Abstract
Mitochondria-associated ER membrane (MAM) is a structure where these calcium-regulating organelles form close physical contact sites for efficient Ca2+ crosstalk. Despite the central importance of MAM Ca2+ dynamics in diverse biological processes, directly and specifically measuring Ca2+ concentrations inside MAM is technically challenging. Here, we develop MAM-Calflux, a MAM-specific BRET-based Ca2+ indicator. The successful application of the bimolecular fluorescence complementation (BiFC) concept highlights Ca2+-responsive BRET signals in MAM. The BiFC strategy imparts dual functionality as a Ca2+ indicator and quantitative structural marker specific for MAM. As a ratiometric Ca2+ indicator, MAM-Calflux estimates steady-state MAM Ca2+ levels. Finally, it enables the visualization of uneven intracellular distribution of MAM Ca2+ and the elucidation of abnormally accumulated MAM Ca2+ from the neurons of Parkinson’s disease mouse model in both steady-state and stimulated conditions. Therefore, we propose that MAM-Calflux can be a versatile tool for ratiometrically measuring dynamic inter-organellar Ca2+ communication.
Subject terms: Calcium, Organelles, Biosensors, Fluorescence imaging, Fluorescent proteins
MAM Ca2+ dynamics play an important role in diverse biological processes, but directly and specifically measuring Ca2+ concentrations in this region is technically challenging. Here the authors report a MAM-specific BRET-based Ca2+ indicator called MAM-Calflux, which works as both a Ca2+ indicator and a structural marker due to its ratiometric nature.
Introduction
Mitochondria and endoplasmic reticulum (ER) form close contact sites spanning ~10–30 nm and are called mitochondria-associated ER membranes (MAM) or mitochondria-ER contacts (MERC)1,2. MAM is a signaling hub that governs ER-mitochondria Ca2+ transfer3–6, lipid synthesis and exchange7, and mitochondrial structure dynamics2 to regulate autophagy4,8, ER stress response9, inflammasome activation10, and apoptosis11. Accumulating evidence implies that MAM is rearranged in response to diseases such as Parkinson’s disease (PD)12,13, Alzheimer’s disease (AD)14,15, amyotrophic lateral sclerosis (ALS)16,17, and type 2 diabetes mellitus (T2DM)18; yet the underlying mechanisms are largely unexplored.
MAM serves as a ground for dynamic Ca2+ transfer from the ER to mitochondria, thus playing an important role in diverse biological processes. Ca2+ indicators, which are located at the mitochondrial matrix or cytosolic part of the outer mitochondrial membrane (OMM), determined that, upon Ca2+ release from the ER, the mitochondria are then exposed to a higher concentration of Ca2+ compared with that in the cytosol; this microdomain with high-Ca2+ concentration is the MAM3,14. The MAM is concentrated with several proteins responsible for MAM Ca2+ transfer, including inositol triphosphate receptors (IP3Rs) and polycystin-2 (PKD2), which are involved in Ca2+ release from the ER, and voltage-dependent anion channels (VDACs), which are involved in Ca2+ influx into the mitochondria. In addition, the MAM structure is maintained by tethering proteins such as glucose-regulated protein 75 (GRP75) and DJ-11,13, which also participate in regulating MAM Ca2+ transfer. Accumulated Ca2+ on the mitochondrial surface activates enzymes responsible for cellular bioenergetics and mitochondrial transport9,19,20. MAM Ca2+ transfer is also important for inhibiting autophagy4,8, stimulating Drp1-dependent mitochondrial division and pro-apoptotic signaling2,21, and buffering local cytosolic Ca2+ concentration5. Despite its importance, there is a limitation in the methodology used to directly and specifically measure MAM Ca2+, resulting in a poor understanding of MAM Ca2+ physiology.
Genetically encoded calcium indicators (GECIs) are powerful tools for understanding Ca2+-mediated cellular functions, particularly in cell biology and neuroscience. GCaMP, one of the most abundantly utilized GECI, is composed of a calmodulin-binding sequence of myosin light chain kinase (M13), circularly permutated green fluorescent protein (GFP), and calmodulin. The binding of Ca2+ to calmodulin and M13 induces conformational changes, which increase the GFP fluorescence intensity22. Over the past decades, significant progress has been made in the development of GECIs with longer wavelengths, higher signal-to-noise ratios, and faster kinetics23,24. In addition, ratiometric versions of GECIs were invented to overcome the limitations of GCaMPs whose basal signal intensities are multiplied in an expression-level-dependent manner. Ratiometric GECIs enable the quantitation of steady-state Ca2+ concentrations, such as in GCaMP-R based on the GCaMP/RFP ratio25, Twitch based on fluorescence resonance energy transfer (FRET)26, and Nanolantern or CalfluxVTN based on bioluminescence resonance energy transfer (BRET)27. Efforts have been made to understand intracellular Ca2+ distribution by developing organelle- or compartment-specific GECIs, such as for mitochondria28, ER29, and synapses30. However, limitations exist in directly and specifically investigating MAM Ca2+, and hence, indirect estimations are made by comparing Ca2+ dynamics separately measured from the ER and mitochondria6,31. Thus, the development of a MAM-specific Ca2+ sensor represents the effort to better understand the physiology of MAM Ca2+ dynamics.
In this study, we devise a modified version of CalfluxVTN which is specific for MAM, named MAM-Calflux. MAM-Calflux can directly probe MAM-specific Ca2+ concentration by applying bimolecular fluorescence complementation (BiFC). Venus fluorescence is only operative where the ER and mitochondria are closely tethered, allowing the MAM-specific observation of Ca2+-responsive BRET signals. The specificity of the Venus to the MAM also enables the use of this sensor as a quantitative structural marker, facilitating the dual functionality. MAM-Calflux demonstrates an uneven distribution of MAM Ca2+ dynamics. Furthermore, we apply this sensor to estimate MAM Ca2+ dynamics in the neurons of multiple neurodegenerative disease mouse models.
Results
MAM-Calflux, a BRET-based MAM-specific calcium indicator
To establish a MAM-specific Ca2+ indicator, we applied the concept of bimolecular fluorescence complementation (BiFC)32 to a BRET-based Ca2+ indicator, CalfluxVTN27, with organelle targeting sequences. CalfluxVTN was separated into two fragments: 1–173 aa Venus fluorescence domain (VN173) and 156–228 aa Venus domain (VC155) conjugated with troponin C and Nanoluciferase (NanoLuc) domains. Linker sequences of optimized lengths were used to insert the ER-targeting sequence (Sac1 521–587 aa) and mitochondria-targeting sequence (AKAP 1–30 aa) into VN173 and VC155 fragments, respectively (Fig. 1a). According to the principle of BiFC assays, the Venus fragment is only fused at the sites where the mitochondrial and ER membranes are closely juxtaposed. This is to ensure that the BRET signal of MAM-Calflux is visible only at the MAM after binding of Ca2+ to troponin C, which triggers a conformational change due to the close apposition between NanoLuc and fused Venus. Thus, MAM-Calflux functions as a MAM-specific Ca2+ sensor (Fig. 1b). Additionally, after Venus is fused at the MAM, it can be visualized by the excitation-based fluorescence imaging instead of the bioluminescence- and Ca2+-dependent BRET. Therefore, MAM-Calflux can also be utilized as a MAM-specific structural marker using Venus fluorescence imaging without a NanoLuc substrate, thus displaying dual functionality (Fig. 1c).
Fig. 1. MAM-Calflux, a BRET-based MAM-specific calcium indicator.
a Schematic design of MAM-Calflux constructs. Based on the CalfluxVTN construct, Venus was divided into two fragments, Venus-N (1–173 aa of Venus, VN) and Venus-C (156–228 aa of Venus, VC). Venus-N was conjugated with ER-targeting sequence (521–587 aa of Sac1) and Venus-C with troponin C (TC), and Nanoluciferase (NanoLuc) was conjugated with mitochondria-targeting sequence (1–30 aa of AKAP). b Schematic representation of the working principle of MAM-Calflux function depending on Ca2+ binding. At the MAM site, Venus-N and Venus-C associate with each other to form a functional fluorescence domain. NanoLuc emits a bioluminescence signal (460 nm) in the presence of the substrate (furimazine). When Ca2+ binds to TC, the conformational change results in drawing the fused Venus and NanoLuc into close proximity, followed by energy transfer and emission of the Venus signal (525 nm). c A dual functionality of MAM-Calflux. In addition to its application in Ca2+ sensing (left), the presence of functional Venus in the MAM region enables MAM-Calflux as a MAM structural marker through direct laser excitation of Venus (right). d–f Intracellular localization of MAM-Calflux. Transfected HeLa cells were immunostained with TOM20 and Calnexin as markers for mitochondria and ER, respectively (d). The 3D rendering image (e) and the line profile plot (f) represent MAM-Calflux localization between mitochondria and ER. Data representative of three experimental repeats. g–h Ca2+-dependent BRET from in vitro purified split-version of CalfluxVTN was measured using a microplate reader system. Luminescence wavelength scan (g) and the sigmoidal curve fitting of BRET ratios upon various free-Ca2+ concentrations (h). (n = 16, 16, 15, 16, 16, 12, 13, 16, 15, 16 microplate wells for 39.00, 1.350, 0.602, 0.351, 0.225, 0.150, 0.100, 0.065, 0.038, 0.017 μM free-Ca2+ groups, respectively, and n = 16 microplate wells for no Ca2+ group). The scale bars represent 10 μm for d and (e, left) and 1 μm for (e, right) and (f). All results for g, h are presented as mean ± SEM. Source data from f–h are provided as a Source Data file.
