Summary
Marek’s disease virus (MDV) is a highly oncogenic alphaherpesvirus that infects immune cells and causes a deadly lymphoproliferative disease in chickens. Cytokines and monoclonal antibodies promote the survival of chicken lymphocytes in vitro. Here, we describe protocols for the isolation, maintenance, and efficient MDV infection of primary chicken lymphocytes and lymphocyte cell lines. This facilitates the investigation of key aspects of the MDV life cycle in the primary target cells of viral replication, latency, genome integration, and reactivation.
For complete details on the use and execution of this protocol, please refer to Schermuly et al.,1 Bertzbach et al. (2019),2 and You et al.3 For a comprehensive background on MDV, please see Osterrieder et al.4 and Bertzbach et al. (2020).5
Subject areas: Cell Biology, Cell isolation, Cell-based Assays, Microbiology
Graphical abstract
Highlights
-
•
In vitro infection system for primary target cells of MDV
-
•
MDV infection of primary lymphocytes and lymphocyte cell lines
-
•
Facilitates evaluation of MDV lytic replication, latency, and transformation
-
•
Reduces need for animal experiments to investigate this oncogenic herpesvirus
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Marek’s disease virus (MDV) is a highly oncogenic alphaherpesvirus that infects immune cells and causes a deadly lymphoproliferative disease in chickens. Cytokines and monoclonal antibodies promote the survival of chicken lymphocytes in vitro. Here, we describe protocols for the isolation, maintenance, and efficient MDV infection of primary chicken lymphocytes and lymphocyte cell lines. This facilitates the investigation of key aspects of the MDV life cycle in the primary target cells of viral replication, latency, genome integration, and reactivation.
Before you begin
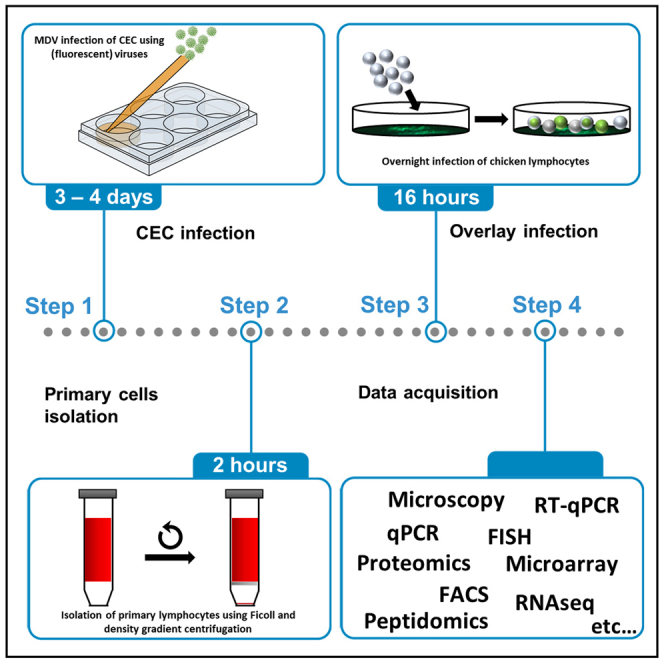
MDV is a highly cell-associated avian alphaherpesvirus that spreads exclusively from cell to cell in vitro and in vivo, while the feather follicle epithelium is the only site where cell-free virus is released. The protocol below describes the specific steps for the infection of primary chicken B-cells with MDV in vitro. However, we have also used this protocol to infect primary chicken T-cells as well as chicken B- (DT40) and T-cell lines (CU91 and other REV-transformed chicken T-cells3). These cell lines are available upon request and highly infected chicken embryo cell (CEC) monolayers need to be prepared as described below (importantly, also check Hernandez et al.6 or Schat et al.7 for information on CEC isolation and maintenance). Infection of the lymphocytes can be achieved by two strategies, by “overlay infection” or by “co-seeding” as described below.
