Abstract
This updated systematic review and meta-analysis of randomized and observational studies published up to April 2023 assessed the relative performance of high-dose inactivated influenza vaccine (HD-IIV) and standard-dose influenza vaccines (SD-IIV) against influenza-associated outcomes in older adults (≥65 years).
The analysis included studies conducted over 12 influenza seasons (2009/2010 to 2019/2020, 2021/2022), including over 45 million individuals aged ≥ 65 years, and showed that HD-IIV provided significantly better protection than SD-IIV against influenza-like illness and influenza-related hospitalizations, as well as cardiovascular, cardiorespiratory, and all-cause hospitalizations. Subgroup analyses showed HD-IIV consistently provided better protection than SD-IIV against influenza outcomes across the age range (65+, 75+ 85+ years), and regardless of the predominantly circulating influenza strain and vaccine antigenic match/mismatch.
Randomized studies continue to drive high-quality evidence on the effectiveness of high-dose inactivated influenza vaccine relative to SD-IIV against severe influenza outcomes in adults aged ≥ 65 years, supported by observational data.
Keywords: High dose influenza vaccine, Effectiveness, Elderly, Hospitalization, Influenza, Meta-analysis
Introduction
The burden of influenza and influenza-related complications is much higher in older adults (≥65 years) than in other age groups [1], yet little is known about how the burden changes with advanced age beyond 65 years. A systematic review and meta-analysis covering the period 1967 to 2011 highlighted the non-optimal protection against seasonal influenza in older people [2].
Among seasonal influenza vaccines administered to people aged ≥ 65 years during the 2018/19 influenza season in the US, nearly two-thirds received high-dose (HD), inactivated vaccine (Fluzone High-Dose, Sanofi Pasteur Inc) [3]. The trivalent version of the HD vaccine (HD-IIV3) was first approved in the US in 2009, and was available until the end of the 2019/20 influenza season, after which it was replaced by the quadrivalent (HD-IIV4) version which was approved in 2019 [3]. A systematic review and meta-analysis of HD-IIV3 based on 15 studies conducted during influenza seasons between 2009/10 and 2018/19 in the US showed that the relative vaccine effectiveness (rVE) for HD-IIV3 versus standard-dose (SD) IIV was 15.9% (95% CI 4.1%, 26.3%) against ILI and was 8.4% (5.7%; 11.0%) against all-cause hospital admissions [4]. The analysis showed that HD-IIV3 provided significantly better protection than SD-IIV at reducing influenza infections and associated complications regardless of dominant circulating strain and antigenic match.
For the 2022/23 influenza season, the Advisory Committee on Immunization Practices (ACIP) recommended that adults aged ≥ 65 years preferentially receive quadrivalent high-dose inactivated influenza vaccine (HD-IIV4), quadrivalent recombinant influenza vaccine (RIV4), or quadrivalent adjuvanted inactivated influenza vaccine (aIIV4) [5], [6]. This recommendation, based on a Grading of Recommendations Assessment, Development and Evaluation (GRADE) review performed by ACIP in June 2022, stated that ‘the most data, for the most outcomes, are available to support the high dose vaccine.’ [1], [5].
In this updated meta-analysis, we build upon the existing evidence for HD-IIV3/4 [7] with newly published studies, additional outcomes, and age subgroup analyses.
Methods
Systematic review
The methods of this systematic review and meta-analysis have been previously published [7]. This report provides an update including studies published up to 30 April 2023. A PRISMA framework was used to identify randomized and observational studies evaluating the rVE of HD-IIV versus SD-IIV against influenza-related outcomes in adults aged ≥ 65 years.
The primary objective was to estimate the pooled rVEs across influenza seasons against: laboratory-confirmed or probable ILI (visits with a rapid influenza diagnostic test followed by prescription of antiviral medication); hospitalizations due to influenza, pneumonia, cardiorespiratory, cardiovascular, and all-cause admissions; and hospitalizations/ER visits due to influenza or pneumonia. To improve specificity of the non-influenza outcomes, studies using administrative databases were included only if admission or discharge diagnostic codes of interest were used for the principal or secondary diagnosis.
Secondary objectives were to estimate stratified rVE estimates, including: by age (65–74 years, 74–85 years, ≥ 75 years, and ≥ 85 years); during A/H3N2– or A/H1N1-predominant seasons; during antigenically-matched or mismatched seasons. Data on the predominant circulating strain and antigenic match/mismatch of the vaccine was evaluated using US CDC viral surveillance data [1]. Additional sensitivity analyses were performed using only randomized and observational studies to estimate the pooled rVE.
