Highlights
-
•
The supplementation of N-acetylcysteine (NAC), resveratrol (RES) and ascorbic acid (AA) as adjunct therapy markedly reduced the concentrations of oxidative stress indices and intestinal injury in canine parvovirus enteritis (CPVE).
-
•
Additionally, NAC and RES supplementations to CPV-infected dogs resulted in pronounced improvement in total leukocyte count and neutrophil count during treatment of CPVE.
-
•
NAC and RES may be considered as potential antioxidants for the management of CPVE along with supportive therapy.
Keywords: Antioxidant, Canine parvovirus, Oxidative stress, N-acetylcysteine, Resveratrol
Abstract
A prospective randomized controlled clinical study was conducted to determine whether antioxidant supplementation as an adjunct therapy alters hemogram, oxidative stress, serum intestinal fatty acid binding protein-2 (IFABP-2) level, fecal viral load, clinical score (CS) and survivability in outpatient canine parvovirus enteritis (CPVE) dogs. The dogs with CPVE were randomized to one of the five treatment groups: supportive treatment (ST) alone, ST with N-acetylcysteine (ST+NAC), resveratrol (ST+RES), coenzyme Q10 (ST+CoQ10) or ascorbic acid (ST+AA). The primary outcome measures were reduction of CS and fecal HA titre, and enhancement of survivability. Secondary outcome measures were reduction of oxidative stress indices and IFABP-2 level from day 0 to day 7. The mean CS and HA titre were significantly (P < 0.05) decreased from day 0 to 7 in ST and all antioxidant groups. The supplementations of NAC, RES and AA along with ST markedly (P < 0.05) reduced the concentrations of malondialdehyde, nitric oxide and IFABP-2 on day 7 as compared to ST alone. Additionally, NAC and RES supplementations markedly (P < 0.05) improved the total leukocyte count and neutrophil count in CPVE-affected dogs. NAC and RES could serve as better antioxidants for the amelioration of oxidative stress in CPVE but, the antioxidants did not confer any additional benefits in reduction of CS, fecal HA tire, or survivability when compared with ST alone.
1. Introduction
Canine parvovirus enteritis (CPVE), an acute viral disease of dogs, is characterized by vomition and diarrhea with or without blood (Mazzaferro, 2020). The pathogenesis of canine parvovirus type 2 (CPV-2) infections attributes to virus-induced destruction of rapidly dividing cells of intestine, thymus, lymph nodes, and bone marrow. The infection in crypts of intestine causes disruption of mucosal barrier and villous atrophy, resulting in malabsorption, severe dehydration/hypovolemia. The intestinal fatty acid binding protein (IFABP), a low molecular weight intracellular protein found specifically and abundantly in epithelial cells of the small intestine's mucosal layer, is considered as a classical marker of gut injury and a potential biomarker for predicting survival in dogs with CPVE (Kokesova et al., 2019; Eregowda et al., 2020). Asides intestinal pathology, CPVE leads to marked pancytopenia, endotoxemia, systemic inflammatory response syndrome (SIRS), resulting in multi-organ dysfunction (Petini et al., 2020; Paul et al., 2023). The epidemiological studies in different parts of India reported that the overall prevalence of CPVE in dogs with out-patient care ranged from 28.00% to 70.73% (Behera et al., 2015; Chethan et al., 2021; Singh et al., 2021; Kalita et al., 2022; Geetha & Selvaraju, 2023). The overall prognosis for survival of dogs depends on type of therapy, and individual patient response to treatment (Kalli et al., 2010; Ling et al., 2012). Although the provision of in-hospital care for CPVE seems to be associated with better clinical outcome, the modified outpatient protocol with diligent supportive care with consistent monitoring is still a reasonable alternative where hospitals cannot offer hospitalization facility due to inadequate infrastructure and high cost of hospital stay (Venn et al., 2017).
In absence of any effective antiviral drug, the core treatment criteria is based on supportive therapy targeting restoration of fluid and electrolyte balance, prevention of secondary bacterial infection and palliation of symptoms. Nevertheless, survival rates can be as high as 64–92% with intensive treatment (Humm & Hughes, 2009). Although the beneficial effects of recombinant feline interferon-ω (rFeIFN-ω), recombinant canine granulocyte colony stimulating factor (rcG-CSF) and immune plasma against CPV infection have been reported, the commercial unavailability in all locations and exorbitant cost make these therapeutics inaccessible for regular use (De Mari et al., 2003; Duffy et al., 2010; Bragg et al., 2012; Acciacca et al., 2020; Mazzaferro, 2020).
A redox imbalance resulting from disturbances of oxidant-antioxidant equilibrium is one of the key pathways of pathogenesis of viral diseases. In CPV infection, a marked oxidative stress is associated with increase in the levels of reactive oxygen species (ROS)/reactive nitrogen species (RNS) and decrease in the reserves of antioxidants (Gaykwad et al., 2018). Earlier studies showed that the pathophysiology of CPVE is strongly associated with an imbalance of oxidative equilibrium and endocrine responses (Schoeman et al., 2013; Kocaturk et al., 2015). Antioxidants are compounds that have the ability to mitigate the oxidative damage conferred by ROS/RNS (Walia et al., 2021). Given the broad impact of oxidative stress in CPVE, inclusion of antioxidants in supportive therapy is a promising treatment option (Hagen et al., 2019). An earlier meta-analysis study found that antioxidant treatment significantly reduced mortality in severely ill human patients (Manzanares et al., 2012).
N-acetylcysteine (NAC), a precursor of glutathione, is a sulfur-containing amino acid cysteine metabolite (Devrim-Lanpir et al., 2021). Therapeutic efficacy of NAC has been documented in viral diseases (Guerrero et al., 2014; Sun et al., 2020). Resveratrol (RES), a naturally occurring antioxidant present in the roots of Polygonum cupsidatum, is known for its antioxidant and other promising biological activities (Nashine et al., 2020; Chopra et al., 2022). The RES supplementation ameliorates the pathogenesis of porcine rotavirus infection by its antioxidant activity owing to its ability of scavenging ROS/RNS species (Cui et al., 2018). Coenzyme Q10 (CoQ10), also known as ubiquinone, is recognized as an antioxidant and free radical scavenger. Intestinal protective action of CoQ10 by its antioxidant activity has been demonstrated in Escherichia coli diarrhea in calves (Garkhal et al., 2017; Gutierrez-Mariscal et al., 2020). Ascorbic acid (AA), a water-soluble vitamin, effectively neutralizes free radicals and prevents lipid peroxidation (Kianian et al., 2020). The supplementation of AA to healthy dogs showed improvements in antioxidant capacity and immune responses (Hesta et al., 2009). Till date, only few studies have been cited on the beneficial effects of antioxidants against CPVE (Kataria et al., 2020a, 2020b). The aim of this study was to determine whether supplementation of antioxidants alters the hemogram, oxidative stress, IFABP-2 level, clinical score (CS), fecal viral load and survivability in client-owned outpatient dogs with CPVE. The primary outcome measure was reduction of CS, fecal HA titre, and enhancement of survivability. Secondary outcome measures were reduction of oxidative stress indices and IFABP-2 level from day 0 to day 7. We hypothesized that supplementation of antioxidants as an adjunct therapy will produce favourable response on hemogram and oxidative stress, attenuate IFABP-2 level, CS, fecal virus load and enhance survivability in outpatient dogs with CPVE.
2. Materials and methods
2.1. Animals
This was a prospective, case-controlled study on dogs naturally infected with CPVE. Client-owned dogs with naturally occurring CPVE presented to Teaching Veterinary Clinical Complex (TVCC) of the institute between August 2017 to March 2018 were considered for inclusion. Only unvaccinated puppies with the history of vomition, diarrhea with or without blood were included. Inclusion criteria for the CPV infected dogs: dogs of either breed or sex, unvaccinated, between the ages of 2 weeks and 6 months, weighing more than 1 kg, and fecal samples positive for CPV by valid Antigen Rapid CPV Ag Test (BioNote, Inc. 22 Samsung 1-ro 4-gil, Hwaseong-si, Gyeonggi-do 18,449, Republic of Korea), hemagglutination test and polymerase chain reaction (PCR) targeting VP2 gene.
