Abstract
Postmenopausal osteoporosis caused by estrogen deficiency affects millions of women worldwide. By influencing both osteoblast and osteoclast development, NOD-like receptor thermoprotein structural domain-associated protein 3 (NLRP3) is a key player in the etiology of osteoporosis (OP). The purpose of this research was to look into the mechanism of action of NLRP3 in osteoporosis caused by a lack of estrogen, highlighting that NLRP3 induces osteoblast pyroptosis and thus inflammatory responses in de-ovulated mice, thereby inhibiting osteogenic differentiation and participating in the development of osteoporosis. In de-ovulated mice, we found an enhanced inflammatory response and suppression of osteogenic activity. In vitro experiments, we found a significant increase in markers of cell pyroptosis and inflammatory responses and a significant decrease in markers of osteogenic differentiation in osteoblasts from de-ovulated mice. However, knockdown of the NLRP3 gene inhibited this cell pyroptosis and improved osteogenic differentiation of osteoblasts. Our findings indicate a potential therapeutic potential for the treatment of estrogen deficiency-induced osteoporosis by demonstrating the critical role that NLRP3 inflammatory vesicles and their downstream-mediated cellular pyroptosis play in bone differentiation.
Keywords: NLRP3, Ovariectomy, Pyroptosis, Inflammation, Osteoporosis
Graphical abstract

Highlights
-
•
NLRP3 promotes inflammatory responses in de-ovulated mice.
-
•
NLRP3 is involved in the inhibition of osteogenic differentiation in de-ovalized mice.
-
•
NLRP3 promotes osteoblast pyroptosis in de-ovulated mice, leading to the release of inflammatory factors.
-
•
NLRP3 promotes osteoblast pyroptosis in de-ovalized mice, leading to inhibition of osteogenic differentiation.
1. Introduction
Osteoporosis is a metabolic illness characterized by decreased bone density and microarchitecture degradation, which can lead to increased bone fragility [1]. The risk of osteoporosis depends on the amount of bone gained during skeletal growth and development, with peak bone mass in adulthood, followed by gradual loss of bone mass with age [2]. Osteoporosis is usually seen in the elderly and occurs especially in postmenopausal women [3]. By promoting osteoblast differentiation and reducing osteoclast activity, estrogen has been shown to be important in the control of bone remodeling. Lack of estrogen in postmenopausal women can result in excessively resorbing bone without sufficient fresh bone growth, leading to bone loss and accelerating the onset of osteoporosis [4]. Despite the introduction of postmenopausal hormone treatment into the clinical care of osteoporosis, it only lowers a tiny proportion of osteoporosis and related fractures [5,6]. Because to the aging population across the world, osteoporosis is more common and has a significant negative impact on both individuals and society. Although osteoporosis may be prevented, it is challenging to identify those who are at risk for developing it. Osteoporosis is simple to diagnose but challenging to cure. Given the huge health and economic burden of osteoporosis [7], osteoporosis prevention and treatment are critical.
NLRP3 inflammatory vesicles are one of the best characterized multiprotein complexes due to its key role in immunity [8]. Innate immunity and inflammation are mediated by NLRP3 inflammatory vesicles. Caspase-1 is triggered when pre-Caspase-1 and apoptosis-associated speckle-like protein (ASC) are pulled together to create NLRP3 inflammatory vesicles [[9], [10], [11]], causing IL-1β and IL-18 to mature and generate an inflammatory response. NLRP3 is important in a number of chronic illnesses. It has been shown that overexpression of NLRP3 inflammatory vesicles can lead to skeletal abnormalities [12,13], but the mechanisms of how NLRP3 affects the skeleton are not well established.
Cell pyroptosis is a sort of programmed death mediated by gasdermin that induces cell expansion until the cell membrane ruptures and the cell contents are expelled, resulting in a substantial inflammatory response [14]. Cell pyroptosis can be triggered by caspase-1 or caspase-11, which is activated by NLRP3 inflammatory vesicles, and activated caspase-1 is able to cleave gasdermin-d (GSDMD), a key actuator, to induce cell pyroptosis [15]. It has been shown that NLRP3 inflammatory vesicle-induced pyroptosis is associated with infectious bone damage [16,17]. Thus, we can know that NLRP3-induced osteoblast pyroptosis affects bone formation.
Osteoblasts originate from multipotent bone marrow stromal stem cells (MSCs). MSCs are a class of stem cells with the potential for self-proliferation and multidirectional differentiation and can differentiate into bone, cartilage, muscle, ligament, tendon and adipose tissues in response to different environments and triggers. In response to osteogenesis-related hormones, cytokines and various other inducers, MSCs can differentiate in a directed manner to form a spectrum of osteoblasts, including osteogenic cells, osteoblast precursor cells, mature osteoblasts and end-stage osteocytes. There are no clear boundaries between the different stages of the cell population and they are a continuous process of development. Osteoblasts are the material basis for osteogenesis and bone formation and are the most important functional cells in the continuous renewal of bone. The dynamic balance between bone formation by osteoblasts and bone resorption by osteoclasts is the basis for the maintenance of a normal state of bone reconstruction. The pathogenesis of osteoclast-mediated bone loss disease has been more comprehensively studied, whereas the pathogenesis of osteoblast-mediated bone formation disease is still not clearly understood. The study of osteoblast proliferation, differentiation characteristics, changes in activity and the regulation of osteoblast differentiation is important for exploring the mechanisms of bone diseases and finding targets for the treatment of related diseases.