First, we confirmed the organelle-specific localization of each of the two MAM-Calflux fragments. The expression of ER-targeted VN173 (MAM-Calflux-N) with the soluble form of VC155 resulted in a Venus fluorescent pattern that significantly overlapped with that of Sec61β-mCherry, an ER marker (Supplementary Fig. 1a, b). When mitochondria-targeted VC155 with troponin C and NanoLuc (MAM-Calflux-C) was transfected with the soluble form of VN173, Venus distribution significantly overlapped with that of TOM20, a mitochondrial marker (Supplementary Fig. 1c, d). Finally, co-expression of MAM-Calflux-N and MAM-Calflux-C resulted in the localization of Venus at the site where ER and mitochondrial markers overlapped, confirming the MAM-specific localization of MAM-Calflux (Fig. 1d–f). Since the self-assembly between fragments in the BiFC strategy was reported33, which can affect MAM formation, we optimized the experimental condition for the MAM-Calflux. MAM-Calflux expression did not affect MAM structures by 17 h after transfection (Supplementary Fig. 1e), but enhanced MAM structure by 48 h, providing optimal experimental time window (within 16–24 h after transfection) for measurement of MAM Ca2+ using MAM-Calflux.
To validate the Ca2+ response of MAM-Calflux, we measured the intensities of luminescence and BRET-based Venus fluorescence with or without Ca2+ using the spectrometry after treatment with furimazine, a substrate for NanoLuc, and ionomycin, a membrane-permeabilizing agent. In HeLa cells, either the co-expression of MAM-Calflux-N and MAM-Calflux-C or the single expression of MAM-Calflux-C resulted in a bioluminescent signal peak at 450–470 nm wavelength, confirming that NanoLuc functionality was maintained despite Venus cleavage (Supplementary Fig. 2a). MAM-Calflux had a significant emission peak at 520–530 nm wavelength, which was diminished by the Ca2+ chelator EGTA, indicating the existence of a Ca2+-responsive BRET signal (Supplementary Fig. 2a, b). MAM-Calflux-C, which contains intact NanoLuc but lacks the functional Venus, only had a bioluminescent signal without the BRET signal, implying that the BRET signal necessitates the fusion of Venus fragments. Rising BRET signals were observed as CaCl2 concentrations increased, confirming the capacity of MAM-Calflux to detect MAM Ca2+ (Supplementary Fig. 2c, d). The Ca2+ response kinetics of the sensor was also determined in vitro using purified proteins with various concentrations of free-Ca2+ via spectrometry (Fig. 1g, h).
Furthermore, we performed a microscopic image analysis of the BRET signal. Since the luminescence signal is much weaker than the fluorescence signal, we utilized an EMCCD camera with a beam splitter (dual-view) to acquire images of the bioluminescence (Em460) and BRET-based Venus (Em525) signals simultaneously in a single frame (Supplementary Fig. 3a–b). Since MAM-Calflux measures Ca2+ levels in MAM regions, we quantitated the BRET ratio after masking MAM regions based on Venus excitation signals (Supplementary Fig. 3c). Time-lapse imaging verified that MAM-Calflux stably emitted Em460 and Em525 signals after ~2 min of furimazine treatment, which was maintained for at least 10 min (Supplementary Fig. 3d). Notably, even though the Em525 signal was significantly detected, it was negligible compared with the excitation-based Venus fluorescent signal (Venus excitation) (Supplementary Fig. 4). This allowed us to simultaneously observe the MAM structure based on Venus excitation and the MAM Ca2+ concentration based on Em460/Em525 signals while the furimazine exists, emphasizing its dual functionality as a MAM marker and MAM Ca2+ indicator.
MAM-Calflux visualizes the changes in MAM-calcium dynamics
Next, we attempted to validate the MAM-Calflux response to changes in MAM Ca2+. We treated the cells with histamine, which indirectly increases ER Ca2+ release through IP3Rs3, followed by CaCl2 and ionomycin, which directly induce excessive calcium influx. Time-lapse imaging detected an immediate increase in the BRET ratio (Fig. 2a–c). Meanwhile, Venus excitation was not affected by histamine and CaCl2/ionomycin, implying that the MAM structure was not significantly affected and the increased BRET ratio was specific for Ca2+ influx (Fig. 2d, e). The BRET ratio was restored within a few minutes after histamine treatment, while the increased BRET ratio after CaCl2/ionomycin treatment was maintained for >3 min (until the end of imaging). This indicates that the restored BRET ratio after histamine treatment presumably demonstrated active Ca2+ restoration in MAM.
Fig. 2. MAM-Calflux visualizes the changes in MAM-specific calcium dynamics.
a–e Time-lapse imaging of MAM-Calflux with serial treatments of 10 μM histamine and 10 μM ionomycin with 1 mM CaCl2. Representative images (a) and time-dependent plots for BRET ratio (b) and Venus excitation (d). Bar graphs represent the BRET ratio (c) and Venus excitation intensities (e) before and after histamine and CaCl2/ionomycin treatments. (n = 15 cells for serial treatment group). The scale bar represents 20 μm. All results are presented as means ± SEM for b, d and box plots representing median and interquartile range with whiskers min/max value and the cross representing mean value for c, e. All P values were calculated using two-tailed Student’s t test comparing before and after each treatment for c, e. Source data from b–e are provided as a Source Data file.
