Abstract
Chagas disease causes a problematic pathology that can lead to megacolon and heart disease, and can even cause the death of the patient. Current therapies for this disease are the same as they were 50 years ago, are not fully effective and have strong side effects. The lack of a safe and effective therapy makes it necessary to search for new, less toxic and totally effective compounds against this parasite. In this work, the antichagasic activity of 46 novel cyanomethyl vinyl ether derivatives was studied. In addition, to elucidate the type of cell death that these compounds produce in parasites, several events related to programmed cell death were studied. The results highlight four more selective compounds, E63, E64, E74 and E83, which also appear to trigger programmed cell death, and are therefore postulated as good candidates to use in future therapeutics for Chagas disease.
Keywords: Chagas, Trypanosoma cruzi, Cell death, Activity, Cyano
Graphical abstract
Highlights
-
•
Treatments for Chagas disease have not changed in the last 50 years.
-
•
New, more active and less toxic therapies for Chagas disease are needed.
-
•
The activity of cyanomethyl vinyl ethers against Trypanosoma cruzi is demonstrated.
-
•
The cyano group is postulated as promising for antichagasic activity.
1. Introduction
Chagas disease or American trypanosomiasis is one of the neglected tropical diseases due to its characteristics and its relationship with areas of poverty. Endemic in 21 Latin American countries, including South America, Central America and Mexico, it affects between 6 and 8 million people in these areas. In recent decades, due to globalization and immigration, the number of cases outside endemic areas has been increasing, with an estimated 300,000 infected people in the United States (Bern y Montgomery, 2009; Organization, 2018; Stanaway y Roth, 2015).
Discovered by Carlos Chagas in 1909 and caused by the protozoan parasite Trypanosoma cruzi, this disease is mainly transmitted by the triatomine, although other less common routes of transmission also occur, which are the cause of cases outside endemic areas, such as congenital transmission, transfusions, or transplants (Chagas, 1909; Guarner, 2019; Rassi et al., 2010).
Chagas disease presents two phases, the initial phase, known as the acute stage, and the late phase, known as the chronic stage, separated by the indeterminate phase (Tanowitz et al., 1992). The acute phase is characterized by the absence of symptoms, in few cases there is indeterminate symptomatology (fever, sweating, muscle aches, general malaise, heart condition, among others), which is easily confused with other pathologies, although sometimes the most characteristic sign of the disease occurs, inflammation in the bite area (inoculation chagoma or Romaña sign) (Coura, 2007). Following this initial acute phase, which can last approximately 2–8 weeks, the patient enters the indeterminate phase, characterized by the absence of symptoms, but with positive serology (Linhares-Lacerda et al., 2018). Most patients usually settle at this indeterminate stage until death. But about 20–30% of these patients develop the chronic phase, characterized by the presence of cardiac and digestive problems (megacolon and megaesophagus). This phase can appear years, even decades after the acute phase (Echavarría et al., 2021)(Echavarría et al., 2021). It is important to mention that the symptomatology may vary according to genetics and geographic area, which makes its clinical diagnosis and treatment even more complicated (Campbell et al., 2004; Miles et al., 1981; Urbina, 2010).
As the third most common parasitic disease worldwide, it is important to emphasize its prevention. This prevention should be carried out in three main approaches. Firstly, by avoiding transmission (vector control, transfusions, transplants), secondly, by improving the detection of cases, thus allowing early treatment (periodic analysis, antiparasitic treatment), and lastly, by improving the patient's quality of life once the disease has developed (treatment of symptoms, health monitoring) (Abad-Franch et al., 2010; Rassi et al., 2009).
Current treatments for Chagas disease are the same as in the 1960s, benznidazole and nifurtimox, whose activity varies according to the geographical area. Treatments with these drugs are prolonged and, in addition, they are toxic, producing many side effects. These side effects mainly include gastrointestinal and central nervous system problems and, in the case of benznidazole, the appearance of allergic dermatitis. All these effects often make it necessary to stop the treatment (Pérez-Molina y Molina, 2018; Rassi et al., 2012). These side effects, in addition to their variability in efficacy, make it necessary to develop new, more effective, and less toxic therapies for this pathology.
Despite being a disease discovered more than 100 years ago by Carlos Chagas, there is still a lack of research on the progression of the disease and its mode of action, which makes the search for new drugs very complicated for researchers.
Over the past 50 years, efforts have been made to develop new treatments for both the acute and chronic phases of Chagas disease. Although no new drugs currently used in the treatment of Chagas disease have been developed, many promising candidates for further study of trypanosomiasis have been developed. These candidates can be divided into two main groups, a first group comprising compounds of synthetic and semi-synthetic origin, and a second group containing products of natural origin. Compounds of synthetic or semi-synthetic origin include, among others, sterol biosynthesis inhibitors, such as ravuconazole (Urbina et al., 2003) or fenarimol analogues (Keenan et al., 2013), antimicrobial peptides, such as AS-48 bacteriocin (Martín-Escolano et al., 2020), cruzipain inhibitors, such as K777 (Barr et al., 2005), nitro-heterocyclic compounds, such as fexinidazole (Raether y Seidenath, 1983) or nitrotriazoles (Papadopoulou et al., 2016), or quinolones (Nefertiti et al., 2018). Compounds of natural origin include flavonoids from Delphinium staphisagria (Marín et al., 2011) or Arrabidaea brachypoda (da Rocha et al., 2014), artemisinins from Artemisia annua (Mishina et al., 2007), lignans from Piper jericoense (García-Huertas et al., 2018) or Zanthoxylum naranjillo (Bastos et al., 1999), celastroloids from Maytenus chiapensis (Núñez et al., 2021) or indolocarbazoles from Streptomyces sanyensis (Cartuche et al., 2020).
The use of compounds based on nitrile or cyano groups has been developed for decades against Trypanosoma spp. (Bethencourt-Estrella et al., 2021; Quilles et al., 2020). In addition, these compounds have been linked to the inhibition of cystein proteases, more specifically against cruzain (Beaulieu et al., 2010; Burtoloso et al., 2017; Fonseca Lameiro et al., 2021).
In this study the activity and cytotoxicity of 46 novel cyanomethyl vinyl ethers derivatives were tested. In addition, the mechanism of action was studied to determine the type of cell death that these compounds produce in the parasites.