Institutional permissions
It is important to note that these procedures were carried out strictly in accordance with the European legislation governing animal experiments (Directive 2010/63/EU) which states that animals solely used to collect organs are not subject to any ethical regulations in Europe (Article 3). Depending on the institution, this may vary and we urge readers to obtain relevant permissions before implementing this protocol.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-chicken TCR alpha/beta (Vß1) | Cihak et al.8 and Southern Biotech | 8240-01 |
| Bacterial and virus strains | ||
| Fluorescently tagged MDV | Bertzbach et al.9 and Jarosinski et al.10 | N/A |
| Experimental models: Cell lines | ||
| Chicken embryo cells | Schat et al.7 | N/A |
| Primary chicken bursal B cells | Schermuly et al.1 | N/A |
| Primary chicken thymic T cells | Schermuly et al.1 | N/A |
| DT40 | Baba et al.11 | N/A |
| CU91 | Schat et al.12 | N/A |
| MT3 cells (855-19) | You et al.3 and Vychodil et al.13 | N/A |
| Other | ||
| Lymphocyte separating solution, density 1.077 g/mL | Capricorn | LSM-A |
| RPMI 1640 | PAN Biotech | P04-17500 |
| IMDM | Gibco | 31980022 |
| MEM | PAN Biotech | P04-00509 |
| Penicillin/streptomycin | AppliChem | A1837 and A1852 |
| FBS | PAN Biotech | P30-1506 |
| Chicken serum | Gibco | 16110-082 |
| Sodium pyruvate | PAN Biotech | P04-43100 |
| Non-essential amino acids | PAN Biotech | P08-32100 |
| Chicken CD40L | Tregaskes et al.,14 Kothlow et al.,15 and Dulwich et al.16 | N/A |
Materials and equipment
Growth media
Store at 4°C, pre-warm to 37°C in a water bath before usage.
-
•
CEC medium
| MEM | N/A |
| FBS | 10% |
| Antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) | 1% |
-
•
Overlay infection medium
| RPMI | N/A |
| FBS | 10% |
| Sodium pyruvate | 1.5% |
| Non-essential amino acids | 1.5% |
| Antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) | 1% |
-
•
Co-seeding infection medium
| IMDM | N/A |
| FBS | 8% |
| Chicken serum | 2% |
| Antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) | 1% |
Step-by-step method details
Propagation of MDV-infected CEC monolayers
Timing: 3–4 days
Because of the highly species-specific and cell-associated nature of MDV, it is necessary to utilize susceptible cells that are easily infectable in vitro to initiate infection in primary chicken lymphocytes or lymphocyte cell lines. The subsequent step involves the propagation of MDV-infected CEC monolayers.
-
1.
Propagate CEC monolayers to a confluency of 80–90% (usually 1–2 days, depending on the cell passage used).
-
2.
Trypsinize cells and mix 1–2 × 106 of the CEC with MDV-infected CEC (3 × 104 plaque-forming units [pfu]) per 6-well (∼9.6 m2).
-
3.
Seed cell mixture in 6-wells and incubate at 37°C under a 5% CO2 atmosphere for 3–4 days.
Alternatives: Prepare frozen MDV stocks with very high titers (1 mL with ± 1 × 106 pfu), as efficient infection of lymphocytes can also be achieved by co-seeding of B- or T-cells with these stocks.
CRITICAL: The CEC monolayers have to be intact and highly infected (Figure 1). To obtain highly infected frozen MDV stocks, infect larger cell culture dishes (e.g. 15 cm dishes) with 3 × 104 pfu for 3–4 days until the culture is completely infected. Next, trypsinize the cells and use them to infect fresh or passaged CEC (at a 1-to-5 ratio) for another 2–3 days until almost all cells are infected.