In summary, we hypothesize that in the absence of estrogen, NLRP3 induces osteoblast pyroptosis and thus mediates an intense inflammatory response that inhibits osteogenic differentiation, which in turn is involved in the development of osteoporosis. We first determined the role of NLRP3 in de-ovulated mice and then investigated the effect of NLRP3 on osteoblasts. We found that NLRP3 leads to increased levels of inflammation in de-ovulated mice, which leads to inhibition of osteogenic differentiation; NLRP3 can be observed in cellular experiments to promote osteoblast pyroptosis in de-ovulated mice, leading to increased levels of inflammation in osteoblasts, which leads to inhibition of osteogenic differentiation of osteoblasts.
2. Materials and methods
2.1. Animal experiments
All experiments were approved by the ethics committee. Female C57BL/6J mice aged eight weeks were kept in regular animal room settings (temperature 22 ± 1°C, humidity 55 ± 5%). The de-ovulatory group (OVX), NLRP3 knockout group (NK), the de-ovulatory group with NLRP3 knockout group (OVX + NK), and the control group (NC) were each randomly assigned 15 mice. The control group underwent sham surgery, and the de-ovulatory and de-ovulatory + NLRP3 knockout groups underwent bilateral oophorectomy under pentobarbital sodium (60 mg/kg) anesthesia. After 8 weeks, the neck was broken and executed with sodium pentobarbital anesthesia, and then the tibia or femur stem was quickly removed on ice, the periosteum and cartilage parts at both ends were removed, and the marrow cavity was repeatedly rinsed with distilled water to rinse the blood remaining on the bone surface, after which the femur or tibia was placed in a 1.5 ml EP tube, marked and placed in a freezing box, pre-frozen in a -20°C refrigerator for 30 min and followed by backup storage in a -80°C refrigerator.
2.2. Immunohistochemical analyses
To create paraffin slices, mouse femur samples were first fixed in 4% paraformaldehyde and then decalcified in 10% ethylenediaminetetraacetic acid (EDTA, pH 7.0).The sections were subsequently dewaxed, graded rehydrated, rinsed, and repaired with high pressure antigen. After then, the slices underwent a 15-min dropwise incubation with 3% hydrogen peroxide to deactivate endogenous peroxidase. Primary antibody (1:100) was incubated overnight at 4°C, rewarmed the next day, rinsed, incubated with secondary antibody, and rinsed again. Color development was performed using the DAB color development kit (Abcam, Eugene, USA) and the reaction was terminated with tap water, followed by re-staining with hematoxylin, dehydration, transparency, neutral gum sealing, and observation under a light microscope.
2.3. Western blot analysis
Using a mortar and pestle, mouse femurs were crushed in liquid nitrogen. The powdered bone tissue was then put to centrifuge tubes along with 200 μl of protein extraction reagent (Wanleibio, Beijing, China), mixed thoroughly, and then placed for 30 min in an ice bath. This was followed by shaking on a vortex mixer for 20 s every 5 min. Centrifugation was then performed for 15 min at 4 °C at 12000 rpm. The BCA Protein Assay Kit (Beyotime Biotechnology, Shanghai, China) was used to measure the concentration of bone tissue proteins in the supernatant after it had been transferred to a fresh centrifuge tube. The samples were evaluated by Western blotting, as described in Ref. [18].
In 6-well plates, mouse osteoblasts were washed with PBS, 120 μl of cell lysate (Biosharp, Beijing, China) was added, treated with ultrasound, and centrifuged at 12000 rpm/min for 15 min at 4°C. The supernatant was retained in order to get total osteoblast protein. The BCA protein assay kit (Beyotime Biotechnology, Shanghai, China) was used to measure the protein concentration. According to Ref. [19], Western blotting was employed to assess the samples (Table 1).
Table 1.
Antibodies for Western blotting.
| Empty Cell | Dilution ratio | Company | Catalog number |
|---|---|---|---|
| RUNX2 | 1:1000 | Cell Signaling Technology | 12556 |
| OPN | 1:1000 | Cell Signaling Technology | 27927 |
| OCN | 1:500 | BIOSS ANTIBODIES | Bs-4917R |
| Caspase-1 | 1:1000 | Cell Signaling Technology | 24232 |
| IL-1β | 1:1000 | Cell Signaling Technology | 31202 |
| IL-18 | 1:500 | BIOSS ANTIBODIES | Bs-0529R |
| GSDMD | 1:1000 | Cell Signaling Technology | 69469 |
| TNF-α | 1:1000 | Cell Signaling Technology | 11948 |
| GAPDH | 1:1000 | Cell Signaling Technology | 5174 |
| NLRP3 | 1:1000 | Abcam | ab263899 |
2.4. H&E staining
Paraformaldehyde was used to fix mouse femurs, followed by a decalcification step in 10% EDTA (pH 7.0) and paraffin wax embedding. For morphological analysis of the tissues, paraffin sections of femoral tissues were immersed in xylene I, xylene II respectively in order to remove paraffin, then subjected to hydration reaction, 10 min of hematoxylin staining, washed for 10 min with tap water in order to remove floating colors, rinsed with distilled water for 2 min, then split with 0.5% ethanol hydrochloride for 3s, 10 min of tap water rinsing, washed for 2 min with distilled water, low magnification microscopy, followed by eosin staining for 30s, then dehydrated, transparent, sealed with treacle, and finally observed under light microscopy.
