals, quartz, silicon dioxide, heavy metals, pollen, fungi, viruses, bacteria, and other pollutants).[9]
Fossil fuels in addition to increasing greenhouse gases, also affect our environment through other ways. They are used to make synthetic materials such as plastics and resins, which are used in a myriad of products. It is estimated that 140,000 chemicals and mixtures of chemicals have been created by humans, most of which did not exist previously.[10]
These climate changes have indirect effects, such as food and water shortage, increases in geographical spread of infectious vectors, loss of habitats and biodiversity, human displacement and migration, political and social instability and health service disruption (Figure 1). For example, increases in the prevalence of allergy has been observed between migrants and native-born and between second and first generation migrants.[11] All these factors affect human immune health.
Figure 1:
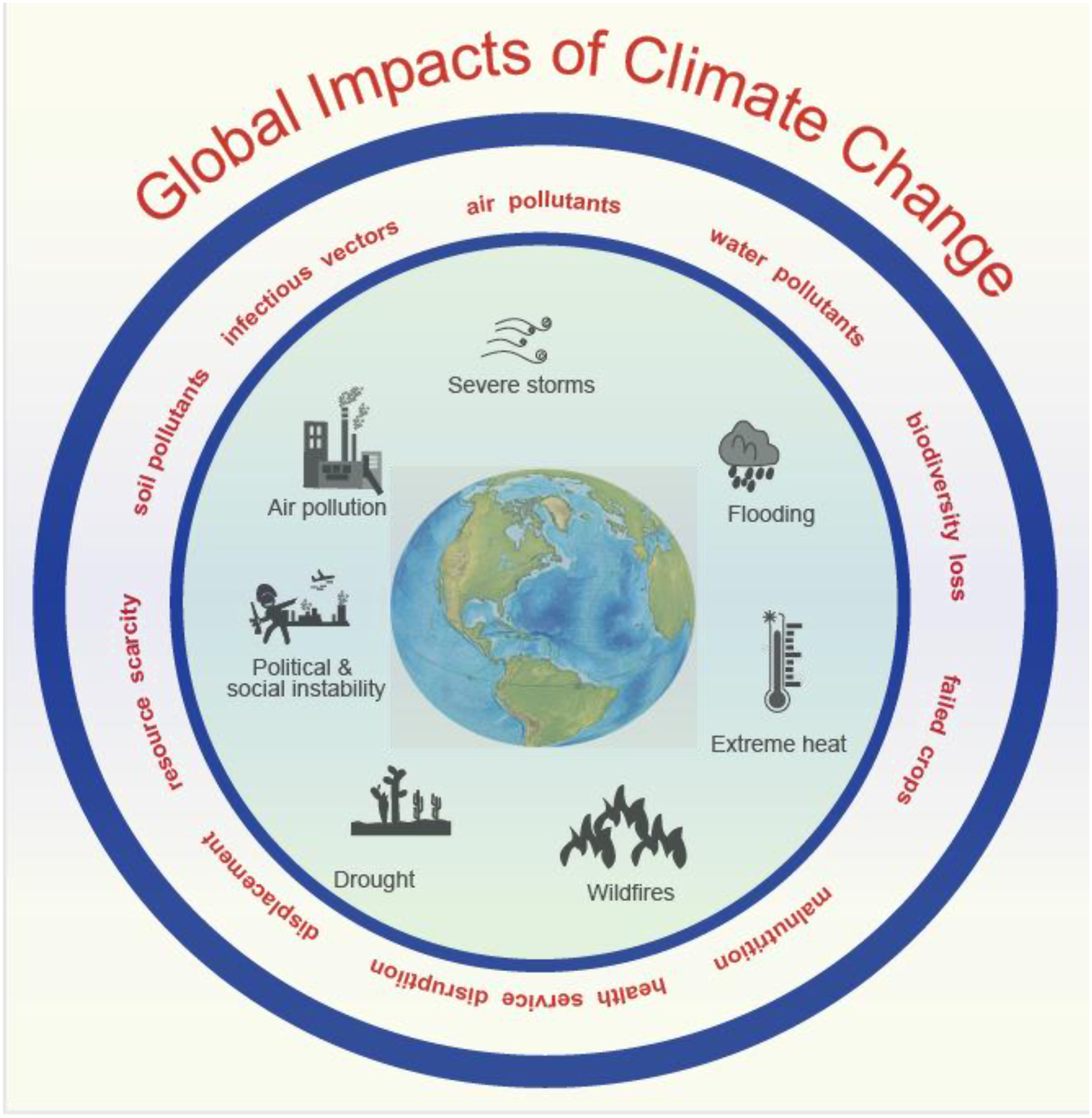
Global impacts of climate change. These factors directly and indirectly affect human health, including immune health and allergic diseases.
Epidemiology of asthma and allergic diseases in relation to climate change
Recent decades have seen increases in respiratory disorders and allergic diseases such as allergic asthma, allergic rhinitis, food allergy, and atopic dermatitis. There has been a 2–3-fold increase in asthma prevalence in the latter part of the 20th century.[12] Currently, food allergy is estimated at 10%, atopic dermatitis at 20%, allergic asthma at 8%, and allergic rhinitis between 30%.[13–16] These increases have been linked to climate change. Further, studies have shown that climate change factors act synergistically with air pollutants to directly and indirectly increase incidence and exacerbation of allergic diseases.
Increased risk of asthma exacerbation in terms of asthma-associated emergency room visits and hospital admissions has been associated with O3, nitrogen dioxide, and sulfur dioxide.[17] Studies conducted in regions with frequent sandstorms suggest an increased prevalence of asthma between 15% to 25%.[18] In Kuwait, dust storm with PM10>200 μg/m3 were associated with respiratory disease in children.[19] During the 2013 wildfire season in Oregon, a 10 μg/m3 increase in wildfire smoke increased risk in asthma diagnosis at emergency departments, office visit, and outpatient visits and was associated with increased asthma rescue inhaler medication fills.[20] A study in Thailand found that chronic smoke exposure decreased forced expiratory volume (FEV1)/ forced vital capacity (FVC) ratio compared with the general Thai population suggesting that long-term smoke exposure induces obstructive lung abnormality.[21] While most studies of wildfire smoke exposure have focused on respiratory effects, one study found an association between atopic dermatitis and itch-related dermatology visits with wildfire smoke exposure. A study conducted in a hospital located 175 miles from the Camp Fire in Paradise, CA, found that a 10-μg/m3 increase in weekly mean PM2.5 concentration was associated with a 7.7% increase in weekly pediatric itch clinic visits. The rates of visits for atopic dermatitis during the Camp Fire was 1.49 and 1.15 for pediatric and adult patients, respectively.[22]
Pollen and mold spores have increased in conjunction with the rising incidence of allergies and asthma. Higher temperatures, CO2, and O3 are increasing pollen concentrations, geographical distribution, and allergenicity. [23] In the San Francisco Bay Area, between 2002–2019, the average increase in duration for tree pollens was found to be 0.47 weeks and 0.51 weeks for mold spores. The common ragweed (Ambrosia artemisiifolia L) has spread from Central to Northern and Eastern Europe.[24] Higher CO2 levels increase photosynthesis in plants leading to increased pollen production.[25] Under controlled climate chamber conditions, a study by Choi et al found that Amb a 1, a ragweed allergen, increased by 230% and 272% at CO2 concentrations of 600 and 1000ppm, respectively, compared to more recent CO2 concentrations of 380ppm.[26] NO2 and O3 has been shown to alter the protein structure of Pla a 3 allergen, a tree allergen, via nitrification and oxidation enhancing its immunogenicity and stability.[27]
