Abstract
Use of the American Society of Anesthesiologists (ASA) physical status classification is important for periprocedural risk stratification. However, the collective effect after adjustment for the Society for Vascular Surgery (SVS) medical comorbidity grading system on long-term all-cause mortality, complications, and discharge disposition is unknown. We examined these associations in patients after thoracic endograft placement. Data from three thoracic endovascular aortic repair (TEVAR) trials through 5 years of follow-up were included. Patients with acute complicated type B dissection (n = 50), traumatic transection (n = 101), or descending thoracic aneurysm (n = 66) were analyzed. The patients were stratified into three groups according to the ASA class: I-II, III, and IV. Multivariable proportional hazards regression models were used to examine the effect of ASA class on 5-year mortality, complications, and rehospitalizations after adjustment for SVS risk score and potential confounders. The largest proportion of patients treated by TEVAR across the ASA groups (n = 217) was ASA IV (n = 97; 44.7%; P < .001), followed by ASA III (n = 83; 38.2%) and ASA I-II (n = 37; 17.1%). Among the ASA groups, the ASA I-II patients were, on average, 6 years younger than those with ASA III and 3 years older than those with ASA IV (ASA I-II: age, 54.3 ± 22.0 years; ASA III: age, 60.0 ± 19.7 years; ASA IV: age, 51.0 ± 18.4 years; P = .009). Multivariable adjusted 5-year outcome models showed that ASA class IV, independent of the SVS score, conferred an increased risk of mortality (hazard ratio [HR], 3.83; 95% confidence interval [CI], 1.19-12.25; P = .0239) and complications (HR, 4.53; 95% CI, 1.69-12.13; P = .0027) but not rehospitalization (HR, 1.84; 95% CI, 0.93-3.68; P = .0817) compared with ASA class I-II. Procedural ASA class is associated with long-term outcomes among post-TEVAR patients, independent of the SVS score. The ASA class and SVS score remain important to patient counseling and postoperative outcomes beyond the index operation.
Keywords: Complications, Discharge, Mortality, Rehospitalization, Thoracic endovascular repair
The American Society of Anesthesiologists (ASA) physical status classification system is used to stratify patients by their preoperative physiologic state and can inform the potential degree of complexity and periprocedural risk.1, 2, 3, 4 Knowledge of the ASA class can help anticipate and provide an early signal for perioperative needs such as blood transfusion, critical care admission, the need for additional testing, frailty, and level of deconditioning––all of which collectively predict for periprocedural risk.3,5 ASA class has contributed to outcomes at 30 and 90 days.6,7 In endovascular repair of abdominal aortic aneurysms, the ASA classification is less predictive but, when combined with the psoas muscle area, could be useful,7 although this has not been independently and unequivocally borne out.2,8,9
Several scoring systems have been used in both open vascular and abdominal endovascular surgery patients to aid in decision-making. These scoring systems include the Society for Vascular Surgery (SVS) medical comorbidity grading system, the APACHE (acute physiology and chronic health evaluation) score, and V-POSSUM (vascular physiologic and operative severity score for the numeration of mortality and morbidity).10, 11, 12 Currently, no effective scoring system is available to predict the mid- or long-term clinical outcomes after endovascular treatment of thoracic aortic disease (thoracic endovascular aortic repair [TEVAR]).
We postulated that for TEVAR performed for acute and chronic pathology, the ASA classification, combined with the SVS grading score, could be an important predictor of long-term mortality, complications, and rehospitalization.
Methods
We analyzed previously collected data approved by the Food and Drug Administration and the institutional review board at each participating institution in the clinical trials. All the patients had provided written informed consent before their procedures. The ASA class was assigned by each site's procedural anesthesiologist. ASA class I indicates a normal, healthy patient; ASA class II, a patient with mild systemic disease; ASA class III, a patient with severe systemic disease and substantive functional limitations; and ASA class IV, a patient with severe systemic disease that is a constant threat to life. For the purposes of the present study, ASA classes I and II, which describe a similar clinical substrate, were combined into one group. The SVS/American Association for Vascular Surgery medical comorbidity grading system scores major (ie, cardiac, pulmonary, renal) and minor (ie, age, hypertension) risk factors individually from 0 to 3, with specified weights applied to each factor's score. The sum of the scores is divided by 10 to restore the 0- to 3-point scale.13 Patients from three separate multicenter investigational device exemption (IDE) trials using the cTAG (conformable TAG thoracic endoprosthesis; W.L. Gore & Associates) were stratified into three groups according to their ASA class assigned at the index procedure. The three IDE trials treated acute type B dissection, traumatic transection, and thoracic aneurysm and enrolled patients between October 2009 and November 2013. The full study design, inclusion and exclusion criteria, treatment, and outcomes data have been previously described.14, 15, 16 All trials were registered at the ClinicalTrials.gov website (trial identifiers, NCT00908388 for acute type B dissection, NCT00917852 for traumatic transection, and NCT00874250 for thoracic aneurysm; available at: clinicaltrials.gov). Data from a clinically established and commercially available device from these closed clinical trials provided the best opportunity to examine the long-term outcomes.
Data were entered into an electronic database by the enrolling site and an independent core laboratory (AortaCore Aortic Imaging Laboratory). The outcomes of interest were all-cause mortality, complications (ie, combined reinterventions, stroke, paraplegia or paraparesis, type of endoleak), and rehospitalization. Although the ASA class is thought to be associated with short-term outcomes, we had limited 30-day events and could not confidently estimate these effects. Endoleaks were reported by the core laboratory and censored at the last contrast-enhanced computed tomography follow-up. All other outcomes were reported by the trial site and censored at the last follow-up.
