Abstract
Human endogenous retroviruses (HERVs) have evolved from exogenous retroviruses and account for approximately 8% of the human genome. A growing number of findings suggest that the abnormal expression of HERV genes is associated with schizophrenia, multiple sclerosis, endometriosis, breast cancer, bladder cancer and other diseases. HERV-W env (syncytin-1) is a membrane glycoprotein which plays an important role in placental development. It includes embryo implantation, fusion of syncytiotrophoblasts and of fertilized eggs, and immune response. The abnormal expression of syncytin-1 is related to placental development-related diseases such as preeclampsia, infertility, and intrauterine growth restriction, as well as tumors such as neuroblastoma, endometrial cancer, and endometriosis. This review mainly focused on the molecular interactions of syncytin-1 in placental development-related diseases and tumors, to explore whether syncytin-1 can be an emerging biological marker and potential therapeutic target.
Keywords: Syncytin-1, Biomarker, Molecular mechanism, Signal pathway, Placenta
Introduction
Human endogenous retroviruses were acquired through multiple infections and integration of extinct exogenous retroviruses during primate evolution. This ancestral infection specifically affects the germline, and the resulting endogenous retroviral proviruses can transmit vertically. HERVs become a stable part of the human genome by continuous Mendelian inheritance, accounting for approximately 8% of human DNA [1, 2]. According to the types of tRNA recognized by the primer-binding site [3], HERV can be divided into at least 31 families, including HERV-W, HERV-T [4], HERV-K [5], HERV-F [6], HERV-E [7] and so on. Although HERV family members have not been identified as replication-competent, they share substantial structure similarity with exogenous retroviruses, for example, HERV-K retains three of the four intact ORFs with the potential to encode proteins or peptides [8]. Most genes in HERV families are silenced, but some genes remain their function. Studies have found abnormal expression of HERV is associated with various diseases such as prostate cancer [9], breast cancer [10], AIDS [11], colorectal cancer [12], multiple sclerosis [13] and neurodegenerative disorders [14].
The classical HERV provirus structure consists of two identical long terminal repeats (LTRs) and four typical viral genes, gag, pro, pol and env (Fig. 1A) [15]. LTRs have the promoter and transcription termination signals to regulate the expression of the virus gene and adjacent gene [16–21]. The gag gene encodes a viral core structural protein [22]. It can participate in viral RNA encapsidation and particle formation [23]. The abnormal expression of gag may be associated with pituitary adenomas [24] and lichen planus [25]. The pol gene encodes viral enzymes such as reverse transcriptase and integrase [26–28], which abnormal expression may be related to mixed connective tissue disease and systemic sclerosis [29]. The env gene encodes viral envelope glycoprotein, which is important for receptor recognition and membrane fusion [28]. Its abnormal expression is greatly associated with lymphoma [30], melanoma [31], non-small cell lung cancer [32], schizophrenia [33] and leukemia [34].
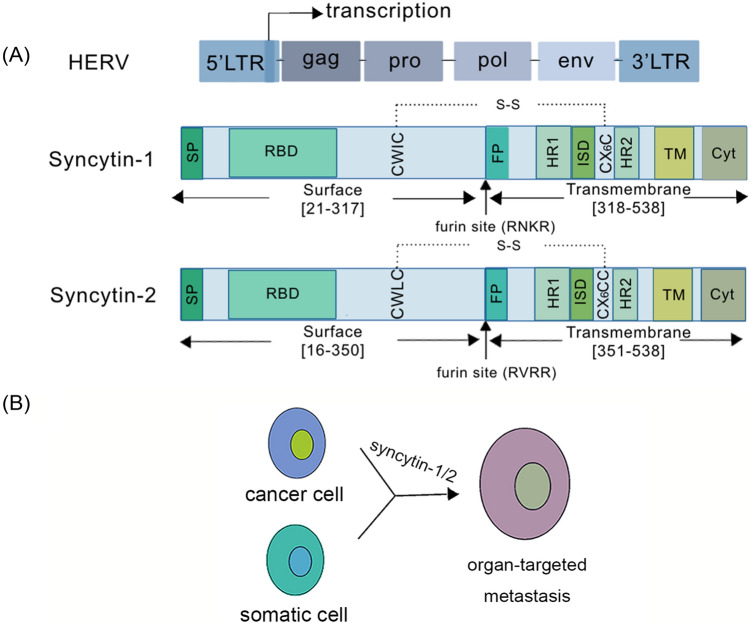
Fig. 1.
Structure and function of syncytin-1. A The general structure of HERV consists mainly of two identical LTR sequences and four typical viral genes, gag, pro, pol and env. And there is a transcriptional promoter at the end of the 5'LTR. The structure of syncytin-1 and syncytin-2 mainly includes SU domains and TM domains. B Syncytin-1 could promote the fusion of somatic cells and cancer cells
Env is a membrane glycoprotein and forms the spike glycoprotein on the surface of retroviral particles [35]. Syncytin-1 is an env protein of the HERV-W family, which receptor is solute carrier family 1 Member 4 and 5 (SLC1A4 and SLC1A5) [36], and syncytin-2 is an env protein of the HERV-FRD family, which receptor is major facilitator superfamily domain containing 2A (MFSD2A) [37]. They are homologous membrane glycoproteins with a similar structure, involved in forming the placental syncytiotrophoblast (Fig. 1A) [38]. Syncytin-1/2 could promote cell fusion effect between tumor cells and normal cells, and might be associated with tumor metastasis (Fig. 1B). Therefore, both syncytin-1 and syncytin-2 can play important roles in pathological and physiological processes.
According to published articles, no systematic analysis of the molecular mechanisms of syncytin-1 in placental development-related diseases and tumors has been reported. Therefore, we will focus on the networks and signal pathways of syncytin-1 during the placental development related diseases and tumors.
The structure and function of syncytin-1
Syncytin-1 is a highly fusogenic membrane glycoprotein of the HERV-W, specifically expressed in the placental trophectoderm [39, 40]. It is mainly localized at locus 7q21.2 [41], and also located in the acrosomal region and equatorial segment of the sperm [42]. The endoprotease furin can cleave the syncytin-1 polypeptide into surface (SU) domains and transmembrane (TM) domains at the RNKR site, which are linked by the intersubunit disulfide bond (S–S) [38]. A signal peptide (SP) and a receptor-binding domain (RBD) are in the amino-terminal protein of the SU domain. And the SU domain is responsible for receptor recognition and binding. The TM domain contains an immunosuppressive domain (ISD), which has immunosuppressive properties. This property could be involved in materno-fetal tolerance toward fetal tissue with the father's gene to survive in the mother's body [43]. The carboxyl-terminal R peptide of the TM domain, which cleaves during virion maturation, can activate the fusion function of the TM domain (Fig. 1A) [15, 29, 40, 44, 45]. Moreover, syncytin-1 can interact with SLC1A4 and SLC1A5, and mediate the formation of syncytium [46], which is the channel of fetal-maternal exchanges [47]. And syncytin-1 can induce infected cells to resist reinfection when interacting with the SLC1A5 receptor on cells. This phenomenon is known as superinfection interference [48]. In summary, the structure of syncytin-1 determines its fusion and immune properties.
The regulation mechanisms of syncytin-1 in physiological processes
Studies found that syncytin-1 and its receptor SLC1A5 are localized at the acrosomal region and equatorial segment of the sperm, and SLC1A5 is also expressed in human oocytes [42]. Therefore, the interaction of syncytin-1 and its receptor could promote membrane fusion of sperm and egg cells, facilitating the formation of fertilized eggs. Syncytin-1 is also expressed in the trophectoderm underlying the inner cell mass in human trophoblast cells [49], indicating that syncytin-1 could play a vital role in embryo implantation. In addition, syncytin-1 shows dynamic expression during embryonic (fetal) development. The syncytin-1 mRNA is significantly increased since the late first trimester (9–12 weeks of gestation) compared to the early first trimester (5–7 weeks of gestation), but then significantly decreased in the late third trimester (37–40 weeks of gestation) [50]. As we all know, the main functions of the placenta are transport, metabolism, protection and endocrine [51]. These changes during different periods of gestation reflect the relationship between fetal development and placental demand. In a word, it is the structure and function of syncytin-1 that determine its importance in placental development.
The role of syncytin-1 in placental development could be affected by various factors. The expression of syncytin-1 is influenced by methylation of CpG islands in the 5′LTR [52], and DNMTs is involved in DNA methylation [53]. Transient DNA hypomethylation in the first trimester of pregnancy can promote syncytin-1-mediated cell fusion and differentiation, while DNA hypermethylation at term reduces syncytin-1-mediated cell fusion [54]. In addition, changes in posttranslational modification of histones are also regulating the expression of syncytin-1. Interestingly, histone modification and DNA methylation often coexist and affect each other [55]. Acetylate glia cells missing a (GCM1) is essential for placental development [56, 57], and it can promote syncytin-1-mediated trophoblast fusion [58]. Its transcript and phosphorylation levels of the GCM1 gene were activated by the cyclic adenosine monophosphate(cAMP)-protein kinase A(PKA) signal pathway and CREB-binding protein (CBP) plays an auxiliary role in this process. In addition, the increased acetylation, decreased ubiquitination, and improved stability of GCM1 protein were regulated by CBP. The changes in GCM1 expression and structure could upregulate syncytin-1-mediated trophoblast fusion [59]. Moreover, human chorionic gonadotrophin (hCG) and corticotropin-releasing hormone (CRH) all can activate cAMP-PKA signal pathway, regulating the expression of syncytin-1 [60–62]. Syncytin-1 is involved in the proliferation of cytotrophoblasts through cell cycle [28], and also mediates the fusion of the villous cytotrophoblast into multinucleated syncytiotrophoblasts [36, 63, 64], the insufficient expression levels of syncytin-1 may lead to defects in the structure and function of the syncytium. In addition, studies have shown that syncytin-1 could also have non-fusogenic activities by participating in the proliferation, differentiation and apoptosis of the trophoblast [65]. In summary, the expression of syncytin-1 is regulated by methylation, cAMP-PKA-GCM1 signal pathway. Syncytin-1 maintains the homeostasis balance in syncytium through its non-fusion and fusion functions, and plays a vital role in the human placenta.