A systemic review and meta-analysis found that an increase in 10 grass pollen grains per cubic meter of exposure was associated with a statistically significant increase in asthma emergency department presentations.[28] In some studies, Alternaria, Cladosporium, and Aspergillus were associated with increased hospital admissions for asthma. [29] A random, retrospective allergy chart review found that mold reactivity increased from 16% to 55% post-hurricane. Further, the post hurricane population included more patients with asthma or lower respiratory symptoms. [30] A study in Austria, found that O3 has an effect on the severity of symptoms of pollen allergy sufferers during the pollen season.[31]
The occurrence of cyanobacteria, also known as blue-green algae, in water systems is a growing public health and environmental concern.[32] Cyanobacteria can form harmful algal blooms (HABs), which are expected to increase in number as climate temperature increases.[33, 34]. Since warm water provides a better environment for the algae to grow, they can increase their biomass forming scum on the water’s surface.[35] A study found that fresh water, marine and terrestrial cyanobacteria displayed distinct allergen characteristics and that cyanobacterial antigen-specific IgE levels in the plasma of allergic donors and mediator release from sensitized human FcεR1-transfected rat basophilic leukemia cells were significantly higher compared to non-allergic controls.[36]
Immune Mechanisms underlying environmental effects on allergies and asthma
In normal healthy individuals, immune tolerance is dominant and is an active process. Immune tolerance has been associated with exposure to pets and farm animals, breast feeding, rural living, probiotics, diversity of diet, protection of the skin barrier, and fiber-rich food, all of which increase microbial exposures. These exposures educate the immune system to build immune tolerance. Evidence suggests that the first year of a child’s life is a critical window where immune tolerance is developed. Both the innate and adaptive immune systems play a key role in maintaining homeostasis and health by promoting tolerance to innocuous substances and allergic reactions to noxious foreign substances. In tolerance, T regulatory cells are upregulated. Antigens are first processed by dendritic cells and presented to naïve T cells. Dendritic cells secrete co-stimulatory molecules such as TGF-β, IL-10, retinoic acid, indoleamine 2,3, dioxygenase, and retinal aldehyde dehydrogenase. These promote the differentiation of naïve T cells into CD4+ Treg cells, which further secrete IL-10 and TGF-β. Further, these cytokines promote class switching of B cells to secrete IgG4 and IgA. IgG4 is thought to compete with IgE to dampen allergic response and IgA binds antigens by either preventing attachment of the antigen to the epithelium or by promoting agglutination of antigen. Overall, tolerance is thought to be brought about by one of more of the following mechanisms: Suppression of Th2 cells, increases in Tregs, decreased production of IgE and increased production of IgG4 and IgA production by B cells, increases in IL-10 and TGF-β cytokines, and suppression of basophil, eosinophil, and mast cell activation.[37, 38]. (Figure 2)
Figure 2:
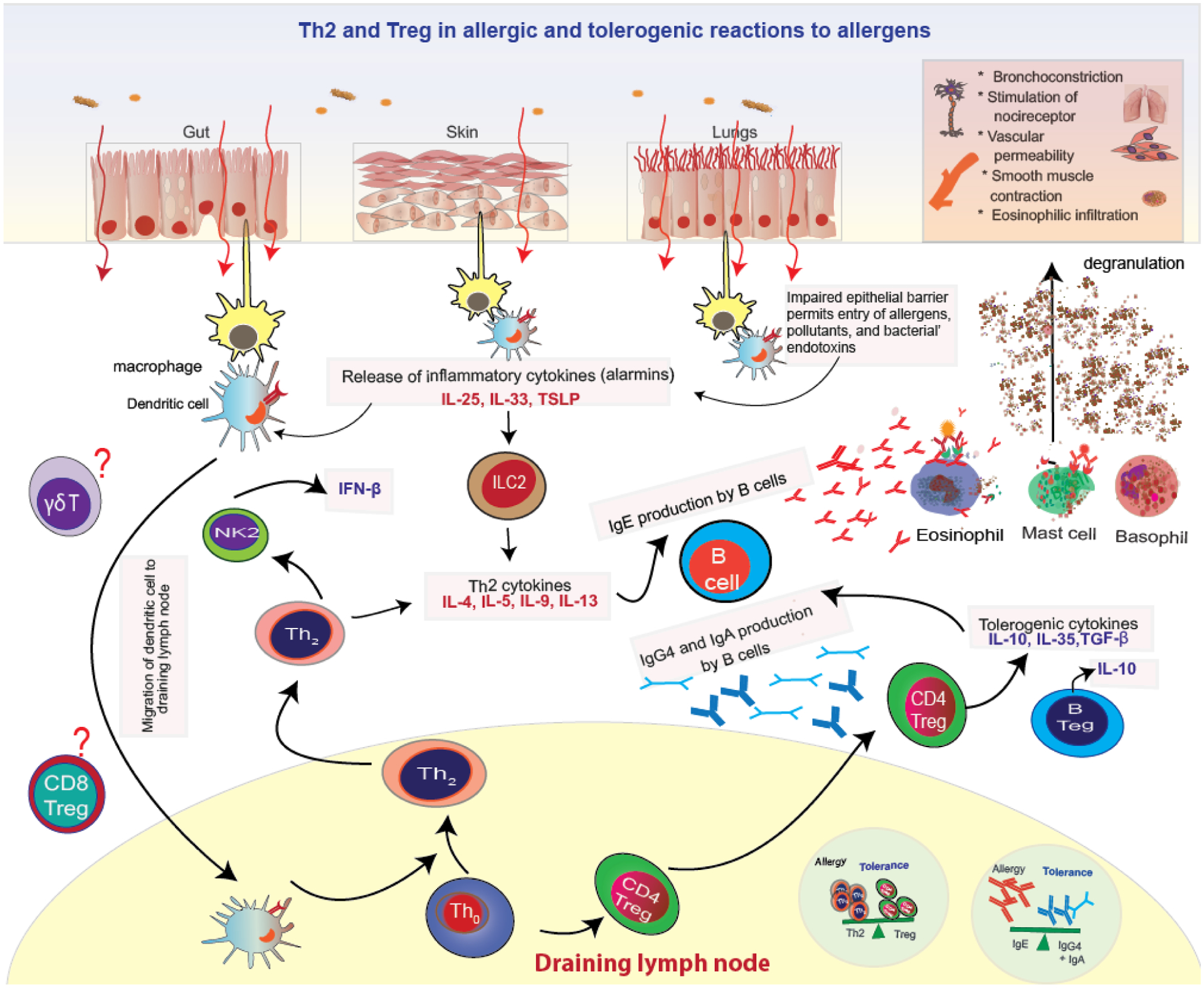
Th2 and Treg allergic and tolerogenic reactions on encountering allergens
In the last few decades, immune deviation leading to allergic reactions has rapidly increased due to multiple factors such as increased hygiene and decreased exposure to common environmental microbes and pathogens and increased exposure to pollutants. While genetic factors play a role in allergic diseases, the increase in the prevalence of allergic diseases in the last few decades is too rapid to be explained by heritable genetic changes and these rapid changes are now attributed to environmental factors, which mediate their effects through epigenetic changes. Pollution and climate change events increase epithelial barrier permeability, microbial dysbiosis, and alter immune responses (innate and adaptive). Climate change associated events such as air pollution, flooding, heat stress and water pollution affect allergic diseases and asthma. Secondary effects such as migration and human displacement and water and food insecurity also affect allergic diseases and asthma.
Air pollution
Major air pollutants that affect allergies and asthma include particulate matter, greenhouse gases, pollen, and mold spores. Air pollutants have increased dramatically due to increases in intensity and frequency of wildfires and sandstorms. Anthropogenic pollutants are also increasing with increased human activity.
