Percutaneous chest tube placement is a relatively common procedure that can be performed by multiple specialties. Chest tube insertion is frequently performed by interventional radiologists (IRs) due to the advantage of imaging guidance including ultrasound (US), fluoroscopy, and computed tomography (CT) which allow for precise placement not achieved with bedside insertion techniques. Chest tube management is increasingly conducted by IRs, signifying the importance of a greater understanding of chest tube management and troubleshooting, especially for IR trainees. This manuscript is specifically tailored for the IR trainees to provide an overview of chest tube management. The practical considerations detailing the entire chest tube process are covered such as indications, technique, relevant anatomy, collection devices, commonly used catheters, postprocedural complications, lytic therapy, and troubleshooting. This guide aims to help the IR trainees feel more confident in chest tube placement and periprocedural care.
Preprocedural Evaluation and Planning
Indications
Chest tube insertion, also known as tube thoracostomy, can be performed for the evacuation of various air and/or fluid collections from the pleural space. Following chest tube placement, evacuation of air and fluid within the pleural cavity allows for lung reexpansion and return to normal ventilatory function. 1 The role of chest tubes in the management of pleural effusions depends on the underlying cause, such as blood (hemothorax), purulent fluid (empyema), and lymphatic fluid (chylothorax). In the setting of hemothorax, the chest tube placement will decompress the pleural cavity, and information provided by fluid appearance and output from the tube can guide the need for blood transfusion, endovascular embolization, or surgical intervention. 2 3 Following cardiothoracic surgeries, chest tubes ensure adequate postoperative drainage of fluid, blood, and air. In instances of multiloculated parapneumonic effusions and empyema, drainage may be slow or inadequate due to fibrinous septations. In these situations, an indwelling chest tube can be used to instill intrapleural fibrinolytic agents to enhance drainage. 4 For malignant pleural effusions, chest tube drainage provides symptomatic relief and allows for chemical pleurodesis or the obliteration of the space between visceral and parietal pleural layers. Various chemicals such as talc, doxycycline, or bleomycin can be instilled via the chest tube to create adhesion between pleural layers and prevent malignant fluid accumulation. 1 Chest tubes can also be used to drain chylothorax due to thoracic duct injury from trauma or surgery. 2 Resolution of a chylothorax usually entails additional treatment including dietary changes, octreotide, and lymphatic embolization. 5
Contraindications
Potential contraindications for chest tube placement need to be considered in the context of the risks versus benefits of the procedure. The relative contraindications of chest tube placement include a highly loculated pleural effusion, bleeding diathesis, and overlying skin or soft tissue infection. Pleural adhesions can partially obliterate the pleural space creating difficult placement of a chest tube into pockets of fluid or gas, which may not be well visualized even with imaging guidance. 2 Chest tube placement is considered a low-risk procedure based on the Society of Interventional Radiology (SIR) guidelines and can be safely performed with an international normalized ratio (INR) < 3.0 and platelet counts of at least 20,000 per μL. Importantly, most anticoagulants do not need to be withheld as long as the target INR is reached. 6 A recent meta-analysis of over 5,000 patients with uncorrected coagulopathies undergoing either US-guided thoracentesis or chest tube placement confirmed the low risk of bleeding in these procedures. 7 A chest tube should not be inserted at a site with an overlying infection such as cellulitis or a Herpes zoster infection. 2 In addition to these relative contraindications, there are certain clinical scenarios in which chest tubes should be avoided, if possible, which are detailed in Table 1 . In these scenarios, other management strategies may have superior efficacy over chest tube placement. 8 9 10 11 12
Table 1. Clinical scenarios in which to avoid chest tube placement.
| Clinical scenarios | Alternate therapy | Potential reasons to place a chest tube |
|---|---|---|
| Pleural effusion secondary to congestive heart failure | • Diuresis • Additional medical therapy to treat heart failure |
Facilitate weaning off the mechanical ventilator |
| Hepatic hydrothorax | • Thoracentesis • TIPS • Liver transplantation |
Symptomatic, large pleural effusion with immediate plans for TIPS or liver transplantation |
| Pneumothorax ex vacuo | • Conservative, patients are typically asymptomatic • Allow pleural fluid to reaccumulate and air will be reabsorbed |
Enlarging pneumothorax as seen on serial chest radiographs |
| Trapped lung and pleural effusion | • Conservative • Decortication |
Indwelling pleural catheter for palliation with symptomatic malignant effusions associated with trapped lung |
| Endobronchial obstruction and pleural effusion | • Treat endobronchial tumor • Palliative care |
|
| Mediastinal emphysema | • Increase inspired oxygen concentration • Mechanical ventilator changes • Antitussive medications |
Decompress a pneumothorax |
Abbreviation: TIPS, transjugular intravascular portosystemic shunt.