Studies have shown that syncytin-1 can be present in trophoblast/placenta-derived microvesicles and shed from the placenta into the maternal circulation, involved in microvesicles-mediated activation of immune cells and immune cell responses to lipopolysaccharide stimulation [66]. The syncytin-1 protein not only affects early innate immune response but also influences adaptive response, therefore, increasing the susceptibility to infection [67]. The leukocytic syncytin-1 expressed in all four leukocyte types, blasts, granulocytes, lymphocytes and monocytes [68], and syncytin-1 can also promote the activation of monocytes [69]. Syncytin-1 in astrocytes and microglia can trigger the activation of c-reactive protein through the toll-like receptor 3 (TLR3)-Interleukin-6 (IL-6) signal pathway, inducing inflammatory responses [33]. In conclusion, syncytin-1, in addition to its role in the placenta, could also be involved in the occurrence of infections and immune responses.
Syncytin-1 and placental development-associated diseases
Syncytin-1 and preeclampsia
Expression of syncytin-1 in preeclampsia
Preeclampsia (PE) can lead to systemic vascular damage in liver, kidney and brain failure as the main manifestation, and is mainly characterized by hypertension and proteinuria during pregnancy [70]. Both poor trophoblast [71] and vascular dysfunction [72] are important pathological features of PE. Studies suggest that suboptimal maternal cardiovascular performance can lead to placental malperfusion, causing the occurrence of PE [73]. And PE is the main cause of maternal and fetal morbidity and mortality worldwide, accounting for approximately 4.6% of all births [74] and causing about 42,000 maternal deaths annually [75]. At present, the main established treatment for PE is to terminate the pregnancy and preterm labor, so the identification of reliable biomarkers is of great significance for the early detection and prevention of PE.
The syncytium is a continuous layer of syncytiotrophoblasts whose formation is mediated by syncytin-1 and has an important role in maternal–fetal circulation. Zhuang et al. performed RT-PCR analysis on placental tissues from 31 to 41 weeks, including 12 normal placental tissues and 8 PE placental tissues, and found syncytin-1 hypermethylation and decreased levels of gene expression in PE human placenta compared to normal human placenta [76]. Lee et al. performed in-situ hybridization and immunohistochemical analysis of 11 cases of 9–21 weeks early gestation human placental tissues and a total of 17 normal placental tissues and 21 PE placental tissues after delivery. They found that the expression of the syncytin-1 protein is down-regulated and the protein localization is abnormal in PE. Syncytin-1 protein is located in the plasma membrane of syncytiotrophoblast basal cells in normal placental tissue, while that in PE is localized in the apical syncytiotrophoblast microchorion [77]. Vargas et al. set up two experimental groups (moderated PE, n = 9 and severe PE, n = 7) and a control group (normal placenta, n = 8). They found a fusion dysfunction of primary trophoblast cells in PE patients through cell fusion assay. And the mRNA and protein levels of syncytin-1 all decreased in PE compared to normal placenta tissues by RT-PCR and Western blot (WB) [78]. In human primary cytotrophoblast cells and BeWo trophoblast cell lines, Ruebner et al. showed that the expression of syncytin-1 in PE is high [79]. Conversely, Holder et al. found the transcript level of syncytin-1 is higher in PE placentas than that in normal term placentas (Table 1) [80]. The different expression of the syncytin-1 gene in these studies is mainly due to the following reasons. Firstly, an anti-syncytin-1 SU polyclonal antibody (H-280, rabbit, Santa Cruz) was used in the Holder et al. study [80], but two polyclonal anti-syncytin-1 antibodies (GeneTex, Orbigen) were used in Lee et al. study [77] and Vargas et al. study respectively [78]. Secondly, it may be due to the different periods in which the placenta samples were taken. First and second trimester placentas and term placentas were used in experiments with high expression of syncytin-1. Zhuang et al. proved that the expression of syncytin-1 is low with placentas in gestational age ranging from 31 to 41 weeks. In addition, racial differences should also be discussed. In conclusion, whether syncytin-1 can be used as a potential marker of PE needs further study.
Table 1.
Relationship between expression of syncytin-1 and preeclampsia
| Type of cancer | Specimen | Stage(w)/Number of cases | Area | Methods | Upstream factors | Expression | Ref. |
|---|---|---|---|---|---|---|---|
| Preeclampsia | Tissue |
9-21w/11 late/(N:17,P:21) |
USA Belgium |
in-situ hybridization IHC |
unk | Low | [77] |
| Cell | unk/unk | Germany |
RT-PCR sqPCR ELISA WB |
PPARγ/RXRα | Low | [79] | |
| Tissue |
N/(n = 8) MPE/(n = 9) SPE/(n = 7) |
Canada |
cell fusion assay RT-PCR WB |
unk | Low | [78] | |
| Tissue | 31-41w/(N:12,P:8) | USA | RT-PCR |
DNMT1 DNMT3B3 Hypermethylation |
Low | [76] | |
| Tissue |
First trimester/unk second trimester/unk term/unk |
UK |
qRT-PCR WB |
unk | Slightly high | [80] |
unk unknown; P preeclampsia; N normal; CT cytotrophoblast; WB Western Blot; MPE moderated preeclampsia; SPE severe preeclampsia
The role and mechanism of syncytin-1 in preeclampsia
Syncytin-1 can play a role in PE through multiple pathways (Fig. 2). Research shows that the placental syncytin-1 protein in PE is located in the apical syncytiotrophoblast microchorion, while it is normally located in the plasma membrane of the syncytiotrophoblast [77]. These results suggest that abnormal protein localization is associated with the occurrence of PE.
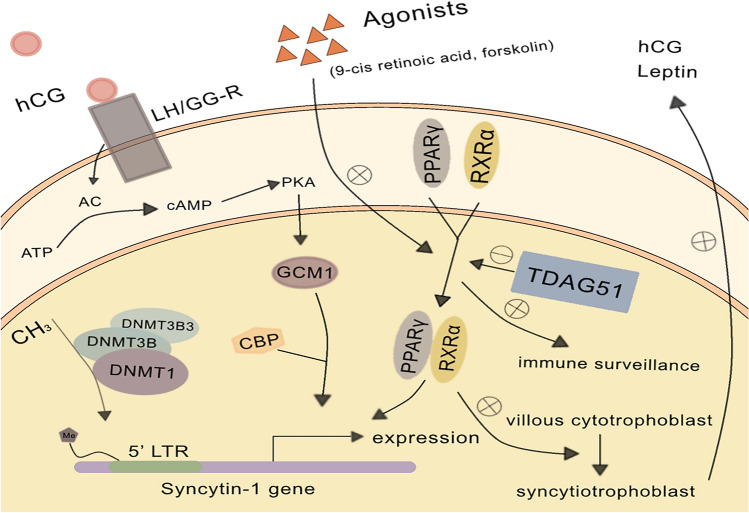
Fig. 2.
Molecular mechanisms and signal pathways of syncytin-1 expression in preeclampsia (PE) Induction of DNA methylation by the DNA methyltransferases DNMT1 and DNMT3B can reduce syncytin-1 expression. Human chorionic gonadotrophin (hCG) could regulate syncytin-1 expression by activating cAMP-PKA-GCM1 signal pathway. PPARγ and RXRα can form heterodimers, and their activation allows muscle-invasive bladder cancer to evade immune surveillance. PPARγ/RXRα nuclear hormone receptor activators such as 9-cis retinoic acid and forskolin can also reduce the expression of syncytin-1 and promote the fusion of villous cytotrophoblast into syncytiotrophoblast. T-cell death-associated gene 51 (TDAG51) is a potential inhibitor of PPARγ/RXRα
Syncytin-1 is regulated by nuclear hormone receptor peroxisome proliferator-activated receptor γ (PPARγ)/retinoid X receptor α (RXRα). The nuclear receptor PPARγ and RXR can form heterodimers and localize in the promoter region to play multiple roles. T-cell death-associated gene 51 (TDAG51) is a negative regulator of PPARγ, competitively binds PPARγ with RXRα, blocks the formation of heterodimers, and thereby inhibits adipogenesis [81]. PPARγ/RXRα genome activation in muscle-invasive bladder cancer can evade immune surveillance [82]. Ruebner et al. found that PPARγ/RXRα activators such as troglitazone, forskolin, 9-cis retinoic acid could increase the expression of syncytin-1 in cytotrophoblast cells, while the inhibitor SB203580 decreased the expression of syncytin-1. The expression of syncytin-1 was significantly decreased in BeWo cell lines treated with activator troglitazone and inhibitor SB203580, which was significantly increased by treatment with inhibitors 9-cis retinoic acid and forskolin. PPARγ/RXRα signal gene expression analysis was performed on the placental tissues, and it was found that the mRNA level and protein expression of syncytin-1 in PE were lower than those in normal placental tissue. Therefore, PPARγ/RXRα signaling directly upregulates syncytin-1 expression and cell fusion in human primary cytotrophoblast and BeWo trophoblasts, promoting the formation of syncytiotrophoblast and the development of the placenta [79]. hCG, leptin, resistin and xenobiotic transporter ABCG2 are all specific PPARγ target genes for placental development. hCG could associate with the fusion of cytotrophoblast cells [83]. Leptin can be produced in syncytiotrophoblast and endothelial cells of the placenta [84, 85]. Maternal placental leptin protein and mRNA levels are significantly increased in PE [86]. ABCG2 is down-regulated in pregnancies with PE further complicated by HELLP (hemolysis, elevated liver enzymes and low platelets) syndrome [87]. Consequently, syncytin-1 could promote the fusion of villous cytotrophoblast into syncytiotrophoblast, and the synthesis of hCG and leptin through regulation by PPARγ/RXRα.