Note: These cells can then either be directly used for co-seeding infections or frozen at −80°C or LN2 using a cryoprotective agent such as DMSO following standard protocols.17
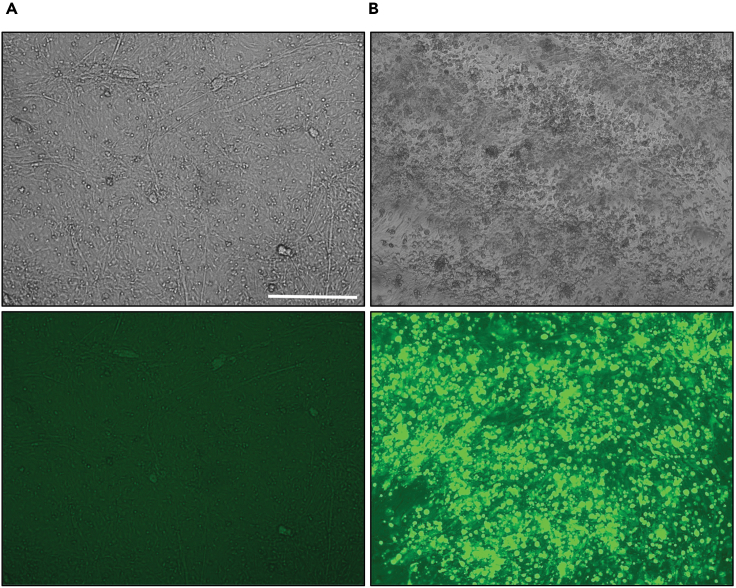
Figure 1.
MDV infection of CEC
(A and B) Representative bright-field and fluorescence microscopy images of (A) mock-infected and (B) highly infected CEC monolayers. Cells were infected with 3 × 104 pfu of a GFP-tagged MDV. The images were captured on day 4 post-infection using a Zeiss Axio Vert.A1 microscope with a magnification of 100x (scale bar: 100 μm).
The infected CEC stocks can be used for titrations, overlay infections and infection by co-seeding. It is key that the infected cells of the virus stocks are highly infected but also possess a high viability.
Isolation of primary chicken B- and T-lymphocytes
Timing: 3 h
The following steps describe the process for isolating primary chicken B-cells from the bursa of Fabricius (or T-cells from the thymus) as described by Martin et al.,18 Schermuly et al.,1 and Bertzbach et al.2
-
4.
Homogenize the bursa/thymus through a 40 μm cell strainer to obtain a uniform single-cell suspension.
-
5.
Leave at room temperature for 10 min to ensure cell clumps and cells with higher densities to sediment (i.e., inter alia, connective tissue cells, fibroblasts, erythrocytes).
-
6.
Next, carefully apply the supernatant (suspension cells) onto a lymphocyte separating solution (density 1.077 g/mL) and spin for 12 min at 650 × g with slow acceleration and deactivated deceleration.
-
7.
Carefully transfer lymphocytes at the interphase into a new tube.
-
8.
Wash lymphocytes with PBS, and maintain cells in the respective growth medium at 41°C and 5% CO2.
-
9.
Activate cells following the same descriptions (Martin et al.,18 Schermuly et al.,1 and Bertzbach et al.2) using recombinant soluble chicken CD40L (2 μg/mL) for B-cells and anti-chicken TCR alpha/beta (Vß1) antibody (5 μg/mL; e.g., clone TCR-2-coated plate).
Alternatives: Grow suspension cell cultures of chicken B- or T-cell lines.
CRITICAL: The primary lymphocytes should be isolated just before their in vitro infection (ideally within 1 h before infection). The cultures of the lymphocyte cell lines should have a high viability (>90%).
Overlay infection using MDV-infected CEC monolayers
Timing: 3–4 days
As the first alternative, we present a detailed step-by-step protocol for infecting chicken lymphocytes via an overlay infection method using MDV-infected CEC monolayers.
-
10.
Grow highly infected CEC monolayers (using 30,000 pfu per 6-well).
-
11.
Microscopically monitor virus replication for 3–4 days until the whole monolayer is infected.
-
12.
Seed 1 × 106 to 1 × 107 of the lymphocytes per well onto the infected CEC and incubate at 41°C and 5% CO2 for 16 h.
Note: For optimal contact with infected CEC and even distribution of the lymphocytes, gently sway the cell culture dish 1–2 h post seeding of the lymphocytes.
-
13.
Carefully remove suspension cells with the cell culture supernatant and pellet them at 250 × g for 5 min.
Note: For a better lymphocyte yield, a PBS-wash can be used to collect lymphocytes that remained on the CEC monolayer after the first harvest of the suspension cells.
-
14.