Statistical analysis
For the separate study ASA groups, analysis of variance was used to compare the mean ± standard deviation for continuous variables and the χ2 or Fisher exact test to investigate the association between categorical variables. Kaplan-Meier estimates with 95% confidence intervals (CIs) were computed for 30 days, 90 days, 180 days, and annually through 5 years after the procedure. The log-rank test was used to determine significant differences in survival and incidence curves. The independent association of ASA status on the 5-year outcomes of mortality, complications, and rehospitalization was modeled using multivariable proportional hazards regression. Multivariable model covariates included aortic-treated cohort, age, gender, race, procedure time, blood loss, left subclavian coverage, and total SVS risk score. The SVS risk score was not applicable for the traumatic dissection cohort; therefore, a missing level dummy variable was used for that cohort. Covariates were determined a priori using clinical judgment and by identifying unbalanced populations across the ASA status groups in descriptive tables. The number of covariates that could be included in the models was restricted by the number of end point events being modeled. Interactions of ASA status and study pathology cohort were tested for all 5-year outcomes to ensure the results could be pooled across the three cohorts. Missing data were minimal (one patient had a missing blood loss value) and were not included in the multivariable models. All statistical analyses were performed using by SAS software, version 9.4 (SAS Institute).
Results
A total of 217 patients from three separate multicenter IDE trials using the cTAG thoracic endoprosthesis (W.L. Gore & Associates) were included and stratified into acute type B dissection (n = 50), traumatic dissection (n = 101), and thoracic aneurysm (n = 66) cohorts.
The largest proportion of patients treated by TEVAR across the ASA groups was ASA IV (n = 97; 44.7%; P < .001), followed by ASA III (n = 83; 38.2%) and ASA I-II (n = 37; 17.1%). ASA IV was also the largest proportion of patients treated for dissection and trauma (data not shown). The 37 patients with ASA I-II were 6 years younger than those with ASA III (n = 83) and 3 years older than those with ASA IV (n = 97; ASA I-II: age, 54.3 ± 22.0 years; ASA III: age, 60.0 ± 19.7 years; ASA IV: age, 51.0 ± 18.4 years; P = .009; Table I). White men were the most frequently treated patients (Table I). Polytrauma with an injury severity score >17 was seen in 88.5% of the ASA IV cohort (Table I). Anatomic characteristics such as aneurysm diameter and length and neck diameter and length were radiographically similar between the sites and core laboratory measurements (Table I). The procedural duration was similar across all ASA groups (P = .664; Table II). Most patients underwent a cutdown of the femoral artery under general anesthesia (Table II). Although complications were highest in the ASA IV group compared with the lower ASA groups (Table III), overall rehospitalization was more frequent in the ASA III and ASA IV groups (Table III). The SVS grading score was similar across all three groups (Table IV). All-cause 30-day mortality was low at 4.6%, and no difference was found in the mortality rate across the groups at 30 and 90 days (Table IV). Reintervention at 5 years was higher in the ASA IV group (P = .082) and the incidence of endoleaks of any type was higher in the ASA III group (P = .011; Table III).
Table I.
Patient characteristics stratified by American Society of Anesthesiologists (ASA) classificationa
| Characteristic | Total (n = 217) | ASA class |
P value | ||
|---|---|---|---|---|---|
| I-II (n = 37) | III (n = 83) | IV (n = 97) | |||
| Patient age, years | 55.0 ± 19.9 | 54.3 ± 22.0 | 60.0 ± 19.7 | 51.0 ± 18.4 | .009 |
| Female gender | 66 (30.4) | 10 (27.0) | 29 (34.9) | 27 (27.8) | .520 |
| Race | .811 | ||||
| White | 160 (73.7) | 28 (75.7) | 63 (75.9) | 69 (71.1) | |
| Black | 40 (18.4) | 5 (13.5) | 16 (19.3) | 19 (19.6) | |
| Asian/Oriental | 5 (2.3) | 1 (2.7) | 1 (1.2) | 3 (3.1) | |
| Native American/Alaska Native | 2 (0.9) | 0 (0.0) | 0 (0.0) | 2 (2.1) | |
| Native Hawaiian/other Pacific Islander | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (1.0) | |
| Other | 9 (4.1) | 3 (8.1) | 3 (3.6) | 3 (3.1) | |
