Abstract
Daylight is an important factor necessary for the proper embryonic development of birds, which raises the question, what happens when birds nest in relatively dim sites? The study experimentally tested whether there is a relationship between light conditions at the nesting site and the protoporphyrin-based pigmentation in the eggshell of the Great Tit (Parus major). We hypothesised that at lower light levels, eggs are less pigmented to increase the amount of light reaching the embryo. Our study system consisted of two types of nest boxes: "dark", in which the only source of light was the entrance hole, and "bright", which had two additional side windows. Photographs of clutches taken during the incubation period were used to quantify eggshell pigmentation. Multispectral image analyses were performed to measure variables correlating with protoporphyrin content, such as spot brightness, average spot size, spotting coverage, and spot red chroma. Repeatability analysis indicated that eggshell colouration characteristics were significantly and moderately repeatable between eggs from a single clutch, which suggests that they are under genetic and environmental control. However, none of the pigmentation traits differed significantly between the two types of nest boxes. We speculate about other ecological aspects that might have influenced the observed variability in eggshell pigmentation.
Subject terms: Behavioural ecology, Evolutionary ecology, Zoology
Introduction
Eggshells of many bird species exhibit a great variety of colours and patterns, which are effects of pigment deposition1. In most cases, eggs are pigmented by biliverdin, which produces a blue-green hue, and/or protoporphyrin, which gives a rusty-brown colour1, but there are also a few rare pigments2,3. Over the last decades, several not mutually exclusive hypotheses have been developed to explain the evolution of variation in eggshell colouration. These include eggshell strengthening4, camouflage5,6, recognising own clutch and protection against brood parasitism7,8, thermoregulation9,10, and sexual selection11, to mention just the most common hypotheses.
An interesting study12 drew attention to the fact that the eggshell transmits natural light, which eventually reaches the embryo. Moreover, there are many premises that light-filtering eggshells may affect developing birds in many ways12. In many cases, the effect of light can be regarded as positive. For example, it accelerates embryo development, provides energy for DNA repair processes (photo-reactivation), promotes functional lateralisation of cerebral hemispheres, and induces the antibacterial function of eggshell pigments on the egg surface12. On the other hand, UV-B radiation (290–320 nm) is a harmful factor because it damages the DNA of the embryo12. Thus, eggshell pigmentation and thickness form a filter modulating the amount and spectrum of light that reaches the embryo inside the egg13. The transmission of light through the calcium matrix increases linearly with light wavelength, while pigments act as filters blocking some wavebands and letting others through13. In general, the overall light transmittance decreases with increasing pigmentation and eggshell thickness13.
Bird nest sites are characterised by a wide variety of light conditions, which results from nest construction (domed and cavity nests vs. open cups), as well as from vegetation structures13. Therefore, depending on the nest light conditions, various processes can shape the evolution of the appearance of eggshells in different species. Open nesters need to limit the amount of harmful UV-B light reaching the embryo and avoid overheating14,15, whereas dim light conditions inside cavities should promote higher transmission of light through eggshells, to trigger the beneficial effect of light on the embryo. In addition, brighter colours could increase the contrast between eggs and nest background, and thus visibility to the parents13. These predictions have been supported by the results of comparative analyses. Cavity-nesting species generally tend to lay less pigmented or unpigmented eggs, while open nesters’ eggs are often variously patterned14,16–18.
In this study, we have made the first attempt to test whether light conditions in the nest site influence eggshell colouration at the population level in a hole-nesting passerine. We used an experimental setup with nest boxes, in which we controlled the amount of entering sunlight. Boxes were occupied by a secondary hole-nesting species, the Great Tit (Parus major), which lays eggs with a light-coloured eggshell background variously spotted with protoporphyrin19. Birds could choose to nest in standard (“dark”) or artificially brightened (“bright”) boxes. Our main aim was to test experimentally whether eggshell pigmentation depends on light intensity inside the nest box, while controlling for several ecological traits that could potentially affect eggshell colouration. In particular, we estimated the effect of eggshell thickness, the first egg laying date, and the clutch size on the variation of eggshell patterning. We expected that females breeding in dark nest boxes would have less pigmented eggshells, to compensate for the light deficiency in dimmer conditions. Moreover, we calculated the intra-clutch repeatability of eggshell patterning, to assess to what extent the pattern is consistent between eggs laid by the same female.
Results
Effect of nest box type on eggshell pigmentation
Generalised Additive Mixed Models (GAMMs) revealed that none of the eggshell colouration characteristics differed significantly between dark and bright nest boxes: percent spots (z = 1.66, p = 0.10), spot brightness (z = − 0.06, p = 0.95), average spot size (t = 0.69, p = 0.49) and spot Rchroma (z = -0.46, p = 0.64) (Table 1).
Table 1.