Preprocedural Antibiotics
Prophylactic antibiotics are not required for most instances of chest tube placement. In a patient with symptoms and signs of empyema, broad-spectrum intravenous antibiotics should be provided that addresses the suspected infection in the pleural space and/or lung. Broad-spectrum antibiotics for empyema should cover suspected pleuropulmonary infectious organisms such as anaerobes, Staphylococcus aureus , Streptococcus pneumoniae , Hemophilus influenzae , Klebsiella pneumoniae , and Escherichia coli . 13
Imaging Guidance
Chest tubes can be placed with or without real-time imaging guidance. Modalities such as US or CT are helpful when the tubes need to be inserted into small or difficult-to-access collections. 14 US is more commonly used over CT due to its availability, convenience, and lack of ionizing radiation. There are several circumstances in which preprocedural CT imaging is useful such as multiple catheter placements in complex or multifocal collections and complex gas-containing collections. 2 Preprocedural CT can also be considered if there is a need to simultaneously evaluate the concomitant chest disease in the patient. Fluoroscopy is often used in conjunction with US or CT to evaluate the extent of drainable fluid in pleural collections and delineate any fistulous tracts. Fluoroscopy can be used as a standalone imaging guidance modality for gas or fluid collections large enough that only bony landmarks are needed for initial access.
Commercially Available Chest Tubes and Possible Catheter Modifications
Table 2 summarizes commercially available catheters commonly used for chest tube placement and their key features. Catheter shapes are straight with a curved tip, straight with a hooked tip, and pigtail (looped tip). The range of tube diameters is 5 to 40 Fr. Two intraprocedural catheter modifications can be performed to enhance certain catheter functions: the manual creation of additional catheter side holes and the removal of the retention string on locking pigtail catheters. The manual creation of additional catheter side holes increases the surface area in contact with the pleural collection, facilitating more efficient and effective drainage. Care must be taken to not introduce excessive side holes which may extend across the pleura or into the thoracic soft tissue and result in increased pain or risk of subcutaneous emphysema or cellulitis, respectively. The creation of excessively large side holes decreases the catheter's structural integrity which may result in catheter breakage and retained catheter fragments. The use of a 10- to 16-Fr biliary catheter as a chest tube provides additional factory-created side holes. The second potential chest tube modification is the removal of the retention string from the locking pigtail catheter which allows for more efficient catheter removal at the bedside. This is especially useful if non-IR clinicians will be performing the catheter removal and pulling the pigtail catheter without the release of the retention string that locks the pigtail. Removal of the retention string before placement may lower the risk of pneumothorax upon tube removal as the catheter does not need to be cut (open to the atmosphere) to release the locking string mechanism. Finally, there is no string to get caught on the lung or pleura upon tube removal, reducing the risk of lung injury. The main drawback associated with removing the retention string on locking pigtail catheters is the increased risk of the catheter falling out inadvertently. There are commercially available nonlocking pigtail catheters that may be used as chest tubes.
Table 2. Commonly used tubes, sizes, and features.
| Chest tube type | Shape | French | Length | Features |
|---|---|---|---|---|
| Gordon Large-Bore Drainage Catheter (Cook) | Straight with a curved tip | 16–22 Fr | 40 cm | • Caliber larger than expected given French size • More compliable than other straight chest tubes |
| Multipurpose Drainage Catheter (Cook) | Straight with looped tip (pigtail) | 8.5–16 Fr | 25 cm, 45 cm, 60 cm | • Pigtail end, 25 mm • Available with or without a locking mechanism • Radiopaque band on proximal area of the loop |
| Dawson Mueller Drainage Catheter (Cook) | Straight with looped tip (pigtail) | 5–14 Fr | 15 cm, 25 cm | • Locking pigtail end, 10 mm • Facilitates drainage in small cavities |
| Thal-Quick Abscess Drainage Set (Cook) | Straight with a hooked tip | 12–28 Fr | 33 cm, 41 cm | • Large bore, clear catheter allows for fluid to be seen during drainage • Large, oval side holes allow for increased drainage |
| Expel Multi-Purpose Drainage Catheter Series (Boston Scientific) | Available in loop, J-tip, curved tip | • Loop: 6–14 Fr • J-tip: 6–14 Fr • Curved: 16–26 Fr |
• Loop: 15 cm, 25 cm, 45 cm • J-tip: 20 cm, 25 cm • Curved: 30 cm, 40 cm |
• Variety of tip configurations • Locking mechanism available • Increased buckling resistance |
| ARGYLE PVC Thoracic Catheter (Cardinal Health) | Straight and right-angle | • Straight: 12–40 Fr • Right-angle: 16–40 Fr |
51 cm | • Clear tube construction • The beveled connector can be used to create a funnel connector |
| Origin General-Purpose (UreSil) | Straight with J-tip | 6–16 Fr | 18–21 cm | • Clear hub for visualization • Large side holes facilitate increased drainage • Nonlocking |
| THORA-VENT (UreSil) | Straight | 11–13 Fr | 10 cm, 13 cm | • Included suction tubing set allows for active monitoring of pneumothorax during treatment • May allow for increased ambulation |
Optimal Chest Tube Caliber
The IR physicians need to anticipate a sufficient chest tube caliber for a pneumothorax or pleural effusion by integrating clinical, drainage, and patient tolerance factors. Smaller caliber chest tubes are less painful and perform noninferior to larger caliber tubes in many clinical settings. 15 However, smaller caliber tubes come with an increased risk of catheter clogging that necessitates exchange for a larger caliber tube, resulting in another procedure for the patient and an increased length of hospitalization. Thus, there is a compromise between greater clinical efficacy (larger caliber tube) and less patient discomfort (smaller caliber tube) that should be balanced on a case-by-case basis. In pneumothorax, the size of the visceral pleural air leak and the underlying integrity of the lung parenchyma must be considered when selecting the correct tube size. A large pneumothorax in patients with either underlying emphysema or bronchopleural fistulae may need a larger caliber (14–18 Fr) chest tube since greater volumes of air need to be removed from the pleural space to achieve pleural apposition. In contrast, a pneumothorax after a percutaneous needle lung biopsy in a patient without parenchymal lung disease may only require a 10- to 12-Fr chest tube for 1 to 2 days. Similarly, a nonloculated serous pleural effusion may be drained using a 10- to 12-Fr chest tube, whereas a larger bore (14–28 Fr) chest tube may be considered for effective drainage of a hemothorax. 16
Parapneumonic effusions and empyemas should be initially drained with a larger diameter (14–18 Fr) chest tube. The use of suction applied to the chest tube, injection of lytic medications, or daily saline flushes through the chest tube may promote catheter lumen patency and reduce the need for larger caliber chest tubes. 2 When dealing with smaller (<10 cm diameter) loculated pleural fluid collections, there may not be enough anatomic space for an angled, large caliber chest tube with extended side holes. In these circumstances, a 12- to 16-Fr pigtail catheter has a smaller “side-hole footprint” and may be ideal for the limited pleural space available in small loculated collections.