| Injury severity score | 31.5 ± 14.5 | 27.4 ± 13.6 | 29.4 ± 15.4 | 33.4 ± 14.3 | .234 |
| Polytrauma | .314 | ||||
| Yes (ISS >17) | 85 (84.2) | 13 (76.5) | 18 (78.3) | 54 (88.5) | |
| No (ISS ≤17) | 16 (15.8) | 4 (23.5) | 5 (21.7) | 7 (11.5) | |
| NA | 116 | 20 | 60 | 36 | |
| Maximum diameter measured at enrolling sites, mm | |||||
| Aneurysm/lesion | 40.0 ± 16.8 (n = 50) | 42.4 ± 17.5 (n = 4) | 45.8 ± 17.4 (n = 20) | 33.6 ± 13.6 (n = 26) | <.001 |
| True lumen | 24.5 ± 8.9 (n = 167) | 19.5 ± 8.3 (n = 33) | 23.1 ± 10.6 (n = 63) | 26.3 ± 7.4 (n = 71) | .246 |
| False lumen | 28.0 ± 8.5 (n = 167) | 25.5 ± 5.3 (n = 33) | 26.9 ± 8.2 (n = 63) | 29.2 ± 9.2 (n = 71) | .539 |
| Overall transverse | 40.2 ± 8.1 (n = 167) | 37.7 ± 5.4 (n = 33) | 40.4 ± 10.1 (n = 63) | 40.4 ± 6.8 (n = 71) | .824 |
| Length measured at enrolling sites, mm | |||||
| Proximal neck (aneurysm or lesion; LCC) | 5.9 ± 8.3 (n = 3) | 7.9 ± 17.4 (n = 1) | 6.3 ± 4.8 (n = 0) | 4.8 ± 4.4 (n = 2) | .131 |
| Distal neck | 15.1 ± 7.4 (n = 1) | 13.1 ± 6.0 (n = 0) | 14.0 ± 8.6 (n = 0) | 16.8 ± 6.5 (n = 1) | .007 |
| Aneurysm/lesion | 5.6 ± 5.7 (n = 50) | 5.6 ± 5.5 (n = 4) | 7.2 ± 6.4 (n = 20) | 4.1 ± 4.7 (n = 26) | .007 |
| Dissection | 35.3 ± 15.4 (n = 170) | 34.7 ± 18.7 (n = 33) | 35.1 ± 17.8 (n = 63) | 35.6 ± 13.3 (n = 74) | .992 |
| Maximum diameter measured at core laboratory (axial), mm | |||||
| Aneurysm/lesion | 43.6 ± 17.7 (n = 50) | 43.7 ± 17.4 (n = 4) | 51.0 ± 18.6 (n = 20) | 36.9 ± 14.2 26 | < .001 |
| Overall transverse | 44.5 ± 10.5 (n = 168) | 37.4 ± 5.5 (n = 33) | 44.2 ± 12.0 (n = 63) | 45.9 ± 9.6 72 | .327 |
| True lumen | 20.7 ± 7.3 (n = 168) | 22.4 ± 2.3 (n = 33) | 20.2 ± 9.1 (n = 63) | 20.7 ± 6.3 72 | .867 |
| False lumen | 24.5 ± 6.7 (n = 168) | 17.3 ± 8.0 (n = 33) | 25.4 ± 7.7 (n = 63) | 24.9 ± 5.1 72 | .075 |
| Maximum false lumen area, mm2 (core laboratory) | |||||
| Dissected DTA | 904.2 ± 387.1 (n = 168) | 620.7 ± 309.4 (n = 33) | 804.0 ± 262.8 (n = 63) | 1029.7 ± 441.4 72 | .044 |
| Dissected aorta | 904.2 ± 387.1 (n = 168) | 620.7 ± 309.4 (n = 33) | 804.0 ± 262.8 (n = 63) | 1029.7 ± 441.4 72 | .044 |
DTA, Descending thoracic aneurysm; ISS, injury severity score; LCC, left common carotid artery; NA, not applicable.
Data presented as number (%) or mean ± standard deviation, with number of missing values in parentheses.
Continuous variables compared using one-way analysis of variance and categorical variables using the χ2 or Fisher exact test.
Table II.
Operative characteristics stratified by American Society of Anesthesiologists (ASA) classificationa
| Operative characteristic | Total (n = 217) | ASA class |
P value | ||
|---|---|---|---|---|---|
| I-II (n = 37) | III (n = 83) | IV (n = 97) | |||
| Procedure duration, minutes | 127.3 ± 70.4 | 121.6 ± 74.4 | 124.3 ± 69.2 | 132.0 ± 70.3 | .664 |
| EBL, mL | 196.6 ± 258.0 | 142.8 ± 113.6 | 205.0 ± 321.1 | 210.0 ± 235.3 | .378 |
| Intubation | 188 (86.6) | 27 (73.0) | 71 (85.5) | 90 (92.8) | .013 |
| LSA procedure | .412 | ||||
| None | 186 (85.7) | 32 (86.5) | 71 (85.5) | 83 (85.6) | |
| Transposed | 7 (3.2) | 2 (5.4) | 4 (4.8) | 1 (1.0) | |
| Bypassed | 24 (11.1) | 3 (8.1) | 8 (9.6) | 13 (13.4) | |
| Access method | .215 | ||||
| Percutaneous | 45 (20.7) | 13 (35.1) | 15 (18.1) | 17 (17.5) | |
| Cutdown | 156 (71.9) | 23 (62.2) | 61 (73.5) | 72 (74.2) | |
| Cutdown and conduit | 16 (7.4) | 1 (2.7) | 7 (8.4) | 8 (8.2) | |
| Access site | .855 | ||||
| Femoral artery | 197 (90.8) | 35 (94.6) | 73 (88.0) | 89 (91.8) | |
| Iliac artery | 18 (8.3) | 2 (5.4) | 9 (10.8) | 7 (7.2) | |
| Infrarenal aorta | 2 (0.9) | 0 (0.0) | 1 (1.2) | 1 (1.0) | |
| Anesthesia | .220 | ||||
| General | 212 (97.7) | 35 (94.6) | 82 (98.8) | 95 (97.9) | |
| Regional | 2 (0.9) | 1 (2.7) | 1 (1.2) | 0 (0.0) | |
| Local | 3 (1.4) | 1 (2.7) | 0 (0.0) | 2 (2.1) | |
| Adjunctive techniques to prevent paraplegia | .080 | ||||
| CSF drainage | 36 (55.4) | 12 (85.7) | 17 (50.0) | 7 (41.2) | |
| Induced hypertension | 9 (13.8) | 1 (7.1) | 4 (11.8) | 4 (23.5) | |
| Other | 20 (30.8) | 1 (7.1) | 13 (38.2) | 6 (35.3) | |
| Missing | 152 | 23 | 49 | 80 | |
CSF, Cerebrospinal fluid; EBL, estimated blood loss; LSA, left subclavian artery.
Data presented as mean ± standard deviation or number (%).
Continuous variables compared using one-way analysis of variance and categorical variables using the χ2 or Fisher exact test.
Table III.