DNA methyltransferase 3 Beta 3 (DNMT3B3) is an inactive isoform, it can stimulate the activities of DNMT3B in vitro [88]. Studies have found that the methylation level of PE is higher than that in normal placental tissues. With the high expression of DNMT3B and DNA methyltransferase 1 (DNMT1), the 5′LTR region of syncytin-1 is hypermethylated, and the expression of syncytin-1 is decreased [76]. Studies have shown a correlation between low expression of syncytin-1 and high apoptosis of cells [89]. This could also be a cause of abnormal placental development in patients with PE.
Based on most experimental results, the expression of syncytin-1 is down-regulated in PE. PPARγ/RXRα, DNMT1 and DNMT3B3 are all factors to affect the expression of syncytin-1. But the controversial results still need to be studied.
Syncytin-1 and other diseases related to placental development
The abnormal expression of syncytin-1 is also associated with other placental developmental related diseases in addition to PE. Studies have found that the expression of syncytin-1 and its receptor SLC1A5 have significantly decreased in samples with asthenozoospermic, oligozoospermic and oligoasthenozoospermic than normozoospermic samples [90], suggesting that decreased expression of syncytin-1 and SLC1A5 could be a cause of infertility. So, it might be one way to treat infertility in the future by increasing their expression. The mRNA and protein levels of syncytin-1 are lower in intrauterine growth restriction (IUGR) placentas than in normal placentas, inducing cell fusion abnormally and apoptosis increasingly [39, 79, 89, 91]. Syncytin-1 glycoprotein is significantly enhanced at the apical of the syncytiotrophoblast of hydatidiform moles compared with the normal placenta [92], indicating that the abnormal expression and localization of syncytin-1 lead to abnormal placental development. Therefore, syncytin-1 can potentially be a molecular marker for these diseases, but further investigation of the specific molecular mechanisms is needed.
Syncytin-1 and tumors
Syncytin-1 and neuroblastoma
Expression of syncytin-1 in neuroblastoma
Neuroblastoma (NB) is the most common extracranial solid tumor in infants and children, accounting for more than 15% of cancer-related deaths in children [93]. And it is caused by developing sympathetic NB cells with a high degree of malignancy and unique biological heterogeneity [94]. Radiation therapy is now the standard of care for high-risk NB, and this treatment is important for local lesion clearance and prevention of local recurrence [95], but the detection of other metastatic lesions in vivo and the degree of prognosis remain unclear. Treatment failure and poor prognosis are often marked by resistance to chemotherapy or immunotherapy in patients with advanced metastatic NB [96]. Therefore, the identification of reliable biomarkers is of great significance for understanding and treating NB.
All these studies have shown that syncytin-1 is highly expressed in NB cell lines (Table 2). Chen et al. and Li et al. all found that the expression of syncytin-1 in NB cell lines SH-SY5Y and IMR-32 was significantly higher than that in normal cells [97, 98]. Hu et al. came to the same conclusion in NB cell lines SH-SY5Y, IMR-32, SK-N-DZ [99], Wieland et al. in SH-SY5Y, IMR-32, SiMa [96], and Liu et al. in SH-SY5Y [100]. Although syncytin-1 is overexpressed in NB cell lines, further studies in NB tumor tissues are needed in the future.
Table 2.
Relationship between high expression of syncytin-1 and neuroblastoma
| Type of cancer | Specimen | Cell lines | Methods | Upstream factors |
Downstream factors | Signal pathway | Oncogenic effect | Ref. |
|---|---|---|---|---|---|---|---|---|
| Neuroblastoma | Cell | SH-SY5Y |
Semi-qPCR qRT-PCR WB |
Caffeine aspirin |
unk | unk | Proliferation | [100] |
| Cell |
SH-SY5Y SK-N-AS SK-N-DZ |
qRT-PCR |
Oxygen tension DNA methylation |
unk | unk | Proliferation | [99] | |
| Cell |
SH-SY5Y IMR-32 SiMa |
qRT-PCR |
Culture medium micro-environmnt |
unk | unk |
Proliferation invasion |
[96] | |
| Cell |
SH-SY5Y IMR-32 |
qPCR WB |
unk | Calcium influx |
TRPC3 DISC1 |
Proliferation | [97] | |
| Cell |
SH-SY5Y IMR-32 |
qRT-PCR WB |
unk | Calcium influx | SK3 |
Proliferation migration |
[98] |
unk unknown; WB Western Blot
The role and mechanism of syncytin-1 in neuroblastoma
Syncytin-1 induces tumorigenesis by multiple factors (Fig. 3). Firstly, the study found that aspirin and caffeine could increase the mRNA level and protein expression of syncytin-1 in the SH-SY5Y cell line. And the luciferase activity assay showed that caffeine could also induce the activation of HERV-W environmental promoter, improve the transcription level of syncytin-1, thus promoting the proliferation of NB cells [100]. Several studies suggested that caffeine could play roles in different pathways, such as releasing calcium stored [101], activating the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway [102], inhibiting cellular mTOR/P70S6K/4E-BP1 [103] or inducing vascular endothelial growth factor expression [104]. Secondly, treatment of the SK-N-DZ cell line with hypoxia-reoxygenation or DNA methylation inhibitor 5-azacytidine could increase the expression of syncytin-1 [99]. Thirdly, changes in the medium micro-environment can also affect the expression of syncytin-1. The expression of syncytin-1 in the NB cell line SH-SY5Y was significantly increased in serum-free stem cell medium, and RNA analysis found that the overexpression of HERV was associated with the overexpression of the immune checkpoint molecule CD200 in NB tumors [96], indicating that serum-free stem cell medium promotes NB cell invasion [105]. Therefore, caffeine, aspirin, oxygen tension, DNA methylation and the medium micro-environment changes both can affect the expression of syncytin-1.
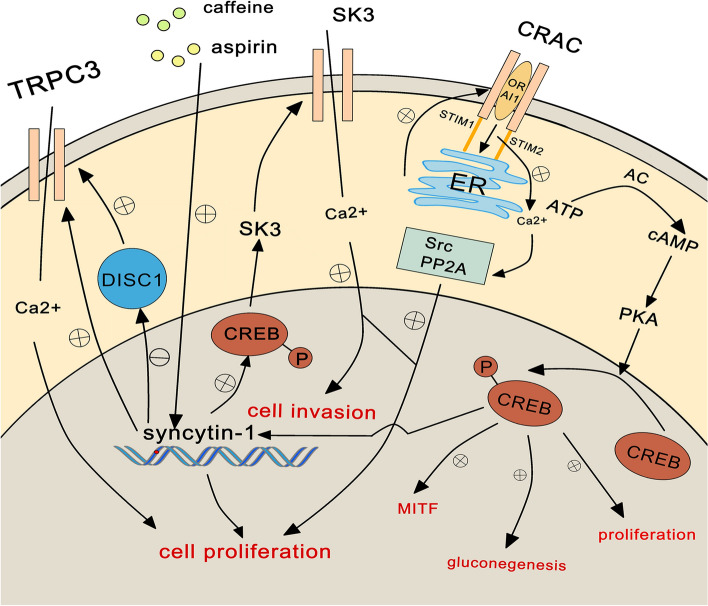
Fig. 3.
Molecular mechanisms and signal pathways of syncytin-1 overexpression in neuroblastoma (NB) Aspirin and caffeine can promote the transcription of syncytin-1, thereby promoting the proliferation of NB cells. The stromal interaction molecule 1 (STIM1) protein in the endoplasmic reticulum (ER) recognizes the depletion of stored calcium and activates the plasma membrane calcium ion channel (ORAI1) in the plasma membrane, establishing a calcium release-activated calcium channel (CRAC), causing calcium influx. The calcium that re-enters the cytoplasm is stored in the endoplasmic reticulum (ER). As the concentration of calcium in the cytoplasm increases, protein kinases such as Src and PP2A are activated, thus promoting cell proliferation. Syncytin-1 can induce calcium influx by activating the TRPC3 channel, SK3 channel and down-regulating DISC1 expression to promote the proliferation and invasion of NB cells. Phosphorylated CREB can also induce tumor cell proliferation, gluconeogenesis and promote microphthalmia-associated transcription factor (MITF) expression
Syncytin-1 affects NB progression through multiple molecular mechanisms. Store-operated calcium entry mechanism is one of the mechanisms of calcium influx. The stromal interaction molecule 1 protein in the endoplasmic reticulum recognizes the depletion of stored calcium and activates the calcium release-activated calcium channel protein 1 in the plasma membrane, establishing a calcium release-activated calcium channel, causing calcium influx. Calcium re-entering the cytoplasm is stored in the endoplasmic reticulum. As the concentration of calcium in the cytoplasm increases, protein kinases such as tyrosine-protein kinase (Src) and protein phosphatase 2 (PP2A) are activated, thus promoting cell proliferation and other processes [106, 107]. Syncytin-1 can promote the proliferation of NB cells by affecting calcium influx. Whole-cell patch clamp experiments showed that in NB cell lines SH-SY5Y and IMR-32, syncytin-1 could promote calcium influx by directly activating transient receptor potential channel 3 (TRPC3) channels and indirectly activating TRPC3 channels by downregulating the expression of DISC1, thereby promoting cell proliferation [97]. And syncytin-1 could also activate the phosphorylated cyclic-AMP response binding protein (CREB) site on the small conductance Ca2+ -activated K+ channel protein 3 (SK3) promoter, causing downstream gene SK3 transcription, and inducing calcium influx via a voltage-independent pathway, finally causing proliferation and migration of NB cells [98]. CREB is phosphorylated and activated by PKA, inducing several physiological processes through the formation of homodimers or heterodimers, such as upregulating microphthalmia-associated transcription factor [108, 109], promoting gluconeogenesis [110] and the proliferation of cancer cells. Furthermore, different types of transient receptor potential channel proteins overexpression were also found in pancreatic, ovarian and lung cancers, and overexpression of voltage-gated calcium channels has been found in testicular, prostate, colorectal, gastric cancers [111]. All in all, syncytin-1 can cause calcium influx by acting on TRPC3, CREB, SK3 and so on, prompting the proliferation and migration of NB cells.