MAM-Calflux measures changes in the steady-state Ca2+ level at MAM
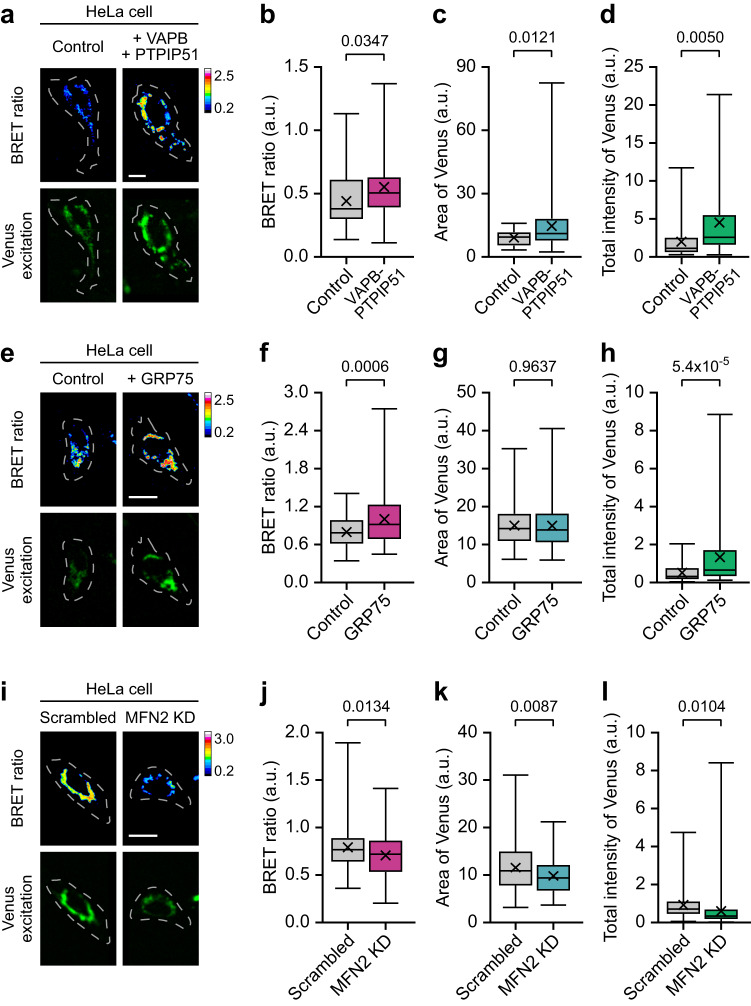
Compared with single fluorescent protein-based calcium indicators, including GCaMPs, FRET-based or BRET-based calcium indicators have the advantage that they are relatively independent from their expression levels because Ca2+ concentration is calculated as ratiometric signals26. This advantage allowed us to estimate steady-state MAM Ca2+ levels via MAM-Calflux imaging without any external Ca2+ stimulation. Thus, we introduced the conditions that were reported to regulate MAM contact size and examined the basal levels of the BRET ratio and Venus excitation of the MAM Ca2+ sensor and MAM marker, respectively. VAPB and PTPIP51 form a tethering complex that mediates the formation of the MAM structures8,16. The over-expression of VAPB and PTPIP51 increased both Venus excitation and BRET ratio in HeLa cells and primary cultured mouse neurons (Fig. 3a–d and Supplementary Fig. 5a–d), implying that both the MAM structure extent and MAM Ca2+ level, respectively, were increased by this tethering complex. In addition, VAPB-PTPIP51 expression increased histamine-induced Ca2+ influx in MAM and mitochondria without affecting cytosolic Ca2+ influx and ER Ca2+ release (Supplementary Fig. 5e–l and Supplementary Fig. 6). GRP75 is a chaperone tethering IP3R and VDAC1 mediating MAM Ca2+ crosstalk1. The BRET signals and total Venus intensities were upregulated by the over-expression of GRP75 (Fig. 3e–h), meaning that GRP75 increased MAM Ca2+ levels and MAM structure closeness but did not change the MAM structure area. This was recapitulated by staurosporine (STS) 2 h treatment (Supplementary Fig. 7), which induces early phase of apoptosis along with increased intracellular Ca2+ influx and MAM formation34,35. In contrast, the knockdown of a tethering protein MFN2 diminished basal MAM Ca2+ levels with low amounts of MAM structures (Fig. 3i–l). These results support the capacity of MAM-Calflux to determine changes in steady-state MAM Ca2+ level as well as in MAM structure.
Fig. 3. MAM-Calflux measures changes in the steady-state Ca2+ level at MAM.
a–d Increased MAM Ca2+ levels and enhanced MAM structure formation measured by MAM-Calflux upon over-expression of VAPB-PTPIP51 tethering complex in HeLa cells. Representative images (a) and bar graphs representing BRET ratio (b), MAM area (c), and total Venus excitation intensities (d). Dashed lines in a represent the cell morphologies. (n = 45 for control HeLa cells, n = 33 for VAPB-PTPIP51 expressing HeLa cells). e–h Increased MAM Ca2+ levels and intact MAM structure measured by MAM-Calflux upon over-expression of GRP75 in HeLa cells. Representative images (e) and bar graphs representing BRET ratio (f), MAM area (g), and total Venus excitation intensities (h). (n = 68 for control HeLa cells, n = 82 for GRP75 expressing HeLa cells). i–l Decreased MAM Ca2+ levels and reduced MAM structure formation measured by MAM-Calflux upon knockdown of MFN2 in HeLa cells. Representative images (i) and bar graphs representing BRET ratio (j), MAM area (k), and total Venus excitation intensities (l). (n = 108 for control HeLa cells, n = 99 for MFN2 knockdown HeLa cells). Dashed lines in a, e, and i outline the cell morphologies. The scale bars represent 10 μm. All results are presented as box plots representing the median and interquartile range with whiskers min/max value and the cross representing the mean value. All P-values were calculated using two-tailed Student’s t test for b–d, f–h, and j–l. Source data from b–d, f–h, and j–l are provided as a Source Data file.
MAM-Calflux can determine the local distribution of MAM Ca2+ dynamics
Targeting GECIs to the mitochondrial surface helped visualize the uneven Ca2+ distribution on high-Ca2+ microdomains, which are largely relevant to the MAM14,36. In addition, local Ca2+ buffering at specific regions, especially the cell periphery and synapses, is regarded as an important role of mitochondria, ER, and MAM in maintaining their respective cellular functions37–39. However, whether MAM Ca2+ dynamics is locally determined independent of MAM structure has not yet been directly explored.
Based on the dual functionality of MAM-Calflux as a structural marker and a Ca2+ sensor, we hypothesized that MAM-Calflux can distinguish the intracellular localization of MAM structures and the distribution of Ca2+ concentrations among them. Line profile analysis in primary cultured neurons, which are highly polarized and necessitate the local distribution of organelles and Ca2+ concentration40,41, identified synchronized peak distributions of MAM Ca2+ intensity compared with that of MAM marker intensity (Supplementary Fig. 8a, b). Meanwhile, comparing MAM Ca2+ peak intensities between regions with corresponding MAM structural closeness, we found significant differences among them implying the local distribution of MAM Ca2+ dynamics among the MAM regions (Supplementary Fig. 8b). Furthermore, during time-lapse imaging of MAM-Calflux-expressing HeLa cells, we visualized the uneven distribution of Venus excitation and BRET ratio at the steady-state (pre-treatment) (Supplementary Fig. 8c). Notably, after histamine treatment, we observed that the BRET ratio was unevenly increased, and some MAM regions responded more strongly than others (Supplementary Fig. 8d), as previously suggested37. In contrast, the Venus excitation pattern was not largely affected by histamine, indicating that the uneven increase in the BRET signal was not induced by MAM structural changes (Supplementary Fig. 8e). Moreover, the direct Ca2+ influx due to CaCl2 increased Ca2+ signals in a general manner (Supplementary Fig. 8f, g), implying that the uneven increase of the BRET signal due to histamine could be attributed to the different IP3R-mediated Ca2+ responses between MAM regions.
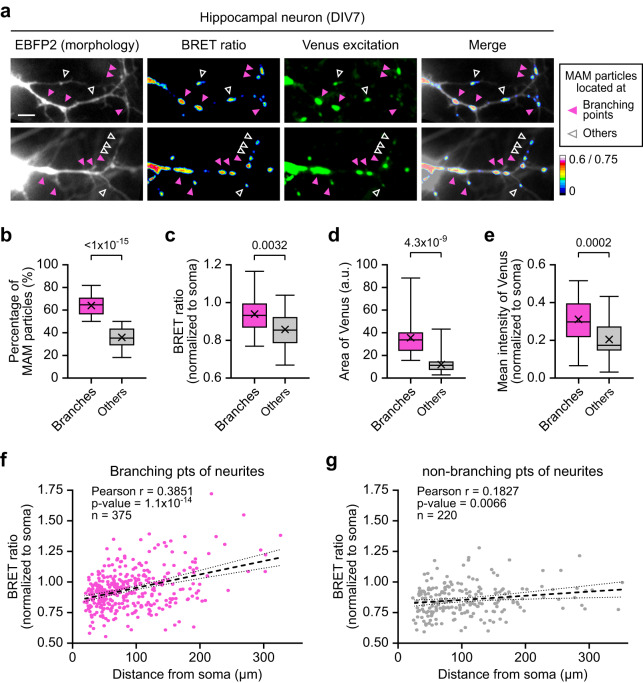
Next, we analyzed MAM Ca2+ distribution in neuronal processes in DIV7 hippocampal neurons. We quantitated BRET signals in each MAM puncta and found a positive correlation between the BRET ratio and the distance from the soma (Supplementary Fig. 9). Moreover, approximately two-thirds of the MAM puncta in neurites were located at the branching points (Fig. 4a, b) and exhibited relatively higher Ca2+ levels and larger MAM areas (Fig. 4c–e). When we separately analyzed the correlation between the BRET ratio and the distance from the soma, MAM puncta at branching points had a stronger positive correlation than the ones at non-branching points (Fig. 4f, g). These results support that MAM accumulates at neurite branching points with pronounced Ca2+ dynamics that may be necessary properties such as local Ca2+ buffering42,43. These findings support the potential of MAM-Calflux in understanding the intracellular distribution of MAM structure and MAM Ca2+ dynamics in various cellular processes.