Data abstraction and meta-analysis
Articles were included if they were primary research studies (randomized controlled trials [RCTs] and observational studies) conducted in adults aged ≥ 65 years where the interventions included HD-IIV and SD-IIV and reported on the rVE against clinical (not immunogenicity) outcomes. A modified Downs and Black critical appraisal tool for randomized and non-randomized studies was used to assess study quality: excellent (25–27), good (19–24), fair (14–18), and poor (≤ 13) [8]. Data abstraction was performed as previously described [7], and the odds ratios (ORs) and standard error (SE) were calculated. Egger’s test was used to quantify potential asymmetry between the ORs of effect measures and the SEs [9]. Higgins’ I2 statistic was used to assess study heterogeneity [10].
Results from individual seasons were stratified based on clinical outcomes, characteristics of study subjects, and influenza season. Meta-analyses were then performed to estimate pooled rVEs of HD-IIV versus SD-IIV. Pooled ORs across multiple studies and influenza seasons were calculated using a random-effects model with DerSimonian–Laird estimators, and rVE was calculated for the pooled estimates with 95% confidence intervals (CI).
Results
Characteristics of identified studies
A flowchart of the publications included in the analysis is shown in Supplement 1. This updated analysis included six new studies (Table 1), beyond the 15 included in the 2020 analysis (Supplement 2)[11], [12], [13], [14], [15], [16]. No evidence of publication bias was detected with funnel plots/Egger’s test (Supplement 3).
Table 1.
Overview of new studies identified for inclusion in the 2022 systematic review.
| Citation, year | Design, country | Influenza seasons | Dominant strain (% of all circulating strains); antigenic match of dominant strain with the vaccine | Size of population aged ≥ 65 years | Influenza-related outcomes | Study quality (Downs and Black Score) |
|---|---|---|---|---|---|---|
| VanAalst, 2021 [16] | Retrospective cohort study, US | 2010–11 2011–12 2012–13 2013–14 2014–15 |
A/H3N2 (84.0%); 96.8% match A/H3N2 (87.0%); 82.0% match A/H3N2 (86.4%); 99.6% mismatch A/H1N1 (57.2%); 99.9% match A/H3N2 (90.6%); 18.6% mismatch |
VHA adults HD: 158,636 SD: 3,480,288 |
Cardiovascular, respiratory, cardiorespiratory hospitalization | Good (19) |
| Machado, 2021 [14] | Retrospective cohort study, US | 2012–13 2013–14 2014–15 2015–16 2016–17 2017–18 |
A/H3N2 (86.4%); 99.6% mismatch A/H1N1 (57.2%); 99.9% match A/H3N2 (90.6%); 18.6% mismatch A/H1N1 (48.4%); 99.9% match A/H3N2 (79.0%); 96.6% mismatch A/H3N2 (69.4%); 93.4% mismatch |
Adults from MarketScan® databases HD: 728,223 SD: 1,633,093 |
Influenza / pneumonia hospital/ER visit | Good (20) |
| Balasubramani, 2020 [11] | Test-negative case control study, US | 2015–16 2016–17 2017–18 2018–19 |
A/H1N1 (48.4%); 99.9% match A/H3N2 (79.0%); 96.6% mismatch A/H3N2 (69.4%); 93.4% mismatch A/H3N2 (53.3%) 11.0% mismatch |
HAIVEN patients HD: 3,861 SD: 2,993 |
Influenza-confirmed acute respiratory illness | Good (21) |
| Izurieta, 2021 [12] | Retrospective cohort study, US | 2019–20 | A/H1N1 (71.0%); 96.0% match | Medicare beneficiaries HD: 7,173,433 SD: 1,584,451 |
Influenza-related hospital encounters / inpatient stays | Good (19) |
| Saade, 2022 [15] (NCT01815268) | Single-blind, pragmatic, comparative effectiveness, cluster RCT | 2013–14 | A/H1N1 (57.2%); 99.9% match |
Residents ≥ 65 in NHs
|
- Hospital admissions related to cardiovascular, pulmonary and influenza-like conditions - Major acute cardiovascular events or respiratory admissions - Hospital admission by any cause |
Good (24) |
| Johansen, 2023 [13] (NCT05048589) |
Pragmatic, open-label, active-controlled, randomized feasibility trial | 2021–22 | A/H3N2 (78.7%); 21.0% mismatch | Danish adults 65–79
|
- Hospitalization for pneumonia or influenza, respiratory or cardiorespiratory disease - All-cause mortality |
Good (24) |
HD, high-dose; IIV, inactivated influenza vaccine; SD, standard dose, VHA, veteran health administration.