2.5. Mouse bone marrow mesenchymal stem cells(BMSC)Isolation and culture
Bone marrow cells (1 × 107) from the femur and tibia of four groups of mice were taken and inoculated in 100 mm cell culture dishes and incubated at 37°C with 5% CO2 for 3 h to allow adherent cells to adhere. The non-adherent cells were subsequently removed by giving the BMSCs two PBS rinses. In α-MEM medium containing 15% fetal bovine serum, 100 U/mL penicillin, and 100 g/mL streptomycin, BMSCs were cultivated. (Procell Life Science&Technology Co.,Ltd, Wuhan, China). Every three days, the fluid was replaced. After cell fusion reached 80%–90%, trypsinization was performed on the cells before plating them onto new culture dishes as the first passage. The BMSCs at passage three were employed in the investigation that followed.
2.6. Osteoblast differentiation induction
On 24-well plates, 1 × 104 mouse BMSCs were put, and they were cultured in α-MEM medium that had 10% fetal bovine serum, 10 nM dexamethasone, 50 g/mL ascorbic acid, and 10 mM glycerophosphate added (All from Sigma-Aldrich, Louis, USA).
2.7. ALP alkaline phosphatase staining
The BMSCs were differentiated into osteoblasts on day 7, and the osteoblasts were stained using the ALP staining kit (Beijing leagene biotech.co.,ltd, Beijing, China). The sterile slides were placed in culture dishes, and the appropriate amount of cell suspension was added for cell crawling when the cells were passaged, and then removed after the cells grew all over the slides. Cell crawls were rinsed with PBS and fixed with cold propanol for 10 min, then rinsed several times with distilled water. After that, they were incubated in incubation solution (3% sodium β-glycerophosphate 5 ml, 2% sodium barbiturate 5 ml, distilled water 10 ml, 2% CaCl2 10 ml, 2% MgSO4 1 ml) for 5h at 37°C. After incubation, they were rinsed 3 times with tap water, immersed in 2% cobalt nitrate for 5min, washed 3 times with distilled water, after which they were immersed in 1% ammonium sulfide for 2 min, and finally rinsed with tap water and sealed by natural drying.
2.8. Total RNA isolation and quantitative real-time RT–PCR
Mouse osteoblasts were subjected to total RNA extraction using the TRIzol kit (Invitrogen, Waltham, USA). We used the PrimeScript RT kit (Takara, Otsu, Japan) to reverse-transcribe the RNA into single-stranded cDNA. The steps were carried out exactly as instructed. Real-time qPCR was performed in samples with a total system of 20 μL. mRNA expression was compared with the CT method (2-ΔΔCT). Every experiment was carried out three times (Table 2).
Table 2.
Primer sequences for real-time RT–PCR.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GSDMD-N | 5′- CAGTGCTCCAGAACCAGGC -3′ | 5′- CCCTTGCCTTCACCCTTCAA -3′ |
| Caspase-1 | 5′- CTATGGACAAGGCACGGGAC -3′ | 5′- TCAGCTGATGGAGCTGATTGA -3′ |
| IL-1β | 5′- ATGCCACCTTTTGACAGTGATG -3′ | 5′- TGATGTGCTGCTGCGAGATT -3′ |
| IL-18 | 5′- ACTTTGGCCGACTTCACTGT -3′ | 5′- GGGGTTCACTGGCACTTTGA -3′ |
| OCN | 5′- TCACAGATGCCAAGCCCAG -3′; | 5′- GGGACTGAGGCTCCAAGGTA -3′ |
| RUNX2 | 5′- GGGAACCAAGAAGGCACAGA -3′ | 5′- ACTTGGTGCAGAGTTCAGGG -3′ |
2.9. Cell pyroptosis assay
The pyroptosis of mouse osteoblasts was evaluated using the lactate dehydrogenase (LDH) release kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Cell culture supernatants were collected in order to assay the cells for lactate dehydrogenase activity to assess the integrity of the cell membrane. An enzyme marker used in spectrophotometry had its absorbance measured at 450 nm. LDH activity was calculated by the formula = (OD of assay group - OD of control group)/(OD of standard group - OD of blank group) × concentration of standard × N × 1000.