Fig. 4. MAM-Calflux can determine the local distribution of MAM-specific calcium dynamics.
a–e MAM-Calflux visualized the MAM puncta enriched at the branching points of developing neurites with higher Ca2+ levels. Representative images (a) for BRET signals upon the MAM puncta at the neurites of DIV7 hippocampal neurons. The magenta-filled arrowheads indicate MAM puncta at the branching points, and the empty white arrowheads indicate MAM puncta at the non-branching points. The fraction of MAM puncta at branching points in each neuron (b) was higher than the non-branching point. BRET ratio (c), MAM area (d), and mean Venus excitation intensities (e) of MAM puncta at the branching points were higher than others. (n = 27 neurons). f–g Correlation between BRET signals and distance from the center of soma among the MAM puncta at the branching points (f) and non-branching points (g). The scale bar represents 10 μm. All results are presented as box plots representing the median and interquartile range with whiskers min/max value and the cross representing the mean value. All P values were calculated using two-tailed Student’s t test for b–e and Pearson correlation coefficient analysis with two-tailed P values for f, g. For f, g, each dot represents each MAM puncta (n = 375 for branching points (f) and n = 220 for non-branching points (g) from 27 individual neurons). For f, g, black dashed lines represent simple linear regression with black dotted lines representing 95% confidence intervals. Source data from b–g are provided as a Source Data file.
MAM-Calflux can monitor altered MAM-calcium physiology in neurodegenerative disease model neurons
Recent studies suggest that neurodegenerative diseases, especially PD, AD, and ALS, are closely related to abnormal regulation of the MAM structure and thereby its functions. However, a deeper understanding of MAM Ca2+ physiology in these diseases is still required44. We introduced MAM-Calflux into the neurons of mouse models pertaining to these diseases to quantitate steady-state Ca2+ levels inside the MAM region, which was not previously achievable.
5xFAD is an AD mouse model that expresses disease-linked mutant forms of human APP and PSEN145. At 7 days in vitro (DIV), the primary cultured 5xFAD hippocampal neurons had a decreased BRET ratio and Venus excitation intensities, indicating that both MAM Ca2+ levels and MAM extent were affected (Fig. 5a–d). Moreover, MAM Ca2+ levels were reverted in those neurons by expressing the VAPB-PTPIP51 complex, thereby reverting MAM structure, indicating that decreased steady-state MAM Ca2+ levels in part arising from loosened MAM (Fig. 5e–i). PINK1 (PTEN-induced kinase 1) is a serine/threonine kinase responsible for mitochondrial quality control, and its loss-of-function mutations cause autosomal recessive early-onset PD46; accumulating evidence indicates an association between PINK1 and MAM biology47,48. PINK1 KO neurons had decreased MAM Ca2+ concentrations, whereas MAM structure was not significantly altered (Supplementary Fig. 10a–d). SOD1 (superoxide dismutase 1) is the second most frequent gene associated with familial ALS, and the accumulation of mutant SOD1 proteins in the MAM has been reported17. SOD1*G93A transgenic mouse neurons, which ectopically express human SOD1 with ALS-linked mutation, had a minimal effect on the Ca2+ dynamics and MAM structure (Supplementary Fig. 10e–h).
Fig. 5. MAM-Calflux can monitor altered MAM structure and MAM Ca2+ levels in Alzheimer’s disease model neurons.
a–d MAM-Calflux detected the decreased MAM Ca2+ levels and MAM integrity in the soma of primary cultured 5xFAD tg mouse neurons. Representative images (a) and bar graphs representing BRET ratio (b), MAM area (c), and total Venus excitation intensities (d). (n = 70 for wild-type neurons and n = 76 for 5xFAD tg neurons). e Representative images (left) and Manders’ overlap coefficients (right) of colocalization between mitochondria (TOM20) and ER (Calreticulin) among 5xFAD tg neurons with VAPB-PTPIP51 tethering complex over-expression. (n = 50 for wild-type neurons, n = 43 for 5xFAD tg neurons, and n = 48 for VAPB-PTPIP51 expressing 5xFAD tg neurons). f–i Representative images (f) and graphs representing BRET ratio (g), MAM area (h), and total Venus excitation intensities (i). (n = 48 for wild-type neurons, n = 54 for 5xFAD tg neurons, and n = 54 for VAPB-PTPIP51 expressing 5xFAD tg neurons). Dashed lines in a and f represent neuronal morphologies. The scale bars represent 10 μm. All results are presented as box plots representing the median and ianterquartile range with whiskers min/max value and the cross representing the mean value. All P values were calculated using two-tailed Student’s t test for b–d and one-way ANOVA with Bonferroni’s multiple comparison tests for e and g–i. Source data from b–e and h–i are provided as a Source Data file.
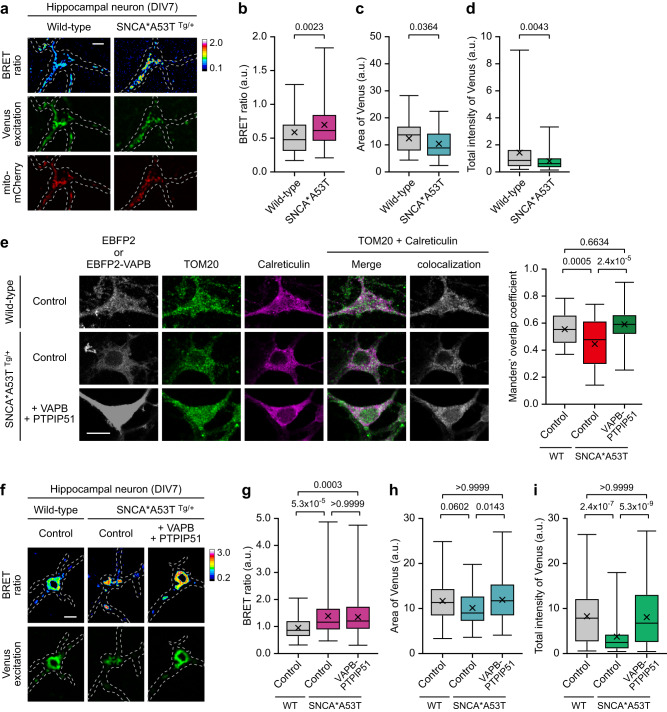
Alpha-synuclein (SNCA or α-syn) protein, which is located at the presynapse and regulates synaptic vesicles, is one of the most prominent causes of PD, as evidenced by its presence in Lewy bodies and missense mutations discovered in familial PD49. Ectopic expression of disease-linked SNCA mutants or excessive delivery of SNCA proteins has been reported to reduce ER-mitochondria contact and Ca2+ transfer12,50. When we examined MAM-Calflux in SNCA*A53T transgenic mouse neurons, which over-express the PD-linked A53T mutant form of human SNCA51,52, MAM steady-state Ca2+ levels were significantly enhanced (Fig. 6a, b). In contrast, the intensity of MAM structure was diminished (Fig. 6c, d), which is consistent with previous reports. BRET signals were not reverted by expressing the VAPB-PTPIP51 complex. However, it recovered Venus signals, indicating that increased steady-state MAM Ca2+ levels were largely independent of the loosened MAM structure (Fig. 6e–i). These results indicate that excessive α-syn proteins reduce the MAM structural extent, resulting in abnormal Ca2+ accumulation in MAM regions with possible link to PD pathogenesis.
Fig. 6. MAM-Calflux can determine the altered MAM-calcium physiology in the α-syn-mediated Parkinson’s disease model neurons.
a–d MAM-Calflux detected the increased MAM steady-state Ca2+ levels with diminished structure integrity in primary cultured SNCA*A53T tg mouse neurons at DIV7. Representative images (a) and bar graphs representing BRET ratio (b), MAM area (c), and total Venus excitation intensities (d). (n = 71 for wild-type neurons and n = 66 for SNCA*A53T tg neurons). e Representative images (left) and Manders’ overlap coefficients (right) of colocalization between mitochondria (TOM20) and ER (Calreticulin) among SNCA*A53T tg neurons with VAPB-PTPIP51 tethering complex over-expression. (n = 55 for wild-type neurons, n = 43 for SNCA*A53T tg neurons, and n = 36 for VAPB-PTPIP51 expressing SNCA*A53T tg neurons). f–i Representative images (f) and graphs representing BRET ratio (g), MAM area (h), and total Venus excitation intensities (i). Dashed lines in f represent the neuronal morphologies. (n = 77 for wild-type neurons, n = 95 for SNCA*A53T tg neurons, and n = 92 for VAPB-PTPIP51 expressing SNCA*A53T tg neurons). Dashed lines in a and f represent the neuronal morphologies. The scale bars represent 10 μm. All results are presented as box plots representing the median and interquartile range with whiskers min/max value and the cross representing the mean value. All P values were calculated using two-tailed Student’s t test for b–d and one-way ANOVA with Bonferroni’s multiple comparison tests for e and g–i. Source data from b–e and g–i are provided as a Source Data file.