2.2. Study design, treatment protocol and monitoring
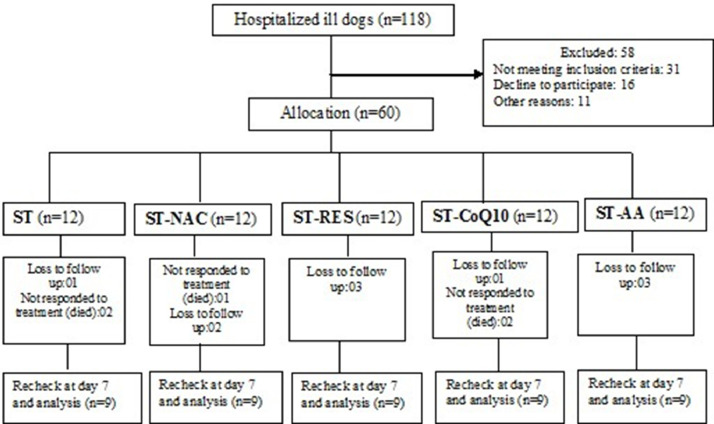
A total of 118 dogs (age group 1.5–6 months) with a history of weakness, reduced appetite, diarrhea/hemorrhagic diarrhea, vomition, dehydration with or without pyrexia were included in the study. Out of 118 diarrheic dogs, 60 were confirmed for CPV infection by rapid antigen test, HA test and PCR. The CPV-2 positive dogs were randomized into one of the five treatment groups, twelve puppies with CPVE were enrolled in each group. Each participated animal was subjected to thorough physical and clinical examination. The percentage of dehydration was estimated by skin tent test, capillary refill time, position of eyeball, color and moisture of mucous membranes, respiratory rate, heart rate, pulse rate and intensity, rectal temperature and packed cell volume (PCV). Twelve CPV-positive dogs received supportive treatment (ST) consisting of IV fluids [Fluid therapy was initiated with isotonic Ringer's lactate (RL) solution as the initial choice for intravascular volume restoration and rehydration. Dextrose normal saline (DNS), potassium chloride and hetastarch were also given as per the requirement. Dose: Fluid deficit was calculated by using the formula, body weight (kg) ×% of dehydration = volume (L) and the ongoing losses. A maintenance requirement was given at the rate of 60 mL/kg], broad spectrum gut acting antibiotic (ceftriaxone-tazobactum @ 25 mg/Kg, IV, q12hr), antiemetic (ondansetron @ 0.2 mg/Kg, IV, q12hr), antacid (pantoprazole @ 1 mg/Kg, IV, q24hr) as well as hemostatic agent (ethamsylate @ 10 mg/kg, IV, q12hr) whenever indicated for seven consecutive days, whereas twelve CPV-positive dogs in group ST+NAC, ST+RES, ST+CoQ10 and ST+AA received ST along with NAC (MUCOMELT™, Venus Remedies Limited, Himachal Pradesh, India) at the dose rate of 70 mg/Kg body weight once daily (IV route), RES (Mega Resveratrol™, Candlewood Stars Inc., Danbury, CT 06,810, USA) at the dose rate of 10 mg/Kg body weight once daily (oral route), CoQ10 (CoQ10™, Zenith nutrition, NJ 08,817, USA) at the dose rate of 10 mg/Kg body weight once daily (oral route) and AA (PAIL-C™, Pail Pharma Private Ltd, Pune, India) at the dose rate of 30 mg/Kg body weight once daily (IV route), respectively for seven days. None of the dogs received any early enteral nutrition during the study period. A computerised random number generator was used to construct a treatment allocation table. The treatment events and observations were supervised by veterinary clinicians. The entire treatment regimen for all of the dogs could not be completed due to a lack of follow-up and non-responding to treatment, so the data of nine cases that completed treatment schedule were included in each group. A total of 45 dogs finally participated and completed the treatment protocol in the study. The present research work has been conducted in line with the CONSORT criteria (Schulz et al., 2011). The baseline demographic information of each group at enrolment is summarized in Table 1 and the trial flowchart is depicted in Fig. 1.
Table 1.
The demographic information of CPV-infected ill dogs at enrollment (Day 0). Age and weight reported as median and ranges.
| Demographics | ST (n = 12) | ST+NAC (n = 12) | ST+RES (n = 12) | ST+CoQ10 (n = 12) | ST+AA (n = 12) |
|---|---|---|---|---|---|
| Age (Months) | 3.5 | 3.55 | 3.5 | 2.55 | 3.25 |
| (1.5–5.5) | (1.0–5.5) | (1.0–5.0) | (0.5–5.5) | (1.5–6.0) | |
| Pure/Mixed breed | 5/7 | 5/7 | 6/6 | 3/9 | 4/8 |
| Sex (M/F) | 8/4 | 9/3 | 7/5 | 8/4 | 9/3 |
| Weight (Kg) | 5.5 | 4.8 | 6.3 | 4.45 | 5.05 |
| (3.6–17.8) | (2.0–16.8) | (1.0–23.6) | (1.4–17.9) | (1.5–22.7) |
ST, supportive treatment; NAC, N-acetylcysteine; RES, resveratrol; CoQ10, coenzyme Q10; AA, ascorbic acid; M, male; F, female.
Fig. 1.
Study flow diagram for clinical study (“lost to follow-up” refers to not return for scheduled rechecks).
2.3. Diagnosis of CPV infection by PCR
The fecal samples collected in a sterile swab were immersed immediately in a tube containing phosphate buffer saline (1 × PBS; pH 7.3 ± 0.1) and stored at −20 °C until further analysis. The fecal samples were centrifuged at 2670 g for 5 min at 4 °C and 200 μL supernatant was taken and processed for deoxyribonucleic acid (DNA) extraction. Total genomic DNA was extracted from all the collected fecal samples using DNA extraction kit (QIAamp DNA Mini Kit,Qiagen, Hilden, Germany) as per manufacturer's instructions. The diagnosis of CPV-2 infection was carried out by direct detection of viral genome in fecal sample by using PCR primers [CPV(X)-F 5′TGATTGTAAACCATGTAGACTA3’ (from 804 to 825) and Reverse- CPV(X)-R 5′TAAGTCAGTATCAAATTCTTTATC3’ (from 1434 to 1411)] targeting VP2 gene fragment as per the method described earlier (Chander et al., 2016).
2.4. Blood sampling
Approximately, 4.0 mL blood was drawn from each dog by venipuncture of cephalic or lateral saphenous vein into vials with and without dipotassium ethylenediaminetetraacetic acid. Whole blood was used for hematology and the blood without anticoagulant was left to clot at room temperature and serum was separated after centrifugation at 2370 g for 5 min at 4 °C. Blood samples were collected before initiation of treatment (day 0) and thereafter on day 3 and 7.
2.5. Laboratory analysis
2.5.1. Hemogram
The hemoglobin (Hb), packed cell volume (PCV), total leukocyte count (TLC) and total thrombocyte count were measured by using an automated blood cell counter (MS4-e, France). Differential leukocyte count (DLC) was performed manually.
2.5.2. Quantification of oxidative stress indices in serum
The lipid peroxidation was measured by determining the serum malondialdehyde (MDA) concentration by the double heating method based on spectrophotometric measurement of the purple color produced by the reaction of thiobarbituric acid (TBA) with MDA (Draper & Hadley, 1990). The nitric oxide (NO) level in the serum was determined by reduction of nitrate with activated copper-cadmium alloy and zinc sulfate followed by color development with Griess reagent (Sastry et al., 2002). The commercially available kit (EZAssayTM GST Activity Estimation Kit, HiMedia Laboratories, Mumbai, India) was used to measure the glutathione S-transferase (GST) activity in the serum.
2.5.3. Quantification of intestinal FABP2 in serum
The commercially available enzyme-linked immunosorbent assay (ELISA) kit [Fatty Acid Binding Protein 2, Intestinal (FABP2), Cloud-Clone Corp. Houston, USA] was used to measure the concentration of IFABP-2 in serum following manufacturer's instructions.