2. Materials and methods
2.1. Compounds
The 46 tested novel compounds, included in Table 1, were synthetized as described by Delgado-Hernández et al., (2021) (Delgado-Hernández et al., 2021). These 46 cyanomethyl vinyl ethers were dissolved in dimethyl sulfoxide (DMSO, Merck, Darmstadt, Germany) and stored at −20 °C in the dark. When adding the compounds to the parasites, care must be taken not to exceed 1% DMSO, as higher concentrations could be toxic to the parasites.
Table 1.
Molecular structure of the cyanomethyl vinyl ethers derivatives. BENZ: benznidazole.
| ID | Molecular structure | ID | Molecular structure | ID | Molecular structure |
|---|---|---|---|---|---|
| E51 |  |
E67 | 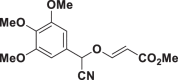 |
E83 |  |
| E52 | 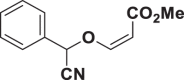 |
E68 | 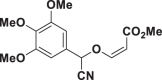 |
E84 | 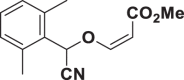 |
| E53 |  |
E69 | 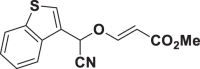 |
E85 | 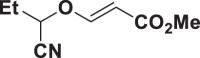 |
| E54 | 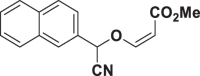 |
E70 |  |
E86 |  |
| E55 | 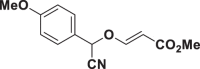 |
E71 |  |
E87 |  |
| E56 |  |
E72 | 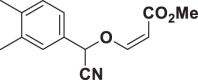 |
E88 |  |
| E57 | 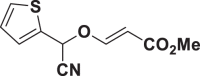 |
E73 | 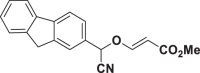 |
E89 |  |
| E58 | 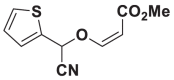 |
E74 |  |
E90 | 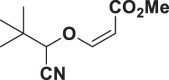 |
| E59 |  |
E75 | 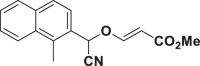 |
E91 | 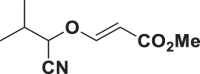 |
| E60 |  |
E76 |  |
E92 | 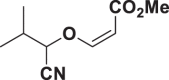 |
| E61 | 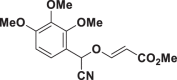 |
E77 | 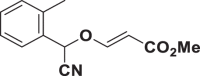 |
E93 |  |
| E62 | 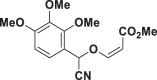 |
E78 |  |
E94 |  |
| E63 | 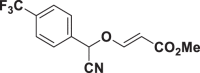 |
E79 |  |
E95 | |
| E64 | 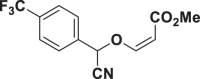 |
E80 | 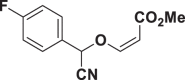 |
E96 |  |
| E65 | 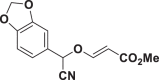 |
E81 |  |
BENZ | 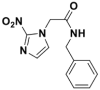 |
| E66 | 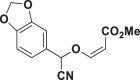 |
E82 | 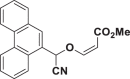 |
2.2. Cultures
The trypanocidal analysis were developed using epimastigotes of Trypanosoma cruzi (Y strain) cultured in Liver Infusion Tryptose medium (LIT) supplemented with 10% of fetal bovine serum (FBS) at 26 °C. The cytotoxicity assays were performed using murine macrophages J774A.1 (ATCC #TIB-67) cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% FBS at 37 °C and 5% CO2 atmosphere.
2.3. Activity against epimastigote stage
The studies of activity against the epimastigote stage were performed using a 96 wells plate, serial dilutions of the cyanomethyl vinyl ethers were added with a quantity of 105 epimastigotes of T. cruzi, in a final volume of 200 μl of LIT per well. To see the fluorescence reaction a 10% of alamarBlue Cell Viability Reagent® (Thermo Fisher Scientific, Madrid, Spain) was added. After 3 days of incubation, which is the time when the parasite culture is in its exponential growth phase, i.e. maximum division and minimum elimination, the fluorescence was determined using the EnSpire Multimode Plate Reader® (PerkinElmer, ThermoFisher Scientific, Madrid, Spain). After corroborating at microscope that the parasites responsible of the colorimetric change are in process of death, the inhibitory concentration 50 (IC50) was calculated using a nonlinear regression analysis (Núñez et al., 2021).
2.4. Studies of cytotoxicity on murine cells
To determine de cytotoxicity concentration 50 (CC50) the same colorimetric assay based on the alamarBlue reagent was done. In this case, 104 cells per well were added previously until full cell adhesion is achieved to the 96 well plate, after that, serial dilutions of the compounds were added in a final volume of 100 μl of RPMI 1640 medium (Gibco, Waltham, MA, USA). Medium should be changed from DMEM as it may interfere with the fluorescence readings with alamarBlue, due to its colour intensity. After 24 h at 37 °C with 5% CO2, which is sufficient time for the macrophage culture to reach its exponential growth phase, fluorescence was measured using the EnSpire Multimode Plate Reader® (PerkinElmer, ThermoFisher Scientific, Madrid, Spain). Finally, with the CC50 and the IC50 the selectivity index was calculated (CC50/IC50) (Cartuche et al., 2020).
2.5. Activity against amastigote stage
To determine the activity of the compounds against the amastigote stage of T. cruzi, the same colorimetric method based on the reduction of alamarBlue was used. A culture of epimastigotes was incubated for 4 days in LIT medium. In a 96 wells plate, 104 murine macrophages were added per well at a final volume of 50 μl in DMEM medium. After a minimum of 2 h of incubation at 37 °C with 5% CO2, 5 × 104 parasites were added to each well (ratio 5:1 parasite:macrophage) and incubated for 24 h at 37 °C with 5% CO2. Following this incubation, wells were washed 3 times with fresh medium to eliminate the non-internalized parasites, and serial dilutions of the compounds were added at a final volume of 100 μl of DMEM. After an incubation of 24 h at 37 °C with 5% CO2, the DMEM was removed and 30 μl of SDS at 0.05% in LIT was added for 30 s to induce the lysis of macrophages, following this, 170 μl of fresh LIT was quickly added to reach a final volume of 200 μl. Finally, 10% of alamarBlue was added, and after 72 h of incubation at 26 °C, the fluorescence was measured using the EnSpire Multimode Plate Reader® (PerkinElmer, ThermoFisher Scientific, Madrid, Spain) and the IC50 of the compounds was calculated using a nonlinear regression analysis.
