Abstract
Objectives
In this era of bacterial resistance, avoiding inappropriate use of antibiotic treatments is of major importance. Respiratory tract infections are frequent among older patients, and differentiating viral from bacterial infections is a challenge. The aim of our study was to evaluate the impact of recently available respiratory PCR testing on antimicrobial prescription in geriatric acute care.
Methods
We performed a retrospective study, including all hospitalized geriatric patients who had had multiplex respiratory PCR testing prescribed from 1st October 2018 to 30th September 2019. The PCR test comprised a respiratory viral panel (RVP) and a respiratory bacterial panel (RBP). PCR testing could be prescribed at any time during hospitalization by geriatricians. Our primary endpoint was antibiotic prescription after viral multiplex PCR testing results.
Results
All in all, 193 patients were included, 88 (45.6%) of whom had positive RVP, while none had positive RBP. Patients with positive RVP had significantly fewer antibiotic prescriptions following test results than patients with negative RVP (odds ratio (OR) 0.41, 95% confidence interval (CI) 0.22-0.77; p=0.004). Among positive-RVP patients, factors associated with antibiotic continuation were presence of radiological infiltrate (OR 12.02, 95%CI 3.07-30.29), and detected Respiratory Syncytial Virus (OR 7.54, 95%CI 1.74-32.65). That said, discontinuation of antibiotic treatment seems safe.
Conclusion
In this population, the impact of viral detection by respiratory multiplex PCR on antibiotic therapy was low. It could be optimized by means of clearly formulated local guidelines, qualified staff and specific training by infectious disease specialists. Cost-effectiveness studies are necessary.
Keywords: respiratory tract infection, antibiotics, multiplex PCR assay, stewardship
1. Background
Antibiotic exposure is associated with the emergence of antimicrobial resistance. It is consequently of major importance to avoid inappropriate use. The geriatric population is a particularly exposed to antibiotics, of which consumption remains stable over time [1].
Respiratory tract infections (RTIs) are, frequent especially among older patients, and it is challenging to differentiate viral from bacterial infections [2], [3].
Before the COVID-19 pandemic, the main pathogens involved in RTIs were Streptococcus pneumoniae, Mycoplasma pneumoniae, Haemophilus influenzae, Legionella pneumophila, as well as Influenza viruses, Respiratory Syncytial Virus (RSV), Human Metapneumovirus, Parainfluenza viruses, and rhinovirus [4], [5].
According to several studies, about one third of RTIs may be due to viruses alone [4], [5], [6], [7]. Multiplex respiratory PCR testing through viral detection is therefore expected to reduce antibiotic prescription. However, its real-life impact is unclear, especially among older patients for whom pneumonia diagnosis is challenging, and who are usually highly exposed to antibiotic treatments due to their frequent bacterial infections and overall frailty [8], [9], [10].
The aim of our study was to evaluate the impact of newly available multiplex respiratory virus testing on antimicrobial prescriptions delivered in geriatric acute care units by geriatricians without guidelines or specialized training, before the COVID-19 era.
2. Material and methods:
We performed a retrospective study including all patients hospitalized with RTI and who had multiplex respiratory PCR testing prescribed from 1st October 2018 to 30 September 2019, in five geriatric units with a total of 138 beds in two French University hospital centers. PCR testing could be prescribed by geriatricians at any time during hospitalization. Results were available in less than 24h, the physicians in charge were free to modify antimicrobial prescription accordingly, and no specific guidelines were available at the time, as the tool had just been rendered available in our hospital.
Exclusion criteria were age under 75 years, absence of PCR testing, and absence of confirmed RTI. The medical charts were reviewed by two independent geriatricians (AL and BD) to assess diagnosis of RTI. Patients could only be included once.
Using a standard questionnaire, the data collected were: age, co-morbidities, autonomy, the clinical and paraclinical aspects, results of the multiplex PCR testing, and the antibiotic prescription. Glomerular filtration rate (eGFR) was estimated by the Cockroft and Gault formula.