2.5.4. Assessment of clinical outcome
The clinical recovery was evaluated by assessing the CS and measuring virus load in feces on day 0, 3, 5 and 7 after initiation of treatment. The clinical signs of CPVE dogs were noted everyday and the overall clinical score was calculated by using an earlier score system (1 to rectal temperature < 37.3 °C; 0 to rectal temperature of 37.4–39.4 °C; 1 to rectal temperature of 39.5–39.9 °C; 2 to rectal temperature of 40.0–40.5 °C; 3 to rectal temperature >40.6 °C; 1 to stool with mucus; 2 to watery stool; 3 to stool with blood; 1 to anorexia; 1 to depression; 1 to lethargy; 1 to vomiting and 1 to coughing) (Van Nguyen et al., 2006). The fecal viral load was measured by hemagglutination (HA test) as per the standard method (Muthuraj et al., 2016).
2.6. Statistical analysis
The sample sizes of the groups were small. The data were checked for normal distribution using the Shapiro-Wilk test. The data were analysed using non-parametric descriptive statistics since the data were not normally distributed. The data have been reported as 75th percentile, and minimum and maximum values. Statistical package for the social sciences (SPSS) version 20 was used for analysis of data. A P < 0.05 was considered statistically significant.
3. Results
3.1. Diagnosis of CPV infection
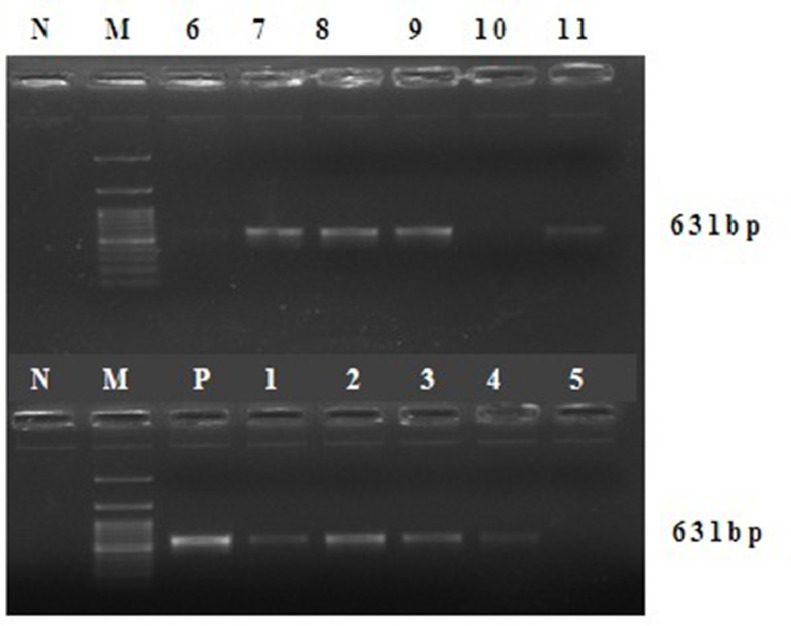
Out of 118 dogs with gastroenteritis, 60 were found positive for CPV-2 infection by PCR as the products yielded expected size of 631 bp in agarose gel under UV transillumination (Fig. 2).
Fig. 2.
Agarose gel electrophoresis of the PCR products for identification of VP2 gene of CPV in fecal samples collected from dogs CPVE. Lane M denotes 100-bp DNA ladder, lane N denotes negative control (no template), lane P denotes positive control (Megavac P vaccine, Indian Immunologicals Ltd, Hyderabad, India, containing tissue culture adapted strain of CPV), lanes 1–4, 7–9 and 11 denote positive, and lanes 5–6 and 10 denote negative for canine parvovirus in fecal samples.
3.2. Effect of treatment on hemogram
The Hb and PCV values were significantly (P < 0.05) reduced on day 3 from pre-treatment values in ST and all antioxidant treatment groups. The neutrophil count was significantly (P < 0.05) increased on day 3 from pre-treatment value in CPVE-affected dogs that received ST+NAC and ST+RES, but no significant changes were recorded in other treatment groups. On day 7, the TLC and neutrophil count were significantly (P < 0.05) increased in all CPVE-affected groups that received either ST or ST along with antioxidants. The comparison of hemogram values among the treatment groups showed that TLC and neutrophil count were significantly (P < 0.05) higher in ST+NAC and ST+RES groups as compared to other treatment groups on day 7 (Table 2).
Table 2.
Changes in hemogram at days 0, 3 and 7 in CPV-infected ill dogs treated with supportive treatment (ST) alone or ST with antioxidants (NAC, RES, CoQ10 and AA). The data have been reported as 75th percentile, and minimum and maximum values.
| Groups (n = 9) | Day 0 | Day 3 | Day7 |
|---|---|---|---|
| Hb (g/dL) | |||
| ST | 14.650a | 12.647b | 12.620b |
| (11.560–14.860) | (09.890–12.670) | (10.390–12.980) | |
| ST+NAC | 14.680a | 13.060b | 13.022b |
| (12.690–14.890) | (10.210–13.210) | (10.560–13.120) | |
| ST+RES | 14.597a | 12.770b | 12.642b |
| (13.460–14.650) | (10.980–13.010) | (11.020–13.010) | |
| ST+CoQ10 | 14.807a | 13.025b | 12.650b |
| (11.290–14.980) | (09.450–13.040) | (10.010–12.980) | |
| ST+AA | 15.050a | 13.095b | 12.897b |
| (09.890–15.260) | (09.780–13.980) | (11.020–12.980) | |
| PCV (%) | |||
| ST | 42.902a | 34.745b | 34.152b |
| (34.680–43.870) | (29.850–35.240) | (30.140–34.250) | |
| ST+NAC | 44.990a | 36.250b | 34.252b |
| (33.540–45.350) | (29.570–36.250) | (33.250–34.260) | |
| ST+RES | 42.555a | 37.215b | 36.302b |
| (33.250–43.470) | (29.870–38.250) | (32.470–38.870) | |
| ST+CoQ10 | 41.805a | 36.895b | 36.292b |
| (35.870–42.480) | (31.250–37.870) | (29.580–36.450) | |
| ST+AA | 44.965a | 37.077b | 36.732b |
| (38.250–45.250) | (29.870–38.570) | (31.020–37.580) | |
| TLC (× 103 cells/µL) | |||
| ST | 06.245a | 06.345a | 06.342Ab |
| (02.580–06.350) | (02.880–06.450) | (03.510–08.120) | |
| ST+NAC | 06.265a | 06.517a | 07.335Bb |
| (02.080–07.210) | (02.280–07.410) | (06.080–07.680) | |
| ST+RES | 05.187a | 05.587a | 07.162Bb |
| (02.040–06.890) | (02.340–06.990) | (06.340–07.290) | |
| ST+CoQ10 | 06.112a | 06.202a | 06.435Ab |
| (02.240–07.590) | (02.440–07.650) | (03.710–08.220) | |
| ST+AA | 06.892a | 06.657a | 06.437Ab |
| (01.860–07.440) | (02.860–07.010) | (03.910–08.320) | |
| Neutrophils (× 103 cells/µL) | |||
| ST | 01.826a | 01.936a | 02.354Ab |
| (0.770–01.905) | (0.772–02.257) | (01.017–03.491) | |
| ST+NAC | 01.789a | 02.048b | 03.267Bc |
| (0.416–01.802) | (0.570–02.223) | (02.492–03.456) | |
| ST+RES | 01.310a | 01.508b | 03.038Bc |
| (0.469–01.791) | (0.655–01.887) | (02.390–03.186) | |
| ST+CoQ10 | 01.833a | 01.961a | 02.463Ab |
| (0.537–02.277) | (0.610–02.524) | (01.075–03.370) | |
| ST+AA | 02.118a | 02.098a | 02.445Ab |
| (0.446–02.232) | (0.800–02.313) | (01.133–03.411) | |
| Lymphocytes (× 103 cells/µL) | |||
| ST | 03.700 | 03.726 | 03.452 |
| (01.419–03.810) | (01.526–03.735) | (02.141–03.978) | |
| ST+NAC | 03.831 | 03.863 | 03.436 |
