Main text
Gene therapy of hematopoietic stem cells (HSCs) has been successfully applied to ameliorate monogenic blood disorders, such as severe combined immunodeficiencies and β-hemoglobinopathies. However, the treatment is resource intensive and limited to patients with access to specialized facilities. The development of in vivo HSC gene therapy could help enable wider distribution, including to the Global South, where diseases such as sickle cell disease are especially prevalent. Previously, Andre Lieber and colleagues developed in vivo HSC gene therapy protocols using helper-dependent adenoviral vectors and demonstrated proof-of-concept in mice and non-human primates.1,2,3 Their approach relies on the mobilization of HSCs from the bone marrow niche followed by the intravenous administration of the adenoviral vector, which includes a transposase to stably integrate the therapeutic transgene into the host genome, and in vivo selection of transduced cells using low-dose chemotherapy.4 By in vivo delivery, these protocols circumvent the need for HSC harvest, ex vivo HSC cultivation, and myeloablative conditioning, which may greatly reduce the toxicity and complexity of the procedure.
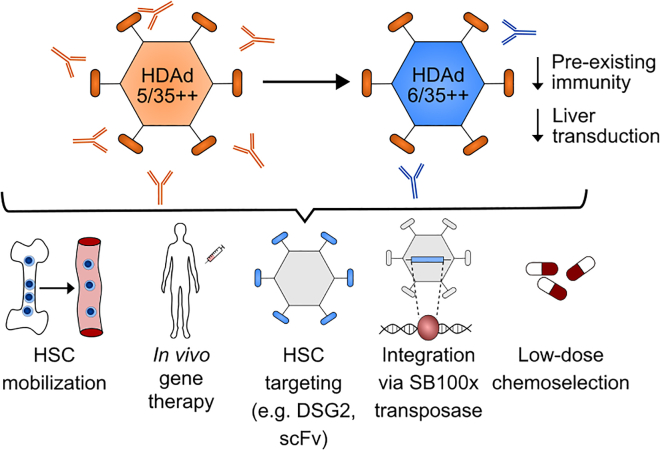
Previously, this research group had focused on the HDAd5/35++ modified helper-dependent adenoviral vector system. This vector platform is based on species C Ad5 serotype and has been modified with mutated Ad35 fiber knobs to increase affinity to CD46 entry receptor, a target antigen that is highly expressed on HSCs. In the present study (see Figure 1), Wang et al. switched to the species C Ad6 serotype, which has a similar genome structure compared with Ad5 but is associated with reduced prevalence of neutralizing antibodies in humans.5 The authors demonstrate that Ad6- and Ad5-based vectors transduce human and rhesus CD34+ hematopoietic stem and progenitor cells (HSPCs) at similar rates in vitro (on average 25%). The novel HDAd6/35++ vector was tested in short-term and long-term in vivo gene therapy experiments using a hCD46-transgenic mouse model. In both settings, HDAd6/35++ performed comparably or slightly better than the original HDAd5/35++ vector. Wang et al. used humanized NBSGW mice as an additional model, demonstrating that the HDAd6/35++ novel vector platform is capable of transducing in vivo human HSCs and not only hCD46-transgenic murine HSCs. Again both vector platforms performed comparably (8% transduced CD34+ cells in the bone marrow in a short-term experiment). Overall, the transduction rates were higher in CD34+ HSPCs compared with lineage-committed populations, which the authors attributed to the higher density of hCD46 on HSPCs. Finally, the vectors were tested in Ad5 pre-immunized mice, which mimic pre-existing immunity against HDAd5/35++. In this context, the novel HDAd6/35++ outperformed the HDAd5/35++ vector, confirming that neutralizing antibodies against serotype Ad5 are not cross-reactive. This lack of cross-reactivity could encourage future experiments to test if sequential dosing with different serotypes might improve HSC gene transfer.
Figure 1.
Swapping the serotype of HDAd5/35++ adenoviral vectors to HDAd6/35++ overcomes the limitation of pre-existing immunity and reduces off-target transduction of hepatocytes
Upon mobilization of HSCs from the bone marrow to the peripheral blood, this novel vector platform is suitable to transduce HSCs for in vivo gene therapy. HSC specificity can be modulated by introduction of specific targeting moieties on the viral particle. Using the Sleeping Beauty SB100x transposase, the adenoviral vector genome may be stably integrated into the host genome of transduced cells, which can be subsequently enriched to a therapeutic level using low-dose chemotherapy selection.
Besides better evading pre-existing immunity, the novel HDAd6/35++ vector also showed less liver off-target tissue transduction compared with HDAd5/35++ (liver vector copy number of 0.5 compared with 4, respectively).5 The reduction in off-target tissue transduction could be important to improve the safety and efficacy of the gene therapy. Moreover, by avoiding sequestration of viral vectors by off-target tissues, viral vector doses could potentially be decreased to achieve requisite transduction levels in HSCs, which could help reduce the overall cost of the therapy.