Discussion
Although Ca2+ transfer was assumed to be one of the major functions of MAM, specific and direct measurement of Ca2+ concentration in the MAM was not possible due to technical limitations. Instead, MAM Ca2+ has been evaluated by measuring cytosolic Ca2+ levels near the OMM14,36 or by comparing Ca2+ dynamics in the ER and mitochondria after stimulation of Ca2+ crosstalk6,31. Csordas et al. presented an advanced methodology utilizing rapamycin-inducible bridge-forming modules (RiBFM) to guide OMM-targeted Pericam toward the ER; thus, the signal was more specific to MAM, although this still could not exclude non-MAM Ca2+ signals and had a chance of artificial increase of MAM structures53. In this study, we modified the fluorescent domain of the ratiometric calcium indicator to be specifically located inside the MAM, which could directly and specifically measure steady-state MAM Ca2+ levels without external stimuli. Its ratiometric character enables the estimation of MAM Ca2+ physiology with cell-to-cell or region-to-region comparisons. For instance, when we compared steady-state BRET ratios of different cell types which were almost simultaneously evaluated, such as HeLa control groups and primary cultured hippocampal neuron control groups in Fig. 3a–d, Supplementary Fig. 5a–d, and Supplementary Fig. 7, the neuronal cells exhibited higher MAM Ca2+ signals than that of HeLa cells, although the MAM structure intensities (Venus excitation) were similar. This implies that MAM in neuronal cells contains more activated Ca2+ transfer machinery or a higher frequency of MAM Ca2+ crosstalk. Therefore, the availability of data on the MAM-Calflux BRET ratio values corresponding to Ca2+ concentrations is critical for the precise measurement of MAM Ca2+ concentrations. Further applications of MAM-Calflux, such as comparisons of MAM Ca2+ concentrations in various cellular compartments and in response to various extracellular conditions, would help comprehend MAM Ca2+ physiology.
The specific characteristics of the BRET-based calcium indicator coupled with the BiFC strategy have imparted the dual functionality in MAM-Calflux as a MAM Ca2+ indicator and MAM structural marker (Fig. 1c). While given the substrate, the bioluminescence signal and BRET-based Venus signal indicate MAM Ca2+ concentrations. Independent of the substrate, laser excitation-based fluorescent imaging of Venus specifically exhibits the MAM structure since the Venus BiFC fragments can be fused only at MAM. Moreover, we verified that the detected BRET-based Venus signal was negligible compared with that of the excitation-based Venus fluorescence signal; thus, the MAM Ca2+ level and MAM structure could be simultaneously analyzed even during time-lapse imaging. Taking advantage of this dual functionality, we confirmed the previously reported local distribution of MAM Ca2+ physiology36,53. Under basal conditions, the MAM Ca2+ level was distinct from the MAM marker intensity, especially in polarized cells such as neurons (Fig. 4 and Supplementary Fig. 8a, b). On the one hand, MAM puncta stalled at the neurite branching points of developing neurons with higher Ca2+ levels than those of nearby MAMs. Emerging evidence emphasizes the importance of local Ca2+ modulation for proper axon/dendrite arborization54 and the positioning of organelles and cytoskeletons at the branching points42,43. Therefore, our findings are likely to provide insights into the roles of the local MAM-mediated Ca2+ dynamics in neuronal process development. We also observed that some MAM regions exhibited particularly strong Ca2+ signals during histamine-stimulated ER Ca2+ release by IP3Rs (Supplementary Fig. 8c–e). This possibly supports previous findings that IP3R clusters exist for local retuning Ca2+ activity and that IP3R isoforms differentially regulate MAM and local Ca2+ transfer55,56. Therefore, we suggest that further investigation using MAM-Calflux can elucidate the local MAM Ca2+ physiology under various conditions independent of local MAM structure formation.
In addition, the dual functionality of MAM-Calflux could help distinguish whether the change in MAM Ca2+ concentration is caused by the MAM structural change or by other factors such as altered Ca2+ transporter activity. Since the MAM provides the physical site for Ca2+ crosstalk between the ER and mitochondria, it is plausible to expect that a tighter MAM structure resulting in greater MAM Ca2+ dynamics. When the MAM structure was enhanced by STS treatment or ectopic expression of tethering proteins, MAM-Calflux demonstrated an increase in the steady-state MAM Ca2+ level (Supplementary Fig. 7 and Fig. 3). In contrast, alterations in the Ca2+ machinery affect the MAM Ca2+ dynamics without affecting the MAM structure. For instance, the knockout of B-cell lymphoma-extra large (Bcl-xL), an activator of voltage-dependent anion-selective channel protein 1 (VDAC1), decreased IP3R-mediated ER-mitochondria Ca2+ transfer. In contrast, the MAM structure was not affected57. We introduced MAM-Calflux into SNCA*A53T mouse neurons, indicating that the steady-state MAM Ca2+ level was significantly higher than that of the wild-type, whereas the MAM structural intensity decreased (Fig. 6). These upregulated Ca2+ levels were not affected by VAPB-PTPIP51 expression, indicating that this is independent of the loosened MAM structure. Indeed, α-syn was reported to interact with and block VDAC1, reducing Ca2+ influx to mitochondria and leading to the accumulation of Ca2+ on the MAM side58. In addition, α-syn aggregation binds to sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and increases cytosolic Ca2+ level at a later stage59. Therefore, based on results from MAM-Calflux, we hypothesize that the pathogenic α-syn decreases Ca2+ uptake by mitochondria, resulting in the abnormal accumulation of Ca2+ in the MAM regardless of its loosened structure. Likewise, defining both MAM structure and MAM Ca2+ by the dual functionality of MAM-Calflux can provide more insights into understanding MAM Ca2+ physiology in various cellular or disease-relevant conditions.
The MAM-specific localization and sustained Ca2+ response of MAM-Calflux proves that this strategy can be employed in developing calcium indicators specific for other inter-organelle contacts by making appropriate substitutions for organelle-specific localization signals of MAM-Calflux and optimizing the linker lengths60. For instance, the plasma membrane (PM)-ER contact serves for the efficient transfer of extracellular Ca2+ to the ER lumen and activates ER Ca2+ release under specific conditions such as muscular contraction61. Thus, interrogating Ca2+ crosstalk between the PM and ER would be informative. Dolman et al. determined that the mitochondria may be responsible for the Ca2+ gradient in trans- and cis-Golgi by close contact with Golgi apparatus62. Since Ca2+ dynamics in the Golgi affect its structures and functions, especially secretory pathways and local Ca2+ buffering63, the interaction between mitochondria and the Golgi apparatus is another possible candidate to examine Ca2+ crosstalk. Calcium indicators specific to these inter-organelle structures may advance our understanding of organellar Ca2+ dynamics and organelle-to-organelle Ca2+ crosstalk within the cell.
Although the MAM-Calflux is a versatile tool for studying MAM-specific Ca2+ physiology, a number of further improvements can be made. First, the split fragments of Venus by BiFC have been reported to undergo spontaneous self-assembly, although this was reduced when each fragment was fused to other proteins64,65. Self-assembly of Venus in MAM-Calflux would ectopically enhance MAM structure formation, resulting in the over-estimation of MAM Ca2+ basal levels. Indeed, we observed that expression of MAM-Calflux for long periods can affect MAM structure formation, requiring careful design of experimental conditions, such as expression period and levels. This may be partially overcome by modifying the strategy for Venus fragmentation in MAM-Calflux, for instance, introducing mutations in Venus66,67, changing Venus split residues68, and utilizing dimerization-dependent fluorescent protein69. Employing low-expression promoters or inducible expression systems will also be beneficial. Also, a relatively low bioluminescence signal and BRET-based Venus signal can restrict the frame-to-frame interval of time-lapse imaging. In our imaging condition, we achieved the shortest interval of 5 sec to observe MAM Ca2+ dynamics against external stimuli. Still, this interval may be insufficient to precisely analyze Ca2+ dynamics in some conditions, indicating room for further improvement in conjunction with the conventional BRET system27.
Methods
Ethical Statement
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Pohang University of Science and Technology (POSTECH-2022-0085). All experiments were performed in accordance with the approved guidelines.