2.6. Chromatin condensation assay
To analyze the presence of chromatin condensation the Vybrant® Apoptosis Assay Kit n◦5, Hoechst 33342/Propidium Iodide (ThermoFisher Scientific, Madrid, Spain) were used. Epimastigotes were incubated with the inhibitory concentration 90 (IC90) of the compounds. After 24 h the solution was centrifuged (825 g, 10 min, 4 °C), resuspended in 50 μl of buffer with Hoechst (5 μg/mL) and propidium iodide (PI) (1 μg/mL) and incubated for 20 min at 26 °C. To see the results EVOS® FL Cell Imaging System (ThermoFisher Scientific, Madrid, Spain) was used. To determine the cell concentration of DAPI light cube and RFP light cube were processed with FIJI ImageJ 2.0 software, in triplicate using 40x images. A negative control (without any treatment) and a reference treatment (benznidazole) were added (Bethencourt-Estrella et al., 2022).
2.7. Plasmatic membrane permeability assay
To determine the alterations in the plasmatic membrane permeability the SYTOX® Green nucleic acid stain fluorescent dye (ThermoFisher Scientific, MA, USA) was used. This probe is normally impermeable to intact plasma membranes, but is able to penetrate cells with increased permeability, reaching the nucleus and binding to nucleic acids, resulting in a strong increase in its fluorescence. The epimastigotes were incubated with the IC90 of the compounds for 24 h at 26 °C. Then, the solution was centrifuged (825 g, 10 min, 4 °C) and resuspended in 50 μl of buffer with 1 μM concentration of the kit. The measure of green cells concentration was developed using the Countess II FL (ThermoFisher Scientific, Madrid, Spain) and the images were done using GFP light cube in the EVOS® FL Cell Imaging System (ThermoFisher Scientific, Madrid, Spain). A positive control (Triton 0.5% for 30 min), a reference treatment (benznidazole) and a negative control (without any treatment) were added (López-Arencibia et al., 2019b).
2.8. ATP alterations assay
The alterations in the ATP levels were determined using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, WI, USA). After an incubation of 24 h of epimastigotes with the IC90 of the compounds, the solution was centrifuged (825 g, 10 min, 4 °C) and resuspended in 25 μl of buffer and 25 μl of the kit. After 10 min of incubation, the luminescence was measured using the EnSpire Multimode Plate Reader® (PerkinElmer, ThermoFisher Scientific, Madrid, Spain) in a white plate. A positive control (sodium azide 20 mM for 3 h), a reference treatment (benznidazole) and a negative control (without any treatment) were added (López-Arencibia et al., 2019a).
2.9. Mitochondrial membrane potential alterations assay
To determine the alterations in the mitochondrial membrane potential the JC-1 Mitochondrial Membrane Potential Assay Kit® (Cayman Chemical, MI, USA) was used. The epimastigotes were incubated with the IC90 of the compounds for 24 h at 26 °C. After centrifuge (825 g, 10 min, 4 °C) and resuspend in 50 μl, 5 μl of the kit were added. Then, the fluorescence was measured using the EnSpire Multimode Plate Reader® (PerkinElmer, ThermoFisher Scientific, Madrid, Spain) in a black plate. A positive control (Carbonyl Cyanide Chlorophenylhydrazone, CCCP 100 μM for 3 h), a reference treatment (benznidazole) and a negative control (without any treatment) were added (Bethencourt-Estrella et al., 2022).
2.10. Presence of reactive oxygen species assay
The presence of reactive oxygen species (ROS) was measured using the CellROX® Deep Red Reagent (Thermo Fisher Scientific, Madrid, Spain). After incubating the epimastigotes with the IC90 of the compounds at 26 °C for 24 h, centrifuging (825 g, 10 min, 4 °C) and resuspending in 50 μl of buffer, the kit was added at 5 μM concentration for 30 min at 26 °C. The measure of red cells concentration was carried out using the Countess II FL (ThermoFisher Scientific, Madrid, Spain) and the images were done using Cy5 light cube in the EVOS® FL Cell Imaging System (ThermoFisher Scientific, Madrid, Spain) (Bethencourt-Estrella et al., 2021).
2.11. Phosphatidylserine externalization assay
The externalization of phosphatidylserine from the inner to the outer part of the plasma membrane is another characteristic event of apoptosis. This was determined using the Tali™ Apoptosis Kit- Annexin V Alexa Fluor® 488 (ThermoFisher Scientific, Madrid, Spain). This kit containing Annexin and PI divides cells into 3 populations: live (unstained), dead (stained with PI) or apoptotic (stained with annexin). The cells were quantified using the cytometer Tali® (ThermoFisher Scientific, Madrid, Spain) (López-Arencibia et al., 2021).
2.12. Statistical methods
The IC50 and CC50 were calculated using a non-linear regression analysis with 95% confidence, with the statistical software GraphPad Prism 9.0.0. All experiments were done in duplicate in three different days and expressed as mean ± standard desviation. After verifying the normal distribution of the data by Shapiro-Wilk test, a Tukey's test was used, considering significant values of p < 0.05. The results obtained from the mechanism of cell death induced were processed performing a one-way ANOVA, the GraphPad Prism 9.0.0 was also used.
3. Results
3.1. Activity against epimastigote stage
The results of activity against the epimastigote stage of T. cruzi are presented in Table 2, the inhibitory concentration 50 were expressed in μM as mean ± standard desviation.
Table 2.
Activity of the cyanomethyl vinyl ethers derivatives against epimastigote stage of Trypanosoma cruzi, represented as inhibitory concentration 50 (IC50 in μM). BENZ: benznidazole.
| ID | IC50 (μM) | ID | IC50 (μM) | ID | IC50 (μM) |
|---|---|---|---|---|---|
| E51 | 39.96 ± 3.73 | E67 | >150 | E83 | 11.25 ± 1.47 |
| E52 | 50.09 ± 0.28 | E68 | >150 | E84 | 67.6 ± 1.35 |
| E53 | 8.84 ± 0.37 | E69 | >150 | E85 | >150 |
| E54 | 13.4 ± 1.24 | E70 | >150 | E86 | >150 |
| E55 | 36.24 ± 0.65 | E71 | >150 | E87 | >150 |
| E56 | 49.1 ± 2.79 | E72 | >150 | E88 | >150 |
| E57 | >150 | E73 | 38.48 ± 3.51 | E89 | >150 |
| E58 | >150 | E74 | 8.88 ± 0.95 | E90 | >150 |
| E59 | >150 | E75 | 10.1 ± 1.74 | E91 | >150 |
| E60 | >150 | E76 | 10.27 ± 1.64 | E92 | >150 |
| E61 | >150 | E77 | >150 | E93 | >150 |
| E62 | >150 | E78 | >150 | E94 | >150 |
| E63 | 5.43 ± 0.14 | E79 | >150 | E95 | >150 |
| E64 | 8.91 ± 1.75 | E80 | >150 | E96 | >150 |
| E65 | 47.77 ± 1.3 | E81 | >150 | ||
| E66 | 46.62 ± 5.21 | E82 | >150 | BENZ | 6.92 ± 0.77 |
3.2. Studies of cytotoxicity
The results of cytotoxicity against murine macrophages are presented in Table 3, the cytotoxicity concentration 50 were expressed in μM as mean ± standard desviation.
