Summary
Age-related loss of intestinal barrier function has been documented across species, but the causes remain unknown. The intestinal barrier is maintained by tight junctions (TJs) in mammals and septate junctions (SJs) in insects. Specialized TJs/SJs, called tricellular junctions (TCJs), are located at the nexus of three adjacent cells, and we have shown that aging results in changes to TCJs in intestines of adult Drosophila melanogaster. We now demonstrate that localization of the TCJ protein bark beetle (Bark) decreases in aged flies. Depletion of bark from enterocytes in young flies led to hallmarks of intestinal aging and shortened lifespan, whereas depletion of bark in progenitor cells reduced Notch activity, biasing differentiation toward the secretory lineage. Our data implicate Bark in EC maturation and maintenance of intestinal barrier integrity. Understanding the assembly and maintenance of TCJs to ensure barrier integrity may lead to strategies to improve tissue integrity when function is compromised.
Subject areas: Entomology, Molecular biology, Cell biology
Graphical abstract

Highlights
-
•
Bark is lost from the tricellular junction in ECs of old flies
-
•
Bark is required in enterocytes (ECs) to maintain intestinal homeostasis
-
•
Bark is required in enteroblasts (EBs) for proper differentiation of progenitor cells
-
•
Loss of bark may contribute to age-related loss of intestinal homeostasis
Entomology; Molecular biology; Cell biology
Introduction
The intestinal barrier allows selective paracellular transport of water, ions, and nutrients while maintaining food and microbes inside the intestinal lumen.1 Barrier integrity is crucial to intestinal homeostasis because leakage of potentially harmful antigens or microbes could be detrimental to interstitial tissues. There is a strong correlation between aging and decline in intestinal barrier integrity across multiple species, including Drosophila melanogaster.2,3,4,5,6,7 Age-associated loss of the intestinal barrier in Drosophila is associated with systemic metabolic defects, such as changes in insulin/insulin-like growth factor signaling, intestinal dysbiosis, chronic expression of inflammatory genes, and an increase in proliferation of intestinal stem cells (ISCs).7,8,9 Intercellular occluding junctions, referred to as tight junctions (TJs) in vertebrates and septate junctions (SJs) in arthropods, play a major role in maintenance of the intestinal barrier.10,11,12 Although TJs and SJs appear different ultrastructurally, they share many homologous proteins, and both TJs and SJs restrict passive, paracellular transport. TJs use a branched network of independently sealing strands to create a semipermeable barrier at “kissing points” between adjacent membranes, whereas SJs reduce net solute diffusion by increasing diffusional distance across the junction with bridge-like structures called septa.13,14 In Drosophila, pleated SJs (pSJs) are found in epithelia derived from ectoderm, whereas smooth SJs (sSJs) are found in endoderm-derived tissues, such as the midgut.15,16,17 Similar to TJs, sSJs in Drosophila are located apical to adherens junctions,17 making the Drosophila midgut a tractable model system for studying mammalian TJs, such as those that maintain the mammalian intestinal barrier.
The Drosophila posterior midgut (PMG) is functionally analogous to the human small intestine.18 The PMG epithelium is composed primarily of absorptive enterocytes (ECs), polyploid cells with microvilli that extend into the gut lumen and facilitate the absorption of nutrients. ECs and secretory enteroendocrine (EE) cells are maintained by a pool of ISCs that are able to self-renew by producing new ISCs. ISCs primarily generate daughter enteroblasts (EBs), which then differentiate into either mature ECs or EE cells.18 EB fate is specified by activation of the Notch signaling pathway,19,20 leading to the expression of the transcription factor klumpfuss (klu) in EBs. EE cell and EC fate decisions become stochastic on loss of lineage specifying klu, and EB fates can be tracked using specific klu-based lineage tracing tools (e.g. kluReDDM).21,22
In aged flies, ISC proliferation increases dramatically, as measured by an increase in cells that are positive for the mitotic marker phospho-histone H3 (pHH3).3,23,24,25,26 The increase in progenitor cells is coupled with a delay in differentiation, resulting in an accumulation of cells co-expressing the ISC/EB markers Escargot, Delta, and reporters of the Notch signaling pathway.3 Smooth SJs are found between adjacent ECs and between ECs and EE cells, and our lab has demonstrated previously that integrity of sSJs between ECs is required for maintenance of the intestinal barrier in the midgut.10 Thus, additional intestinal phenotypes exhibited by aged flies include loss of sSJ physical integrity (visible gaps between membranes)10 and loss of the intestinal barrier.7 Indeed, electron micrographs of ECs in aged flies revealed gaps between adjacent ECs, suggesting a loss of sSJ integrity over time.10 Gaps in SJs correlated with changes in localization of key sSJ components in PMGs from old flies. Immunofluorescence (IF) imaging revealed decreased staining intensity for several sSJ components at the junction, relative to the cytoplasm, with a concomitant increase in staining intensity in the cytoplasm, relative to what was observed for cells in intestines from young flies.10
In Drosophila, specialized junctions, referred to as tricellular junctions, are found where three adjacent cells meet. Similar to sSJ proteins, the tricellular septate junction (tSJ) protein Gliotactin (Gli) was lost from the tSJ and increasingly localized to cytoplasmic puncta in ECs from aged flies.10 Bark beetle (Bark), another tSJ-specific protein, also referred to as Anakonda, is required for proper Gli localization to the tSJ in the fly embryonic epithelium. Bark and Gli colocalize at the tSJ in both pSJs and sSJs.27,28 Because Gli is required at tSJs between ECs to maintain intestinal homeostasis,10 and because proper Gli localization was disrupted in aged flies, we wanted to determine whether Bark also plays a role in the adult intestine. Here, we show that Bark localizes to the tSJ and that it is required in both EBs and mature ECs to maintain intestinal homeostasis.