In summary, syncytin-1 overexpression can be induced by extracellular factors such as drugs and culture micro-environment, and syncytin-1 overexpression can also activate channels that promote calcium influx and thus have an effect on NB. These results illustrate that syncytin-1 has the potential to become a prognostic marker for NB, but more definite mechanisms need to be further investigated.
The relation between syncytin-1 and endometrial cancer/endometriosis
Endometrial cancer (EnCa) is one of the most common malignant invasive cancers in the world, often occurring in women with postmenopausal bleeding [112]. Studies have shown that the expression of syncytin-1 in EnCa tissues and cells is higher than that in normal tissues and cells (Table 3). Strick et al. found that both steroid hormones and cAMP can induce a significant increase in syncytin-1 mRNA and protein expression in EnCa cells. Steroid hormone-induced syncytin-1 promotes cell proliferation, and syncytin-1 promotes cell–cell fusion in the absence of transforming growth factor β. Similarly, cAMP also induces syncytin-1 to promote cell–cell fusion [113]. In addition, hypomethylation of the HERV-W 5′LTR region can increase syncytin-1 expression, and the tumor staging and histological grading of EnCa can also have an impact on syncytin-1 expression [114]. The overexpression of syncytin-1 promotes EnCa cell proliferation by rapidly transitioning from G2 to the M phase. And it also promotes cell migration and invasion by inducing the expression of epithelial-mesenchymal transition (EMT)-related gene proteins, such as waveform protein and E-cadherin protein. This result suggested that syncytin-1 as a fusogenic gene could promote cell migration and invasion by inducing EMT progress. Kaplan–Meier analysis found that high syncytin-1 expression was often associated with poor survival and prognosis [115]. This suggests that syncytin-1 is involved in the early oncogenic process of EnCa.
Table 3.
Relationship between high expression of syncytin-1 and endometrial cancer
| Type of cancer | Specimen | Number of cases | Methods | Upstream factors | Downstream factors | Oncogenic effect | Relationship between high expression of syncytin-1 and endometrial cancer: | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical parameter | Relevance | ||||||||
| Endometrial cancer | Tissue | 48/(E:24,N:24) |
qPCR RT-PCR Northern Blot |
Steroids CAMP |
unk |
Fusion proliferation |
unk | unk | [113] |
| Tissue | 67/(E:38,N:29) | qPCR | Hypomethylation | unk | Proliferation |
TNM staging |
+ | [114] | |
| Histological grading | + | ||||||||
| Tissue cell | 167/(E:130,N:37) | RT-PCR | unk |
Waveform E-adhesion |
Fusion proliferation migration |
unk | unk | [115] | |
unk unknown; E endometrial cancer; N normal; + positive correlation
Endometriosis is an estrogen-dependent chronic gynecological disease, it is characterized by the presence of endometrial tissue outside its normal location [116]. Similar to EnCa, syncytin-1 may also influence the development of endometriosis lesions. Zhou et al. found that syncytin-1 is overexpressed in endometriosis tissues via hypomethylation in the LTR promoter region. The alternations in DNMT3B isoforms can result in hypomethylation of the syncytin-1 promoter, and the overexpression of syncytin-1 can upregulate the human somatomammotropin (hCS) gene [117]. In addition, the RNA levels of syncytin-1 were increased only in eutopic endometrium from patients with endometriosis [118]. This suggests that syncytin-1 may play an essential role in the morphological development of the human endometrium.
Correlation between syncytin-1 and testicular cancer/seminoma
Testicular cancer is a common solid malignant tumor in young men. Testicular germ cell tumors (TGCT) account for about 98% of all testicular malignancies, and seminomas account for about 60% of TGCT [119]. Gimenez et al. found in the custom HERV genechip microarray that syncytin-1 mRNA expression was not observed in normal testicular tissues, while the expression of syncytin-1 was up-regulated in testicular cancer tissues [120]. Another study found that the mRNA levels of syncytin-1 in seminoma tissues were higher than those in seminoma-matched controls and non-seminoma GCTs. The expression of tet methylcytosine dioxygenase 1 is highly increased in most seminomas, which results in hypomethylation of the syncytin-1 promoter, increasing the expression of syncytin-1. The interaction of syncytin-1 and its receptor SLC1A4/SLC1A5, and the transcriptional factor glial cells missing transcription factor 1 (GCM1) are all able to influence most tumorigenesis, nevertheless, the interaction has not been found in TGCT or seminomas [121]. So, the overexpression of syncytin-1 may be associated with the development of testicular cancer and seminoma, but further research is needed.
Syncytin-1 and other tumors
The expression of syncytin-1 is high in NB cell lines, EnCa, testicular cancer and seminoma, and also abnormally expressed in other tumors. Studies have shown that when cell apoptosis or necrosis, chromosomal DNA is cleaved into large amounts of circulating free DNA, which is released into the serum and plasma [122, 123]. In non-small cell lung cancer (NSCLC), the syncytin-1 gene is increased expression due to chromosome activation and nucleosome depolymerization. Thus, the circulating free DNA of syncytin-1 is increased in the serum of NSCLC patients [124]. And the expression of syncytin-1 was significantly higher in NSCLC tissues than in para-carcinoma tissues. The hypomethylation of the syncytin-1 promoter could promote the expression of syncytin-1 in NSCLC [32]. The transcription factor SP1 can promote the expression of syncytin-1 in NSCLC cells. And the downregulation of the SP1/Syncytin-1 axis can reverse the epithelial-mesenchymal transition process by inhibiting the activity of Akt and Erk1/2 signal pathways in NSCLC cells, inhibiting cell proliferation and migration, promoting cell apoptosis [125]. Our previous study found that the expression of syncytin-1 in urothelial cell carcinoma tissues of the bladder is more than in tumor-adjacent tissues. The transcriptional activator c-Myb interacts with the mutated 3'-LTR of syncytin-1, upregulating the levels of mRNA and protein of syncytin-1, inducing urothelial cell carcinoma tumorigenesis and tumor cell proliferation [126]. Syncytin-1 expresses in breast cancer cells, and the corresponding D-type retroviral receptor SLC1A5 expresses in endothelial cells. Syncytin-1 can facilitate breast cancer-endothelial cell fusions. The downregulation of syncytin-1 expression highly inhibits cell fusion. And inhibitory syncytin peptides can also inhibit cell fusion. Therefore, syncytin-1 could be a potential therapeutic target to inhibit tumor cell fusion [127]. The expression of syncytin-1 is higher in colorectal cancer tissues than in normal tissues [128]. Li et al. found that arsenic trioxide (ATO) can increase the expression of transcription factor GCM1, which in turn increases the expression of syncytin-1 and its receptor SLC1A5, mediating cell fusion, inducing the formation of the polyploid giant cancer cells (PGCCs) in colorectal cancer, promoting the migration, proliferation and invasion of colorectal cancer cells. The expression level of syncytin-1 gradually increased with tumor grades increasingly. It was thought that the expression of syncytin-1 could be closely related to the pathological grade, clinical stage and distant metastasis of colorectal cancer tissues [129]. Syncytin-1 is also expressed in mycosis fungoides, a primary cutaneous T-cell lymphoma [130, 131]. Interestingly, the mRNA and protein levels of syncytin-1 in pancreatic cancer are lower than in normal tissues. And the decrease of syncytin-1 expression is related to the hypermethylation of CpG sites on 5′LTR [132]. Taken together, the expression of syncytin-1 is high in NSCLC, urothelial cell carcinoma tissues of the bladder, breast cancer and colorectal cancer, but low in pancreatic cancer. And syncytin-1 has the potential to be a molecular marker for their diagnosis in the future.
Syncytin-2 and diseases
Syncytin-2 is an env glycoprotein of the HERV-FRD family. It plays an important role in placental development through its involvement in the formation of the syncytiotrophoblast [133]. The abnormal expression of syncytin-2 has also been associated with human diseases. The MFSD2A is a syncytin-2-mediated cell fusion cognate receptor. Studies have found that syncytin-2 is expressed in the breast cancer cell line MCF-7. Interestingly, the ectopic expression of the transcription factor GCM1 can upregulate the activity of the MFSD2A promoter, promote the expression of MFSD2A genes, and also bind to GCM1-binding sites (GBSs) in syncytin-2 gene (SYN2GBS) at the CpG site of 5'-LTR to induce syncytin-2 hypomethylation, transactivate the promoter of syncytin-2, and promote its transcription. The interaction of syncytin-2 and MFSD2A can eventually promote the fusion of MCF-7 cells [134]. Furthermore, the high expression of syncytn-2 is also associated with colorectal cancer [128], EnCa [114] and seminoma [121]. Therefore, the physiological and pathological role of syncytin-2 predicts that it could become an important molecular biological marker in the future.