Probable or laboratory-confirmed ILI
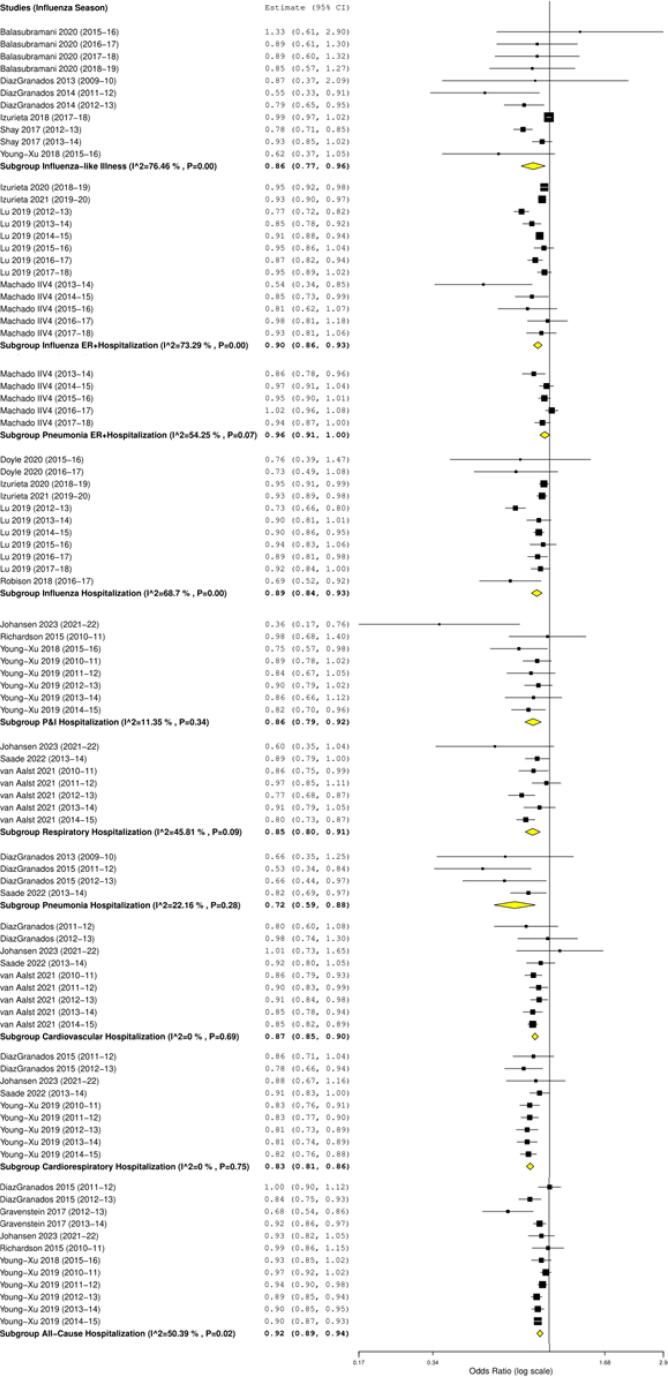
Based on 11 studies the rVE of HD-IIV3 versus SD-IIV against ILI was 14.3% (95% CI 4.2%, 23.3%; p = 0.007) across all seasons. For A/H3N2-dominant seasons, the rVE was 16.3% (95% CI 2.5%, 28.2%; p = 0.022), and for A/H1N1-dominant seasons the rVE was 8.0% (95% CI −3.7%, 18.4%; p = 0.170) (Table 2; Fig. 1).
Table 2.
Pooled relative vaccine efficacy/effectiveness of HD-IIV3 versus SD-IIV against influenza-related outcomes.