Our primary endpoint was antibiotic prescription (initiation, continuation or discontinuation) after viral multiplex PCR testing results. Secondary endpoints were defined as follows: clinical cure by end of hospitalization, recurrence before day 30, death during hospitalization, absence of rehospitalization before Day 30. Cure was defined as absence of clinical and biological signs of infection without additional antibiotic treatment, and was confirmed by the physician in charge. Recurrence was defined by the onset of a new episode of viral or bacterial RTI after planned end of antibiotic course.
The standard of care in geriatric patients with RTI without PCR was antibiotic treatment prescribed by the physician in charge.
The multiplex real-time PCR kit used was FILMARRAY® Respiratory 2.1 plus panel (bioMérieux®, Craponne, France). This test consists in a respiratory viral panel (RVP) that detects 15 viruses (adenovirus, coronavirus 229E, HKU1, NL63, and OC43, human metapneumovirus, rhinovirus/enterovirus, influenza A and B viruses, MERS-CoV, parainfluenza viruses 1-4, Respiratory Syncytial Virus), and a respiratory bacterial panel (RBP), which detects 4 bacteria (Mycoplasma pneumoniae, Chlamydia pneumoniae, Bordetella pertussis, Bordetella parapertussis). Results are available in less than 24 hours.
Continuous variables are presented as means, standard deviation, median, range and interquartile range for continuous characteristics, and by tabular descriptions for qualitative characteristics.
Through a multi-stage approach, we first identified the factors associated in our study population with antibiotic prescription. These factors associated were assessed in a bivariate analysis using Chi-square test or Fisher’s exact test for categorical variables, and Student t-test for continuous variables. All reported P values ≤ 0.05 were considered statistically significant.
The variables considered were: the results of PCR tests, demographic and medical characteristics, and all clinical, biological and radiological data from Day 0 of antibiotic treatment. A multivariable analysis by logistic regression was then performed using all variables from the bivariate analysis that had a P-value ≤ 0.2, as well as age, sex and Charlson score. The final model was obtained using backward stepwise regression with 0.10 thresholds. Using the same procedure, we then identified the factors associated with antibiotic prescription in the subgroup of patients with a positive PCR test.
Results are expressed as odds ratio (OR) with a confidence interval (CI) of 95%.
Analyses were performed using Stata software, version 15 (StataCorp, US).
3. Results:
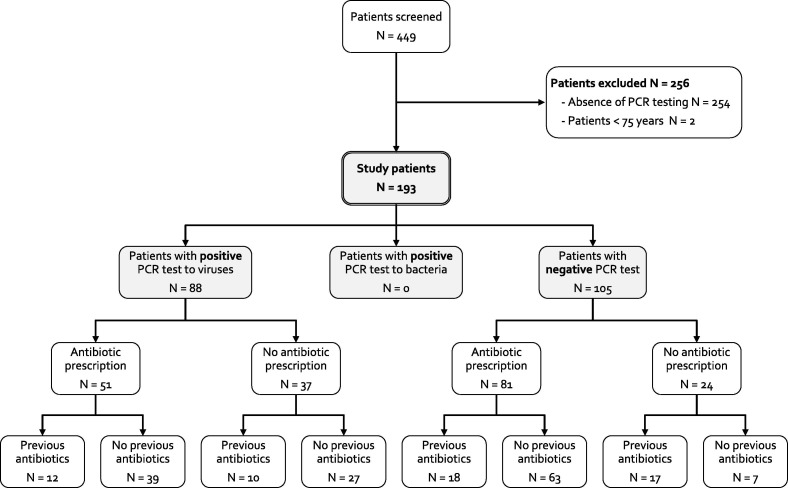
During the study period, 449 patients were hospitalized with RTI according to the hospital database; 254 were excluded due to absence of PCR testing and 2 patients were less than 75 years old (flow chart in Figure 1 ).
Figure 1.
Study flow chart
All in all, 193 patients were included; their demographics and baseline characteristics are presented in Table 1 .
Table 1.