Results
Bark is expressed in the adult intestine
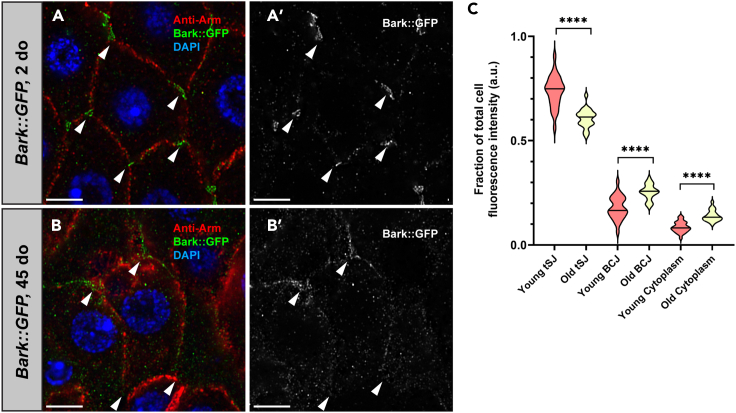
Bark is a type I transmembrane protein with a tripartite extracellular domain; it has been proposed that Bark forms a trimer, which acts as a diaphragm in the center of the tSJ canal.27 Bark was demonstrated to be required for proper localization of Gli and maturation of the pSJ in the embryonic epithelium.27 To determine whether Bark has a role in the adult intestine, PMGs from young (2 do) flies expressing a GFP-tagged form of Bark (hereafter referred to as Bark::GFP, see STAR Methods)29 were stained with anti-Bark antibody.28 Bark localized to tSJs in both the PMG and hindgut, similar to what was observed in the embryonic epithelium27 (Figures 1, S1, and 2A). Specifically, in the PMG, Bark localized to the tSJ between adjacent ECs and ECs and EE cells. By contrast, in aged (40-day-old, do) flies, Bark localization decreased at the tSJ between adjacent ECs and appeared to spread along the bicellular junction, with modest accumulation in the cytoplasm (Figures 2B, 2B′, and 2C). Loss of Bark from the tSJ in PMGs from aged flies is similar to what was observed for Gli; however, previous studies indicated significantly more accumulation of Gli in the cytoplasm, when compared to Bark.10
Figure 1.
Anakonda/Bark beetle (Bark) localizes to the tricellular septate junction (tSJ) in the adult Drosophila intestine
(A–A″) Representative staining for Bark (antisera, red) (Bark::GFP, GFP, green) in the posterior midgut (M) of young (2 do) flies. Pylorus (P) and hindgut (H) to the right. DNA is stained with DAPI (blue). Scale bars, 50 μm.
(B–B″) High magnification view of an enterocyte (EC) showing staining of endogenous Bark protein and GFP-tagged Bark at the tSJ (arrowheads). Scale bars, 5 μm.
(C–C″) High magnification view of an EC showing GFP-tagged Bark colocalizes with the bicellular septate junction (bSJ) protein Discs large 1 (Dlg1, red) at the tSJ (arrowheads). Scale bars, 5 μm.
Figure 2.
Bark is lost from the tSJ in aged flies
(A) In young (2 do) flies, Bark localizes to the tSJ (arrowheads) in ECs, whereas it is no longer detected at the tSJ in the aged (45 do) flies. Scale bars, 5 μm.
(B and B′) Adherens junctions (Armadillo, Arm, red); Bark (GFP, green); DNA (DAPI, blue). Scale bars, 5 μm.
(C) Quantification reveals that Bark is increased at the bicellular septate junction (bSJ) or in the cytoplasm of old flies. 3 cells were analyzed per PMG. young, n = 26; old, n = 22. 3 cells were measured per PMG. Each clearly visible tSJ in the cell was measured. 6 measurements were taken of the BCJ and cytoplasm in each cell, respectively. Quantification of fluorescence intensity ratios for Bark (C) in the bicellular junction (BCJ), tSJ, and cytoplasm. Black lines indicate median; statistical significance determined by Mann-Whitney test. ns, not significant; ∗, p < 0.05; ∗∗, p < 0.001; ∗∗∗∗, p < 0.0001.
Given the previously described relationship between Bark and Gli, where Bark is required for recruitment of Gli to the tSJ in developing embryonic epithelia,27 we wanted to determine whether Bark is required for Gli localization in the adult PMG. As mutations in bark are embryonic lethal, we took advantage of an inducible gene expression system, GeneSwitch (GS), to deplete bark from ECs, specifically in young adult flies. The GeneSwitch (GS) system permits cell type-specific gene expression following feeding flies the progesterone analog mifepristone (RU-486).30,31 Similar to previously published results in the embryonic epithelium,27 RNAi-mediated depletion of bark in ECs in midguts of young flies resulted in a decrease in Gli::GFP staining intensity at the tSJ (Figures 3A and 3B”). However, in contrast to the findings in the embryonic epithelium, depletion of Gli resulted in an increase in Bark::GFP staining intensity at the bicellular junction, somewhat reminiscent of the localization observed in guts from aged flies (Figures 2B–2B’ and 3C–3D″). A similar, mutually dependent relationship between Gli and Bark has been observed in the pupal notum.32 In addition, depletion of either bark or Gli non-autonomously increased ISC proliferation (Figures 3E, 3F and 4A–4C) and Glidepletion reduced Bark::GFP staining at TCJ (Figure 3G), as observed in old flies (Figure 2C). Taken together, these data suggest that the mechanisms for assembly and maintenance of the tSJ may differ by tissue type and/or with age.
Figure 3.
Depletion of Gli results in loss of Bark from the tSJ in the adult posterior midgut
(A–A″) In young outcrossed fly PMGs, Gli localizes exclusively to the tSJ. Scale bars, 20 μm.
(B–B″) EC-specific depletion of Bark protein by 5966GS;bark RNAiGD expression causes mislocalization of Gli to cytoplasmic puncta, indicating Bark is required for Gli localization to the tSJ in the PMG. Genotypes: w; Gli::GFP; bark RNAiGD (control); w; Gli::GFP; 5966GS;bark RNAiGD. All flies are fed RU-486 for 7 days to induce transgene expression. Scale bars, 20 μm.
(C–C″) In young outcrossed (7 do) fly PMGs, Bark is localized exclusively to the tSJ. Scale bars, 20 μm.
(D–D″) Depletion of Gli expression in ECs of young (7 do) flies leads to loss of Bark from the tSJ and an increase in Bark on the bicellular junction and cytoplasm. Genotypes: w; 5966GS (control); w; Bark::GFP, 5966GS;Gli RNAi. All flies are fed RU-486 for 5 days to induce transgene expression. Bark (GFP, green); Armadillo (Arm, adherens junctions, red); DNA (DAPI, blue). Scale bars, 20 μm.
(E) Quantification of mitotic events in the posterior midguts of 5966GS;bark RNAiGD and outcrossed controls. Median ±SEM; statistical significance was determined by Mann-Whitney test. ∗∗∗∗, p < 0.0001.
(F) Quantification of mitotic events in the posterior midguts of 5966GS;Gli RNAi and outcrossed controls. Median ±SEM; statistical significance was determined by Mann-Whitney test. ∗∗∗∗, p < 0.0001.