Animals
Pregnant B6 mice used for primary hippocampal neuron culture were purchased from Hyochang Science (Daegu, South Korea). SNCA*A53T mice were a generous gift from Dr. Won-Jong Oh (Korea Brain Research Institute)52. PINK1-deficient mice were a generous gift from Dr. Xiaoxi Zhuang (Chicago University) and Dr. Sang Myun Park (Ajou University School of Medicine)70. 5xFAD mice were a generous gift from Dr. Kyong-Tai Kim (Pohang University of Science and Technology)45. SOD1*G93A mice71 were purchased from The Jackson Laboratory (Stock No. 002726) and maintained on a C57BL/6 background. The animals were group-housed under diurnal light conditions (12 hr light, 12 hr dark cycle) and had free access to food and water. (temperature 22 °C ± 2 °C, humidity 50% ± 5%).
Antibodies and plasmids
Anti-TOM20 mouse monoclonal antibodies (Cat# ab56783, Abcam, and Cat# sc-17764, Santa Cruz Biotechnology), anti-Calnexin (Cat# ab22595, Abcam), and anti-Calreticulin (Cat# ab2907, Abcam) were used for immunocytochemistry experiments for 1:50–1:200. For immunostaining, Alexa Fluor 568 or 647 conjugated goat anti-mouse IgG (Cat# A-11004 and Cat# A-21236, Molecular Probes) and Alexa Fluor 647 conjugated goat anti-rabbit IgG (Cat# A-21244, Molecular Probes) were used as secondary antibodies for 1:200.
pT7-CalfluxVTN, which was a gift from Carl Johnson (Addgene plasmid # 83926)27, was served as the template to design MAM-Calflux. A 173 aa N-terminal fragment of Venus domain of CalfluxVTN, VN173, was conjugated with a 103 aa linker and the ER-targeting sequence (mSac1 521–587 aa) at its C-terminus. A fragment containing the C-terminal part of Venus, VC155, and following Troponin C and NanoLuc domains was conjugated with the mitochondria-targeting sequence (mAkap1 1–30 aa) and a 57 aa linker at its N-terminus. Sec61b-mCherry was a gift from Gia Voeltz (Addgene plasmid # 49155)72. pCMV R-CEPIA1er was a gift from Masamitsu Iino (Addgene plasmid # 58216)29. The core sequence of human MFN2 shRNA was GGAAGAGCACCGTGATCAATG73.
Cell culture and transfection
HeLa (ATCC # CCL-2) cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) (HyClone) supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco) and 1% penicillin/streptomycin (Gibco). The cell-line was authenticated using STR profiling method and tested negative for mycoplasma contamination. For imaging samples, ~1.5 × 105 cells/mL of cells were seeded on the 18-mm or 25-mm cover glass in 12-well or six-well plates, respectively. The cells were transfected using transfection reagents, either polyethylenimine (PEI) or Lipofectamine 2000 (Thermo Fisher Scientific). For transfection in a 12-well plate, 300–400 ng of MAM-Calflux-P2A construct and each 400-600 ng of other plasmids, such as EBFP2-N1, EBFP2-hVAPB, FLAG-hPTPIP51, MYC-hGRP75, and pLL3.7-mRFP-MFN2 shRNA, were mixed in 100 μL Opti-MEM with 4 μL PEI or 1 μL Lipofectamine 2000.
Primary cultures of hippocampal neurons were established by isolating E16 B6 mouse embryo hippocampal tissues in Hanks’ balanced salt solution (HBSS) (Gibco) and dissociating the tissues in 0.25% trypsin (Sigma-Aldrich) and 0.1% DNase I (Sigma-Aldrich) for 10 min at 37°C. Cells were resuspended in Neurobasal medium (Gibco) supplemented with 10 mM HEPES (pH 7.4) and 10% (v/v) horse serum to a final cell concentration of 4.0 × 105 cells/mL. The cells were plated on glass coverslips pre-coated with poly-D-lysine and laminin. After 2 h of plating, the cell medium was replaced with Neurobasal medium containing 2 mM glutamine, 2% (v/v) B27 supplement (Gibco), and 1% (v/v) penicillin/streptomycin. The neurons were transfected with Lipofectamine 2000, and the medium was replaced with the culture medium 2 h after transfection. For transfection in a 12-well plate, 500–600 ng of MAM-Calflux-P2A construct and each 800–1000 ng of other plasmids were mixed in 100 μL Opti-MEM with 2 μL Lipofectamine 2000.
Immunocytochemistry
For immunocytochemistry, the cells were fixed with 4% paraformaldehyde in PBS or 4% paraformaldehyde and 4% sucrose in PBS for 20 min and washed with PBS for three times. Cells were permeabilized with 0.2% Triton-X-100 in PBS for 5 min and blocked with 5% goat serum or 5% BSA in PBS for >30 min. For protein staining, the cells were incubated with primary antibodies diluted in the blocking solution for 1 h at room temperature or overnight at 4°C, washed with PBS for three times, and treated with secondary antibodies diluted in the blocking solution for 1 h at room temperature. Cell images were acquired using FV3000 confocal laser scanning microscope (Olympus) and processed using cellSens 2.3 software (Olympus) and ImageJ 1.52p (Fiji) software (National Institute of Health)74. Images were deconvolved using the advanced constrained iterative (CI) algorithm-based deconvolution program of cellSens software. Colocalization between ER and mitochondrial markers was quantitated using cellSens software, Imaris 9.2 software (Bitplane), or Fiji software.
BRET measurement using plate reader system
To obtain in vitro purified Calflux proteins, the His6-tagged split-version of CalfluxVTN (lacking targeting sequences and linkers in MAM-Calflux) was introduced to the BL21 strain and cultured at 37 °C in LB medium containing 100 mg/mL ampicillin, until an OD600nm of 0.6 was achieved. Subsequently, a 16-hour induction was performed using 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 16 °C, followed by cell harvesting via centrifugation and snap-freezing in liquid nitrogen. The resulting cell pellets were resuspended in lysis buffer (20 mM Tris pH 8.0, 100 mM NaCl, 2 mM EDTA, 1% Triton-X, protease inhibitor, 1 mg/ml lysozyme, pH 7.4), incubated for 30 min at 37 °C, and sonicated (5 min at 20% amplitude, 5 s on, 2 s off). Cellular debris was removed through centrifugation at 7000 × g for 15 min at 4 °C, and the resulting clear supernatant was combined with Ni-NTA agarose beads in phosphate-buffered saline (PBS), followed by a 4-hour incubation at 4 °C. The beads were subsequently washed with a washing buffer (20 mM Tris pH 8.0, 100 mM NaCl, 20 mM imidazole), and the His-tagged proteins were eluted using an elution buffer (20 mM Tris pH 8.0, 100 mM NaCl, 300 mM imidazole). Finally, the proteins were dialyzed in a dialysis buffer (20 mM Tris-HCl pH 8.0, 100 mM NaCl, 3% glycerol, and 1 mM DTT). In vitro purified Calflux-N term and Calflux-C term were mixed to calcium calibration buffer being 1 μM and 0.05 μM for final concentrations, respectively. Calcium calibration buffer was made by mix of Ca-EGTA (10 mM CaEGTA, 100 mM KCl, and 30 mM MOPS pH 7.2) and zero calcium buffer (10 mM K2EGTA, 100 mM KCl, and 30 mM MOPS pH 7.2).
For live cell samples, HeLa cells in six-well plate were transfected with MAM-Calflux-C or MAM-Calflux. After 6 h, the cells were detached using trypsin-EDTA, resuspended in complete DMEM, and transferred to a white-bottom 96-well plate. For emission wavelength scan, after 12-16 h, the medium was replaced with Ca2+-free HBSS (Cat# 14175-095, Gibco) with 2 μM ionomycin and either 5 mM EGTA or variable concentrations of CaCl2 from 0.001 to 100 mM for 10–15 min.
Luciferase was activated by treating the cells with 50 μM of furimazine (Cat# AOB36539, Aobious) substrate. After stabilization for 2 minutes, the emission intensities of wavelengths from 400 to 600 nm with 2 nm intervals were measured at 37 °C using a multi-functional microplate reader (Tecan Infinite M200 pro).