Demographics and baseline characteristics of the study population
| PatientsN = 193 | |
|---|---|
| Age (mean ± SD, years) | 89.7 ± 6.2 |
| Female | 122 (63.2) |
| Comorbidities | |
| Major neuro-cognitive disorder | 110 (57.3) |
| Atrial fibrillation | 60 (31.1) |
| Dysphagia | 47 (24.4) |
| Congestive heart failure | 45 (23.3) |
| COPD | 42 (21.8) |
| Chronic kidney disease | 38 (19.7) |
| Ischemic heart disease | 37 (19.2) |
| Cerebrovascular disease | 36 (18.7) |
| Diabetes mellitus | 31 (16.1) |
| Cancer | 26 (13.5) |
| Obesity | 13 (10.2) |
| Peripheral vascular disease | 14 (7.3) |
| Blood disorder | 14 (7.3) |
| Charlson comorbidity index (mean ± SD) | 6.7 ± 1.7 |
| BMI (mean ± SD, kg/m2) | 22.8 ± 5.7 |
| MMSE (mean, SD) | 19.7 ± 11.2 |
| Antibiotics prescribed before PCR testing | 57 (29.5%) |
| Clinical signs | |
| Systolic blood pressure < 90 mmHg | 9 (4.7) |
| Temperature ≥ 38.3°C | 31 (16.1) |
| Oxygen saturation < 94% | 71 (36.8) |
| Oxygen flow rate ≥ 1L/min | 66 (34.2) |
| Cough | 131 (67.9) |
| Crackles | 80 (41.5) |
| Heart failure | 70 (36.3) |
| Sputum | 44 (22.8) |
| Confusion | 33 (17.1) |
| Biological parameters | |
| Albumin level < 35 g/L | 142 (74.0) |
| Neutrophils count ≥ 7.5 G/L | 99 (51.6) |
| Lymphocytes count < 1.0 G/L | 89 (46.1) |
| C-Reactive Protein level ≥ 5 mg/L | 180 (94.2) |
| Renal failure* | |
| Absent (eGFR > 90 mL /min/1.73m2) | 6 (3.1) |
| Mild (eGFR < 90 mL/min/1.73m2) | 94 (48.7) |
| Moderate (eGFR < 60 mL/min/1.73m2) | 80 (41.5) |
| Severe (eGFR < 30 mL/min/1.73m2) | 13 (6.7) |
| Radiological infiltrate | 70 (36.3) |
| PCR results | |
| Positive | 88 (45.6) |
| Flu virus (A) | 36 (40.9) |
| Rhinovirus | 20 (22.7) |
| Respiratory Syncitial Virus | 15 (17.1) |
| Bacteria | 0 (0) |
Data are n (%) unless otherwise stated.
* according to Cockroft and Gault
BMI: body-mass index; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; MMSE: mini-mental state examination; PCR: polymerase chain reaction; SD: standard deviation
Antibiotic prescriptions were found among 57 (29.5%) patients before PCR testing.
The most commonly used antibiotics were: amoxicillin-clavulanate (n=82; 62.1%), third-generation cephalosporins (n=22; 16.7%) and third-generation cephalosporins associated with metronidazole (n=12; 9.1%). Mean antibiotic duration was 6.3 ± 4.0 (min 1; max 42) days.
Death during hospitalization occurred in 24 (12.4%) cases, and recurrence of RTI before day 30 in 32 (23.5%) cases.
Following PCR test results, PCR testing had a significant impact on antibiotic prescription among the study population. In fact, antibiotic prescriptions were found in 81/105 (77.1%) patients with negative PCR tests, and in 51/88 (58.0%) of patients with positive RVPs (OR 0.41, 95%CI 0.22-0.77; p = 0.004).
Moreover, among patients with no antibiotic prescription prior to PCR testing, antibiotic treatments were prescribed to 63/70 (90.0%) patients with negative PCR test and to 39/66 (59.0%) with positive RVPs (OR 0.16, 95%CI 0.06-0.41; p < 0.0001). On the contrary, among patients taking antibiotics prior to PCR testing, no significant impact on antibiotic prescription was observed: 18/35 (51.4%) patients with negative PCR test vs. 12/22 (54.5%) with positive RVPs (OR 1.13, 95%CI 0.40-3.33; p = 0.82).
While mean antibiotic treatment duration was shorter in the group of patients with positive RVPs compared with the group of patients with negative PCR tests, the difference was not significant (5.7 days ± 2.8 vs. 6.7 days ± 4.6, p = 0.1).
