Abstract
Cyclophorus saturnus is an edible land snail traditionally harvested for human food, yet little is known about its nutritional value, especially in Thailand. This study aimed to investigate its nutritional potential as an alternative food resource. In the present study, proximate composition, essential mineral content, amino acid, and lipid profiles of the meat were evaluated. Proximate analysis showed that C. saturnus contained 80.04% moisture, 11.88% protein, 6.04% carbohydrate, and 0.93% fat, with 80.01 kcal/100 g fresh matter. For minerals, calcium was the most abundant element in the meat. Its protein contained glutamic and aspartic as the major amino acids, while it was not a good source of tryptophan and methionine but was considered a very rich source of other essential amino acids (amino acid scores greater than 100). Its lipid fraction showed a higher proportion of mono and polyunsaturated fatty acids (MUFA and PUFA, 67.69%) and a lower proportion of saturated fatty acids (SFA) (32.31%). The PUFA/SFA ratio (1.56), hypocholesterolemic/hypercholesterolemic ratio (HH; 5.58), atherogenicity index (AI; 0.48), and thrombogenicity index (TI; 0.20) are considered nutritionally healthy for humans. Overall, this study demonstrates the nutritional potential of C. saturnus to serve as a nutritious part of the human diet and as an alternative ingredient in food systems; therefore, its production and consumption should be more extensively promoted.
Keywords: Proximate composition, Mineral contents, Fatty acids, Amino acids, Recommended daily intake, Estimated daily intake
1. Introduction
Land snails are a specialized food item that has long been prized by numerous countries, particularly Thailand, China, Taiwan, and Western European nations [1]. In recent years, snail farming has grown significantly worldwide due to the economic importance of land snails and growing demand for edible use and cosmetics [2]. According to estimates, 43,000 tons of snails were consumed globally in 2016, and 50,000 tons are expected to be consumed by the end of 2025 [3]. Snail meat has been reported to be rich in protein and have a low fat and cholesterol content that make it meet the demands of modern nutrition [[4], [5], [6]]. It has also been reported that snail meat is a valuable source of essential fatty acids, amino acids, and minerals [4,7]. Due to its nutritional benefits, snail meat has been considered a healthy alternative source of dietary protein for human consumption in many parts of the world [8].
In Thailand, land snails especially Cyclophorus genus are commonly used as an ingredient for regional cuisine such as Larb and Tom Yum Hoi [9]. The land snails of the genus Cyclophorus are predominantly active during the wet season and widely distributed in many regions of South East Asia including Thailand, particularly in humid regions of mountainous areas [10]. Typically, they are harvested by hand-picking during the rainy season. Cyclophorus snails have an operculum and a thick, massive pyramidal or turbinated shell with stripe patterns of brown, white and black colors on the surface [11]. The maximum size range of the snails can be between 20 and 25 mm in height and 30–40 mm in width. An adult female snail typically lays eggs (40–150 eggs per hatching) in moist soil, decaying logs, or dead leaves, and 5–8 months are required for larvae to develop into adults after hatching [9]. Some of their foods include fungi, lichen, decaying plants as well as fresh plants such as vegetables, tubers and flowers [11]. Among the various species of edible Cyclophorus snails, Cyclophorus saturnus, locally known as the “Hoihom or Fragrant snail,” is one of the most common species that is plentiful and consumed in Thailand because its meat has a unique aroma and good flavor when cooked [12,13].
As an alternative protein source for human beings, the nutritional potential of edible snails has recently received more attention. Some reports are available on the nutritional value of different species of land snails such as Helix pomatia [7,14], Cornu aspersum, Eobania vermiculata [4], Theba pisana [5]. However, to the best of our knowledge, no data are available on the nutritional properties of C. saturnus. Understanding their nutritional characteristics would make it easier to identify the components necessary for their marketability and promotion that could give it considerable economic value in the future. Therefore, the aim of this work was to investigate the nutritional potential of C. saturnus including fatty acids and amino acids as well as proximate and mineral compositions in the edible part of the snails collected from Thailand. Such snail would allow the development of many values added products to address the modern consumers who are increasingly looking for a nutritionally interesting material and reveal a rich-in-nutrients food item.
2. Materials and methods
2.1. Snail sample preparation and ethics statement
Snail samples (C. saturnus) with mixed sex were collected from Phu Phan Mountain range, northeastern Thailand. Snails were kept in plastic bags with ice during transport to laboratory and then stored at −20 °C until analysis. The samples were prepared according to the modified procedures of Galluzzo et al. [4]. A total of 200 specimens with mean individual weights of 5.09 ± 0.50 g without considering snail gender was used in this study. The edible portion of snail muscle was taken from the shell, homogenized, and used for chemical analyses with three replications performed for each analysis. The Institute of Animals for Scientific Development (IAD) of Thailand's guidelines for the use of animals in research were followed strictly during the study. The handling of land snails in this study was authorized by the Mahasarakham University ethics council (IACUC-MSU-21/2023).
2.2. Proximate and energy value analysis
The moisture content was analyzed by drying until reaching a constant weight at 105 °C in an oven, according to the methods specified by the Association of Official Analytical Chemists [15]. Then the loss of weight on drying was used to calculate the amount of moisture in snail samples and presented as the % of fresh matter (FM). Protein assay was performed using an auto Kjeldahl System (Buchi B-324/I-437, Uster, Switzerland) following the Kjeldahl procedure [15]. Protein content was calculated using a determined nitrogen content with a conversion factor of 6.25 (N x 6.25). The lipid in snail samples was quantified using the Soxhlet extraction apparatus (Gerhardt GmbH, Königswinter, Germany) using hexane as an extraction solvent [15]. Ash was measured by the incineration of samples at 550 °C in a muffle furnace for 7 h [15]. The total carbohydrate content was obtained by calculation using a simple subtraction method, while the energy value of the snail samples was calculated using energy conversion factors and presented as kcal/100 g FM as the sum of the percentage composition of lipids, protein, and total carbohydrate, multiplying by factors of 9, 4, and 4, respectively, as described by Gomot [16].
2.3. Mineral composition analysis and estimated daily intakes (EDIs)
Snail meat samples were assessed for various mineral elements including calcium (Ca), magnesium (Mg), sodium (Na), iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu) using atomic absorption spectrophotometry (AAS, ZEEnit 700 P, Analytik Jena, Germany), as described by Nkansah et al. [6] with some modifications. Briefly, snail meat sample was dried at 105 °C. Ten milliliters of nitric acid (HNO3) were pipetted into a digestion flask containing 1 g of dried sample and heated in a fume hood until the fumes disappeared. The cooled mixture was then mixed with 4 mL of 70% perchloric acid (HClO4) and heated again to obtain a clear liquid before it was cooled and diluted to 50 mL with distilled water. A clear aliquot was taken for absorbance measurement using AAS. The calibration curve of each mineral standard was generated, and the mineral content in sample was calculated, with results expressed as mg/100 g of dried sample (DM). Colorimetric determination of total phosphorus (P) by the molybdate method was carried out after calcination and incineration of snail meat sample using UV/Vis Spectrophotometer (SPECORD 210 PLUS, Analytik Jena, Germany), according to the AOAC procedure [15].
The body weight of the consumers, the daily snail consumption rate, and the element concentration all contribute to estimated daily intakes (EDIs) of trace elements from snail consumption. Estimation was done using Equation (1) [17].
| EDIs = C metal x C factor x D food intake/ body weight | (1) |
Where C metal, C factor and D food intake are the mean essential metal concentration in the various snail species (mg/100 g), the conversion factor (0.085), the daily consumption rate of snail (kg/day), respectively. In this investigation, the body weight was estimated at 70 kg.