The epithelial cells of the skin and lungs are the first line of defense against air pollutants. With increasing environmental pollutants, there is an increasing number of assaults on barrier surfaces. Many of the compounds encountered by these surfaces are novel synthetic ones, which humans have never encountered before. These environmental assaults lead to impairment of the epithelial barrier and the release of the proinflammatory epidermal cytokines (Type 2 alarmins) thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 by damaged keratinocytes, which orchestrate immune allergic responses. These alarmins activate dendritic cells and drive differentiation of naïve CD4+ T cells to Th2 cells, which produce Th2 type cytokines (IL-4, IL-5, IL-9, and IL-13). ILC2s are also stimulated by alarmins and secrete considerable levels of IL-5, IL-13, as well as IL-4 and IL-9, and thus also drive Th2 response. [39–41]These type 2 cytokines promote tissue mast cells, basophils, and eosinophil accumulation, IgE class switching by B cells, and production of IgE. Allergen-specific IgE antibodies then bind to FcεRI receptors on mast cells or basophils, leading to sensitization to the specific allergen. These cells are now activated and crosslinking of FcεRI-bound IgE antibodies on subsequent allergen exposure leads with degranulation of these cells. leading to the release of histamine, prostaglandins D2, leukotrienes, and tryptase and other inflammatory mediators. Symptoms of an allergic reaction include vasoconstriction, eosinophilic infiltration, bronchoconstriction, smooth muscle contraction, and stimulation of nocireceptor.[38] (Figure 2)
The role of Th2 and Tregs in allergic reactions and immune tolerance are the most well researched. Another cell more recently implicated in tolerance are Bregs and Natural killer 1 (NK1) cells. Bregs secrete the immunomodulatory cytokines IL-10, TGF-β, and IL-35 and play a role in immune tolerance.[42] NK1 cells produce IFN-β and suppress IgE production from B cells.[43] In addition to Th2 cells, research in animal models suggests a significant role of γδ T cells in regulation of IgE production and influx of eosinophils to airways. A number of different γδ T cell subsets appear to exist capable of a rapid response to a range of stimuli. Further research on these pathways are needed.[44] (Figure 2)
PM, volatile organic compounds, tobacco smoke, wildfire smoke, traffic-related air pollution, and heat stress can impact skin barrier integrity and form reactive oxygen species increasing the risk of atopic dermatitis and other allergic diseases [45–48] PM also activates NF-kB and NLRP3 inflammasome signaling and production of pro-inflammatory mediators including IL-1, IL-2, IL-6, IL-8, IL-12, TNF-α and could act synergistically with allergens to mediate allergic response.[49] Epigenetics also plays a role. Children exposed to air pollution had increased methylation of FOXP3, two times the risk of asthma diagnosis,[50] and up to 4 times higher asthma severity scores [51]. Fire fighters exposed to wildfires show increased pulmonary and systemic inflammation, and serum taken from fire fighters 12 hours after exposure has increased IL-6 and IL-12 and decreased IL-10.[52, 53] The aryl hydrocarbon receptor (AhR) has also been implicated in asthma. Particulate matter and other air pollutants such as polycyclic aromatic hydrocarbons activate AhR and induce MUC5AC expression and mucus hypersecretion.[54] (Figure 3).
Figure 3:
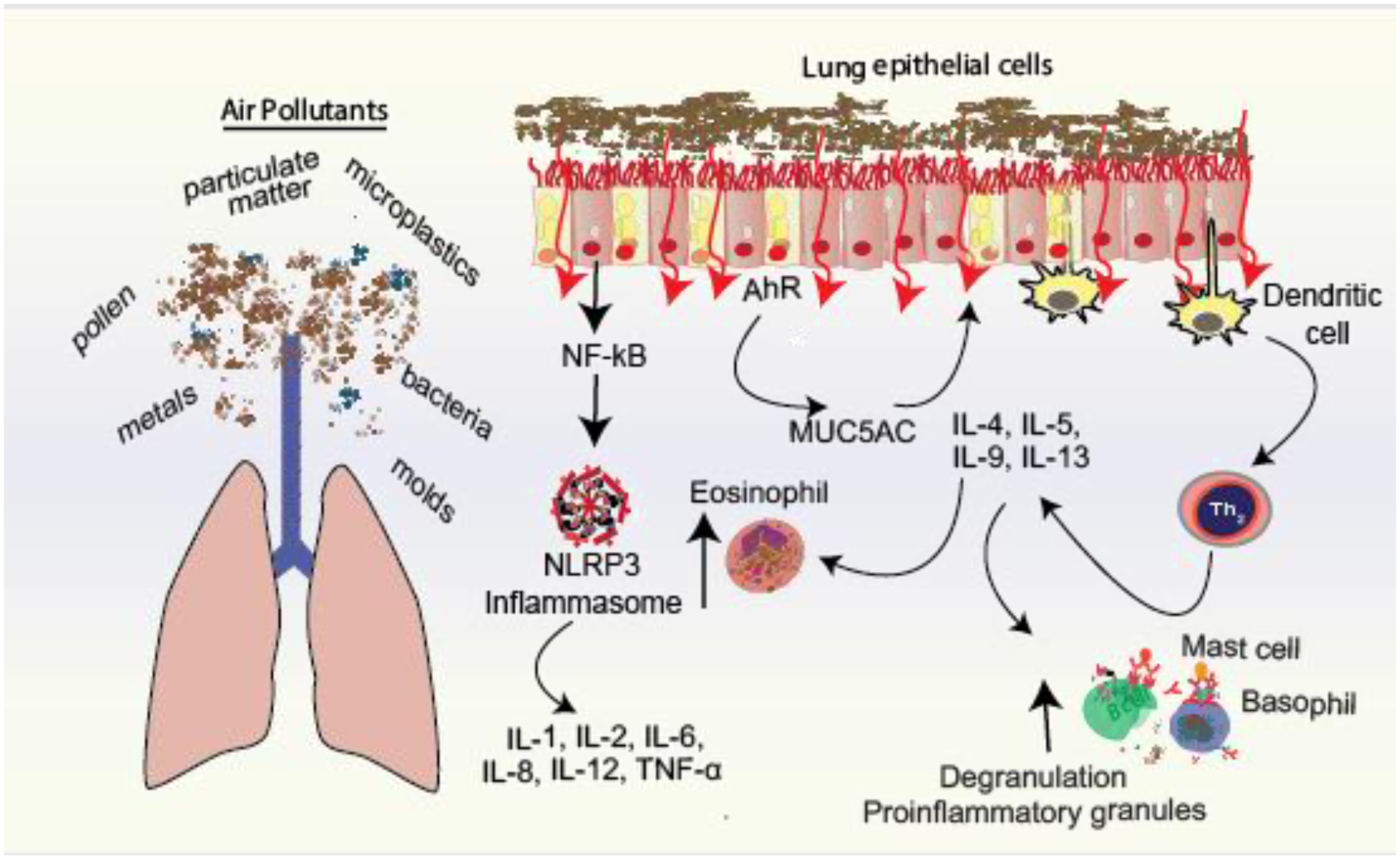
Mechanistic pathways by which air pollutants mediate proinflammatory cytokines in the lungs. IL, interleukin; TNF, tumor necrosis factor; NF-kB, Nuclear factor kappa B, AhR, aryl hydrocarbon receptor
Air pollution can alter pollen morphology, protein content, or release. In addition, pollen and fungal spores also release other compounds that can act as adjuvants in mediating allergy. The outer surface of the pollen contains a complex mixture of pigments, waxes, lipids, aromatics and proteins. It has been found that air pollution enhances release of pollen-associated lipid mediators (PALMs) and significantly higher levels were found for pollen collected near roads with heavy traffic. Further PALMs from ragweed pollen extract enhanced IgE production in Th2 primed B cells.[55, 56] In addition, bacteria and bacterial endotoxins have been associated with highly allergenic pollen. In vitro cell cultures using selected bacterial isolates from hazel pollen induced a potent concentration-dependent release of chemokine IL-8 and MCP-1, chemokines that are responsible for the recruitment of granulocytes [57] O3 increases symptom severity in those with pollen allergy during the extended birch, grass, and ragweed pollen seasons (which can begin as early as mid-December and subside only in October of the following year) [58].
Thunderstorms and Flooding
Global warming causes more water to evaporate increasing moisture content in the air.[59] Extreme precipitation events, which used to occur once every decade are now occurring 30% more frequently.[60] A 2020 analysis showed that the likelihood of a tropical storm developing into a Category 3 or higher hurricane are increasing by 8% per decade.[61]
Sea level rise, warmer temperatures, and increased rainfall facilitate growth of molds. Following Hurricane María (2017) in Puerto Rico, high levels of indoor filamentous fungi, such as Aspergillus species, was observed one year after the event. [62] In damp and poorly ventilated buildings, concentrations of bioaerosols such as fungi (e.g., molds and yeasts), fungal spores, and hyphae, as well as allergens, bacteria, spores, and microbial toxins are high. [63] They can also contain pro-inflammatory components, such as mycotoxins, dust mite allergens, algae, amoebae, and viruses.[64] Following hurricane Katrina in 2015 in New Orleans, the heat and wet debris provided ideal breeding grounds for molds. Homes with greater flood damage demonstrated higher levels of mold growth compared with homes with little or no flooding. Many of the molds identified were allergenic, including Alternaria, Aspergillus, Cladosporium, Curvularia, and Penicillium.[65]
The synergistic effects of thunderstorms and pollen on allergy and asthma is well documented. During certain thunderstorms, the number of individuals presenting with asthma was found to suddenly and significantly increase.[66] This phenomenon has been termed thunderstorms asthma (TA). The 2016 TA event in Melbourne, Australia, resulted in nearly 10,000 hospital emergency department presentations for asthma and 10 deaths.[67] A Canadian study found a doubling of fungal spore counts and more than a 15% increase in pediatric asthma emergency room visits on thunderstorm days.[68] TA is relatively rare. Since 1983, there have been only 29 reported events; however, these events are likely to increase in frequency with global warming.[69]
During a thunderstorm, pollen and/or mold spores are swept upwards by warm updrafts where they are broken by osmotic shock into smaller more allergenic fragments by either an electric charge or by absorption of moisture into smaller more allergenic fragments.[70] Ruptured pollen fragments are more easily inhaled due to their small size inducing bronchial hyperresponsiveness and exacerbations of allergic rhinitis and asthma. Even non-asthmatic individuals with only seasonal rhinitis are at greater risk of having an asthma attack during such thunderstorms.[71] Figure 4 depicts some of the climate change events that affect allergies and asthma.