| Outcome |
All seasons HD vs SD rVE (95% CI) |
Predominant circulating straina HD vs SD rVE (95% CI) |
Antigenic similarity with predominant circulating strainb HD vs SD rVE (95% CI) |
Study designc HD vs SD rVE (95% CI) |
|||
|---|---|---|---|---|---|---|---|
| A/H3N2-dominant | A/H1N1- dominant | Matched seasons | Mismatched seasons | RCTs | Observational studies | ||
| Influenza-like illnessd | n = 11 14.3% (4.2%, 23.3%) p = 0.007 |
n = 7 16.3% (2.5%, 28.2%) p = 0.022 |
n = 4 8.0% (−3.7%, 18.4%) p = 0.170 |
n = 4 20.4% (−10.7%, 42.7%) p = 0.175 |
n = 7 13.7% (0.0%, 25.5%) p = 0.050 |
n = 3 24.1% (10.0%, 36.1%) p = 0.002 |
n = 8 11.1% (−0.1%, 21.0%) p = 0.051 |
| Hospitalization/ER | |||||||
| Influenzae | n = 13 10.4% (6.8%, 13.9%) p < 0.001 |
n = 8 10.3% (5.4%, 15.0%) p < 0.001 |
n = 5 11.0% (3.8%, 17.6%) p = 0.003 |
n = 5 11.0% (3.8%, 17.6%) p = 0.003 |
n = 8 10.3% (5.4%, 15.0%) p < 0.001 |
– | n = 13 10.4% (6.8%, 13.9%) p < 0.001 |
| Pneumoniaf | n = 5 4.4% (−0.1%, 8.6%) p = 0.053 |
n = 3 2.2% (−2.8%, 6.9%) p = 0.384 |
n = 2 8.4% (−0.7%, 16.7%) p = 0.069 |
n = 2 8.4% (−0.7%, 16.7%) p = 0.069 |
n = 3 2.2% (−2.8%, 6.9%) p = 0.384 |
– | n = 5 4.4% (−0.1%, 8.6%) p = 0.053 |
| Hospitalization | |||||||
| Influenzae | n = 11 11.2% (7.4%, 14.8%) p < 0.001 |
n = 7 13.7% (7.0%, 20.0%) p < 0.001 |
n = 4 7.2% (3.3%, 11.0%) p < 0.001 |
n = 4 7.2% (3.3%, 11.0%) p < 0.001 |
n = 7 13.7% (7.0%, 20.0%) p < 0.001 |
– | n = 11 11.2% (7.4%, 14.8%) p < 0.001 |
| Pneumoniaf | n = 5 27.8% (12.5%, 40.5%) p < 0.001 |
n = 2 39.9% (19.3%, 55.3%) p < 0.001 |
n = 2 19.1% (5.0%, 31.2%) p = 0.010 |
n = 3 28.7% (6.0%, 45.9%) p = 0.016 |
– | n = 4 27.8% (12.5%, 40.5%) p < 0.001 |
|
| Pneumonia/ Influenzag |
n = 8 14.4% (6.8%, 20.6%) p < 0.001 |
n = 6 13.7% (5.3%, 21.4%) p = 0.002 |
n = 2 19.6% (3.0%, 33.4%) p = 0.023 |
n = 5 13.5% (5.0%, 21.3%) p = 0.002 |
n = 3 19.3% (−0.3%, 35.1%) p = 0.053 |
– | n = 7 13.4% (7.3%, 19.2%) p < 0.001 |
| Respiratory | n = 7 14.7% (8.5%, 20.4%) p < 0.001 |
n = 5 16.6% (8.4%, 24.1%) p < 0.001 |
n = 2 10.3% (1.9%, 17.9%) p = 0.018 |
n = 5 9.9% (4.5%, 14.9%) p < 0.001 |
n = 3 21.3% (15.6%, 26.7%) p < 0.001 |
n = 2 19.6% (−12.8%, 42.8%) p = 0.207 |
n = 5 14.8% (7.6%, 21.5%) p < 0.001 |
| Cardiovascular | n = 9 12.8% (10.2%, 15.3%) p < 0.001 |
n = 7 12.8% (10.0%, 15.6%) p < 0.001 |
n = 2 12.6% (5.8%, 18.9%) p < 0.001 |
n = 5 12.5% (8.4%, 16.4%) p < 0.001 |
n = 4 12.6% (8.6%, 16.4%) p < 0.001 |
n = 4 7.8% (−2.5, 17.0%) p = 0.132 |
n = 5 13.2% (10.5%, 15.8%) p < 0.001 |
| Cardiorespiratory | n = 9 16.7% (13.8%, 19.5%) p < 0.001 |
n = 7 17.6% (14.2%, 20.9%) p < 0.001 |
n = 2 14.1% (3.7%, 23.4%) p = 0.009 |
n = 5 15.6% (11.8%, 19.2%) p < 0.001 |
n = 4 18.4% (13.8%, 22.9%) p < 0.001 |
n = 4 12.2% (5.6%, 18.3%) p < 0.001 |
n = 5 17.9% (14.7%, 21.0%) p < 0.001 |
| All-cause | n = 12 8.2% (5.5%, 10.8%) p < 0.001 |
n = 9 8.0% (4.4%, 11.6%) p < 0.001 |
n = 3 8.9% (5.4%, 12.2%) p < 0.001 |
n = 7 6.1% (3.6%, 8.4%) p < 0.001 |
n = 5 12.6% (7.8%, 17.2%) p < 0.001 |
n = 5 10.6% (2.7%, 17.8%) p = 0.009 |
n = 7 7.8% (5.3%, 10.3%) p < 0.001 |
CDC, Centers for Disease Control and prevention; CI, confidence interval; ER, emergency room; HD, high-dose; IIV, inactivated influenza vaccine; RCT, randomized controlled trial; rVE, relative vaccine efficacy/effectiveness; SD, standard dose.