2.4. Fatty acid composition analysis
Snail meat samples’ fatty acid profiles were determined following a modified version of the previous methods [18,19]. Fatty acid methyl esters (FAMEs) were prepared from the lipid extract of snail sample using saponification followed by methylation procedures. The extract (1 mL) was saponified with 4 mL of 0.5 M NaOH in methanol at 80 °C for 60 min, and then, the methylation of fatty acids was performed by the addition of 5 mL of methanolic BF3 reagent at 80 °C for 20 min. The obtained mixture was cooled and added to saturated NaCl (5 mL). FAMEs were subsequently extracted with 2 mL of iso-octane (2, 2, 4-trimethylpentane) and transferred into an amber glass autosampler vial before analysis of the fatty acid composition using gas chromatography (430-GC model, Bruker, Billerica, MA, USA) equipped with a flame ionization detector (GC-FID). Chromatographic separation was performed on a fused silica Rt-2560 GC column (100 m × 0.25 mm ID, 0.50 μm phase; Restek Corporation, Bellefonte PA, USA) with a 1 mL/min flow rate, using nitrogen as the carrier gas. Individual fatty acids were identified and calculated based on a comparison of their retention times to the FAME standards and reported as a percentage of fatty acids in lipid fraction as well as mg of individual fatty acids per 100 g of fresh snail meat. The nutritional quality indexes of lipids, including the total saturated fatty acids (ΣSFA), total monounsaturated fatty acids (ΣMUFA), total polyunsaturated fatty acids (ΣPUFA), total n3 PUFA (Σn3), total n6 PUFA (Σn6), ΣPUFA/ΣSFA, and n3 PUFA/n6 PUFA ratio, were calculated based on the fatty acid composition, while the hypocholesterolemic/hypercholesterolemic (HH) ratio, atherogenicity index (AI), and thrombogenicity index (TI) were estimated using Equations (2)–(4) [20].
| HH = (cis − C18:1 + ΣPUFA)/ (C12:0 + C14:0 + C16:0) | (2) |
| AI = [C12:0 + (4 × C14:0) + C16:0]/ ΣUFA | (3) |
| TI = (C14:0 + C16:0 + C18:0)/ [(0.5 × ΣMUFA) + (0.5 × Σn6) + (3 × Σn3) + (n3/n6)] | (4) |
2.5. Amino acid profile analysis
The amino acid composition of the snail meat samples was determined using an automatic amino acid analyzer (ARACUS, MembraPure, Hennigsdorf, Germany) following the method of Wangkahart et al. [21] with minor modifications. Snail samples were hydrolyzed at 110 °C for 24 h with 6 M HCl containing 0.5% 2-mercaptoethanol, followed by using a nitrogen blower to eliminate HCl from the digested solution. The residual amount was then redissolved with 0.02 M HCl and subsequently passed through a 0.45-μm syringe filter prior to analysis of the amino acid composition. A separate study was used to determine the tryptophan content. The weighted samples were hydrolyzed for 20 h at 110 °C in 4 M LiOH containing 95 mM ascorbic acid. The hydrolysate was then neutralized with 6 M HCl, centrifuged, and the supernatant was then passed through a 0.45-μm syringe filter and examined via an automatic amino acid analyzer.
The composition was then computed by comparison with the retention time and peak areas of the standards and expressed as mg/g fresh meat and mg/g of protein (DM). The essential amino acid score (as a percentage of adequacy) of the snail meat sample was calculated based on the reference amino acid pattern for adults [22] using the following equation:
| Amino acid score = Amino acid content in sample × 100/Reference amino acid pattern | (5) |
3. Results and discussion
3.1. Proximate composition and energy value
The data from the proximate analysis and energy value of the C. saturnus sample, reported on both a fresh and dry matter basis, are shown in Table 1.
Table 1.
Proximate composition and energy value of C. saturnus.
| Composition | % (FM) | % (DM) |
|---|---|---|
| Moisture | 80.04 ± 0.39 | – |
| Protein | 11.88 ± 0.03 | 59.51 ± 0.15 |
| Fat | 0.93 ± 0.08 | 4.66 ± 0.40 |
| Ash | 1.12 ± 0.02 | 5.61 ± 0.10 |
| Carbohydrate | 6.04 ± 0.30 | 30.26 ± 1.50 |
| Energy (kcal/100 g) | 80.01 ± 1.36 | 400.85 ± 6.81 |
Values are expressed as mean ± standard error of the mean (SEM).
FM = fresh matter basis, DM = dry matter basis.
3.1.1. Moisture content and dry matter
The amount of water is one of the key factors indicating the sensory quality of the flesh in terms of texture, tenderness and juiciness [[23], [24], [25]]. Moisture was the main ingredient in fresh C. saturnus meat, 80.04% of the content, and 19.96% dry matter was identified. The moisture value of the C. saturnus meat sample was in the range of the moisture content reported for different species of land snails (75.20–84.91% FM) by previous studies, according to Pissia, Matsakidou, and Kiosseoglou [8]. The observed value of moisture content in C. saturnus meat was close to the result previously reported in land snail H. pomatia from Turkey (80.80% FM) [14] and C. haughtoni from Thailand (81.85%) [9], while the lower levels were recorded in Archachatina Marginata (73.67% FM) and Achatina from Nigeria (75.28% FM) [26]. In other fresh meats were reported to contain water as the main component such as beef (73.1%), and lamb (72.9%) but lower than that found in C. saturnus meat.
3.1.2. Protein content
The C. saturnus sample was found to contain 11.88% protein FM, which could make it a good source of protein compared with the protein values found in cereal grains, 7–18% FM [27]. Previous research reported that different species of land snails collected from different locations contained protein values varying between 10.08% and 20.76% FM [8,26,28]. This is the first report on the content of protein in C. saturnus snails from Thailand, and the amount is slightly lower than that reported for C. haughtoni (14.07% FM), a land snail in the same genus found in Thailand [9] and Helix aspersa (12.87% FM), from Turkey [29]. Milinsk et al. [30] also reported a content of protein ranging from 9.50% to 12.56% FM in H. aspersa, a farmed snail fed a different feed formula. The protein content of C. saturnus was found to be lower than the levels found in conventional meats, including pork, beef, and chicken (17.3–24.1% FM) [31], and fish (15–20% FM) [32].
3.1.3. Fat content
In this study, the content of fat of C. saturnus (0.93% FM) was close to the fat value reported by Babalola and Akinsoyinu [26] for Limicolaria species (1.05% FM), a common edible land snail found in Nigeria. However, previous studies showed that the fat content in edible snail meat could be as low as 0.38% FM, as recorded for C. haughtoni collected from Thailand [9] and 0.41% FM for H. aspersa collected from Turkey [29]; in addition, the content was reported as 2.44% FM in Archachatina marginata obtained from Nigeria by Babalola and Akinsoyinu [26]. The fat content in C. saturnus was considered as low as 0.93% when compared to the lean portion in some raw meats such as pork loin (4.7% FM), beef loin (3.3–7.6% FM), duck meat (6.2% FM) and chicken breast (1.2–8.9% FM) [31]; therefore, it can be an alternative meat for people with fat-related diseases.
3.1.4. Ash content
The mean total ash detected in C. saturnus was 1.12% FM, which is within the range of ash content (between 1.07% and 2.33% FM) previously described in the literature for several species of edible land snails collected from different sites [8].
3.1.5. Total carbohydrate content
The percentage of total carbohydrates found in C. saturnus was 6.04% FM, as shown in Table 1. This value was slightly lower than the content of total carbohydrate reported by Babalola and Akinsoyinu [26] (6.91% FM) for Limicolaria sp., a land snail obtained from a natural area in Oyo state, Nigeria. Caetano et al. [5] demonstrated that snails contained a variety of carbohydrate types, including monomers like glucose and reserve polysaccharides, primarily glycogen. Different values of total carbohydrate, estimated indirectly, were observed for different land snail species. For C. haughtoni, the land snail species of the same Cyclophorus genus that was collected from Thailand showed a lower value of total carbohydrate [9]. Babalola and Akinsoyinu [26] reported levels of total carbohydrates in wild snail meat of 1.8% FM for A. marginata and 7.25% FM for Achatina fulica, while Milinsk et al. [30] reported that between 7.07% and 10.01% FM of total carbohydrates were observed in the meat of farmed snails (H. aspersa maxima) fed different diets. Moniruzzaman et al. [33] demonstrated that freshwater snails and clams from Bangladesh contained carbohydrates in amounts of 30.2–57.3% DM, higher than C. saturnus (30.26% DM). However, very low values of total carbohydrates were also reported by Fagbuaro et al. [34], with less than 1% FM for several land snail species including A. marginata ovum, A. marginata saturalis, Achatina, and Limicolaria spp. Variation in the amount of total carbohydrate in land snails could be related to several factors including the snail's species, diet, and location [5].
3.1.6. Energy value
The energy value obtained for C. saturnus meat was 80.01 kcal/100 g FM, as shown in Table 1. This value was higher than that reported in the literature for H. aspersa maxima meat (66 kcal/100 g FM) by Gomot [16], while it was within the range of energy value (76.92–87.08 kcal/100 g FM) observed for the same species of snail meat by Milinsk et al. [30]. The energy value of C. saturnus meat was in the range of the values recorded for freshwater mollusk species, between 346 and 436 kcal/100 g DM [33]. However, C. saturnus meat was low in calories even compared with other lean cuts of meat such as chicken breast (108–176 kcal/100 g FM), pork loin (131 kcal/100 g FM), and beef loin (114 kcal/100 g FM) [31]. Therefore, due to the reasonable amount of protein, the consumption of snail meat could help to reduce excessive intake of carbohydrates and fats.