Table 3.
Cytotoxicity of the cyanomethyl vinyl ethers derivatives against murine macrophages, represented as cytotoxic concentration 50 (CC50 in μM). ND: not determined. BENZ: benznidazole.
| ID | CC50 (μM) | ID | CC50 (μM) | ID | CC50 (μM) |
|---|---|---|---|---|---|
| E51 | 709.44 ± 20.48 | E67 | ND | E83 | 339.82 ± 6.36 |
| E52 | >700 | E68 | ND | E84 | >300 |
| E53 | 47.47 ± 52.24 | E69 | ND | E85 | ND |
| E54 | 200.46 ± 6.04 | E70 | ND | E86 | ND |
| E55 | >700 | E71 | ND | E87 | ND |
| E56 | >700 | E72 | ND | E88 | ND |
| E57 | ND | E73 | >300 | E89 | ND |
| E58 | ND | E74 | >1300 | E90 | ND |
| E59 | ND | E75 | 96.76 ± 23.36 | E91 | ND |
| E60 | ND | E76 | 157.98 ± 18.99 | E92 | ND |
| E61 | ND | E77 | ND | E93 | ND |
| E62 | ND | E78 | ND | E94 | ND |
| E63 | 167.13 ± 4.13 | E79 | ND | E95 | ND |
| E64 | >300 | E80 | ND | E96 | ND |
| E65 | >700 | E81 | ND | ||
| E66 | >700 | E82 | ND | BENZ | 399.91 ± 1.4 μM |
3.3. Selectivity index against epimastigote stage
The selectivity indexes of the epimastigote stage were calculated as the ratio CC50/IC50, these values were included in Table 4. The most selective cyanomethyl vinyl ethers were selected to do the different analysis to elucidate the type of cell death produced in the parasite.
Table 4.
Selectivity index (SI) of the cyanomethyl vinyl ethers derivatives against epimastigote stage of Trypanosoma cruzi. ND: not determined. BENZ: benznidazole.
| ID | SI | ID | SI | ID | SI |
|---|---|---|---|---|---|
| E51 | 11.52 | E67 | ND | E83 | 30.2 |
| E52 | >13.97 | E68 | ND | E84 | 4.4 |
| E53 | 5.37 | E69 | ND | E85 | ND |
| E54 | 15.0 | E70 | ND | E86 | ND |
| E55 | >19.3 | E71 | ND | E87 | ND |
| E56 | >14.3 | E72 | ND | E88 | ND |
| E57 | ND | E73 | 7.8 | E89 | ND |
| E58 | ND | E74 | 146.4 | E90 | ND |
| E59 | ND | E75 | 9.6 | E91 | ND |
| E60 | ND | E76 | 15.4 | E92 | ND |
| E61 | ND | E77 | ND | E93 | ND |
| E62 | ND | E78 | ND | E94 | ND |
| E63 | 30.8 | E79 | ND | E95 | ND |
| E64 | >33.7 | E80 | ND | E96 | ND |
| E65 | >14.7 | E81 | ND | ||
| E66 | >15.0 | E82 | ND | BENZ | 57.8 |
3.4. Activity against amastigote stage
The results of activity against the amastigote stage of T. cruzi of the most selective compounds against amastigote stage were presented in Table 5, the inhibitory concentration 50 were expressed in μM as mean ± standard desviation.
Table 5.
Activity of the cyanomethyl vinyl ethers derivatives against amastigote stage of Trypanosoma cruzi, represented as inhibitory concentration 50 (IC50 in μM). BENZ: benznidazole.
| ID | IC50 (μM) | ID | IC50 (μM) | ID | IC50 (μM) |
|---|---|---|---|---|---|
| E63 | 16.11 ± 0.70 | E74 | 38.62 ± 8.88 | ||
| E64 | 9.94 ± 0.80 | E83 | 23.27 ± 2.93 | BENZ | 2.67 ± 0.39 |
3.5. Selectivity index against amastigote stage
The selectivity indexes against the amastigote stage of the most selective cyanomethyl vinyl ethers were presented in Table 6.
Table 6.
Selectivity index of the cyanomethyl vinyl ethers derivatives against amastigote stage of Trypanosoma cruzi. BENZ: benznidazole.
| ID | SI | ID | SI | ID | SI |
|---|---|---|---|---|---|
| E63 | 10.4 | E74 | 33.7 | ||
| E64 | 30.2 | E83 | 14.6 | BENZ | 149.8 |
3.6. Chromatin condensation analysis
The presence of chromatin condensation and dead cells was expressed in mean fluorescence intensity in Fig. 1. The images of DAPI and RFP light cubes taken EVOS® FL Cell Imaging System were included in figure S1 and S.2.
Fig. 1.
Results of the chromatin condensation and the number of dead cells represented as mean fluorescence intensity, benznidazole was used as the reference treatment. A Tukey test with the GraphPad.PRISM® 9.0 software was done to test the statistical differences between means. (non-significant [ns]; p < 0.05 [*]; p < 0.001 [***]; p <0.0001 [****]).
