Abstract
Rhabdomyosarcoma (RMS) is the most common mesenchymal tumor in children and adolescents, with 10% of cases occurring in the orbits. RMS should be suspected whenever children present with rapidly progressing unilateral exophthalmos. Its symptoms depend on the lesion's origin and location. We report the clinical case of a 19-year-old male patient admitted to the hospital because of blurred vision and bulging eyes that gradually increased over several months. Magnetic resonance imaging showed a mass located mainly in the left orbit, pushing and deforming but not invading the eyeball. The lesion had grown into the left ethmoid sinus wall. The histopathological incisional biopsy results were with alveolar RMS.
Keywords: Orbital rhabdomyosarcoma, Soft-tissue sarcoma, Magnetic resonance imaging
Introduction
Rhabdomyosarcoma (RMS) is a mesenchymal cell malignancy representing 5% of all pediatric malignancies, usually seen in the first decade of life, especially between the ages of 5-7 [1]. Histopathological classifications of RMS include Embryonal, alveolar, pleomorphic and spindle cell subtypes. The Embryonal subtype is the most common, and the alveolar subtype is the least common, with PAX3/7-FOXO1 fusion gene amplification in most cases and has the worst prognosis. The presenting symptom is usually rapidly progressing unilateral exophthalmos. The rapid growth of lesions can mimic other infections or tumors in the orbit. Computed tomography (CT) and magnetic resonance imaging (MRI) are 2 necessary imaging modalities for determining the location, size, and extent of invasion and evaluating recurrence or residual lesions after treatment. Understanding the clinical, imaging, and histopathological features is essential for the early diagnosis and management of these lesions.
Case report
A 19-year-old male patient with no remarkable medical history was admitted to our hospital because of bulging eyes and increasingly blurred vision over the past few months. No family history of cancer, and no history of chemical or radiation exposure was found. The patient was examined at the eye hospital with the initial diagnosis of an orbital tumor, then referred for transfer to our hospital. At this time, the tumor was still small with 3 cm in diameter and had not affected the vison. However, due to the Coronavirus disease (COVID-19) pandemic, he did not attend. After 6 months, the lesion rapidly increased in size with bleeding; the patient came to our hospital for examination.
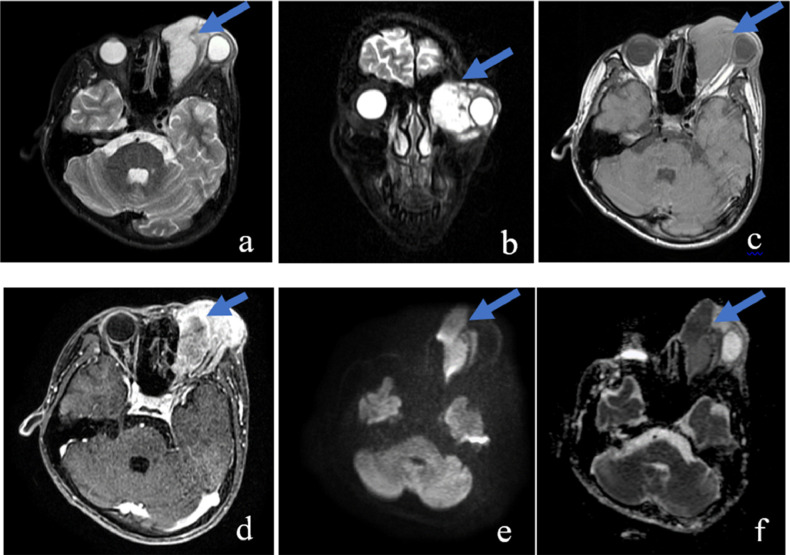
A large lesion measuring 60 × 44 × 50 mm located mainly in the extraconal of the left orbit was found on MRI. It showed isointensity with the extraocular muscle in the T1-weighted (T1W) image, hyperintensity in the short tau inversion recovery (STIR) image, heterogeneous enhancement postcontrast, and no internal cysts or calcification. The lesion's border with the medial rectus muscle and ethmoid sinus's inner wall was unclear. It compressed, causing ocular deformity but not invading the eyeball, intra-conal space, or intracranial. The lesion had a strongly restricted diffusion on diffusion-weighted imaging (DWI)/apparent diffusion coefficient (ADC) images with a minimum ADC of 0.7 × 10−3 mm2/s (Fig. 1). Systemic workup including complete blood count, urinalysis, serum electrolytes, bone marrow aspiration, lumbar puncture, bone scintigraphy, cervical and abdominal ultrasound, abdominal CT and chest CT was unremarkable.