3.2. Macro mineral compositions
The macro mineral compositions on a fresh and dry matter basis and the EDIs of Ca, P, and Mg are presented in Table 2. Among all the minerals measured, Ca was the most abundant in C. saturnus meat, (865 mg/100 g DM or 172.65 mg/100 g FM); these results agree with the findings of earlier studies on the major elements in other land snail species [5]. Nkansah et al. [6] also reported the content of Ca, the most plentiful mineral, in the meat of three snail species from Ghana, A. marginata (701.79 mg/100 g DM), A. achatina (656.9 mg/100 g DM), and A. fulica (402.09 mg/100 g DM), while Kalio and Etela [35] reported values in the range of 144–175 mg/100 g FM in A. marginata. Additionally, C. saturnus meat was an excellent source of Ca compared to some food products (on a fresh matter basis) such as fish (9–103 mg/100 g FM) [36], whole milk (124 mg/100 g FM), chicken eggs (64 mg/100 g FM) [37], chicken (12 mg/100 g FM), pork (6 mg/100 g FM), and beef (15 mg/100 g FM) [38]. Ca estimated EDIs were low when compared to their acceptable daily intake levels [39,40].
Table 2.
Mineral composition and estimated daily intakes of trace elements from the intake of C. saturnus.
| Parameter | mg/100 g FM | mg/100 g DM | EDIs |
|---|---|---|---|
| Macro mineral | |||
| Calcium (Ca) | 172.65 ± 0.98 | 865.00 ± 5.00 | 5.25E-3 |
| Phosphorus (P) | 100.80 ± 0.98 | 505.00 ± 5.00 | 3.06E-3 |
| Magnesium (Mg) | 36.93 ± 0.98 | 185.00 ± 5.00 | 6.07E-6 |
| Trace mineral | |||
| Sodium (Na) | 22.98 ± 0.03 | 115.15 ± 0.15 | 6.99E-4 |
| Iron (Fe) | 8.97 ± 0.04 | 44.96 ± 0.22 | 6.06E-6 |
| Zinc (Zn) | 3.32 ± 0.01 | 16.63 ± 0.04 | 1.01E-4 |
| Copper (Cu) | 1.06 ± 0.01 | 5.29 ± 0.04 | 3.21E-5 |
| Manganese (Mn) | 0.42 ± 0.01 | 2.09 ± 0.02 | 1.26E-5 |
Values are expressed as mean ± standard error of the mean (SEM).
FM = fresh matter, DM = dry matter, EDIs = Estimated daily intakes.
P was the next most abundant mineral element in C. saturnus meat. Both elements are essential minerals involved in several physiological processes in the human body, including bone formation [41]. In this study, the content of P (505 mg/100 g DM; 100.8 mg/100 g FM) was comparable to that of the wild Roman snail, H. pomatia (104.52 mg/100 g FM) [14], and higher than the values of 268.53, 241.9, and 61.29 mg/100 g DM reported in the meats of A. marginata, A. achatina, and A. fulica, respectively [6]. However, a higher concentration of P was previously reported by Fagbuaro et al. [34] for different species of giant land snail meat, in the range of 123.23–153.89 mg/100 g FM, which was higher than the value recorded in this study on the basis of fresh matter (Table 2). C. saturnus meat contains P at a lower level than traditional meats such as chicken, beef, and pork, with values in the range of 173–229 mg/100 g FM [38]. P estimated EDIs were low when compared to their acceptable daily intake levels [39,40].
Mg is also important to reduce blood pressure levels and improve cardiovascular health [42]. The result obtained in this study was 185 mg/100 g DM (36.93 mg/100 g FM), lower than that in the literature for H. pomatia (54.05 mg/100 g FM) [14]. Comparing the result of Mg on a fresh basis obtained in this study with other meats such as fresh fish of different species (16–109 mg/100 g FM) [36], lamb (19 mg/100 g FM), chicken (25 mg/100 g FM), and pork (25 mg/100 g FM) [38], it can be suggested that C. saturnus meat is a rich source of Mg. Finally, Mg estimated EDIs were low when compared to their acceptable daily intake levels [39,40].
3.3. Trace mineral elements
The trace mineral elements on a fresh and dry matter basis and the EDIs of Na, Fe, Zn, Cu and Mn are presented in Table 2. Considering the content of Na on a wet basis in snail meat, C. saturnus provided a lower concentration of Na (22.57 mg/100 g FM) than that found for other species of land snails (50.8–60.94 mg/100 g FM) [34] and for Otala lactea (60.1 mg/100 g FM) and T. pisana (92.2 mg/100 g FM) species [5]. The content obtained was also low when compared to traditional meats such as chicken, pork, and lamb, which contain 77, 55, and 46 mg/100 g FM, respectively [38].
Additionally, C. saturnus meat contained a considerable amount of Fe (44.96 mg/100 g DM; 8.97 mg/100 g FM) when compared to the content found in Roman land snail species (H. pomatia) (1.71 mg/100 g FM) [14] and the most popular snail species in Africa, including A. marginata (6.33 mg/100 g DM), A. achatina (5.75 mg/100 g DM), and A. fulica (26.64 mg/100 g DM) [6]. However, Gomot [16] observed a high content of Fe of the deshelled part in different species of reared snails, ranging between 30.4 and 78 mg/100 g DM. Fe is a crucial mineral involved in a range of metabolic activities in humans, including oxygen transport, hemoglobin formation, DNA synthesis, immune response, and energy production [43]. Flesh foods are rich in absorbable iron, recommended to prevent iron deficiency, particularly for children, teenagers, and women of reproductive age [43]. The presence of Fe in C. saturnus meat (8.81 mg/100 g on a fresh matter basis) was also high when compared with that in other fresh meats such as lamb (1.64 mg/100 g), chicken (0.89 mg/100 g), and pork (1.01 mg/100 g) [38], indicating that C. saturnus meat is an excellent source of Fe.
Meat products have also been reported as good sources of Zn, with the trace mineral playing numerous biological roles in human metabolism such as a cofactor of several enzymes [28]. The mean percentage of Zn obtained in this study was 3.32 mg/100 g FM, which was higher than the values reported by Adeyeye [28] for A. marginata (1.44 mg/100 g FM), Archatina sp. (1.87 mg/100 g FM), and Limicolaria sp. (1.23 mg/100 g FM), land snail species collected from Nigeria, whereas Kalio and Etela [35] reported a higher Zn content in the range of 7.5–9.6 mg/100 g FM for A. marginata, a farmed snail fed different diets. Our study indicated that C. saturnus meat provided a Zn content close to that of raw beef and mutton at 3.6 and 3.8 mg/100 g FM; however, it was higher than that recorded in raw pork (1.6–2.7 mg/100 g FM), chicken (0.8–1 mg/100 g FM) [31], and fish (0.38–0.99 mg/100 g FM) [36].
Low levels of other minerals present in C. saturnus meat included Cu and Mn (Table 2). Both Cu and Mn are trace minerals required for numerous biological functions in the human body [6]. When compared to other land snail species such as A. marginata [35], with Cu and Mn values ranging from 0.56 to 0.76 and 0.15–0.25 mg/100 g FM, respectively, C. saturnus meat was superior in Cu (1.06 mg/100 g FM) and Mn (0.42 mg/100 g FM). According to data reported by Wright et al. [44], high concentrations of Cu and Mn were recorded in oysters (raw) in the ranges of 1.58–2.86 and 0.30–0.64 mg/100 g meat, respectively. They also reported lower levels than in conventional meats such as pork, chicken, and beef, with Cu content in the range of 0.05–0.09 mg/100 g fresh meat and Mn levels below the detection limit of assay (less than 0.01 mg/100 g) [44]. The level of Mn in C. saturnus (as reported in dry weight; Table 2) was considered close to the value in Pomacea canaliculata snails from a commercial farm in the Republic of Korea (2.0 mg/100 g DM), whereas P. canaliculata contained a higher concentration of Cu (7.1 mg/100 g DM) [1]. Overall, Na, Fe, Zn, Cu and Mn estimated EDIs were low when compared to their acceptable daily intake levels [39,40].