Kaplan Meier estimates among patients stratified by American Society of Anesthesiologists (ASA) classificationa
| Kaplan-Meier estimates | Total (n = 217) | ASA class |
P value | ||
|---|---|---|---|---|---|
| I-II (n = 37) | III (n = 83) | IV (n = 97) | |||
| All-cause mortality | .2224 | ||||
| 30 Days | 10 (4.6) | 2 (5.5) | 2 (2.4) | 6 (6.2) | |
| 90 Days | 14 (6.5) | 2 (5.5) | 4 (4.8) | 8 (8.4) | |
| 5 Years | 46 (24.8) | 4 (11.8) | 20 (27.1) | 22 (27.3) | |
| Complication (any) | .0972 | ||||
| 30 Days | 21 (9.9) | 2 (5.6) | 6 (7.3) | 13 (13.9) | |
| 90 Days | 23 (10.9) | 2 (5.6) | 7 (8.5) | 14 (15.1) | |
| 5 Years | 53 (36.4) | 5 (17.5) | 22 (37.2) | 26 (43.8) | |
| Rehospitalization | .2086 | ||||
| 30 Days | 13 (6.3) | 0 (0) | 8 (9.8) | 5 (5.6) | |
| 90 Days | 26 (12.8) | 1 (2.9) | 13 (16.1) | 12 (13.6) | |
| 5 Years | 96 (54.1) | 12 (38.6) | 42 (57.1) | 42 (57.4) | |
Data presented as number (%).
Kaplan-Meier estimates and log-rank P values for overall difference across all follow-up points.
Table IV.
Outcomes among patients stratified by American Society of Anesthesiologists (ASA) classificationa
| Outcome | Total (n = 217) | ASA class |
P value | ||
|---|---|---|---|---|---|
| I-II (n = 37) | III (n = 83) | IV (n = 97) | |||
| Total SVS score (missing data) | 6.2 ± 3.0 (n = 101) | 5.5 ± 3.0 (n = 17) | 5.9 ± 2.7 (n = 23) | 7.1 ± 3.3 (n = 61) | .100 |
| 30 Days | |||||
| Death | 10 (4.6) | 2 (5.4) | 2 (2.4) | 6 (6.2) | .517 |
| Complication (any type) | 23 (10.6) | 2 (5.4) | 7 (8.4) | 14 (14.4) | .270 |
| Rehospitalization | 26 (12.0) | 1 (2.7) | 13 (15.7) | 12 (12.4) | .120 |
| 90 Days | |||||
| Death | 14 (6.5) | 2 (5.4) | 4 (4.8) | 8 (8.2) | .622 |
| Complication (any type) | 23 (10.6) | 2 (5.4) | 7 (8.4) | 14 (14.4) | .270 |
| Rehospitalization | 26 (12.0) | 1 (2.7) | 13 (15.7) | 12 (12.4) | .120 |
| 5 Years | |||||
| Reintervention | 14 (6.5) | 2 (5.4) | 2 (2.4) | 10 (10.3) | .082 |
| Stroke | 21 (9.7) | 2 (5.4) | 9 (10.8) | 10 (10.3) | .749 |
| Paraplegia | 4 (1.8) | 1 (2.7) | 1 (1.2) | 2 (2.1) | .824 |
| Type I endoleak | 9 (4.1) | 0 (0.0) | 5 (6.0) | 4 (4.1) | .397 |
| Endoleak, any type | 34 (16.1) | 1 (2.9) | 20 (24.4) | 13 (13.8) | .011 |
SVS, Society for Vascular Surgery.
Data presented as mean ± standard deviation or number (%).
Continuous variables compared using one-way analysis of variance and categorical variables using the χ2 or Fisher exact test.
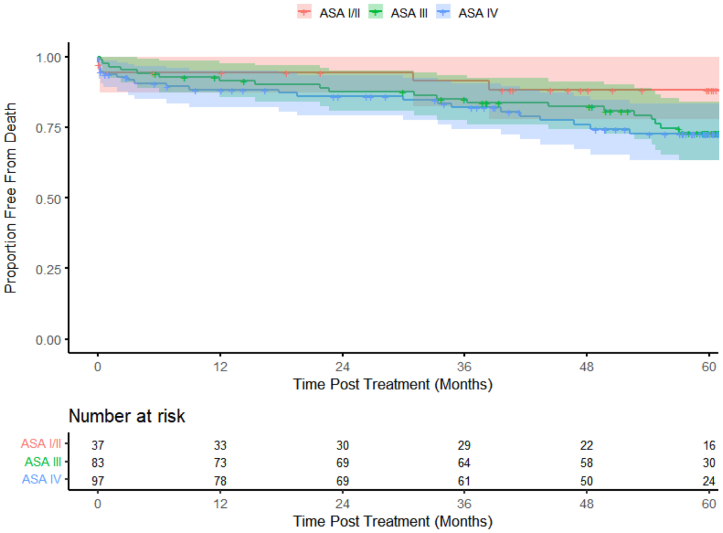
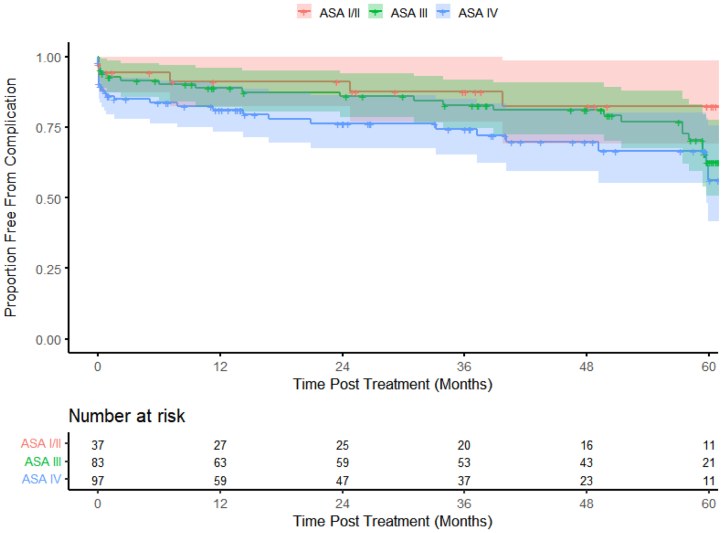
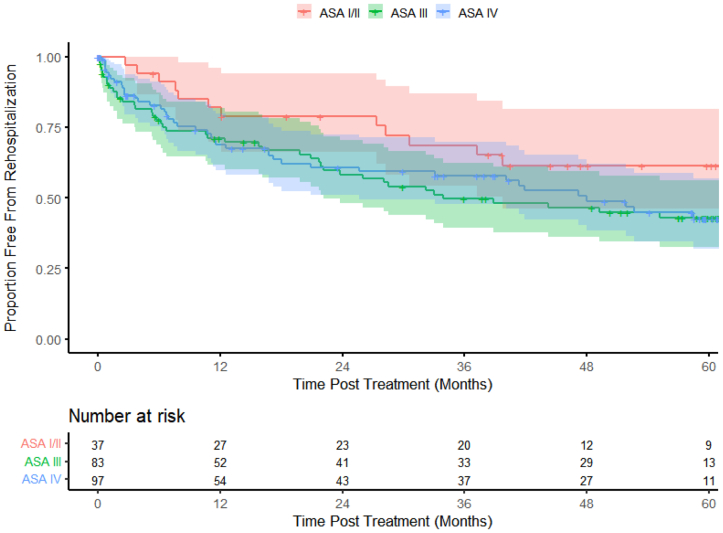