(G) Quantification of fluorescence intensity ratios for Bark in the bicellular junction (BCJ), tSJ, and cytoplasm. Black lines indicate median; Statistical significance determined by Mann-Whitney test. ∗∗, p < 0.01.
Figure 4.
Bark is required at the tSJ in enterocytes to maintain intestinal homeostasis
(A and B) Depletion of Bark in ECs of young (2 do) flies by 5966GS;bark RNAiGD expression for 5 days results in an increase in mitotic activity (n = 54), compared to outcrossed controls (n = 48). Genotypes: (A) w; esg-GFP, 5966GS (B) w; esg-GFP, 5966GS;bark RNAiGD. All flies are fed RU-486 to induce transgene expression. ISCs/EBs (GFP, green); mitotic cells (phosphorylated histone H3, pHH3, red), DNA (DAPI, blue). Scale bars, 20 μm.
(C) Quantification of mitotic events. w; esg-GFP, 5966GS;bark RNAiKK flies are also included (n = 42). Error bars represent mean with SEM. Median ±SEM; statistical significance was determined by Mann-Whitney test. ∗∗∗∗, p < 0.0001.
(D and E) Intestinal barrier integrity assay shows loss of barrier function upon depletion of Bark (red, n = 301) and (D) statistically significant (∗∗∗∗) shortening of lifespan, when compared to outcrossed controls (blue, n = 299). Genotypes: Red, w; esg-GFP, 5966GS>UAS-bark RNAiGD; blue, w; esg-GFP, 5966GS. ∗, p < 0.05; ∗∗∗, p < 0.001, ∗∗∗∗, p < 0.0001. Statistical significance determined by Fisher’s exact test (barrier integrity assay) and non-parametric log rank Mantel-Cox test (lifespan assay).
bark is required in ECs for the maintenance of intestinal homeostasis
Given that the changes in Gli::GFP on depletion of bark, and vice versa, resemble what occurs at the tSJ in an aged midgut, we wanted to determine whether depletion from ECs in PMGs from young flies led to other aging phenotypes. Following depletion of bark from ECs in young flies for 5 days, the number of pHH3+ cells was significantly higher than in controls (Figures 3E, 3F and 4 A–4C), suggesting that Bark is required in ECs to maintain intestinal homeostasis, similar to Gli (Figure 3F).10 Immunostaining confirmed depletion of Bark protein from ECs using the 5966GS driver, validating the RNAi line (Figures S2A and S2B). In addition, the number of pHH3+ cells increased with extended induction times (Figures S2E–S2G). Similar results were obtained using additional, independent RNAi lines (Figures 4C, S2C–S2D’, S2H–S2K), as well as alternative, inducible systems to deplete bark from ECs (i.e., Myo1A-GAL4ts, Figures S2H–S2K). As a result of increased ISC proliferation, depletion of bark in ECs also increased total cell number (Figure S2J) and progenitor cell numbers, as indicated by esg+ cells (Figure S2K), suggesting disrupted intestinal homeostasis.
Next, we wanted to determine whether Bark is required for maintenance of the intestinal barrier, similar to Gli.10 The GeneSwitch system was used to deplete bark in ECs throughout the adult lifespan. Integrity of the intestinal barrier and lifespan were assayed in parallel by feeding the flies a non-absorbable, non-toxic blue food dye as described previously.7,33 In flies with an intact intestinal barrier, the dye remains in the lumen of the digestive tract; however, when the intestinal barrier is severely compromised, the dye visibly leaks into the hemolymph.33 Consistent with a model in which Bark plays an integral role in tSJs, depletion of bark in ECs led to significant loss of the intestinal barrier after 19 days. Furthermore, as has been demonstrated previously, loss of barrier integrity correlated with shortened lifespan, when compared to control flies (Figures 4D and 4E).7 These data indicate that Bark is required in ECs for regulating intestinal homeostasis, maintaining the intestinal barrier, and for a normal lifespan.
bark is required in EBs for proper differentiation
One hallmark of the aging PMG is accumulation of EB-like cells that express hallmarks of both ISCs and ECs.3,24 Although Bark expression was detected at tSJs between ECs and EEs, single cell sequencing data revealed expression of bark in EBs.34,35 Therefore, we wanted to investigate a role for bark in progenitor cells committed to the EC lineage by combining conditional expression of bark RNAi with an established lineage tracing approach, ReDDM.21,22 Briefly, kluReDDM utilizes different fluorophore stabilities in combination with EB-specific manipulation and tracing of differentiated ECs (see STAR Methods for more information).21,22 Depleting bark using kluReDDM resulted in accumulation of klu+ EBs (Figures 5A, 5B’’’ and 5E), although not at the expense of EC differentiation (Figures 5F and 5G). Similar results were obtained when bark was depleted from ISCs and EBs using another ISC/EB driver (esgReDDM) (Figure S3).36 Furthermore, specific depletion of bark from EBs reduced lifespan compared to outcrossed controls (Figures 5J and 5K) and increased loss of epithelial barrier integrity, similar to depletion in ECs. Importantly, reduction in lifespan was not caused by persistent depletion of bark in ECs, as traced, newly differentiated ECs show Bark::GFP localized at the TCJ (Figures S4A–S4B”). Depletion of bark in EBs also led to an increase in newly generated EEs, identified by antibody staining for the EE marker Prospero (Pros+, Figure 5H), and a non-autonomous increase in ISC proliferation (pHH3+, Figures 5C, 5D’ and 5I). Similar to our observations of ECs in intestines from aged flies,10 EB-specific depletion of bark reduced immunofluorescence staining intensity of the SJ marker Dlg1 at the bSJ (Figures S4C–S4E).
Figure 5.
EB-specific depletion of bark results in EB accumulation, EE fate plasticity, and increases epithelial permeability
(A–B‴) Confocal images of control PMGs (A-A‴) and >bark RNAiKK (B-B‴) in the R5 region after 7 days of tracing. Scale bar 20 μm.
(C–D′) Knockdown of bark (D-D′) leads to an increase in the number of proliferating cells (marked by pHH3) as compared to the controls (C-C’). Scale bar 10 μm.
(E–H) Quantifications of the number of EBs (E), new ECs (F), and traced EEs (H) on bark knockdown after 7 days of tracing. Depletion of bark induces EB to EE differentiation (insert, GFP−/RFP+/anti-Pros+, H) but no significant change in the number of ECs (GFP−/RFP+/anti-Dlg1+, F). (Control n = 17, >bark RNAiKK n = 13). Mean ± SD; statistical significance was determined by Mann-Whitney test. ∗∗∗∗, p < 0.001. In addition, bark knockdown increased the number of progenitor cells (GFP+/RFP+, E). Mean ± SD; statistical significance was determined by Mann-Whitney test. ∗∗∗∗, p < 0.001. (G) EC/EB ratio decreases upon bark knockdown. (Control n = 17, >bark RNAiKK n = 13). Mean ± SD; statistical significance was determined by Mann-Whitney test. ∗, p < 0.05.