Figure 4:
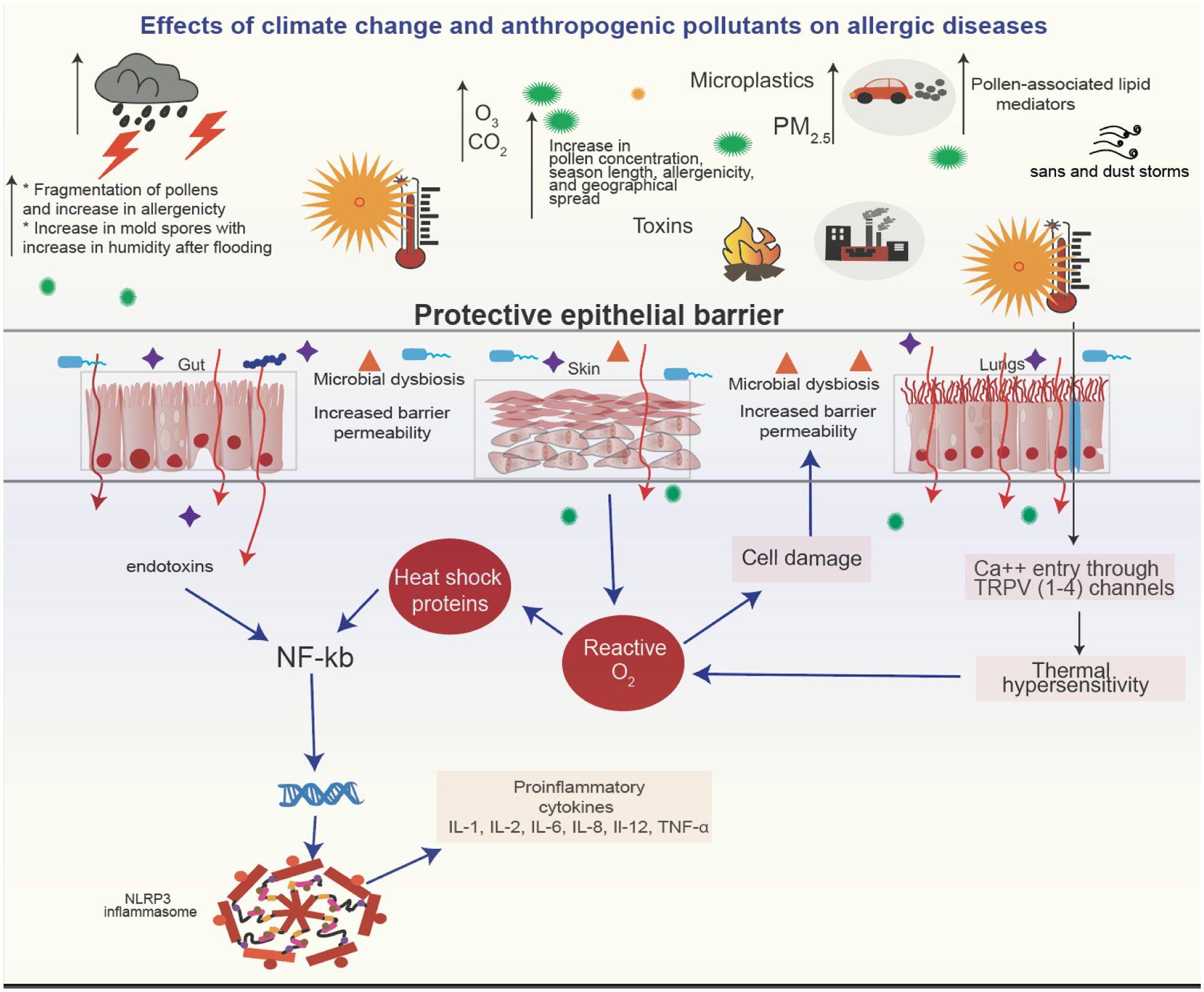
Mechanisms by which climate change events mediate allergic response independently and synergistically in conjunction with allergens.
Heat Stress
In 2018, an increase of 220 million heatwave exposure events were observed compared to the average number of heatwave events between 1986–2005.[72] Studies have shown that high temperatures are associated with lower lung function in those with asthma.[73] A study in Maryland, USA, found that exposure to extreme heat was associated with a 3% increased risk of hospitalization for asthma and that the risk was considerably higher when the analysis was restricted to the summer season. A systematic review and meta-analysis found that pooled relative risks for asthma attacks in extreme heat was 1.07.[74] However, another systematic review of six studies evaluating the association between air pollution, pollen, and heat was inconclusive.[75]
Heat stress mediates asthma and allergy by multiple pathways. During heat stress, blood is redirected from the central organs to the periphery increasing membrane fluidity and facilitating heat loss. Studies on heat stress in humans have mainly been conducted during exertional heat stress. Studies in humans during exertional heat stress and in domestic animals show that intestinal integrity is compromised leading to intestinal permeability (leaky gut).[76]. At high temperatures, disruption of claudins, occludins, and junctional adhesion molecules, which are the major transmembrane proteins of the tight junctions, occur leading to increase in intestinal epithelial permeability and translocation of luminal antigens including endotoxins such as lipopolysaccharides (LPS). Binding of toll like receptor 4 (TLR4) to LPS activates the transcription factor nuclear factor κB (NF-κB). Nf-kB is also activated due to release of reactive oxygen species (ROS) and subsequent production of heat shock proteins (HSPs) during heat stress. NF-KB mediates its effects through the inflammasome NLRP3 and subsequent release of pro-inflammatory mediators such as prostaglandin 2 (PGE2), tumor necrosis factor (TNF)-α, IL-1β, IL-6, IFN-γ, and C-reactive protein (CRP). [77–79]
An additional mechanism that has been postulated by which heat stress mediates its effects on asthma and allergic disease are transient receptor potentials (TRPs), a family of Ca2+-permeable, non-selective cation channels, found in epidermal keratinocytes allowing sensation to a range of temperatures. There are a number of different TRP channels and research in their varying roles is still ongoing. Four thermo-TRPs have been characterized to date that respond to heat: TRPVs1-4 are activated by varying levels of heat. TRPV1 is activated at ≥42°C, TRPV2 at ≥52°C, TRPV3 at 32°C~39°C, and TRPV4 at 27°C~34°C.[80] TRPV1 channels have been found overexpressed in the airways of patients with refractory asthma.[81] In a murine model of asthma, TRPV1 antagonist or TRPV1 siRNA was accompanied by reduction of airway hyperresponsiveness and airway inflammation with reduction of inflammatory cytokines, such as TSLP, IL-25, IL-33, IL-4, IL-5, and IL-13.[82] In pulmonary inflammatory diseases, TRPV4 has been linked with fungal sensitization and asthma in children.[83] Extreme heat has also been shown to induce bronchoconstriction and trigger asthma symptoms by stimulating cholinergic reflex pathway and vagal bronchopulmonary C-fiber sensory nerves.[73] (Figure 5)
Figure 5:
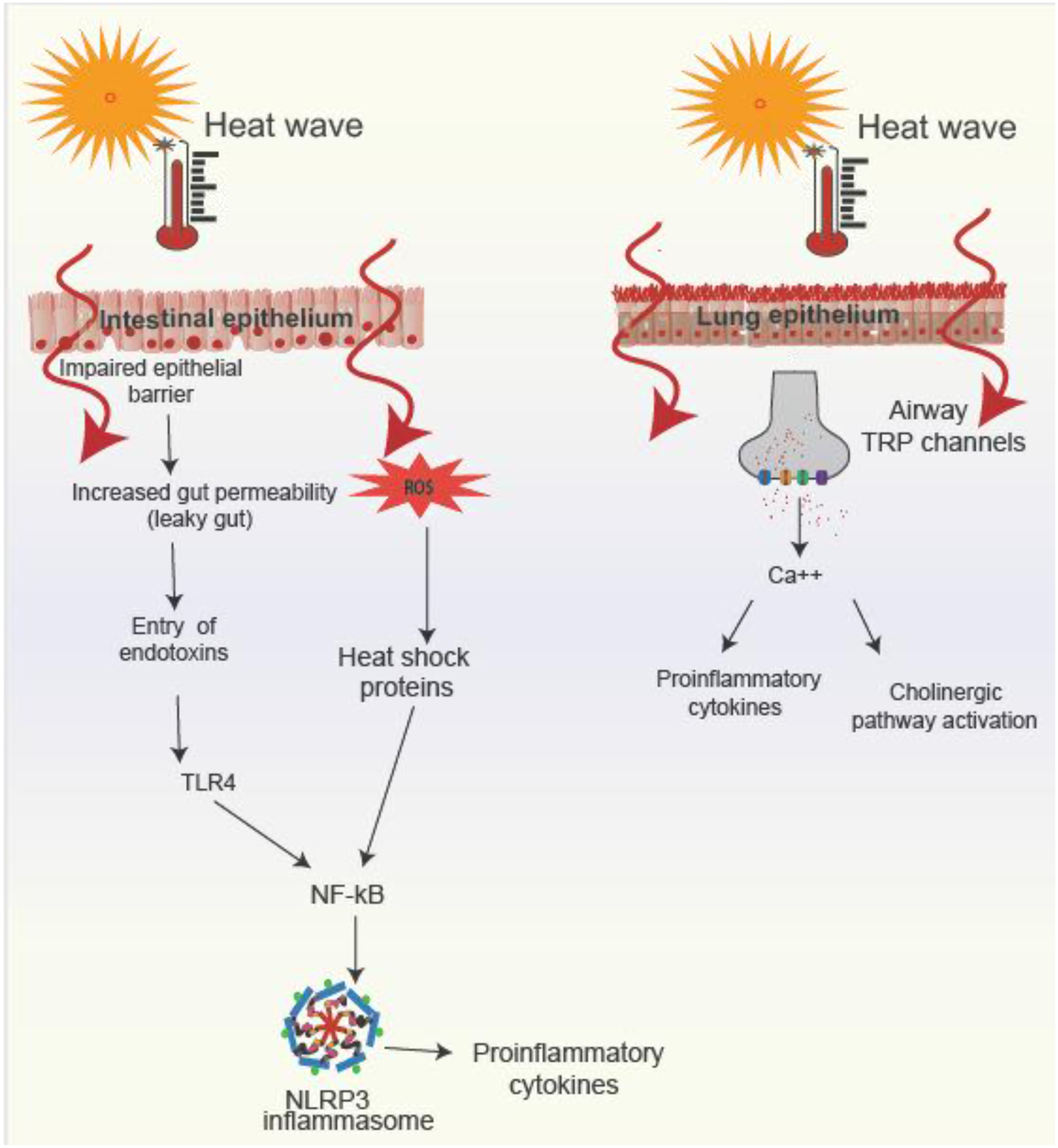
Mechanisms by which heat stress mediates allergic diseases and asthma
Water and food insecurity
Climate warming is increasing drought, food and water insecurity. Over 2 billion people live in countries which are water stressed. These numbers are expected to increase with climate change and population growth. Additionally, water is increasingly contaminated with metals and other chemicals. Common drinking water pollutants include metals, such as arsenic, lead, and mercury, and organic chemicals such as poly and per- and polyfluoroalkyl substances (PFAS) and phthalates, have been identified as pollutants of concern. [84, 85] It is approximated that about 200 million people are currently being exposed to arsenic through contaminated groundwater. A study in Bangladesh found that arsenic exposure levels was positively associated with IL-4, IL-5, IL-13, and eotaxin.