The Kaplan-Meier curves showed that the mortality curves started to separate at ∼18 months; however, at 5 years, the ASA III and ASA IV patients had similar survival compared with the ASA I-II patients (log-rank P = .2224; Fig 1). The incidence of complications was higher in the ASA IV group beginning almost immediately after the procedure compared with the other ASA groups (log-rank P = .0972; Fig 2). Rehospitalization was lower for the ASA I-II patients than for the ASA III and IV cohorts (Fig 3). The ASA I-II patients experienced an initial bout of readmissions after the index procedure through year 1, followed by stabilization for 12 to 18 months, and again after year 3 (Fig 3). In contrast, the ASA III and ASA IV patients demonstrated a similar need for rehospitalization over time (log-rank P = .2086; Fig 3).
Fig 1.
Kaplan-Meier estimates showing long-term mortality over 5 years (color bands represent 95% confidence intervals [CIs]).
Fig 2.
Kaplan-Meier estimates showing long-term complications over 5 years (color bands represent 95% confidence intervals [CIs]).
Fig 3.
Kaplan-Meier estimates showing long-term rehospitalization over 5 years (color bands represent 95% confidence intervals [CIs]).
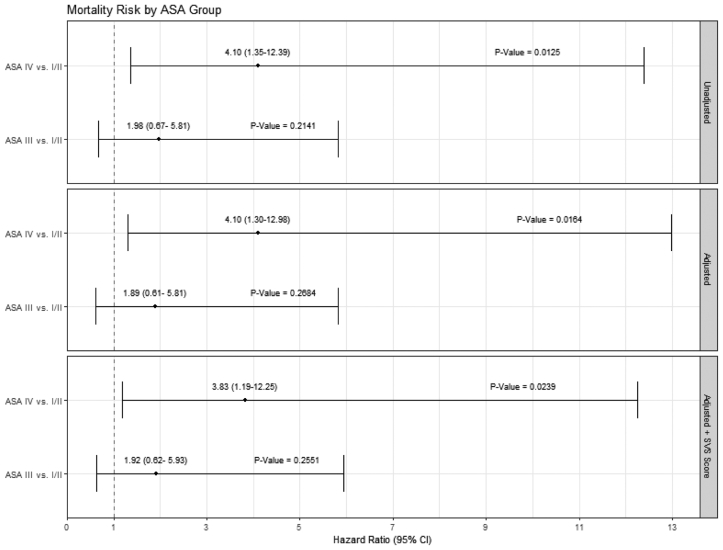
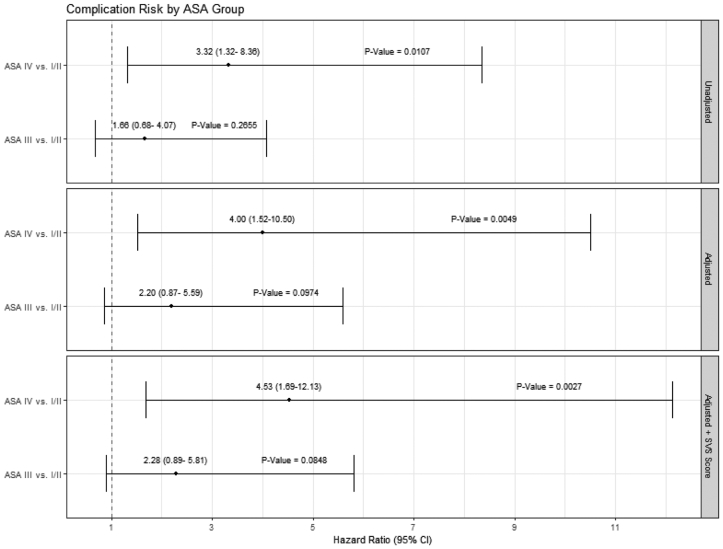
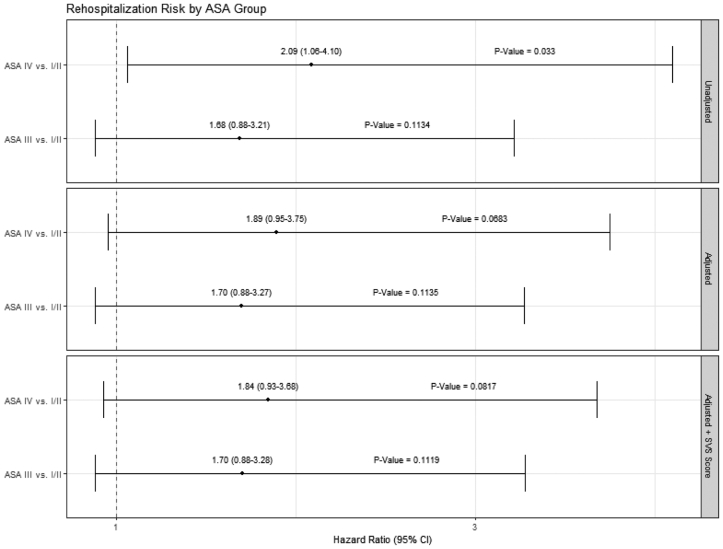
Our analysis results suggest that following TEVAR for acute dissection, traumatic dissection, and thoracic aneurysm, ASA III and ASA IV patients appear to have clinically similar experiences over time regarding mortality, complications, and rehospitalization. We added the SVS score to determine the additional contribution of the ASA class to the physiologic state and compared ASA III and ASA IV patients to ASA I-II patients. Forest plots of the multivariable model results show that the ASA IV patients had a higher hazard proportion of mortality compared with the ASA I-II patients before and after multivariable adjustments and persisted after adding the SVS score (Fig 4). In contrast, after adjustment, the ASA I-II and ASA III patients had similar hazard ratios for mortality. This pattern was also noted in the postprocedure complication rates (Fig 5). Rehospitalization showed a trend toward similar rates across the ASA patient groups with and without SVS adjustments; notably, the 95% CIs for the hazard ratios were just below the unity line (Fig 6). The SVS score was not independently associated with an increased risk for any outcome assessed in our TEVAR proportional hazard models (data not shown).
Fig 4.
Forest plots for mortality after covariate and Society for Vascular Surgery (SVS) score adjustments (dashed line indicates unity).
Fig 5.
Forest plots for complication risk after covariate and Society for Vascular Surgery (SVS) score adjustments (dashed line indicates unity).
Fig 6.
Forest plots for rehospitalization risk after covariate and Society for Vascular Surgery (SVS) score adjustments (dashed line indicates unity).
Discussion
