Learning Objectives
Upon completion of this activity, participants will be able to:
Distinguish the prevalence of intraoperative suprachoroidal hemorrhage (AISH) after cataract surgery.
Identify the most significant risk factor for AISH after cataract surgery.
Compare different techniques of anesthesia for cataract surgery in the context of their associated risk for AISH.
Evaluate other patient factors that can affect the risk for AISH after cataract surgery.
Accreditation Statements
In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and Springer Nature. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Credit hours
1.0

Release date: May 12, 2023
Expiration date: May 12, 2024
Post-test link: https://www.medscape.org/eye/posttest989929
EDITOR
Sobha Sivaprasad, MD, Editor, Eye
Journal CME author disclosure information
Charles P. Vega has disclosed the following relevant financial relationships: Consultant or advisor for Boehringer Ingelheim Pharmaceuticals, Inc.; GlaxoSmithKline; Johnson & Johnson Pharmaceutical Research & Development, L.L.C.
Subject terms: Epidemiology, Eye diseases
Abstract
Objective
To establish the incidence of acute intraoperative suprachoroidal haemorrhage (AISH) during cataract surgery and identify the risk factors for this complication.
Methods
Data from the Royal College of Ophthalmologists’ National Ophthalmology Database was analysed. During the 11-year study period, from 01/04/2010 to 31/03/2021, 709 083 operations performed on 498 170 patients from 65 centres were eligible for inclusion.
Results
AISH occurred in 0.03% (204/709 083, approximately 1 in 3 500) of eligible cataract operations performed during the study period. Posterior capsule rupture was the risk factor most strongly associated with AISH (OR: 17.6, 95% CI: 12.4–24.9, p < 0.001). Other ocular risk factors identified were raised intraocular pressure (IOP) preoperatively (OR: 3.7, 95% CI: 2.5–5.5, p < 0.001), glaucoma (OR: 1.7, 95% CI: 1.2–2.4, p = 0.004). Risk increased with age and patients aged over 90 years were at greatest risk (OR: 6.7, 95% CI: 3.5–12.8, p < 0.001). The addition of intracameral anaesthetic when performing surgery under topical anaesthetic appears to be protective (OR: 0.5, 95% CI: 0.3–0.8, p = 0.003), compared to topical anaesthetic alone. There was a 16-fold increase in the incidence of vision loss when AISH occurred.
Conclusions
The risk of AISH during modern cataract surgery is approximately 1 in 3 500 and is associated with a significant increase in the risk of vision loss should it occur. Posterior capsule rupture is the risk factor most strongly associated with AISH. Preoperative IOP control is a modifiable risk factor. The use of intracameral anaesthesia may reduce the risk of AISH.
Introduction
Acute intraoperative suprachoroidal haemorrhage (AISH) is a rare but sight-threatening complication of cataract surgery [1–3]. Acute hypotony during surgery can cause a ciliochoroidal effusion and subsequent rupture of stretched posterior ciliary arteries [4–7]. Multiple risk factors for AISH have been identified, including older age, cardiovascular disease, glaucoma, increased axial length and posterior capsule rupture [7, 8]. AISH is a cause of irreversible vision loss following cataract surgery: a large epidemiological study found that a final visual acuity of 6/12 or better was achieved in only 40% of patients who developed this complication [3].
Previous studies have reported an incidence of 0.03–0.06% for AISH occurring during cataract surgery using phacoemulsification [1, 9]. However, there have been substantial changes in surgical and anaesthetic techniques in the past two decades since these studies were published. The administration of oral anticoagulants to our patient population has also changed, with an increase in direct oral anticoagulant (DOAC) prescriptions and a corresponding decrease in warfarin use [10].
This study aims to establish an estimated incidence of AISH occurring during modern cataract surgery and identify risk factors for this complication, using data submitted to the Royal College of Ophthalmologists (RCOphth) National Ophthalmology Database (NOD).
Methods
Data source
The RCOphth NOD is open to centres performing both National Health Service (NHS)-funded and private cataract surgery in England, Northern Ireland, Scotland, Wales, and the Channel Islands, in traditional NHS centres, independent sector treatment centres (ISTC) and private providers (contributing centres are supplied as supplemental information). The data is recorded on electronic medical record systems (EMR) or in-house databases and submitted annually for cataract operations using phacoemulsification to treat patients aged 18 years or older, where the primary intention was cataract surgery and not combined ‘cataract + other’ surgery, unless the ‘other’ surgery formed part of the cataract operation (e.g. an operative manoeuvre to increase the size of the pupil). Further information on audit eligible cataract operations can be found on the audit website (www.nodaudit.org.uk). The data was recorded on either the Medisoft EMR system (Medisoft Ophthalmology, Medisoft Limited, Leeds, UK, www.medisoft.co.uk), the Open Eyes EMR system (www.openeyes.org.uk) or in-house databases compliant with the RCOphth minimum national cataract dataset [11].
Eligible cataract operations were performed between 01/04/2010 and 31/03/2021, which equate to 11 NHS years, where each NHS year runs from 1st April to 31st March. The operations were performed in RCOphth NOD contributing centres with at least 50 eligible operations that supplied data for anaesthesia (type, needle, medication and complication).
Excluded were operations: without details for anaesthesia and operations where general anaesthesia was used; without details for preoperative intraocular pressure (IOP); without details for axial length (AL) and axial length smaller than 18 mm; without information if an intraocular lens implant (IOL) was inserted; and data from centres with <2% of patients recorded as taking anticoagulant medication - due to concerns about the accuracy of medication records in these centres.
The grade of operating surgeon was categorised as consultant surgeons, career grade non-consultant surgeons (associate specialists, staff grades and trust doctors), more experienced trainee surgeons (fellows, registrars and specialty trainees years 3–7), and less experienced trainee surgeons (senior house officer, specialty trainee years 1–2 and foundation doctors years 1 and 2).
The anaesthetic technique was categorised as topical anaesthesia alone, combined topical and intracameral anaesthesia, sub-Tenon’s block and peribulbar/retrobulbar block. In cases of multiple category allocations, the more invasive/advanced technique was considered to have been used.