The most pressing challenge in the in vivo HSC gene therapy field is to achieve higher HSC-specific transduction to create an efficient and cost-effective gene therapy product. Unfortunately, there is not a single, unique antigen for HSCs. CD46 as well as other antigens such as CD117 and CD90 are highly expressed on HSCs but also on other hematopoietic and non-hematopoietic cell types, which ultimately results in potential off-target cell transduction and sequestration of viral particles. In this vein, recent work from the Lieber lab has explored desmoglein 2 as an alternative receptor for in vivo gene therapy of NHP CD34+ cells, showcasing the flexibility of the adenoviral vector system for different HSC target receptors.6 Other efforts have investigated the co-expression of multiple targeting moieties such as through scFv domains on enveloped particles or protein switches to achieve cell type-specific targeting.7,8
An area of concern regarding adenoviral vectors is their immunogenicity, which may necessitate immunosuppressive treatment. Relatively high MOIs and vector doses may also be required to achieve cell transduction (2,000 viral particles/cell in vitro or doses of 1012 viral particles/kg body weight). Similar to retroviral vectors, there is a small chance of insertional mutagenesis associated with transposase-mediated semirandom integration, which might only be avoided by targeted integration or genome editing. Also the requirement for in vivo selection with MGMT-P140K overexpression and low-dose chemotherapy (O6BG/BCNU) to achieve high gene marking rates raises questions about possible long-term adverse events due to chemotherapy. Notwithstanding these remaining challenges, the current study5 represents a forward step toward the holy grail of in vivo gene therapy that could be distributed worldwide.
Acknowledgments
Declaration of interests
The authors declare no competing interests.
References
- 1.Wang H., Georgakopoulou A., Psatha N., Li C., Capsali C., Samal H.B., Anagnostopoulos A., Ehrhardt A., Izsvák Z., Papayannopoulou T., et al. In vivo hematopoietic stem cell gene therapy ameliorates murine thalassemia intermedia. J. Clin. Invest. 2019;129:598–615. doi: 10.1172/JCI122836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C., Wang H., Georgakopoulou A., Gil S., Yannaki E., Lieber A. In vivo HSC gene therapy using a Bi-modular HDAd5/35++ vector cures sickle cell disease in a mouse model. Mol. Ther. 2021;29:822–837. doi: 10.1016/j.ymthe.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C., Wang H., Gil S., Germond A., Fountain C., Baldessari A., Kim J., Liu Z., Georgakopoulou A., Radtke S., et al. Safe and efficient in vivo hematopoietic stem cell transduction in nonhuman primates using HDAd5/35++ vectors. Mol. Ther. Methods Clin. Dev. 2022;24:127–141. doi: 10.1016/j.omtm.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H., Richter M., Psatha N., Li C., Kim J., Liu J., Ehrhardt A., Nilsson S.K., Cao B., Palmer D., et al. A combined in vivo HSC transduction/selection approach results in efficient and stable gene expression in peripheral blood cells in mice. Mol. Ther. Methods Clin. Dev. 2018;8:52–64. doi: 10.1016/j.omtm.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H., Georgakopoulou A., Zhang W., Kim J., Gil S., Ehrhardt A., Lieber A. HDAd6/35++ - a new helper-dependent adenovirus vector platform for in vivo transduction of hematopoietic stem cells. Molecular Therapy - Methods & Clinical Development. 2023;29:213–226. doi: 10.1016/j.omtm.2023.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H., Germond A., Li C., Gil S., Kim J., Kiem H.P., Lieber A. In vivo HSC transduction in rhesus macaques with an HDAd5/3+ vector targeting desmoglein 2 and transiently overexpressing cxcr4. Blood Adv. 2022;6:4360–4372. doi: 10.1182/bloodadvances.2022007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton J.R., Chen E., Perez B.S., Sandoval Espinoza C.R., Kang M.H., Trinidad M., Doudna J.A. Programmable enveloped delivery vehicles for human genome engineering in vivo. bioRxiv. 2023 doi: 10.1038/s41587-023-02085-z. https://www.biorxiv.org/content/biorxiv/early/2023/04/02/2022.08.24.505004 Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lajoie M.J., Boyken S.E., Salter A.I., Bruffey J., Rajan A., Langan R.A., Olshefsky A., Muhunthan V., Bick M.J., Gewe M., et al. Designed protein logic to target cells with precise combinations of surface antigens. Science. 2020;369:1637–1643. doi: 10.1126/science.aba6527. [DOI] [PMC free article] [PubMed] [Google Scholar]