(I) Quantification of the number of mitotic cells on bark depletion after 7 days of tracing. (control n = 11, >bark RNAiKK n = 15). Mean ± SD; statistical significance was determined by Mann-Whitney test. ∗∗, p < 0.01.
(J) Survival (in percentage) over time on bark knockdown using kluReDDM. Survival curves were plotted by combining data from >80 flies per one genotype group: Control (blue, n = 89), bark RNAiKK (red, n = 109). bark knockdown in EBs reduces lifespan significantly. Statistical significance determined by non-parametric log rank Mantel-Cox test. ∗∗∗∗, p < 0.0001.
(K) Intestinal barrier integrity assay shows loss of barrier function upon depletion of Bark (red, n = 110) when compared to outcrossed controls (blue, n = 81). Statistical significance determined by Fisher’s exact test.
(L) kluReDDM (w; UAS-CD8::GFP, tub-GAL80ts; klu-GAL4; UAS-H2B::RFP) tracing. Expression of two fluorophores (CD8::GFP and H2B::RFP) is driven by EB-specific driver klu-GAL4. EBs differentiating to epithelial ECs lose CD8::GFP, while stable H2B::RFP persists. The expression of UAS-driven transgenes is temporally controlled by a ubiquitously expressed temperature-sensitive GAL80ts repressor, which is inactivated by temperature shift to 29°C.
Cell fate decisions after ISC division strongly depend on the Notch signaling pathway, whereby low Notch activation was shown to drive EE differentiation.19,20,37 By using Su(H)-GBE-GFP as a reporter of Notch activation,38 we found that depletion of bark using klu-GAL4 led to a reduction in Su(H)-GBE-GFP fluorescence intensity (Figures 6A–6C) and smaller EB nuclei (Figure 6D). Indeed, intestines of aged flies (45 do) contain more EBs (Figures 6E, 6F and 6H) with reduced Notch-activity (Figure 6G), phenocopying bark depletion from EBs. Therefore, our data indicate that on bark depletion, EBs demonstrate an increased propensity to differentiate along the EE lineage, which has previously been observed on loss of the lineage marker klu in EBs.21,22 Taken together, our findings suggest that Bark plays a role during EB to EC differentiation and that depletion of bark leads to accumulation of EBs with reduced Notch activity, increasing differentiation toward EE fate.
Figure 6.
Knockdown of bark in ISCs/EBs results in reduced Notch pathway activation, similar to aging
(A–B′) Expression of H2B::RFP is driven by ISC- and EB- driver esg-GAL4. H2B::RFP persists in epithelial ECs differentiated from EBs. Gbe+Su(H)GFP is a readout of Notch activity and a marker of the EB lineage (arrowheads, B-B′) Scale bar, 10 um.
(C and D) Quantification of GFP fluorescence intensity and (D) EB nuclear size following 7 days of bark depletion. Knockdown of bark decreases GFP fluorescence intensity (C) and EB nuclear size (GFP+ cells, D) compared to controls (control n = 73, >bark RNAiKK n = 70). Mean ± SD; statistical significance determined by Mann-Whitney test. ∗∗∗∗, p < 0.0001.
(E and F) In aged (45 do) flies (F), EB number (GFP+ cells) is higher than in young (2 do) flies (E). (arrowheads, B-B′) Scale bar, 10 um.
(G and H) Quantification of GFP fluorescence intensity and (H) EB number following 7 days of bark depletion. Knockdown of bark decreases GFP fluorescence intensity (control n = 27, >bark RNAiKK n = 30) (G) and increases EB number (GFP+ cells, control n = 3, >bark RNAiKK n = 3) compared to controls (H). Mean ± SD; statistical significance determined by Student’s t test. ∗, p < 0.05, ∗∗∗∗, p < 0.0001.
Discussion
Occluding junctions, known as tight junctions in mammals and septate junctions in arthropods, play a significant role in maintaining the intestinal barrier,1,10,11 which passively restricts paracellular flow across the intestinal epithelium. Of interest, loss of the intestinal barrier has been described as a result of aging and is associated with impending death across species.7 Owing to the strong correlation between aging and loss of the intestinal barrier,2,3,4,5,6,7 we wanted to characterize the impact of aging on occluding junctions, using the Drosophila intestine as a model system. Here, we describe the expression, localization and role of the tSJ component Bark in the adult PMG.
Bark localized to the tSJ in both the PMG and hindgut (Figures 1, and S1); however, analysis of PMGs from aged flies indicated that Bark was lost from the tSJ over time (Figure 2). Concomitant with the decreased staining intensity at the tSJ, we observed an increase in staining along the bicellular junction (Figure 2). Depletion of bark from ECs in young flies led to an increase in ISC proliferation, loss of the intestinal barrier, and shortened lifespan (Figures 3 and 4). These results are similar to what we reported previously for another tSJ component, Gli.10 Although our study utilizes RNAi-mediated depletion of tSJ components, we have shown previously that transcription of bark does not decrease with age in the PMG10; therefore, our approach may not accurately recapitulate the impact of aging on the tSJ. However, these studies are a relevant first step toward understanding the importance of Bark and other SJ proteins in intestinal homeostasis.
Our finding that Bark is lost from the tSJ in PMGs from aged flies, in combination with the observation that depletion of bark from ECs in young flies recapitulates some prevalent intestinal phenotypes that are apparent with age, supports the hypothesis that loss of SJ components may contribute to age-related loss of intestinal barrier function.10,11 One possible mechanism leading to age-related changes in the localization of SJ proteins could be alterations in vesicular trafficking, including increased endocytosis of SJ proteins and/or decreased delivery of recycled or newly synthesized SJ proteins to the plasma membrane. Manipulation of transport machinery, rather than production of SJ proteins, could be explored as an approach to restore Bark and other SJ proteins at the SJ in aged flies; however, manipulation of transport machinery would likely result in pleiotropic, unrelated phenotypes.