Based on US CDC national surveillance data.
Based on US CDC data on viral antigenic characterization comparing reference vaccine strains with circulating viruses.
Individual-level randomized and cluster-randomized studies.
Probable/laboratory confirmed influenza-like illness.
ICD-9-CM 487 coded hospitalizations.
ICD-9-CM 480–486 coded hospitalizations.
ICD-9-CM 480–488 coded hospitalizations.
Fig. 1.
Forest plots of the pooled OR of HD-IIV3 versus SD-IIV against influenza-related outcomes.
In eight of the 12 seasons, there was a mismatch between vaccine and circulating strains, including during 2018/19, when half of the circulating strains were A/H3N2, of which 11.0% were mismatched with the vaccine strain[1]. Sub-analyses showed that the rVE of HD versus SD against ILI was 20.4% (95% CI −10.7%, 42.7%; p=0.175) in matched seasons and 13.7% (95% CI 0.0%, 25.5%; p = 0.050) in mismatched seasons. Apart from the antigenic mismatch of pandemic A/H1N1 virus with the seasonal vaccine in 2009/10, all of the other mismatched seasons were dominated by A/H3N2 viruses that had mutated.
Hospitalizations and ER visits
HD-IIV was more effective than SD-IIV for the prevention of influenza-related hospitalizations, with an rVE of 11.2% (7.4%, 14.8%; p<0.001) for all seasons and 13.7% (7.0%, 20.0%; p<0.001) for A/H3N2-dominant seasons. Included in this updated analysis were respiratory-related hospitalizations and cardiovascular-related hospitalizations, where pooled rVEs across seasons were 14.7% (8.5%, 20.4%; p < 0.001) and 12.8% (10.2%, 15.3%; p<0.001), respectively.
Further outcomes reported in this update were influenza- or pneumonia-related hospitalizations/ER visits. Based on 13 observational studies of HD-IIV3 versus SD-IIV, rVE against influenza-related hospitalizations/ER visits was 10.4% (6.8%, 13.9%; p=0.001) across all seasons (Table 2; Fig. 1). HD-IIV3 was more effective than SD-IIV for protection against influenza-related hospitalizations/ER visits during matched and mismatched season, and during A/H3N2– and A/H1N1-dominant seasons (Table 2).
Subgroup analysis by age
The rVE was consistent across all age ranges. Among 65–74 year-olds, the rVE for HD-IIV3 versus SD-IIV against ILI was 21.1% (12.4%, 28.9%; p<0.001); among those aged ≥75 years, the rVE was 24.8% (12.3%, 35.6%; p<0.001) (Table 3). For influenza-related hospitalizations, HD-IIV3 was more effective than SD-IIV with a rVE of 8.7% (1.5%, 15.2%; p=0.018) in those aged 65–74 years; however, rVE increased with age to 12.2% (7.3%, 16.9%; p<0.001) in those aged ≥75 years, and to 16.0% (9.8%, 21.8%; p<0.001) in those aged ≥85 years. A similar trend was observed for rVE against influenza-related hospitalization/ER visit.
Table 3.
Pooled relative vaccine efficacy/effectiveness of HD-IIV3 versus SD-IIV against influenza-related outcomes according to age.
| Outcome |
Age subgroup rVE (95% CI) |
|||
|---|---|---|---|---|
| 65–74 years | 75–84 years | ≥75 years | ≥85 years | |
| Influenza-like illness | n = 2 21.1% (12.4%, 28.9%) p < 0.001 |
n = 2 21.9% (7.8%, 33.9%) p = 0.004 |
n = 3 24.8% (12.3%, 35.6%) p < 0.001 |
– |
| Influenza-related | ||||
| Hospitalization/ER visit | n = 6 4.6% (-1.7%, 10.5%) p = 0.146 |
n = 6 9.0% (3.1%, 14.5%) p = 0.003 |
n = 12 12.0% (7.8%, 16.0%) p < 0.001 |
n = 6 14.9% (9.4%, 20.1%) p < 0.001 |
| Hospitalization | n = 7 8.7% (1.5%, 15.2%) p = 0.018 |
n = 7 8.3% (1.4%, 14.7%) p = 0.019 |
n = 13 12.2% (7.3%, 16.9%) p < 0.001 |
n = 6 16% (9.8%, 21.8%) p < 0.001 |
CI, confidence interval; ER, emergency room; HD, high-dose; IIV, inactivated influenza vaccine; rVE, relative vaccine efficacy/effectiveness; SD, standard dose.