Summary table for Generalized Additive Mixed Models (GAMMs) fitted to Great Tit eggshell pigmentation traits and accounted for the nest box type (standard “dark” and artificially brightened “bright” nest boxes), clutch size, date of the first egg and eggshell thickness.
| Parametric coefficients | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Term | Percent spots | Spot Rchroma | Average spot size | Spot brightness | ||||||||||||
| Estimate | SE | z | P | Estimate | SE | z | p | Estimate | SE | t | P | Estimate | SE | z | p | |
| (Intercept) | −1.69 | 0.24 | −7.12 | < 0.01 | −0.54 | 0.07 | −8.06 | < 0.01 | 2.96 | 0.64 | 4.64 | < 0.01 | −1.05 | 0.56 | −1.87 | 0.06 |
| Clutch size | 0.04 | 0.02 | 2.02 | 0.04 | 0.00 | 0.01 | −0.41 | 0.68 | 0.05 | 0.03 | 1.97 | 0.05 | −0.03 | 0.03 | −0.85 | 0.40 |
| Nest box type | 0.12 | 0.07 | 1.66 | 0.10 | −0.01 | 0.02 | −0.46 | 0.64 | 0.06 | 0.09 | 0.69 | 0.49 | 0.01 | 0.11 | 0.06 | 0.95 |
| Thickness | 239.92 | 88.38 | 2.72 | 0.007 | 233.40 | 68.46 | 3.41 | < 0.001 | ||||||||
| Approximate significance of smooth terms | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| edf | Ref.df | Chi-sq | p | edf | Ref.df | Chi-sq | p | edf | Ref.df | F | p | edf | Ref.df | Chi-sq | p | |
| s(First egg date) | 1 | 1 | 3.22 | 0.07 | 4.38 | 4.43 | 17.64 | 0.002 | 1.00 | 1.00 | 0.98 | 0.32 | 1.00 | 1.00 | 0.04 | 0.85 |
| s(Nest box ID) | 25.4 | 28.00 | 280.22 | < 0.01 | 23.46 | 28.00 | 493.01 | < 0.01 | 24.63 | 28.00 | 7.12 | < 0.01 | 27.03 | 28.00 | 806.12 | < 0.01 |
| s(Thickness) | 2.40 | 3.10 | 4.67 | 0.22 | 1.62 | 2.05 | 0.92 | 0.61 | ||||||||
| R2adj = 0.536 | R2adj = 0.789 | R2adj = 0.442 | R2adj = 0.726 | |||||||||||||
Significant values are in bold.
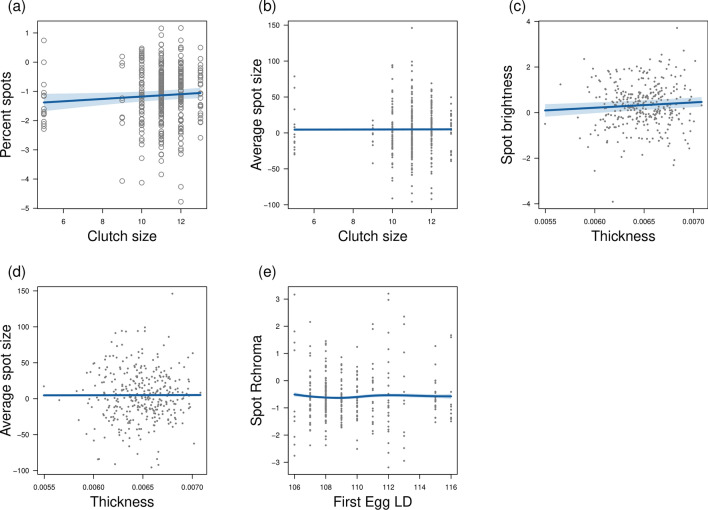
However, data showed (Table 1 and Supplementary Fig. S1) that clutch size has a positive effect on percent spots (z = 2.02, p = 0.04) (Fig. 1a) and a marginally significant effect on average spot size (t = 1.97, p = 0.05) (Fig. 1b). We also found a positive association between eggshell thickness and both spot brightness (z = 3.41, p < 0.001) (Fig. 1c) and average spot size (t = 2.72, p = 0.007) (Fig. 1d). Besides, data revealed a nonlinear relationship (chi-square = 17.64, p = 0.002) between the laying date of the first egg and spot Rchroma (Fig. 1e).
Figure 1.
(a–e) Conditional plots visualising significant effects of Great Tit egg characteristics on pigmentation traits presented on the scale of the linear predictor. Shaded areas indicate 95% confidence intervals and points represent partial residuals. LD = laying date.
Repeatability of traits
All studied egg traits were significantly repeatable within clutches (Table 2), but egg dimensions were on average more repeatable than colouration traits (0.778 vs. 0.638). According to the classification of repeatability values20, the repeatability of all egg dimensions was good (R ≥ 0.75). Among eggshell pigmentation traits, spot brightness and percent spots were moderately repeatable (0.75 > R > 0.50), spot Rchroma was the most repeatable of all the traits studied (R = 0.852), whereas average spot size had poor repeatability (R < 0.50).