Anticoagulant medications of interest were aspirin only, antiplatelet agents (dypiridamole, clopidogrel, prasugrel, ticagrelor, ticlopidine), warfarin, direct oral anticoagulants (DOAC) (dabigatran, rivaroxaban, edoxaban, apixaban) and none of the above. For patients taking aspirin plus anticoagulants, warfarin or DOACs, the medication of use was assumed to be the non-aspirin medication.
Visual acuity loss was calculated as a difference between postoperative and preoperative visual acuity and defined according to the rules displayed in Table 1. Preoperative visual acuity (VA) was defined as the best recorded distance VA (corrected or uncorrected but not pinhole) that is closest to the date of surgery, including the day of surgery and within 6 months prior to surgery. Postoperative visual acuity was defined as the best measurement of distance VA (corrected, uncorrected or pinhole) between 8 days and 6 months after the surgery.
Table 1.
Definition of visual loss.
| Pre-operative VA | Postoperative VA loss |
|---|---|
| <1.00 LogMAR | A loss of ≥0.30 LogMAR |
| ≥1.00 to <CF | Postoperative VA of HM, NPL or PL |
| CF | Postoperative VA of NPL or PL |
| HM | Postoperative VA of NPL |
| PL | VA loss not considered |
| NPL | VA loss not considered |
CF count fingers, HM hand movements, PL perception of light, NPL no perception of light.
The lead clinician and Caldicott Guardian (responsible nominee for data protection) at each centre provided written approval for anonymised data extraction. Anonymised database analyses of this type do not require ethical permission due to being viewed as audit or service evaluation (see http://www.hra.nhs.uk/research-community/before-you-apply/determinewhether-your-study-is-research/). This study was conducted in accordance with the declaration of Helsinki, and the UK’s Data Protection Act.
Model fitting
Logistic regression was used to model the factors influencing the risk of developing suprachoroidal haemorrhage where all candidate variables were assessed by fitting a univariate model for each variable and performing a chi square test. Variables considered statistically significant from univariate testing at the 10% level were considered in the multivariate model.
The final model was fitted by forwards selection on the variables going from the ‘null’ model to the ‘best fitting’ model. Models were compared using a likelihood-ratio test and the Akaike Information Criterion.
Hypertension was not considered in the multivariate modelling due to poor quality of data provided, and pupil size was not used due to the subjective nature of the variable and poor quality of data provided.
Robust standard errors were calculated using clustering on individual surgeons and surgeon’s grade. All analyses were performed using STATA 17 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC). The Royal College of Ophthalmologists is the data controller for the NOD Cataract Audit, and as such, applications can be made to the RCOphth NOD for access to the database by emailing noaproject@rcophth.ac.uk.
Results
Sample and demographics
Within the study period, 709 083 operations performed on 498 170 patients from 65 centres were eligible for analysis, where 564 691 (79.6%) operations were performed in traditional NHS institutions and 144 392 (20.4%) operations in ISTCs.
The operations were performed by 2 680 surgeons, 727 of whom had data for more than one surgeon grade, where 1 160 consultant surgeons performed 503 779 (71.1%) operations, 286 career grade non-consultant surgeons performed 52 646 (7.4%) operations, 1 471 more experienced trainee surgeons performed 127 629 (18.0%) operations and 490 less experienced surgeons performed 25 029 (3.5%) operations.
During the study period, 222 568 (44.7%) patients had surgery to both eyes including 547 (0.1%) patients who underwent immediate sequential bilateral cataract surgery (ISBCS). For the ISBCS patients, 250 (45.7%) were male, 297 (54.3%) were female, the median age at surgery was 72.8 years (IQR: 63.6–79.2 years), 93 (17.0%) patients had diabetes mellitus and 135 (24.7%) patients were recorded as taking anticoagulant medication.
For non-ISBCS patients, 213 104 (42.8%) patients were male, 284 283 (57.1%) were female and the gender was not stated for 236 (<0.1%) patients. Excluding ISBCS patients, first treated eye surgery was performed on 390 357 (55.1%) eyes where the median age at surgery was 76.4 years (IQR: 69.4–82.3 years), 81 483 (20.9%) operations were in patients with diabetes mellitus and 120 775 (30.9%) operations in patients who were recorded as taking anticoagulant medication. Second treated eye surgery was performed on 317 632 (44.9%) eyes where the median age at surgery was 77.4 years (IQR: 70.7–82.9 years), 69 001 (21.7%) operations were in patients with diabetes mellitus and 104 063 (32.8%) operations were in patients who were recorded as taking anticoagulant medication.
Topical anaesthesia alone was used in 88 720 (12.5%) operations, combined topical and intracameral anaesthesia in 326 668 (46.1%) operations, sub-Tenon’s anaesthesia in 263 207 (37.1%) operations, and peribulbar/retrobulbar anaesthesia in 30 488 (4.3%).
Glaucoma was present as a comorbidity in 69 528 (9.8%) eyes. A brunescent/white/mature cataract was documented in 33 923 (4.8%) eyes. During surgery, 9 223 (1.3%) eyes experienced posterior capsular rupture (PCR) and 1 231 (0.2%) eyes had no IOL inserted.
Suprachoroidal haemorrhage
Acute intraoperative suprachoroidal haemorrhage occurred in 204 (0.03%) eyes, with no cases for ISBCS patients. Of the AISH cases, 74 (36.3%) were male and 130 (63.7%) were female. The median age was 80.6 years (IQR: 75.0–86.1 years) and 72 (35.3%) of eyes were recorded as being left aphakic.
Risk factor modelling
From the univariate analysis, the patient’s gender (p = 0.22), first or second eye (p = 0.45) and use of anticoagulant medication (p = 0.61) were found to have little statistically significant association with AISH. Variables which were statistically significant at the 10% level from univariate analysis were considered for multivariate modelling where the only covariate considered that did not make the final model was the presence of a brunescent/white/mature cataract. The covariates remaining in the final model were: the patient’s age and diabetic status at surgery, the grade of operating surgeon, type of anaesthesia used, axial length, preoperative IOP, the incision size, the occurrence of PCR, and the presence of glaucoma (Table 2).
Table 2.