Staining of mouse osteoblasts with Hoechst 33342/PI double staining kit (KeyGEN BioTECH, Jiangsu, China). Following walling, mouse osteoblasts were transplanted onto 24-well plates at a density of 1 × 104 cells per well. The cells were then double stained with Hoechst 33342/PI. 3 μL of Hoechst 33342 was used to stain the cells for 10 min at 37 °C, then with 3 μL PI at room temperature for 10 min before being examined under a fluorescence microscope.
2.10. Statistical analysis
Every value is shown as the mean ± SEM. Multiple comparisons were statistically analyzed using one-way ANOVA. P<0.05 was used to determine significant differences. Data analysis was carried out using GraphPad Prism 8.0 software.
3. Results
3.1. NLRP3 causes elevated levels of inflammation in de-ovulated mice
First, we analyzed the role of NLRP3 in devitalized mice. We used immunohistochemistry and Western bloting to detect TNF-α, IL-1β and IL-18, and found that TNF-α, IL-1β and IL-18 levels in femoral tissues of de-ovulated mice were higher than those in the control group (Fig. 2A-B), indicating an inflammatory response in de-ovulated mice. Meanwhile, the expression levels of TNF-α, IL-1β and IL-18 were lower in femoral tissues of de-ovulated + NLRP3 knockout mice compared with de-ovulated mice (Fig. 2A-B), indicating that NLRP3 mediates this inflammatory response. These results suggest that NLRP3 can lead to elevated levels of inflammation in devitalized mice.
Fig. 2.
(A) Immunohistochemical staining analysis of protein expression levels of TNF-α, IL-1β and IL-18 in mouse femoral tissues, scale bar = 200 μm. (B) Western blot analysis images and associated histograms of TNF-α, IL-1β and IL-18 proteins in mouse femoral tissues. All experiments were performed independently at least three times. (***P<0.001, ****P<0.0001 vs Control group; #P<0.05, ###P<0.001, ####P<0.0001 vs OVX group).
3.2. NLRP3 is involved in the inhibition of osteogenic differentiation in de-ovalized mice
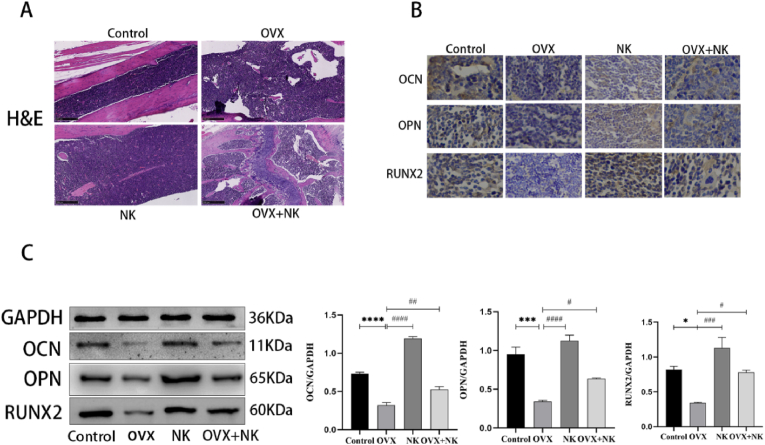
Next, we investigated the role of NLRP3 in de-ovulated mice's osteogenic development. To observe the morphology of bone tissue in mice, we performed H&E staining of bone tissue, and the results showed (Fig. 3A) that bone trabeculae were unevenly distributed and sparsely arranged with a low number of trabeculae in de-ovulated mice, while bone trabeculae were more evenly distributed and closely arranged with a higher number of trabeculae in de-ovulated + NLRP3 knockout mice. We employed immunohistochemistry and Western Blotting to find the osteogenic markers OCN, OPN, and Runx2 in de-ovulated mice to further confirm the impact of NLRP3 on osteogenic differentiation. The findings demonstrated that OCN, OPN, and Runx2 protein expression levels were substantially reduced in femoral tissues of de-ovulated mice, whereas they were significantly higher in femoral tissues of NLRP3 knockout mice compared to de-ovulated mice, and slightly higher in femoral tissues of de-ovulated + NLRP3 knockout mice compared to de-ovulated mice. All of these findings imply that NLRP3 is a factor in the suppression of osteogenic development in de-ovalized mice.
Fig. 3.
(A) H&E staining analysis of femur structure, scale bar = 250 μm. (B) Immunohistochemical staining analysis of OCN, OPN and RUNX2 expression content in mouse femur tissue, scale bar = 200 μm. (C) Western blot analysis images and correlation histograms of OCN, OPN and RUNX2 proteins in mouse femur tissue. Every experiment was carried out at least three times independently. (*P<0.05, ***P<0.001, ****P<0.0001 vs Control group; #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001 vs OVX group).