Fig. 1.
On MRI, a large lesion (arrow) measuring 60 × 44 × 50 mm located in the left orbit was hyperintense in the STIR image (A, B), isointense with extraocular muscle in the T1W image (C), and heterogeneously enhanced postcontrast (D). It caused an ocular compression deformity but did not invade the eyeball, intra-conal space, or intracranial. The lesion had a strong restricted diffusion in DWI/ADC images with a minimum ADC of 0.7 × 10−3 mm2/s (E, F).
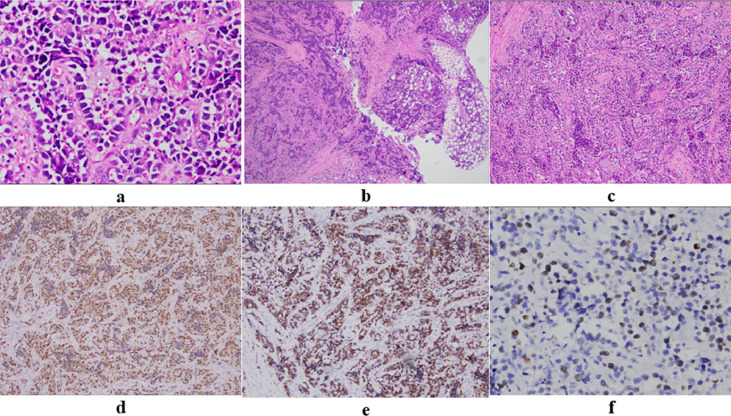
The patient then underwent an incisional biopsy. Microscopically, the tumor was highly cellular, characterized by primitive round cells with scant cytoplasm and large hyperchromatic, sometimes pleomorphic nuclei. Tumor cells were arranged in nests or alveolars separated by paucicellular fibrovascular septa. Some areas showed a solid pattern. Tumor cells showed immunoreactivity for desmin and myogenin. The nuclear immunostaining for myogenin was strong and diffuse, unlike the focal staining pattern of other RMS subtypes. Mitotic figures were easily identified, and the Ki-67 index was ∼40%. Histopathology and immunohistochemical staining were consistent with alveolar RMS and PAX3/7- FOXO1 fusion not amplified by Fluorescence in situ hybridization (FISH) analysis (Fig. 2). According to European Pediatric Soft Tissue Sarcoma Study Group (EPSSG) and tumor board meeting (medical oncologist, radiologist, radiation oncologists, pathologist, surgeon), the patient was diagnosed with Alveolar RMS high risk group G, T2bN0M0. According to EPSSG protocol, IVA regimen (ifosfamide, dactinomycin and Vincristin) was applied. The patient was treated with 3 courses of chemotherapy and showed a partial response of over 70% (Fig. 3); we decided to receive chemotherapy combined with concurrent chemoradiotherapy according to EPSSG guideline, then maintenance treatment for 6 months with vinorelbine - cyclophosphamide. The patient has finished treatment with total of 52 weeks, and the tumor has achieved complete response. After 1 year of follow-up, the patient was completely stable, no recurrence was seen.
Fig. 2.
(A–C) Hematoxylin and eosin staining showed the lesion's border was infiltrative (A; 400 ×) and characterized by primitive round cells with scant cytoplasm and large hyperchromatic nuclei (B; 40 ×). The tumor cells were arranged in alveolars separated by paucicellular fibrovascular septa, often with loss of cellular cohesion in the center (C; 100 ×). (C–E) Immunohistochemistry showed that the nuclear expression of myogenin and desmin was strong and diffuse (D, E; 100 ×). Ki-67 staining (F; 400 ×) showed a proliferative index of 40%.