Table 2.
Results of the repeatability analysis of Great Tit egg dimensions and pigmentation traits.
| Trait | Repeatability | Upper 95% CI | Lower 95% CI | p |
|---|---|---|---|---|
| Egg volume | 0.818 | 0.707 | 0.886 | < 0.001 |
| Egg length | 0.769 | 0.646 | 0.844 | < 0.001 |
| Egg width | 0.747 | 0.616 | 0.834 | < 0.001 |
| Spot Rchroma | 0.794 | 0.680 | 0.859 | < 0.001 |
| Spot brightness | 0.738 | 0.609 | 0.818 | < 0.001 |
| Percent spots | 0.564 | 0.415 | 0.676 | < 0.001 |
| Average spot size | 0.456 | 0.298 | 0.583 | < 0.001 |
Discussion
Contrary to our predictions, we found no significant effect of light conditions in the nest site on variation in eggshell pigmentation. We are confident that the obtained results are not artefacts due to the flawed methodology of eggshell colouration measurements. Standardised digital photography is an accurate and objective method widely used to score eggshell pigmentation19,21. Digital photography has an advantage over other methods because it is not based on subjective patterning scores22 and, contrary to spectrophotometry, it is capable of embracing the entire egg surface, including eggs with complicated patterning23. Moreover, our previous study showed that the light intensity manipulation used in this study was efficient enough to create distinctly different light conditions. The light intensity in the “bright” boxes is about 50 times higher than in the “dark” ones24.
The results of our study did not indicate any linkage between nest site light conditions and eggshell pigmentation, regardless of potential mechanisms of such a relationship. All measures of eggshell colouration were significantly intra-female repeatable, and the repeatability of spot red chroma could be even classified as high20. Our study confirms results from earlier studies on the Great Tit25 and other species with egg pigmentation based on protoporphyrin26, which indicated that the repeatability of eggshell colouration traits ranges approximately from 0.5 to 0.9. Since repeatability reflects the upper limit of heritability of phenotypic variation27, we can conclude that eggshell colouration in the studied Great Tit population is under both environmental and genetic control28. Another study concerning this issue was carried out on the Great Tit population in Wytham Woods, which showed that eggshell patterning traits present a certain level of heritability and are rather weakly influenced by environmental conditions28. However, a direct comparison with our study is not possible, as the variation of eggshell patterning was assessed using different methods. Moreover, in the present study, we applied within-clutch repeatability, whereas in Wytham Woods between-clutch repeatability was used. Furthermore, these two populations live in distant geographical locations that may differ in the mechanisms responsible for eggshell patterning28.
The high variability of eggshell colouration in some bird species is supposed to evolve as an adaptation to particular environmental conditions. A classic example is the Common Cuckoo (Cuculus canorus), with several genetic lineages of females specialised to produce eggs coloured to match eggs of their preferred hosts29. A similar mechanism exists even in species with more subtle variation in eggshell colouration. A recent study has shown that females inherit a preference for particular nest habitat, which provides the best camouflage for a given eggshell pigmentation30. Assuming the great importance of light for embryo development, an analogous selection pressure on eggshell pigmentation in Great Tit with regard to light intensity in the nesting site could be expected.
Despite the obvious variability in eggshell colouration, we found no differences in colouration traits between dark and bright nest boxes. It is possible that the potential benefits of better light transmission are not significant enough to cause different levels of pigment deposition between dark and bright nest boxes or that other functions of pigmentation are more important in the case of the Great Tit. On the other hand, certain benefits of improved illumination have been observed. It is known that the population of Great Tits shows clear preferences for brighter nest boxes24. In addition, offspring raised in bright nest boxes are in a slightly better condition compared to dark boxes conspecifics, as they have higher immunocompetence and fledge earlier31. The lack of relationship between eggshell colouration and nest box type revealed by our experiment could also result from the sensibility of the studied species to light. It is probable that even small amounts of light passing through the eggshell may induce beneficial effects on the embryo. Although this hypothesis awaits future studies, such a possibility is likely since the cavity-nesters have evolved in a very constrained light regime32. Another important aspect is the behavioural response of Great Tits to nest box illumination. Individuals from the studied population preferred to nest in bright nest boxes, but birds from dark nest boxes actively compensated for the light deficiency by building significantly higher nests (i.e. closer to the entrance hole)24. Thus, the response to varied light levels could be rather behavioural than physiological.