The ASA classification is the most widely used medical comorbidity scoring system for surgical patients, and, to the best of our knowledge, we are the first to report an independent association of ASA class and SVS score for patients undergoing TEVAR. Our study shows that a combination of ASA class and SVS grading score can be used as a risk stratification tool and informs long-term outcomes after TEVAR. A comprehensive long-term outlook for patients treated with TEVAR by ASA class has not been examined. Thus, we have shown an important association between the procedural ASA class and SVS score among diverse and complex aortic pathologies––acute aortic dissection, traumatic transection, and chronic descending thoracic aortic aneurysm. We considered the shorter term outcomes, because they are more likely to be associated with ASA class; however, given the very few events, we were not able to estimate effects. We found that the ASA classification was associated with and independently conferred risk of all-cause mortality, complications, and reintervention after TEVAR. This finding is important for clinicians when counseling patients, planning operations, and managing complications.
Dijkstra et al9 described their 1-year experience with the Endurant stent graft (Medtronic Vascular) in treating abdominal aortic aneurysms. They compared the ASA classification and SVS medical comorbidity grading system and concluded that the SVS comorbidity grading system, more so than the ASA classification system, predicted major adverse events and 1-year survival.9 They reported a <2% mortality and excellent results overall at 1 year. In contrast, we could not find an association between the SVS score and all-cause mortality, complications, or rehospitalization for thoracic endograft recipients. Conners et al5 reported outcomes from 167 single-center patients who underwent EVAR and found no differences in the technical and clinical success rates, complications, or 30-day mortality when stratified by ASA class.
Regarding the ASA physical status itself, Mak et al17 used survey data from anesthetists in Hong Kong to compare 10 hypothetical patients and reported only fair interobserver consistency in the use of the ASA class. Taken together, several groups and disciplines have challenged the validity of the procedural ASA classification system, suggesting the need for more objective grading, revised recommendations, and improved consistency. Thoracic aortic pathologies are complex and often have differential laminar flow characteristics in malperfusion syndromes, making them difficult to risk stratify using the ASA class alone, nor should they be, because the ASA classification system was not developed for this purpose. Our overall procedural mortality (30-day) was low in a complex subset of patients not controlled for anesthetic management (in the clinical trials). Albeit our patients represent a diverse group from a select group based on clinical trial inclusion, our series represents a complicated and challenging anatomic problem treated at various centers across the United States. In contrast to the EVAR results, we found that for TEVAR, a higher ASA class affects mortality, complications, and reinterventions.