Considering the patients with positive RVPs (n = 88), their demographics and baseline characteristics are presented in Table 2 , along with their outcomes according to the existence of antibiotic prescriptions following PCR test results.Table 3.
Table 2.
Demographics and baseline characteristics of patients with positive PCR tests, and their outcome according to the presence of antibiotic prescription following PCR test results (n=88)
| No antibiotic prescriptionN = 37 | Antibiotic prescriptionN = 51 | P-value | |
|---|---|---|---|
| Age (mean ± SD, years) | 90.4 ± 7.3 | 90.2 ± 5.5 | 0.888 |
| Female | 29 (75.7) | 34 (66.7) | 0.361 |
| Major neuro-cognitive disorder | 25 (67.6) | 39 (76.5) | 0.468 |
| Charlson comorbidity index (mean ± SD) | 6.6 ± 2.1 | 6.8 ± 1.6 | 0.744 |
| BMI (mean ± SD, kg/m2) | 22.3 ± 6.2 | 22.1 ± 4.9 | 0.922 |
| Antibiotics prescribed before PCR testing | 10 (27.0) | 12 (23.5) | 0.708 |
| Clinical signs | |||
| Systolic blood pressure < 90 mmHg | 1 (2.7) | 4 (7.8) | NA |
| Temperature ≥ 38.3°C | 5 (13.5) | 9 (17.7) | 0.061 |
| Oxygen saturation < 94% | 16 (43.2) | 22 (43.1) | 0.992 |
| Oxygen flow rate ≥ 1L/min | 13 (35.1) | 20 (39.2) | 0.696 |
| Cough | 26 (70.3) | 40 (78.4) | 0.383 |
| Sputum | 9 (24.3) | 14 (27.5) | 0.742 |
| Confusion | 6 (16.2) | 13 (25.5) | 0.297 |
| Heart failure | 12 (32.4) | 22 (43.1) | 0.309 |
| Crackles | 10 (27.0) | 20 (39.2) | 0.234 |
| Biological parameters | |||
| Albumin level < 35 g/L | 22 (59.5) | 38 (74.5) | 0.135 |
| Neutrophils count ≥ 7.5 G/L | 10 (27.0) | 24 (47.1) | 0.057 |
| Lymphocytes count < 1.0 G/L | 18 (48.7) | 28 (54.9) | 0.562 |
| C-Reactive Protein level ≥ 5 mg/L | 31 (83.8) | 49 (96.1) | 0.048 |
| Renal failure* | |||
| Absent (eGFR > 90 mL /min/1.73m2) | 1 (2.7) | 1 (2.0.) | 0.818 |
| Mild (eGFR < 90 mL/min/1.73m2) | 16 (43.2) | 24 (47.1) | 0.723 |
| Moderate (eGFR < 60 mL/min/1.73m2) | 19 (51.4) | 19 (37.3) | 0.188 |
| Severe (eGFR < 30 mL/min/1.73m2) | 1 (2.7) | 7 (13.7) | 0.076 |
| Pulmonary edema | 9 (27.3) | 11 (21.6) | 0.549 |
| Pleural effusion | 5 (15.2) | 2 (3.9) | 0.106 |
| Radiological infiltrate | 3 (8.1) | 21 (41.2) | 0.001 |
| PCR results | |||
| Flu virus (A) | 20 (54.1) | 16 (31.4) | 0.033 |
| Rhinovirus | 6 (16.2) | 14 (27.5) | 0.214 |
| Respiratory Syncitial Virus | 3 (8.1) | 12 (23.5) | 0.084 |
| Metapneumovirus | 3 (8.1) | 4 (7.8) | 1.000 |
| Outcome | |||
| Clinical cure at end of hospitalization | 30 (81.1) | 40 (78.4) | 0.761 |
| Recurrence at Day 30 | 1 (2.7) | 2 (3.9) | > 1.000 |
| Death during hospitalization | 5 (13.5) | 8 (15.7) | 1.000 |
| Death all cause at Day 30 | 7 (24.1) | 10 (31.3) | 0.580 |
| Absence of rehospitalization at Day 30 | 7 (24.1) | 5 (22.7) | 1.000 |
Data are n (%) unless otherwise stated.