Our study was the first attempt to experimentally investigate the relationships between solar radiation in the nest site and eggshell pigmentation in wild birds. However, there are some correlative studies that link egg colouration intensity with the protection of the embryo from UV-B radiation. The first one was devoted to populations of Village Weaver (Ploceus cucullatus), which builds a woven domed nest, whose walls partly transmit sunlight. It has been demonstrated that populations exposed to a higher intensity of solar UV radiation have more pigmented eggs, as compared to a population from area situated at higher latitudes and with more frequent cloud cover33. Similarly, in a ground-nesting species, Kentish Plover (Charadrius alexandrinus), clutches laid in areas of higher UV radiation were darker34. Also Reed Warbler (Acrocephalus scirpaceus) eggshells were brighter when the clutch was laid during heavy rains, and hence, presumably, under lower illumination due to cloud cover35. The above studies suggest that eggshell pigmentation has a protective function, which is important in species breeding in open areas or areas where a threat from UV radiation is high. It is important to keep in mind, however, that due to the correlative nature of these studies, other factors could interfere with eggshell pigmentation, aside from light, e.g. geographical location, ambient temperature, precipitation or food availability.
The observed variation in eggshell pigmentation could also reflect female body condition, which was not controlled in our study. One study reported36 that lighter Great Tit females lay more pigmented eggs, but this relationship was not confirmed in other studies concerning e.g. body size25,37,38. Some studies indicate that females that lay darker eggs may be less healthy and suffer from physiological stress, because of the oxidative properties of protoporphyrin38,39, and this could in turn have a negative impact on the quality of eggs and chicks40. Another factor that could potentially affect eggshell pigmentation is female age. In the Blue Tit (Cyanistes caeruleus), a closely related species, older females tend to lay brighter eggs39.
Our study revealed that eggs from larger clutches tended to have bigger spots, which covered a slightly higher percentage of the eggshell surface. Previous research provided mixed results on this relationship. For example, an opposite relationship was shown38: bigger clutches had lower concentrations of protoporphyrin, whereas other study36 did not find any significant relationship. A possible explanation of the pattern found in the present study is that females laying bigger clutches are more deficient in calcium and use the pigment as a structural reinforcement of the eggshell4. This hypothesis, however, assumes that more protoporphyrin should be built into the eggs with thinner shells4,41. We found that the thickness and eggshell pigmentation traits are correlated: eggs with bigger and brighter spots had significantly thicker eggshells. Thus, our results partly support and partly contradict the eggshell reinforcement hypothesis. One possible interpretation is that females in superior conditions can afford to produce eggshells that are both thick and intensively pigmented. It is also possible that in eggs with thicker eggshells, the pigment gets into the deeper shell layers, and this could result in brighter spots but still keep its efficient filtering function. Alternatively, the trade-off between the number of spots and their colouration is possible. Assuming that protoporphyrin is a limited resource, females that produce more densely spotted eggs must reduce their colour intensity.
We found that the spot red chroma intensity varied nonlinearly with the first egg laying date, as it decreased from the beginning of the season to the 19th of April, next increased until the 22nd of April, and slightly decreased later (Supplementary Fig. S1). Similar studies on the same species have shown that egg pigmentation intensity and spotting coverage decrease during the season28,42, or did not find any relationship36. Moreover, Great Tits tend to produce more pigmented eggs in poorer environmental conditions: during colder weather, food shortage, and higher breeding density42. Although we did not measure these variables, the red chroma fluctuating pattern may be explained by changing environmental conditions during the season, especially varying temperature.
One of the limitations of our study is the fact that the experiment was conducted on a Great Tit population breeding in nest boxes, where light conditions may significantly differ from those experienced in natural hollows. Although this topic remains largely unexplored, it is presumed that natural hollows are generally very dark, at least at dawn43,44. On the other hand, they can be brighter, depending on the time of day and size, shape, orientation of the entrance, and whether there are additional openings such as cracks and crevices in the trunk44. Therefore, we decided to use two extreme light intensities to investigate its potential impact, although these may not accurately reflect the conditions Great Tits naturally experience in the nesting site. However, the use of artificial cavities has a number of advantages over natural hollows, which are scarce in most managed forests. They provide unobstructed and quick access to the nest and eggs, the possibility to monitor broods45, and to manipulate physical factors, e.g., light or temperature46. Another limitation of our study is that it was performed during only one season. Conducting the experiment in subsequent years under varied environmental conditions could provide a more comprehensive understanding of how eggshell pigmentation interacts with light conditions at the nesting site. Moreover, the design of our study was inadequate to check whether light intensity in the nesting site could shape eggshell pigmentation on a longer time scale, since we investigated only one generation of birds and lacked pedigree data. Our Great Tit population was exposed to extreme light conditions for a period of time that was too short (three years since the nest boxes were hung) to cause significant evolutionary changes. Additionally, studies on other cavity-nesting species may be beneficial, as light sensitivity may be species-specific.
In the present study, we found no relationship between light intensity at the nesting site and eggshell pigmentation traits in the Great Tit. Repeatability values of eggshell pigmentation characteristics suggest that they may depend on both genetic and environmental factors. Many other factors, such as the breeding female condition, which was not controlled in this study, could contribute to much of the observed variation in eggshell colouration. Moreover, our study did not find unequivocal support for the eggshell reinforcement function of protoporphyrin. Further research is required to test the sensibility of embryos of secondary hole nesters to dim light and its long-term effect on their condition. Finally, long-term investigations embracing several female generations breeding in conditions of natural cavity illumination are necessary to test whether there exists any interaction between cavity illumination level, nest choice, and intensity of eggshell pigmentation.