Resuspend the pellet in growth medium and culture the resuspended cells at 41°C and 5% CO2 to allow the remaining carryover CEC to adhere overnight.
Note: Alternatively, the cells can be directly FACS-sorted to obtain a pure infected lymphocyte population.
CRITICAL: As mentioned above, the CEC monolayers have to be intact and highly infected. The primary lymphocytes should be isolated just before the overlay infection. The cultures of the lymphocyte cell lines should have a high viability (>90%, measured e.g. by FACS).
Infection via co-seeding of lymphocytes with frozen MDV-infected CEC
Timing: 17–25 h
As a second alternative, we present step-by-step details for an infection through co-seeding of lymphocytes with frozen MDV-infected CEC.
-
15.
Thaw the frozen cryovial with the infected CEC in a water bath at 37°C (shake the cryovial until only very small ice crystals are left).
-
16.
Transfer the content of the cryovial into a 50 mL falcon.
-
17.
Add PBS or medium without FBS (very slowly, drop by drop, continuously shake the falcon tube – the cells are quite fragile) and fill the tube up to 50 mL.
-
18.
Centrifuge at 250 × g for 10 min at room temperature.
-
19.
Carefully remove (decant) supernatant.
-
20.
Resuspend the infected cell pellet in 5 mL of your lymphocyte suspension (1 × 107 cells per mL).
Note: Alternatively, the lymphocytes can be directly seeded into the wells before adding the respective amount of suspended infected CEC.
-
21.
Plate CEC/lymphocyte mix in wells of a 24-well plate, 1 mL of the suspension per well.
-
22.
Incubate the cells at 41°C and 5% CO2 for 16–24 h.
-
23.
Proceed with FACS or any other downstream analysis, following steps 13–14 of the overlay protocol above.
CRITICAL: The CEC should be highly viable at freezing and have a high pfu. The freezing media (with its DMSO) has to be removed completely during the washing (steps 15–19) to avoid carryover resulting in lymphocyte death. Again, the primary lymphocytes should be isolated just before the infection. The cultures of the lymphocyte cell lines should have the highest viabilities (>90%).
Expected outcomes
This protocol describes MDV infections of primary chicken lymphocytes and/or lymphocyte cell lines such as CU91 and DT40. To obtain a sufficient number of infected cells for downstream analyses, infection rates and cell survival have to be balanced. Optimally, the lymphocyte infection yields infection rates of up to 40–60% of the viable lymphocytes, with a high viability of the entire culture (Figure 2). These cells can be harvested and used for various downstream analyses. Indeed, a variety of studies have been conducted with lymphocytes that were infected using this protocol, including FACS, FISH, and microarrays, as well as transcriptome-, proteome- and peptidome analyses.2,3,13,19,20,21,22
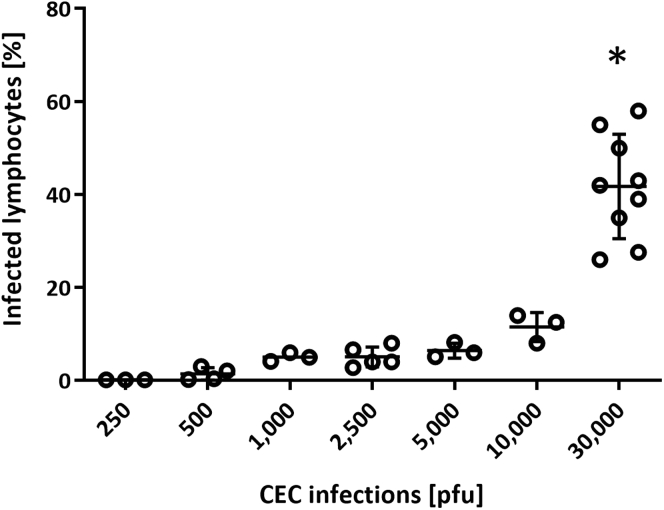
Figure 2.