3.3. NLRP3 induces osteoblast pyroptosis
As NLRP3 is implicated in the prevention of osteogenic differentiation in de-ovulated mice, we isolated bone marrow mesenchymal stem cells from four groups of mice, stimulated osteogenic differentiation, and examined whether ALP labeling caused osteogenic differentiation (Fig. 1B). ALP is a marker that responds to the level of catabolism in bone tissue and plays a key role in calcification. Calcium ions are deposited on collagen under the action of ALP, completing the process of matrix mineralisation. Bone tissue is formed by calcification of the bone matrix, which is synthesised and secreted by osteoblasts; ALP activity reflects, to a certain extent, the degree of differentiation and functional status of osteoblasts. ALP staining is a qualitative assay for revealing the pattern of changes in osteogenic activity during the induction process. Aberrant activation of the NLRP3 inflammasome can lead to osteoblast pyroptosis by upregulating the expression of caspase-1 and gasdermin D (GSDMD), which in turn release inflammatory factors. Seven days after the induction of osteogenic differentiation in mouse BMSCs cells, we detected caspase-1 and GSDMD proteins using Western blotting and qRT-PCR (Fig. 4A-B), and the findings revealed that the OVX group's expression of Caspase-1 and GSDMD-N was considerably greater than the Control group's (P<0.05), whereas Caspase-1 and GSDMD-N expression was lower in the OVX + NK group than in the OVX group (P<0.05), it was considerably lower in the NK group as compared to the OVX group in the NK group(P<0.05). And these results suggest that depletion of ovaries led to abnormal activation of NLRP3 in mouse osteoblasts, which induced cellular pyroptosis. We also employed the LDH release assay kit to identify cellular LDH activity to further assess the amount of cell pyroptosis (Fig. 4C), and the results showed that the OVX group had higher LDH activity than the OVX + NK group (P<0.05). Hoechst 33342/PI double staining revealed (Fig. 4D) that the PI fluorescence intensity was considerably greater in the OVX group compared to the Control group, and that the PI fluorescence intensity was lower in the OVX + NK group compared to the OVX group. These results reconfirmed that NLRP3 inflammatory vesicles can induce osteoblast pyroptosis in the absence of estrogen.
Fig. 1.
(A) Western blot analysis images and associated histograms of NLRP3 proteins in mouse femoral tissues,in order to confirm that the NLRP3 gene has been knocked out. (B) BMSCs were extracted from mice femurs and cultivated for 7 days in osteogenic differentiation induction media. Cells were stained with ALP for pictures. A large number of brownish-black particles are seen microscopically and appear strongly positive, Scale bar = 200 μm. All experiments were performed independently at least three times. (***P<0.001 vs Control group; ###P<0.001 vs OVX group).
Fig. 4.
(A) Western blot analysis images and correlation histograms of Caspase-1, GSDMD-N proteins in osteoblasts of mouse BMSCs cells after 7 days of osteogenic differentiation induction. (B) BMSCs cells after induction of osteogenic differentiation were subjected to qRT-PCR to compare the relative expression levels of Caspase-1, GSDMD-N mRNA in each group. (C) LDH activity was measured in the supernatant of osteoblasts. (D) Fluorescence microscopy images of osteoblasts subjected to Hoechst 33342/PI double staining, scale bar = 200 μm. All experiments were performed independently at least three times. (****P<0.0001 vs Control group; ###P<0.001, ####P<0.0001 vs OVX group).
3.4. NLRP3-induced osteoblast pyroptosis leads to high levels of inflammation
To investigate how osteoblast pyroptosis affects osteogenic differentiation, we further examined inflammation-related proteins in osteoblasts using Western Blotting and qRT-PCR for IL-1β and IL-18 (Fig. 5A-B), and the findings demonstrated that IL-1β and IL-18 expression was considerably higher in de-ovulated mice's osteoblasts, whereas it was substantially lower in NLRP3 knockout mice's osteoblasts than in de-ovulated mice's osteoblasts. Conversely, IL-1β and IL-18 expression levels were lower in de-ovulated + NLRP3 knockout mice's osteoblasts than in de-ovulated mice alone. These outcomes indicate that in the absence of estrogen, abnormal activation of NLRP3-induced cellular pyroptosis promotes cellular inflammatory responses.
Fig. 5.
(A) Western blot analysis images and correlation histograms of IL-1β and IL-18 proteins in osteoblasts from mouse BMSCs cells 7 days after osteogenic differentiation induction. (B) BMSCs cells after induction of osteogenic differentiation were subjected to qRT-PCR to compare the relative expression levels of IL-1β and IL-18 mRNA in each group. All experiments were carried out at least three times independently. (***P<0.001, ****P<0.0001 vs Control group; ##P<0.01, ####P<0.0001 vs OVX group).
3.5. High inflammatory levels due to NLRP3 inhibit osteoblast differentiation
The effect of NLRP3-induced hyperinflammation levels on osteogenic differentiation was then observed using the genes OCN and Runx2, which are connected to osteogenesis. Our analysis of them using Western Blotting and qRT-PCR revealed (Fig. 6A-B) that osteogenic differentiation was inhibited in osteoblasts of de-ovulated mice, and the expression levels of OCN and Runx2 were significantly decreased, whereas the expression levels of OCN and Runx2 were substantially increased in osteoblasts of NLRP3 knockout mice. Osteoblasts from de-ovulated + NLRP3 knockout mice had greater amounts of OCN and Runx2 expression than osteoblasts from de-ovulated animals. In conclusion, in the absence of estrogen, the high level of inflammation caused by NLRP3-induced cell pyroptosis inhibited the osteogenic differentiation of osteoblasts.