Chest Tube Positioning
In a supine patient, simple pleural fluid collects dependently in the posterior pleural space. Positioning the chest tube within the dependent posterior location allows that fluid to be more effectively removed ( Fig. 1 ). The best strategy to accomplish posterior tube placement is to enter the pleural space along the posterior axillary line and orient the needle, guidewire, and chest tube posteriorly. The procedural workspace needed to insert a posterior pleural space chest tube is often improved by rotating the supine patient in a contralateral direction to better visualize a posterolateral entry site. Entry of a chest tube through the lateral chest and aimed posteriorly does not guarantee that the catheter tip will end up in the posterior pleural space to drain pleural fluid. The tip of the chest tube may accidentally reside within the major fissure or even the anterior pleural space, thus failing to evacuate the pleural effusion. Once the guidewire is adjacent to the spine on the anteroposterior fluoroscopy view, the guidewire should remain close to the spine with an oblique view. A single chest tube is usually effective for parapneumonic effusion or empyema ( Fig. 1 ). If the preprocedure CT demonstrates a large pleural effusion with multiple loculations, two chest tubes can promote more efficient drainage ( Fig. 1 ).
Fig. 1.
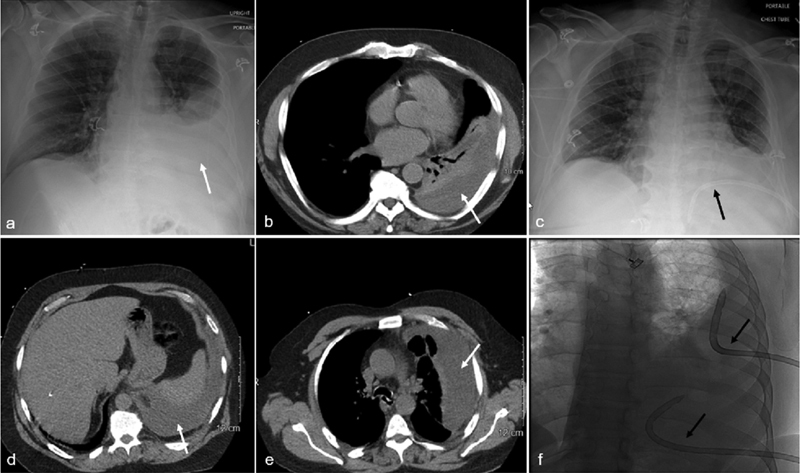
Anatomic positioning of chest tubes. A 65-year-old male with a loculated left parapneumonic pleural effusion. Chest radiograph ( a ) and CT of the chest ( b ) show the posterior pleural fluid collection (white arrow). A 16-Fr Gordon angled chest tube (white arrow) is placed into the dependent posterior inferior left pleural space (black arrow) ( c ). A 55-year-old male with Staphylococcus and Bacteroides empyema. CT of the chest ( d, e ) shows a large, loculated left pleural collection (white arrow). Due to the size and extent of loculations of the empyema, two chest tubes are placed to efficiently drain the infected fluid along with intracatheter lytic therapy. A fluoroscopic image ( f ) depicts an 18-Fr Gordon angled chest tube in the superior lateral left pleural space and another tube in the posterior inferior left pleural collection (black arrows).
In a supine patient, simple pleural gas collects nondependently in the anterior pleural space. A simple pneumothorax is evacuated optimally with the chest tube in the anterior pleural space ( Fig. 2 ). This is most likely achieved by accessing the pleural space along the anterior axillary line or by entering more anteriorly while remaining lateral to the internal thoracic artery and vein. When placing an anterior left chest tube, the operator must confirm the position of the pericardium and heart through the use of imaging, particularly in the presence of cardiomegaly and/or pericardial effusion.
Fig. 2.