Univariate analysis of potential risk factors for suprachoroidal haemorrhage for patient, ocular and surgical covariates.
| N (row %) | AISH | No AISH | Overall | p value |
|---|---|---|---|---|
| Number of operations | 204 (0.03) | 708,879 | 709,083 | |
| Patient Factors | ||||
| Gender | ||||
| Male | 74 (0.02) | 299,023 | 299,097 | |
| Female | 130 (0.03) | 409,584 | 409,714 | 0.222 |
| Not stated | 0 (0.00) | 272 | 272 | |
| Age at surgery in years | ||||
| <70 | 20 (0.01) | 177,207 | 177,227 | <0.001 |
| 70–74 | 31 (0.03) | 123,415 | 123,446 | |
| 75–79 | 44 (0.03) | 150,801 | 150,845 | |
| 80–84 | 41 (0.03) | 144,307 | 144,348 | |
| 85–89 | 43 (0.05) | 85,022 | 85,065 | |
| >=90 | 25 (0.09) | 28,127 | 28,152 | |
| Diabetic Status | ||||
| Not diabetic | 174 (0.03) | 558,239 | 558,413 | 0.022 |
| Has diabetes mellitus | 30 (0.02) | 150,640 | 150,670 | |
| Anticoagulant Use | ||||
| No | 131 (0.03) | 483,844 | 483,975 | 0.215 |
| Yes | 73 (0.03) | 225,035 | 225,108 | |
| Eye Factors | ||||
| Age | ||||
| IOP | ||||
| First/Second eyea | ||||
| First treated eye | 118 (0.03) | 391,333 | 391,451 | 0.449 |
| Second treated eye | 86 (0.03) | 317,546 | 317,632 | |
| Axial Length (mm) | ||||
| <21 (short) | 28 (0.05) | 59,174 | 59,202 | |
| 21–26 (normal) | 167 (0.03) | 615,001 | 615,168 | 0.021 |
| >26 (long) | 9 (0.03) | 34,704 | 34,713 | |
| Glaucoma | ||||
| No | 157 (0.02) | 639,398 | 639,555 | 0.001 |
| Yes | 47 (0.07) | 69,481 | 69,528 | |
| Brunescent/white/mature cataract | ||||
| No | 184 (0.03) | 674,976 | 675,160 | 0.001 |
| Yes | 20 (0.06) | 33,903 | 33,923 | |
| IOP | ||||
| <10 (low) | 4 (0.02) | 19,462 | 19,466 | |
| 10–21 (normal) | 160 (0.02) | 653,709 | 653,869 | <0.001 |
| >21 (high) | 40 (0.11) | 35,708 | 35,748 | |
| Operative Factors | ||||
| Surgeon’s grade | ||||
| Consultants | 132 (0.03) | 503,647 | 503,779 | <0.001 |
| Career grade non-consultants | 7 (0.01) | 52,639 | 52,646 | |
| Experienced trainees | 62 (0.05) | 127,567 | 127,629 | |
| Inexperienced trainees | 3 (0.01) | 25,026 | 25,029 | |
| PCR | ||||
| No | 159 (0.02) | 699,701 | 699,860 | <0.001 |
| Yes | 45 (0.49) | 9178 | 9223 | |
| Anaesthesia type | ||||
| Topical | 32 (0.04) | 88,688 | 88,720 | <0.001 |
| Intracameral | 58 (0.02) | 326,610 | 326,668 | |
| Sub-Tenon’s | 103 (0.04) | 263,104 | 263,207 | |
| Peribulbar/Retrobulbar | 11 (0.04) | 30,477 | 30,488 | |
| Incision size (mm) | ||||
| <22 mm | 10 (0.04) | 24,374 | 24,384 | |
| 2.2–2.75 mm | 117 (0.03) | 427,918 | 428,035 | <0.001 |
| 2.75–3.2 mm | 51 (0.02) | 221,537 | 221,588 | |
| >3.2 mm | 26 (0.07) | 35,050 | 35,076 | |
N = 709 083 operations performed in 65 centres by 2680 surgeons.
aAll ISBCS cases are included with first treated eyes.
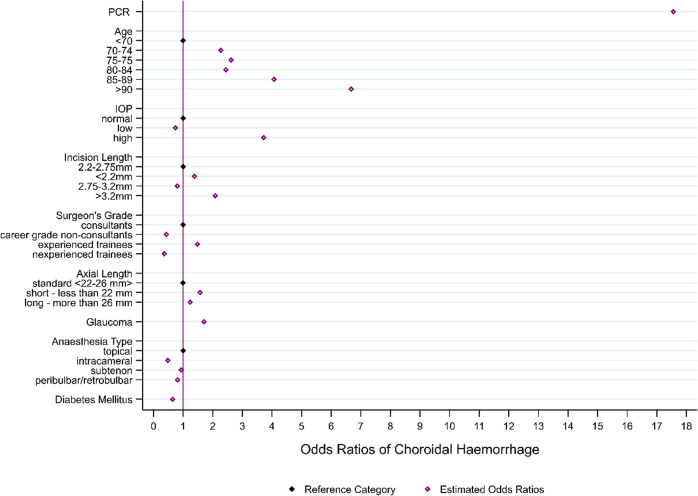
The most influential risk factors identified by the model (Fig. 1) were PCR (OR: 17.6; 95% CI: 12.4 to 24.9), age of 90 years and above (OR: 6.7; 95% CI: 3.5 to 12.8), age 85–89 years (OR: 4.1; 95% CI: 2.2 to 7.5) and high IOP (IOP > 21; OR: 3.7; 95% CI: 2.5 to 5.5). The other covariates increasing risk of AISH were: larger incision size, short axial length, age between 70 and 84, the surgeon’s grade (more experienced trainees only) and the presence of glaucoma. The use of intracameral anaesthesia (OR: 0.5; 95% CI: 0.3 to 0.8), and presence of diabetes mellitus (OR: 0.6; 95% CI: 0.4 to 0.9) were found to have a potential protective effect (Table 3; Fig. 2).
Fig. 1. Suprachoroidal haemorrhage risk factor model odds ratios.
N = 709 083 operations performed in 65 centres by 2680 surgeons.
Table 3.