Conclusion
Syncytin-1 can promote the fusion of villous cytotrophoblast into syncytiotrophoblast, and play an essential role in human placenta development. And it also promotes tumor cell proliferation, anti-apoptotic, invasion, and migration through its fusogenic and non-fusogenic activities. Syncytin-1 abnormally expressed in placental development related diseases and tumors, including PE, infertility, IUGR, hydatidiform moles, NB, EnCa, endometriosis, testicular cancer, seminoma and NSCLC. Caffeine, aspirin, medium micro-environment, steroid hormones, cAMP, PPARγ/RXRα agonists and inhibitors, ATO, DNA methylation levels can all affect the transcription level of syncytin-1. And syncytin-1 can also affect various signal pathways to induce tumorigenesis, such as TRPC3, DISC1, SK3, Akt and Erk1/2, promoting the proliferation and invasion of tumor cells and improving the ability of distant metastasis. The following points need to be further considered deeply in this review. (1) Syncytin-1 is highly expressed in both NB, EnCa, endometriosis, testicular cancer, seminoma, NSCLC, urothelial cell carcinoma tissues of the bladder and colorectal cancer. But it is low expressed in pancreatic cancer, infertility and IUGR. Though studies have shown that syncytin-1 expression is associated with PE, high or low expression of syncytin-1 in PE remains controversial. So, it is necessary to further explore the more specific molecular mechanisms of syncytin-1 through experiments. (2) The expression of syncytin-1 is high in NB, EnCa, testicular cancer and other diseases, can syncytin-1 be directly used as a diagnostic marker for diseases? (3) Syncytin-1 is a membrane glycoprotein, which may inhibit tumorigenesis by applying inhibitor drugs to inhibit the activity and signal pathways of syncytin-1 and its receptors. In conclusion, whether syncytin-1 can become a new biological marker and potential therapeutic target and whether it can be more effective than the currently confirmed target requires further experimental and clinical research.
Future direction
Molecularly targeted therapy has increasingly become a research hotspot. Although syncytin-1 plays a role in placental development related diseases and tumors, its molecular mechanisms and signal pathways need further study. Syncytin-1 is a membrane glycoprotein, so future studies could be focused on exploring antibody inhibitor drugs targeting syncytin-1 and its receptors, to inhibit the occurrence of diseases. Furthermore, studies have shown that syncytin-1 can be detected in the blood, which may serve as a diagnostic marker in the future. Syncytin-1 can participate in the formation of placental syncytiotrophoblasts through cell fusion, and could also play an important role in proliferation, migration and invasion of tumor, so its specific mechanisms need to be further explored in the future.
Acknowledgements
The authors thank Dr. Qian Wang of Columbia University for revising and polishing our manuscript.
Author contributions
HY, ZZ and QW contributed to the conception and design of the study. QW wrote the first draft of the manuscript. YS modified the grammar. QB organized and checked the database. YS, NZ and XL wrote parts of the manuscript. MW and JW drew figures. LL drew tables. All authors read and approved the final manuscript.
Funding
This work was supported by the grants from the Shandong Provincial Natural Science Foundation (No. ZR2020MH078 and No. ZR2020MH070), Shandong Province Medicine and Health Science and Technology Development Plan Project (No. 2019WS368 and No. 2017WS143), Doctoral Setup Foundation of Jining Medical University (No. 2017JYQD23), and the College Students’ Innovative Entrepreneurial Training Plan Program of Jining Medical University (No. cx2021094 and No. cx2021011).
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhankui Zhao, Email: zhaozhankuimd@mail.jnmc.edu.cn.
Honglian Yu, Email: yuhonglian@mail.jnmc.edu.cn, Email: yhonglian23@126.com.
References
- 1.Belshaw R, Pereira V, Katzourakis A, Talbot G, Paces J, Burt A, et al. Long-term reinfection of the human genome by endogenous retroviruses. Proc Natl Acad Sci USA. 2004;101(14):4894–4899. doi: 10.1073/pnas.0307800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 3.Cohen M, Larsson E. Human endogenous retroviruses. BioEssays. 1988;9(6):191–196. doi: 10.1002/bies.950090603. [DOI] [PubMed] [Google Scholar]
- 4.Yi JM, Kim HS. Expression and phylogenetic analyses of human endogenous retrovirus HC2 belonging to the HERV-T family in human tissues and cancer cells. J Hum Genet. 2007;52(4):285–296. doi: 10.1007/s10038-007-0115-8. [DOI] [PubMed] [Google Scholar]
- 5.Dervan E, Bhattacharyya DD, McAuliffe JD, Khan FH, Glynn SA. Ancient adversary—HERV-K (HML-2) in cancer. Front Oncol. 2021;11:658489. doi: 10.3389/fonc.2021.658489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjellman C, Sjögren HO, Salford LG, Widegren B. HERV-F (XA34) is a full-length human endogenous retrovirus expressed in placental and fetal tissues. Gene. 1999;239(1):99–107. doi: 10.1016/S0378-1119(99)00372-8. [DOI] [PubMed] [Google Scholar]
- 7.Le Dantec C, Vallet S, Brooks WH, Renaudineau Y. Human endogenous retrovirus group E and its involvement in diseases. Viruses. 2015;7(3):1238–1257. doi: 10.3390/v7031238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vargiu L, Rodriguez-Tomé P, Sperber GO, Cadeddu M, Grandi N, Blikstad V, et al. Classification and characterization of human endogenous retroviruses; mosaic forms are common. Retrovirology. 2016;13:7. doi: 10.1186/s12977-015-0232-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezaei SD, Hayward JA, Norden S, Pedersen J, Mills J, Hearps AC, et al. HERV-K gag RNA and protein levels are elevated in malignant regions of the prostate in males with prostate cancer. Viruses. 2021;13(3):449. doi: 10.3390/v13030449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavakolian S, Goudarzi H, Faghihloo E. Evaluating the expression level of HERV-K env, np9, rec and gag in breast tissue. Infect Agent Cancer. 2019;14:42. doi: 10.1186/s13027-019-0260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monde K, Terasawa H, Nakano Y, Soheilian F, Nagashima K, Maeda Y, et al. Molecular mechanisms by which HERV-K Gag interferes with HIV-1 Gag assembly and particle infectivity. Retrovirology. 2017;14(1):27. doi: 10.1186/s12977-017-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullins CS, Huhns M, Krohn M, Peters S, Cheynet V, Oriol G, et al. Generation, characterization and application of antibodies directed against HERV-H gag protein in colorectal samples. PLoS ONE. 2016;11(4):e0153349. doi: 10.1371/journal.pone.0153349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perron H, Lazarini F, Ruprecht K, Pechoux-Longin C, Seilhean D, Sazdovitch V, et al. Human endogenous retrovirus (HERV)-W ENV and GAG proteins: physiological expression in human brain and pathophysiological modulation in multiple sclerosis lesions. J Neurovirol. 2005;11(1):23–33. doi: 10.1080/13550280590901741. [DOI] [PubMed] [Google Scholar]
- 14.Christensen T. HERVs in neuropathogenesis. J Neuroimmune Pharmacol. 2010;5(3):326–335. doi: 10.1007/s11481-010-9214-y. [DOI] [PubMed] [Google Scholar]
- 15.Johnson WE. Origins and evolutionary consequences of ancient endogenous retroviruses. Nat Rev Microbiol. 2019;17(6):355–370. doi: 10.1038/s41579-019-0189-2. [DOI] [PubMed] [Google Scholar]
- 16.Pradhan RK, Ramakrishna W. Transposons: unexpected players in cancer. Gene. 2022;808:145975. doi: 10.1016/j.gene.2021.145975. [DOI] [PubMed] [Google Scholar]
- 17.Karn J, Stoltzfus CM. Transcriptional and posttranscriptional regulation of HIV-1 gene expression. Cold Spring Harb Perspect Med. 2012;2(2):a006916. doi: 10.1101/cshperspect.a006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Yan L, Jiang J, Wang Y, Jiang Y, Yan T, et al. The structure and retrotransposition mechanism of LTR-retrotransposons in the asexual yeast Candida albicans. Virulence. 2014;5(6):655–664. doi: 10.4161/viru.32180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casacuberta JM, Santiago N. Plant LTR-retrotransposons and MITEs: control of transposition and impact on the evolution of plant genes and genomes. Gene. 2003;311:1–11. doi: 10.1016/S0378-1119(03)00557-2. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm M, Wilhelm FX. Reverse transcription of retroviruses and LTR retrotransposons. Cell Mol Life Sci. 2001;58(9):1246–1262. doi: 10.1007/PL00000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keshet E, Schiff R, Itin A. Mouse retrotransposons: a cellular reservoir of long terminal repeat (LTR) elements with diverse transcriptional specificities. Adv Cancer Res. 1991;56:215–251. doi: 10.1016/S0065-230X(08)60482-0. [DOI] [PubMed] [Google Scholar]
- 22.Krebs AS, Mendonca LM, Zhang P. Structural analysis of retrovirus assembly and maturation. Viruses. 2021;14(1):54. doi: 10.3390/v14010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prudhomme S, Bonnaud B, Mallet F. Endogenous retroviruses and animal reproduction. Cytogenet Genome Res. 2005;110(1–4):353–364. doi: 10.1159/000084967. [DOI] [PubMed] [Google Scholar]