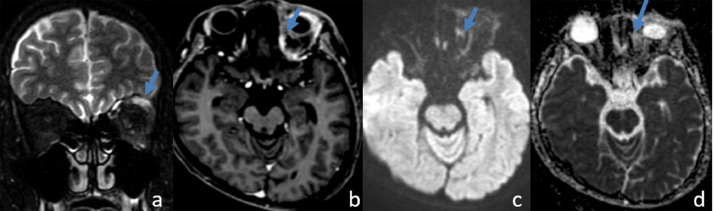
Fig. 3.
MRI after 3 courses of chemotherapy shows that the tumor (arrow) decreased the size significantly.
Discussion
Orbital RMS accounts for 25%-35% of head and neck RMS and 10%-20% of all RMS [2]. The etiology of RMS is still unknown; however, some reports have shown that risk factors such as X-rays exposure, parental drug use, vaginal bleeding during pregnancy, and childhood allergies are associated with an increased risk of developing the disease [3]. In addition, RMS is also associated with several familial syndromes such as Li-Fraumeni, Neurofibromatosis type I, Costello, Noonan syndrome, Beckwith-Wiedemann, and DICER1 syndrome [3]. Clinical manifestations of orbital RMS usually include unilateral proptosis, globe displacement, strabismus, eyelid swelling, red eye, ptosis, chemosis or limited ocular movement, which can sometimes cause facial paralysis. The acute onset and rapidly progressive nature of the disease may mimic infectious or inflammatory etiologies. When a child has a rapid progressing unilateral exophthalmos, RMS should be taken into consideration [4]. These tumors are often diagnosed before distant metastases, rarely involving local lymph nodes, possibly because the orbit has a small lymphatic system [5,6]. However, lung and bone metastases were reported in some untreated cases. Locally, orbital RMS can extend intracranially and infiltrate the orbital bones [2]. Compared to localized disease, metastatic orbital RMS has a less favorable prognosis. Nevertheless, a joint European-North American pooled study found the orbital site to be favorable [5,7].
Imaging plays an important role in lesion diagnosis. CT is dominant in the evaluation of associated bone lesions. However, MRI is preferred because it is better at showing the orbital soft tissues in detail, providing exact location, vascular flow characteristics, enhancement patterns, intracranial invasion without any ionizing radiation risk. Moreover, MRI with DWI sequence is an ideal modality to detect residual lesions and follow-up post chemoradiotherapy.
Orbital RMS is commonly classified as extraconal lesions because they tend to arise from the extraocular musculature or eyelid, although they can involve both intra and extraconal in some cases [8]. In early stages, RMS presents as a well-defined, homogeneous soft mass lesion homogenous with the muscle and without bone destruction. Advanced lesions often are demarcated, invade surrounding structures, have internal calcifications (although not a common feature), and destroy adjacent necrosis. After injection, it enhances moderately to markedly with rare ring enhancement.
On MRI, the lesion is isointense with the extraocular muscle in T1W images. In T2-weighted images, the lesion is hyperintense with the extraocular muscle and intraorbital fat but may also be isointense or hypointense due to its high cell density. Focal hyperintensity may be seen on T1- and T2-weighted images due to bleeding. Comparison of pre- and postinjection images helps detect intracranial invasion and adjacent paranasal sinuses. In some cases, especially in children aged <2 years, the lesion is strongly enhanced because of its rich vascularity, which can easily lead to misdiagnosis as capillary hemangioma. DWI is a valuable sequence in the differential diagnosis of RMS from other lesions. Some studies have suggested that the ADC is useful in the differential diagnosis between benign and malignant orbital lesions with a cut-off value of 1.14 ± 0.33 × 10−3 mm2/s [9]. In our case, with a low ADC value (0.7 × 10−3 mm2/s), the lesion tends to be malignant.