Suprachoroidal haemorrhage risk factor model estimates.
| Risk factor estimates | Odds Ratio | Odds Ratio 95% CI | Coefficient | p value |
|---|---|---|---|---|
| PCR | 17.562 | (12.402, 24.869) | 2.866 | <0.001 |
| Age | ||||
| >70a | REF | |||
| 70–74 | 2.276 | (1.265, 4.097) | 0.823 | 0.006 |
| 75–79 | 2.616 | (1.456, 4.702) | 0.962 | 0.001 |
| 80–84 | 2.445 | (1.321, 4.527) | 0.894 | 0.004 |
| 85–89 | 4.069 | (2.203, 7.516) | 1.403 | <0.001 |
| >=90 | 6.675 | (3.469, 12.842) | 1.898 | <0.001 |
| IOP | ||||
| Normala | REF | |||
| Low | 0.740 | (0.245, 2.236) | −0.301 | 0.593 |
| High | 3.723 | (2.506, 5.532) | 1.315 | <0.001 |
| Incision size | ||||
| 2.2–2.75 mma | REF | |||
| <22 mm | 1.388 | (0.701, 2.748) | 0.328 | 0.347 |
| 2.75–3.2 mm | 0.804 | (0.565, 1.142) | −0.219 | 0.223 |
| >3.2 mm | 2.087 | (1.332, 3.269) | 0.736 | 0.001 |
| Axial Length | ||||
| 22–26 mma | REF | |||
| <22 mm | 1.572 | (1.061, 2.328) | 0.452 | 0.024 |
| >26 mm | 1.236 | (0.560, 2.729) | 0.212 | 0.599 |
| Surgeon’s grade | ||||
| Consultantsa | REF | |||
| Career grade non-consultants | 0.486 | (0.170, 1.115) | −0.831 | 0.083 |
| Experienced trainees | 0.935 | (1.060, 2.066) | 0.392 | 0.021 |
| Inexperienced trainees | 0.810 | (0.121, 1.082) | −1.016 | 0.069 |
| Glaucoma | 1.704 | (1.189, 2.440) | 0.533 | 0.004 |
| Anaesthesia type | ||||
| Topicala | REF | |||
| Intracameral | 0.486 | (0.301, 0.785) | −0.721 | 0.003 |
| Subtenon | 0.935 | (0.594, 1.470) | −0.068 | 0.770 |
| Peribulbar/Retrobulbar | 0.810 | (0.351, 1.871) | −0.211 | 0.622 |
| Diabetic Status | 0.643 | (0.441, 0.938) | −0.442 | 0.022 |
| Constant | <0.001 | (<0.001, <0.001) | −9.200 | <0.001 |
| N (row %) | AISH | No AISH | Overall | p value |
|---|---|---|---|---|
| Number of operations | 204 (0.03) | 708,879 | 709,083 | |
| Patient Factors | ||||
| Gender | ||||
| Male | 74 (0.02) | 299,023 | 299,097 | |
| Female | 130 (0.03) | 409,584 | 409,714 | 0.222 |
| Not stated | 0 (0.00) | 272 | 272 | |
| Age at surgery in years | ||||
| <70 | 20 (0.01) | 177,207 | 177,227 | <0.001 |
| 70–74 | 31 (0.03) | 123,415 | 123,446 | |
| 75–79 | 44 (0.03) | 150,801 | 150,845 | |
| 80–84 | 41 (0.03) | 144,307 | 144,348 | |
| 85–89 | 43 (0.05) | 85,022 | 85,065 | |
| >=90 | 25 (0.09) | 28,127 | 28,152 | |
| Diabetic Status | ||||
| Not diabetic | 174 (0.03) | 558,239 | 558,413 | 0.022 |
| Has diabetes mellitus | 30 (0.02) | 150,640 | 150,670 | |
| Anticoagulant Use | ||||
| No | 131 (0.03) | 483,844 | 483,975 | 0.215 |
| Yes | 73 (0.03) | 225,035 | 225,108 | |
| Eye Factors | ||||
| Age | ||||
| IOP | ||||
| First/Second eyea | ||||
| First treated eye | 118 (0.03) | 391,333 | 391,451 | 0.449 |
| Second treated eye | 86 (0.03) | 317,546 | 317,632 | |
| Axial Length (mm) | ||||
| <21 (short) | 28 (0.05) | 59,174 | 59,202 | |
| 21–26 (normal) | 167 (0.03) | 615,001 | 615,168 | 0.021 |
| >26 (long) | 9 (0.03) | 34,704 | 34,713 | |
| Glaucoma | ||||
| No | 157 (0.02) | 639,398 | 639,555 | 0.001 |
| Yes | 47 (0.07) | 69,481 | 69,528 | |
| Brunescent/white/mature cataract | ||||
| No | 184 (0.03) | 674,976 | 675,160 | 0.001 |
| Yes | 20 (0.06) | 33,903 | 33,923 | |
| IOP | ||||
| <10 (low) | 4 (0.02) | 19,462 | 19,466 | |
| 10–21 (normal) | 160 (0.02) | 653,709 | 653,869 | <0.001 |
| >21 (high) | 40 (0.11) | 35,708 | 35,748 | |
| Operative Factors | ||||
| Surgeon’s grade | ||||
| Consultants | 132 (0.03) | 503,647 | 503,779 | <0.001 |
| Career grade non-consultants | 7 (0.01) | 52,639 | 52,646 | |
| Experienced trainees | 62 (0.05) | 127,567 | 127,629 | |
| Inexperienced trainees | 3 (0.01) | 25,026 | 25,029 | |
| PCR | ||||
| No | 159 (0.02) | 699,701 | 699,860 | <0.001 |
| Yes | 45 (0.49) | 9178 | 9223 | |
| Anaesthesia type | ||||
| Topical | 32 (0.04) | 88,688 | 88,720 | <0.001 |
| Intracameral | 58 (0.02) | 326,610 | 326,668 | |
| Sub-Tenon’s | 103 (0.04) | 263,104 | 263,207 | |
| Peribulbar/Retrobulbar | 11 (0.04) | 30,477 | 30,488 | |
| Incision size (mm) | ||||
| <22 mm | 10 (0.04) | 24,374 | 24,384 | |
| 2.2–2.75 mm | 117 (0.03) | 427,918 | 428,035 | <0.001 |
| 2.75–3.2 mm | 51 (0.02) | 221,537 | 221,588 | |
| >3.2 mm | 26 (0.07) | 35,050 | 35,076 | |
N = 709,083 operations performed in 65 centres by 2680 surgeons.
aReference category.