* according to Cockroft and Gault
BMI: body-mass index; eGFR: estimated glomerular filtration rate; PCR: polymerase chain reaction; SD: standard deviation
Table 3.
Multivariate analysis of factors associated with antibiotic prescription among the PCR positive population
| aOR (95%CI) | P-value | |
|---|---|---|
| Age (mean ± SD, years) | 0.97 (0.89-1.05) | 0.38 |
| Female | 0.58 (0.19-1.73) | 0.33 |
| Charlson comorbidity index (mean ± SD) | 0.98 (0.75-1.27) | 0.87 |
| Radiological infiltrate | 12.02 (3.07-47.08) | 0.004 |
| Positive PCR test to Respiratory Syncitial Virus | 7.54 (1.74-32.65) | 0.01 |
SD: standard deviation; aOR: adjusted odds ratio; CI: confidence interval
In the multivariate analysis, the only factors associated with antibiotic prescription were radiological infiltrate (adjusted OR 12.02, 95%CI 3.07-47.08; p = 0.004) and RSV identification (adjusted OR 7.54, 95%CI 1.74-32.65; p = 0.007).
When comparing clinical presentation between the RSV-positive patients (n = 15) and the patients with PCR tests positive for other viruses (n = 73), RSV-positive patients had significantly more purulent sputum production (p = 0.02).
Finally, among patients with positive RVP, discontinuation of antibiotic treatment was not significantly associated with recurrence, mortality, or length of stay (Table 2).
4. Discussion:
Our study showed that respiratory PCR testing has limited impact on antibiotic prescription in older patients with suspected RTI. Even positive RVP did not lead to systematic antibiotic discontinuation, especially when patients were already being treated with antibiotics. X-ray results seem to be more relevant for physicians in their decisions on antibiotic prescription. Finally, none of our study patients had positive RBP, which meant that our study focused on the impact of RVP (rather than RBP) on antibiotic prescriptions.
Most clinical signs and symptoms in acute RTIs are not related to the pathogen, especially among older patients. Over recent years, highly sensitive and specific PCR tests have become the gold standard diagnostic method for viral RTIs.
That said, indications for respiratory multiplex PCR remain an area of uncertainty in medical research [11]. In patients with community-acquired pneumonia, the sensitivity and specificity of a test depend on the type of respiratory sample used: nasopharyngeal, sputum, aspiration, or bronchoalveolar lavage [12]. Unfortunately, the limited availability and high cost of these tests restrict their use.
Furthermore, little is known about the real-life impact on routine clinical practice of respiratory PCR testing for RTI in the elderly population, which is already subject to high exposure of antibiotics, frequently leading to multidrug-resistant organism infections [13], especially if the availability of this test is not associated with local recommendations and/or specific training with ID specialists.
In our study, the impact of PCR testing was low. Antibiotic modifications seemed to depend primarily on the presence of X-ray infiltration.
In the literature, the impact of multiplex PCR testing in cases of RTI on antibiotic prescription usually depends on a variety of factors, including not only duration of symptoms, comorbidities, immunosuppression, but also the availability of other ancillary test results at the time of presentation, such as chest X-ray (as in our study) [14].
In addition, RTI prevalence varies widely over seasons, leading to inconsistently positive or negative predictive values [14].
One study compared the outcomes in adult patients with positive RVP during two consecutive influenza seasons [15], with infections being diagnosed by conventional methods in the first season and by PCR testing in the second season. A multivariate logistic regression found that a diagnosis of influenza by PCR was associated with lower duration of antimicrobial use (p = 0.032), which suggests that PCR testing could improve the management of patients with suspected viral RTIs.
In an emergency department (ED), a retrospective study analyzed the clinical impact of rapid viral and bacterial respiratory testing among adult patients with respiratory symptoms [16]. In patients with positive viral PCR testing, antibiotic initiation was lower in patients with normal chest X-ray compared to those with abnormal chest imaging (57.2% and 93.0%, respectively; p < 0.001). This shows that as in our study, thoracic X-rays were taken into account in the antibiotic prescription decision.