BRET imaging using EMCCD equipped fluorescence microscope
HeLa or primary cultured neurons on the cover glass were transfected with MAM-Calflux and EBFP2-N1, EBFP2-hVAPB and FLAG-hPTPIP51, and Myc-hGRP75. For MFN2 knockdown, HeLa cells were transfected with pLL3.7-mRFP-MFN2 shRNA, moved to a cover glass after 24–30 h of transfection, and again transfected with MAM-Calflux after 24–30 h of seeding. After 18–24 h, the cover glass was transferred to a live imaging chamber, and the cell medium was replaced with Ca2+-free HBSS with 10 mM HEPES and 50 μM furimazine. To maintain a sufficient concentration of furimazine, at least 250 μL of buffer was used for the 18-mm coverslip. After 5 min of stabilization with furimazine, BRET imaging was performed with an inverted fluorescence microscope (IX71, Olympus) using ×20 (UApoN340 ×20/0.70 W infinite/0.17/FN22), ×40 (UPlanSApo ×40/0.95 infinite/0.11-0.23/FN26.5), and ×60 (UPlanSApo ×60/1.35 Oil infinite/0.17/FN26.5) objective lenses at 37 °C. To get a 525/460 nm ratio image, a dual-view beam splitter (Photometrics) was installed in front of the EMCCD camera (Cat# C9100-13, Hamamatsu). To obtain the BFP/RFP and Venus images separately, a triple dichroic mirror (59001bs, Chroma) with dual-view emission filters for 525 nm (AT525/30 m, Cat# 223164, Chroma) and for 460 nm and 600 nm (59003 m, Cat# 365631, Chroma) were used. Images were acquired using MetaMorph 7.7 software (Molecular Devices) with multi-dimensional acquisition for the Em525 and Em460 dual image, Venus excitation image, and BFP excitation image, sequentially. Each image was obtained at 690 kHz, 11×, gain 100, binning 1, 10 s for Em460/Em525 imaging and 690 kHz, 1×, 500 ms for Venus excitation imaging. These conditions prevented the crosstalk of signals from Em525 into Venus excitation images. The BFP excitation imaging was obtained at 2.75 MHz, 1×, 300 ms. The images were analyzed using Fiji with the use of “Template Matching” plug-in to align Em460 and Em525 images, “Subtract Background” function or “BRET Analyzer 1.0.7” plug-in to subtract background signals, and “BRET Analyzer 1.0.7” plug-in to produce BRET ratio images. To calculate mean BRET ratio after masking MAM regions, “Threshold” and “Analyze Particles” functions were used over Venus excitation images to determine MAM-specific region-of-interests (ROIs).
Live Ca2+ imaging using MAM-Calflux or mitochondria-, cytosolic-, and ER-specific Ca2+ sensors
Live calcium imaging was performed as previously described, with some modifications6,75. To measure the Ca2+ responses of subcellular organelles, HeLa cells or primary cultured hippocampal neurons were transfected with MAM-Calflux and EBFP2-N1, cyto-GCaMP6s and R-CEPIA1er, or mito-GCaMP6s and R-CEPIA1er constructs on DIV6. The cells were loaded with Ca2+-free HBSS and sequentially exposed to 10 μM histamine and 10 μM Ca2+-free ionomycin (Cat# I9657, Sigma-Aldrich) with 1 mM CaCl2. For a single treatment of histamine, cells were loaded with HBSS containing 1 mM MgCl2 and 2 mM CaCl2. To maintain a sufficient concentration of furimazine during time-lapse imaging, at least 250 μL of buffer was used for the 18-mm coverslip, and images were acquired within 20–25 min.
BRET images were recorded in MetaMorph software with multi-dimensional acquisition and time-lapse functions for the Em525 and Em460 dual image, the Venus excitation image, and the BFP excitation image, sequentially. In total 25–35 frames were acquired, and imaging conditions and interval times were adjusted to finally obtain ~15 seconds between frames. After image processing, the amplitude (ΔB/B0) of each neuron was calculated as (B−B0)/B0, where B0 is the baseline BRET ratio signal averaged over five frames before stimulation and B is the peak intensity of the BRET ratio in the response.
The fluorescence intensities were recorded at 37 °C with supplying 5% CO2 gas in FV31S-DT software at intervals of 2 seconds for 200 seconds in total using FV3000 confocal laser scanning microscope (Olympus) with UPLSAPO ×20/0.75 NA objective. Background fluorescence was subtracted and the amplitude (ΔF/F0) of each neuron was calculated as (F−F0)/F0, where F0 is the baseline mito-GCaMP6s, cyto-GCaMP6s, or R-CEPIA1er fluorescence signal averaged over 20 seconds before the stimulation and F is the peak intensity of fluorescence in the response.
Statistics and reproducibility
All graphs are presented as the mean ± SEM or box plots representing the median and interquartile range with whiskers min/max value and the cross representing the mean value. The statistical significance of the data was analyzed using two-tailed Student’s t test for comparisons between two groups and one-way ANOVA followed by Bonferroni’s post-hoc test for comparisons among multiple groups. All statistical analysis, correlation analysis, and fitting with a sigmoidal curve model or a simple linear regression model were calculated using Prism 9.4 (GraphPad).
Sample sizes for all statistical evaluations are indicated in Figure legends. No statistical method was used to predetermine sample size. Number of cells per group was chosen based on sample size in the calcium measurement experiment with microscopic images which meets or exceed previous studies6,60. No data were excluded from the analyses. All sample images were acquired in random order within the sets and all cells were chosen at the random site. Experimenters were blinded to each group allocation during the image analysis by removing any sample information in file names.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by funds from a Samsung Science and Technology Foundation grant (SSTF-BA1601-14) and the National Research Foundation of Korea (NRF-2020M3E5E2039894, NRF-2021R1A2C3010639, and NRF-2017R1A5A1015366 to S.K.P.). This study was also supported by KBRI Basic Research Program funded by the Korean Ministry of Science and ICT (21-BR-03-01 to S.K.P.) and Korea Initiative for Fostering University of Research and Innovation Program (NRF-2020M3H1A1075314 to Y.W).
Source data
Author contributions
E.C., Y.W., Y.S., and S.K.P. conceptualized the study. E.C., Y.W., B.K.S., S.J.K., and T.T.M.N. performed the experiments and analyzed the data. E.C., Y.W., Y.S., B.K.S., T.T.M.N., J.Y.Y., T.D.N., S.B.L., D.J.M., and S.K.P. participated in designing the methodology. E.C., Y.W., Y.S., and S.K.P. provided experimental resources and assisted with the final preparation of the manuscript. S.K.P. supervised the study. E.C., Y.W., and S.K.P. wrote the manuscript. All authors read and approved the final version of the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data are available in the main text or the supplementary materials. Source data for all figures are provided with the paper. Materials are available from the corresponding author. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Eunbyul Cho, Youngsik Woo.
Contributor Information
Youngsik Woo, Email: youngsik.woo@postech.ac.kr.
Sang Ki Park, Email: skpark@postech.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-39343-2.