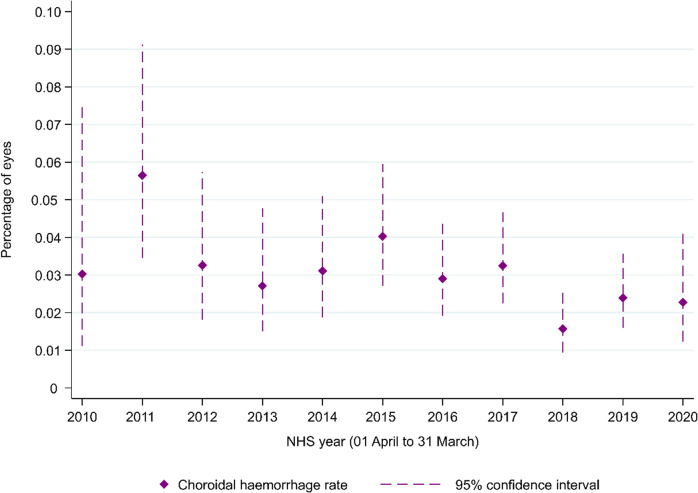
Fig. 2. Suprachoroidal haemorrhage rates for each NHS year between 2010 and 2020, where the NHS year runs from 1st April to 31st March by NHS year.
Operations in 2010 (N = 16,519); 2012 (N = 31,865); 2012 (N = 39,877); 2013 (N = 47,950); 2014 (N = 54,656); 2015 (N = 67,032); 2016 (N = 86,094); 2017 (N = 95,417); 2018 (N = 108,290); 2019 (108,600); 2020 (N = 52,783).
The area under the receiver operating curve for the final model was 79.8% (95% CI: 76.5 to 83.2).
Using the risk factor model, the probability of AISH is 0.010% for a non-diabetic patient aged less than 70 with normal preoperative IOP, standard axial length and no glaucoma, where the surgery is performed by a consultant surgeon with topical anaesthesia, standard incision size and where PCR is avoided. This risk lowers to 0.005% when intracameral anaesthesia is used and increases to 0.177% if topical anaesthesia is used and PCR occurs.
The probability of AISH in the highest risk eyes (non-diabetic patients above 90 years old with a diagnosis of glaucoma, high preoperative IOP and short axial length, where the surgery is performed by a more experienced trainee under topical anaesthesia and using a larger incision size) is 19.8% when PCR occurs during surgery. The risk substantially decreases to 1.4% when PCR is avoided.
Anaesthetic technique
Using the risk factor model, the median probability of AISH, dependent on the risk factors, is 0.2% (range 0.001–26.7%) for topical anaesthesia alone, 0.1% (range 0.001–15.1%) for combined topical and intracameral anaesthesia, 0.2% (range 0.001–25.4%) for sub-Tenon’s anaesthesia and 0.1% (range 0.001–22.8%) for peribulbar/retrobulbar anaesthesia operations.
Anticoagulant medications
31.7% (225 108) of operations were performed on eyes of patients recorded as taking one or more anticoagulants. There was no statistically significant difference in the risk of AISH (risk ratio 1.2 and 95% CI 0.9–1.6, p = 0.22) found between patients recorded as taking and those not recorded as taking anticoagulants.
Visual Acuity Loss
Both a preoperative and postoperative visual acuity measurement was recorded for 559 740 (78.9%) eyes. Of these, 157 (0.03%) experienced AISH. The risk of visual loss was approximately 16 times higher when AISH occurred than when was it was avoided (risk ratio = 15.8: 95% CI 9.9–25.2, p < 0.001).
Discussion
In this large national study, we found that suprachoroidal haemorrhage was most likely to occur for patients older than 85 years, in eyes with raised IOP preoperatively and that experienced posterior capsular rupture during surgery. Other influential risk factors were short axial length, the presence of glaucoma and the surgeon’s grade (more experienced specialty trainees). The use of intracameral anaesthesia and presence of diabetes mellitus demonstrated a potential protective effect.
Incidence and demographics
We report an incidence of 0.03% (204/709 083, approximately 1 in 3 500) for AISH occurring during cataract surgery using phacoemulsification. This is consistent with previously reported incidences of 0.03–0.06% for phacoemulsification surgery [1, 9], and considerably lower than that reported for extracapsular cataract extraction (ECCE) surgery (0.13%) [1].
Acute intraoperative suprachoroidal haemorrhage was reported more frequently in female patients, but this is reflective of the demographics of the study population (58% female vs 42% male) and sex was not found to be a risk factor for AISH on univariate analysis. The median age of 81 years is similar to that previously reported in a UK population of patients with AISH [7]. Increasing age was significantly associated with a greater risk of AISH, with the greatest risk in patients aged over 90 years. It is likely that senile arteriosclerosis and therefore increased fragility of the ciliary and choroidal vessels accounts for this [7, 12].
Systemic risk factors
Our study found there was no difference in the rate of AISH between patients recorded as taking and not recorded as taking anticoagulant medications. The use of oral anticoagulants can rarely be associated with spontaneous suprachoroidal haemorrhage [13, 14]. However, the mechanism of a spontaneous haemorrhage is different to that of AISH, and in some cases may be explained by a bleed from a choroidal neovascular membrane that extends because of iatrogenic coagulopathy, and then causes rupture of the stretched posterior ciliary arteries [13]. Anticoagulation would not be expected to precipitate the mechanical rupture of vessels, and our findings are in keeping with previous studies that did not demonstrate an increased risk of AISH in therapeutically anticoagulated patients [2, 7]. If an AISH occurs and the patient is on an anticoagulant medication, the bleeding may be expected to be more extensive. Patients on antiplatelets may also have systemic arteriopathy and therefore more fragile vessels that are more likely to rupture when stretched [15]. We attempted to ascertain whether or not patients who were recorded as taking anticoagulant medications and developed AISH had a worse visual outcome than those patients not recorded as taking anticoagulants, but the sample size was insufficient for statistical analysis.
Our study found there was a small reduction in the proportion of patients who were recorded as taking anticoagulants (31.7%), compared to a study of the Cataract National Dataset from 2001–2006 in which 34.7% of patients were recorded as taking one or more anticoagulant medication, with aspirin being the most commonly prescribed anticoagulant [16]. This is likely due to the fact that aspirin is no longer recommended for the primary prevention of cardiovascular disease, due to the risk of major bleeding [17, 18].