- 24.Buslei R, Strissel PL, Henke C, Schey R, Lang N, Ruebner M, et al. Activation and regulation of endogenous retroviral genes in the human pituitary gland and related endocrine tumours. Neuropathol Appl Neurobiol. 2015;41(2):180–200. doi: 10.1111/nan.12136. [DOI] [PubMed] [Google Scholar]
- 25.Nogueira MA, Gavioli CF, Pereira NZ, de Carvalho GC, Domingues R, Aoki V, et al. Human endogenous retrovirus expression is inversely related with the up-regulation of interferon-inducible genes in the skin of patients with lichen planus. Arch Dermatol Res. 2015;307(3):259–264. doi: 10.1007/s00403-014-1524-0. [DOI] [PubMed] [Google Scholar]
- 26.Coffin JM. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- 27.Hanson HM, Willkomm NA, Yang H, Mansky LM. Human retrovirus genomic RNA packaging. Viruses. 2022;14(5):1094. doi: 10.3390/v14051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soygur B, Sati L. The role of syncytins in human reproduction and reproductive organ cancers. Reproduction. 2016;152(5):R167–178. doi: 10.1530/REP-16-0031. [DOI] [PubMed] [Google Scholar]
- 29.Prokop J, Jagodzinski PP. Identification of retroviral conserved pol sequences in serum of mixed connective tissue disease and systemic sclerosis patients. Biomed Pharmacother. 2004;58(1):61–64. doi: 10.1016/j.biopha.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Gross H, Barth S, Pfuhl T, Willnecker V, Spurk A, Gurtsevitch V, et al. The NP9 protein encoded by the human endogenous retrovirus HERV-K(HML-2) negatively regulates gene activation of the Epstein-Barr virus nuclear antigen 2 (EBNA2) Int J Cancer. 2011;129(5):1105–1115. doi: 10.1002/ijc.25760. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Kaye S, Gore ME, McClure MO, Bunker CB. The role of human endogenous retroviruses in melanoma. Br J Dermatol. 2009;161(6):1225–1231. doi: 10.1111/j.1365-2133.2009.09415.x. [DOI] [PubMed] [Google Scholar]
- 32.Fu Y, Zhuang X, Xia X, Li X, Xiao K, Liu X. Correlation between promoter hypomethylation and increased expression of syncytin-1 in non-small cell lung cancer. Int J Gen Med. 2021;14:957–965. doi: 10.2147/IJGM.S294392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Liu Z, Wang P, Li S, Zeng J, Tu X, et al. Syncytin-1, an endogenous retroviral protein, triggers the activation of CRP via TLR3 signal cascade in glial cells. Brain Behav Immun. 2018;67:324–334. doi: 10.1016/j.bbi.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Chen T, Meng Z, Gan Y, Wang X, Xu F, Gu Y, et al. The viral oncogene Np9 acts as a critical molecular switch for co-activating beta-catenin, ERK, Akt and Notch1 and promoting the growth of human leukemia stem/progenitor cells. Leukemia. 2013;27(7):1469–1478. doi: 10.1038/leu.2013.8. [DOI] [PubMed] [Google Scholar]
- 35.Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, et al. Paleovirology of 'syncytins', retroviral env genes exapted for a role in placentation. Philos Trans R Soc Lond B Biol Sci. 2013;368(1626):20120507. doi: 10.1098/rstb.2012.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74(7):3321–3329. doi: 10.1128/JVI.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esnault C, Priet S, Ribet D, Vernochet C, Bruls T, Lavialle C, et al. A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc Natl Acad Sci USA. 2008;105(45):17532–17537. doi: 10.1073/pnas.0807413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CP, Chen LF, Yang SR, Chen CY, Ko CC, Chang GD, et al. Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol Reprod. 2008;79(5):815–823. doi: 10.1095/biolreprod.108.069765. [DOI] [PubMed] [Google Scholar]
- 39.Huang Q, Chen H, Li J, Oliver M, Ma X, Byck D, et al. Epigenetic and non-epigenetic regulation of syncytin-1 expression in human placenta and cancer tissues. Cell Signal. 2014;26(3):648–656. doi: 10.1016/j.cellsig.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Shi J, Liu S. Recent advances in the study of active endogenous retrovirus envelope glycoproteins in the mammalian placenta. Virol Sin. 2015;30(4):239–248. doi: 10.1007/s12250-015-3617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grandi N, Cadeddu M, Blomberg J, Tramontano E. Contribution of type W human endogenous retroviruses to the human genome: characterization of HERV-W proviral insertions and processed pseudogenes. Retrovirology. 2016;13(1):67. doi: 10.1186/s12977-016-0301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjerregaard B, Lemmen JG, Petersen MR, Østrup E, Iversen LH, Almstrup K, et al. Syncytin-1 and its receptor is present in human gametes. J Assist Reprod Genet. 2014;31(5):533–539. doi: 10.1007/s10815-014-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupressoir A, Lavialle C, Heidmann T. From ancestral infectious retroviruses to bona fide cellular genes: role of the captured syncytins in placentation. Placenta. 2012;33(9):663–671. doi: 10.1016/j.placenta.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Durnaoglu S, Lee SK, Ahnn J. Syncytin, envelope protein of human endogenous retrovirus (HERV): no longer ‘fossil’ in human genome. Anim Cells Syst (Seoul) 2021;25(6):358–368. doi: 10.1080/19768354.2021.2019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lokossou AG, Toudic C, Barbeau B. Implication of human endogenous retrovirus envelope proteins in placental functions. Viruses. 2014;6(11):4609–4627. doi: 10.3390/v6114609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavillette D, Marin M, Ruggieri A, Mallet F, Cosset FL, Kabat D. The envelope glycoprotein of human endogenous retrovirus type W uses a divergent family of amino acid transporters/cell surface receptors. J Virol. 2002;76(13):6442–6452. doi: 10.1128/JVI.76.13.6442-6452.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roland CS, Hu J, Ren CE, Chen H, Li J, Varvoutis MS, et al. Morphological changes of placental syncytium and their implications for the pathogenesis of preeclampsia. Cell Mol Life Sci. 2016;73(2):365–376. doi: 10.1007/s00018-015-2069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinha A, Johnson WE. Retroviruses of the RDR superinfection interference group: ancient origins and broad host distribution of a promiscuous Env gene. Curr Opin Virol. 2017;25:105–112. doi: 10.1016/j.coviro.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Soygur B, Moore H. Expression of Syncytin 1 (HERV-W), in the preimplantation human blastocyst, embryonic stem cells and trophoblast cells derived in vitro. Hum Reprod. 2016;31(7):1455–1461. doi: 10.1093/humrep/dew097. [DOI] [PubMed] [Google Scholar]
- 50.Chen CP, Wang KG, Chen CY, Yu C, Chuang HC, Chen H. Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. BJOG. 2006;113(2):152–158. doi: 10.1111/j.1471-0528.2005.00843.x. [DOI] [PubMed] [Google Scholar]
- 51.Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114(5–6):397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 52.Matousková M, Blazková J, Pajer P, Pavlícek A, Hejnar J. CpG methylation suppresses transcriptional activity of human syncytin-1 in non-placental tissues. Exp Cell Res. 2006;312(7):1011–1020. doi: 10.1016/j.yexcr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Gao Y, He Z, Wang Z, Luo Y, Sun H, Zhou Y, et al. Increased expression and altered methylation of HERVWE1 in the human placentas of smaller fetuses from monozygotic, dichorionic, discordant twins. PLoS ONE. 2012;7(3):e33503. doi: 10.1371/journal.pone.0033503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bolze PA, Mommert M, Mallet F. Contribution of syncytins and other endogenous retroviral envelopes to human placenta pathologies. Prog Mol Biol Transl Sci. 2017;145:111–162. doi: 10.1016/bs.pmbts.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 55.Gerbaud P, Pidoux G. Review: an overview of molecular events occurring in human trophoblast fusion. Placenta. 2015;36(Suppl 1):S35–42. doi: 10.1016/j.placenta.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Schreiber J, Riethmacher-Sonnenberg E, Riethmacher D, Tuerk EE, Enderich J, Bösl MR, et al. Placental failure in mice lacking the mammalian homolog of glial cells missing. GCMa Mol Cell Biol. 2000;20(7):2466–2474. doi: 10.1128/MCB.20.7.2466-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet. 2000;25(3):311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- 58.Yu C, Shen K, Lin M, Chen P, Lin C, Chang G-D, et al. GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem. 2002;277(51):50062–50068. doi: 10.1074/jbc.M209316200. [DOI] [PubMed] [Google Scholar]
- 59.Chang CW, Chuang HC, Yu C, Yao TP, Chen H. Stimulation of GCMa transcriptional activity by cyclic AMP/protein kinase A signaling is attributed to CBP-mediated acetylation of GCMa. Mol Cell Biol. 2005;25(19):8401–8414. doi: 10.1128/MCB.25.19.8401-8414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132(3):1387–1395. doi: 10.1210/endo.132.3.7679981. [DOI] [PubMed] [Google Scholar]
- 61.Knerr I, Schubert SW, Wich C, Amann K, Aigner T, Vogler T, et al. Stimulation of GCMa and syncytin via cAMP mediated PKA signaling in human trophoblastic cells under normoxic and hypoxic conditions. FEBS Lett. 2005;579(18):3991–3998. doi: 10.1016/j.febslet.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 62.Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, et al. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;16(7):1638–1651. doi: 10.1210/mend.16.7.0863. [DOI] [PubMed] [Google Scholar]