Methods
Study area and experimental design
The experiment was performed in 2018 in Wielkopolski National Park, near Poznań in western Poland (52°16′07″N, 16°47′53″E). The study area (48.35 ha) was covered by deciduous and mixed forests47. A total number of 159 nest boxes (width 12.0 cm, length 16.0 cm, height 40.0 cm; entrance diameter 3.3 cm) were evenly distributed over the study area in 2014. The side walls of each nest box were equipped with a semi-transparent, round resin window (5.0 cm) with a moveable opaque black plastic shutter. By using shutters, we could make the boxes bright (with open shutters) or dark (with closed shutters). For more detailed information on the nest box construction, see45. In total, we analysed data from 14 dark and 18 bright nest boxes. From the beginning of March, nest boxes were regularly monitored every 3–5 days. Trail cameras were placed in the nest boxes to determine the date of the first laid egg and the clutch size45.
Photographs
Approximately in the middle of the incubation period photographs of clutches were taken. Eggs were gently removed from the nest and placed on a black holder made of a black plastic mass and featured with a scale (mm), and reflection standards (99% and 60%, Labsphere, NH USA, Fig. 2). We took photographs only during sunny weather, in a patch of sunlight. In order to disperse light and illuminate the eggs evenly, they were surrounded with a sheet of white polytetrafluoroethylene (PTFE), 0.5 mm thick. The holder was attached to a 15-cm tripod to keep the eggs in a fixed position. If a clutch was larger than 12 eggs, two shots were taken. The photos were taken with Canon 7D Mark II SLR with Canon Zoom EF-S 18–135 mm (1.35–5.6) lens. The camera was placed on a tripod with the lens pointing down at a 90-degree angle. All shots were done with a focal length of 85 mm, aperture f/8 and ISO 640. To eliminate camera shake, we took photos with the self-timer function. For each photo, the automatic exposure bracketing function (AEB, −1, 0, +1 EV) was used and all images were saved as RAW files. After this procedure, the eggs were returned to the nest box without delay. All methods were approved by the Regional Directorate for Environmental Protection, the Ministry of Climate and Environment, and performed in accordance with Polish law.
Figure 2.

Example photo of eggs from one Great Tit clutch.
Digital image analysis
The collected RAW photos were screened in RAWTherapee software to select the shots with the best exposure. Then, we processed the photos in ImageJ software48 with MICA Toolbox plug-in, version 1.2221. First, we selected grey standards to normalise the images and set a scale. Next, we selected regions of interest (i.e. eggs) and recorded information about egg dimensions (length, width, volume, and surface area). Furthermore, we estimated the percentage of egg surface occupied by spots by creating a binary mask. To prepare the mask, we used a built-in ImageJ thresholding function with the following settings: 20-px radius and Phansalkar method. We worked out the settings empirically, visually comparing the mask with the original image of an egg in the green channel. We selected the green channel as it had been recognised to match the spectral sensitivity of avian double cones49. The average spot size was calculated using an ImageJ built-in function "Analyse Particles", and the spot reflectance was estimated by customising Batch Multispectral Image Analysis function from MICA Toolbox. This way, we computed the spot brightness as a sum of the reflectance in the red, green, and blue channels (it is thus the overall brightness in the whole range of wavelengths to which the camera is sensitive), and the red chroma of spots as the reflectance in the red channel divided by brightness. For the purpose of the analysis, spot brightness was divided by 300 to obtain values within the 0–1 range. All our custom-written macro files used during the digital image analysis are available at the GitHub repository.
Statistical analysis
The following eggshell pigmentation characteristics were used as response variables: the percentage of spotting coverage (percent spots), the average size of spots (average spot size), the red chroma of spots (spot Rchroma), and the brightness of spots (spot brightness). Our choice was based on the results of previous studies, stating that these variables correlate significantly with protoporphyrin content16,38. We tested the effect of nest box type on pigmentation, while controlling for the date of the first egg laid (expressed as day number since the beginning of the year), clutch size, and eggshell thickness. The latter was calculated using the formula (1)50,51.
| 1 |
Generalised Additive Mixed Models (GAMMs)52 were used to test whether the nest box type affects eggshell pigmentation traits. We assumed that percent spots, spot brightness, and spot Rchroma originate from a beta distribution with the logit link function, while average spot size followed a Gaussian distribution with the logarithmic link function. The ID of the nest box was used as a random effect. The date of the first egg laid and the eggshell thickness were fitted as smooth terms, but when they were significant at 1.0 effective degrees of freedom, they were refitted as parametric terms. GAMMs were fitted using a function from the mgcv package52, diagnosed with tools from the DHARMa package53, and visualised by the plotting function provided by the visreg package54. Within-clutch repeatability of egg dimensions, average spot size, as well as arcsine-transformed percent spots, spot brightness, and spot Rchroma was calculated as an intra-class correlation coefficient, using the LMM-based approach for Gaussian data and functions from the rptR package55. Statistical analyses were conducted in R 4.2 software56.