Patients with diabetes mellitus were found to be less likely to develop AISH than non-diabetic patients. Previous studies demonstrated that patients with generalised atherosclerosis and those taking cardiovascular medications were at increased risk of AISH, but did not find a significant association between AISH and a history of diabetes [7, 8]. The reduced risk for diabetic patients that is apparent in our study population may be related to diabetic choroidopathy. Choroidal vascularity is diminished with increasing severity of diabetic retinopathy, both at the posterior pole and peripheral choroid [19–21]. Compromised choroidal blood flow may reduce the likelihood of a ciliochoroidal effusion developing during intraoperative hypotony, thereby preventing mechanical stretch and rupture of the posterior ciliary arteries.
Ocular risk factors
A previous diagnosis of glaucoma and elevated IOP preoperatively were demonstrated to increase the risk of AISH. Glaucoma is an established risk factor for AISH during cataract surgery [7, 8, 12, 22]. Two mechanisms are proposed to increase the risk of SCH: sustained elevated intraocular pressure (IOP) causes vascular necrosis of the choroidal and posterior ciliary arteries [4, 22], and elevated preoperative IOP creates a relatively greater fall in pressure during intraoperative fluctuations [2, 22]. One case-control study found that raised IOP preoperatively was a risk factor, but a previous diagnosis of glaucoma was not [2]. This may suggest that well-controlled glaucoma represents less of a risk for AISH.
Our study found that extremes of axial length (<22 mm and >26 mm) were associated with an increased risk of AISH at the 10% significance level on univariate analysis. However, only short axial length was statistically significant on multivariate analysis. Increased axial length has previously been associated with AISH [2, 8]. A longer axial length is associated with more fragile blood vessels, a larger vitreous cavity and less support for the retina and choroid from the vitreous body [23]. Thin sclera is more elastic, meaning posterior ciliary arteries are more vulnerable to tangential shearing forces [12]. Previous studies have compared mean values for axial length between cases of AISH and controls, and found the mean axial length to be longer in eyes that developed AISH [2, 8]. However, in this study we divided axial length into categorical variables and found that short eyes (<22 mm) had a significantly increased risk of AISH compared to medium eyes (22–26 mm), whereas long eyes (>26 mm) did not. Shorter eyes are more prone to intraoperative complications than medium or long eyes [24], and prolonged or complicated surgery may in turn increase the likelihood of AISH. In this study, longer eyes had an odds ratio >1 for AISH, but this did not reach statistical significance.
Surgical risk factors
Posterior capsular rupture represents the greatest increase in risk of AISH. The odds ratio in our study (OR 17.6) was much greater than that reported in the previous study by Ling et al. (OR 3.9) [7]. Posterior capsule rupture with vitreous prolapse necessitates an anterior vitrectomy and often significantly prolongs surgery, which may increase the likelihood of intraoperative hypotony [7]. Increased manipulation of the anterior segment, such as extension of the corneal wound to insert a 3-piece intraocular lens, also increases the likelihood of intraocular pressure fluctuations. An intact posterior capsule may have a role in tamponading a suprachoroidal haemorrhage by preventing vitreous prolapse and thus limiting the space for an evolving haemorrhage to expand into [25]. An alternative explanation for this association would be the shallowing of the anterior chamber resultant from AISH leading to posterior capsule rupture, hence the direction of causality could be in either direction or both.
Our study found that the use of intracameral anaesthesia, compared to topical anaesthesia alone, appeared to have a protective effect, with a lower incidence of AISH in this group. Previous studies have failed to show any statistically significant difference in risk of AISH between topical, sub-Tenon’s and peribulbar blocks [1, 7]. However, it has been suggested that of the local anaesthetic techniques, peribulbar anaesthesia would be expected to increase the risk of AISH by increasing preoperative IOP [1, 26].
It is unclear why intracameral anaesthesia appears to reduce the likelihood of AISH. It may relate to patient comfort intraoperatively and the action of orbicularis oculi. Sub-Tenon’s and peribulbar blocks may anaesthetise the orbicularis oculi muscle to some extent, meaning that patients cannot exert as much pressure on the lid speculum by squeezing their eyelids intraoperatively. This is not the case with topical anaesthesia and so, in a patient who is uncooperative or perceives pain intraoperatively, there may be sudden egress of fluid form the anterior chamber due to external pressure exerted on the globe and therefore acute hypotony. A systematic review of studies comparing topical anaesthesia plus intracameral lidocaine to topical anaesthesia alone found that the addition of intracameral lidocaine reduced the likelihood of intraoperative pain [27]. It is therefore possible that intracameral anaesthesia reduces the likelihood of anterior chamber collapse by ensuring patients are more comfortable intraoperatively. This theory would be supported by the previous finding that the addition of adrenaline to the anaesthetic administered for a lid block is also protective from AISH, likely by prolonging the duration of lid akinesia [8]. A possible confounder would be the administration of intracameral phenylephrine along with intracameral lidocaine. The effect of intracameral phenylephrine on the choroidal circulation has not been studied, but topical phenylephrine has been shown to affect blood flow in the posterior segment [28]. In our study, data relating to the administration of intracameral phenylephrine was insufficient for statistical analysis of the association between this and AISH. Due to dataset limitations, the recording of intracameral lidocaine includes when it was given as a combination therapy of lidocaine, phenylephrine and tropicamide (Mydrane, Thea Pharmaceuticals Ltd) without the ability to differentiate from lidocaine alone.
There were no documented cases of AISH in patients undergoing ISBCS. However, the occurrence of clinically-apparent AISH in the first eye during planned ISBCS would be an absolute contraindication to proceeding with second eye surgery in the same sitting.
Our study found that more experienced trainees had an increased risk of AISH, compared to consultant surgeons, but less experienced trainees did not. This may be due to experienced trainees operating on more challenging cases than more junior trainees would, with an increased risk of other intraoperative complications or prolonged surgery. Career grade non-consultants had a lower risk of AISH, compared to consultants. The reason for this is unclear, but may again be related to case mix.