| (01.310–04.614) | (01.322–04.446) | (02.979–03.610) | |
| ST+RES | 03.406 | 03.537 | 03.804 |
| (01.326–04.478) | (01.404–04.473) | (02.853–03.844) | |
| ST+CoQ10 | 03.709 | 03.664 | 03.463 |
| (01.433–04.554) | (01.537–04.360) | (02.211–04.192) | |
| ST+AA | 03.984 | 03.875 | 03.476 |
| (01.209–04.464) | (01.526–03.925) | (02.285–04.243) | |
| Monocytes (× 103 cells/µL) | |||
| ST | 0.390 | 0.365 | 0.389 |
| (0.206–0.434) | (0.201–0.441) | (0.210–0.406) | |
| ST+NAC | 0.358 | 0.344 | 0.366 |
| (0.208–0.504) | (0.223–0.444) | (0.284–0.384) | |
| ST+RES | 0.358 | 0.352 | 0.363 |
| (0.183–0.413) | (0.187–0.419) | (0.291–0.387) | |
| ST+CoQ10 | 0.345 | 0.327 | 0.392 |
| (0.179–0.455) | (0.195–0.459) | (0.222–0.411) | |
| ST+AA | 0.412 | 0.394 | 0.409 |
| (0.130–0.469) | (0.171–0.420) | (0.234–0.416) | |
| Basophils (× 103 cells/µL) | |||
| ST | 0.039AB | 0.039B | 0.055A |
| (0.000–0.062) | (0.000–0.063) | (0.000–0.057) | |
| ST+NAC | 0.047AB | 0.044B | 0.091A |
| (0.000–0.059) | (0.000–0.045) | (0.000–0.153) | |
| ST+RES | 0.024B | 0.029B | 0.063A |
| (0.000–0.035) | (0.000–0.047) | (0.000–0.065) | |
| ST+CoQ10 | 0.061AB | 0.062AB | 0.056A |
| (0.000–0.075) | (0.000–0.076) | (0.000–0.058) | |
| ST+AA | 0.053AB | 0.052B | 0.057A |
| (0.000–0.067) | (0.000–0.070) | (0.000–0.058) | |
| Eosinophils (× 103 cells/µL) | |||
| ST | 0.265 | 0.269 | 0.251 |
| (0.206–0.434) | (0.201–0.441) | (0.210–0.406) | |
| ST+NAC | 0.265 | 0.260 | 0.357 |
| (0.208–0.504) | (0.223–0.444) | (0.284–0.384) | |
| ST+RES | 0.190a | 0.216a | 0.294b |
| (0.183–0.413) | (0.187–0.419) | (0.291–0.387) | |
| ST+CoQ10 | 0.242a | 0.245ab | 0.253b |
| (0.179–0.455) | (0.195–0.459) | (0.222–0.411) | |
| ST+AA | 0.317 | 0.327 | 0.259 |
| (0.130–0.469) | (0.171–0.420) | (0.234–0.416) | |
| Thrombocytes (× 105 cells/µL) | |||
| ST | 03.267 | 03.142 | 03.015 |
| (01.750–03.320) | (01.820–03.210) | (01.870–03.120) | |
| ST+NAC | 02.907 | 02.925 | 02.905 |
| (02.460–03.020) | (02.410–02.940) | (02.979–03.610) | |
| ST+RES | 02.905 | 02.860 | 02.872 |
| (01.870–03.100) | (01.920–02.950) | (02.853–03.844) | |
| ST+CoQ10 | 02.787 | 02.742 | 02.855 |
| (02.260–02.870) | (02.300–02.840) | (02.211–04.192) | |
| ST+AA | 02.862 | 02.807 | 02.750 |
| (02.010–03.020) | (01.970–02.950) | (02.285–04.243) | |
The 75th percentile values followed by the different uppercase letters (A, B) and lowercase letters (a, b, c) differ significantly among the groups and days, respectively at P = 0.05. ST, supportive treatment; NAC, N-acetylcysteine; RES, resveratrol; CoQ10, coenzyme Q10; AA, ascorbic acid; Hb, hemoglobin; PCV, packed cell volume; TLC, total leukocyte count.
3.3. Effect of treatment on biomarkers of oxidative stress
The concentrations of MDA, NO and GST were significantly (P < 0.05) reduced on day 7 as compared to pre-treatment values in CPVE-affected dogs that received either ST or ST along with antioxidants. When the mean oxidative stress indices were compared between the treatment groups, it was found that the MDA, NO and GST concentrations were significantly (P < 0.05) lower on day 7 in ST+NAC, ST+RES and ST+AA groups as compared to ST and ST+CoQ10 groups (Table 3).
Table 3.
Changes of oxidative stress indices (MDA, NO and GSH) at days 0, 3 and 7 in CPV-infected ill dogs treated with supportive treatment (ST) alone or ST with antioxidants (NAC, RES, CoQ10 and AA). The data have been reported as 75th percentile, and minimum and maximum values.
| Groups (n = 9) | Day 0 | Day 3 | Day7 |
|---|---|---|---|
| MDA (nM/mL) | |||
| ST | 09.125a | 09.097a | 06.382Ab |
| (08.640–09.140) | (08.710–09.150) | (05.990–06.570) | |
| ST+NAC | 09.155a | 09.135a | 05.140Bb |
| (08.350–09.260) | (08.310–09.240) | (04.850–05.200) | |
| ST+RES | 09.177a | 09.157a | 05.175Bb |
| (08.620–09.350) | (08.630–09.330) | (04.870–05.190) | |
| ST+CoQ10 | 09.345a | 09.325a | 06.377Ab |
| (08.240–09.750) | (08.260–09.730) | (05.990–06.550) | |
| ST+AA | 09.375a | 09.362a | 05.132Bb |
| (07.890–09.450) | (07.870–09.460) | (04.890–05.170) | |
| NO (μM/mL) | |||
| ST | 09.267a | 09.257a | 06.770Ab |
| (08.970–09.320) | (09.080–09.310) | (06.240–07.040) | |
| ST+NAC | 09.537a | 09.515a | 05.492Bb |
| (08.850–09.620) | (08.790–09.240) | (05.010–05.620) | |
| ST+RES | 09.392a | 09.500a | 05.557Bb |
| (08.980–09.520) | (08.630–09.560) | (04.960–05.640) | |
| ST+CoQ10 | 09.545a | 09.825a | 06.870Ab |
| (08.620–10.550) | (08.470–10.230) | (05.970–07.050) | |
| ST+AA | 09.875a | 09.862a | 05.580Bb |
| (07.890–09.950) | (07.870–09.960) | (04.880–05.670) | |
| GST (μmol/mL/min) | |||
| ST | 0.932a | 0.942a | 0.657Ab |
| (0.790–0.940) | (0.820–0.950) | (0.530–0.680) | |
| ST+NAC | 0.965a | 0.947a | 0.475Bb |
| (0.810–0.980) | (0.780–0.970) | (0.410–0.490) | |
| ST+RES | 0.930a | 0.925a | 0.485Bb |
| (0.790–01.020) | (0.820–01.000) | (0.420–0.500) | |
| ST+CoQ10 | 01.022a | 01.010a | 0.665Ab |
| (0.780–01.030) | (0.770–01.010) | (0.530–0.680) | |
| ST+AA | 0.920a | 0.927a | 0.487Bb |
| (0.790–0.980) | (0.820–0.980) | (0.410–0.510) | |
The 75th percentile values followed by the different uppercase letters (A, B) and lowercase letters (a, b) differ significantly among the groups and days, respectively at P = 0.05. MDA, malondialdehyde; NO, nitric oxide; GSH, glutathione S-transferase; ST, supportive treatment; NAC, N-acetylcysteine; RES, resveratrol; CoQ10, coenzyme Q10; AA, ascorbic acid.