3.7. Plasmatic membrane permeability analysis
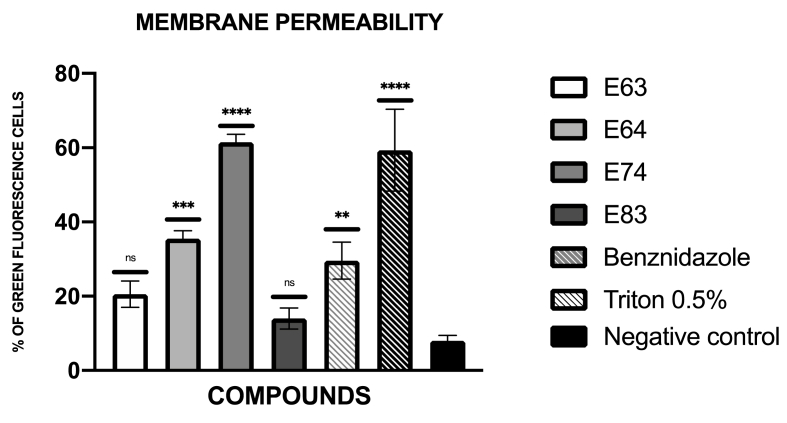
The presence of plasmatic membrane permeability was expressed in percentage relative to the negative control. These results were included in Fig. 2. The images of GFP light channel obtained with the EVOS® FL Cell Imaging System were included in figure S3.
Fig. 2.
Results of the plasma membrane permeability represented as the percentage of green cells, benznidazole was used as the reference treatment, and Triton at 0.5% was used as positive control (C+). A Tukey test with the GraphPad.PRISM® 9.0 software was done to test the statistical differences between means. (non-significant [ns]; p < 0.01 [**]; p < 0.001 [***]; p <0.0001 [****]).
3.8. ATP alterations analysis
The levels of ATP were expressed in percentage relative to the negative control and included in Fig. 3.
Fig. 3.
Results of the decrease in the ATP levels represented as the percentage relative to negative control (C-), benznidazole was used as the reference treatment, and sodium azide was used as positive control (C+). A Tukey test with the GraphPad.PRISM® 9.0 software was done to test the statistical differences between means. (p <0.0001 [****]).
3.9. Mitochondrial membrane potential alterations analysis
The results of alterations in the mitochondrial membrane potential were expressed in percentage relative to the negative control and included in Fig. 4.
Fig. 4.
Results of mitochondrial membrane potential alterations (△Ψm) represented as the percentage relative to negative control (C-), benznidazole was used as the reference treatment, and CCCP was used as positive control (C+). A Tukey test with the GraphPad.PRISM® 9.0 software was done to test the statistical differences between means. (p < 0.001 [***]; p <0.0001 [****]).
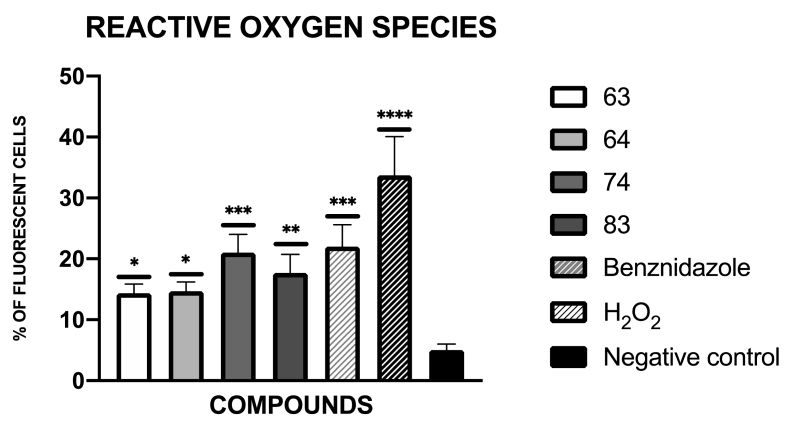
3.10. Presence of reactive oxygen species analysis
The presence of reactive oxygen species was presented in Fig. 5, as a percentage relative to the negative control. The images obtained with the Cy5 light cube in the EVOS® FL Cell Imaging System were included in figure S4.
Fig. 5.
Results of reactive oxygen species represented as percentage of fluorescent cells, benznidazole was used as the reference treatment, and H2O2 was used as positive control (C+). A Tukey test with the GraphPad.PRISM® 9.0 software was done to test the statistical differences between means. (p < 0.05 [*]; p < 0.01 [**]; p < 0.001 [***]; p <0.0001 [****]).
3.11. Phosphatidylserine externalization analysis
The externalization of phosphatidylserine was expressed as percentage of live (black), dead (red) and apoptotic (green) cells. The percentage of stained cells obtained with the Tali® was presented in Fig. 6.
Fig. 6.
Results of phosphatidylserine exposure represented as percentage of apoptotic (green), dead (red) and live (black) cells, benznidazole was used as the reference treatment. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
From the 46 cyanomethyl vinyl ethers studied 16 presented activity against epimastigote stage of T. cruzi at the range between 5.43 and 67.6 μM. These most active compounds were selected to study the cytotoxicity and to determine the activity against amastigote stage. Out of these compounds, four of them, E63, E64, E74 and E83, showed a good selectivity index against epimastigote stage, close to the reference treatment, benznidazole. Furthermore, E74 showed a selectivity index almost three times higher than benznidazole. These four compounds showed activity against the amastigote stage with selectivity indexes between 10.4 and 33.7.
Comparing the results of activity against the different forms of the parasite, cyanomethylvinyl ethers show better activity against the epimastigote forms of the parasite. This is corroborated by the idea that, because they contain cyano groups, they act on cruzipain, which is expressed to a greater extent in the epimastigote forms than in the amastigote forms (Beaulieu et al., 2010; Burtoloso et al., 2017).
These four most selective compounds were selected to study the type of mechanism they perform for the elimination of the parasite. For this purpose, different events characteristic of apoptotic-like death were studied. Events studied include chromatin condensation, decreased cellular ATP level, altered plasma membrane permeability, decreased mitochondrial membrane potential, exposure to phosphatidylserine and accumulation of reactive oxygen species. All of these are characteristic of apoptotic cell death (Basmaciyan et al., 2019; Basmaciyan y Casanova, 2019; Bethencourt-Estrella et al., 2022; López-Arencibia et al., 2019a; Menna-Barreto, 2019).
In this step, to demonstrate that these four most selective compounds produced apoptotic cell death, the results shown that these four compounds produce the above-mentioned events characteristic of this type of programmed cell death. It can therefore be concluded that they appear to be good trypanocides, as they will not produce the problems caused by necrotic cell death.
Regarding the IC50 values, the configuration of the double bond does not seem to play an important role because the activity of each pair of isomers is very similar in most of the cases. In fact, there are no cases where one of the isomers lacks activity (>150) while its counterpart has a significant activity (<100).