Fig. 6.
(A) Western blot analysis images and correlation histograms of OCN and RUNX2 proteins in mouse BMSCs cells after 7 days of osteogenic differentiation induction in osteoblasts. (B) BMSCs cells after induction of osteogenic differentiation were subjected to qRT-PCR to compare the relative expression levels of OCN and RUNX2 mRNA in each group. All experiments were performed independently at least three times. (***P<0.001, ****P<0.0001 vs Control group; #P<0.05, ##P<0.01, ####P<0.0001 vs OVX group).
4. Discussion
Estrogen is a key hormone that controls bone metabolism. Estrogen deficiency can promote osteoclast differentiation both directly and indirectly by stimulating nuclear factor - ligands (RANKL) on osteoblasts, T cells, and B cells, which in turn promotes bone resorption and aids in the development of OP [20]. Estrogen insufficiency throws off the delicate balance between osteoblasts and osteoclasts, increasing osteoblast death and impeding osteoblast development via elevated reactive oxygen species (ROS) and nuclear factor kappa-B (NF–B) pathways. Because of this, there is a relative deficiency in bone synthesis, which causes bone loss [21,22]. In addition to the effects of estrogen on bone metabolism, chronic inflammation promotes bone loss and contributes to the development of OP [23]. NLRP3 inflammatory vesicles are supramolecular complexes that are concentrated in the cytoplasm that affect the innate immune response and inflammatory response. These complexes can sense a variety of pathogens as well as signals related to injury, which activates Caspase-1 and IL-1β maturation [24], mediates the onset of inflammatory responses, and contributes to the development of OP. It has been shown that aging and chronic inflammation-related metabolic disorders of various types, including gout, are directly connected to aberrant activation of NLRP3 inflammatory vesicles [[25], [26], [27], [28]]. Moreover, low estrogen levels can induce a low-grade inflammatory response in living organisms, resulting in pro-inflammatory molecules that activate OP by altering the production and transcription of osteogenic and osteoclastic factors [29,30]. In the pathophysiology of OP, levels of several inflammatory factors, such as IL-1β, IL-6, and TNF-α, are markedly elevated [31]. One of the main members of the IL-1 family, IL-1β [32], is crucial in bone loss that occurs after estrogen insufficiency [33,34]. Currently, the treatment of postmenopausal osteoporosis is mainly focused on two aspects, increasing bone formation or reducing bone resorption [35]. The pathogenesis of postmenopausal osteoporosis explored in this study provides new ideas for future treatment.
In animal experiments, we found that NLRP3 caused elevated levels of inflammation in de-ovulated mice. H&E results showed that bone trabeculae were unevenly distributed and sparsely arranged with a low number of bone trabeculae in de-ovulated mice, whereas bone trabeculae were more evenly distributed and closely arranged with a higher number of bone trabeculae in ovariectomized + NLRP3 knockout mice, indicating that the morphology of bone tissue was impaired in de-ovulated mice, and the knockout of NLRP3 gene improved the extent of this impairment. Since Runx2 is often referred to as the master switch for osteogenic differentiation and OCN and OPN are important osteogenic markers [36], we examined these osteogenic markers. Osteogenic marker expression levels were significantly lower in de-ovulated mice, while higher levels were observed in de-ovulated + NLRP3 knockout mice. Our findings imply that NLRP3 enhances the inflammatory response in de-ovulated mice, inhibits osteogenic differentiation, and hence plays a role in osteoporosis development.
In our research, we discovered that induced osteoblasts made from OVX-BMSCs and the femurs of OVX mice both have activated NLRP3. Our study also found that the OVX-BMSCs group activated Caspase-1, GSDMD-N, IL-1β, and IL-18 compared to the Control-BMSCs group, indicating that NLRP3 induces osteoblast pyroptosis in de-ovalized mice, thereby promoting the inflammatory response. In contrast, GSDMD, Caspase-1, IL-1β, IL-18 expression was slightly lower in the OVX + NK-BMSCs group compared with the OVX-BMSCs group, indicating that NLRP3 knockdown reversed this cell pyroptosis and thus suppressed the inflammatory response. These results suggest that depletion of ovaries activates NLRP3 induces cell pyroptosis and promotes the inflammatory response.
In our study, de-ovulation also resulted in impaired osteogenic potential of bone marrow mesenchymal stem cells, and the expression of osteoblast markers Runx2 and OCN was lower in the OVX-BMSCs group compared to the Control-BMSCs group, implying that osteoblastic differentiation of osteoblasts was lower in de-ovulated mice, which was consistent with previous studies [[37], [38], [39]].The OVX + NK-BMSCs group was more capable of osteogenic differentiation than the OVX-BMSCs group, and the expression of osteoblast markers Runx2 and OCN was higher, indicating that NLRP3 was involved in the osteogenic differentiation of mouse BMSCs, and this result remained consistent with the study [40]. Taken together we can conclude that the osteogenic differentiation ability of bone marrow BMSCs in ovariectomized mice is inhibited and its inhibition is regulated by NLRP3, which is similar with the findings of a recent study [41]. At the same time, our findings further imply that cell pyroptosis decreases osteoblasts' ability to differentiate by inducing an inflammatory response. Taking the whole cellular experiments together we can conclude that NLRP3 induces the pyroptosis of osteoblasts in ovariectomized mice, promoting the inflammatory response and thus inhibiting the osteogenic differentiation of osteoblasts.