3.4. Effect of treatment on IFABP-2
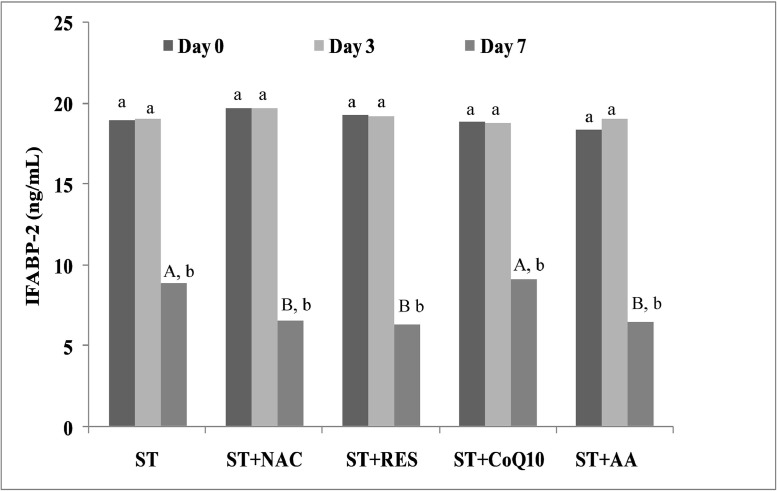
The IFABP-2 concentration was significantly (P < 0.05) decreased on day 7 in CPVE-affected dogs in response to ST or ST along with antioxidants. When the IFABP-2 concentration was compared between the treatment groups, it was observed that the IFABP-2 concentration was significantly (P < 0.05) lower on day 7 in ST+NAC, ST+RES and ST+AA groups as compared to ST and ST+CoQ10 groups (Fig. 3).
Fig. 3.
Changes of concentrations of intestinal fatty acid binding protein-2 (IFABP-2) in serum at days 0, 3 and 7 in CPV-infected ill dogs treated with supportive treatment (ST) alone or ST with antioxidants (NAC, RES, CoQ10 and AA). Data have been reported as 75th percentile. The 75th percentile values followed by the different uppercase letters (A, B) and lowercase letters (a, b) differ significantly among the groups and days, respectively at P = 0.05.
ST, supportive treatment; NAC, N-acetylcysteine; RES, resveratrol; CoQ10, coenzyme Q10; AA, ascorbic acid.
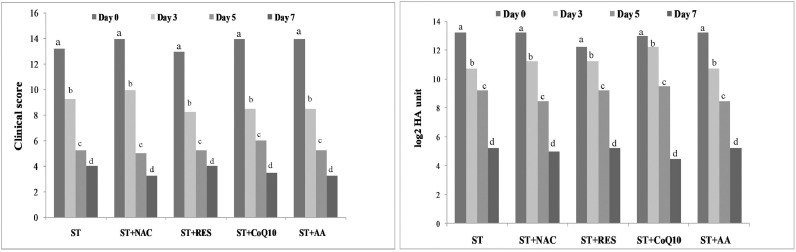
3.5. Effect of treatment on CS, fecal viral load and survivability
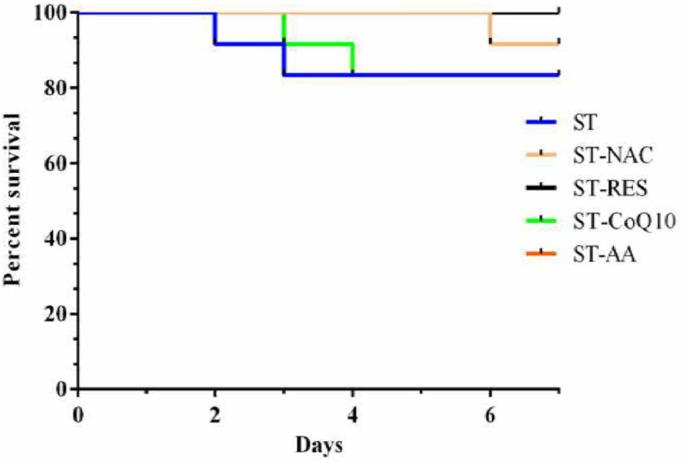
The CS and HA titre were significantly dropped on day 3, 5 and 7 from pre-treatment values in CPVE-affected dogs in response to ST or ST along with antioxidants. The clinical score and HA titre did not differ significantly among the treatment groups on day 3, 5 and 7 (Fig. 4). Two dogs in each ST and ST-CoQ10 groups and one dog in ST-NAC group did not respond to treatments and died. The Log-rank (Mantel-Cox) test showed no significant difference in survivability of CPVE dogs across the groups (Chi square, 4.4048; df, 4; p value, 0.353) (Fig. 5).
Fig. 4.
Changes of clinical score and hemagglutination (HA) titre (log2 HA unit) at days 0, 3, 5 and 7 in CPV-infected ill dogs treated with supportive treatment (ST) alone or ST with antioxidants (NAC, RES, CoQ10 and AA). Data have been reported as 75th percentile. The 75th percentile values followed by the different lowercase letters (a, b,c, d) differ significantly among the days at P = 0.05.
ST, supportive treatment; NAC, N-acetylcysteine; RES, resveratrol; CoQ10, coenzyme Q10; AA, ascorbic acid.
Fig. 5.
Survivability of dogs with CPVE at day 7 in response to supportive treatment (ST) alone or ST with antioxidants (NAC, RES, CoQ10 and AA) The comparison by Log-rank (Mantel-Cox) test shows no significant difference among the groups (Chi square, 4.4048; df, 4; P value, 0.353).
4. Discussion
This is the first detailed report to document the efficacy of antioxidants as adjunct therapy in outpatient dogs with CPVE. The marked elevation of mean Hb and PCV in dogs with CPVE is attributed to hemoconcentration due to decreased water intake and excessive fluid loss during the episodes of vomiting and diarrhea (Botha et al., 2020). The decrease of mean Hb and PCV values on day 3 and 7 from pre-treatment values in all the treatment groups could be the result of rehydration from fluid therapy. In CPVE, the leukopenia and neutropenia are attributed to the cytotoxic effects of virus on hematopoietic progenitor cells of bone marrow and lymphoproliferative organs, resulting in insufficient compensation for the inflamed gastrointestinal tract's massive demand for neutrophils (Goddard et al., 2008; Gaykwad et al., 2018; Mazzaferro, 2020). The leukopenia and neutropenia are considered as potential prognostic indicators to predict the outcome of CPVE (Goddard et al., 2008). Therefore, improvement of leukocyte and neutrophil counts in peripheral circulation is utmost important to prevent the CPV associated mortality of dogs. Although a marked improvement of absolute TLC and neutrophil count were noted on day 7 in all the treatment groups, the ST+NAC and ST+RES groups had significantly (P<0.05) greater values than other treatment groups. Both NAC and RES have been shown to raise the mitotic index, frequency, and total number of hematopoietic stem cells and myeloid progenitors in the bone marrow in previous in vivo and in vitro studies (Berniakovich et al., 2012; Rimmelé et al., 2014).
Redox imbalance contributes to tissue injury and organ dysfunction via oxidation of enzymes and proteins, peroxidation of cell membranes and induction of pro-inflammatory cytokines. MDA, the principal and most studied product of polyunsaturated fatty acid peroxidation, is a classical marker of free radical-induced cell damage (Mohideen et al., 2021). NO, an important signaling molecule under physiological concentrations, becomes a free radical to generate peroxynitrite anion (ONOO) under stress conditions and leads to cell membrane damage (Radi, 2018). GST, a metabolic isozyme, plays a vital role in defense mechanisms against oxidative injury. GST protects cells from oxidative injury by detoxifying the secondary ROS produced when ROS react with cellular constituents (Veal et al., 2002). In the current study, increased MDA and NO concentrations in serum of infected dogs at pre-treatment as compared to post-treatment stage indicates profound oxidative stress in CPVE. The elevation of serum GST activity in CPVE-affected dogs is probably due to a compensatory mechanism leading to up-regulation of antioxidant enzymes (Panda et al., 2009). CPV-induced oxidative stress is mediated through damage of mitochondrial membrane, non-structural protein 1 (NS1) induced DNA damage besides liberation of pro-inflammatory cytokines (Arora et al., 2021). Here, the decrease in mean MDA, NO and GST values was more pronounced in ST+NAC, ST+RES and ST+AA groups as compared to ST and ST+CoQ10 groups. It is reported that NAC and RES are excellent free radical scavengers that help to maintain cellular redox balance by increasing the production of antioxidant enzymes (catalase, heme oxygenase, superoxide dismutase and glutathione peroxidise) (Kavas et al., 2013; Gaykwad et al., 2018). Whereas, NAC inhibits inducible nitric oxide synthase (iNOS), lowers pro-inflammatory cytokine expression/release, prevents lipid peroxidation, and replenishes non-enzymatic antioxidant reserves (Daiber et al., 2019). The AA protects cellular lipids, proteins and DNA from oxidatation and NO associated injury by inhibiting the expression of the subunit p47phox (Zeng et al., 2020). The beneficial effects of antioxidant treatments against viral diseases have been reported in earlier studies (Proskurnina et al., 2020).