Subgroup analysis by study type
In RCTs, the rVE for HD-IIV3 versus SD-IIV against ILI was 24.1% (10.0%, 36.1%; p=0.002; three studies); in observational studies, the rVE was 11.1% (-0.1%, 21.0%; p=0.051; eight studies) (Table 1). The largest RCT of HD-IIV3 versus SD-IIV against laboratory-confirmed influenza, which included 32,000 participants in the USA and Canada over the 2011/12 and 2012/13 influenza seasons and met the pre-defined criteria for superiority, demonstrated an rVE of 24.2% (9.7%, 36.5%) [17].
Discussion
This meta-analysis of studies conducted over 12 influenza seasons, including over 45 million individuals aged ≥65 years, showed that HD-IIV provided significantly better protection than SD-IIV against ILI and influenza-related hospitalizations, as well as pneumonia-related hospitalizations, and cardiovascular, cardiorespiratory, and all-cause hospitalizations. New outcomes in the updated analysis were hospitalizations/ER visits and CV hospitalizations, against which HD-IIV provided better protection than SD-IIV. Furthermore, HD-IIV was more effective than SD-IIV against ILI during A/H3N2-dominant seasons and during seasons with vaccine mismatches.
This updated analysis included a subgroup analysis by age. HD-IIV provided better protection than SD-IIV against ILI in those aged 65–74 years and ≥75 years, and provided better protection against hospitalization/ER visits among those aged ≥75 years and ≥85 years, but not among those aged 65–74 years. Overall, the trend of protection for HD-IIV over SD-IIV against severe influenza outcomes across the age groups is consistent with and well supported by prior research [18], [19].
A recent study assessing the rVE of HD-IIV versus SD-IIV against cardiopulmonary outcomes was not included in this analysis [20]. This RCT, assessing rVE against all-cause death or cardiopulmonary hospitalization over three seasons (2016/17–2019/20) among 5,373 high-risk patients aged 18+ years with recent acute myocardial infarction or heart failure hospitalization and ≥1 additional risk factor, reported no significant difference between the two groups [20]. This study was excluded as the population was not exclusively aged ≥65 years, results were not stratified by age and, moreover, these findings were not generalizable to the general population, given the high-risk status of all enrolled participants.
A key difficulty in developing improved vaccines for adults aged ≥65 years is recruiting large enough populations in RCTs to establish causal relationships with the clinical benefits, as exemplified by two adjuvanted vaccine trials that failed to meet their pre-specified endpoints [21], [22]. To overcome these challenges, new studies of HD-IIV4 versus SD-IIV4 aim to integrate individual randomization into routine vaccination practice while simultaneously leveraging real-world registry-based data collection for all follow-up [23]. Conducted in Denmark during the 2021/22 season, the DANFLU-1 trial (NCT05048589), which was included in this systematic review and meta-analysis, established the feasibility of this approach, randomizing >12,000 participants aged ≥65 years and showing reduced rates of hospitalization for influenza or pneumonia (rVE 64.4% [24.2%, 84.6%]) and all-cause mortality (48.9% [24.2%, 84.6%]) among those receiving HD-IIV4 [23]. The ongoing DANFLU-2 trial (NCT05517174) aims to build upon DANFLU-1, assessing the rVE of HD-IIV4 versus SD-IIV4 against severe influenza outcomes in a fully-powered, multi-season study among >200,000 adults aged ≥65 years in Denmark [24].
Limitations
There are several limitations to the data in the study, including the high degree of statistical heterogeneity observed in several of the pooled rVE estimates and the inclusion of unmeasured confounders, such as health-seeking behaviour or selection bias, that could affect the findings of the observational studies, as previously detailed [4].
Conclusions
Evidence from 12 influenza seasons from randomized and real-world studies in >45 million study participants (including >29 million HD-IIV recipients) continues to show that HD-IIV was more effective than SD-IIV at reducing influenza and associated serious outcomes in people aged ≥65 years, irrespective of age or characteristics of the influenza season. This is consistent with GRADE analyses performed by several health authorities, including the US (CDC/ACIP), Europe (ECDC), Germany (STIKO), Australia (NCIRS/ATAGI), and Canada (NACI), each highlighting greater quality and volume of data supporting the improved protection conferred by HD-IIV over SD-IIV against influenza among those aged ≥65 years [5], [25], [26], [27], [28].
Studies are ongoing to strengthen the estimate of the efficacy and effectiveness of HD-IIV relative to SD-IIV against severe outcomes, including hospitalisation as primary endpoints in older adults, thanks to large-scale randomized pragmatic trials, integrating randomization into routine clinical practice while leveraging registry-based data collection for follow-up.