Infection of chicken lymphocytes with various pfu
Exemplarity lymphocyte infection using our protocol for the overlay infection with MDV-infected CEC monolayers. Lymphocytes were seeded on the infected monolayers for 16 h, and the number of viable and infected lymphocytes was subsequently quantified by FACS (n ≥ 3). Error bars indicate standard deviations. The asterisk indicates significance (∗p < 0.05, Kruskal–Wallis test). These data have previously been published in You et al.3
Quantification and statistical analysis
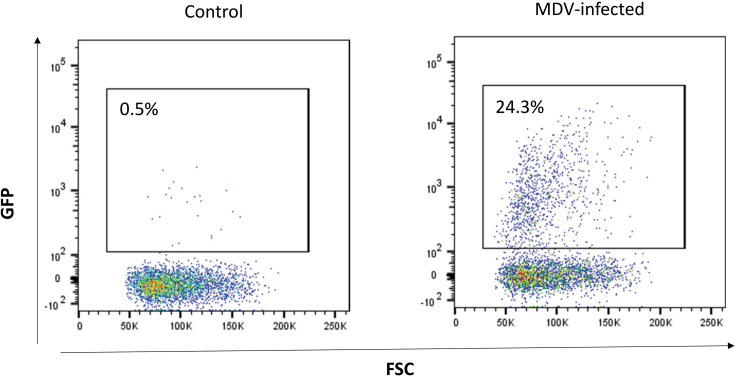
We routinely perform FACS analyses to quantify viable and infected lymphocytes using a viability dye (e.g., eBioscience Fixable Viability Dye eFluor 780) and fluorescent viral reporter viruses3,10,13 that allow detection of infected cells (Figure 3). As primary B- and T-cells are activated, they should begin to undergo blast transformation (higher FSC/SSC).
Figure 3.
Representative plot of bursal B cells that were infected with a GFP-tagged MDV using the co-seeding protocol
The infection level was assessed at 24 h post-infection by flow cytometry. Samples were gated for viable lymphocyte singlets (Fixable Viability Dye eFluor 780-negative).
Limitations
This protocol allows in vitro characterization of MDV infection of chicken lymphocytes. Therefore, animal experimentation can be complemented or even replaced by this method. The described experiments, however, might obviously not reflect the identical aspects observed in vivo including MDV-induced tumor formation.
Troubleshooting
Problem 1
Low CEC viability (apparent through high cell granulation and subsequent CEC detachment from cell culture dishes; step “propagation of MDV-infected CEC monolayers”).
Potential solution
-
•
Use low-passage CEC.
-
•
Optimize preparation of fresh CEC.
-
•
Increase the pfu for CEC infection while simultaneously reducing the incubation time from 4 to 3 days. Check cells daily under the microscope. We also recommend a flow cytometry check to assess the amount of infected (fluorescent) cells.
Problem 2
Low CEC infection rates (step “propagation of MDV-infected CEC monolayers”).
Potential solution
-
•
Optimize CEC infection protocol following the critical steps in the “propagation of MDV-infected CEC monolayers” section above.
-
•
Use low-passage CEC, ideally passages 1–2.
Problem 3
Low viability of primary chicken lymphocytes pre- or post-infection (steps “isolation of primary chicken B- and T-lymphocytes”, “overlay infection using MDV-infected CEC monolayers”, and “infection via co-seeding of lymphocytes with frozen MDV-infected CEC”).
Potential solution
-
•
Reduce the time between organ removal (bursa of Fabricius/thymus) and lymphocyte isolation.
-
•
Isolate primary cells at room temperature (instead of on ice).
-
•
Use functional CD40L/TCR-2 to activate primary lymphocytes.
-
•
Wash frozen CEC meticulously (infection via co-seeding).
Problem 4
Low viability of chicken lymphocyte cell lines pre- or post-infection (steps “isolation of primary chicken B- and T-lymphocytes”, “overlay infection using MDV-infected CEC monolayers”, and “infection via co-seeding of lymphocytes with frozen MDV-infected CEC”).
Potential solution
-
•
Heat-inactivate serum that is used as a supplement for growth media.
-
•
Split cells regularly to dilute out dead cells and cell debris.
-
•
Wash frozen CEC meticulously before infection (infection via co-seeding).