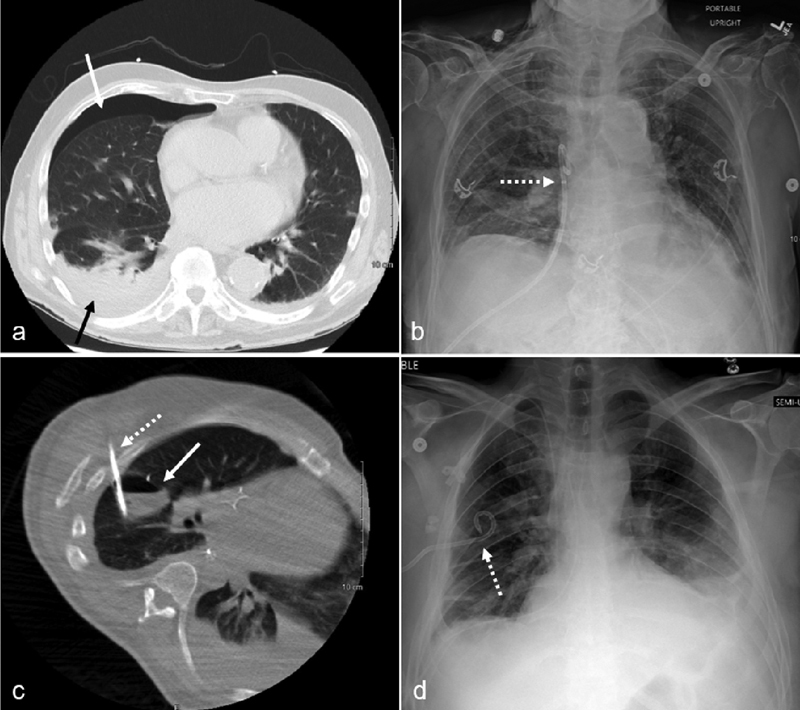
An 85-year-old male presented with a delayed presentation of a right pneumothorax following parathyroid surgery. Chest CT ( a ) demonstrates a small right pneumothorax (solid white arrow), small pleural effusion (solid black arrow), and basilar atelectasis. A 12-Fr, nonlocking pigtail catheter (dotted arrow) was placed into the anterior right pleural space using CT guidance as seen on the subsequent chest radiography ( b ). A 38-year-old male with an infected hydropneumothorax following an Ivor-Lewis esophagectomy. Chest CT during drainage procedure (c) shows a loculated gas and fluid collection in the right major fissure (solid white arrow). Using CT guidance, a 12-Fr locking pigtail chest tube (dotted white arrows) was placed into the intrafissural collection as demonstrated by CT (c) and chest radiograph ( d ).
Suboptimal chest tube locations include near the scapula, close to the spine, and into the major fissure. When placing a chest tube along the posterolateral chest, catheter entry adjacent to the inferior scapula may lead to chest tube dislodgement. Typically, chest tube insertion is performed in the supine position with the ipsilateral upper extremity abducted, displacing the inferior tip of the scapula superiorly. When the patient adducts the upper extremity following the procedure, the inferior tip of the scapula will return to its inferior anatomic position and may move or retract the posterolateral chest tube out of position. Chest tube mispositioning by the scapula is avoided by using a more inferior or lateral entry point relative to the position of the inferior scapula. When a loculated posteromedial pleural effusion requires chest tube drainage, if possible, the catheter trajectory should avoid traversal of the paraspinal muscles and the extreme medial intercostal space. A higher risk of bleeding is associated with crossing the blood vessel-rich paraspinal muscles or with injury to the relatively large intercostal vessels in the medial intercostal space ( Fig. 3 ). 17 A supine patient laying directly on a posteromedial chest tube may cause tube kinking and migration and often leads to increased pain for the patient. With a chest tube positioned in the major fissure, removal of intrapleural fluid and gas is typically limited. 2 However, there may be rare instances in which a loculated major fissure collection may be drained by a chest tube ( Fig. 2 ). Extrapleural collections can be effectively drained by a chest tube. 18
Fig. 3.
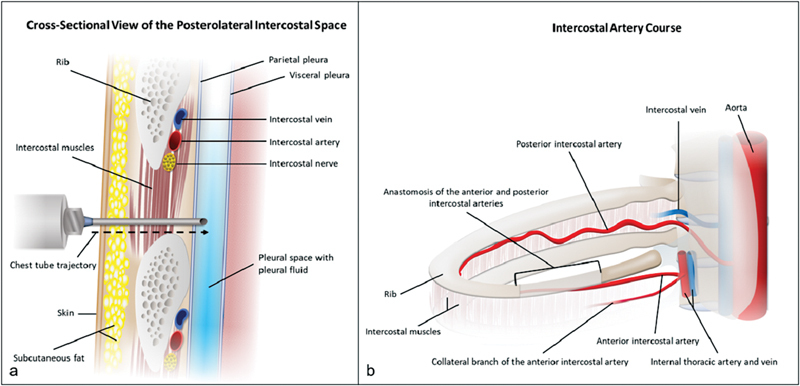
Anatomy of the intercostal space and the intercostal artery. The cross-sectional view of the posterolateral intercostal space ( a ) reveals the neurovascular bundle is along the costal groove. The percutaneous chest tube trajectory through the intercostal space is ideally along the superior border of the rib which minimizes injury to the intercostal nerve and vessels. The intercostal artery courses along the inferior border of the rib except in the paravertebral region ( b ). Needle access superior to the rib in the paravertebral region may not avoid injury to the intercostal artery since the posterior intercostal artery courses in the mid and lower intercostal space near the spine.