References
- 1.Szabadkai G, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman JR, et al. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzuto R, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas C, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SJ, et al. DISC1 modulates neuronal stress responses by gate-keeping ER-mitochondria Ca(2+) transfer through the MAM. Cell Rep. 2017;21:2748–2759. doi: 10.1016/j.celrep.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 7.Kornmann B, et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Suaga P, et al. The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr. Biol. 2017;27:371–385. doi: 10.1016/j.cub.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bravo R, et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J. Cell Sci. 2011;124:2143–2152. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 11.Giorgi C, et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330:1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paillusson S, et al. alpha-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca(2+) homeostasis and mitochondrial ATP production. Acta Neuropathol. 2017;134:129–149. doi: 10.1007/s00401-017-1704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, et al. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc. Natl. Acad. Sci. USA. 2019;116:25322–25328. doi: 10.1073/pnas.1906565116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zampese E, et al. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc. Natl. Acad. Sci. USA. 2011;108:2777–2782. doi: 10.1073/pnas.1100735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martino Adami PV, et al. Perturbed mitochondria-ER contacts in live neurons that model the amyloid pathology of Alzheimer’s disease. J. Cell Sci. 2019;132:jcs229906. doi: 10.1242/jcs.229906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoica R, et al. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat. Commun. 2014;5:3996. doi: 10.1038/ncomms4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe S, et al. Mitochondria-associated membrane collapse is a common pathomechanism in SIGMAR1- and SOD1-linked ALS. EMBO Mol. Med. 2016;8:1421–1437. doi: 10.15252/emmm.201606403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thivolet C, Vial G, Cassel R, Rieusset J, Madec AM. Reduction of endoplasmic reticulum- mitochondria interactions in beta cells from patients with type 2 diabetes. PLoS One. 2017;12:e0182027. doi: 10.1371/journal.pone.0182027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Modi S, et al. Miro clusters regulate ER-mitochondria contact sites and link cristae organization to the mitochondrial transport machinery. Nat. Commun. 2019;10:4399. doi: 10.1038/s41467-019-12382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 22.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, et al. An expanded palette of genetically encoded Ca(2)(+) indicators. Science. 2011;333:1888–1891. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dana H, et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods. 2019;16:649–657. doi: 10.1038/s41592-019-0435-6. [DOI] [PubMed] [Google Scholar]
- 25.Cho JH, et al. The GCaMP-R family of genetically encoded ratiometric calcium indicators. ACS Chem. Biol. 2017;12:1066–1074. doi: 10.1021/acschembio.6b00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thestrup T, et al. Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nat. Methods. 2014;11:175–182. doi: 10.1038/nmeth.2773. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, et al. Coupling optogenetic stimulation with NanoLuc-based luminescence (BRET) Ca(++) sensing. Nat. Commun. 2016;7:13268. doi: 10.1038/ncomms13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patron M, et al. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki J, et al. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dreosti E, Odermatt B, Dorostkar MM, Lagnado L. A genetically encoded reporter of synaptic activity in vivo. Nat. Methods. 2009;6:883–889. doi: 10.1038/nmeth.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 32.Kerppola TK. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat. Protoc. 2006;1:1278–1286. doi: 10.1038/nprot.2006.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shyu YJ, Hu CD. Fluorescence complementation: an emerging tool for biological research. Trends Biotechnol. 2008;26:622–630. doi: 10.1016/j.tibtech.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Yang Z, Zhao X, Xu J, Shang W, Tong C. A novel fluorescent reporter detects plastic remodeling of mitochondria-ER contact sites. J. Cell Sci. 2018;131:jcs208686. doi: 10.1242/jcs.208686. [DOI] [PubMed] [Google Scholar]
- 35.Lalier L, et al. TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis. Cell Death Dis. 2021;12:182. doi: 10.1038/s41419-021-03471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giacomello M, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Collins TJ, Lipp P, Berridge MJ, Bootman MD. Mitochondrial Ca(2+) uptake depends on the spatial and temporal profile of cytosolic Ca(2+) signals. J. Biol. Chem. 2001;276:26411–26420. doi: 10.1074/jbc.M101101200. [DOI] [PubMed] [Google Scholar]
- 38.de Juan-Sanz J, et al. Axonal endoplasmic reticulum Ca(2+) content controls release probability in CNS nerve terminals. Neuron. 2017;93:867–881 e866. doi: 10.1016/j.neuron.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaccaro V, Devine MJ, Higgs NF, Kittler JT. Miro1-dependent mitochondrial positioning drives the rescaling of presynaptic Ca2+ signals during homeostatic plasticity. EMBO Rep. 2017;18:231–240. doi: 10.15252/embr.201642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez OA, Couve A. The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends Cell Biol. 2011;21:219–227. doi: 10.1016/j.tcb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Mandal A, Drerup CM. Axonal transport and mitochondrial function in neurons. Front. Cell Neurosci. 2019;13:373. doi: 10.3389/fncel.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep. 2013;5:1564–1575. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodakuntla S, Nedozralova H, Basnet N, Mizuno N. Cytoskeleton and membrane organization at axon branches. Front Cell Dev. Biol. 2021;9:707486. doi: 10.3389/fcell.2021.707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paillusson S, et al. There’s something wrong with my MAM; the ER-mitochondria axis and neurodegenerative diseases. Trends Neurosci. 2016;39:146–157. doi: 10.1016/j.tins.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oakley H, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valente EM, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 47.Lee KS, et al. Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc. Natl. Acad. Sci. USA. 2018;115:E8844–E8853. doi: 10.1073/pnas.1721136115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parrado-Fernandez C, et al. Reduction of PINK1 or DJ-1 impair mitochondrial motility in neurites and alter ER-mitochondria contacts. J. Cell. Mol. Med. 2018;22:5439–5449. doi: 10.1111/jcmm.13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 50.Cali T, et al. splitGFP technology reveals dose-dependent ER-mitochondria interface modulation by alpha-synuclein A53T and A30P mutants. Cells. 2019;8:1072. doi: 10.3390/cells8091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 52.Giasson BI, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/S0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 53.Csordas G, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hutchins BI, Kalil K. Differential outgrowth of axons and their branches is regulated by localized calcium transients. J. Neurosci. 2008;28:143–153. doi: 10.1523/JNEUROSCI.4548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taufiq Ur R, Skupin A, Falcke M, Taylor CW. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+ Nature. 2009;458:655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartok A, et al. IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nat. Commun. 2019;10:3726. doi: 10.1038/s41467-019-11646-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang H, et al. An interaction between Bcl-xL and the voltage-dependent anion channel (VDAC) promotes mitochondrial Ca2+ uptake. J. Biol. Chem. 2013;288:19870–19881. doi: 10.1074/jbc.M112.448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rostovtseva TK, et al. Alpha-synuclein shows high affinity interaction with voltage-dependent anion channel, suggesting mechanisms of mitochondrial regulation and toxicity in Parkinson disease. J. Biol. Chem. 2015;290:18467–18477. doi: 10.1074/jbc.M115.641746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Betzer C, et al. Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation. EMBO Rep. 2018;19:e44617. doi: 10.15252/embr.201744617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vallese F, et al. An expanded palette of improved SPLICS reporters detects multiple organelle contacts in vitro and in vivo. Nat. Commun. 2020;11:6069. doi: 10.1038/s41467-020-19892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stefan CJ, Manford AG, Emr SD. ER-PM connections: sites of information transfer and inter-organelle communication. Curr. Opin. Cell Biol. 2013;25:434–442. doi: 10.1016/j.ceb.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dolman NJ, et al. Stable Golgi-mitochondria complexes and formation of Golgi Ca(2+) gradients in pancreatic acinar cells. J. Biol. Chem. 2005;280:15794–15799. doi: 10.1074/jbc.M412694200. [DOI] [PubMed] [Google Scholar]
- 63.Lissandron V, Podini P, Pizzo P, Pozzan T. Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc. Natl. Acad. Sci. USA. 2010;107:9198–9203. doi: 10.1073/pnas.1004702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gohl C, Banovic D, Grevelhorster A, Bogdan S. WAVE forms hetero- and homo-oligomeric complexes at integrin junctions in Drosophila visualized by bimolecular fluorescence complementation. J. Biol. Chem. 2010;285:40171–40179. doi: 10.1074/jbc.M110.139337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hudry B, Viala S, Graba Y, Merabet S. Visualization of protein interactions in living Drosophila embryos by the bimolecular fluorescence complementation assay. BMC Biol. 2011;9:5. doi: 10.1186/1741-7007-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kodama Y, Hu CD. An improved bimolecular fluorescence complementation assay with a high signal-to-noise ratio. Biotechniques. 2010;49:793–805. doi: 10.2144/000113519. [DOI] [PubMed] [Google Scholar]
- 67.Nakagawa C, Inahata K, Nishimura S, Sugimoto K. Improvement of a Venus-based bimolecular fluorescence complementation assay to visualize bFos-bJun interaction in living cells. Biosci. Biotechnol. Biochem. 2011;75:1399–1401. doi: 10.1271/bbb.110189. [DOI] [PubMed] [Google Scholar]
- 68.Ohashi K, Kiuchi T, Shoji K, Sampei K, Mizuno K. Visualization of cofilin-actin and Ras-Raf interactions by bimolecular fluorescence complementation assays using a new pair of split Venus fragments. Biotechniques. 2012;52:45–50. doi: 10.2144/000113777. [DOI] [PubMed] [Google Scholar]
- 69.Alford SC, Ding Y, Simmen T, Campbell RE. Dimerization-dependent green and yellow fluorescent proteins. ACS Synth. Biol. 2012;1:569–575. doi: 10.1021/sb300050j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiong H, et al. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J. Clin. Invest. 2009;119:650–660. doi: 10.1172/JCI37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tu PH, et al. Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc. Natl. Acad. Sci. USA. 1996;93:3155–3160. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zurek N, Sparks L, Voeltz G. Reticulon short hairpin transmembrane domains are used to shape ER tubules. Traffic. 2011;12:28–41. doi: 10.1111/j.1600-0854.2010.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng L, Yi S, Yin X, Li Y, Luan Q. MFN2 knockdown promotes osteogenic differentiation of iPSC-MSCs through aerobic glycolysis mediated by the Wnt/beta-catenin signaling pathway. Stem Cell Res. Ther. 2022;13:162. doi: 10.1186/s13287-022-02836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suh BK, et al. Schizophrenia-associated dysbindin modulates axonal mitochondrial movement in cooperation with p150(glued) Mol. Brain. 2021;14:14. doi: 10.1186/s13041-020-00720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials. Source data for all figures are provided with the paper. Materials are available from the corresponding author. Source data are provided with this paper.