Approaching the eye lesions, the combination of age, medical history, clinical symptoms, location, and especially using compartment – based approach is necessary for narrowing the diagnostic considerations and determining the most appropriate management strategy [8]. All in this case, the lesion is located in extraconal space; some diagnoses may be listed besides RMS such as orbital cellulitis, idiopathic orbital inflammation, capillary hemangioma, lymphangioma, Langerhans cell histiocytosis (LCH), and lymphoma. Orbital cellulitis which is a common cause of proptosis in children is often misdiagnosed as orbital RMS because of mimic clinical symptoms [10]. However, the absence of systemic symptoms like fever and lethargic behavior is more indicative of orbital RMS. On MRI, orbital cellulitis often shows thickening and infiltrated fat, contrast enhancement without restricted diffusion [10]. Orbital cellulitis may be complicated by an abscess that presents with ring-shaped enhancement and restricted diffusion in the center. Idiopathic orbital inflammation (IOI) often called pseudotumor, which is the third most common orbital disease, typically cause an increase in oculomotor muscle size [11]. On MRI, IOI shows an infiltrative mass, less often a focal mass, with contrast enhancement and lacks restricted diffusion with mean ADC value of 1.15 (± 0.37) × 10−3 mm2/s [11,12]. In contrast, RMS with strongly restricted diffusion – like this case- is usually close to extraocular muscles without enlargement of the muscle belly. IOP shows an outstanding response to corticosteroids. In some case with atypical presentation, trial corticosteroid therapy helps differentially diagnose idiopathic inflammatory orbital pseudotumors and tumors [13].
Orbital capillary hemangioma is common in neonates during the first month of life and progresses slowly, so that can appear in young adult. The DWI sequence can help differentiate these 2 lesion types; RMS is predominantly restricted on DWI/ADC images, while capillary hemangioma is not [14]. In our case, excluding hemangioma from the diagnosis is pretty simple because of the age and strongly diffuse restricted lesion on DWI. Orbital lymphangioma often has many small cystic structures with visible fluid inside, which is rare in RMS [15]. Orbital LCH is rare, accounting for <1% of orbital space-occupying lesions in children and adults, with a peak incidence from 1 to 3 years of age. Most orbital LCH lesions involve the frontal bone, typically anteriorly in the superior or superolateral wall. They can be confused with an invasive orbital RMS since they destroy bone and invade the orbit via a direct route [16].
One of another differential diagnose in this patient is lymphoma. Orbital lymphoma may be primary or secondary to systemic lymphoma. While these tumors tend to affect adults aged 50-70 or older, they can also affect younger individuals. The most common orbital lymphoma site is the lacrimal gland. Lymphoma's characteristics on T1W images are similar to those of RMS, with the degree of enhancement varying from moderate to strong and restricted diffusion on DWI. However, lymphoma commonly presents as a homogeneously enhancing mass and typically envelops anatomic orbital structures without invasion or depressing the eyeball [17].
Histopathology is vital for definitive diagnosis when orbital RMS is suspected on imaging. Incisional or excisional biopsy is performed in most cases to ensure a diagnosis. RMS is histopathological classified into 4 subtypes (Embryonal, alveolar, pleomorphic and spindle cell) with different epidemiological characteristics. Embryonal RMS accounts for 50%-70% of orbit RMS cases and can be found in other locations, such as the genital or retroperitoneal regions. Embryonal RMS has 2 peak ages: a higher peak at 0-5 years and a lower peak at adolescence [5,18]. Alveolar RMS accounts for 20%-30% of orbit RMS, occurs at any age but is more common in adolescents and young adults [5]. PAX3/7-FOXO1 fusion gene is a signature genetic change of Alveolar RMS, accounting for 80% [19]. Pleomorphic and spindle cell RMS extremely rare appear in the orbit. In addition, RMS pathology has been demonstrated to be an independent prognostic factor. Overall, Embryonal RMS survival was better than in patients with pleomorphic and alveolar RMS, with 5-year mortality rates of 26.7% compared with 45,4% and 53.3%, respectively [20].