Procedural Description
Moderate Sedation and Anesthetics
The use of moderate sedation is recommended when inserting a chest tube. Anesthesia support should be considered in patients with hemodynamic instability, respiratory distress, a significantly compromised function of the contralateral lung (e.g., preexisting pulmonary or cardiovascular disease, contralateral pleural effusion), or American Society of Anesthesiologists (ASA) Class IV or higher. The parietal pleura is the most pain-sensitive structure traversed during transthoracic procedures and insufficient administration of local anesthesia prior to chest tube placement is often an underoptimized step to limit insertional and postprocedure pain. A short-acting local anesthetic, such as lidocaine 1 or 2%, is often used. Following chest tube insertion, injection of additional long-acting local anesthetic agents, such as bupivacaine 0.25 or 0.5%, can provide extended pain relief following the procedure.
Applied Anatomy and Technique
It is conventionally taught that needle placement into the intercostal space should be along the superior border of the rib, in order to minimize the risk of injury to the intercostal neurovascular bundle that courses in the costal groove along the undersurface of the rib. 19 The cross-sectional anatomy of the intercostal space is illustrated in Fig. 3 . Older patients with tortuous intercostal arteries and posterior access closer to the spinous process are associated with an increased risk for traversing the intercostal artery. 20
Chest tube placement is typically accomplished using the Seldinger technique while blunt dissection through the pleura and trocar technique are infrequently used in IR. Seldinger technique allows for the placement of an 18-gauge needle into a fluid or gas pleural collection using image guidance. An 0.035-inch guidewire is inserted through the needle and into the pleural space. The location of the guidewire is imaged using fluoroscopy or CT. If needed, a 5-Fr Kumpe catheter can reposition the guidewire under fluoroscopic guidance for optimal chest tube location. The use of a 21-gauge needle for access into the pleural space is valuable for delicate needle trajectories, near the spleen, and in patients with higher bleeding risks.
Fluid aspiration through the needle provides an early indicator of the nature of the pleural fluid or in the setting of pneumothorax, and aspiration of air provides additional confirmation of needle placement into the pleural space. The guidewire should move with low resistance across the pleural space, as observed with fluoroscopy. Limited initial guidewire purchase and mobility by fluoroscopy suggests a nonideal location such as entry into a highly loculated pleural space, within the lung parenchyma, or in the extrapleural space. Another needle access entry site for a better intrapleural location should be sought. If the guidewire location is uncertain to be in the pleural space, cone beam CT may be utilized for problem-solving.
Following guidewire placement, the track is serially dilated prior to insertion of the chest tube catheter over a plastic or metal stiffener. After placement, the chest tube is connected to a drainage system. In the setting of pleural effusion, the fluid should spontaneously flow through the tube. If fluid only can be aspirated with a syringe, the chest tube may need to be repositioned to improve flow. In the setting of pneumothorax, bubbling through the chest tube drainage system (CTDS) is expected initially. Following lung reexpansion, continued bubbling through the drainage system indicates an air leak through the visceral pleura (bronchopleural fistula). The catheter should be secured to prevent kinking or dislodgement after clinical and/or imaging confirmation of appropriate chest tube placement. Suturing should be performed with one or two, nonabsorbable sutures (e.g., 0 silk, 0 PROLENE). A transparent dressing is preferable to allow the insertion site to be visually inspected by nursing staff. Use of the omental tape technique or a FLEXI-TRAK Anchoring Device will provide additional anchor points to the skin, promote chest tube stability, and avoid kinking and excessive tension at the tube insertion site.
Immediate Postprocedure Management, Chest Tube Drainage Systems, and Troubleshooting
Immediately following chest tube placement, drainage of intrapleural air and/or fluid usually results in prompt symptomatic and clinical improvement for the patient. The newly placed chest tube and reapposition of pleural surfaces often lead to pleuritic chest pain. Reassurance and intravenous narcotic analgesics are often successful. Rapid drainage of large amounts of pleural fluid or air may cause respiratory distress, hypoxemia, and even the development of reexpansion pulmonary edema. The initial removal of pleural fluid should be limited to 1,000 to 1,500 mL to avoid pulmonary edema, 2 and the chest tube should be clamped for 1 to 2 hours before unclamping again. Similar to thoracentesis, the presence of repetitive coughing and chest tightness or pain alerts the operator to limit intrapleural fluid drainage. The use of chest tube gravity drainage without negative pressure applied (water seal) initially may allow more time for the lung to reexpand and improve patient tolerance.
Clinical deterioration of the patient may occur following any chest tube placement for intrapleural fluid or air evacuation. An urgent chest X-ray can further evaluate respiratory distress which could reveal pneumothorax, pulmonary edema, hemothorax, or chest tube malposition. Patients with impending respiratory failure demonstrate hypoxemia and signs of increased work of breathing. Immediate patient assessment and treatment include airway assessment, vital signs, more upright patient positioning to improve breathing mechanics, and titration of inspired oxygen according to continuous oximetry. Selected patients may require an escalation of their level of hospital care.