In our study, RSV-positive patients had significantly more purulent sputum production. These results could partly explain why, in the multivariable analysis, patients with a positive RSV confirmed by PCR were significantly associated with antibiotic prescriptions (p = 0.007).
In a Canadian study, in which 800 patients admitted for suspicion of RTI were tested using a 12-virus respiratory panel, positive testing with Influenza virus was associated with shorter length of hospital stay (mean 12.4 ± 15.6 vs. 14.8 ± 16.1 for patients with negative testing), and appropriate antiviral treatment prescriptions [17]. In a multivariable analysis, antibiotic discontinuation was significantly associated with radiographic suspicion of bacterial pneumonia (OR 0.59, 95%CI 0.39-0.90), but was not significantly associated with an Influenza virus–positive test result (OR 1.38, 95%CI 0.89-2.16). Positive testing for viruses other than influenza virus was not associated with significantly different outcomes.
A review of respiratory viral panels was recently performed by Wils et al. [18]. According to nine studies, active antimicrobial stewardship (e.g., education on how to interpret the results of respiratory viral panels) led to reduced antibiotic prescription in patients with viral RTIs. Furthermore, three other studies found a positive impact on antibiotic therapy in a population without suspected bacterial pneumonia (e.g., absence of radiological evidence of bacterial infection or low procalcitonin (PCT) level). Moreover, the review suggested reduced antibiotic prescription associated with the rapid testing and obtaining of results.
Finally, improved antiviral use and enhanced application of infection control measures as a result of RVP testing were found in all studies.
Recently, a multicenter randomized controlled trial (RCT) among hospitalized patients with pneumonia at risk of Gram-negative bacterial infection that evaluated bacterial multiplex PCR assay of bronchoalveolar lavage showed decreased duration of inappropriate antibiotic treatments [19]. On the contrary, another RCT among severe SARS-CoV-2 pneumonia patients showed that compared with routine practice, an algorithm strategy involving respiratory multiplex PCR and procalcitonin level did not modify antibiotic exposure or clinical outcomes [20].
Surprisingly, in our study, no positive results on the respiratory bacterial PCR panel were observed on 193 patients. This could be explained by the panel of intracellular bacteria tested with this assay, which is not appropriate to community-acquired pneumonia epidemiology among older patients. Coverage of significant RTI pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, even in the elderly population, should have a positive impact on antimicrobial stewardship. Rapid molecular rapid testing for respiratory viruses should be used with appropriate testing covering significant pathogens according to the targeted population.
As regards the elderly population, a prospective cohort study in an ED evaluated the impact of RVP testing associated with serum PCT level [21]. Positive RVPs were significantly associated with a higher proportion of antibiotic discontinuation or de-escalation (26.0% vs 16.1%, p = 0.007), use of antivirals (8.9% vs 0.6%, p < 0.001), and shorter duration of intravenous antibiotics (10.0 vs 14.5 days, p < 0.001). Hence, combining rapid RVP testing and PCT monitoring could be a useful strategy in this population.
Our study had several limitations. Firstly, it was a retrospective study with a small sample size. Moreover, in this pre-COVID era, PCR testing was not protocolized among patients with suspected RTIs and was mostly performed after initiation of antibiotic treatment. There were no local guidelines for test prescriptions and consequences of the results. Finally, the diagnosis of RTI was not certain, as most patients were afebrile and few had X-ray infiltrations. Furthermore, a larger panel of microorganisms, especially bacterial agents, may have a greater impact on antibiotic prescriptions.
All in all, despite several limitations, respiratory virus multiplex PCR has several advantages, including a short turnaround time, the ability to detect bacteria after antibiotic treatment, and the ability to detect viruses, which could potentially facilitate discontinuation of unnecessary antibiotic treatment [19].
Therefore, we believe that respiratory multiplex PCR should be used only when its results are sure to impact antimicrobial treatment. In cases of high suspicion or during epidemic periods, PCR detection of RSV, Influenza, or metapneumovirus should be used. Moreover, detection of bacteria should only be used in cases of severe pneumonia and in patients with a high risk of infection due to multidrug-resistant organisms.