- 63.Gong R, Peng X, Kang S, Feng H, Huang J, Zhang W, et al. Structural characterization of the fusion core in syncytin, envelope protein of human endogenous retrovirus family W. Biochem Biophys Res Commun. 2005;331(4):1193–1200. doi: 10.1016/j.bbrc.2005.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403(6771):785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 65.Bu C, Wang Z, Ren Y, Chen D, Jiang SW. Syncytin-1 nonfusogenic activities modulate inflammation and contribute to preeclampsia pathogenesis. Cell Mol Life Sci. 2022;79(6):290. doi: 10.1007/s00018-022-04294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holder BS, Tower CL, Forbes K, Mulla MJ, Aplin JD, Abrahams VM. Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology. 2012;136(2):184–191. doi: 10.1111/j.1365-2567.2012.03568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tolosa JM, Parsons KS, Hansbro PM, Smith R, Wark PA. The placental protein syncytin-1 impairs antiviral responses and exaggerates inflammatory responses to influenza. PLoS ONE. 2015;10(4):e0118629. doi: 10.1371/journal.pone.0118629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Y, Zhu H, Song J, Jiang Y, Ouyang H, Huang R, et al. Upregulation of leukocytic syncytin-1 in acute myeloid leukemia patients. Med Sci Monit. 2016;22:2392–2403. doi: 10.12659/MSM.899303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Montojo M, Rodriguez-Martin E, Ramos-Mozo P, Ortega-Madueno I, Dominguez-Mozo MI, Arias-Leal A, et al. Syncytin-1/HERV-W envelope is an early activation marker of leukocytes and is upregulated in multiple sclerosis patients. Eur J Immunol. 2020;50(5):685–694. doi: 10.1002/eji.201948423. [DOI] [PubMed] [Google Scholar]
- 70.Bergallo M, Tovo PA, Bertino E, Dapra V, Pirra A, Montanari P, et al. Human endogenous retroviruses HERV-H, HERV-W and HERV-K in preeclampsia. Minerva Ginecol. 2019;71(3):260–262. doi: 10.23736/S0026-4784.19.04321-1. [DOI] [PubMed] [Google Scholar]
- 71.Fantone S, Mazzucchelli R, Giannubilo SR, Ciavattini A, Marzioni D, Tossetta G. AT-rich interactive domain 1A protein expression in normal and pathological pregnancies complicated by preeclampsia. Histochem Cell Biol. 2020;154(3):339–346. doi: 10.1007/s00418-020-01892-8. [DOI] [PubMed] [Google Scholar]
- 72.Opichka MA, Rappelt MW, Gutterman DD, Grobe JL, McIntosh JJ. Vascular dysfunction in preeclampsia. Cells. 2021;10(11):3055. doi: 10.3390/cells10113055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melchiorre K, Giorgione V, Thilaganathan B. The placenta and preeclampsia: villain or victim? Am J Obstet Gynecol. 2022;226(2S):S954–S962. doi: 10.1016/j.ajog.2020.10.024. [DOI] [PubMed] [Google Scholar]
- 74.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021;398(10297):341–354. doi: 10.1016/S0140-6736(20)32335-7. [DOI] [PubMed] [Google Scholar]
- 76.Zhuang XW, Li J, Brost BC, Xia XY, Chen HB, Wang CX, et al. Decreased expression and altered methylation of syncytin-1 gene in human placentas associated with preeclampsia. Curr Pharm Des. 2014;20(11):1796–1802. doi: 10.2174/13816128113199990541. [DOI] [PubMed] [Google Scholar]
- 77.Lee X, Keith JC, Jr, Stumm N, Moutsatsos I, McCoy JM, Crum CP, et al. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta. 2001;22(10):808–812. doi: 10.1053/plac.2001.0722. [DOI] [PubMed] [Google Scholar]
- 78.Vargas A, Toufaily C, LeBellego F, Rassart É, Lafond J, Barbeau B. Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod Sci. 2011;18(11):1085–1091. doi: 10.1177/1933719111404608. [DOI] [PubMed] [Google Scholar]
- 79.Ruebner M, Langbein M, Strissel PL, Henke C, Schmidt D, Goecke TW, et al. Regulation of the human endogenous retroviral Syncytin-1 and cell-cell fusion by the nuclear hormone receptors PPARgamma/RXRalpha in placentogenesis. J Cell Biochem. 2012;113(7):2383–2396. doi: 10.1002/jcb.24110. [DOI] [PubMed] [Google Scholar]
- 80.Holder BS, Tower CL, Abrahams VM, Aplin JD. Syncytin 1 in the human placenta. Placenta. 2012;33(6):460–466. doi: 10.1016/j.placenta.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Kim S, Lee N, Park ES, Yun H, Ha TU, Jeon H, et al. T-cell death associated gene 51 is a novel negative regulator of PPARgamma that inhibits PPARgamma-RXRalpha heterodimer formation in adipogenesis. Mol Cells. 2021;44(1):1–12. doi: 10.14348/molcells.2020.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Korpal M, Puyang X, Jeremy WuZ, Seiler R, Furman C, Oo HZ, et al. Evasion of immunosurveillance by genomic alterations of PPARgamma/RXRalpha in bladder cancer. Nat Commun. 2017;8(1):103. doi: 10.1038/s41467-017-00147-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brouillet S, Hoffmann P, Chauvet S, Salomon A, Chamboredon S, Sergent F, et al. Revisiting the role of hCG: new regulation of the angiogenic factor EG-VEGF and its receptors. Cell Mol Life Sci. 2012;69(9):1537–1550. doi: 10.1007/s00018-011-0889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sagawa N, Yura S, Itoh H, Kakui K, Takemura M, Nuamah MA, et al. Possible role of placental leptin in pregnancy: a review. Endocrine. 2002;19(1):65–71. doi: 10.1385/ENDO:19:1:65. [DOI] [PubMed] [Google Scholar]
- 85.Iwagaki S, Yokoyama Y, Tang L, Takahashi Y, Nakagawa Y, Tamaya T. Augmentation of leptin and hypoxia-inducible factor 1alpha mRNAs in the pre-eclamptic placenta. Gynecol Endocrinol. 2004;18(5):263–268. doi: 10.1080/0951359042000196277. [DOI] [PubMed] [Google Scholar]
- 86.Rao S, Kumari A, Sharma M, Kabi BC. Predicting maternal serum adiponectin and leptin level as biomarkers of pre-eclampsia: a prospective study. J Obstet Gynaecol India. 2021;71(1):58–65. doi: 10.1007/s13224-020-01378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jebbink J, Veenboer G, Boussata S, Keijser R, Kremer AE, Elferink RO, et al. Total bile acids in the maternal and fetal compartment in relation to placental ABCG2 expression in preeclamptic pregnancies complicated by HELLP syndrome. Biochim Biophys Acta. 2015;1852(1):131–136. doi: 10.1016/j.bbadis.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 88.Zeng Y, Ren R, Kaur G, Hardikar S, Ying Z, Babcock L, et al. The inactive Dnmt3b3 isoform preferentially enhances Dnmt3b-mediated DNA methylation. Genes Dev. 2020;34(21–22):1546–1558. doi: 10.1101/gad.341925.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Langbein M, Strick R, Strissel PL, Vogt N, Parsch H, Beckmann MW, et al. Impaired cytotrophoblast cell-cell fusion is associated with reduced Syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev. 2008;75(1):175–183. doi: 10.1002/mrd.20729. [DOI] [PubMed] [Google Scholar]
- 90.Tas GG, Soygur B, Kutlu O, Sati L. A comprehensive investigation of human endogenous retroviral syncytin proteins and their receptors in men with normozoospermia and impaired semen quality. J Assist Reprod Genet. 2023;40(1):97–111. doi: 10.1007/s10815-022-02673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruebner M, Strissel PL, Langbein M, Fahlbusch F, Wachter DL, Faschingbauer F, et al. Impaired cell fusion and differentiation in placentae from patients with intrauterine growth restriction correlate with reduced levels of HERV envelope genes. J Mol Med (Berl) 2010;88(11):1143–1156. doi: 10.1007/s00109-010-0656-8. [DOI] [PubMed] [Google Scholar]
- 92.Bolze PA, Patrier S, Cheynet V, Oriol G, Massardier J, Hajri T, et al. Expression patterns of ERVWE1/Syncytin-1 and other placentally expressed human endogenous retroviruses along the malignant transformation process of hydatidiform moles. Placenta. 2016;39:116–124. doi: 10.1016/j.placenta.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 93.Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin North Am. 2010;24(1):65–86. doi: 10.1016/j.hoc.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 94.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 95.Zhao Q, Liu Y, Zhang Y, Meng L, Wei J, Wang B, et al. Role and toxicity of radiation therapy in neuroblastoma patients: a literature review. Crit Rev Oncol Hematol. 2020;149:102924. doi: 10.1016/j.critrevonc.2020.102924. [DOI] [PubMed] [Google Scholar]
- 96.Wieland L, Engel K, Volkmer I, Kruger A, Posern G, Kornhuber ME, et al. Overexpression of endogenous retroviruses and malignancy markers in neuroblastoma cell lines by medium-induced microenvironmental changes. Front Oncol. 2021;11:637522. doi: 10.3389/fonc.2021.637522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y, Yan Q, Zhou P, Li S, Zhu F. HERV-W env regulates calcium influx via activating TRPC3 channel together with depressing DISC1 in human neuroblastoma cells. J Neurovirol. 2019;25(1):101–113. doi: 10.1007/s13365-018-0692-7. [DOI] [PubMed] [Google Scholar]