In addition to analyzing a role for Bark in ECs, we show that EB-specific depletion of bark results in accumulation of EB-like cells, resembling defects observed in flies that are aged or have damaged intestinal epithelia.39,40 The accumulation of EB-like cells correlated with a decrease in lifespan (Figures 5B and 5E), as reported previously.36 EBs depleted for bark are capable of forming ECs in normal numbers (Figure 5F), although cells originating from Bark-depleted EBs are abnormally small (Figures 6A–6B and 6D), show irregular Dlg1 localization (Figures S4C–S4E) and fate is shifted toward the EE lineage (Figure 5H). Therefore, we speculate that depletion of bark from EBs results in failure to form proper contact with existing ECs, which delays EB differentiation, leading to their accumulation. Interestingly, Bark expression following transient knockdown of bark in EBs is rescued in new (i.e., linage-traced) ECs, as they show normal Bark::GFP at tSJ. Our findings indicate an essential role for Bark during tSJ establishment when differentiating EBs integrate into the intestinal epithelium. Future studies will focus on an ultrastructural study of Bark localization in late EBs, achieving a better understanding of how Bark may participate in recently identified apical membrane initiation sites41 whereas EBs undergo epithelial integration, acting to establish and preserve intestinal barrier function.
In our evaluation of the relationship between Gli and Bark protein in the PMG tSJ, we found that depletion of one component results in changes in staining intensity of the other at tSJs in adult PMGs (Figure 3). Our findings are different from previous studies that examined the relationship between Bark and Gli in the embryonic epithelium,27 which found Bark is required for Gli to localize to the TCJ, whereas the reverse was not true. However, another study revealed Gli and Bark are mutually dependent for localization to the tSJ in the pupal notum.32 Differences could be because of relationships between Gli and Bark in embryonic versus adult tissues, the use of null alleles versus RNAi-mediated depletion, time of RNAi depletion, and/or the use of antibodies targeting the proteins directly, rather than GFP tags. An ultrastructural study could better pinpoint the nature of Gli-Bark interaction in the PMG. Investigation of the relationship between Gli and Bark to another tSJ protein, M632,42 in the Drosophila PMG may also help further illuminate these differing results.
In summary, we have found that Bark protein is required in the tSJs of ECs and EBs in the Drosophila posterior midgut to maintain homeostasis with respect to ISC proliferation, EB fate, intestinal barrier function, and lifespan. Because Bark also mislocalizes from the tSJ in the aged fly PMG, our study suggests that this mislocalization may contribute to the overall intestinal aging phenotype. In addition, Bark and the tSJ protein Gliotactin appear mutually dependent on one another for proper tSJ localization in the adult PMG, unlike in the embryonic epithelium.27 Given the importance of intestinal barrier function, and age-related intestinal barrier loss across species,2,3,4,5,6,7 our discovery of an essential role for Bark during homeostatic cellular turnover sheds light on fascinating future research lines. This study could help generate insight into the role of tSJs in the human gut and possible pathologies associated with tSJ dysfunction.
Limitations of the study
Because RNAi-based knockdowns may not be fully penetrant, a potential caveat is that depletion of Bark may not fully recapitulate aging phenotypes. In addition, because Bark protein is not readily detected in EBs, it is difficult to determine exactly what the role of Bark may be in EBs. However, we speculate that expression of Bark at the EB-EC transition is critical for the initial assembly of tricellular junctions in the adult intestine.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-Armadillo | DSHB | Cat #N2 7A1, RRID:AB_528089 |
| Guinea pig polyclonal anti-Bark | Laboratory of Reinhard Schuh (MPI for Multidisciplinary Sciences) | N/A |
| Mouse monoclonal anti-Dlg1 | DSHB | Cat #4F3, RRID:AB_528203 |
| Chicken polyclonal anti-GFP | Aves Labs | Cat #GFP-1010, RRID:AB_2307313 |
| Chicken polyclonal anti-GFP | Abcam | Cat #ab13970, RRID:AB_300798 |
| Rabbit polyclonal anti-GFP | Molecular Probes | Cat #A-11122, RRID:AB_221569 |
| Rabbit polyclonal anti-pHH3 | Millipore | Cat #06-570, RRID:AB_310177 |
| Mouse monoclonal anti-Pros | DSHB | Cat #MR1A, RRID:AB_528440 |
| Goat anti-chicken Alexa488 | Invitrogen | Cat #A-11039, RRID:AB_142924 |
| Goat anti-guinea pig Alexa568 | Invitrogen | Cat #A-11075, RRID:AB_141954 |
| Goat Anti-mouse Alexa568 | Invitrogen | Cat #A-11004, RRID:AB_2534072 |
| Goat anti-mouse Alexa647 | Invitrogen | Cat #A-21235, RRID:AB_2535804 |
| Donkey anti-rabbit Alexa647 | Invitrogen | Cat #A-31573, RRID:AB_2536183 |
| Chemicals, peptides, and recombinant proteins | ||
| RU-486 | Sigma | Cat #M8046 |
| FD&C Blue Dye no. 1 | Spectrum Chemical | Cat #FD110 |
| Erioglaucine Disodium Salt (E133) | BLD Pharmatech Ltd., Shanghai, China | Cat #3844-45-9 |
| Experimental models: Organisms/strains | ||
| D. melanogaster: tub-GAL80ts | Bloomington Drosophila Stock Center | BDSC: 7017 |
| D. melanogaster: Gli::GFP | Kyoto Stock Center | DGRC #115-332 |
| D. melanogaster: w1118 | Laboratory of D. Walker (U. of California- Los Angeles) | N/A |
| D. melanogaster: Su(H)lacZ; esg:GFP, 5966GAL4GS | Laboratory of B. Ohlstein (UT Southwestern) | N/A |
| D. melanogaster: 5966-GAL4GS | Laboratory of H. Jasper (Buck Institute) | N/A |
| D. melanogaster: Myo1A-GAL4 | Laboratory of H. Jasper (Buck Institute) | N/A |
| D. melanogaster: P{bark.Ty1-Tev-SGFP-FLAG} | Laboratory of S. Luschnig (U. of Münster) | N/A |
| D. melanogaster: yw hsflp; Sco/Cyo Dfd-GMR nuGFP; UAS-bark attP2y (L057) | Laboratory of S. Luschnig (U. of Münster) | N/A |
| D. melanogaster: Gbe+Su(H)GFP | Laboratory of T. Klein (U. of Düsseldorf) | N/A |
| D. melanogaster: w; esg-GAL4,UAS-CD8::GFP; UAS-H2B::RFP, tub-GAL80ts | Antonello et al. 2015 | N/A |
| D. melanogaster: w; UAS-CD8::GFP, tub-GAL80ts; klu-GAL4, UAS-H2B::RFP | Reiff at al. 2019 | N/A |
| D. melanogaster: w; UAS-CD8::GFP, tub-GAL80ts; klu-GAL4, UAS-H2B::RFP | Laboratory of T. Reiff (U. of Düsseldorf) | N/A |
| D. melanogaster: w; UAS-CD8::RFP, tub-GAL80ts; klu-GAL4, UAS-H2B::RFP | Laboratory of T. Reiff (U. of Düsseldorf) | N/A |
| D. melanogaster: esg-GAL4; UAS-H2B::RFP | Laboratory of Tobias Reiff (U. of Düsseldorf) | N/A |
| D. melanogaster: esg-GFP; UAS-H2B::RFP | Laboratory of Tobias Reiff (U. of Düsseldorf) | N/A |
| D. melanogaster: UAS-bark-RNAiGD | Vienna Drosophila Resource Center | v52608 |
| D. melanogaster: UAS-bark-RNAiKK | Vienna Drosophila Resource Center | v107348 |
| D. melanogaster: UAS-Gli RNAi | Vienna Drosophila Resource Center | V37115 |
| Software and algorithms | ||
| Prism v8.0.2 | Graphpad | N/A |
| Illustrator CC 2020 | Adobe | N/A |
| ImageJ 1.51n | Wayne Rasband, NIH | https://imagej.nih.gov/ij |
| ZEN Blue v.3.3 | Zeiss | N/A |
| ZEN Black v.2.0 | Zeiss | N/A |
| Canvas X-Pro | Canvas X-Pro 2019 | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Leanne Jones (leanne.jones@ucsf.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Set 1: Fig. 1C, Figs. 5-6, Fig. EV2H-K, Figs. EV3-EV4.