Problem 5
Low infection rates in chicken lymphocytes (steps “overlay infection using MDV-infected CEC monolayers” and “infection via co-seeding of lymphocytes with frozen MDV-infected CEC”).
Potential solution
-
•
Use highly viable lymphocytes for infection.
Note: infection rate decreases if MDV-infected CEC were cultured too long (overlay infection) or MDV stocks harvested too late (co-seeding) as both decrease CEC viability.
-
•
Ensure the use of highly infected and viable CEC.
-
•
Avoid excessive resuspension of primary lymphocytes when dissolving the CEC pellet. Lymphocytes can also be added to the plate first, followed by the infected CEC (see step 20, co-seeding infection).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Benedikt B. Kaufer (benedikt.kaufer@fu-berlin.de).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze datasets/code.
Acknowledgments
We thank Drs. Andelé M. Conradie and Tereza Vychodil (FUB) for refinement of this protocol. This research was funded by the Volkswagen Foundation Lichtenberg grant A112662 awarded to B.B.K. and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) in the framework of the Research Unit ImmunoChick (FOR5130) project KA 3492/9-1 awarded to S.H. and B.B.K. We acknowledge support from the Open Access Publication Fund of the Freie Universität Berlin.
Author contributions
Conceptualization, visualization, writing, editing, L.D.B.; conceptualization, editing, M.K., Y.Y., L.K., M.A.S., A.K.; conceptualization, editing, supervision, S.H.; conceptualization, editing, supervision, B.B.K.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Sonja Härtle, Email: sonja.haertle@lmu.de.
Benedikt B. Kaufer, Email: benedikt.kaufer@fu-berlin.de.
References
- 1.Schermuly J., Greco A., Härtle S., Osterrieder N., Kaufer B.B., Kaspers B. In vitro model for lytic replication, latency, and transformation of an oncogenic alphaherpesvirus. Proc. Natl. Acad. Sci. USA. 2015;112:7279–7284. doi: 10.1073/pnas.1424420112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertzbach L.D., Pfaff F., Pauker V.I., Kheimar A.M., Höper D., Härtle S., Karger A., Kaufer B.B. The transcriptional landscape of Marek's disease virus in primary chicken B cells reveals novel splice variants and genes. Viruses. 2019;11 doi: 10.3390/v11030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You Y., Vychodil T., Aimola G., Previdelli R.L., Göbel T.W., Bertzbach L.D., Kaufer B.B. A cell culture system to investigate Marek’s disease virus integration into host chromosomes. Microorganisms. 2021;9:2489. doi: 10.3390/microorganisms9122489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osterrieder N., Kamil J.P., Schumacher D., Tischer B.K., Trapp S. Marek's disease virus: from miasma to model. Nat. Rev. Microbiol. 2006;4:283–294. doi: 10.1038/nrmicro1382. [DOI] [PubMed] [Google Scholar]
- 5.Bertzbach L.D., Conradie A.M., You Y., Kaufer B.B. Latest Insights into Marek's disease virus pathogenesis and tumorigenesis. Cancers. 2020;12 doi: 10.3390/cancers12030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez R., Brown D.T. Growth and maintenance of chick embryo fibroblasts (CEF). Curr Protoc Microbiol Appendix. 2010;4:4I. doi: 10.1002/9780471729259.mca04is17. [DOI] [PubMed] [Google Scholar]
- 7.Schat K., Sellers H. In: A Laboratory Manual for the Identification and Characterization of Avian Pathogens. Dufour-Zavala L., Swayne D., Glisson J., Pearson J., Reed W., Jackwood M., Woolcock P., editors. American Association of Avian Pathologists); 2008. Cell-culture methods; pp. 195–203. [Google Scholar]
- 8.Cihak J., Ziegler-Heitbrock H.W., Trainer H., Schranner I., Merkenschlager M., Lösch U. Characterization and functional properties of a novel monoclonal antibody which identifies a T cell receptor in chickens. Eur. J. Immunol. 1988;18:533–537. doi: 10.1002/eji.1830180407. [DOI] [PubMed] [Google Scholar]