The chest tube is connected to an air- and water-tight closed CTDS, which is placed below the drainage site to prevent fluid to siphon back into the pleural space. In most patients, −20 cm H 2 O suction is applied to the chest tube to promote intrapleural drainage through the chest tube. There are commercially available CTDSs, such as Atrium Oasis (Getinge, Merrimack, NH), Pleur-evac (Teleflex, Morrisville, NC), and Altitude (Cardinal Health, Dublin, OH). CTDSs generally have three interconnected chambers: a collection chamber, a water-seal chamber, and a suction control chamber. There are wet (water column) and dry (valve regulator) suction control mechanisms used for different CTDSs, which provide negative pressure at up to 25 cm H 2 O for wet devices and up to 40 cm H 2 O for dry devices. The main advantage of the dry mechanism CTDS is that a device tip-over will not result in spillage of the fluid chamber into the water-seal chamber. The wall suction is kept at least negative 80 mm Hg (which is over 108 cm H 2 O) to assure that the desired suction is delivered.
Postprocedural Considerations
Complications
Complication rates for tube thoracostomy are highly variable, ranging from 1 to 40%, with a recent meta-analysis reporting a 19% complication rate, 21 but serious complications are rare. 2 This wide variation in chest tube complications is clarified by the classification of complications: insertional, positional, removal, infective/immunologic, and instructional, educational, or equipment related. 21 22 Insertional complications such as bleeding, injury to surrounding anatomic structures (e.g., spleen, liver), and extrapleural placement are infrequently encountered. 21 More commonly seen are positional complications when a tube does not perform adequately, such as tube kinking, obstruction, or entrapment in the major fissure. Positional complications often necessitate the placement of a new tube, sometimes with operative intervention. 23 Postremoval complications involving pneumothorax, bleeding, or spontaneous tube dislodgement are less common. Causation of cellulitis at the chest tube insertion site or pleural space infection is highly unexpected with the use of sterile technique. 21 23 Equipment and clinical team educational-related adverse events are rare at most institutions. 23
Daily Patient Assessment
Chest tube patients need to be seen each day and more frequently as needed. The patient's vital signs, oximetry, chest tube output, and the appearance of the fluid should be reviewed. The examination should include lung auscultation and palpation for chest wall crepitus (subcutaneous emphysema). The chest tube entry site, dressing, and chest tube connections should be inspected to identify potential chest tube kinking, leaks, and retraction. There should be tidal breathing fluctuation in the water seal chamber. If no fluctuation is seen with breathing, the chest tube has either evacuated the air or fluid in the pleural space, or the chest tube or drainage tubing is occluded. The patient can be asked to cough; if there is bubbling into the water seal chamber, then there is an air leak. The chest tube should not be removed when an air leak (bronchopleural fistula) is detected during the bedside evaluation, even if there is no pneumothorax seen on the chest radiograph. Imaging follow-up includes a chest X-ray within 24 hours of chest tube placement. Daily imaging is not always needed, particularly if bedside assessment provides data that the chest tube is stable in position and is functioning.
Role of Lytic Therapy
Intrapleural lytic therapy is most commonly used in the setting of loculated pleural fluid collections such as complex parapneumonic effusions or empyemas, but it can also be used to treat retained hemothorax. 1 Catheters may become clogged by fibrinous exudates and drain poorly, resulting in a decreased ability to clear the infectious debris and possibly requiring surgical intervention. Evidence suggests that the combination of intrapleural tissue plasminogen activator (alteplase) and DNase (dornase alfa, Pulmozyme; Genentech, Inc, South San Francisco, CA) via the chest tube may improve drainage of infected material or retained hemothorax and likely improves the clinical course of patients. This increased drainage is associated with a decreased hospital stay as well as a reduced need for thoracic surgery interventions. 1 24 First-line treatment for retained hemothorax is early video-assisted thoracoscopic surgery, but lytic therapy can be considered as an adjunct or alternative treatment, especially in poor surgical candidates. 25 Our institution's approach to intrapleural lytic therapy is to administer the combination of alteplase 10 mg and dornase alfa 5 mg via the chest tube and clamp the tube for 2 hours, perform twice a day for 3 days, and stop if there is bleeding from the chest tube.