Set 2: Fig. 1A-B, Figs. 2–4, Fig. EV1, Fig. EV2A-G.
Set 1
Fly food contained 7.12% corn meal, 4.5% malt extract, 4% sugar beet syrup, 1.68% dried yeast, 0.95% soy flour, 0.5% agarose, 0.45% propionic acid, and 0.15% NIPAGIN powder (antimycotic agent). GAL80ts flies were kept at 18°C (permissive temperature) until shifted to 29°. Otherwise, flies and crosses were kept at 25°C. All analyses were performed on 3-7 day old (do) mated females that were shifted to 29°C for 7 days for tracing. A maximum of 20 flies (females and males) were housed in one fly vial.
Set 2
Flies were cultured in vials containing standard cornmeal medium (1.1% agar, 2.9% baker’s yeast, 9.2% maltose, and 7.1% cornmeal; all concentrations given in wt/vol). Propionic acid (0.5%) and TegoSept (methylparaben, Sigma, 0.16%) were added to adjust pH and prevent fungal growth, respectively. No more than 30 flies were housed per vial. All experiments were conducted at 25°C. Newly eclosed adults were kept for an additional 1–2 days before inducing transcription activation by placement on food containing the steroid hormone mifepristone (RU-486, Sigma) in a 25 μg/ml concentration and flipped every 2 days thereafter. All analyses for these studies were performed on female flies, as age-related gut pathology has been well established in females.3,7
Method details
Immunohistochemistry
Set 1
Dissected guts were fixed in 4% PFA in 1XPBS for 45 min. After fixation the guts were washed with 1XPBS for 10 min and stained with primary antibodies, diluted in 0.5% PBT (0.5% Triton (Sigma-Aldrich) in 1XPBS) + 5% normal goat serum (Thermo Fisher Scientific, Berman, Germany). Primary antibody staining was performed at 4°C overnight on an orbital shaker. Primary antibodies used were mouse anti-Dlg1 (DSHB, 1:250), chicken anti-GFP (Abcam, 1:250), rabbit anti-pHH3 (Millipore, 1:200), and mouse anti-Pros (DSHB, 1:250). On the next day, guts were washed with 1XPBS for 10 min and incubated with secondary antibodies and DAPI (1:1000; 100 μg/mL stock solution in 0.18 M Tris pH 7.4; DAPI No. 18860, Serva, Heidelberg) for at least 3 h at RT. After washing with 1XPBS for 10 min the stained guts were mounted in Fluoromount-G Mounting Medium (Electron Microscopy Sciences). Stained posterior midguts were imaged in the R5 region using an LSM 710 confocal microscope (Carl Zeiss) with ‘Plan-Apochromat 20 × /0.8 M27′ and ‘C-Apochromat 40 × /1.20 W Corr M27′ objectives. Image resolution was set to at least 2048 × 2048 pixels. Focal planes were combined into Z-stacks and images were then processed by Fiji software. Final images were assembled using Canvas X-Pro.
Set 2
All images were taken in the P3–P4 regions of the Drosophila PMG, located by centering the pyloric ring in a ×40 field of view (fov) and moving 1–2 fov toward the anterior. PMGs were dissected into ice-cold PBS/4% formaldehyde and incubated for 1 h in fixative at room temperature. Samples were then washed three times, for 10 min each, in PBT (PBS containing 0.5% Triton X-100), 10 min in Na-deoxycholate (0.3%) in PBT (PBS with 0.3% Triton X-100), and incubated in block (PBT-0.5% bovine serum albumin) for 30 min at room temperature. Samples were immunostained with primary antibodies overnight at 4°C. Primary antibodies used were mouse anti-Armadillo (DSHB, 1:20), guinea pig anti-Bark (gift from Reinhard Schuh, 1:1000), mouse anti-Dlg1 (DSHB, 1:250), chicken anti-GFP (Aves Labs, 1:500), chicken anti-GFP (Abcam, 1:200), rabbit anti-GFP (Molecular Probes, 1:3000), and rabbit anti-pHH3 (Millipore, 1:200). Samples were washed 3 × 10 min at room temperature in PBT (PBS containing 0.5% Triton X-100), incubated with secondary antibodies (1:500, Invitrogen) at room temperature for 2 h, washed 3 × 10 min with PBT and mounted in Vecta-Shield/DAPI (Vector Laboratories, H-1200). Images were acquired on a Zeiss LSM780 or LSM880 inverted confocal microscope (UCLA MCDB/BSCRC Microscopy Core), and on a Zeiss Axio Observer Z1 with Apotome 2 using the ZEN Black v.2.0 (Zeiss) software. Images were processed with Fiji/ImageJ and Zen Blue v.3.3 (Zeiss) software. Final figures were assembled using Adobe Illustrator.