This study found that an incision size >3.2 mm was associated with the occurrence of AISH. However, an incision >3.2 mm likely indicates complicated surgery requiring a modified technique such as placement of a 3-piece sulcus IOL. For sub-3.2 mm wounds, there was no relationship between the size of incision and the risk of AISH. A stable anterior chamber should be maintained throughout routine small incision phacoemulsification surgery and this accounts for the lower rate of AISH when compared to ECCE surgery.
Outcomes
There is a high risk of significant vision loss postoperatively when AISH occurs during cataract surgery, with a final visual acuity of <6/12 reported in 33–60% of patients in previous studies [1–3]. Our study found that the risk of vision loss following cataract surgery was approximately 16 times higher when a suprachoroidal haemorrhage occurred.
Management and prevention
AISH can be managed conservatively, or by surgical drainage with or without pars plana vitrectomy [29, 30]. Treating elevated IOP preoperatively is a notable modifiable risk factor. It is also important to maintain a positive IOP throughout surgery and minimise pressure fluctuations [31].
Limitations
This study has several limitations. First, the data is heavily influenced by small event rates and a tiny outcome rate in a large sample. In this analysis, 44.7% patients had both eyes undergo cataract surgery which can introduce patient level correlation impacting on statistical comparisons. This is further complicated by potential ocular level correlation from certain ocular conditions that can develop as bilateral disease, and the possibility that age-related ocular conditions are more prevalent in second treated eyes.
It is possible that not all recorded first treated eye operations were the patient’s actual first eye surgery, as the patients could have their first eye surgery prior to a centre adopting an electronic data collection system, or performed in a different centre, and at present the RCOphth NOD cannot link patients’ data if collected at different centres.
It is not possible to account for all the sources of variation between centres, patients and possible correlations, consequently the interpretation of p-values require caution as they are likely to be too low, especially for some covariates with extremely low event rates.
Finally, the RCOphth NOD database does not have the same rigour as data validation in prospective clinical trials could have, such as information on patients’ medications owing to inconsistent medication data entry on the EMR. However, the feasibility of a clinical trial for suprachoroidal haemorrhage is challenging due to the need for a large sample size and it would prove to be a prohibitively costly endeavour to capture such rare incidents. Therefore, the RCOphth NOD database is a suitable alternative as it enables de-identified clinical data extraction directly from electronic health records, and provides a real-world insight to surgical practices across different sites in England, Wales and Guernsey.
In conclusion, AISH is a rare complication of modern cataract surgery in the UK, occurring at a rate of approximately 1 in every 3 500 cases, but is associated with a 16-fold increase in the likelihood of postoperative vision loss. This study is the first to demonstrate that the addition of intracameral anaesthetic to cases performed under topical anaesthesia appears to have a protective effect.
Summary
What was known before
Acute intraoperative suprachoroidal haemorrhage (AISH) is a rare but potentially blinding complication of cataract surgery.
Previously identified risk factors for AISH include: increasing age, raised preoperative IOP, glaucoma, posterior capsule rupture.
What this study adds
The incidence of AISH during modern cataract surgery in a UK population is approximately 1 in 3500.
There is a 16-fold increase in the risk of vision loss postoperatively if AISH occurs.
The addition of intracameral anaesthesia to topical anaesthesia may reduce the likelihood of AISH.
Posterior capsule rupture represents the greatest risk for development of AISH in our study population.
Supplementary information
Acknowledgements
It is with gratitude that we remember our friend and colleague Robert Johnston, who sadly died in September 2016. Without his inspirational vision, determination and career-long commitment to quality improvement in ophthalmology this work would not have been possible. We acknowledge the support of the hospitals that participated in this National Ophthalmology Database Audit study and thank our medical and non-medical colleagues for the considerable time and effort devoted to data collection.
Author contributions
All authors participated in initial discussions regarding study design and definitions. All authors reviewed initial drafts and approved final manuscript. SS and MHG-G prepared first draft (MHGG methods and results; SS intro and discussion). PD collaborated with MHGG for the statistical analysis and PD also is responsible for source database. JB oversaw manuscript preparation commenting on interim drafts.
Funding
The National Cataract Audit is currently funded through participation fees from centres as well as unrestricted financial contributions from Alcon and Bausch + Lomb.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02514-y.
References
- 1.Eriksson A, Koranyi G, Seregard S, Philipson B. Risk of acute suprachoroidal hemorrhage with phacoemulsification. J Cataract Refract Surg. 1998;24:793–800. doi: 10.1016/S0886-3350(98)80133-8. [DOI] [PubMed] [Google Scholar]
- 2.Beatty S, Lotery A, Kent D, O’Driscoll A, Kilmartin DJ, Wallace D, et al. Acute intraoperative suprachoroidal haemorrhage in ocular surgery. Eye. 1998;12:815–20. doi: 10.1038/eye.1998.210. [DOI] [PubMed] [Google Scholar]
- 3.Ling R, Cole M, James C, Kamalarajah S, Foot B, Shaw S. Suprachoroidal haemorrhage complicating cataract surgery in the UK: epidemiology, clinical features, management, and outcomes. Br J Ophthalmol. 2004;88:478–80. doi: 10.1136/bjo.2003.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manschot WA. The pathology of expulsive hemorrhage. Am J Ophthalmol. 1955;40:15–24. doi: 10.1016/0002-9394(55)92116-4. [DOI] [PubMed] [Google Scholar]
- 5.Wolter JR, Garfinkel RA. Ciliochoroidal effusion as precursor of suprachoroidal hemorrhage: a pathologic study. Ophthalmic Surg. 1988;19:344–9. [PubMed] [Google Scholar]
- 6.Reynolds MG, Haimovici R, Flynn HW, DiBernardo C, Byrne SF, Feuer W. Suprachoroidal hemorrhage. Clinical features and results of secondary surgical management. Ophthalmology. 1993;100:460–5. doi: 10.1016/S0161-6420(93)31621-0. [DOI] [PubMed] [Google Scholar]
- 7.Ling R, Kamalarajah S, Cole M, James C, Shaw S. Suprachoroidal haemorrhage complicating cataract surgery in the UK: a case control study of risk factors. Br J Ophthalmol. 2004;88:474–7. doi: 10.1136/bjo.2003.026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speaker MG, Guerriero PN, Met JA, Coad CT, Berger A, Marmor M. A case-control study of risk factors for intraoperative suprachoroidal expulsive hemorrhage. Ophthalmology. 1991;98:202–10. doi: 10.1016/S0161-6420(91)32316-9. [DOI] [PubMed] [Google Scholar]
- 9.Davison JA. Acute intraoperative suprachoroidal hemorrhage in capsular bag phacoemulsification. J Cataract Refract Surg. 1993;19:534–7. doi: 10.1016/S0886-3350(13)80618-9. [DOI] [PubMed] [Google Scholar]
- 10.Afzal S, Zaidi STR, Merchant HA, Babar Z-U-D, Hasan SS. Prescribing trends of oral anticoagulants in England over the last decade: a focus on new and old drugs and adverse events reporting. J Thromb Thrombolysis. 2021;52:646–53. doi: 10.1007/s11239-021-02416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical Data Sets. The Royal College of Ophthalmologists. https://www.rcophth.ac.uk/standards-and-guidance/audit-and-data/clinical-data-sets/. Accessed 5 July 2022.