[86] Further, a study found that in utero exposure to arsenic was associated with a higher risk of infection during the first year of life, particularly infections requiring medical treatment, and with diarrhea and respiratory symptom. [87] Climate change is also likely to increase food insecurity due to changing weather patterns such as drought, heat waves, and heavy rainfall. It is estimated that nearly 670 million people will still be undernourished in 2030 – 8 percent of the world population.[88] Rising CO2 concentrations are linked to a reduction in the nutritional quality of major cereal crops, which affect immune health.[89] Climate-induced migration and forced displacement due to water and food insecurity expose humans to allergens and infectious vectors that they have never encountered before. For example, climate change has enabled the lone star tick to expand its geographical habitat. The tick is responsible for sensitizing individuals to red meat, also termed alpha-gal syndrome. When it bites an individual, it transmits a sugar called alpha-gal sensitizing the person to alpha-gal, which is found in mammalian meat. On consuming mammalian meat, these sensitized individuals undergo an allergic reaction.[90]
Conclusion
Climate change is altering our environment and affecting human health, including allergies and asthma. The human body is exposed to higher concentrations of natural pollutants as well as many novel synthetic chemicals. Environmental assaults have been shown to increase epithelial barrier permeability, microbial dysbiosis, and allergic responses to innocuous environmental factors. There have been great advances in understanding the mechanism underlying immune tolerance and how the environment mediates allergic reactions. The pathways underlying Th2 mediated allergy is well elucidated. Further work on other pathways and how pollutants, climatic factors, and the microbiome synergistically work together is needed.
Highlights.
Fossil fuels are increasing greenhouse gas concentrations.
Greenhouse gases have increased global temperatures by 1.1°C higher than in 1880
CO2 levels are high at 421 ppm, levels last seen four million years ago.
Climate change is adversely affecting immune health
Prevalence of allergies and asthma have increased with climate change
Funding:
Supported by the Air pollution disrupts Inflammasome Regulation in HEart And Lung Total Health (AIRHEALTH) NIH grant NHLBI R01HL081521 and NIEHS grant R01ES032253.
Competing Interest Statement
Dr. Nadeau reports grants from National Institute of Allergy and Infectious Diseases (NIAID), National Heart, Lung, and Blood Institute (NHLBI), National Institute of Environmental Health Sciences (NIEHS), and Food Allergy Research & Education (FARE); Stock options from IgGenix, Seed Health, ClostraBio, Cour, Alladapt, Clostrabio, and ImmuneID; Director of the World Allergy Organization Center of Excellence for Stanford; Advisor at Cour Pharma; Consultant for Excellergy, Red tree ventures ,Before Brands, Alladapt, Cour, Latitude, Regeneron, and IgGenix; Co-founder of Before Brands, Alladapt, Latitude, and IgGenix; National Scientific Committee member at Immune Tolerance Network (ITN), and National Institutes of Health (NIH) clinical research centers; patents include, “Mixed allergen composition and methods for using the same,” “Granulocyte-based methods for detecting and monitoring immune system disorders,” and “Methods and Assays for Detecting and Quantifying Pure Subpopulations of White Blood Cells in Immune System Disorders.” All other authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- [1].Earth Observatory, World of Change: Global Temperatures, 2022. https://earthobservatory.nasa.gov/world-of-change/global-temperatures. (Accessed December 6 2022).
- [2].de la Vega E, Chalk TB, Wilson PA, Bysani RP, Foster GL, Atmospheric CO2 during the Mid-Piacenzian Warm Period and the M2 glaciation, Scientific Reports 10(1) (2020) 11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].National Oceanic and Atmospheric Administration, Carbon dioxide now more than 50% higher than pre-industrial levels, 2020. https://www.noaa.gov/news-release/carbon-dioxide-now-more-than-50-higher-than-pre-industrial-levels. (Accessed December 6 2022).