RMS treatment aims to prevent local tumor progression and distant metastasis. In the past, RMS's overall survival rate (OS) was 25%-30%, with the primary treatment being surgical tumor resection. Nowadays, a multidisciplinary approach including surgery, chemotherapy, and radiation therapy is preferred in the treatment of orbital RMS that has improved the OS rate to approximately 90% [1]. Chemotherapy and radiotherapy protocol is usually planned based on the internationally accepted staging system of the “Intergroup Rhabdomyosarcoma Study Group” Or European Pediatric Soft Tissue Sarcoma Study Group. Chemotherapy is considered as a backbone in the treatment of RMS with 2 main chemotherapy regimens used include the VAC regimen (vincristine, actinomycin D, and cyclophosphamide) and (2) the IVA (ifosfamide, vincristine, and actinomycin D). Some poor prognostic factors for orbital RMS include the age of onset (younger than 1 year or ≥ 10 years), alveolar type, tumor size (>5cm), periorbital structures invasion, and distant organ metastasis, PAX3/7-FOXO1 fusion positive [1,19]. Regarding this patient, although orbit was assigned as a favorable site, he had a poor prognosis because the age of onset was more than ten years old, tumor size > 5 cm and alveolar type. Moreover, the late arrival of patients to the hospital due to COVID-19 also delays treatment, causing rapid tumor growth with bleeding complications. If the patient was treated immediately with an initial tumor size of 3 cm (good prognosis factor) without affecting vision, total tumor resection could be initiated at the beginning, prolonging the patient's survival time and helping to preserve vision. Fortunately, the patient had PAX3/7-FOXO1 fusion negative, so the tumor responded well to chemotherapy, and the patient is currently stable after 1 year of treatment.
Conclusions
Orbital RMS is a common primary malignancy in children, often presenting with rapidly progressing protrusion. Differential diagnosis from other intraorbital lesions remains challenging. A multidimensional approach involving clinical features, imaging, and pathology is necessary for early diagnosis and good management of orbital RMS.
Author's contributions
Ta HN, Vu LM, and Nguyen MD: Case file retrieval and case summary preparation. Ta HN, Vu LM, and Nguyen MD: preparation of manuscript and editing. All authors read and approved the final manuscript.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Our institution does not require ethical approval for reporting individual cases or case series.
Patient consent
Informed consent for patient information to be published in this article was obtained.
Footnotes
Competing Interests: The authors do not report any conflicts of interest.
References
- 1.Korkmaz I, Yaman B, Ceylan N, Kantar M, Kamer S, Palamar M. Orbital rhabdomyosarcoma: Review. Eur Eye Res. 2023;3(1):26–31. doi: 10.14744/eer.2022.02996. [DOI] [Google Scholar]
- 2.Shields JA, Shields CL. Rhabdomyosarcoma: review for the ophthalmologist. Surv Ophthalmol. 2003;48(1):39–57. doi: 10.1016/s0039-6257(02)00415-0. [DOI] [PubMed] [Google Scholar]
- 3.Skapek SX, Ferrari A, Gupta A, Lupo PJ, Butler E, Shipley J, et al. Rhabdomyosarcoma. Nat Rev Dis Primers. 2019;5(1):1. doi: 10.1038/s41572-018-0051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karcioglu ZA, Hadjistilianou D, Rozans M, DeFrancesco S. Orbital rhabdomyosarcoma. Cancer Control. 2004;11(5):328–333. doi: 10.1177/107327480401100507. [DOI] [PubMed] [Google Scholar]