Analyzing patients with ASA III (n = 17) and ASA IV (n = 14) undergoing TEVAR to treat aneurysms, Neuhauser et al18 reported that at a mean follow-up of 15 months, significant morbidity and mortality should be anticipated. Their series had 42% emergent cases, with an incidence of type I endoleaks of 23%. Baumgart et al,19 from the West German Heart Center in Essen, Germany, reported their outcomes for 84 patients after TEVAR with a mean follow-up of 21 ± 18 months. They found that ASA IV (n = 8) and ASA V (n = 7) were associated with significantly worse outcomes.19 Unsurprisingly, the analysis at 5 years from the ENGAGE (Endurant stent graft natural selection global postmarket) registry among only octogenarians after EVAR found that both ASA III and ASA IV vs ASA I were associated with increased all-cause mortality.20 Consistent with these prior findings for endovascular aneurysm repair patients, we have extended the current understanding and report that a higher ASA class is also independently associated with increased all-cause mortality and complications in the long term in patients with acute complicated type B dissection and traumatic dissection. We posit that in a more heterogeneous series, ASA class would continue to remain important.
Reddy et al21 examined a health maintenance organization database among ASA III and higher patients undergoing total hip (n = 1742) and total knee (n = 3283) arthroplasty with same-day home discharge (n = 1742) and found the risk of 90-day adverse events (ie, emergency department visits, unplanned readmissions, complications, mortality) was similar to that for those patients with an in-patient stay. In contrast, among spine surgery patients, ASA III and IV classes were associated with higher rates of nonhome discharge (adjusted odds ratio, 5.0; 95% CI, 3.1-8.1).8 Insomuch as these two patient populations are different (ie, joints vs spine) it is more likely that the technical procedure had greater effects on the outcomes than did the ASA class alone. In our study, we had a significantly different ASA patient mix statistically and showed that the Kaplan-Meier estimates generally separate patients into two broad categories—ASA I-II (healthier) and ASA III-IV (sicker). In our study and similar to the orthopedic cohorts, the technical operation is different across ASA groups, suggesting that endovascular technology in TEVAR is safe and effective for acute and chronic conditions over time. Our data also hinted at a period of stability after the initial episodes of care between the 2- and 4-year marks among the ASA groups. Although clinical decision making for urgent and/or emergent interventions is not informed by our results, the readmission rate, reintervention rate, follow-up, and need for close monitoring with surveillance are supported. Furthermore, as clinicians plan for reinterventions, their counseling with their patients could be influenced.
Koh et al10 recently demonstrated that frailty scoring is important to 30-day mortality, morbidity, nonhome discharge, adverse events, failure to rescue, and care required after discharge following EVAR. A ruptured abdominal aortic aneurysm is challenging to manage, and two separate groups of investigators one decade apart have reported that risk stratification systems such as POSSUM (physiologic and operative severity score for the enumeration of mortality), APACHE II (acute physiologic and chronic health evaluation) and Glasgow aneurysm score have low predictive capacity to identify and risk stratify patients with abdominal aortic pathologies.22,23 As such, a more concentrated endovascular (for thoracic, abdominal, and thoracoabdominal aortic surgery)-specific ASA classification scale and SVS grading score as a supplemental component require strong consideration.
Our analysis has limitations, given our ability to analyze data collected during three IDE trials. Retrospectively examining the contribution of ASA class and SVS score in prior operations and limited to three thoracic aortic conditions is a challenging undertaking, particularly considering the different physiologic conditions between elective aneurysms, emergency dissections, and traumatic disruptions. The use of a clinical trial database to understand this association only scratches the surface of the issue and is only a starting point to understanding these relationships and significance. Although clinical trial data collection is arduous, data collection and reporting error can be expected. However, coding errors were expectedly and homogenously distributed across the three groups, affecting equally the study populations in this evaluation.
The procedural ASA class and SVS grade assignment is associated with long-term outcomes among post-TEVAR patients. Although the periprocedural outcomes are good among these patients, our findings suggest an important role for ASA class and SVS grade that lasts beyond the initial operation. Patient selection and counseling during the preoperative course and important postoperative management considerations can be informed by these data. Surgeons treating patients with TEVAR should be aware that the initial documented clinical scores for a procedure will continue to inform long-term outcomes.
Footnotes
Author conflict of interest: B.C.T., C.R., and K.J.R. are employees of W.L. Gore & Associates. C.M.B., M.A.F., and W.D.J. have consultant agreements with W.L. Gore & Associates. N.C.E. has no conflicts of interest.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Kraev A.I., McGinn J., Etkin Y., Turner J.W., Landis G.S. Improving the power of the American Society of Anesthesiology classification system to risk stratify vascular surgery patients based on National surgical Quality Improvement project-defined functional status. Ann Vasc Surg. 2018;52:153–157. doi: 10.1016/j.avsg.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Bartha E., Ahlstrand R., Bell M., et al. ASA classification and surgical severity grading used to identify a high-risk population, a multicenter prospective cohort study in Swedish tertiary hospitals. Acta Anaesthesiol Scand. 2021;65:1168–1177. doi: 10.1111/aas.13932. [DOI] [PubMed] [Google Scholar]