3.4. Fatty acid composition
The fatty acid profiles for C. saturnus meat consider three main types: saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs), as shown in Table 3. Fifteen fatty acids were detected in C. saturnus meat, including seven SFAs, two MUFAs, and six PUFAs. Interestingly, the most prevalent type of fatty acid in C. saturnus meat was PUFA (50.49% of lipid fraction or 469.56 mg/100 g fresh snail meat), followed by SFA (32.31% or 300.47 mg/100 g fresh snail meat) and MUFA (17.20% or 159.96 mg/100 g fresh snail meat), respectively. The highest proportion of PUFA was also reported by Galluzzo et al. [4] in other species of land snail (T. pisana, C. aspersum, and E. vermiculata), as up to 48% of the total fatty acids. In addition, Milinsk et al. [30] revealed that the meat of farmed snail H. aspersa maxima fed with different feeds had high proportions of PUFA in the range of 49.9–57.1%, while proportions of MUFA (20.7–23.8%) were not significantly different from those of SFA (20.2–26.6%). For meats from main animal species such as lamb, pork, beef, and chicken, the levels of total PUFA were 5.80, 11.86, 14.42, and 30.74%, respectively; however, these meats contained high levels of MUFA as well as SFA [38]. C. saturnus snail meat was rich in unsaturated fatty acids (UFA) and low in SFA, which is more desirable and nutritionally beneficial due to the potential health benefits, i.e., lowering the risk of various metabolic diseases [45].
Table 3.
Fatty acid composition and concentration obtained from the edible part of C. saturnus.
| Fatty acid | % of total fatty acids | mg/100 g fresh meat |
|---|---|---|
| Myristic acid (C14:0) | 0.66 ± 0.04 | 6.11 ± 0.41 |
| Pentadecylic acid (C15:0) | 1.32 ± 0.08 | 12.26 ± 0.73 |
| Palmitic acid (C16:0) | 10.96 ± 0.38 | 101.96 ± 3.55 |
| Margaric acid (C17:0) | 4.21 ± 0.15 | 39.14 ± 1.35 |
| Stearic acid (C18:0) | 11.58 ± 0.03 | 107.68 ± 0.32 |
| Arachidic acid (C20:0) | 1.51 ± 0.15 | 14.05 ± 1.44 |
| Heneicosylic acid (C21:0) | 2.07 ± 0.22 | 19.27 ± 2.06 |
| Total saturated fatty acids (Σ SFA) | 32.31 | 300.47 |
| Oleic acid (C18:1 n9c) | 14.32 ± 0.26 | 133.19 ± 2.37 |
| Gondoic acid (C20:1) | 2.88 ± 0.01 | 26.77 ± 0.02 |
| Total monounsaturated fatty acids (Σ MUFA) | 17.20 | 159.96 |
| Linoleic acid (C18:2 n6c) | 13.60 ± 0.09 | 126.43 ± 0.85 |
| γ-Linolenic acid (C18:3 n6) | 6.53 ± 0.42 | 60.72 ± 3.86 |
| Eicosadienoic acid (C20:2) | 6.60 ± 0.05 | 61.34 ± 0.51 |
| Eicosatrienoic acid (C20:3 n3) | 3.24 ± 0.30 | 30.15 ± 2.79 |
| Arachidonic acid (C20:4 n6) | 17.86 ± 0.24 | 166.06 ± 1.58 |
| Eicosapentaenoic acid (C20:5 n3; EPA) | 2.67 ± 0.05 | 24.86 ± 0.51 |
| Total polyunsaturated fatty acids (Σ PUFA) | 50.49 | 469.56 |
| PUFA/SFA ratio | 1.56 | |
| Σn6 | 44.58 | |
| Σn3 | 5.92 | |
| n6/n3 ratio | 7.54 | |
| HH | 5.58 | |
| TI | 0.48 | |
| AI | 0.20 |
Values are expressed as mean ± standard error of the mean (SEM).
Considering each type of fatty acid, stearic acid (C18:0) and palmitic acid (C16:0) were the major representatives of SFA, about 35.84% and 33.93% of the total SFA, respectively, while oleic acid (C18:1 n9c) was the most predominant MUFA (about 83% of the total MUFA). These findings are in agreement with previous studies on the predominance of these fatty acids in land snail meat such as H. pomatia [14], H. aspersa [29], T. pisana, C. aspersum, and E. vermiculata [4]. Evidence indicates that higher SFA intake—in particular, myristic, palmitic, and lauric acids—is associated with an elevation of low-density lipoprotein cholesterol levels and increased risk of cardiovascular disease, while stearic acid decreases such effects [46].
Arachidonic acid (C20:4 n6) and linoleic acid (C18:2 n6c) were recorded as the major PUFA, constituting up to 35.4% and 26.9% of the total PUFA present in C. saturnus, respectively. The predominance of linoleic and arachidonic acids was also reported in other snail species such as H. aspersa [29], T. pisana [4], and specifically Lunella undulata, a turban snail with a high content of arachidonic acid (34.37% of total PUFA) [47]. It has been well documented that linoleic and arachidonic acids are the dominant n6-PUFA present in the human diet, especially in animal source foods, and have a wide range of biological activities and health effects such as linoleic acid's reduction of blood cholesterol and LDL cholesterol concentration and arachidonic acid's role in brain development and function [38,45,48]. In this study, eicosatrienoic acid (ETE) (C20:3 n3) and eicosapentaenoic acid (EPA) (C20:5 n3) were the major n3 PUFA found in C. saturnus meat in small amounts (5.92%) compared to the total n6 PUFA content (44.58%). This finding was consistent with that of other snail meat species containing a small amount of n3 PUFA such as H. pomatia (2.51%) [14] and H. aspersa maxima (5.51–6.19%) [30]. The dietary intake of long-chain n3 PUFAs such as EPA is linked to a reduced risk of noncommunicable diseases due to the essential role in various physiological mechanisms such as anti-inflammatory effects, neurologic and visual development, and lowering blood triacylglycerol levels [45].
Regarding a nutritional quality index, the ratio of n6/n3 PUFA is a classic index frequently used in the evaluation of dietary fat and oil quality, with optimal values recommended as a healthy diet ranging from 1 to 4 [49]. The ratio of n6/n3 in snail meat reported by Milinsk et al. [50] for farmed H. aspersa maxima ranged from 5.01 to 7.05. High n6/n3 ratios were also reported in edible land snails from different farms in Poland, with values between 6.12 and 10.20 for C. aspersa maxima, and between 6.56 and 10.26 for C. aspersum [7]. Additionally, the ratio of n6/n3 in C. saturnus meat was comparable to other traditional meats, such as some fish species (5.07–8.79) [51], skinless chicken (7.81) and pork (7.4) [52].
Additionally, the PUFA/SFA ratio is a typical value used for the evaluation of the nutritional quality of dietary foods; it is employed to estimate how food affects cardiovascular health, with higher values being associated with more beneficial health impacts [20]. It has been suggested that undesirable values of PUFA/SFA ratio are under 0.45 [53]; however, high values could lead to oxidative stress [54]. The favorable values of PUFA/SFA that lower the risk of cardiovascular diseases are in the range of 1–1.50, as suggested by Kang et al. [55]. The PUFA/SFA ratio of C. saturnus meat determined in the present study was 1.56, which was considered nutritionally healthy for humans. Szkucik et al. [7] reported these values in land snail species cultured on different farms, varying between 1.10 and 1.55 for C. aspersa maxima and 1.40 and 1.59 for C. aspersum. Higher PUFA/SFA values were reported in edible land snails collected from Italy, including T. pisana (2.32), C. aspersum (1.75), and E. vermiculata (1.82) [4]. However, a low PUFA/SFA ratio was recorded by Özogul et al. [14] for Roman land snails (H. pomatia), exhibiting a value of 0.68. For meats from other animal species, PUFA/SFA values ranged between 0.20 and 2.10 in different shellfish species, while the values in fish varied between 0.95 and 1.60 [56]. Pork, beef, lamb, and chicken showed lower PUFA/SFA values of 0.31, 0.35, 0.12, and 0.95, respectively [38].
The hypocholesterolemic/hypercholesterolemic (HH) ratio is one of the nutritional quality indices used to assess dietary lipid profiles; it is frequently used to evaluate the impact of particular fatty acids and hypercholesterolemic fatty acids on cholesterol metabolism [20]. Diets with a high ratio of HH are more nutritionally desirable and better for health since they contain a higher level of hypocholesterolemic fatty acids (oleic acid and PUFA) than hypercholesterolemic fatty acids (myristic, lauric, and palmitic acids) [56]. In this study, C. saturnus showed an index of 5.58, which was greater than the values found for shellfish (0.21–4.75) [56] and some fish species such as raw Kutum roach (3.59) [57] due to a lower proportion of C14:0 and C16:0 (Table 3).