Funding
This work was supported by Sanofi. The funder was involved in the study design, data collection, data analysis, data interpretation, writing of the report, and the decision to submit the paper for publication.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and were derived from manuscripts available in the public domain.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: JKHL, GKLL, JKY, MML, and SIS are employees of Sanofi, the manufacturer of the high-dose influenza vaccine, and may hold shares and/or stock options in the company.
Acknowledgements
We thank Rolan Vaisman, Amandeep Khurana, and Bruce Seet for assistance with manuscript preparation. Medical writing support was provided by Steven Goodrick and Annick Moon, on behalf of inScience Communications, Springer Healthcare Ltd, funded by Sanofi. The authors would like to thank Isabel Gregoire for manuscript coordination on behalf of Sanofi.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2023.100327.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Centers for Disease Control and Prevention. FluView Interactive. Available at: http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html. Accessed October 2022.
- 2.Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 3.Sanofi.: FDA approves Fluzone® High-Dose Quadrivalent (Influenza Vaccine) for adults 65 years of age and older. Available at: https://www.news.sanofi.us/2019-11-04-FDA-approves-Fluzone-R-High-Dose-Quadrivalent-Influenza-Vaccine-for-adults-65-years-of-age-and-older [Accessed Oct 2022].
- 4.Lee J.K.H., Lam G.K.L., Shin T., Samson S.I., Greenberg D.P., Chit A. Efficacy and effectiveness of high-dose influenza vaccine in older adults by circulating strain and antigenic match: An updated systematic review and meta-analysis. Vaccine. 2021;39(Suppl 1):A24–A35. doi: 10.1016/j.vaccine.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Advisory Committee on Immunization Practices.: Influenza Vaccines for Persons Aged ≥65 Years: Evidence to Recommendations (EtR) Framework. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf [Accessed Nov 2022]. 2022.
- 6.Grohskopf L.A., Blanton L.H., Ferdinands J.M., Chung J.R., Broder K.R., Talbot H.K., et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 Influenza Season. MMWR Recomm Rep. 2022;71(1):1–28. doi: 10.15585/mmwr.rr7101a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J.K.H., Lam G.K.L., Shin T., Kim J., Krishnan A., Greenberg D.P., et al. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: a systematic review and meta-analysis. Expert Rev Vaccines. 2018;17(5):435–443. doi: 10.1080/14760584.2018.1471989. [DOI] [PubMed] [Google Scholar]
- 8.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irwig L., Macaskill P., et al. Bias in meta-analysis detected by a simple, graphical test. Graphical test is itself biased. BMJ (Clinical research ed) 1998;316(7129):470. author reply 470-471. [PMC free article] [PubMed] [Google Scholar]
- 10.Cochrane Org.: Random-effects (DerSimonian and Laird) method for meta-analysis. Cochrane Handbook. Available at. http://handbook.cochrane.org/chapter_9/9_4_3_1_random_effects_dersimonian_and_laird_method_for.htm.
- 11.Balasubramani G.K., Choi W.S., Nowalk M.P., Zimmerman R.K., Monto A.S., Martin E.T., et al. Relative effectiveness of high dose versus standard dose influenza vaccines in older adult outpatients over four seasons, 2015–16 to 2018–19. Vaccine. 2020;38(42):6562–6569. doi: 10.1016/j.vaccine.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izurieta H.S., Lu M., Kelman J., Lu Y., Lindaas A., Loc J., et al. Comparative Effectiveness of Influenza Vaccines Among US Medicare Beneficiaries Ages 65 Years and Older During the 2019–2020 Season. Clin Infect Dis. 2021;73(11):e4251–e4259. doi: 10.1093/cid/ciaa1727. [DOI] [PubMed] [Google Scholar]
- 13.Johansen N.D., Modin D., Nealon J., Samson S., Salamand C., Loiacono M.M., et al. A Pragmatic Randomized Feasibility Trial of Influenza Vaccines. NEJM Evidence. 2023;2(2) doi: 10.1056/EVIDoa2200206. [DOI] [PubMed] [Google Scholar]
- 14.Machado M.A.A., Moura C.S., Abrahamowicz M., Ward B.J., Pilote L., Bernatsky S. Relative effectiveness of influenza vaccines in elderly persons in the United States, 2012/2013-2017/2018 seasons. NPJ Vaccines. 2021;6(1):108. doi: 10.1038/s41541-021-00373-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saade E.A., Abul Y., McConeghy K., Edward Davidson H., Han L., Joyce N., et al. High-dose influenza vaccines for the prevention of hospitalization due to cardiovascular events in older adults in the nursing home: Post-hoc analysis of a cluster-randomized trial. Vaccine. 2022;40(47):6700–6705. doi: 10.1016/j.vaccine.2022.09.085. [DOI] [PubMed] [Google Scholar]