The most significant differences between pairs of isomers are 73–74, where the Z-isomer is over four times more active than the E-isomer, and 83–84, where the E-isomer is this time six times more active than the Z-isomer. There is no evident explanation for the enhanced activity for one of the two isomers in each case.
The difference in the IC50 values of these cyanomethyl vinyl ethers is therefore mainly due to the substituent present at the sp3 carbon other than the cyano group. While aliphatic (85–96) or heteroaromatic (57–58, 69–70) have no significant activity, some aromatic substituents present IC50 in the 1–70 μM range. Among all the aromatic substituents, again, there is no clear explanation on how the electronic nature of the aromatic ring affects the biological activity but there are numerous examples in which the IC50 values are in the 5–15 μM range.
Paying attention to the structures of these compounds, it seems that part of their activity comes from the cyano group, but clearly the other substituents modify the activity of these compounds. This is corroborated with previous literature, where antikinetoplastid activity from compounds with cyano groups has already been reported. One example is the study by Gerpe et al. where the best results of IC50 against epimastigotes of two different strains of Trypanosoma cruzi were obtained as values of 7 and 16 μM, and another example of work is that of Ancizu et al. where the lowest IC50 values were around 10 and 19 μM (Ancizu et al., 2009; Gerpe et al., 2006). All this, when compared to the present work, highlights the better activity of the ethers, which presents up to 8 compounds with values in these ranges, where compound E63 stands out for its activity with an IC50 value of 5.43 μM. Therefore, this study confirms the literature, and puts compounds with cyano groups as a line to continue the search for possible trypanocidal compounds (Barea et al., 2012; Chao et al., 2019; Farahat et al., 2018; Torrence et al., 2006).
5. Conclusions
In conclusion, compounds E63, E64, E74 and E83 show in vitro activity against Trypanosoma cruzi, as well as apoptotic cell death, and are therefore promising antichagasic compounds with which to continue studies.
Author contributions
Conceptualization, J.E.P. and J.L.-M.; methodology, C.J.B.-E. and A.L.-A.; software, C.J.B.-E.; validation, A.L.-A.; J.E.P. and J.L.-M.; formal analysis, C.J.B.-E., D.S.N.-H. and A.L.-A.; investigation, C.J.B.-E.; resources, S.D.-H.; data curation, C.J.B.-E.; writing—original draft preparation, C.J.B.-E.; writing—review and editing, J.L.-M. and J.E.P; visualization, F.G.-T. and D.T.; supervision, J.E.P.; project administration, J.L.-M.; funding acquisition, J.L.-M. and J.E.P. All authors have read and agreed to the published version of the manuscript.
Note
Supplementary data associated with this article.
Declaration of competing interest
The authors declare no conflicto of interest.
Acknowledgments
This work was funded by projects: CB21/13/00100 CIBER de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III. Cabildo Insular de Tenerife 2023-2028 and Ministerio de Sanidad.Fundación La Caixa-CajaCanarias 2022CLISA26. C.J.B.-E. (TESIS2020010057) and D.S.N.-H.
(TESIS2019010065) was funded by a grant from the Agencia Canaria de Investigación, Innovación y Sociedad de la Información cofunded by FEDER. Grant PID2021-128047NB-I00 was funded by MCIN/AEI/10.13039/501100011033 and by “European Regional Development Fund (ERDF) A way of making Europe”. Grant PDC2022-133706-I00, funded by MCIN/AEI/10.13039/501100011033 and by the “European Union NextGenerationEU/PRTR”. S.D.-H thanks La Laguna University and Ministry of Science and Innovation for a Junior Posdoctoral contract.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2023.05.001.
Contributor Information
Carlos J. Bethencourt-Estrella, Email: cbethene@ull.edu.es.
Samuel Delgado-Hernández, Email: sdelgadh@ull.es.
Atteneri López-Arencibia, Email: atlopez@ull.edu.es.
Desirée San Nicolás-Hernández, Email: dsannico@ull.edu.es.
David Tejedor, Email: dtejedor@ipna.csic.es.
Fernando García-Tellado, Email: fgarcia@ipna.csic.es.
Jacob Lorenzo-Morales, Email: jmlorenz@ull.edu.es.
José E. Piñero, Email: jpinero@ull.edu.es.
Appendix A. Supplementary data
The following is the supplementary data to this article.
References
- Abad-Franch F., Santos W.S., Schofield C.J. Research needs for Chagas disease prevention. Acta Trop. 2010;115:44–54. doi: 10.1016/j.actatropica.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Ancizu S., Moreno E., Torres E., Burguete A., Pérez-Silanes S., Benítez D., Villar R., Solano B., Marín A., Aldana I., Cerecetto H., González M., Monge A. Heterocyclic-2-carboxylic acid (3-cyano-1,4-di-N-oxidequinoxalin-2-yl)amide derivatives as hits for the development of neglected disease drugs. Molecules. 2009;14:2256–2272. doi: 10.3390/molecules14062256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea C., Pabón A., Galiano S., Pérez-Silanes S., Gonzalez G., Deyssard C., Monge A., Deharo E., Aldana I. Antiplasmodial and leishmanicidal activities of 2-cyano-3-(4-phenylpiperazine-1-carboxamido) quinoxaline 1,4-dioxide derivatives. Molecules. 2012;17:9451–9461. doi: 10.3390/molecules17089451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr S.C., Warner K.L., Kornreic B.G., Piscitelli J., Wolfe A., Benet L., McKerrow J.H. A cysteine protease inhibitor protects dogs from cardiac damage during infection by Trypanosoma cruzi. Antimicrob. Agents Chemother. 2005;49:5160–5161. doi: 10.1128/AAC.49.12.5160-5161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basmaciyan L., Casanova M. Cell death in leishmania. Parasite. 2019;26:71. doi: 10.1051/parasite/2019071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basmaciyan L., Robinson D.R., Azas N., Casanova M. (De)glutamylation and cell death in Leishmania parasites. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos J.K., Albuquerque S., Silva M.L. Evaluation of the trypanocidal activity of lignans isolated from the leaves of Zanthoxylum naranjillo. Planta Med. 1999;65:541–544. doi: 10.1055/s-1999-14012. [DOI] [PubMed] [Google Scholar]