Smurf assay
Figure 4
Smurf assay: A stock solution of Erioglaucine Disodium Salt (E133) (BLD Pharmatech Ltd., Shanghai, China) of concentration 320 g/l was prepared. To prepare the food for the Smurf assay, 300 μl of the stock solution was mixed with 4 ml of standard food medium. Flies were checked daily for loss of barrier function.
Figure 5
Flies were maintained on standard medium prepared with FD&C Blue Dye no. 1, added at a concentration of 2.5% (wt/vol). Loss of intestinal barrier function was determined (“Smurf” status) when dye was clearly observed outside the digestive tract.33 Flies were checked three times weekly for loss of barrier function and/or death.
Survival analysis
Flies were kept at 18°C and 3-5 days old flies were then shifted to 29°C. Not more than 10 flies (1:1 ratio of females:males) were kept per vial and flipped every other day to avoid bacterial contamination in the food. Flies were checked daily to record death events. At least 80 flies were assessed per genotype. Survival curves were plotted using Prism GraphPad software.
Quantification and statistical analysis
Quantification of proliferation
Quantification of mitotic events, as measured by pHH3+ cells per gut, was performed by acquiring 4 independent images at 40X magnification per gut in the P3-P4 region.
Quantification of nuclear size and fluorescence intensity
Nuclear size measurements were performed in Fiji by outlining the nucleus manually and measuring the area. Mean intensities of the manually selected nuclear area were measured using Fiji by manually selecting the area of interest.
Quantification of cell counts
GFP positive progenitor cells and RFP positive differentiated cells (EC and/or EE) ofesgReDDM,36 kluReDDM, and Myo-GAL4ts were counted manually.
Quantification of tSJ and cytoplasmic fluorescence intensity
Quantification of antibody fluorescence at the tSJ and cytoplasm was performed with the ZEN Blue software. Images were acquired on a Zeiss LSM880 inverted confocal microscope equipped with AiryScan at 100X. Full z-stacks were used, and anti-Armadillo staining was used to mark membranes. 3 cells were measured per PMG. Each clearly visible tSJ in the cell was measured. 6 measurements of the cytoplasm were taken in each cell.
Quantification of BCJ fluorescence intensity
Figure 3, S4
Quantification of antibody fluorescence at the bSJ was performed with the ZEN Blue software. Images were acquired on a Zeiss LSM880 inverted confocal microscope equipped with AiryScan at 100X. Full z-stacks were used, and anti-Armadillo staining was used to mark membranes. 3 cells were measured per PMG. 6 measurements were taken of the bicellular junction in each cell.
Figure 6
Images were taken in directly adjacent fields of view on each side of the gut in the R5 region. Quantification of progenitor cell number and epithelial renewal were performed manually. Cell size and fluorescence intensity measurements were performed on Fiji (ImageJ 1.51 n, Wayne Rasband, National Institutes of Health, USA) by outlining the cell of interest.
Statistics
GraphPad Prism 8.0.2 was used to run statistical analysis and create graphs of quantifications. For single comparisons, data sets were analyzed by two-sided unpairedt-test or Mann-Whitney test. Multiple comparisons were analyzed by one-way ANOVA or Kruskal-Wallis tests. For survival analyses, Mantel-Cox log-rank test was performed. Significant differences are displayed as ∗ for P ≤ 0.05, ∗∗ for P ≤ 0.01, ∗∗∗ for P ≤ 0.001 and ∗∗∗∗ for P ≤ 0.0001. Experiments were repeated at least twice. No statistical method was used to predetermine sample size. Experiments were not randomized, and researchers were not blinded to allocation during experiments or outcome assessment. Statistical tests and significance used in each figure can also be found in figure legends.
Acknowledgments
The authors thank the Vienna Drosophila RNAi Center (VDRC), the Bloomington Drosophila Stock Center (NIHP400D018537), the Transgenic RNAi Project (TRiP) at Harvard Medical School (NIK/NIGMS R01-GM084947) and Thomas Klein for reagents. We also thank the UCLA MCDB/BSCRC Microscopy Core and Center for Advanced Imaging (CAi) at Heinrich-Heine-Universität Düsseldorf (DFG INST 208/539-1 FUGG) for imaging training and facilities. In addition, we are grateful to Jones laboratory members for comments and feedback on experiments and the manuscript. Special thanks are also given to Volker Hartenstein for sharing laboratory space and equipment. This work was supported by the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at the University of California, Los Angeles and the NIH: R01AG028092, R01DK105442, R01GM135767 (D.L.J.). MG is funded by the Deutsche Forschungsgemeinschaft (DFG-Sachbeihilfe RE 3453/6–1).
Author contributions
R.A.H., M.G., and M.R-D. designed, performed, and analyzed experiments and edited the manuscript. E.E., F.de la T., C.D., and N.H.K. performed experiments. T.R and D.L.J. designed and analyzed experiments and wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper received support from a program designed to increase minority representation in their field of research.
Published: May 19, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106901.