- 9.Bertzbach L.D., van Haarlem D.A., Härtle S., Kaufer B.B., Jansen C.A. Marek's disease virus infection of natural killer cells. Microorganisms. 2019;7:588. doi: 10.3390/microorganisms7120588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarosinski K.W., Arndt S., Kaufer B.B., Osterrieder N. Fluorescently tagged pUL47 of Marek's disease virus reveals differential tissue expression of the tegument protein in vivo. J. Virol. 2012;86:2428–2436. doi: 10.1128/JVI.06719-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baba T.W., Giroir B.P., Humphries E.H. Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology. 1985;144:139–151. doi: 10.1016/0042-6822(85)90312-5. [DOI] [PubMed] [Google Scholar]
- 12.Schat K.A., Pratt W.D., Morgan R., Weinstock D., Calnek B.W. Stable transfection of reticuloendotheliosis virus-transformed lymphoblastoid cell lines. Avian Dis. 1992;36:432–439. [PubMed] [Google Scholar]
- 13.Vychodil T., Wight D.J., Nascimento M., Jolmes F., Korte T., Herrmann A., Kaufer B.B. Visualization of Marek's disease virus genomes in Living cells during lytic replication and latency. Viruses. 2022;14 doi: 10.3390/v14020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tregaskes C.A., Glansbeek H.L., Gill A.C., Hunt L.G., Burnside J., Young J.R. Conservation of biological properties of the CD40 ligand, CD154 in a non-mammalian vertebrate. Dev. Comp. Immunol. 2005;29:361–374. doi: 10.1016/j.dci.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Kothlow S., Morgenroth I., Tregaskes C.A., Kaspers B., Young J.R. CD40 ligand supports the long-term maintenance and differentiation of chicken B cells in culture. Dev. Comp. Immunol. 2008;32:1015–1026. doi: 10.1016/j.dci.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Dulwich K.L., Asfor A.S., Gray A.G., Nair V., Broadbent A.J. An ex vivo chicken primary bursal-cell culture model to study infectious bursal disease virus pathogenesis. J. Vis. Exp. 2018 doi: 10.3791/58489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Springer S.T., Stiff P.C., Jr., Lee H.H. A simplified and effective method for freezing and storage of chick embryo fibroblasts at -79°C. Cryobiology. 1969;6:130–132. doi: 10.1016/s0011-2240(69)80475-x. [DOI] [PubMed] [Google Scholar]
- 18.Martin A., Lillehoj H.S., Kaspers B., Bacon L.D. Antigen-specific T cell proliferation following coccidia infection. Poult. Sci. 1993;72:2084–2094. doi: 10.3382/ps.0722084. [DOI] [PubMed] [Google Scholar]
- 19.Bencherit D., Remy S., Le Vern Y., Vychodil T., Bertzbach L.D., Kaufer B.B., Denesvre C., Trapp-Fragnet L. Induction of DNA Damages upon Marek's disease virus infection: Implication in viral replication and Pathogenesis. J. Virol. 2017;91:e01658-17. doi: 10.1128/JVI.01658-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halabi S., Ghosh M., Stevanović S., Rammensee H.G., Bertzbach L.D., Kaufer B.B., Moncrieffe M.C., Kaspers B., Härtle S., Kaufman J. The dominantly expressed class II molecule from a resistant MHC haplotype presents only a few Marek's disease virus peptides by using an unprecedented binding motif. PLoS Biol. 2021;19:e3001057. doi: 10.1371/journal.pbio.3001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapp-Fragnet L., Schermuly J., Kohn M., Bertzbach L.D., Pfaff F., Denesvre C., Kaufer B.B., Härtle S. Marek's disease virus prolongs survival of primary chicken B-cells by inducing a senescence-like phenotype. PLoS Pathog. 2021;17:e1010006. doi: 10.1371/journal.ppat.1010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chasseur A.S., Trozzi G., Istasse C., Petit A., Rasschaert P., Denesvre C., Kaufer B.B., Bertzbach L.D., Muylkens B., Coupeau D. Marek's disease virus virulence genes encode circular RNAs. J. Virol. 2022;96:e0032122. doi: 10.1128/jvi.00321-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze datasets/code.