- 16.van Aalst R., Russo E.M., Neupane N., Mahmud S.M., Wilschut J., Samson S.I., et al. Comparing the impact of high-dose versus standard dose influenza vaccines on hospitalization cost for cardiovascular and respiratory diseases: Economic assessment in the US Veteran population during 5 respiratory seasons using an instrumental variable method. Vaccine. 2021;39:A51–A55. doi: 10.1016/j.vaccine.2020.05.080. [DOI] [PubMed] [Google Scholar]
- 17.DiazGranados C.A., Dunning A.J., Kimmel M., Kirby D., Treanor J., Collins A., et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 18.Czaja C.A., Miller L., Alden N., Wald H.L., Cummings C.N., Rolfes M.A., et al. Age-Related Differences in Hospitalization Rates, Clinical Presentation, and Outcomes Among Older Adults Hospitalized With Influenza—U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET) Open Forum Infect Dis. 2019;6(7) doi: 10.1093/ofid/ofz225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Y., Chillarige Y., Izurieta H.S., Wei Y., Xu W., Lu M., et al. Effect of Age on Relative Effectiveness of High-Dose Versus Standard-Dose Influenza Vaccines Among US Medicare Beneficiaries Aged ≥65 Years. J Infect Dis. 2019;220(9):1511–1520. doi: 10.1093/infdis/jiz360. [DOI] [PubMed] [Google Scholar]
- 20.Vardeny O., Kim K, Udell J.A., Joseph J., Desai A.S., Farkouh M.E., et al. Effect of High-Dose Trivalent vs Standard-Dose Quadrivalent Influenza Vaccine on Mortality or Cardiopulmonary Hospitalization in Patients With High-risk Cardiovascular Disease: A Randomized Clinical Trial. JAMA. 2021;325(1):39. doi: 10.1001/jama.2020.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beran J., Reynales H., Poder A., Yu C.Y., Pitisuttithum P., Yuan L.L., et al. Prevention of influenza during mismatched seasons in older adults with an MF59-adjuvanted quadrivalent influenza vaccine: a randomised, controlled, multicentre, phase 3 efficacy study. Lancet Infect Dis. 2021;21(7):1027–1037. doi: 10.1016/S1473-3099(20)30694-0. [DOI] [PubMed] [Google Scholar]
- 22.McElhaney J.E., Beran J., Devaster J.-M., Esen M., Launay O., Leroux-Roels G., et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013;13(6):485–496. doi: 10.1016/S1473-3099(13)70046-X. [DOI] [PubMed] [Google Scholar]
- 23.Biering-Sørensen T: Innovative randomised trial hints at mortality benefits with high-dose influenza vaccines. Available at: https://www.escardio.org/The-ESC/Press-Office/Press-releases/Innovative-randomised-trial-hints-at-mortality-benefits-with-high-dose-influenza-vaccines [Accessed Nov 2022]. ESC Congress 2022.
- 24.Clinicaltrials.gov. N: A Pragmatic Randomized Trial to Evaluate the Effectiveness of High-Dose Quadrivalent Influenza Vaccine vs. Standard-Dose Quadrivalent Influenza Vaccine in Older Adults (DANFLU-2). . Updated 26 September 2022.
- 25.European Centre for Disease Prevention and Control.: Systematic review of the efficacy, effectiveness and safety of newer and enhanced seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals aged 18 years and over. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-vaccines-systematic-review-efficacy.pdf [Accessed Nov 2022].
- 26.Pérez-Rubio A., Castrodeza J.J., Eiros J.M. Choice of influenza vaccine in people over 65 years old. Analysis of reports from international vaccination advisory committees. Rev Esp Quimioter. 2021;34(6):631–638. doi: 10.37201/req/076.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinilaite A, Papenburg J, on behalf of the National Advisory Committee on Immunization (NACI). Summary of the National Advisory Committee on Immunization (NACI) Seasonal Influenza Vaccine Statement for 2022–2023. Can Commun Dis Rep 2022; 48(9): 373-382. [PMC free article] [PubMed]
- 28.Australian Technical Advisory Group on Immunisation (ATAGI). Statement on the Administration of Seasonal Influenza Vaccines in 2023. Available at: https://www.health.gov.au/sites/default/files/2023-03/atagi-advice-on-seasonal-influenza-vaccines-in-2023.pdf [Accessed Mar 2023]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and were derived from manuscripts available in the public domain.
Data will be made available on request.