- Beaulieu C., Isabel E., Fortier A., Massé F., Mellon C., Méthot N., Ndao M., Nicoll-Griffith D., Lee D., Park H., Black W.C. Identification of potent and reversible cruzipain inhibitors for the treatment of Chagas disease. Bioorg. Med. Chem. Lett. 2010;20:7444–7449. doi: 10.1016/j.bmcl.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Bern C., Montgomery S.P. An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am. 2009;49:e52–e54. doi: 10.1086/605091. [DOI] [PubMed] [Google Scholar]
- Bethencourt-Estrella C.J., Delgado-Hernández S., López-Arencibia A., San Nicolás-Hernández D., Sifaoui I., Tejedor D., García-Tellado F., Lorenzo-Morales J., Piñero J.E. Acrylonitrile derivatives against trypanosoma cruzi: in vitro activity and programmed cell death study. Pharmaceuticals. 2021;14 doi: 10.3390/ph14060552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethencourt-Estrella C.J., Delgado-Hernández S., López-Arencibia A., San Nicolás-Hernández D., Tejedor D., García-Tellado F., Lorenzo-Morales J., Piñero J.E. In vitro activity and cell death mechanism induced by acrylonitrile derivatives against Leishmania amazonensis. Bioorg. Chem. 2022;124 doi: 10.1016/j.bioorg.2022.105872. [DOI] [PubMed] [Google Scholar]
- Burtoloso A.C.B., de Albuquerque S., Furber M., Gomes J.C., Gonçalez C., Kenny P.W., Leitão A., Montanari C.A., Quilles J.C.J., Ribeiro J.F.R., Rocha J.R. Anti-trypanosomal activity of non-peptidic nitrile-based cysteine protease inhibitors. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D.A., Westenberger S.J., Sturm N.R. The determinants of Chagas disease: connecting parasite and host genetics. Curr. Mol. Med. 2004;4:549–562. doi: 10.2174/1566524043360249. [DOI] [PubMed] [Google Scholar]
- Cartuche L., Sifaoui I., López-Arencibia A., Bethencourt-Estrella C.J., San Nicolás-Hernández D., Lorenzo-Morales J., Piñero J.E., Díaz-Marrero A.R., Fernández J.J. Antikinetoplastid activity of indolocarbazoles from Streptomyces sanyensis. Biomolecules. 2020;10 doi: 10.3390/biom10040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagas C. Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- Chao M.N., Lorenzo-Ocampo M.V., Szajnman S.H., Docampo R., Rodriguez J.B. Further insights of selenium-containing analogues of WC-9 against Trypanosoma cruzi. Bioorg. Med. Chem. 2019;27:1350–1361. doi: 10.1016/j.bmc.2019.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura J.R. Chagas disease: what is known and what is needed--a background article. Mem. Inst. Oswaldo Cruz. 2007;102(Suppl. l):113–122. doi: 10.1590/s0074-02762007000900018. [DOI] [PubMed] [Google Scholar]
- da Rocha C.Q., Queiroz E.F., Meira C.S., Moreira D.R.M., Soares M.B.P., Marcourt L., Vilegas W., Wolfender J.-L. Dimeric flavonoids from Arrabidaea brachypoda and assessment of their anti-Trypanosoma cruzi activity. J. Nat. Prod. 2014;77:1345–1350. doi: 10.1021/np401060j. [DOI] [PubMed] [Google Scholar]
- Delgado-Hernández S., García-Tellado F., Tejedor D. Cyanovinylation of aldehydes: organocatalytic multicomponent synthesis of conjugated cyanomethyl vinyl ethers. Molecules. 2021;26 doi: 10.3390/molecules26144120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echavarría N.G., Echeverría L.E., Stewart M., Gallego C., Saldarriaga C. Chagas disease: chronic Chagas cardiomyopathy. Curr. Probl. Cardiol. 2021;46 doi: 10.1016/j.cpcardiol.2019.100507. [DOI] [PubMed] [Google Scholar]
- Farahat A.A., Ismail M.A., Kumar A., Wenzler T., Brun R., Paul A., Wilson W.D., Boykin D.W. Indole and benzimidazole bichalcophenes: synthesis, DNA binding and antiparasitic activity. Eur. J. Med. Chem. 2018;143:1590–1596. doi: 10.1016/j.ejmech.2017.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca Lameiro R. da, Shamim A., Rosini F., Cendron R., Jatai Batista P.H., Montanari C.A. Synthesis, biochemical evaluation and molecular modeling studies of nonpeptidic nitrile-based fluorinated compounds. Future Med. Chem. 2021;13:25–43. doi: 10.4155/fmc-2020-0057. [DOI] [PubMed] [Google Scholar]
- García-Huertas P., Olmo F., Sánchez-Moreno M., Dominguez J., Chahboun R., Triana-Chávez O. Activity in vitro and in vivo against Trypanosoma cruzi of a furofuran lignan isolated from Piper jericoense. Exp. Parasitol. 2018;189:34–42. doi: 10.1016/j.exppara.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Gerpe A., Aguirre G., Boiani L., Cerecetto H., González M., Olea-Azar C., Rigol C., Maya J.D., Morello A., Piro O.E., Arán V.J., Azqueta A., de Ceráin A.L., Monge A., Rojas M.A., Yaluff G. Indazole N-oxide derivatives as antiprotozoal agents: synthesis, biological evaluation and mechanism of action studies. Bioorg. Med. Chem. 2006;14:3467–3480. doi: 10.1016/j.bmc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Guarner J. Chagas disease as example of a reemerging parasite. Semin. Diagn. Pathol. 2019;36:164–169. doi: 10.1053/j.semdp.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Keenan M., Alexander P.W., Diao H., Best W.M., Khong A., Kerfoot M., Thompson R.C.A., White K.L., Shackleford D.M., Ryan E., Gregg A.D., Charman S.A., von Geldern T.W., Scandale I., Chatelain E. Design, structure-activity relationship and in vivo efficacy of piperazine analogues of fenarimol as inhibitors of Trypanosoma cruzi. Bioorg. Med. Chem. 2013;21:1756–1763. doi: 10.1016/j.bmc.2013.01.050. [DOI] [PubMed] [Google Scholar]