- 3.Enneking F.K., Radhakrishnan N.S., Berg K., Patel S., Wishin J.M., Vasilopoulos T. Patient-centered anesthesia triage system predicts ASA physical status. Anesth Analg. 2017;124:1957–1962. doi: 10.1213/ANE.0000000000001712. [DOI] [PubMed] [Google Scholar]
- 4.Sathiyakumar V., Molina C.S., Thakore R.V., Obremskey W.T., Sethi M.K. ASA score as a predictor of 30-day perioperative readmission in patients with orthopaedic trauma injuries: an NSQIP analysis. J Orthop Trauma. 2015;29:e127–e132. doi: 10.1097/BOT.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 5.Conners M.S., 3rd, Tonnessen B.H., Sternbergh W.C., 3rd, Carter G., Yoselevitz M., Money S.R. Does ASA classification impact success rates of endovascular aneurysm repairs? Ann Vasc Surg. 2002;16:550–555. doi: 10.1007/s10016-001-0274-6. [DOI] [PubMed] [Google Scholar]
- 6.Debopadhaya S., Marmor M.T. Frailty and comorbidity predict 30 day postoperative outcomes, independent of anatomical site of fracture. Arch Orthop Trauma Surg. Published online January 17, 2023 doi: 10.1007/s00402-023-04764-7. [DOI] [PubMed] [Google Scholar]
- 7.Paajanen P., Lindstrom I., Oksala N., et al. Radiographically quantified sarcopenia and traditional cardiovascular risk assessment in predicting long-term mortality after endovascular aortic repair. J Vasc Surg. 2022;76:908–915.e2. doi: 10.1016/j.jvs.2022.03.859. [DOI] [PubMed] [Google Scholar]
- 8.Schupper A.J., Shuman W.H., Baron R.B., et al. Utilization of the American Society of Anesthesiologists (ASA) classification system in evaluating outcomes and costs following deformity spine procedures. Spine Deform. 2021;9:185–190. doi: 10.1007/s43390-020-00176-4. [DOI] [PubMed] [Google Scholar]
- 9.Dijkstra M.L., van Sterkenburg S.M., Lardenoye J.W., Zeebregts C.J., Reijnen M.M. One-year outcomes of endovascular aneurysm repair in high-risk patients using the Endurant stent-graft: comparison of the ASA classification and SVS/AAVS medical comorbidity grading system for the prediction of mortality and adverse events. J Endovasc Ther. 2016;23:574–582. doi: 10.1177/1526602816648455. [DOI] [PubMed] [Google Scholar]
- 10.Koh B.J., Lee Q., Wee I.J., et al. Frailty scoring in vascular and endovascular surgery: a systematic review. Vasc Med. 2022;27:302–307. doi: 10.1177/1358863X221093400. [DOI] [PubMed] [Google Scholar]
- 11.Canning P., Doherty G., Tawfick W., Cindea C.N., Hynes N., Sultan S. Analysing the Society for vascular surgery and American association for vascular surgery scoring systems for outcomes post-endovascular aortic repair. Ir J Med Sci. 2020;189:1005–1013. doi: 10.1007/s11845-019-02160-y. [DOI] [PubMed] [Google Scholar]
- 12.Byrne J.S., Condon E.T., Ahmed M., et al. Surgical audit using the POSSUM scoring tool in vascular surgery patients. Ir J Med Sci. 2009;178:453–456. doi: 10.1007/s11845-009-0280-1. [DOI] [PubMed] [Google Scholar]
- 13.Chaikof E.L., Fillinger M.F., Matsumura J.S., et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–1066. doi: 10.1067/mva.2002.123991. [DOI] [PubMed] [Google Scholar]
- 14.Farber M.A., Giglia J.S., Starnes B.W., et al. Evaluation of the redesigned conformable GORE TAG thoracic endoprosthesis for traumatic aortic transection. J Vasc Surg. 2013;58:651–658. doi: 10.1016/j.jvs.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Cambria R.P., Conrad M.F., Matsumoto A.H., et al. Multicenter clinical trial of the conformable stent graft for the treatment of acute, complicated type B dissection. J Vasc Surg. 2015;62:271–278. doi: 10.1016/j.jvs.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Jordan W.D., Jr., Rovin J., Moainie S., et al. Results of a prospective multicenter trial of CTAG thoracic endograft. J Vasc Surg. 2015;61:589–595. doi: 10.1016/j.jvs.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Mak P.H., Campbell R.C., Irwin M.G. The ASA physical status classification: inter-observer consistency. American Society of Anesthesiologists. Anaesth Intensive Care. 2002;30:633–640. doi: 10.1177/0310057X0203000516. [DOI] [PubMed] [Google Scholar]
- 18.Neuhauser B., Perkmann R., Greiner A., et al. Mid-term results after endovascular repair of the atherosclerotic descending thoracic aortic aneurysm. Eur J Vasc Endovasc Surg. 2004;28:146–153. doi: 10.1016/j.ejvs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Baumgart D., Eggebrecht H., Herold U., et al. Underlying aortic pathology and clinical health status determine success of endovascular stent-grafting for descending thoracic aortic disease. Catheter Cardiovasc Interv. 2006;67:527–534. doi: 10.1002/ccd.20647. [DOI] [PubMed] [Google Scholar]
- 20.Mwipatayi B.P., Oshin O.A., Faraj J., et al. Analysis of Midterm outcomes of endovascular aneurysm repair in octogenarians from the ENGAGE registry. J Endovasc Ther. 2020;27:836–844. doi: 10.1177/1526602820923827. [DOI] [PubMed] [Google Scholar]
- 21.Reddy N.C., Prentice H.A., Paxton E.W., Hinman A.D., Lin A.G., Navarro R.A. Association between same-day discharge total joint arthroplasty and risk of 90-day adverse events in patients with ASA classification of >/=3. J Bone Joint Surg Am. 2021;103:2032–2044. doi: 10.2106/JBJS.20.02110. [DOI] [PubMed] [Google Scholar]
- 22.Lazarides M.K., Arvanitis D.P., Drista H., Staramos D.N., Dayantas J.N. POSSUM and Apache II scores do not predict the outcome of ruptured infrarenal aortic aneurysms. Ann Vasc Surg. 1997;11:155–158. doi: 10.1007/s100169900026. [DOI] [PubMed] [Google Scholar]
- 23.Kapma M., Kahmann O., van Stijn I., Zeebregts C.J., Vahl A. Evaluation of risk prediction models, V-POSSUM and GAS, in patients with acute abdominal aortic rupture treated with EVAR or an open procedure. J Cardiovasc Surg (Torino) 2017;58:439–445. doi: 10.23736/S0021-9509.17.07657-1. [DOI] [PubMed] [Google Scholar]