- 5.Jurdy L, Merks JHM, Pieters BR, Mourits MP, Kloos RJHM, Strackee SD, et al. Orbital rhabdomyosarcomas: a review. Saudi J Ophthalmol. 2013;27(3):167–175. doi: 10.1016/j.sjopt.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freling NJM, Merks JHM, Saeed P, Balm AJM, Bras J, Pieters BR, et al. Imaging findings in craniofacial childhood rhabdomyosarcoma. Pediatr Radiol. 2010;40(11):1723–1738. doi: 10.1007/s00247-010-1787-3. quiz 1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao AA, Naheedy JH, Chen JYY, Robbins SL, Ramkumar HL. A clinical update and radiologic review of pediatric orbital and ocular tumors. J Oncol. 2013 doi: 10.1155/2013/975908. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph AK, Guerin JB, Eckel LJ, Dalvin LA, Keating GF, Liebo GB, et al. Imaging findings of pediatric orbital masses and tumor mimics. RadioGraphics. 2022;42(3):880–897. doi: 10.1148/rg.210116. [DOI] [PubMed] [Google Scholar]
- 9.Jaju A, Rychlik K, Ryan ME. MRI of pediatric orbital masses: role of quantitative diffusion-weighted imaging in differentiating benign from malignant lesions. Clin Neuroradiol. 2020;30(3):615–624. doi: 10.1007/s00062-019-00790-4. [DOI] [PubMed] [Google Scholar]
- 10.Amir SP, Kamaruddin MI, Akib MNR, Sirajuddin J. Orbital cellulitis clinically mimicking rhabdomyosarcoma. IMCRJ. 2019;12:285–289. doi: 10.2147/IMCRJ.S201678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira TA, Saraiva P, Genders SW, Buchem MV, Luyten GPM, Beenakker JW. CT and MR imaging of orbital inflammation. Neuroradiology. 2018;60(12):1253–1266. doi: 10.1007/s00234-018-2103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelgawad MS, Mohamed WMA, Aly RA. Value of diffusion-weighted magnetic resonance imaging (DWI) in differentiating orbital lymphoma from idiopathic orbital inflammatory pseudotumor. Egypt J Radiol Nucl Med. 2022;53(1):235. doi: 10.1186/s43055-022-00918-6. [DOI] [Google Scholar]
- 13.Ding ZX, Lip G, Chong V. Idiopathic orbital pseudotumour. Clin Radiol. 2011;66(9):886–892. doi: 10.1016/j.crad.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh EC, Heran MKS, Peleg A, Rootman J. Imaging of the natural history of an orbital capillary hemangioma. Orbit. 2006;25(1):69–72. doi: 10.1080/01676830500544326. [DOI] [PubMed] [Google Scholar]
- 15.Dhellemmes P, Brevière GM, Degrugillier-Chopinet C, Vinchon M. [Vascular lesions of the orbit in children] Neurochirurgie. 2010;56(2–3):271–280. doi: 10.1016/j.neuchi.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 16.Maccheron LJ, McNab AA, Elder J, Selva D, Martin FJ, Clement CI, et al. Ocular adnexal Langerhans cell histiocytosis clinical features and management. Orbit. 2006;25(3):169–177. doi: 10.1080/01676830600669486. [DOI] [PubMed] [Google Scholar]
- 17.Vogele D, Sollmann N, Beck A, Haggenmüller B, Schmidt SA, Schmitz B, et al. Orbital tumors—clinical, radiologic and histopathologic correlation. Diagnostics. 2022;12(10):2376. doi: 10.3390/diagnostics12102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egas-Bejar D, Huh WW. Rhabdomyosarcoma in adolescent and young adult patients: current perspectives. Adolesc Health Med Ther. 2014;5:115–125. doi: 10.2147/AHMT.S44582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heske CM, Chi YY, Venkatramani R, Li M, Arnold MA, Dasgupta R, et al. Survival outcomes of patients with localized FOXO1 fusion positive rhabdomyosarcoma treated on recent clinical trials: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Cancer. 2021;127(6):946–956. doi: 10.1155/2020/2635486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han D, Li C, Li X, Huang Q, Xu F, Zheng S, et al. Prognostic factors in patients with rhabdomyosarcoma using competing-risks analysis: a study of cases in the SEER database. J Oncol. 2020;2020 doi: 10.1002/cncr.33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.