Atherogenicity index (AI) shows the link between the main classes of SFA and UFA, the former of which include myristic, lauric, and palmitic acids (but not stearic acid), which are regarded as pro-atherogenic, whereas UFA is considered anti-atherogenic [20]. Thrombogenicity index (TI) is also used to describe the thrombogenic potential (tendency for blood clot formation) of fatty acids by describing the interaction between prothrombogenic fatty acids (myristic, lauric, and palmitic acids) and antithrombogenic fatty acids (MUFA and PUFA) [20]. Diets with lower values of AI and TI are considered more nutritionally beneficial for cardiovascular health [1]. In this study, the AI and TI values of C. saturnus fat were 0.48 and 0.20, respectively—slightly lower than the values reported for farmed snail species such as Pomacea canaliculate from the Republic of Korea, which exhibited AI and TI values of 0.55 and 0.41, respectively [1]. For some species of shellfish such as scallops (Chlamys farreri and Patinopecten yessoensis), AI values ranged from 0.29 to 0.37, and TI values ranged from 0.09 to 0.17 [58]. For different fish species, the values of AI were reported to be in the range of 0.21–1.16, and the values of TI ranged from 0.14 to 0.87 [[59], [60], [61]].
3.5. Amino acid composition
The amino acid composition in C. saturnus meat is presented as mg/g meat (on a fresh matter basis) and mg/g of crude protein (on a dry matter basis) in Table 4. The total amino acid content measured was 105.63 mg/g fresh meat (889.3 mg/g of crude protein, DM). The meat contained essential and nonessential amino acids with values of 51.53 and 54.11 mg/g fresh meat, respectively. One of the most significant indicators of protein's nutritional quality is the food's composition and proportion of essential amino acids. It was demonstrated that C. saturnus meat was a good source of essential amino acids (arginine, histidine, isoleucine, leucine, lysine, phenylalanine, threonine, and valine), which constituted 48.78% of the total amino acids detected. Arginine is classified as a conditionally essential amino acid for infants and especially for vulnerable adults experiencing pregnancy, stress, critical illness, or disorders [1,62]. Among the essential amino acids detected, arginine, lysine, and leucine were dominant in the snail meat sample, which corresponded to previous reports on the amino acid profile in the snails Limicolaria sp., A. archatina, A. marginata [63], and P. canaliculata [1]. However, the essential amino acids tryptophan and methionine were not detected in the C. saturnus sample. Both of these amino acids were not also found in another land snail species of genus Cyclophorus (C. fulguratus) collected from Thailand [11], while they were found at a marginal level in P. canaliculata [1]. Previous studies have indicated that methionine is the limiting amino acid in land snails [29,64]. Adeyeye and Afolabi [63] also reported that tryptophan was not found in land snails, Limicolaria sp., A. archatina, and A. marginata.
Table 4.
Amino acid composition of the edible part of C. saturnus.
| Amino acid | % of total amino acids | mg/g of crude protein (DM) | mg/g fresh meat |
|---|---|---|---|
| Essential amino acid | |||
| Argininea | 8.41 ± 0.17 | 74.78 ± 1.51 | 8.88 ± 0.18 |
| Histidine | 2.31 ± 0.01 | 20.56 ± 0.02 | 2.44 ± 0.01 |
| Isoleucine | 5.03 ± 0.01 | 44.70 ± 0.05 | 5.31 ± 0.01 |
| Leucine | 8.15 ± 0.02 | 72.50 ± 0.22 | 8.61 ± 0.03 |
| Lysine | 7.42 ± 0.07 | 66.00 ± 0.65 | 7.84 ± 0.08 |
| Phenylalanine | 5.04 ± 0.21 | 44.82 ± 1.85 | 5.32 ± 0.22 |
| Threonine | 6.80 ± 0.03 | 60.50 ± 0.28 | 7.19 ± 0.03 |
| Valine | 5.61 ± 0.02 | 49.93 ± 0.22 | 5.93 ± 0.03 |
| Non-essential amino acid | |||
| Alanineb | 7.27 ± 0.04 | 64.62 ± 0.34 | 7.68 ± 0.04 |
| Aspartic acidb | 11.66 ± 0.11 | 103.65 ± 0.98 | 12.31 ± 0.12 |
| Glutamic acidb | 14.77 ± 0.18 | 131.31 ± 1.61 | 15.60 ± 0.19 |
| Glycineb | 6.89 ± 0.03 | 61.26 ± 0.30 | 7.28 ± 0.04 |
| Hydroxyproline | 0.93 ± 0.02 | 8.30 ± 0.16 | 0.99 ± 0.02 |
| Proline | 2.56 ± 0.13 | 22.74 ± 1.15 | 2.70 ± 0.14 |
| Serine | 3.23 ± 0.13 | 28.69 ± 1.13 | 3.41 ± 0.13 |
| Tyrosinea | 3.93 ± 0.02 | 34.95 ± 0.22 | 4.15 ± 0.03 |
| ΣEAA | 48.78 | 433.78 | 51.53 |
| ΣNEAA | 51.22 | 455.51 | 54.11 |
| ΣAA | 100 | 889.30 | 105.63 |
Values are expressed as mean ± standard error of the mean (SEM).
Conditionally essential amino acid.
Flavor-enhancing amino acids.
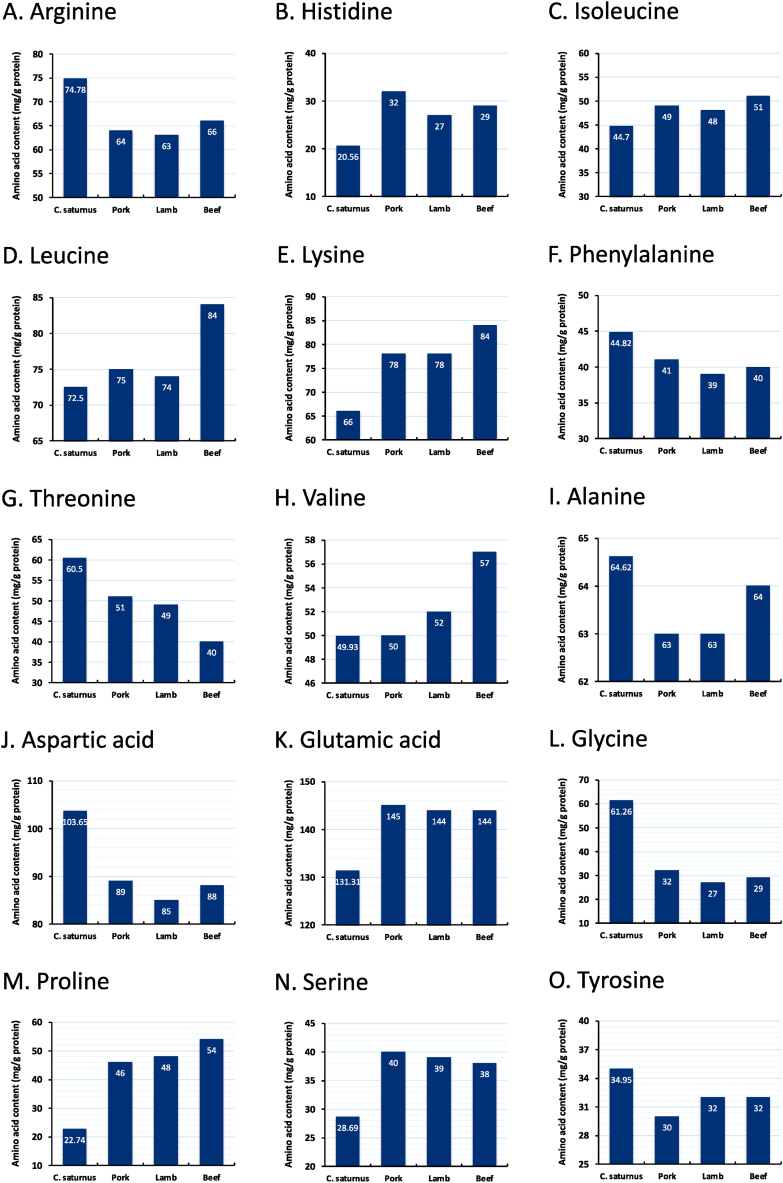
Interestingly, the land snail C. saturnus had a relatively high concentration of flavor-enhancing amino acids, particularly glutamic and aspartic acids (131.31 and 103.65 mg/g protein DM, respectively), which contributes to its pleasant umami taste [65]. Glutamic acid acts as a precursor for the production of glutathione and a neurotransmitter, gamma-aminobutyric acid (GABA), while aspartic acid has physiological functions as nucleic acid and nucleotide constituents [66]. The highest content of glutamic acid was also recorded by Adeyeye and Afolabi [63], for land snails A. archatina (111 mg/g protein DM) and A. marginata (144 mg/g protein DM), while the aspartic acid concentrations of these snails were 69.4 and 72.7 mg/g protein DM, respectively. Additionally, the presence of a high glutamic acid content is in accordance with the previous report for the snail Cookia sulcata (138–141 mg/g protein DM), while this species had lower aspartic acid (69–70 mg/g protein DM) than the value obtained in the present study [67]. In the present study, alanine and glycine were also found in high concentrations, which enhances the pleasant sweet–umami taste of C. saturnus meat [68]. It has also been reported that, because amino acids participate in Maillard reactions when heated during processing conditions, their high concentration may also affect flavor due to the generation of volatile compounds [69]. When compared to conventional meats such as beef, pork, and lamb [70] C. saturnus meat contained comparable amounts of some amino acids, especially arginine (Fig. 1A), phenylalanine (Fig. 1F), threonine (Fig. 1G), alanine (Fig. 1I), aspartic acid (Fig. 1J), glycine (Fig. 1L), and tyrosine (Fig. 1O).