Lastly, the availability of these innovative and expensive tools for exhaustive microbiological diagnosis of RTI should be associated with local guidelines, along with specific staff training to define the best indication and interpretation of results. This process is necessary to optimize their use and impact, and to improve patient care.
5. Conclusion:
In our study, the impact of respiratory multiplex PCR on antibiotic prescription for patients hospitalized due to RTI in an acute geriatric ward was low, probably due to the absence of local guidelines, training and counselling. Nevertheless, discontinuation of antibiotic treatment in the event of positive PCR testing seemed safe enough. Cost-effective study should more precisely evaluate the interest of these tests, and possibly help to safely reduce unnecessary antibiotic exposure in this population. Training of physicians should likewise help to correctly interpret PCR results, lead when appropriate to discontinuation, and finally optimize the use of these expensive new diagnotic tools.
Funding:
This research received no external funding.
Ethics approval:
Considering the retrospective study design, data collection from preexisting medical records, and respect for the anonymity of the patients included (referred to as “Hors Loi Jardé” studies in France), no ethical approval or administrative approval was necessary for this study. This study was submitted to the local Data Protection Officer (DPO), and upon approval, identified in the hospital study registry.
Consent to participate:
No patient included in the study expressed opposition to the use of their clinical data in this retrospective study.
Data availability:
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions:
MP and AD conceived and designed the study. AL, BD, LL and EG collected the data.
AL, MH and CD analyzed the data. All authors contributed to interpretation of the data.
CD and AD drafted the first version of the manuscript. All authors critically reviewed the manuscript and approved the final version.
References:
- 1.Santé Publique France. [Antibiotic consumption in community setting in France between 2010-2020. Preliminary analysis.] [In French] 2020. https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/resistance-aux-antibiotiques/documents/rapport-synthese/consommation-d-antibiotiques-en-secteur-de-ville-en-france-2010-2020 (accessed May 27, 2023).
- 2.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sangil A., Calbo E., Robles A., Benet S., Viladot M.E., Pascual V., et al. Aetiology of community-acquired pneumonia among adults in an H1N1 pandemic year: the role of respiratory viruses. European Journal of Clinical Microbiology & Infectious Diseases : Official Publication of the European Society of Clinical Microbiology. 2012;31:2765–2772. doi: 10.1007/s10096-012-1626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trémolières F. Current epidemiology of microbial low respiratory tract infections. Medecine et Maladies Infectieuses. 2006;36(11–12):546–554. doi: 10.1016/j.medmal.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavia A.T. What is the role of respiratory viruses in community-acquired pneumonia?: What is the best therapy for influenza and other viral causes of community-acquired pneumonia? Infectious Disease Clinics of North America. 2013;27:157–175. doi: 10.1016/j.idc.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain S., Self W.H., Wunderink R.G., Fakhran S., Balk R., Bramley A.M., et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. New England Journal of Medicine. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alimi Y., Lim W.S., Lansbury L., Leonardi-Bee J., Nguyen-Van-Tam J.S. Systematic review of respiratory viral pathogens identified in adults with community-acquired pneumonia in Europe. Journal of Clinical Virology : The Official Publication of the Pan American Society for Clinical Virology. 2017;95:26–35. doi: 10.1016/j.jcv.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavazzi G., Krause K.-H.-H. Ageing and infection. The Lancet Infectious Diseases. 2002;2:659–666. doi: 10.1016/S1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- 9.Chebib N., Cuvelier C., Malézieux-Picard A., Parent T., Roux X., Fassier T., et al. Pneumonia prevention in the elderly patients: the other sides. Aging Clinical and Experimental Research. 2021;33:1091–1100. doi: 10.1007/s40520-019-01437-7. [DOI] [PubMed] [Google Scholar]