Supplemental information
Data and code availability
-
•
All data reported in this paper is available from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Marchiando A.M., Graham W.V., Turner J.R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 2010;5:119–144. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood T.B.L. Intrinsic ageing of gut epithelial stem cells. Mech. Ageing Dev. 2004;125:911–915. doi: 10.1016/j.mad.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Biteau B., Hochmuth C.E., Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren W.y., Wu K.f., Li X., Luo M., Liu H.c., Zhang S.c., Hu Y. Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin. Exp. Res. 2014;26:183–191. doi: 10.1007/s40520-013-0148-0. [DOI] [PubMed] [Google Scholar]
- 5.Schiffrin E.J., Morley J.E., Donnet-Hughes A., Guigoz Y. The inflammatory status of the elderly: the intestinal contribution. Mutat. Res. 2010;690:50–56. doi: 10.1016/j.mrfmmm.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Tran L., Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1045–1056. doi: 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rera M., Clark R.I., Walker D.W. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc. Natl. Acad. Sci. USA. 2012;109:21528–21533. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark R.I., Salazar A., Yamada R., Fitz-Gibbon S., Morselli M., Alcaraz J., Rana A., Rera M., Pellegrini M., Ja W.W., et al. Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep. 2015;12:1656–1667. doi: 10.1016/j.celrep.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo L., Karpac J., Tran S.L., Jasper H. PGRP-SC2 promotes gut immune homeostasis to limit commensal dysbiosis and extend lifespan. Cell. 2014;156:109–122. doi: 10.1016/j.cell.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resnik-Docampo M., Koehler C.L., Clark R.I., Schinaman J.M., Sauer V., Wong D.M., Lewis S., D’Alterio C., Walker D.W., Jones D.L. Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis. Nat. Cell Biol. 2017;19:52–59. doi: 10.1038/ncb3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar A.M., Resnik-Docampo M., Ulgherait M., Clark R.I., Shirasu-Hiza M., Jones D.L., Walker D.W. Intestinal snakeskin limits microbial dysbiosis during aging and promotes longevity. iScience. 2018;9:229–243. doi: 10.1016/j.isci.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumi Y., Furuse K., Furuse M. Septate junctions regulate gut homeostasis through regulation of stem cell proliferation and enterocyte behavior in Drosophila. J. Cell Sci. 2019;132:232108. doi: 10.1242/jcs.232108. [DOI] [PubMed] [Google Scholar]
- 13.Mariano C., Sasaki H., Brites D., Brito M.A. A look at tricellulin and its role in tight junction formation and maintenance. Eur. J. Cell Biol. 2011;90:787–796. doi: 10.1016/j.ejcb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Furuse M., Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Tepass U., Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev. Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- 16.Lane N.J., Skaer H. 1980. Intercellular junctions in insect tissues; pp. 35–213. [Google Scholar]
- 17.Resnik-Docampo M., Sauer V., Schinaman J.M., Clark R.I., Walker D.W., Jones D.L. Keeping it tight: the relationship between bacterial dysbiosis, septate junctions, and the intestinal barrier in Drosophila. Fly. 2018;12:34–40. doi: 10.1080/19336934.2018.1441651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apidianakis Y., Rahme L.G. Drosophila melanogaster as a model for human intestinal infection and pathology. Dis. Model. Mech. 2011;4:21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohlstein B., Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 20.Micchelli C.A., Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 21.Reiff T., Antonello Z.A., Ballesta-Illán E., Mira L., Sala S., Navarro M., Martinez L.M., Dominguez M. Notch and EGFR regulate apoptosis in progenitor cells to ensure gut homeostasis in Drosophila. EMBO J. 2019;38:e101346. doi: 10.15252/embj.2018101346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korzelius J., Azami S., Ronnen-Oron T., Koch P., Baldauf M., Meier E., Rodriguez-Fernandez I.A., Groth M., Sousa-Victor P., Jasper H. The WT1-like transcription factor Klumpfuss maintains lineage commitment of enterocyte progenitors in the Drosophila intestine. Nat. Commun. 2019;10:4123. doi: 10.1038/s41467-019-12003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Jasper H. Gastrointestinal stem cells in health and disease: from flies to humans. Dis. Model. Mech. 2016;9:487–499. doi: 10.1242/dmm.024232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H., Patel P.H., Kohlmaier A., Grenley M.O., McEwen D.G., Edgar B.A. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi Y.J., Hwang M.S., Park J.S., Bae S.K., Kim Y.S., Yoo M.A. Age-related upregulation of Drosophila caudal gene via NF-kappaB in the adult posterior midgut. Biochim. Biophys. Acta. 2008;1780:1093–1100. doi: 10.1016/j.bbagen.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Park J.S., Kim Y.S., Yoo M.A. The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging. 2009;1:637–651. doi: 10.18632/aging.100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byri S., Misra T., Syed Z.A., Bätz T., Shah J., Boril L., Glashauser J., Aegerter-Wilmsen T., Matzat T., Moussian B., et al. The triple-repeat protein anakonda controls epithelial tricellular junction formation in Drosophila. Dev. Cell. 2015;33:535–548. doi: 10.1016/j.devcel.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrandt A., Pflanz R., Behr M., Tarp T., Riedel D., Schuh R. Bark beetle controls epithelial morphogenesis by septate junction maturation in Drosophila. Dev. Biol. 2015;400:237–247. doi: 10.1016/j.ydbio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Sarov M., Barz C., Jambor H., Hein M.Y., Schmied C., Suchold D., Stender B., Janosch S., Kj V.V., Krishnan R.T., et al. A genome-wide resource for the analysis of protein localisation in Drosophila. Elife. 2016;5:e12068. doi: 10.7554/eLife.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 31.Osterwalder T., Yoon K.S., White B.H., Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc. Natl. Acad. Sci. USA. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esmangart de Bournonville T., le Borgne R. Interplay between anakonda, Gliotactin, and M6 for tricellular junction assembly and anchoring of septate junctions in Drosophila epithelium. Curr. Biol. 2020;30:4245–4253. doi: 10.1016/j.cub.2020.07.090. [DOI] [PubMed] [Google Scholar]
- 33.Rera M., Bahadorani S., Cho J., Koehler C.L., Ulgherait M., Hur J.H., Ansari W.S., Lo T., Jones D.L., Walker D.W. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metabol. 2011;14:623–634. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dutta D., Dobson A.J., Houtz P.L., Gläßer C., Revah J., Korzelius J., Patel P.H., Edgar B.A., Buchon N. Regional cell-specific transcriptome mapping reveals regulatory complexity in the adult Drosophila midgut. Cell Rep. 2015;12:346–358. doi: 10.1016/j.celrep.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Hung R.J., Li J.S.S., Liu Y., Perrimon N. Defining cell types and lineage in the Drosophila midgut using single cell transcriptomics. Curr. Opin. Insect Sci. 2021;47:12–17. doi: 10.1016/j.cois.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Antonello Z.A., Reiff T., Ballesta-Illan E., Dominguez M. Robust intestinal homeostasis relies on cellular plasticity in enteroblasts mediated by miR-8–Escargot switch. EMBO J. 2015;34:2025–2041. doi: 10.15252/embj.201591517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohlstein B., Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2005;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 38.Furriols M., Bray S. Dissecting the mechanisms of suppressor of hairless function. Dev. Biol. 2000;227:520–532. doi: 10.1006/dbio.2000.9923. [DOI] [PubMed] [Google Scholar]
- 39.Biteau B., Karpac J., Supoyo S., DeGennaro M., Lehmann R., Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhai Z., Kondo S., Ha N., Boquete J.P., Brunner M., Ueda R., Lemaitre B. Accumulation of differentiating intestinal stem cell progenies drives tumorigenesis. Nat. Commun. 2015;6:10219–10313. doi: 10.1038/ncomms10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J., St Johnston D. De novo apical domain formation inside the Drosophila adult midgut epithelium. Elife. 2022;11:e76366. doi: 10.7554/eLife.76366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wittek A., Hollmann M., Schleutker R., Luschnig S. The transmembrane proteins M6 and anakonda cooperate to initiate tricellular junction assembly in epithelia of Drosophila. Curr. Biol. 2020;30:4254–4262. doi: 10.1016/j.cub.2020.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper is available from the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.