In the current study, NLRP3 inflammatory vesicles, an important component of intrinsic immunity, have an important role in the immune response of the body and in the process of disease development. The capacity of NLRP3 inflammatory vesicles to be triggered by a range of pathogens and danger signals makes them essential in a number of disease processes. Being the focal point of the inflammatory response, NLRP3 inflammatory vesicles may thus provide a novel target for the therapy of a variety of inflammatory illnesses. And our study found the role of NLRP3 in postmenopausal osteoporosis. The mechanism was explored by in vitro experiments, which revealed that NLRP3-induced osteoblast pyroptosis in the absence of estrogen promoted the inflammatory response and thus inhibited osteoblast differentiation. Intriguingly, suppression of NLRP3 inflammatory vesicles prevented significant bone loss in de-ovulatory mice in vivo, suggesting that regulating NLRP3 could be a new approach for treating postmenopausal osteoporosis.
CRediT author statement
Chenchen Yang: Conceptualization, Methodology, Software Analysis, Writing – original draft, Writing – review & editing.
Bing Song: Conceptualization, Supervision, Funding acquisition.
Lixia Han: Validation, Methodology.
Zhize Gao: Critical revision of articles.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the Research Foundation of Education Bureau of Liaoning Province, China (2022290).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2023.101496.
Contributor Information
Chenchen Yang, Email: chenchenyang1995@163.com.
Bing Song, Email: 13634965277@163.com.
Lixia Han, Email: 1451135421@qq.com.
Zhize Gao, Email: 1184171197@qq.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lane J.M., Russell L., Khan S.N. Osteoporosis. Clin. Orthop. Relat. Res. 2000;(372):139–150. doi: 10.1097/00003086-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Yu B., Wang C.Y. Osteoporosis: the result of an 'aged' bone microenvironment. Trends Mol. Med. 2016;22(8):641–644. doi: 10.1016/j.molmed.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crandall C.J., Larson J., Wright N.C., et al. Serial bone density measurement and incident fracture risk discrimination in postmenopausal women. JAMA Intern. Med. 2020;180(9):1232–1240. doi: 10.1001/jamainternmed.2020.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W., Zhou L., Zhou C., et al. GDF11 decreases bone mass by stimulating osteoclastogenesis and inhibiting osteoblast differentiation. Nat. Commun. 2016;7 doi: 10.1038/ncomms12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randell K.M., Honkanen R.J., Kröger H., et al. Does hormone-replacement therapy prevent fractures in early postmenopausal women? J. Bone Miner. Res. 2002;17(3):528–533. doi: 10.1359/jbmr.2002.17.3.528. [DOI] [PubMed] [Google Scholar]
- 6.De Villiers T.J. The role of menopausal hormone therapy in the management of osteoporosis. Climacteric. 2015;18(Suppl 2):19–21. doi: 10.3109/13697137.2015.1099806. [DOI] [PubMed] [Google Scholar]
- 7.Harvey N., Dennison E., Cooper C. Osteoporosis: impact on health and economics. Nat. Rev. Rheumatol. 2010;6(2):99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 8.Swanson K.V., Deng M., Ting J.P.Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jo E.-K., Kim J.K., Shin D.-M., et al. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016;13(2):148–159. doi: 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley N., Jeltema D., Duan Y., et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20(13) doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L., Hauenstein A.V. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol. Aspect. Med. 2020;76 doi: 10.1016/j.mam.2020.100889. [DOI] [PubMed] [Google Scholar]
- 12.Detzen L., Cheat B., Besbes A., et al. NLRP3 is involved in long bone edification and the maturation of osteogenic cells. J. Cell. Physiol. 2021;236(6):4455–4469. doi: 10.1002/jcp.30162. [DOI] [PubMed] [Google Scholar]
- 13.Snouwaert J.N., Mytrang N., Repenning P.W., et al. An NLRP3 mutation causes arthropathy and osteoporosis in humanized mice. Cell Rep. 2016;17(11):3077–3088. doi: 10.1016/j.celrep.2016.11.052. [DOI] [PubMed] [Google Scholar]
- 14.Galluzzi L., Vitale I., Aaronson S.A., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sborgi L., Rühl S., Mulvihill E., et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ran S., Chu M., Gu S., et al. Enterococcus faecalis induces apoptosis and pyroptosis of human osteoblastic MG63 cells via the NLRP3 inflammasome. Int. Endod. J. 2019;52(1):44–53. doi: 10.1111/iej.12965. [DOI] [PubMed] [Google Scholar]
- 17.Zhu X., Zhang K., Lu K., et al. Inhibition of pyroptosis attenuates Staphylococcus aureus-induced bone injury in traumatic osteomyelitis. Ann. Transl. Med. 2019;7(8):170. doi: 10.21037/atm.2019.03.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L., Zhang L., Wang Z., et al. Melatonin suppresses estrogen deficiency-induced osteoporosis and promotes osteoblastogenesis by inactivating the NLRP3 inflammasome. Calcif. Tissue Int. 2018;103(4):400–410. doi: 10.1007/s00223-018-0428-y. [DOI] [PubMed] [Google Scholar]