A significant increase of IFABP-2 concentration in CPVE-affected animals suggests substantial intestinal injury during virus multiplication (Chethan et al., 2017, 2019; Eregowda et al., 2020). In acute CPVE, the enteric form is manifested with the extensive lesions of hyperaemia, hemorrhage, necrosis, diffuse or segmental granulation of the serosa, granulation and atrophy of the mucosa across all the segments of small intestine (de Oliveira et al., 2018). When the IFABP-2 concentration was compared between the groups, it was found that the IFABP-2 level was significantly lower on day 7 in ST+NAC, ST+RES and ST+AA groups than ST and ST+CoQ10 groups. The pro-inflammatory cytokine storm in viral infection causes redox imbalance, leading to the destruction of infected cells and release of viral particles (Ivanov et al., 2011). Here, the prevention of intestinal injury triggered by redox imbalance and pro-inflammatory cytokines might be the possible reason for reduction of IFABP-2 level in NAC, RES and AA antioxidant groups. NAC is reported to reduce the plasma concentration of IFABP in mice with intestinal ischemia (Khadaroo et al., 2014).
In this study, the dogs with CPVE were presented with complains of lethargy, depression, reduced appetite, severe dehydration, vomition, diarrhea/hemorrhagic diarrhea with or without pyrexia. The drop of mean CS in CPVE-affected dogs that received ST or ST along with antioxidants could be due to palliation of symptoms as a treatment response. Regardless of the different treatment groups, all the CPVE-affected groups received need based supportive treatment. The aggressive treatment with crystalloid solutions, synthetic and natural colloids, antimicrobial formulations, antiemetics, analgesics, correction of hypoglycaemia and electrolyte abnormalities, and enteric dietary support is the cornerstone for management of CPVE (Mylonakis et al., 2016). The incubation period of CPV infection ranges from 4 to 14 days. The virus shedding starts a few days before commencement of clinical signs and thereafter decreases slowly 3–4 weeks after exposure (McCaw & Hoskins, 2006). Decrease of HA titre in CPVE-affected animals could be due to the combined effect of cell mediated as well as humoral immune responses of hosts. In this study, no significant difference in survivability was recorded across the treatment groups from day 0 to 7. An earlier study also demonstrated that combination of antioxidant supplementation did not alter clinical score and survival of systemically-ill hospitalised dogs (Hagen et al., 2019).
The present study had some limitations. Small sample size may have limited our power to observe statistical differences. Enrolled dogs represented a wide range of body weight and breed difference that varied in severity of illness. Additionally, the degree of dehydration in individual dogs at hospitalization could not be considered as an inclusion criterion due to heterogeneous patient populations. The different age and status of dehydration influence the compensatory mechanisms of water-electrolyte disturbances and treatment outcome with different fluid therapy. Due to out-patient care, our study was reliant upon self-reported owner compliance. In addition, we could not perform the molecular characterization of virus in all CPV-positive fecal samples in order to investigate the distribution of CPV-2 variants; different virus variants have been documented to influence the progress of CPVE.
5. Conclusion
In conclusion, although the study had some limitations, the findings suggest that the supplementations of NAC, RES and AA along with ST markedly reduced oxidative stress and intestinal injury in CPVE-affected dogs. Only NAC and RES supplementation to CPVE-affected dogs resulted in early relief from leukopenia and neutropenia, which have a significant impact on prognosis. Although the antioxidants had no further benefits in terms of reducing CS or fecal HA titre when compared to ST alone, the study suggests that NAC and RES supplementation may be beneficial during out-patient care of dogs with naturally existing CPVE. The future studies need to focus on determination of optimal antioxidant selection, dose and duration of supplementation to obtain full therapeutic benefits while treating CPV infection.
Ethical approval and consent to participate
Informed consent was obtained from all dog owners before enrolment in the study. The study protocol was approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (No.F.25/17/2019-CPCSEA), India.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Registration of research studies
1. Name of the registry: Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
2. Unique identifying number or registration ID: No.F.25/17/2019-CPCSEA.
Provenance and peer review
Not commissioned, externally peer-reviewed.
CRediT authorship contribution statement
Gollahalli Eregowda Chethan: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Conceptualization. Ujjwal Kumar De: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Conceptualization. Mithilesh Kumar Singh: Methodology, Investigation. Vishal Chander: Methodology, Investigation. Raguvaran Raja: Methodology, Investigation. Babul Rudra Paul: Methodology, Investigation. Om Prakash Choudhary: Writing – review & editing, Data curation. Neeraj Thakur: Writing – review & editing, Data curation. Kalyan Sarma: Validation, Supervision. Hridayesh Prasad: Validation, Supervision.
Declaration of Competing Interest
The authors disclosed no conflict of interest.
Acknowledgements
The authors are highly thankful to the Director, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly for providing the requisite facilities to carry out research work. This work is a part of an institute funded project. CGE thanks ICAR-IVRI for granting fellowship for his research program.
Contributor Information
Gollahalli Eregowda Chethan, Email: chethanvetmed@gmail.com.
Ujjwal Kumar De, Email: ujjwalde@gmail.com.