Fig. 1.
Comparison of some amino acids among C. saturnus meat and conventional meats (Data adopted from Mazhangara et al. [70]). (A) Arginine. (B) Histidine. (C) Isoleucine. (D) Leucine. (E) Lysine. (F) Phenylalanine. (G) Threonine. (H) Valine. (I) Alanine. (J) Aspartic acid. (K) Glutamic acid. (L) Glycine. (M) Proline. (N) Serine. (O) Tyrosine.
The amino acid score, a method of evaluating the quality of protein of C. saturnus meat samples, is shown in Table 5. The amino acid scores were greater than 100 in all essential amino acids, but the level of tryptophan and sulfur-containing amino acid (methionine) was low and unable to detect. However, it appears to be a very valuable source of other essential amino acids, which are considered crucial for human consumption [22].
Table 5.
Amino acid scores of C. saturnus meat and comparison of essential amino acid composition between C. saturnus meat and amino acid reference pattern for adults [22].
| Amino acid | Contents in sample (mg/g of protein) | Reference pattern | Amino acid score |
|---|---|---|---|
| Histidine | 20.56 | 15 | 137.07 |
| Isoleucine | 44.70 | 30 | 149.00 |
| Leucine | 72.50 | 59 | 122.88 |
| Lysine | 66.00 | 45 | 146.67 |
| Phenylalanine + Tyrosine | 79.77 | 38 | 209.92 |
| Threonine | 60.50 | 23 | 263.04 |
| Valine | 49.93 | 39 | 128.03 |
| Tryptophan | ND | 6 | – |
| Methionine | ND | 16 | – |
| Total AA with tyrosine | 393.96 |
ND = Not detected.
3.6. Overview of nutritional properties of C. saturnus meat
The key nutritional data for C. saturnus meat is summarized in Table 6. The respective percentage of recommended daily intake (Thai RDI) [71] for each parameter is also shown taking into account a portion of 100 g of the meat, simulating the official labeling of this food item. Findings indicated that an intake of 100 g of the snail meat could offer 80 kcal, which is equivalent to 4% of the total energy in a 2000 kcal daily diet, and this serving is a great source of protein and minerals including Ca, P, Mg, Fe, Zn, Cu, and Mn, contributing more than 10% of the RDI for each, whereas low contributions (less than 10%) of the Na, energy, fat, and carbohydrate RDI were achieved from this snail portion.
Table 6.
Nutritional properties of C. saturnus meat and respective percentages of the recommended daily intake (%RDI) provided by 100 g of C. saturnus meat.
| Nutrients | Recommended valuea | Content per 100 g fresh meat | %RDI |
|---|---|---|---|
| Energy (kcal) | 2000 | 80.01 | 4.00 |
| Protein (g) | 50 | 11.88 | 23.76 |
| Total fat (g) | 65 | 0.93 | 6.20 |
| Carbohydrate (g) | 300 | 6.04 | 2.01 |
| Calcium (mg) | 800 | 172.65 | 21.58 |
| Phosphorus (mg) | 800 | 100.80 | 12.60 |
| Magnesium (mg) | 350 | 36.93 | 10.55 |
| Sodium (mg) | 2000 | 22.98 | 1.10 |
| Iron (mg) | 15 | 8.97 | 59.83 |
| Zinc (mg) | 15 | 3.32 | 22.13 |
| Copper (mg) | 2 | 1.06 | 52.79 |
| Manganese (mg) | 3.5 | 0.42 | 11.92 |
Thai Recommended Daily Intakes (Thai RDI) for population over 6 years of age based on a 2000 kcal daily energy requirement.
4. Conclusions
This is the first report on the nutritional composition of C. saturnus compared to other edible snail meats and conventional foods of animal origin. Nutritional elements present in the snail meat indicate that C. saturnus can be considered a valuable dietary source of various health-related nutrients. C. saturnus contains a reasonable amount of protein and provides fewer calories due to the lower fat and carbohydrate contents. It is a rich source of essential minerals such as Ca, Mg, Fe, Zn, Cu, and Mn. In terms of the fatty acid profile, C. saturnus contains a high level of UFA and a low content of SFA, and properly balanced PUFA/SFA, n6/n3, HH, AI, and TI ratios that are considered healthy for humans. It is also a good source of essential amino acids arginine, histidine, isoleucine, leucine, lysine, phenylalanine, threonine and valine, while methionine and tryptophan are the limiting amino acids. In addition, C. saturnus contains a high level of flavor-enhancing amino acids that could contribute to the delicious taste of the meat. To enrich sustainable food supplies, it is important to support and promote the production and consumption of this snail species worldwide, especially in developing countries.
Funding
This research project was financially supported by Thailand Science Research and Innovation (TSRI) and Mahasarakham University, Thailand (Grant No. 6506032/2565).
Author contribution statement
Supap Nontasan, Eakapol Wangkahart: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Rachanee Nammatra: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
The data that has been used is confidential.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- 1.Ghosh S., Jung C., Meyer-Rochow V.B. Snail as mini-livestock: nutritional potential of farmed Pomacea canaliculata (ampullariidae), agric. Nat. Resour. 2017;51:504–511. doi: 10.1016/J.ANRES.2017.12.007. [DOI] [Google Scholar]
- 2.Apostolou K., Staikou A., Sotiraki S., Hatziioannou M. An assessment of snail-farm systems based on land use and farm components. Anima. 2021;11:272. doi: 10.3390/ANI11020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Snail Market . 2018. Key Findings and Insights.https://www.fooddive.com/press-release/20180528-global-snail-market-key-findings-and-insights/ access 17th October 2022. [Google Scholar]
- 4.Galluzzo F.G., Cammilleri G., Ulrici A., Calvini R., Pulvirenti A., Lo Cascio G., Macaluso A., Vella A., Cicero N., Amato A., Ferrantelli V. Land snails as a valuable source of fatty acids: a multivariate statistical approach. Foods. 2019;8:676. doi: 10.3390/FOODS8120676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caetano D., Miranda A., Lopes S., Paiva J., Rodrigues A., Videira A., Almeida C.M.M. Nutritional and toxicity profiles of two species of land snail, Theba pisana and Otala lactea, from Morocco. J. Food Compos. Anal. 2021;100 doi: 10.1016/J.JFCA.2021.103893. [DOI] [Google Scholar]
- 6.Nkansah M.A., Agyei E.A., Opoku F. Mineral and proximate composition of the meat and shell of three snail species. Heliyon. 2021;7 doi: 10.1016/J.HELIYON.2021.E08149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szkucik K., Ziomek M., Paszkiewicz W., Drozd Ł., Gondek M., Knysz P. Fatty acid profile in fat obtained from edible part of land snails harvested in Poland. J. Vet. Res. 2018;62:526. doi: 10.2478/JVETRES-2018-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pissia M., Matsakidou A., Kiosseoglou V. Raw materials from snails for food preparation. Futur. Foods. 2021;3 doi: 10.1016/J.FUFO.2021.100034. [DOI] [Google Scholar]
- 9.W. Paisantanakij, S. Aroonsrimorakot, P. Koedrith, U. Kovitvadhi, A. Hambananda, Land snail as alternative food: safety and nutritional perspectives of Cyclophorus haughtoni. In The 3rd International Conference of Multidisciplinary Approaches on UN Sustainable Development Goals UNSDGs 2018, Bangkok, Thailand (p.173).