- 19.Lei L., Sun J., Han J., et al. Interleukin-17 induces pyroptosis in osteoblasts through the NLRP3 inflammasome pathway in vitro. Int. Immunopharm. 2021;96 doi: 10.1016/j.intimp.2021.107781. [DOI] [PubMed] [Google Scholar]
- 20.Eghbali-Fatourechi G., Khosla S., Sanyal A., et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Invest. 2003;111(8):1221–1230. doi: 10.1172/jci17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai X.C., Lu D., Bai J., et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem. Biophys. Res. Commun. 2004;314(1):197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 22.Novack D.V. Role of NF-κB in the skeleton. Cell Res. 2011;21(1):169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redlich K., Smolen J.S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012;11(3):234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 24.Agostini L., Martinon F., Burns K., et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20(3):319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 25.Rovira-Llopis S., Apostolova N., Bañuls C., et al. Mitochondria, the NLRP3 inflammasome, and sirtuins in type 2 diabetes: new therapeutic targets. Antioxidants Redox Signal. 2018;29(8):749–791. doi: 10.1089/ars.2017.7313. [DOI] [PubMed] [Google Scholar]
- 26.Mcallister M.J., Chemaly M., Eakin A.J., et al. NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthritis Cartilage. 2018;26(5):612–619. doi: 10.1016/j.joca.2018.02.901. [DOI] [PubMed] [Google Scholar]
- 27.Rheinheimer J., De Souza B.M., Cardoso N.S., et al. Current role of the NLRP3 inflammasome on obesity and insulin resistance: a systematic review. Metabolism. 2017;74:1–9. doi: 10.1016/j.metabol.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Szekanecz Z., Szamosi S., Kovács G.E., et al. The NLRP3 inflammasome - interleukin 1 pathway as a therapeutic target in gout. Arch. Biochem. Biophys. 2019;670:82–93. doi: 10.1016/j.abb.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Cline-Smith A., Axelbaum A., Shashkova E., et al. Ovariectomy activates chronic low-grade inflammation mediated by memory T cells, which promotes osteoporosis in mice. J. Bone Miner. Res. 2020;35(6):1174–1187. doi: 10.1002/jbmr.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietschmann P., Mechtcheriakova D., Meshcheryakova A., et al. Immunology of osteoporosis: a mini-review. Gerontology. 2016;62(2):128–137. doi: 10.1159/000431091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mclean R.R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 2009;7(4):134–139. doi: 10.1007/s11914-009-0023-2. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello C., Arend W., Sims J., et al. IL-1 family nomenclature. Nat. Immunol. 2010;11(11):973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Percegoni N., Ferreira A.C., Rodrigues C.F., et al. Profile of serum IL-1beta and IL-10 shortly after ovariectomy and estradiol replacement in rats. Horm. Metab. Res. 2009;41(1):50–54. doi: 10.1055/s-0028-1087173. [DOI] [PubMed] [Google Scholar]
- 34.Khosla S. Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68(10):1226–1235. doi: 10.1093/gerona/gls163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu K.N., Lie J.D., Wan C.K.V., et al. Osteoporosis: a review of treatment options. P t. 2018;43(2):92–104. [PMC free article] [PubMed] [Google Scholar]
- 36.Granéli C., Thorfve A., Ruetschi U., et al. Novel markers of osteogenic and adipogenic differentiation of human bone marrow stromal cells identified using a quantitative proteomics approach. Stem Cell Res. 2014;12(1):153–165. doi: 10.1016/j.scr.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Elbaz A., Wu X., Rivas D., et al. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J. Cell Mol. Med. 2010;14(4):982–991. doi: 10.1111/j.1582-4934.2009.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo A., Li K., Tian H.C., et al. FGF19 protects against obesity-induced bone loss by promoting osteogenic differentiation. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112524. [DOI] [PubMed] [Google Scholar]
- 39.Liang B., Shen X., Lan C., et al. Glycolipid toxicity induces osteogenic dysfunction via the TLR4/S100B pathway. Int. Immunopharm. 2021;97 doi: 10.1016/j.intimp.2021.107792. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J., Wei K. Necrosulfonamide reverses pyroptosis-induced inhibition of proliferation and differentiation of osteoblasts through the NLRP3/caspase-1/GSDMD pathway. Exp. Cell Res. 2021;405(2) doi: 10.1016/j.yexcr.2021.112648. [DOI] [PubMed] [Google Scholar]
- 41.Wang L., Chen K., Wan X., et al. NLRP3 inflammasome activation in mesenchymal stem cells inhibits osteogenic differentiation and enhances adipogenic differentiation. Biochem. Biophys. Res. Commun. 2017;484(4):871–877. doi: 10.1016/j.bbrc.2017.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.