References
- Acciacca R.A., Sullivan L.A., Webb T.L., Johnson V., Dow S.W. Clinical evaluation of hyperimmune plasma for treatment of dogs with naturally occurring parvoviral enteritis. Journal of Veterinary Emergency and Critical Care. 2020;30(5):525–533. doi: 10.1111/vec.12987. [DOI] [PubMed] [Google Scholar]
- Arora R., Malla W.A., Tyagi A., Mahajan S., Sajjanar B., Tiwari A.K. Canine parvovirus and its non-structural gene 1 as oncolytic agents: Mechanism of action and induction of anti-tumor immune response. Frontiers in Oncology. 2021:1290. doi: 10.3389/fonc.2021.648873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera M., Panda S.K., Sahoo P.K., Acharya A.P., Patra R.C., Das S., Pati S. Epidemiological study of canine parvovirus infection in and around Bhubaneswar, Odisha, India. Veterinary World. 2015;8(1):33. doi: 10.14202/vetworld.2015.33-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berniakovich I., Laricchia-Robbio L., Belmonte J.C.I. N-acetylcysteine protects induced pluripotent stem cells from in vitro stress: Impact on differentiation outcome. International Journal of Developmental Biology. 2012;56(9):729–735. doi: 10.1387/ijdb.120070ji. [DOI] [PubMed] [Google Scholar]
- Botha W.J., Goddard A., Pazzi P., Whitehead Z. Haemostatic changes associated with fluid resuscitation in canine parvoviral enteritis. Journal of the South African Veterinary Association. 2020;91(1):1–9. doi: 10.4102/jsava.v91i0.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg R.F., Duffy A.L., DeCecco F.A., Chung D.K., Green M.T., Veir J.K., Dow S.W. Clinical evaluation of a single dose of immune plasma for treatment of canine parvovirus infection. Journal of the American Veterinary Medical Association. 2012;240(6):700–704. doi: 10.2460/javma.240.6.700. [DOI] [PubMed] [Google Scholar]
- Chander V., Chakravarti S., Gupta V., Nandi S., Singh M., Badasara S.K., Sharma C., Mittal M., Dandapat S., Gupta V.K. Multiplex amplification refractory mutation system PCR (ARMS-PCR) provides sequencing independent typing of canine parvovirus. Infection, Genetics and Evolution. 2016;46:59–64. doi: 10.1016/j.meegid.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Chethan G.E., Garkhal J., Sircar S., Malik Y.P.S., Mukherjee R., Sahoo N.R., Agarwal R.K., De U.K. Immunomodulatory potential of β-glucan as supportive treatment in porcine rotavirus enteritis. Veterinary Immunology and Immunopathology. 2017;191:36–43. doi: 10.1016/j.vetimm.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Chethan G.E., Kumar De U., Garkhal J., Sircar S., Malik Y.P.S., Sahoo N.R., Verma M.R. Immunomodulating dose of levamisole stimulates innate immune response and prevents intestinal damage in porcine rotavirus diarrhea: A restricted-randomized, single-blinded, and placebo-controlled clinical trial. Tropical Animal Health and Production. 2019;51:1455–1465. doi: 10.1007/s11250-019-01833-1. [DOI] [PubMed] [Google Scholar]
- Chethan G.E., Singh M., Chander V., Singh D., Rajesh J.B., Prasad H., De U.K. Occurrence of canine parvovirus-2 and canine adenovirus-1 infections in dogs: A hospital based study. Indian Journal of Animal Research. 2021;55(2):217–221. [Google Scholar]
- Chopra H., Bibi S., Islam F., Ahmad S.U., Olawale O.A., Alhumaydhi F.A., Marzouki R., Baig A.A., Emran T.B. Emerging trends in the delivery of resveratrol by nanostructures: Applications of nanotechnology in life sciences. Journal of Nanomaterials. 2022;2022:1–17. [Google Scholar]
- Cui Q., Fu Q., Zhao X., Song X., Yu J., Yang Y., Sun K., Bai L., Tian Y., Chen S., Yin Z. Protective effects and immunomodulation on piglets infected with rotavirus following resveratrol supplementation. PloS one. 2018;13(2) doi: 10.1371/journal.pone.0192692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A., Xia N., Steven S., Oelze M., Hanf A., Kröller-Schön S., Li H. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. International Journal of Molecular Sciences. 2019;20(1):187. doi: 10.3390/ijms20010187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mari K., Maynard L., Eun H.M., Lebreux B. Treatment of canine parvoviral enteritis with interferon-omega in a placebo-controlled field trial. Veterinary Record. 2003;152(4):105–108. doi: 10.1136/vr.152.4.105. [DOI] [PubMed] [Google Scholar]
- De Oliveira P.S., Cargnelutti J.F., Masuda E.K., Fighera R.A., Kommers G.D., Silva M.C.D., Weiblen R., Flores E.F. Epidemiological, clinical and pathological features of canine parvovirus 2c infection in dogs from southern Brazil. Pesquisa Veterinária Brasileira. 2018;38:113–118. [Google Scholar]
- Devrim-Lanpir A., Hill L., Knechtle B. How N-acetylcysteine supplementation affects redox regulation, especially at mitohormesis and sarcohormesis level: Current perspective. Antioxidants. 2021;10(2):153. doi: 10.3390/antiox10020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper H.H., Hadley M. Vol. 186. Academic press; 1990. Malondialdehyde determination as index of lipid Peroxidation; pp. 421–431. (Methods in enzymology). [DOI] [PubMed] [Google Scholar]
- Duffy A., Dow S., Ogilvie G., Rao S., Hackett T. Hematologic improvement in dogs with parvovirus infection treated with recombinant canine granulocyte-colony stimulating factor. Journal of Veterinary Pharmacology and Therapeutics. 2010;33(4):352–356. doi: 10.1111/j.1365-2885.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- Eregowda C.G., De U.K., Singh M., Prasad H., Sarma K., Roychoudhury P., Rajesh J.B., Patra M.K., Behera S.K. Assessment of certain biomarkers for predicting survival in response to treatment in dogs naturally infected with canine parvovirus. Microbial pathogenesis. 2020;149 doi: 10.1016/j.micpath.2020.104485. [DOI] [PubMed] [Google Scholar]
- Garkhal J., Chethan G., Gupta V., Qureshi S., Mukherjee R., Dimri U., Gaur G.K., Agarwal R.K., De U.K. Antioxidant potential of coenzyme Q10 in Escherichia coli associated calf diarrhea. Indian Journal of Animal Sciences. 2017;87(6):694–700. [Google Scholar]
- Gaykwad C., Garkhal J., Chethan G.E., Nandi S., De U.K. Amelioration of oxidative stress using N-acetylcysteine in canine parvoviral enteritis. Journal of Veterinary Pharmacology and Therapeutics. 2018;41(1):68–75. doi: 10.1111/jvp.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha M., Selvaraju G. Canine parvoviral enteritis and its determinants-an epidemiological analysis. Indian Journal of Animal Research. 2023;57(2):225–230. [Google Scholar]
- Goddard A., Leisewitz A.L., Christopher M.M., Duncan N.M., Becker P.J. Prognostic usefulness of blood leukocyte changes in canine parvoviral enteritis. Journal of Veterinary Internal Medicine. 2008;22(2):309–316. doi: 10.1111/j.1939-1676.2008.0073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero C.A., Torres D.P., García L.L., Guerrero R.A., Acosta O. N-Acetylcysteine treatment of rotavirus-associated diarrhea in children. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2014;34(11):e333–e340. doi: 10.1002/phar.1489. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mariscal F.M., Arenas-de Larriva A.P., Limia-Perez L., Romero-Cabrera J.L., Yubero-Serrano E.M., López-Miranda J. Coenzyme Q10 supplementation for the reduction of oxidative stress: Clinical implications in the treatment of chronic diseases. International Journal of Molecular Sciences. 2020;21(21):7870. doi: 10.3390/ijms21217870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen D.M., Ekena J.L., Geesaman B.M., Viviano K.R. Antioxidant supplementation during illness in dogs: Effect on oxidative stress and outcome, an exploratory study. Journal of Small Animal Practice. 2019;60(9):543–550. doi: 10.1111/jsap.13050. [DOI] [PubMed] [Google Scholar]
- Hesta M., Ottermans C., Krammer-Lukas S., Zentek J., Hellweg P., Buyse J., Janssens G.P.J. The effect of vitamin C supplementation in healthy dogs on antioxidative capacity and immune parameters. Journal of Animal Physiology and Animal Nutrition. 2009;93(1):26–34. doi: 10.1111/j.1439-0396.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- Humm K.R., Hughes D. In: Small animal critical care medicine. Silverstein D.C., Hopper K., editors. Saunders; St. Louis: 2009. Canine parvovirus infection; pp. 482–485. [Google Scholar]
- Ivanov A.V., Smirnova O.A., Ivanova O.N., Masalova O.V., Kochetkov S.N., Isaguliants M.G. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PloS one. 2011;6(9):e24957. doi: 10.1371/journal.pone.0024957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalita J.C., Prasad A., Verma P., Singh J.L., Arora N. Epidemiology of canine parvovirus infection in and around Pantnagar, Uttarakhand: A retrospective study. The Pharma Innovation Journal. 2022;SP-11(11):24–30. [Google Scholar]