- 10.Nantarat N., Tongkerd P., Sutcharit C., Wade C.M., Naggs F., Panha S. Phylogenetic relationships of the operculate land snail genus Cyclophorus Montfort, 1810 in Thailand. Mol. Phylogenet. Evol. 2014;70:99–111. doi: 10.1016/j.ympev.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Aroonsrimorakot S., Wongsiri S., Whangchai N., Jaturongloumlart S., Ariyadej C. 5th Int. Conf. Agric. Environ. Civ. Eng. 2017. Phuket, Thail.; 2014. Bioaccumulation of heavy metal and food safety of Hoi due (Hemipecta distincta) and Hoi hom (Cyclophorus fulguratus) pp. 14–20.https://erp.mju.ac.th/openFile.aspx?id=MjcyMzky accessed March 25, 2023. [Google Scholar]
- 12.Kongim B. 2008. Cyclophorid Snails.http://www1a.biotec.or.th/BRT/index.php/biodiversity/240cyclophorid-snail accessed October 5, 2022. [Google Scholar]
- 13.Kongim B., Naggs F., Panha S. Karyotypes of operculate land snails of the genus Cyclophorus (Prosobranchia: cyclophoridae) in Thailand. Invertebr. Reprod. Dev. 2010;49:1–8. doi: 10.1080/07924259.2006.9652188. [DOI] [Google Scholar]
- 14.Özogul Y., Özogul F., Olgunoglu A.I. Fatty acid profile and mineral content of the wild snail (Helix pomatia) from the region of the south of the Turkey. Eur. Food Res. Technol. 2005;221:547–549. doi: 10.1007/S00217-005-1191-7. [DOI] [Google Scholar]
- 15.Association of Official Analytical Chemists (AOAC) eighteenth ed. AOAC International; 2005. Official Methods of Analysis. [Google Scholar]
- 16.Gomot A. Biochemical composition of Helix snails: influence of genetic and physiological factors. J. Molluscan Stud. 1998;64:173–181. doi: 10.1093/MOLLUS/64.2.173. [DOI] [Google Scholar]
- 17.Guo J., Yue T., Li X., Yuan Y. Heavy metal levels in kiwifruit orchard soils and trees and its potential health risk assessment in Shaanxi, China. Environ. Sci. Pollut. Res. 2016;23:14560–14566. doi: 10.1007/s11356-016-6620-6. [DOI] [PubMed] [Google Scholar]
- 18.Ridwanudin A., Kasuya H., Haga Y., Kabeya N., Satoh S. Interactive effect of dietary fish oil and pyrimidine nucleotide supplementation on the fatty acid composition of juvenile rainbow trout Oncorhynchus mykiss: enhancement of ARA and DHA contents in the fillet of fish fed-supplemented diet. Aquacult. Res. 2021;52:4934–4945. doi: 10.1111/ARE.15327. [DOI] [Google Scholar]
- 19.Wangkahart E., Bruneel B., Wisetsri T., Nontasan S., Martin S.A., Chantiratikul A. Interactive effects of dietary lipid and nutritional emulsifier supplementation on growth, chemical composition, immune response and lipid metabolism of juvenile Nile tilapia (Oreochromis niloticus) Aquaculture. 2022;546 doi: 10.1016/j.aquaculture.2021.737341. [DOI] [Google Scholar]
- 20.Chen J., Liu H. Nutritional indices for assessing fatty acids: a mini-review. Int. J. Mol. Sci. 2020;21:5695. doi: 10.3390/IJMS21165695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wangkahart E., Kersanté P., Lee P.T., Sanbut O., Nontasan S., Chantiratikul A. Effect of Kera-Stim® 50, a feed additive containing free amino acid mix on growth, antioxidant and immune responses, digestive enzymes, and fatty acid composition in Nile tilapia (Oreochromis niloticus) Aquaculture. 2022;551 doi: 10.1016/j.aquaculture.2021.737874. [DOI] [Google Scholar]
- 22.FAO/WHO/UNU Expert Consultation Protein and amino acid requirements in human nutrition : report of a joint FAO/WHO/UNU expert consultation. WHO Tech. Rep. Ser. ; No. 2007;935 www.who.int/bookorders accessed October 3, 2022. [PubMed] [Google Scholar]
- 23.Chen J., Rosenthal A. Modifying Food Texture Nov. Ingredients Process. Tech. Woodhead Publishing; 2015. Food texture and structure; pp. 3–24. [DOI] [Google Scholar]
- 24.Wangkahart E., Wachiraamonloed S., Lee P.T., Subramani P.A., Qi Z., Wang B. Impacts of Aegle marmelos fruit extract as a medicinal herb on growth performance, antioxidant and immune responses, digestive enzymes, and disease resistance against Streptococcus agalactiae in Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2022;120:402–410. doi: 10.1016/j.fsi.2021.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Wangkahart E., Bruneel B., Chantiratikul A., de Jong M., Pakdeenarong N., Subramani P.A. Optimum dietary sources and levels of selenium improve growth, antioxidant status, and disease resistance: Re-evaluation in a farmed fish species, Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2022;121:172–182. doi: 10.1016/j.fsi.2021.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Babalola O.O., Akinsoyinu A.O. Proximate composition and mineral profile of snail meat from different breeds of land snail in Nigeria. Pakistan J. Nutr. 2009;8:1842–1844. doi: 10.3923/PJN.2009.1842.1844. [DOI] [Google Scholar]
- 27.Poutanen K.S., Kårlund A.O., Gómez-Gallego C., Johansson D.P., Scheers N.M., Marklinder I.M., Eriksen A.K., Silventoinen P.C., Nordlund E., Sozer N., Hanhineva K.J., Kolehmainen M., Landberg R. Grains – a major source of sustainable protein for health. Nutr. Rev. 2022;80:1648–1663. doi: 10.1093/NUTRIT/NUAB084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adeyeye E.I. Waste yield, proximate and mineral composition of three different types of land snails found in Nigeria. Int. J. Food Sci. Nutr. 1996;47:111–116. doi: 10.3109/09637489609012572. [DOI] [PubMed] [Google Scholar]
- 29.Çağıltay F., Erkan N., Tosun D., Selçuk A. Amino acid, fatty acid, vitamin and mineral contents of the edible garden snail (Helix aspersa) J. Fish. 2011;5:354–363. doi: 10.3153/JFSCOM.2011040. [DOI] [Google Scholar]
- 30.Milinsk M.C., das Graças Padre R., Hayashi C., de Oliveira C.C., Visentainer J.V., de Souza N.E., Matsushita M. Effects of feed protein and lipid contents on fatty acid profile of snail (Helix aspersa maxima) meat. J. Food Compos. Anal. 2006;19:212–216. doi: 10.1016/J.JFCA.2004.09.011. [DOI] [Google Scholar]
- 31.Pereira C.C., Vicente R.B. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013;93:586–592. doi: 10.1016/J.MEATSCI.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Erbay E.A., Yesilsu A.F. Fish protein and its derivatives: functionality, biotechnology and health effects. Aquat. Food Stud. 2021;1 https://www.aquaticfood.org/uploads/pdf_1.pdf [Google Scholar]
- 33.Moniruzzaman M., Sku S., Chowdhury P., Tanu M.B., Yeasmine S., Hossen M.N., Min T., Bai S.C., Mahmud Y. Nutritional evaluation of some economically important marine and freshwater mollusc species of Bangladesh. Heliyon. 2021;7 doi: 10.1016/J.HELIYON.2021.E07088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fagbuaro O., Oso J.A., Edward J.B., Ogunleye R.F. Nutritional status of four species of giant land snails in Nigeria. J. Zhejiang Univ. - Sci. B. 2006;7:686. doi: 10.1631/JZUS.2006.B0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalio G.A., Etela I. Nutritional and sensory profiling of the African giant land snail fed commercial-type and leaf-based diets in a rain-forest ecology. Afr. J. Food Nutr. Sci. 2011;11 https://go.gale.com/ps/i.do?p=AONE&sw=w&issn=16845358&v=2.1&it=r&id=GALE%7CA270375417&sid=googleScholar&linkaccess=fulltext accessed September 1, 2022. [Google Scholar]
- 36.Kiczorowska B., Samolińska W., Grela E.R., Bik-Małodzińska M. Nutrient and mineral profile of chosen fresh and smoked fish. Nutrients. 2019;11 doi: 10.3390/NU11071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shkembi B., Huppertz T. Calcium absorption from food products: food matrix effects. Nutrients. 2021;14 doi: 10.3390/NU14010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cobos Á., Díaz O. In: Handb. Food Chem. Cheung P., editor. Springer; Berlin, Heidelberg: 2014. Chemical composition of meat and meat products; pp. 1–32. [DOI] [Google Scholar]
- 39.FAO/WHO . Food and Agriculture Organization of the United Nations; Geneva, World Health Organization (JECFA/73/SC), Rome. 2010. Summary and conclusions of the seventy-third meeting of the joint FAO/WHO expert committee on food additives, geneva, 8–17 june 2010. [Google Scholar]