Supplementary Information
Acknowledgements
We thank Wielkopolski National Park authorities and workers for their generous help during the project. We are grateful to Lechosław Kuczyński, who provided valuable advice regarding statistical analysis. We also thank two anonymous reviewers whose comments greatly improved the manuscript. The study was funded by a grant from the National Science Centre in Poland (no. 2013/09/B/NZ8/03280). KM was financially supported by National Science Centre (NCN) in Poland (grant no. 2018/29/B/NZ8/00066) when taking part in the research. PP was supported by the National Science Centre doctoral scholarship (no. 2019/32/T/NZ8/00256) when taking part in this research. KS was financially supported by the Polish Ministry of Science and Higher Education as a part of the Diamond Grant programme for 2019–2023 (grant no. 0228/DIA/2019/48) during this study.
Author contributions
A.S., P.P., K.M., and K.S. conceived the idea, A.S., P.P., and K.M. collected the field data, K.M. performed the analysis, K.S. contributed with scripts for image analysis, all authors wrote the manuscript and contributed to its later version. All authors gave final approval for publication.
Data availability
Data are deposited in the Zenodo archive https://doi.org/10.5281/zenodo.7653168. All our custom-written macro files used during the digital image analysis are available at the GitHub repository https://github.com/KlaudiaSzala/eggshell-spots.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-36658-4.
References
- 1.Sparks NHC. Eggshell pigments: From formation to deposition. Avian Biol Res. 2011;4:162–167. doi: 10.3184/175815511X13228269481875. [DOI] [Google Scholar]
- 2.Díaz Lora S, et al. Hoopoe Upupa epops male feeding effort is related to female cosmetic egg colouration. J. Avian Biol. 2020 doi: 10.1111/jav.02433. [DOI] [Google Scholar]
- 3.Hamchand R, Hanley D, Prum RO, Brückner C. Expanding the eggshell colour gamut: Uroerythrin and bilirubin from tinamou (Tinamidae) eggshells. Sci Rep. 2020;10:11264. doi: 10.1038/s41598-020-68070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gosler AG, Higham JP, Reynolds SJ. Why are birds’ eggs speckled? Ecol Lett. 2005;8:1105–1113. doi: 10.1111/j.1461-0248.2005.00816.x. [DOI] [Google Scholar]
- 5.Troscianko J, Wilson-Aggarwal J, Stevens M, Spottiswoode CN. Camouflage predicts survival in ground-nesting birds. Sci Rep. 2016;6:19966. doi: 10.1038/srep19966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace, A. R. Darwinism: An Exposition of the Theory of Natural Selection, with Some of Its Applications. (Cambridge University Press, 1889).
- 7.Birkhead TR. Behavioural adaptations to high density nesting in the common guillemot Uria aalge. Anim. Behav. 1978;26:321–331. doi: 10.1016/0003-3472(78)90050-7. [DOI] [Google Scholar]
- 8.Lahti DC, Lahti AR. How precise is egg discrimination in weaverbirds? Anim. Behav. 2002;63:1135–1142. doi: 10.1006/anbe.2002.3009. [DOI] [Google Scholar]
- 9.Bakken GS, Vanderbilt VC, Buttemer WA, Dawson WR. Avian eggs: Thermoregulatory value of very high near-infrared reflectance. Science. 1978;1979(200):321–323. doi: 10.1126/science.200.4339.321. [DOI] [PubMed] [Google Scholar]
- 10.Wisocki PA, et al. The global distribution of avian eggshell colours suggest a thermoregulatory benefit of darker pigmentation. Nat. Ecol. Evol. 2020;4:148–155. doi: 10.1038/s41559-019-1003-2. [DOI] [PubMed] [Google Scholar]
- 11.Moreno J, Osorno JL. Avian egg colour and sexual selection: Does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett. 2003;6:803–806. doi: 10.1046/j.1461-0248.2003.00505.x. [DOI] [Google Scholar]
- 12.Maurer G, Portugal SJ, Cassey P. Review: An embryo’s eye view of avian eggshell pigmentation. J. Avian Biol. 2011;42:494–504. doi: 10.1111/j.1600-048X.2011.05368.x. [DOI] [Google Scholar]
- 13.Maurer G, et al. First light for avian embryos: Eggshell thickness and pigmentation mediate variation in development and UV exposure in wild bird eggs. Funct. Ecol. 2015;29:209–218. doi: 10.1111/1365-2435.12314. [DOI] [Google Scholar]
- 14.Kilner RM. The evolution of egg colour and patterning in birds. Biol. Rev. Camb. Philos. Soc. 2006;81:383–406. doi: 10.1017/S1464793106007044. [DOI] [PubMed] [Google Scholar]