- [4].National Oceanic and Atmospheric Administration, Ocean acidification, 2022. https://www.noaa.gov/education/resource-collections/ocean-coasts/ocean-acidification. (Accessed December 12 2022).
- [5].Ebi KL, Vanos J, Baldwin JW, Bell JE, Hondula DM, Errett NA, Hayes K, Reid CE, Saha S, Spector J, Berry P, Extreme Weather and Climate Change: Population Health and Health System Implications, Annu Rev Public Health 42 (2021) 293–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Beagley J, Belesova K, Boykoff M, Byass P, Cai W, Campbell-Lendrum D, Capstick S, Chambers J, Coleman S, Dalin C, Daly M, Dasandi N, Dasgupta S, Davies M, Di Napoli C, Dominguez-Salas P, Drummond P, Dubrow R, Ebi KL, Eckelman M, Ekins P, Escobar LE, Georgeson L, Golder S, Grace D, Graham H, Haggar P, Hamilton I, Hartinger S, Hess J, Hsu SC, Hughes N, Jankin Mikhaylov S, Jimenez MP, Kelman I, Kennard H, Kiesewetter G, Kinney PL, Kjellstrom T, Kniveton D, Lampard P, Lemke B, Liu Y, Liu Z, Lott M, Lowe R, Martinez-Urtaza J, Maslin M, McAllister L, McGushin A, McMichael C, Milner J, Moradi-Lakeh M, Morrissey K, Munzert S, Murray KA, Neville T, Nilsson M, Sewe MO, Oreszczyn T, Otto M, Owfi F, Pearman O, Pencheon D, Quinn R, Rabbaniha M, Robinson E, Rocklov J, Romanello M, Semenza JC, Sherman J, Shi L, Springmann M, Tabatabaei M, Taylor J, Trinanes J, Shumake-Guillemot J, Vu B, Wilkinson P, Winning M, Gong P, Montgomery H, Costello A, The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises, Lancet 397(10269) (2021) 129–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].United States Environmental Protection Agency, Climate Change Indicators: Heat Waves, 2022. https://www.epa.gov/climate-indicators/climate-change-indicators-heat-waves. (Accessed Dec 7 2022).
- [8].Kok JF, Adebiyi AA, Albani S, Balkanski Y, Checa-Garcia R, Chin M, Colarco PR, Hamilton DS, Huang Y, Ito A, Contribution of the world’s main dust source regions to the global cycle of desert dust, Atmospheric Chemistry and Physics 21(10) (2021) 8169–8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aghababaeian H, Ostadtaghizadeh A, Ardalan A, Asgary A, Akbary M, Yekaninejad MS, Stephens C, Global Health Impacts of Dust Storms: A Systematic Review, Environ Health Insights 15 (2021) 11786302211018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].United Nations Environment Programme, Global chemicals outlook II- From legacies to innovative solutions: Implementing the 2030 agenda for sustainable development, 2019. https://www.unenvironment.org/explore-topics/chemicals-waste/what-we-do/policy-and-governance/global-chemicals-outlook. (Accessed December 10 2022).
- [11].Tham EH, Loo EXL, Zhu Y, Shek LP, Effects of Migration on Allergic Diseases, Int Arch Allergy Immunol 178(2) (2019) 128–140. [DOI] [PubMed] [Google Scholar]
- [12].Custovic A, Chapter 3 - Epidemiology of Allergic Diseases, in: O’Hehir RE, Holgate ST, Sheikh A (Eds.), Middleton’s Allergy Essentials, Elsevier; 2017, pp. 51–72. [Google Scholar]
- [13].Savoure M, Bousquet J, Jaakkola JJK, Jaakkola MS, Jacquemin B, Nadif R, Worldwide prevalence of rhinitis in adults: A review of definitions and temporal evolution, Clin Transl Allergy 12(3) (2022) e12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee HH, Patel KR, Singam V, Rastogi S, Silverberg JI, A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis, J Am Acad Dermatol 80(6) (2019) 1526–1532 e7. [DOI] [PubMed] [Google Scholar]
- [15].Global Health Metrics, Asthma—Level 3 cause, 2020. https://www.thelancet.com/pb-assets/Lancet/gbd/summaries/diseases/asthma.pdf. (Accessed May 9 2022).
- [16].Messina M, Venter C, Recent Surveys on Food Allergy Prevalence, Nutrition Today 55(1) (2020). [Google Scholar]
- [17].Chowdhury S, Haines A, Klingmüller K, Kumar V, Pozzer A, Venkataraman C, Witt C, Lelieveld J, Global and national assessment of the incidence of asthma in children and adolescents from major sources of ambient NO2, Environmental Research Letters 16(3) (2021) 035020. [Google Scholar]
- [18].D’Amato G, Akdis CA, Desert dust and respiratory diseases: Further insights into the epithelial barrier hypothesis, Allergy 77(12) (2022) 3490–3492. [DOI] [PubMed] [Google Scholar]
- [19].Thalib L, Al-Taiar A, Dust storms and the risk of asthma admissions to hospitals in Kuwait, Sci Total Environ 433 (2012) 347–51. [DOI] [PubMed] [Google Scholar]
- [20].Gan RW, Liu J, Ford B, O’Dell K, Vaidyanathan A, Wilson A, Volckens J, Pfister G, Fischer EV, Pierce JR, Magzamen S, The association between wildfire smoke exposure and asthma-specific medical care utilization in Oregon during the 2013 wildfire season, J Expo Sci Environ Epidemiol 30(4) (2020) 618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ontawong A, Saokaew S, Jamroendararasame B, Duangjai A, Impact of long-term exposure wildfire smog on respiratory health outcomes, Expert Rev Respir Med 14(5) (2020) 527–531. [DOI] [PubMed] [Google Scholar]
- [22].Fadadu RP, Grimes B, Jewell NP, Vargo J, Young AT, Abuabara K, Balmes JR, Wei ML, Association of wildfire air pollution and health care use for atopic dermatitis and itch, JAMA dermatology 157(6) (2021) 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lind T, Ekebom A, Alm Kubler K, Ostensson P, Bellander T, Lohmus M, Pollen Season Trends (1973–2013) in Stockholm Area, Sweden, PLoS One 11(11) (2016) e0166887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rauer D, Gilles S, Wimmer M, Frank U, Mueller C, Musiol S, Vafadari B, Aglas L, Ferreira F, Schmitt-Kopplin P, Durner J, Winkler JB, Ernst D, Behrendt H, Schmidt-Weber CB, Traidl-Hoffmann C, Alessandrini F, Ragweed plants grown under elevated CO2 levels produce pollen which elicit stronger allergic lung inflammation, Allergy (2020). [DOI] [PubMed] [Google Scholar]
- [25].Kim KR, Oh JW, Woo SY, Seo YA, Choi YJ, Kim HS, Lee WY, Kim BJ, Does the increase in ambient CO2 concentration elevate allergy risks posed by oak pollen?, Int J Biometeorol 62(9) (2018) 1587–1594. [DOI] [PubMed] [Google Scholar]
- [26].Oh JW, Pollen Allergy in a Changing Planetary Environment, Allergy Asthma Immunol Res 14(2) (2022) 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou S, Wang X, Lu S, Yao C, Zhang L, Rao L, Liu X, Zhang W, Li S, Wang W, Wang Q, Characterization of allergenicity of Platanus pollen allergen a 3 (Pla a 3) after exposure to NO2 and O3, Environ Pollut 278 (2021) 116913. [DOI] [PubMed] [Google Scholar]
- [28].Erbas B, Jazayeri M, Lambert KA, Katelaris CH, Prendergast LA, Tham R, Parrodi MJ, Davies J, Newbigin E, Abramson MJ, Dharmage SC, Outdoor pollen is a trigger of child and adolescent asthma emergency department presentations: A systematic review and meta-analysis, Allergy 73(8) (2018) 1632–1641. [DOI] [PubMed] [Google Scholar]
- [29].O’Driscoll BR, Hopkinson LC, Denning DW, Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions, BMC pulmonary medicine 5(1) (2005) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Saporta D, Hurst D, Increased Sensitization to Mold Allergens Measured by Intradermal Skin Testing following Hurricanes, J Environ Public Health 2017 (2017) 2793820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Berger M, Bastl K, Bastl M, Dirr L, Hutter HP, Moshammer H, Gstottner W, Impact of air pollution on symptom severity during the birch, grass and ragweed pollen period in Vienna, Austria: Importance of O3 in 2010–2018, Environ Pollut 263(Pt A) (2020) 114526. [DOI] [PubMed] [Google Scholar]