- 12.Davison JA. Acute intraoperative suprachoroidal hemorrhage in extracapsular cataract surgery. J Cataract Refract Surg. 1986;12:606–22. doi: 10.1016/S0886-3350(86)80075-X. [DOI] [PubMed] [Google Scholar]
- 13.Knox FA, Johnston PB. Spontaneous suprachoroidal haemorrhage in a patient with age-related macular degeneration on excessive anticoagulation therapy. Eye. 2002;16:669–70. doi: 10.1038/sj.eye.6700109. [DOI] [PubMed] [Google Scholar]
- 14.Akkan Aydogmus FS, Serdar K, Kalayci D, Çelik A. Spontaneous Suprachoroidal Hemorrhage Associated with Iatrogenic Coagulopathy. Retinal Cases Brief Rep. 2019;13:174–5. doi: 10.1097/ICB.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 15.Chandra A, Xing W, Kadhim MR, Williamson TH. Suprachoroidal hemorrhage in pars plana vitrectomy: risk factors and outcomes over 10 years. Ophthalmology. 2014;121:311–7. doi: 10.1016/j.ophtha.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Benzimra JD, Johnston RL, Jaycock P, Galloway PH, Lambert G, Chung AKK, et al. The Cataract National Dataset electronic multicentre audit of 55,567 operations: antiplatelet and anticoagulant medications. Eye. 2009;23:10–6. doi: 10.1038/sj.eye.6703069. [DOI] [PubMed] [Google Scholar]
- 17.Antithrombotic Trialists’ (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raber I, McCarthy CP, Vaduganathan M, Bhatt DL, Wood DA, Cleland JGF, et al. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet. 2019;393:2155–67. doi: 10.1016/S0140-6736(19)30541-0. [DOI] [PubMed] [Google Scholar]
- 19.Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol. 1998;116:589–97. doi: 10.1001/archopht.116.5.589. [DOI] [PubMed] [Google Scholar]
- 20.Kim M, Choi SY, Park Y-H. Quantitative analysis of retinal and choroidal microvascular changes in patients with diabetes. Sci Rep. 2018;8:12146. doi: 10.1038/s41598-018-30699-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell JF, Zhou H, Shi Y, Shen M, Gregori G, Feuer WJ, et al. Longitudinal Analysis of Diabetic Choroidopathy in Proliferative Diabetic Retinopathy Treated with Panretinal Photocoagulation using Widefield Swept-Source Optical Coherence Tomography. Retina. 2022;42:417–25. doi: 10.1097/IAE.0000000000003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samuels B. Postoperative Nonexpulsive Subchoroidal Hemorrhage. Arch Ophthalmol. 1931;6:840–51. doi: 10.1001/archopht.1931.00820070871003. [DOI] [Google Scholar]
- 23.Mo B, Li S-F, Liu Y, Zhou J, Wang S-L, Shi X-Y. Suprachoroidal hemorrhage associated with pars plana vitrectomy. BMC Ophthalmol. 2021;21:295. doi: 10.1186/s12886-021-02062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day AC, Norridge CFE, Donachie PHJ, Barnes B, Sparrow JM. Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 8, cohort analysis of the relationship between intraoperative complications of cataract surgery and axial length. BMJ Open. 2022;12:e053560. doi: 10.1136/bmjopen-2021-053560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes JH, Payne JW, Green WR. Clinicopathologic study of eyes obtained postmortem from a patient 6 and 2 years after operative choroidal hemorrhage. Ophthalmic Surg. 1987;18:667–71. [PubMed] [Google Scholar]
- 26.Stevens J, Giubilei M, Lanigan L. Hykin P. Sub-Tenon, Retrobulbar and Peribulbar Local Anaesthesia: The Effect Upon Intraocular Pressure. Eur J Implant Refractive Surg. 1993;5:25–8. doi: 10.1016/S0955-3681(13)80057-3. [DOI] [Google Scholar]
- 27.Minakaran N, Ezra DG, Allan BD. Topical anaesthesia plus intracameral lidocaine versus topical anaesthesia alone for phacoemulsification cataract surgery in adults. Cochrane Database Syst Rev. 2020;7:CD005276. doi: 10.1002/14651858.CD005276.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takayama J, Mayama C, Mishima A, Nagahara M, Tomidokoro A, Araie M. Topical phenylephrine decreases blood velocity in the optic nerve head and increases resistive index in the retinal arteries. Eye. 2009;23:827–34. doi: 10.1038/eye.2008.142. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Ionides A. Does acute suprachoroidal haemorrhage during phacoemulsification cataract surgery need surgical treatment? Graefes Arch Clin Exp Ophthalmol. 2022;260:3395–6. doi: 10.1007/s00417-022-05689-4. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi A, Jalil A, Sousa DC, Patton N, Dhawahir-Scala F, Charles SJ, et al. Outcomes of suprachoroidal haemorrhage drainage with and without vitrectomy: a 10-year study. Eye. 2021;35:1879–85. doi: 10.1038/s41433-020-01170-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blumenthal M, Grinbaum A, Assia EI. Preventing expulsive hemorrhage using an anterior chamber maintainer to eliminate hypotony. J Cataract Refract Surg. 1997;23:476–9. doi: 10.1016/S0886-3350(97)80202-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.