- Linhares-Lacerda L., Granato A., Gomes-Neto J.F., Conde L., Freire-de-Lima L., de Freitas E.O., Freire-de-Lima C.G., Coutinho Barroso S.P., Jorge de Alcântara Guerra R., Pedrosa R.C., Savino W., Morrot A. Circulating plasma MicroRNA-208a as potential biomarker of chronic indeterminate phase of Chagas disease. Front. Microbiol. 2018;9:269. doi: 10.3389/fmicb.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arencibia A., Reyes-Batlle M., Freijo M.B., Sifaoui I., Bethencourt-Estrella C.J., Rizo-Liendo A., Chiboub O., McNaughton-Smith G., Lorenzo-Morales J., Abad-Grillo T., Piñero J.E. In vitro activity of 1H-phenalen-1-one derivatives against Leishmania spp. and evidence of programmed cell death. Parasites Vectors. 2019;12:601. doi: 10.1186/s13071-019-3854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arencibia A., San Nicolás-Hernández D., Bethencourt-Estrella C.J., Sifaoui I., Reyes-Batlle M., Rodríguez-Expósito R.L., Rizo-Liendo A., Lorenzo-Morales J., Bazzocchi I.L., Piñero J.E., Jiménez I.A. vol. 8. Pathog.; Basel, Switzerland: 2019. (Withanolides from Withania Aristata as Antikinetoplastid Agents through Induction of Programmed Cell Death). [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arencibia A., Sifaoui I., Reyes-Batlle M., Bethencourt-Estrella C.J., San Nicolás-Hernández D., Lorenzo-Morales J., Piñero J.E. Discovery of new chemical tools against leishmania amazonensis via the MMV pathogen box. Pharmaceuticals. 2021;14 doi: 10.3390/ph14121219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín C., Ramírez-Macías I., López-Céspedes A., Olmo F., Villegas N., Díaz J.G., Rosales M.J., Gutiérrez-Sánchez R., Sánchez-Moreno M. In vitro and in vivo trypanocidal activity of flavonoids from Delphinium staphisagria against Chagas disease. J. Nat. Prod. 2011;74:744–750. doi: 10.1021/np1008043. [DOI] [PubMed] [Google Scholar]
- Martín-Escolano R., Cebrián R., Maqueda M., Romero D., Rosales M.J., Sánchez-Moreno M., Marín C. Assessing the effectiveness of AS-48 in experimental mice models of Chagas' disease. J. Antimicrob. Chemother. 2020;75:1537–1545. doi: 10.1093/jac/dkaa030. [DOI] [PubMed] [Google Scholar]
- Menna-Barreto R.F.S. Cell death pathways in pathogenic trypanosomatids: lessons of (over)kill. Cell Death Dis. 2019;10:93. doi: 10.1038/s41419-019-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles M.A., Cedillos R.A., Póvoa M.M., de Souza A.A., Prata A., Macedo V. Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas' disease? Lancet (London, England) 1981;1:1338–1340. doi: 10.1016/s0140-6736(81)92518-6. [DOI] [PubMed] [Google Scholar]
- Mishina Y.V., Krishna S., Haynes R.K., Meade J.C. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrob. Agents Chemother. 2007;51:1852–1854. doi: 10.1128/AAC.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefertiti A.S.G., Batista M.M., Da Silva P.B., Batista D.G.J., Da Silva C.F., Peres R.B., Torres-Santos E.C., Cunha-Junior E.F., Holt E., Boykin D.W., Brun R., Wenzler T., Soeiro M.N.C. In vitro and in vivo studies of the trypanocidal effect of novel quinolines. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01936-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez M.J., Martínez M.L., López-Arencibia A., Bethencourt-Estrella C.J., San Nicolás-Hernández D., Jiménez I.A., Lorenzo-Morales J., Piñero J.E., Bazzocchi I.L. In vitro susceptibility of kinetoplastids to celastroloids from Maytenus chiapensis. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.02236-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization P.A.H. Chagas disease in the Americas: a review of the current public health situation and a vision for the future. Rep. conclusions Recomm. 2018 [Google Scholar]
- Papadopoulou M.V., Bloomer W.D., Rosenzweig H.S., Wilkinson S.R., Szular J., Kaiser M. Nitrotriazole-based acetamides and propanamides with broad spectrum antitrypanosomal activity. Eur. J. Med. Chem. 2016;123:895–904. doi: 10.1016/j.ejmech.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Molina J.A., Molina I. Chagas disease. Lancet (London, England) 2018;391:82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- Quilles J.C.J., Shamim A., Tezuka D.Y., Batista P.H.J., Lopes C.D., de Albuquerque S., Montanari C.A., Leitão A. Dipeptidyl nitrile derivatives suppress the Trypanosoma cruzi in vitro infection. Exp. Parasitol. 2020;219 doi: 10.1016/j.exppara.2020.108032. [DOI] [PubMed] [Google Scholar]
- Raether W., Seidenath H. The activity of fexinidazole (HOE 239) against experimental infections with Trypanosoma cruzi, trichomonads and Entamoeba histolytica. Ann. Trop. Med. Parasitol. 1983;77:13–26. doi: 10.1080/00034983.1983.11811668. [DOI] [PubMed] [Google Scholar]
- Rassi A.J., Dias J.C.P., Marin-Neto J.A., Rassi A. Challenges and opportunities for primary, secondary, and tertiary prevention of Chagas' disease. Heart. 2009;95:524–534. doi: 10.1136/hrt.2008.159624. [DOI] [PubMed] [Google Scholar]
- Rassi A.J., Rassi A., Marcondes de Rezende J. American trypanosomiasis (Chagas disease) Infect. Dis. Clin. 2012;26:275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Rassi A.J., Rassi A., Marin-Neto J.A. Chagas disease. Lancet (London, England) 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Stanaway J.D., Roth G. The burden of Chagas disease: estimates and challenges. Glob. Heart. 2015;10:139–144. doi: 10.1016/j.gheart.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Tanowitz H.B., Kirchhoff L.V., Simon D., Morris S.A., Weiss L.M., Wittner M. Chagas' disease. Clin. Microbiol. Rev. 1992;5:400–419. doi: 10.1128/CMR.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrence P.F., Fan X., Zhang X., Loiseau P.M. Structurally diverse 5-substituted pyrimidine nucleosides as inhibitors of Leishmania donovani promastigotes in vitro. Bioorg. Med. Chem. Lett. 2006;16:5047–5051. doi: 10.1016/j.bmcl.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Urbina J.A. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 2010;115:55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Urbina J.A., Payares G., Sanoja C., Lira R., Romanha A.J. In vitro and in vivo activities of ravuconazole on Trypanosoma cruzi, the causative agent of Chagas disease. Int. J. Antimicrob. Agents. 2003;21:27–38. doi: 10.1016/s0924-8579(02)00273-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.