- [32].Backer LC, Cyanobacterial harmful algal blooms (CyanoHABs): Developing a public health response, Lake and reservoir Management 18(1) (2002) 20–31. [Google Scholar]
- [33].Moore SK, Trainer VL, Mantua NJ, Parker MS, Laws EA, Backer LC, Fleming LE, Impacts of climate variability and future climate change on harmful algal blooms and human health, Environmental health, Springer, 2008, pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chapra SC, Boehlert B, Fant C, Bierman VJ Jr, Henderson J, Mills D, Mas DM, Rennels L, Jantarasami L, Martinich J, Climate change impacts on harmful algal blooms in US freshwaters: a screening-level assessment, Environmental Science & Technology 51(16) (2017) 8933–8943. [DOI] [PubMed] [Google Scholar]
- [35].Paerl HW, Huisman J, Blooms like it hot, Science 320(5872) (2008) 57–58. [DOI] [PubMed] [Google Scholar]
- [36].Lang-Yona N, Kunert AT, Vogel L, Kampf CJ, Bellinghausen I, Saloga J, Schink A, Ziegler K, Lucas K, Schuppan D, Pöschl U, Weber B, Fröhlich-Nowoisky J, Fresh water, marine and terrestrial cyanobacteria display distinct allergen characteristics, Sci Total Environ 612 (2018) 767–774. [DOI] [PubMed] [Google Scholar]
- [37].Soyer OU, Akdis M, Ring J, Behrendt H, Crameri R, Lauener R, Akdis CA, Mechanisms of peripheral tolerance to allergens, Allergy 68(2) (2013) 161–70. [DOI] [PubMed] [Google Scholar]
- [38].Sampath V, Nadeau KC, Newly identified T cell subsets in mechanistic studies of food immunotherapy, J Clin Invest 129(4) (2019) 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cosmi L, Liotta F, Maggi L, Annunziato F, Role of Type 2 Innate Lymphoid Cells in Allergic Diseases, Curr Allergy Asthma Rep 17(10) (2017) 66. [DOI] [PubMed] [Google Scholar]
- [40].Doherty TA, At the bench: understanding group 2 innate lymphoid cells in disease, J Leukoc Biol 97(3) (2015) 455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Roan F, Obata-Ninomiya K, Ziegler SF, Epithelial cell-derived cytokines: more than just signaling the alarm, J Clin Invest 129(4) (2019) 1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fan K, Jin L, Yu S, Roles of regulatory B cells in the pathogenesis of allergic rhinitis, Allergol Immunopathol (Madr) 50(5) (2022) 7–15. [DOI] [PubMed] [Google Scholar]
- [43].Deniz G, van de Veen W, Akdis M, Natural killer cells in patients with allergic diseases, Journal of Allergy and Clinical Immunology 132(3) (2013) 527–535. [DOI] [PubMed] [Google Scholar]
- [44].Zarobkiewicz MK, Wawryk-Gawda E, Kowalska W, Janiszewska M, Bojarska-Junak A, γδ T Lymphocytes in Asthma: a Complicated Picture, Archivum Immunologiae et Therapiae Experimentalis 69(1) (2021) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kantor R, Silverberg JI, Environmental risk factors and their role in the management of atopic dermatitis, Expert review of clinical immunology 13(1) (2017) 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hendricks A, Eichenfield L, Shi V, The impact of airborne pollution on atopic dermatitis: a literature review, British Journal of Dermatology 183(1) (2020) 16–23. [DOI] [PubMed] [Google Scholar]
- [47].Drakaki E, Dessinioti C, Antoniou CV, Air pollution and the skin, Frontiers in Environmental Science 2 (2014) 11. [Google Scholar]
- [48].Parker ER, Mo J, Goodman RS, The Dermatological Manifestations of Extreme Weather Events: A Comprehensive Review of Skin Disease and Vulnerability, The Journal of Climate Change and Health (2022) 100162. [Google Scholar]
- [49].Liu T, Zhang L, Joo D, Sun S-C, NF-κB signaling in inflammation, Signal Transduction and Targeted Therapy 2(1) (2017) 17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Brunst KJ, Leung Y-K, Ryan PH, Hershey GKK, Levin L, Ji H, LeMasters GK, Ho S-M, Forkhead box protein 3 (FOXP3) hypermethylation is associated with diesel exhaust exposure and risk for childhood asthma, Journal of allergy and clinical immunology 131(2) (2013) 592–594. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, Tager I, Ambient air pollution impairs regulatory T-cell function in asthma, Journal of allergy and clinical immunology 126(4) (2010) 845–852. e10. [DOI] [PubMed] [Google Scholar]
- [52].Main LC, Wolkow AP, Tait JL, Della Gatta P, Raines J, Snow R, Aisbett B, Firefighter’s Acute Inflammatory Response to Wildfire Suppression, J Occup Environ Med 62(2) (2020) 145–148. [DOI] [PubMed] [Google Scholar]
- [53].Ferguson MD, Semmens EO, Dumke C, Quindry JC, Ward TJ, Measured Pulmonary and Systemic Markers of Inflammation and Oxidative Stress Following Wildland Firefighter Simulations, J Occup Environ Med 58(4) (2016) 407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Poulain-Godefroy O, Bouté M, Carrard J, Alvarez-Simon D, Tsicopoulos A, de Nadai P, The Aryl Hydrocarbon Receptor in Asthma: Friend or Foe?, Int J Mol Sci 21(22) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Reinmuth-Selzle K, Kampf CJ, Lucas K, Lang-Yona N, Fröhlich-Nowoisky J, Shiraiwa M, Lakey PSJ, Lai S, Liu F, Kunert AT, Ziegler K, Shen F, Sgarbanti R, Weber B, Bellinghausen I, Saloga J, Weller MG, Duschl A, Schuppan D, Pöschl U, Air Pollution and Climate Change Effects on Allergies in the Anthropocene: Abundance, Interaction, and Modification of Allergens and Adjuvants, Environ Sci Technol 51(8) (2017) 4119–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhao F, Elkelish A, Durner J, Lindermayr C, Winkler JB, Ruёff F, Behrendt H, Traidl-Hoffmann C, Holzinger A, Kofler W, Braun P, von Toerne C, Hauck SM, Ernst D, Frank U, Common ragweed (Ambrosia artemisiifolia L.): allergenicity and molecular characterization of pollen after plant exposure to elevated NO2, Plant Cell Environ 39(1) (2016) 147–64. [DOI] [PubMed] [Google Scholar]
- [57].Ambika Manirajan B, Hinrichs AK, Ratering S, Rusch V, Schwiertz A, Geissler-Plaum R, Eichner G, Cardinale M, Kuntz S, Schnell S, Bacterial Species Associated with Highly Allergenic Plant Pollen Yield a High Level of Endotoxins and Induce Chemokine and Cytokine Release from Human A549 Cells, Inflammation 45(6) (2022) 2186–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Berger M, Bastl M, Bouchal J, Dirr L, Berger U, The influence of air pollution on pollen allergy sufferers, Allergologie Select 5 (2021) 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Global Climate Change, A Force of Nature: Hurricanes in a Changing Climate, 2022. https://climate.nasa.gov/news/3184/a-force-of-nature-hurricanes-in-a-changingclimate/#:~:text=Moreover%2C%20according%20to%20Knutson%2C%20most,both%20of%20which%20fuel%20hurricanes. (Accessed Dec 30 2022).
- [60].Intergovernmental Panel on Climate Change, Sixth Assessment Report - physical science basis for climate change, 2021. https://www.ipcc.ch/report/ar6/wg1/#FullReport (Accessed Sep 9 2021).