- Kalli I., Leontides L.S., Mylonakis M.E., Adamama-Moraitou K., Rallis T., Koutinas A.F. Factors affecting the occurrence, duration of hospitalization and final outcome in canine parvovirus infection. Research in Veterinary Science. 2010;89(2):174–178. doi: 10.1016/j.rvsc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Kataria D., Agnihotri D., Jain V., Charaya G., Singh Y. Molecular occurrence and therapeutic management of canine parvovirus infection in dogs. International Journal of Current. Microbiology and Applied Sciences. 2020;9:1770–1779. [Google Scholar]
- Kataria D., Agnihotri D., Kumar S., Lohiya A. Comparative assessment of oxidative stress in dogs regarding CPV infection. The Innovation Journal : The Public Sector Innovation Journal. 2020;9(2):115–117. [Google Scholar]
- Kavas G.Ö., Ayral P.A., Elhan A.H. The effects of resveratrol on oxidant/antioxidant systems and their cofactors in rats. Advances in Clinical and Experimental Medicine. 2013;22(1):151–155. [PubMed] [Google Scholar]
- Khadaroo R.G., Fortis S., Salim S.Y., Streutker C., Churchill T.A., Zhang H. I-FABP as biomarker for the early diagnosis of acute mesenteric ischemia and resultant lung injury. PloS one. 2014;9(12) doi: 10.1371/journal.pone.0115242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianian F., Karimian S.M., Kadkhodaee M., Takzaree N., Seifi B., Sadeghipour H.R. Protective effects of ascorbic acid and calcitriol combination on airway remodelling in ovalbumin-induced chronic asthma. Pharmaceutical Biology. 2020;58(1):107–115. doi: 10.1080/13880209.2019.1710218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaturk M.E.R.İ.Ç., Tvarijonaviciute A., Martinez-Subiela S., Tecles F., Eralp O., Yilmaz Z.E.K.İ., Ceron J.J. Inflammatory and oxidative biomarkers of disease severity in dogs with parvoviral enteritis. Journal of Small Animal Practice. 2015;56(2):119–124. doi: 10.1111/jsap.12250. [DOI] [PubMed] [Google Scholar]
- Kokesova A., Coufal S., Frybova B., Kverka M., Rygl M. The intestinal fatty acid-binding protein as a marker for intestinal damage in gastroschisis. PloS One. 2019;14(1) doi: 10.1371/journal.pone.0210797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling M., Norris J.M., Kelman M., Ward M.P. Risk factors for death from canine parvoviral-related disease in Australia. Veterinary Microbiology. 2012;158(3–4):280–290. doi: 10.1016/j.vetmic.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares W., Dhaliwal R., Jiang X., Murch L., Heyland D.K. Antioxidant micronutrients in the critically ill: A systematic review and meta-analysis. Critical Care. 2012;16:1–13. doi: 10.1186/cc11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferro E.M. Update on canine parvoviral enteritis. Veterinary Clinics: Small Animal Practice. 2020;50(6):1307–1325. doi: 10.1016/j.cvsm.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaw D.L., Hoskins J.D. In: Infectious diseases of the dog and cat. 4th ed. Greene C.E., editor. Saunders; St Louis: 2006. Canine viral enteritis; pp. 63–73. [Google Scholar]
- Mohideen K., Sudhakar U., Balakrishnan T., Almasri M.A., Al-Ahmari M.M., Al Dira H.S., Suhluli M., Dubey A., Mujoo S., Khurshid Z., Raj A.T., Patil S. Malondialdehyde, an oxidative stress marker in oral squamous cell carcinoma—A systematic review and meta-analysis. Current Issues in Molecular Biology. 2021;43(2):1019–1035. doi: 10.3390/cimb43020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuraj, P.G., Thomas, J., Verma, S., Sharma, C., Goswami, T.K., & Singh, M. (2016). Usefulness of haemagglutination test for screening of canine parvovirus infection in dogs.
- Mylonakis M.E., Kalli I., Rallis T.S. Canine parvoviral enteritis: An update on the clinical diagnosis, treatment, and prevention. Veterinary Medicine: Research and Reports. 2016:91–100. doi: 10.2147/VMRR.S80971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashine S., Nesburn A.B., Kuppermann B.D., Kenney M.C. Role of resveratrol in transmitochondrial AMD RPE cells. Nutrients. 2020;12(1):159. doi: 10.3390/nu12010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D., Patra R.C., Nandi S., Swarup D. Oxidative stress indices in gastroenteritis in dogs with canine parvoviral infection. Research in Veterinary Science. 2009;86(1):36–42. doi: 10.1016/j.rvsc.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B.R., De U.K., Sarkar V.K., Gandhar J.S., Patra M.K., Agrawal R.K., Singh M.K., Soni S., Eregowda C.G. Effect of systemic inflammatory response syndrome on thrombocytogram, acute phase proteins, electrolytes, acid-base indices and cytokine expression in naturally canine parvovirus infected dogs. Veterinary Immunology and Immunopathology. 2023;259 doi: 10.1016/j.vetimm.2023.110598. [DOI] [PubMed] [Google Scholar]
- Petini M., Drigo M., Zoia A. Prognostic value of systemic inflammatory response syndrome and serum concentrations of acute phase proteins, cholesterol, and total thyroxine in cats with panleukopenia. Journal of Veterinary Internal Medicine. 2020;34(2):719–724. doi: 10.1111/jvim.15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proskurnina E.V., Izmailov D.Y., Sozarukova M.M., Zhuravleva T.A., Leneva I.A., Poromov A.A. Antioxidant potential of antiviral drug umifenovir. Molecules (Basel, Switzerland) 2020;25(7):1577. doi: 10.3390/molecules25071577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proceedings of the National Academy of Sciences. 2018;115(23):5839–5848. doi: 10.1073/pnas.1804932115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelé P., Lofek-Czubek S., Ghaffari S. Resveratrol increases the bone marrow hematopoietic stem and progenitor cell capacity. American Journal of Hematology. 2014;89(12):E235–E238. doi: 10.1002/ajh.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry K.V.H., Moudgal R.P., Mohan J., Tyagi J.S., Rao G. Spectrophotometric determination of serum nitrite and nitrate by copper–cadmium alloy. Analytical Biochemistry. 2002;306(1):79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- Schoeman J.P., Goddard A., Leisewitz A.L. Biomarkers in canine parvovirus enteritis. New Zealand Veterinary Journal. 2013;61(4):217–222. doi: 10.1080/00480169.2013.776451. [DOI] [PubMed] [Google Scholar]
- Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. International Journal of Surgery. 2011;9:672–677. doi: 10.1016/j.ijsu.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Singh P., Kaur G., Chandra M., Dwivedi P.N. Prevalence and molecular characterization of canine parvovirus. Veterinary World. 2021;14(3):603. doi: 10.14202/vetworld.2021.603-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R., Sun S., Zhang Y., Zhou Y., Shan Y., Li X., Fang W. PCV2 induces reactive oxygen species to promote nucleocytoplasmic translocation of the viral DNA binding protein HMGB1 to enhance its replication. Journal of Virology. 2020;94(13) doi: 10.1128/JVI.00238-20. e00238-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nguyen S., Umeda K., Yokoyama H., Tohya Y., Kodama Y. Passive protection of dogs against clinical disease due to Canine parvovirus-2 by specific antibody from chicken egg yolk. Canadian Journal of Veterinary Research. 2006;70(1):62. [PMC free article] [PubMed] [Google Scholar]
- Veal E.A., Toone W.M., Jones N., Morgan B.A. Distinct roles for glutathione S-transferases in the oxidative stress response in Schizosaccharomyces pombe. Journal of Biological Chemistry. 2002;277(38):35523–35531. doi: 10.1074/jbc.M111548200. [DOI] [PubMed] [Google Scholar]
- Venn E.C., Preisner K., Boscan P.L., Twedt D.C., Sullivan L.A. Evaluation of an outpatient protocol in the treatment of canine parvoviral enteritis. Journal of Veterinary Emergency and Critical Care. 2017;27(1):52–65. doi: 10.1111/vec.12561. [DOI] [PubMed] [Google Scholar]
- Walia V., Kaushik D., Mittal V., Kumar K., Verma R., Parashar J., Akter R., Rahman M.H., Bhatia S., Al-Harassi A., Karthika C., Ashraf G.M. Delineation of neuroprotective effects and possible benefits of antioxidantstherapy for the treatment of Alzheimer's diseases by targeting mitochondrial-derived reactive oxygen species: Bench to bedside. Molecular Neurobiology. 2021:1–24. doi: 10.1007/s12035-021-02617-1. [DOI] [PubMed] [Google Scholar]
- Zeng X., Xu K., Wang J., Xu Y., Qu S. Pretreatment of ascorbic acid inhibits MPTP-induced astrocytic oxidative stress through suppressing NF-κB signaling. Neural Plasticity. 2020;2020 doi: 10.1155/2020/8872296. [DOI] [PMC free article] [PubMed] [Google Scholar]