- 10.Prendki V., Malézieux-Picard A., Azurmendi L., Sanchez J.C., Vuilleumier N., Carballo S., et al. Accuracy of C-reactive protein, procalcitonin, serum amyloid A and neopterin for low-dose CT-scan confirmed pneumonia in elderly patients: A prospective cohort study. PLOS ONE. 2020;15:e0239606. doi: 10.1371/journal.pone.0239606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickens C.I., Wunderink R.G. Clinical impact of bacterial syndromic testing in pneumonia. Lancet Respir Med. 2022;10:816–818. doi: 10.1016/S2213-2600(22)00095-9. [DOI] [PubMed] [Google Scholar]
- 12.Dudoignon E., Coutrot M., Camelena F., Leone M., Dépret F. Multiplex bacterial PCR for antibiotic stewardship in pneumonia. The Lancet Respiratory Medicine. 2022;10:e78. doi: 10.1016/S2213-2600(22)00264-8. [DOI] [PubMed] [Google Scholar]
- 13.de Laroche M., Fellous L., Salomon E., Saadeh D., Duran C., Bouchand F., et al. Bloodstream infections in older population: epidemiology, outcome, and impact of multidrug resistance. European Journal of Clinical Microbiology & Infectious Diseases. 2021;40:1665–1672. doi: 10.1007/s10096-021-04212-7. [DOI] [PubMed] [Google Scholar]
- 14.Hanson K.E., Azar M.M., Banerjee R., Chou A., Colgrove R.C., Ginocchio C.C., et al. Molecular Testing for Acute Respiratory Tract Infections: Clinical and Diagnostic Recommendations From the IDSA’s Diagnostics Committee. Clinical Infectious Diseases. 2020;71:2744–2751. doi: 10.1093/cid/ciaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rappo U., Schuetz A.N., Jenkins S.G., Calfee D.P., Walsh T.J., Wells M.T., et al. Impact of Early Detection of Respiratory Viruses by Multiplex PCR Assay on Clinical Outcomes in Adult Patients. Journal of Clinical Microbiology. 2016;54:2096–2103. doi: 10.1128/JCM.00549-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss Z.F., Cunha C.B., Chambers A.B., Carr A.V., Rochat C., Raglow-Defranco M., et al. Opportunities Revealed for Antimicrobial Stewardship and Clinical Practice with Implementation of a Rapid Respiratory Multiplex Assay. Journal of Clinical Microbiology. 2019:57. doi: 10.1128/JCM.00861-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semret M., Schiller I., Jardin B.A., Frenette C., Loo V.G., Papenburg J., et al. Multiplex Respiratory Virus Testing for Antimicrobial Stewardship: A Prospective Assessment of Antimicrobial Use and Clinical Outcomes Among Hospitalized Adults. The Journal of Infectious Diseases. 2017;216:936–944. doi: 10.1093/infdis/jix288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wils J., Saegeman V., Schuermans A. Impact of multiplexed respiratory viral panels on infection control measures and antimicrobial stewardship: a review of the literature. European Journal of Clinical Microbiology & Infectious Diseases. 2022;41:187–202. doi: 10.1007/s10096-021-04375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darie A.M., Khanna N., Jahn K., Osthoff M., Bassetti S., Osthoff M., et al. Fast multiplex bacterial PCR of bronchoalveolar lavage for antibiotic stewardship in hospitalised patients with pneumonia at risk of Gram-negative bacterial infection (Flagship II): a multicentre, randomised controlled trial. The Lancet Respiratory Medicine. 2022;10:877–887. doi: 10.1016/S2213-2600(22)00086-8. [DOI] [PubMed] [Google Scholar]
- 20.Fartoukh M., Nseir S., Mégarbane B., Cohen Y., Lafarge A., Contou D., et al. Respiratory multiplex PCR and procalcitonin to reduce antibiotic exposure in severe SARS-CoV-2 pneumonia: a multicentre randomized controlled trial. Clinical Microbiology and Infection : The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2023;29(6):734–743. doi: 10.1016/J.CMI.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee C.C., Chang J.C.Y., Mao X.W., Hsu W.T., Chen S.Y., Chen Y.C., et al. Combining Procalcitonin and Rapid Multiplex Respiratory Virus Testing for Antibiotic Stewardship in Older Adult Patients With Severe Acute Respiratory Infection. Journal of the American Medical Directors Association. 2020;21:62–67. doi: 10.1016/j.jamda.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]