- [61].Kossin JP, Knapp KR, Olander TL, Velden CS, Global increase in major tropical cyclone exceedance probability over the past four decades, Proc Natl Acad Sci U S A 117(22) (2020) 11975–11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vélez-Torres LN, Bolaños-Rosero B, Godoy-Vitorino F, Rivera-Mariani FE, Maestre JP, Kinney K, Cavallin H, Hurricane María drives increased indoor proliferation of filamentous fungi in San Juan, Puerto Rico: a two-year culture-based approach, PeerJ 10 (2022) e12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Burge HA, Bioaerosols in the residential environment, Bioaerosols handbook, CRC; Press2020, pp. 579–597. [Google Scholar]
- [64].Löndahl J, Physical and biological properties of bioaerosols, Bioaerosol detection technologies, Springer; 2014, pp. 33–48. [Google Scholar]
- [65].Barbeau DN, Grimsley LF, White LE, El-Dahr JM, Lichtveld M, Mold Exposure and Health Effects Following Hurricanes Katrina and Rita, Annual Review of Public Health 31(1) (2010) 165–178. [DOI] [PubMed] [Google Scholar]
- [66].Rorie A, Poole JA, The Role of Extreme Weather and Climate-Related Events on Asthma Outcomes, Immunol Allergy Clin North Am 41(1) (2021) 73–84. [DOI] [PubMed] [Google Scholar]
- [67].Bannister T, Ebert EE, Williams T, Douglas P, Wain A, Carroll M, Silver J, Newbigin E, Lampugnani ER, Hughes N, Looker C, Mulvenna V, Csutoros D, Jones PJ, Davies JM, Suphioglu C, Beggs PJ, Emmerson KM, Huete A, Nguyen H, A Pilot Forecasting System for Epidemic Thunderstorm Asthma in Southeastern Australia, American Meteorological Society 102(2) (2021) E399–E420. [Google Scholar]
- [68].Dales RE, Cakmak S, Judek S, Dann T, Coates F, Brook JR, Burnett RT, The role of fungal spores in thunderstorm asthma, Chest 123(3) (2003) 745–750. [DOI] [PubMed] [Google Scholar]
- [69].Stewart C, Young NL, Kim ND, Johnston DM, Turner R, Thunderstorm asthma: a review, risks for Aotearoa New Zealand, and health emergency management considerations, N Z Med J 135(1557) (2022) 49–63. [PubMed] [Google Scholar]
- [70].Kevat A, Thunderstorm Asthma: Looking Back and Looking Forward, J Asthma Allergy 13 (2020) 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].D’Amato G, Vitale C, D’Amato M, Cecchi L, Liccardi G, Molino A, Vatrella A, Sanduzzi A, Maesano C, Annesi-Maesano I, Thunderstorm-related asthma: what happens and why, Clinical & Experimental Allergy 46(3) (2016) 390–396. [DOI] [PubMed] [Google Scholar]
- [72].Watts N, Amann M, Arnell N, Ayeb-Karlsson S, Belesova K, Boykoff M, Byass P, Cai W, Campbell-Lendrum D, Capstick S, Chambers J, Dalin C, Daly M, Dasandi N, Davies M, Drummond P, Dubrow R, Ebi KL, Eckelman M, Ekins P, Escobar LE, Fernandez Montoya L, Georgeson L, Graham H, Haggar P, Hamilton I, Hartinger S, Hess J, Kelman I, Kiesewetter G, Kjellstrom T, Kniveton D, Lemke B, Liu Y, Lott M, Lowe R, Sewe MO, Martinez-Urtaza J, Maslin M, McAllister L, McGushin A, Jankin Mikhaylov S, Milner J, Moradi-Lakeh M, Morrissey K, Murray K, Munzert S, Nilsson M, Neville T, Oreszczyn T, Owfi F, Pearman O, Pencheon D, Phung D, Pye S, Quinn R, Rabbaniha M, Robinson E, Rocklov J, Semenza JC, Sherman J, Shumake-Guillemot J, Tabatabaei M, Taylor J, Trinanes J, Wilkinson P, Costello A, Gong P, Montgomery H, The 2019 report of The Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate, Lancet 394(10211) (2019) 1836–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hayes D Jr., Collins PB, Khosravi M, Lin RL, Lee LY, Bronchoconstriction triggered by breathing hot humid air in patients with asthma: role of cholinergic reflex, Am J Respir Crit Care Med 185(11) (2012) 1190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Han A, Deng S, Yu J, Zhang Y, Jalaludin B, Huang C, Asthma triggered by extreme temperatures: From epidemiological evidence to biological plausibility, Environ Res 216(Pt 2) (2023) 114489. [DOI] [PubMed] [Google Scholar]
- [75].Anenberg SC, Haines S, Wang E, Nassikas N, Kinney PL, Synergistic health effects of air pollution, temperature, and pollen exposure: a systematic review of epidemiological evidence, Environmental Health 19(1) (2020) 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bouchama A, Knochel JP, Heat Stroke, New England Journal of Medicine 346(25) (2002) 1978–1988. [DOI] [PubMed] [Google Scholar]
- [77].Heled Y, Fleischmann C, Epstein Y, Cytokines and their role in hyperthermia and heat stroke, J Basic Clin Physiol Pharmacol 24(2) (2013) 85–96. [DOI] [PubMed] [Google Scholar]
- [78].Liu YL, Ding KN, Shen XL, Liu HX, Zhang YA, Liu YQ, He YM, Tang LP, Chronic heat stress promotes liver inflammation in broilers via enhancing NF-κB and NLRP3 signaling pathway, BMC Vet Res 18(1) (2022) 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].de Punder K, Pruimboom L, Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability, Front Immunol 6 (2015) 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Vay L, Gu C, McNaughton PA, The thermo-TRP ion channel family: properties and therapeutic implications, Br J Pharmacol 165(4) (2012) 787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Dumitrache MD, Jieanu AS, Scheau C, Badarau IA, Popescu GDA, Caruntu A, Costache DO, Costache RS, Constantin C, Neagu M, Caruntu C, Comparative effects of capsaicin in chronic obstructive pulmonary disease and asthma (Review), Exp Ther Med 22(3) (2021) 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Choi JY, Lee HY, Hur J, Kim KH, Kang JY, Rhee CK, Lee SY, TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model, Allergy Asthma Immunol Res 10(3) (2018) 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wiesner DL, Merkhofer RM, Ober C, Kujoth GC, Niu M, Keller NP, Gern JE, Brockman-Schneider RA, Evans MD, Jackson DJ, Warner T, Jarjour NN, Esnault SJ, Feldman MB, Freeman M, Mou H, Vyas JM, Klein BS, Club Cell TRPV4 Serves as a Damage Sensor Driving Lung Allergic Inflammation, Cell Host Microbe 27(4) (2020) 614–628.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Quinete N, Hauser-Davis RA, Drinking water pollutants may affect the immune system: concerns regarding COVID-19 health effects, Environ Sci Pollut Res Int 28(1) (2021) 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].World Health Organization, Drinking-water, 2022. https://www.who.int/news-room/fact-sheets/detail/drinking-water. (Accessed Dec 29 2022).
- [86].Rahman A, Islam MS, Tony SR, Siddique AE, Mondal V, Hosen Z, Islam Z, Hossain MI, Rahman M, Anjum A, Paul SK, Hossen F, Sarker MK, Hossain S, Salam KA, Haque A, Hoque MA, Saud ZA, Xin L, Sumi D, Himeno S, Hossain K, T helper 2-driven immune dysfunction in chronic arsenic-exposed individuals and its link to the features of allergic asthma, Toxicology and Applied Pharmacology 420 (2021) 115532. [DOI] [PubMed] [Google Scholar]
- [87].Farzan SF, Li Z, Korrick SA, Spiegelman D, Enelow R, Nadeau K, Baker E, Karagas MR, Infant Infections and Respiratory Symptoms in Relation to in Utero Arsenic Exposure in a U.S. Cohort, Environ Health Perspect 124(6) (2016) 840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Food and Agriculture Organization of the United Nations, The state of food security and nutrition in the world 2022, 2022. https://www.fao.org/3/cc0639en/online/cc0639en.html. (Accessed December 30 2022).
- [89].Haines A, Ebi K, The Imperative for Climate Action to Protect Health, N Engl J Med 380(3) (2019) 263–273. [DOI] [PubMed] [Google Scholar]
- [90].Zurbano-Azqueta L, Antón-Casas E, Duque-Gómez S, Jiménez-Gómez I, Fernández-Pellón L, López-Gutiérrez J, Alpha-gal syndrome. Allergy to red meat and gelatin, Rev Clin Esp (Barc) 222(7) (2022) 401–405. [DOI] [PubMed] [Google Scholar]