- 98.Li S, Liu ZC, Yin SJ, Chen YT, Yu HL, Zeng J, et al. Human endogenous retrovirus W family envelope gene activates the small conductance Ca2+-activated K+ channel in human neuroblastoma cells through CREB. Neuroscience. 2013;247:164–174. doi: 10.1016/j.neuroscience.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 99.Hu L, Uzhameckis D, Hedborg F, Blomberg J. Dynamic and selective HERV RNA expression in neuroblastoma cells subjected to variation in oxygen tension and demethylation. APMIS. 2016;124(1–2):140–149. doi: 10.1111/apm.12494. [DOI] [PubMed] [Google Scholar]
- 100.Liu C, Chen Y, Li S, Yu H, Zeng J, Wang X, et al. Activation of elements in HERV-W family by caffeine and aspirin. Virus Genes. 2013;47(2):219–227. doi: 10.1007/s11262-013-0939-6. [DOI] [PubMed] [Google Scholar]
- 101.Riddoch FC, Rowbotham SE, Brown AM, Redfern CP, Cheek TR. Release and sequestration of Ca2+ by a caffeine- and ryanodine-sensitive store in a sub-population of human SH-SY5Y neuroblastoma cells. Cell Calcium. 2005;38(2):111–120. doi: 10.1016/j.ceca.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 102.Nakaso K, Ito S, Nakashima K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson's disease model of SH-SY5Y cells. Neurosci Lett. 2008;432(2):146–150. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 103.Sangaunchom P, Dharmasaroja P. Caffeine potentiates ethanol-induced neurotoxicity through mTOR/p70S6K/4E-BP1 inhibition in SH-SY5Y cells. Int J Toxicol. 2020;39(2):131–140. doi: 10.1177/1091581819900150. [DOI] [PubMed] [Google Scholar]
- 104.Kakio S, Funakoshi-Tago M, Kobata K, Tamura H. Coffee induces vascular endothelial growth factor (VEGF) expression in human neuroblastama SH-SY5Y cells. Nutr Neurosci. 2017;20(6):336–342. doi: 10.1080/1028415X.2015.1133106. [DOI] [PubMed] [Google Scholar]
- 105.Xin C, Zhu J, Gu S, Yin M, Ma J, Pan C, et al. CD200 is overexpressed in neuroblastoma and regulates tumor immune microenvironment. Cancer Immunol Immunother. 2020;69(11):2333–2343. doi: 10.1007/s00262-020-02589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pinto MC, Kihara AH, Goulart VA, Tonelli FM, Gomes KN, Ulrich H, et al. Calcium signaling and cell proliferation. Cell Signal. 2015;27(11):2139–2149. doi: 10.1016/j.cellsig.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 107.Monteith GR, Prevarskaya N, Roberts-Thomson SJ. The calcium-cancer signalling nexus. Nat Rev Cancer. 2017;17(6):367–380. doi: 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- 108.Jiang Z, Li S, Liu Y, Deng P, Huang J, He G. Sesamin induces melanogenesis by microphthalmia-associated transcription factor and tyrosinase up-regulation via cAMP signaling pathway. Acta Biochim Biophys Sin (Shanghai) 2011;43(10):763–770. doi: 10.1093/abbs/gmr078. [DOI] [PubMed] [Google Scholar]
- 109.Sato S, Roberts K, Gambino G, Cook A, Kouzarides T, Goding CR. CBP/p300 as a co-factor for the microphthalmia transcription factor. Oncogene. 1997;14(25):3083–3092. doi: 10.1038/sj.onc.1201298. [DOI] [PubMed] [Google Scholar]
- 110.Zhu X, Li H, Wu Y, Zhou J, Yang G, Wang W, et al. CREB-upregulated lncRNA MEG3 promotes hepatic gluconeogenesis by regulating miR-302a-3p-CRTC2 axis. J Cell Biochem. 2019;120(3):4192–4202. doi: 10.1002/jcb.27706. [DOI] [PubMed] [Google Scholar]
- 111.Tajada S, Villalobos C. Calcium permeable channels in cancer hallmarks. Front Pharmacol. 2020;11:968. doi: 10.3389/fphar.2020.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clarke MA, Long BJ, Del Mar MA, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(9):1210–1222. doi: 10.1001/jamainternmed.2018.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Strick R, Ackermann S, Langbein M, Swiatek J, Schubert SW, Hashemolhosseini S, et al. Proliferation and cell-cell fusion of endometrial carcinoma are induced by the human endogenous retroviral syncytin-1 and regulated by TGF-beta. J Mol Med (Berl) 2007;85(1):23–38. doi: 10.1007/s00109-006-0104-y. [DOI] [PubMed] [Google Scholar]
- 114.Strissel PL, Ruebner M, Thiel F, Wachter D, Ekici AB, Wolf F, et al. Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: emergence of new molecular targets. Oncotarget. 2012;3(10):1204–1219. doi: 10.18632/oncotarget.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu C, Xu J, Wen F, Yang F, Li X, Geng D, et al. Upregulation of syncytin-1 promotes invasion and metastasis by activating epithelial–mesenchymal transition-related pathway in endometrial carcinoma. Onco Targets Ther. 2019;12:31–40. doi: 10.2147/OTT.S191041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 117.Zhou H, Li J, Podratz KC, Tipton T, Marzolf S, Chen HB, et al. Hypomethylation and activation of syncytin-1 gene in endometriotic tissue. Curr Pharm Des. 2014;20(11):1786–1795. doi: 10.2174/13816128113199990540. [DOI] [PubMed] [Google Scholar]
- 118.Oppelt P, Strick R, Strissel PL, Winzierl K, Beckmann MW, Renner SP. Expression of the human endogenous retroviruse-W envelope gene syncytin in endometriosis lesions. Gynecol Endocrinol. 2009;25(11):741–747. doi: 10.3109/09513590903184142. [DOI] [PubMed] [Google Scholar]
- 119.McGlynn KA, Devesa SS, Sigurdson AJ, Brown LM, Tsao L, Tarone RE. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97(1):63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- 120.Gimenez J, Montgiraud C, Pichon JP, Bonnaud B, Arsac M, Ruel K, et al. Custom human endogenous retroviruses dedicated microarray identifies self-induced HERV-W family elements reactivated in testicular cancer upon methylation control. Nucleic Acids Res. 2010;38(7):2229–2246. doi: 10.1093/nar/gkp1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Benešová M, Trejbalová K, Kovářová D, Vernerová Z, Hron T, Kučerová D, et al. DNA hypomethylation and aberrant expression of the human endogenous retrovirus ERVWE1/syncytin-1 in seminomas. Retrovirology. 2017;14(1):20. doi: 10.1186/s12977-017-0342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van der Vaart M, Pretorius PJ. The origin of circulating free DNA. Clin Chem. 2007;53(12):2215. doi: 10.1373/clinchem.2007.092734. [DOI] [PubMed] [Google Scholar]
- 123.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 124.Zhuang X, Qian J, Xia X, Wang Y, Wang H, Jing L, et al. Serum circulating free DNA of syncytin-1 as a novel molecular marker for early diagnosis of non-small-cell lung cancer. Biomark Med. 2022;16(18):1259–1268. doi: 10.2217/bmm-2022-0499. [DOI] [PubMed] [Google Scholar]
- 125.Li X, Fu Y, Xia X, Zhang X, Xiao K, Zhuang X, et al. Knockdown of SP1/Syncytin1 axis inhibits the proliferation and metastasis through the AKT and ERK1/2 signaling pathways in non-small cell lung cancer. Cancer Med. 2019;8(12):5750–5759. doi: 10.1002/cam4.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu H, Liu T, Zhao Z, Chen Y, Zeng J, Liu S, et al. Mutations in 3'-long terminal repeat of HERV-W family in chromosome 7 upregulate syncytin-1 expression in urothelial cell carcinoma of the bladder through interacting with c-Myb. Oncogene. 2014;33(30):3947–3958. doi: 10.1038/onc.2013.366. [DOI] [PubMed] [Google Scholar]
- 127.Bjerregaard B, Holck S, Christensen IJ, Larsson LI. Syncytin is involved in breast cancer-endothelial cell fusions. Cell Mol Life Sci. 2006;63(16):1906–1911. doi: 10.1007/s00018-006-6201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Díaz-Carballo D, Acikelli AH, Klein J, Jastrow H, Dammann P, Wyganowski T, et al. Therapeutic potential of antiviral drugs targeting chemorefractory colorectal adenocarcinoma cells overexpressing endogenous retroviral elements. J Exp Clin Cancer Res. 2015;34(1):81. doi: 10.1186/s13046-015-0199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Z, Zheng M, Zhang H, Yang X, Fan L, Fu F, et al. Arsenic trioxide promotes tumor progression by inducing the formation of PGCCs and embryonic hemoglobin in colon cancer cells. Front Oncol. 2021;11:720814. doi: 10.3389/fonc.2021.720814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maliniemi P, Vincendeau M, Mayer J, Frank O, Hahtola S, Karenko L, et al. Expression of human endogenous retrovirus-w including syncytin-1 in cutaneous T-cell lymphoma. PLoS ONE. 2013;8(10):e76281. doi: 10.1371/journal.pone.0076281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Laukkanen K, Saarinen M, Mallet F, Aatonen M, Hau A, Ranki A. Cutaneous T-cell lymphoma (CTCL) cell line-derived extracellular vesicles contain herv-w-encoded fusogenic syncytin-1. J Invest Dermatol. 2020;140(7):1466–1469 e1464. doi: 10.1016/j.jid.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 132.Lu Q, Li J, Senkowski C, Tang Z, Wang J, Huang T, et al. Promoter hypermethylation and decreased expression of syncytin-1 in pancreatic adenocarcinomas. PLoS ONE. 2015;10(7):e0134412. doi: 10.1371/journal.pone.0134412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Renard M, Varela PF, Letzelter C, Duquerroy S, Rey FA, Heidmann T. Crystal structure of a pivotal domain of human syncytin-2, a 40 million years old endogenous retrovirus fusogenic envelope gene captured by primates. J Mol Biol. 2005;352(5):1029–1034. doi: 10.1016/j.jmb.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 134.Liang CY, Wang LJ, Chen CP, Chen LF, Chen YH, Chen H. GCM1 regulation of the expression of syncytin 2 and its cognate receptor MFSD2A in human placenta. Biol Reprod. 2010;83(3):387–395. doi: 10.1095/biolreprod.110.083915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.