For therapeutic lysis of loculated pleural effusions, there are current procedural terminology (CPT) codes that may be used for billing. CPT code 32561 may be used for the first day of the instillation of lytic agents via a chest tube for the breakup of a multiloculated pleural effusion. CPT code 32562 may be used for each subsequent day in which there is the instillation of lytic agents via a chest tube. These CPT codes do not apply to the instillation of lytic agents into catheters in other locations, such as the peritoneal cavity. 26
Troubleshooting
After a chest tube has been placed, the provider may find that the amount of fluid drainage is lower than expected. If this happens, some common and easily resolved issues should be investigated first. If the CTDS collection chamber is near full, fluid drainage ceases, and the CTDS should be changed. Another reason for low fluid output from the chest tube is that the chest tube has successfully evacuated the pleural effusion; confirm with imaging. Occasionally, there may be erroneous low chest tube output entered during a single hospital shift. This is confirmed if bedside assessment indicates adequate fluid output. If the chest tube collection device has been inadvertently rotated multiple times, either by caregivers or an ambulatory patient, the chest tube or connecting tubing may become twisted and occluded. Occasionally the chest tube is dressed in a fashion that introduces a kink in the tubing, resulting in ineffective drainage. Drainage could also be impeded by a closed stopcock or clave in line with the chest tube, thick secretions or blood clogging the tube, or chest tube displacement. To improve patency in a clogged chest tube, bedside irrigation with sterile saline can be performed daily, followed by catheter exchange under fluoroscopy if issues persist.
Lack of tidal breathing fluctuation in the chest tube or drainage system may indicate successful pleural space drainage which can be identified by imaging. A chest tube kink or disconnection may be the reason for no tidal fluctuation. If the chest tube is occluded with debris or clot, sterile saline irrigation or exchange of the chest tube may be necessary.
The presence of subcutaneous emphysema usually indicates ineffective evacuation of pleural gas which migrates into the nearby soft tissues. Exchange for a larger caliber chest tube or placement of an additional chest tube is a potential remedy. Subcutaneous emphysema also may be due to chest tube side holes outside of the pleural space so that pleural gas can exit into soft tissues. If this is the case, exchange and reposition the chest tube so that side holes do not extend across the pleura.
Air bubbles through the chest tube and CTDS usually occur from an air leak in the lung. Prior to any chest tube management changes, always exclude the air bubbles which are from tubing disconnection or a hole in the external chest tube. An increase in suction applied to the chest tube should improve the apposition of pleural surfaces to stop the air leak. If the existing chest tube is of a small caliber or distant from the known lung air leak, chest tube exchange and repositioning should be considered. When a bronchopleural fistula persists despite increased applied suction and other interventions, a trial of water seal can be attempted since suction applied to the chest tube may account for the perpetuation of the pleural air leak.
Chest Tube Removal
The appropriate timing for the removal of chest tubes placed to drain pleural effusions remains a subject of debate. Thresholds for removal range from 200 to 500 mL per day. 1 Higher output thresholds for removal in postoperative patients were associated with higher rates of readmission for recurrent symptomatic pleural effusion, and therefore some authors recommend a lower threshold of 200 mL per day for removal. 14 In addition, the serous-appearing drainage and minimal radiologic evidence of residual fluid are factors favoring chest tube removal. Airspace disease may be confused with residual pleural effusion on a chest radiograph, potentially causing a delay in tube removal when the pleural fluid evacuation is satisfactory. Prior to the removal of a chest tube placed for pneumothorax, the chest tube is usually transitioned from suction to water seal to observe for the development of a chest tube air leak and/or pneumothorax. A water seal trial should be brief to not extend the length of hospital stay. Chest tube removal while on suction is acceptable if removal criteria are met. A chest tube clamping trial for pneumothorax is not always favored since it is an unnecessary step if removal criteria have been met and it adds unnecessary risk for the development of a tension pneumothorax.
There are variations in practice regarding chest tube removal techniques. A dose of intravenous narcotic analgesic medication 5 to 10 minutes before chest tube removal improves the tolerance of the procedure. The chest tube is favored to be removed at the end of expiration while the patient is performing a Valsalva maneuver. At this moment, the difference between the pleural pressure and the outside atmospheric pressure is the lowest. A chest tube may also be removed safely during inspiration. Irrespective of the technique used, rapid removal of the chest tube minimizes the time when the chest tube site provides a potential opening that can permit the intake of intrapleural air (which might occur during a quick inspiration as the patient experiences pain). Following removal, the chest tube entry site should quickly be covered with dry or petroleum gauze to create a seal followed by a sterile dressing. 1 2 Suture closure of the chest tube site is not needed unless a large incision was created for the chest tube insertion. In hospitalized patients, a chest radiograph after chest tube removal provides a new baseline study for comparison with subsequent imaging. Postremoval chest radiographs were once a common practice, but current evidence suggests that clinical observation following removal is sufficient. 27
Summary
In summary, chest tube placement can be performed most expertly by IR physicians given the advantages of diagnostic radiology knowledge and procedural guidance using multiple imaging modalities. IRs can play a greater role in patient care by providing more comprehensive chest tube management.
Footnotes
Conflicts of interest The authors would like to state that they do not have any conflicts of interest.