- 15.Lahti DC, Ardia DR. Shedding light on bird egg color: Pigment as parasol and the dark car effect. Am Nat. 2016;187:547–563. doi: 10.1086/685780. [DOI] [PubMed] [Google Scholar]
- 16.Brulez K, et al. Eggshell pigment composition covaries with phylogeny but not with life history or with nesting ecology traits of British passerines. Ecol Evol. 2016;6:1637–1645. doi: 10.1002/ece3.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avilés JM, Pérez-Contreras T, Navarro C, Soler JJ. Dark nests and conspicuousness in color patterns of nestlings of altricial birds. Am Nat. 2008;171:327–338. doi: 10.1086/527493. [DOI] [PubMed] [Google Scholar]
- 18.Lack D. The significance of the colour of Turdine eggs. Ibis. 1958;100:146–166. doi: 10.1111/j.1474-919X.1958.tb08786.x. [DOI] [Google Scholar]
- 19.Wegmann M, Vallat-Michel A, Richner H. An evaluation of different methods for assessing eggshell pigmentation and pigment concentration using great tit eggs. J Avian Biol. 2015;46:597–607. doi: 10.1111/jav.00495. [DOI] [Google Scholar]
- 20.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troscianko J, Stevens M. Image calibration and analysis toolbox: A free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol Evol. 2015;6:1320–1331. doi: 10.1111/2041-210X.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brulez K, et al. Eggshell spot scoring methods cannot be used as a reliable proxy to determine pigment quantity. J. Avian Biol. 2014;45:94–102. doi: 10.1111/j.1600-048X.2013.00236.x. [DOI] [Google Scholar]
- 23.Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko TS. Using digital photography to study animal coloration. Biol J Linn Soc. 2007;90:211–237. doi: 10.1111/j.1095-8312.2007.00725.x. [DOI] [Google Scholar]
- 24.Podkowa P, Surmacki A. The importance of illumination in nest site choice and nest characteristics of cavity nesting birds. Sci. Rep. 2017;7:1329. doi: 10.1038/s41598-017-01430-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giordano M, Costantini D, Pick JL, Tschirren B. Female oxidative status, egg antioxidant protection and eggshell pigmentation: A supplemental feeding experiment in great tits. Behav. Ecol. Sociobiol. 2015;69:777–785. doi: 10.1007/s00265-015-1893-1. [DOI] [Google Scholar]
- 26.Surmacki A, Kuczyński L, Tryjanowski P. Eggshell patterning in the Red-backed Shrike Lanius collurio: Relation to egg size and potential function. Acta Ornithol. 2006;41:145–151. doi: 10.3161/000164506780143861. [DOI] [Google Scholar]
- 27.Christians JK. Avian egg size: Variation within species and inflexibility within individuals. Biol Rev. 2002;77:1–26. doi: 10.1017/S1464793101005784. [DOI] [PubMed] [Google Scholar]
- 28.Gosler AG, Barnett PR, Reynolds SJ. Inheritance and variation in eggshell patterning in the great tit Parus major. Proc. R. Soc. B. 2000;267:2469–2473. doi: 10.1098/rspb.2000.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avilés JM. Egg colour mimicry in the common cuckoo Cuculus canorus as revealed by modelling host retinal function. Proc. R. Soc. B. 2008;275:2345–2352. doi: 10.1098/rspb.2008.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gómez J, et al. Individual egg camouflage is influenced by microhabitat selection and use of nest materials in ground-nesting birds. Behav. Ecol. Sociobiol. 2018;72:142. doi: 10.1007/s00265-018-2558-7. [DOI] [Google Scholar]
- 31.Podkowa P, Surmacki A. The effect of daylight exposure on the immune response and body condition of Great Tit nestlings. J Ornithol. 2023;164:203–216. doi: 10.1007/s10336-022-02017-9. [DOI] [Google Scholar]
- 32.Butler MW, Waite HS. Eggshell biliverdin concentration does not sufficiently predict eggshell coloration. J. Avian Biol. 2016;47:491–499. doi: 10.1111/jav.00842. [DOI] [Google Scholar]
- 33.Lahti DC. Population differentiation and rapid evolution of egg color in accordance with solar radiation. Auk. 2008;125:796–802. doi: 10.1525/auk.2008.07033. [DOI] [Google Scholar]
- 34.Gómez J, et al. Latitudinal variation in biophysical characteristics of avian eggshells to cope with differential effects of solar radiation. Ecol. Evol. 2018;8:8019–8029. doi: 10.1002/ece3.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avilés JM, Stokke BG, Moksnes A, Røskaft E, Møller AP. Environmental conditions influence egg color of reed warblers Acrocephalus scirpaceus and their parasite, the common cuckoo Cuculus canorus. Behav. Ecol. Sociobiol. 2007;61:475–485. doi: 10.1007/s00265-006-0275-0. [DOI] [Google Scholar]