- 40.EC . Off J Eur Union; 2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuff. [Google Scholar]
- 41.Hoag L.D., Dharmarajan T.S. In: Geriatr. Gastroenterol. Pitchumoni C.S., Dharmarajan T., editors. Springer; Cham: 2021. Calcium and phosphorus; pp. 735–763. [DOI] [Google Scholar]
- 42.Houston M.C., Harper K.J. Potassium, magnesium, and calcium: their role in both the cause and treatment of hypertension. J. Clin. Hypertens. 2008;10:11. doi: 10.1111/J.1751-7176.2008.08575.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbaspour N., Hurrell R., Kelishadi R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014;19:174. (accessed August 31, 2022) [PMC free article] [PubMed] [Google Scholar]
- 44.Wright A.C., Fan Y., Baker G.L. Nutritional value and food safety of bivalve molluscan shellfish. J. Shellfish Res. 2018;37:695–708. doi: 10.2983/035.037.0403. [DOI] [Google Scholar]
- 45.Calder P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enteral Nutr. 2015;39:18S–32S. doi: 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]
- 46.Senyilmaz-Tiebe D., Pfaff D.H., Virtue S., Schwarz K.V., Fleming T., Altamura S., Muckenthaler M.U., Okun J.G., Vidal-Puig A., Nawroth P., Teleman A.A. Dietary stearic acid regulates mitochondria in vivo in humans. Nat. Commun. 2018;9 doi: 10.1038/S41467-018-05614-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lah R.A., Smith J., Savins D., Dowell A., Bucher D., Benkendorff K. Investigation of nutritional properties of three species of marine turban snails for human consumption. Food Sci. Nutr. 2017;5:14–30. doi: 10.1002/FSN3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wood J.D., Richardson R.I., Nute G.R., Fisher A.V., Campo M.M., Kasapidou E., Sheard P.R., Enser M. Effects of fatty acids on meat quality: a review. Meat Sci. 2004;66:21–32. doi: 10.1016/S0309-1740(03)00022-6. [DOI] [PubMed] [Google Scholar]
- 49.Simopoulos A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56:365–379. doi: 10.1016/S0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 50.Milinsk M.C., Das Graças Padre R., Hayashi C., De Souza N.E., Matsushita M. Influence of diets enriched with different vegetable oils on the fatty acid profiles of snail Helix aspersa maxima. Food Chem. 2003;82:553–558. doi: 10.1016/S0308-8146(03)00010-4. [DOI] [Google Scholar]
- 51.Moreira A.B., Visentainer J.V., De Souza N.E., Matsushita M. Fatty acids profile and cholesterol contents of three Brazilian Brycon freshwater fishes. J. Food Compos. Anal. 2001;14:565–574. doi: 10.1006/JFCA.2001.1025. [DOI] [Google Scholar]
- 52.Williams P. Nutritional composition of red meat. Nutr. Diet. 2007;64:S113–S119. doi: 10.1111/J.1747-0080.2007.00197.X. [DOI] [Google Scholar]
- 53.Melo D.M., Roseno T.F., Barros W.M., de Faria R.A.P.G., Paglarini C. de S., Faria P.B., Mariotto S., de Souza X.R. Fatty acid profiles and cholesterol content of five species of pacu-pevas from the pantanal region of Mato Grosso, Brazil. J. Food Compos. Anal. 2019;83 doi: 10.1016/J.JFCA.2019.103283. [DOI] [Google Scholar]
- 54.Hayes K.C. Dietary fat and heart health: in search of the ideal fat, Asia Pac. J. Clin. Nutr. 2002;11(Suppl 7):S394–S400. doi: 10.1046/J.1440-6047.11.S.7.13.X. [DOI] [PubMed] [Google Scholar]
- 55.Kang M.J., Shin M.S., Park J.N., Lee S.S. The effects of polyunsaturated:saturated fatty acids ratios and peroxidisability index values of dietary fats on serum lipid profiles and hepatic enzyme activities in rats. Br. J. Nutr. 2005;94:526–532. doi: 10.1079/BJN20051523. [DOI] [PubMed] [Google Scholar]
- 56.Rincón-Cervera M.Á., González-Barriga V., Romero J., Rojas R., López-Arana S. Quantification and distribution of omega-3 fatty acids in South Pacific fish and shellfish species. Foods. 2020;9:233. doi: 10.3390/FOODS9020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosseini H., Mahmoudzadeh M., Rezaei M., Mahmoudzadeh L., Khaksar R., Khosroshahi N.K., Babakhani A. Effect of different cooking methods on minerals, vitamins and nutritional quality indices of kutum roach (Rutilus frisii kutum) Food Chem. 2014;148:86–91. doi: 10.1016/J.FOODCHEM.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 58.Wu Z.X., Hu X.P., Zhou D.Y., Tan Z.F., Liu Y.X., Xie H.K., Rakariyatham K., Shahidi F. Seasonal variation of proximate composition and lipid nutritional value of two species of scallops (Chlamys farreri and Patinopecten yessoensis) Eur. J. Lipid Sci. Technol. 2019;121 doi: 10.1002/EJLT.201800493. [DOI] [Google Scholar]
- 59.González-Félix M.L., Maldonado-Othón C.A., Perez-Velazquez M. Effect of dietary lipid level and replacement of fish oil by soybean oil in compound feeds for the shortfin corvina (Cynoscion parvipinnis) Aquaculture. 2016;454:217–228. doi: 10.1016/J.AQUACULTURE.2015.12.021. [DOI] [Google Scholar]
- 60.Tonial I.B., Francielly De Oliveira D., Coelho A.R., Matsushita M., Augusto F., Coró G., Evelazio De Souza N., Visentainer J.V. Quantification of essential fatty acids and assessment of the nutritional quality indexes of lipids in Tilapia Alevins and Juvenile Tilapia Fish (Oreochromis niloticus) J. Food Res. 2014;3:114. doi: 10.5539/JFR.V3N3P105. [DOI] [Google Scholar]
- 61.Senso L., Suárez M.D., Ruiz-Cara T., García-Gallego M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata) Food Chem. 2007;101:298–307. doi: 10.1016/J.FOODCHEM.2006.01.036. [DOI] [Google Scholar]
- 62.Liang M., Wang Z., Li H., Cai L., Pan J., He H., Wu Q., Tang Y., Ma J., Yang L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018;115:315–328. doi: 10.1016/J.FCT.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 63.Adeyeye E.I., Afolabi E.O. Amino acid composition of three different types of land snails consumed in Nigeria. Food Chem. 2004;85:535–539. doi: 10.1016/S0308-8146(03)00247-4. [DOI] [Google Scholar]
- 64.Çelik M.Y., Duman M.B., Sariipek M., Uzun Gören G., Kaya Öztürk D., Kocatepe D., Karayücel S. Comparison of proximate and amino acid composition between farmed and wild land snails (Cornu aspersum Müller, 1774) J. Aquat. Food Prod. Technol. 2020;29:383–390. doi: 10.1080/10498850.2020.1740850. [DOI] [Google Scholar]
- 65.Tang B., Wei W., Huang Y., Zou X., Yue Y. Growth performance and nutritional composition of Hemifusus ternatanus under artificial culturing conditions. Aquaculture. 2016;459:186–190. doi: 10.1016/J.AQUACULTURE.2016.03.051. [DOI] [Google Scholar]
- 66.Fürst P. Basics in clinical nutrition: proteins and amino acids. e-SPEN, Eur. e-J. Clin. Nutr. Metab. 2009;4:e62–e65. doi: 10.1016/j.eclnm.2008.07.010. [DOI] [Google Scholar]
- 67.Mason S.L., Shi J., Bekhit A.E.D., Gooneratne R. Nutritional and toxicological studies of New Zealand Cookia sulcata. J. Food Compos. Anal. 2014;36:79–84. doi: 10.1016/J.JFCA.2014.08.002. [DOI] [Google Scholar]
- 68.Wen X., Chen A., Wu Y., Yang Y., Xu Y., Xia W., Zhang Y., Cao Y., Chen S. Comparative evaluation of proximate compositions and taste attributes of three Asian hard clams (Meretrix meretrix) with different shell colors. Int. J. Food Prop. 2020;23:400–411. doi: 10.1080/10942912.2020.1733015. [DOI] [Google Scholar]
- 69.Li L., Belloch C., Flores M. The Maillard reaction as source of meat flavor compounds in dry cured meat model systems under mild temperature conditions. Molecules. 2021;26:223. doi: 10.3390/MOLECULES26010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mazhangara I.R., Chivandi E., Mupangwa J.F., Muchenje V. The potential of goat meat in the red meat industry. Sustainability. 2019;11:3671. doi: 10.3390/SU11133671. [DOI] [Google Scholar]
- 71.Thai recommended daily intakes (Thai RDI) 2016. http://food.fda.moph.go.th/Rules/dataRules/4-4-2ThaiRDI.pdf accessed March 28, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.