References
- 1.Anderson D, Chen S A, Godoy L A, Brown L M, Cooke D T. Comprehensive review of chest tube management: a review. JAMA Surg. 2022;157(03):269–274. doi: 10.1001/jamasurg.2021.7050. [DOI] [PubMed] [Google Scholar]
- 2.Hogg J R, Caccavale M, Gillen B. Tube thoracostomy: a review for the interventional radiologist. Semin Intervent Radiol. 2011;28(01):39–47. doi: 10.1055/s-0031-1273939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood K, Wahidi M M. Straightening out chest tubes: what size, what type, and when. Clin Chest Med. 2013;34(01):63–71. doi: 10.1016/j.ccm.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Yu H. Management of pleural effusion, empyema, and lung abscess. Semin Intervent Radiol. 2011;28(01):75–86. doi: 10.1055/s-0031-1273942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrath E E, Blades Z, Anderson P B. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010;104(01):1–8. doi: 10.1016/j.rmed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Patel I J, Rahim S, Davidson J C. Society of Interventional Radiology Consensus Guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions - Part II: Recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(08):1168–11840. doi: 10.1016/j.jvir.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Fong C, Tan C WC, Tan D KY, See K C. Safety of thoracentesis and tube thoracostomy in patients with uncorrected coagulopathy: a systematic review and meta-analysis. Chest. 2021;160(05):1875–1889. doi: 10.1016/j.chest.2021.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Hecker E, Hamouri S, Müller E, Ewig S. Pleural empyema and lung abscess: current treatment options. Zentralbl Chir. 2012;137(03):248–256. doi: 10.1055/s-0031-1284047. [DOI] [PubMed] [Google Scholar]
- 9.Huggins J T, Maldonado F, Chopra A, Rahman N, Light R. Unexpandable lung from pleural disease. Respirology. 2018;23(02):160–167. doi: 10.1111/resp.13199. [DOI] [PubMed] [Google Scholar]
- 10.Krok K L, Cárdenas A. Hepatic hydrothorax. Semin Respir Crit Care Med. 2012;33(01):3–10. doi: 10.1055/s-0032-1301729. [DOI] [PubMed] [Google Scholar]
- 11.Oberg C L, Holden V K, Channick C L. Benign central airway obstruction. Semin Respir Crit Care Med. 2018;39(06):731–746. doi: 10.1055/s-0038-1676574. [DOI] [PubMed] [Google Scholar]
- 12.Staes W, Funaki B. “Ex vacuo” pneumothorax. Semin Intervent Radiol. 2009;26(01):82–85. doi: 10.1055/s-0029-1208386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brook I, Frazier E H. Aerobic and anaerobic microbiology of empyema. A retrospective review in two military hospitals. Chest. 1993;103(05):1502–1507. doi: 10.1378/chest.103.5.1502. [DOI] [PubMed] [Google Scholar]
- 14.Porcel J M. Chest tube drainage of the pleural space: a concise review for pulmonologists. Tuberc Respir Dis (Seoul) 2018;81(02):106–115. doi: 10.4046/trd.2017.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallifax R J, Psallidas I, Rahman N M. Chest drain size: the debate continues. Curr Pulmonol Rep. 2017;6(01):26–29. doi: 10.1007/s13665-017-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Light R W. Pleural controversy: optimal chest tube size for drainage. Respirology. 2011;16(02):244–248. doi: 10.1111/j.1440-1843.2010.01913.x. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths J R, Roberts N. Do junior doctors know where to insert chest drains safely? Postgrad Med J. 2005;81(957):456–458. doi: 10.1136/pgmj.2004.024752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott S, Levis D A, Arellano R S. Chest drainage. Semin Intervent Radiol. 2012;29(04):247–255. doi: 10.1055/s-0032-1330058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helm E J, Rahman N M, Talakoub O, Fox D L, Gleeson F V. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(03):634–639. doi: 10.1378/chest.12-1285. [DOI] [PubMed] [Google Scholar]
- 20.Misura T, Drakopoulos D, Mitrakovic M. Avoiding the intercostal arteries in percutaneous thoracic interventions. J Vasc Interv Radiol. 2022;33(04):416–41900. doi: 10.1016/j.jvir.2021.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez M C, El Khatib M, Prokop L, Zielinski M D, Aho J M. Complications in tube thoracostomy: systematic review and meta-analysis. J Trauma Acute Care Surg. 2018;85(02):410–416. doi: 10.1097/TA.0000000000001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aho J M, Ruparel R K, Rowse P G, Brahmbhatt R D, Jenkins D, Rivera M. Tube thoracostomy: a structured review of case reports and a standardized format for reporting complications. World J Surg. 2015;39(11):2691–2706. doi: 10.1007/s00268-015-3158-6. [DOI] [PubMed] [Google Scholar]
- 23.Vilkki V A, Gunn J M. Complications related to tube thoracostomy in Southwest Finland hospital district between 2004 and 2014. Scand J Surg. 2020;109(04):314–319. doi: 10.1177/1457496919857262. [DOI] [PubMed] [Google Scholar]
- 24.Rahman N M, Maskell N A, West A. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011;365(06):518–526. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]
- 25.Hendriksen B S, Kuroki M T, Armen S B, Reed M F, Taylor M D, Hollenbeak C S. Lytic therapy for retained traumatic hemothorax: a systematic review and meta-analysis. Chest. 2019;155(04):805–815. doi: 10.1016/j.chest.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 26.American Medical Association . USA: AMA; 2021. CPT 2022. [Google Scholar]
- 27.Beattie G, Cohan C M, Chomsky-Higgins K, Tang A, Senekjian L, Victorino G P. Is a chest radiograph after thoracostomy tube removal necessary? A cost-effective analysis. Injury. 2020;51(11):2493–2499. doi: 10.1016/j.injury.2020.07.055. [DOI] [PubMed] [Google Scholar]