- 36.Stoddard, M. C., Fayet, A. L., Kilner, R. M. & Hinde, C. A. Egg speckling patterns do not advertise offspring quality or influence male provisioning in great tits. PLoS One7. 10.1371/journal.pone.0040211 (2012). [DOI] [PMC free article] [PubMed]
- 37.de Coster G, de Neve L, Lens L. Intra-clutch variation in avian eggshell pigmentation covaries with female quality. J. Ornithol. 2013;154:1057–1065. doi: 10.1007/s10336-013-0974-z. [DOI] [Google Scholar]
- 38.Hargitai R, et al. Darker eggshell spotting indicates lower yolk antioxidant level and poorer female quality in the Eurasian Great Tit (Parus major) Auk. 2016;133:131–146. doi: 10.1642/AUK-15-128.1. [DOI] [Google Scholar]
- 39.Martínez-De La Puente J, et al. Are eggshell spottiness and colour indicators of health and condition in blue tits Cyanistes caeruleus? J. Avian Biol. 2007;38:377–384. doi: 10.1111/j.0908-8857.2007.03877.x. [DOI] [Google Scholar]
- 40.Hargitai R, Herényi M, Nagy G, Török J. Eggshell spotting pattern is related to hatching asynchrony, hematocrit value and growth of nestling great tits Parus major. J. Avian Biol. 2018 doi: 10.1111/jav.01827. [DOI] [Google Scholar]
- 41.Hargitai R, Nagy G, Herényi M, Török J. Effects of experimental calcium availability, egg parameters and laying order on Great Tit Parus major eggshell pigmentation patterns. Ibis. 2013;155:561–570. doi: 10.1111/ibi.12054. [DOI] [Google Scholar]
- 42.Hargitai R, et al. Effects of environmental conditions on the egg mass, yolk antioxidant level, eggshell thickness and eggshell spotting patterns of Great Tits (Parus major) J. Ornithol. 2016;157:995–1006. doi: 10.1007/s10336-016-1348-0. [DOI] [Google Scholar]
- 43.Wesolowski T, Maziarz M. Dark tree cavities: A challenge for hole nesting birds? J. Avian Biol. 2012;43:454–460. doi: 10.1111/j.1600-048X.2012.05704.x. [DOI] [Google Scholar]
- 44.Maziarz M, Wesołowski T. Does darkness limit the use of tree cavities for nesting by birds? J. Ornithol. 2014;155:793–799. doi: 10.1007/s10336-014-1069-1. [DOI] [Google Scholar]
- 45.Surmacki A, Podkowa P. The use of trail cameras to monitor species inhabiting artificial nest boxes. Ecol. Evol. 2022;12:1–11. doi: 10.1002/ece3.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryan SM, Bryant DM. Heating nest-boxes reveals an energetic constraint on incubation behaviour in great tits, Parus major. Proc. R. Soc. B: Biol. Sci. 1999;266:157–162. doi: 10.1098/rspb.1999.0616. [DOI] [Google Scholar]
- 47.Kudelska K, Podkowa P, Karaśkiewicz K, Surmacki A. Znaczenie skrzynek lȩgowych dla ptaków obszarów leśnych na przykladzie Wielkopolskiego Parku Narodowego. Sylwan. 2017;161:949–957. [Google Scholar]
- 48.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spottiswoode CN, Stevens M. Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc. Natl. Acad. Sci. USA. 2010;107:8672–8676. doi: 10.1073/pnas.0910486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ar A, Paganelli CV, Reeves RB, Greene DG, Rahn H. The avian egg: Water vapor conductance, shell thickness, and functional pore area. Condor. 1974;76:153–158. doi: 10.2307/1366725. [DOI] [Google Scholar]
- 51.Ojanen M. Composition of the eggs of the great tit (Parus major) and the pied flycatcher (Ficedula hypoleuca) Ann. Zool. Fennici. 1983;20:57–63. [Google Scholar]
- 52.Wood, S. N. Generalized Additive Models: An Introduction with R. (Chapman and Hall/CRC, 2017).
- 53.Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. https://CRAN.R-project.org/package=DHARMa (2022).
- 54.Breheny P, Burchett W. Visualization of regression models using visreg. R J. 2017;9:56–71. doi: 10.32614/RJ-2017-046. [DOI] [Google Scholar]
- 55.Nakagawa S, Schielzeth H. Repeatability for Gaussian and non-Gaussian data: A practical guide for biologists. Biol. Rev. 2010;85:935–956. doi: 10.1111/j.1469-185X.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- 56.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are deposited in the Zenodo archive https://doi.org/10.5281/zenodo.7653168. All our custom-written macro files used during the digital image analysis are available at the GitHub repository https://github.com/KlaudiaSzala/eggshell-spots.