Abstract
Jordan is rich in the flora of ethnobotanical importance. This scoping review aims to highlight the ethnopharmacological value of Jordanian medicinal plants using the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) guidelines. A total of one hundred twenty-four articles published between 2000 and 2022 obtained from PubMed, EBSCO, and Google Scholar databases were included in this review. These plants own several classes of secondary bioactive metabolites, including alkaloids, flavonoids, phenolics, and terpenes. Jordanian plants exhibited potential therapeutic activity against various tumors, bacterial infections, elevated blood glucose levels, hyperlipidemia, platelets aggregation disorders, and gastrointestinal disorders. Phytochemicals’ biological activities depend on their structures, parts used, methods of extraction, and evaluation model. In conclusion, this review highlights the need of researching Jordan's abundant naturally occurring medicinal plants and their phytochemicals as novel lead molecules in drug discovery and development. Studying active phytochemicals for disease treatment will help develop drugs for safe treatment and cure in the future.
Keywords: Jordan, Medical plants, Phytochemicals, Cancer, Diabetes, Antimicrobial
1. Introduction
The use of plants and herbs as medicine has increased remarkably worldwide. Many people use these preparations and products to treat various health-related challenges, needs, and illnesses across different countries. Literature also reported that around 80% of the Arab population uses herbal medicine in treating and preventing disorders [1].
Jordan is a Middle Eastern, Arab country with a landscape divided into six biogeographic regions: Mediterranean, Mediterranean-Irano-Turanian, Saharo Arabian, Irano-Turanian, Sudanian, and Saharo-Arabian, bridging between Asia, Africa, and Europe. Consequently, this environmental landscape diversity has influenced the natural habitats and plant flora biodiversity [[2], [3], [4]]. The Jordanian culture is known for its rich heritage of folk medicine, which is common under-researched practice [5]. Ajloune, Jordan, for instance, a well-known city for its heritage of plants due to climatic and fertile soil, exhibits the highest use value of medicinal herbs and plants in treating digestive and dental illnesses. Studies have demonstrated that Jordanians tend to use phytomedicines mostly as self-medication for treating diabetes, dyslipidemia, hypertension, and cancer and in fertility [6]. The availability of plants, the presence of modern medical facilities, and the culture of the society in the region all affect the utilization of plants and herbs for medicinal purposes. Inherently, various concerns were also underlined regarding the use of herbs as medicines, these herbs’ efficacy, potential side effects, and herb-drug interaction [7].
It was reported that around 80% of the population in Arab culture use herbal medicine to treat and prevent disease. The Jordanian culture is rich in the precious heritage of folk medicine which is a common under-researched practice. Jordanian have used phytomedicines mostly as a self-medication for treatment of diabetes, dyslipidemia, hypertension, caner and in fertility [8]. Table 1 shows the ethnobotanical use of herbs in Jordan.
Table 1.
Ethnobotanical use of some Jordanian plant species.
| Plant Name | Traditional use | Reference | Plant name | Traditional use | Reference |
|---|---|---|---|---|---|
| Achillea santolina L | Antispasmodic, diabetes Carminative, depurative stomachaches. | [9,10] | Pistacia atlantica Boiss | Edible | [11] |
| Chrysanthemum coronarium L. | Abdominal pain | [12] | Pistacia palaestina Boiss. | Antispasmodic | [12] |
| Ducrosia flabellifolia Boiss. | Analgesic, cold, antianxiety, antimicrobial | [13] | Salvia triloba L.f. | Spasm, carminative, common cold, enhance memory, antidandruff | [12] |
| Inula viscosa L. Ait. | Anthelmintic, lung disorder, muscle relaxant | [9,10] | Salvia ceratophylla L. | Abdominal pain, cancer headaches, coughs stomachaches, asthma microbial infections, and other pulmonary and urinary disorders | [14] |
| Laurus nobilis L. | Cancer, skin disease | [15] | Salvia syriaca L. | infectious diseases and stomach disorders as well as the common cold | [16] |
| Mandragora autumnalis Bertol. | Sedative, and cough | [12] | Silybum marianum L | Liver disease | [9] |
| Origanum syriacum L. | Abdominal pain, aperative, carminative, cough | [12] | Tamarix aphylla L.H.Karst. | Fever | [15] |
| Ocimum basilicum L. | depression, migraine, cardiac tonic | [9] | Viscum cruciatum Sieb | Cancer | [17] |
Furthermore, Jordan has rich flora expressed in a variety of plant species. It was reported that Jordan has about 2543 species belonging to 142 different families and 868 genera [7]. Table 1 presents famous Jordanian flora in addition to their traditional uses. This calls for more structured research regarding these diverse species, as this might open new doors for research on Jordanian phytochemicals. It might also shed light on some under-researched domains pertaining to Jordanian flora. Therefore, this review investigates Jordanian plants and herbs’ traditional uses and the phytochemical content by describing the ethnopharmacological use, biological activity, secondary metabolite, and mechanisms of action of phytochemicals when applicable. Aiming to examine and explore the literature to report the biological activities of Jordanian herbs to construct an evidence-based medicine for the Jordanian plant species.
2. Method
A scoping review was found to be appropriate to meet the aims of this study as it represents an overview of Jordan’s medicinal plants and their utilization. Using the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) guidelines, an extensive search of Scopus, PubMed, and EBSCO. A separate Google Scholar search was conducted in an effort to locate supplementary articles. In addition, hand-searched literature articles were also included to investigate all current relevant studies comprehensively in order to achieve an in-depth exploration of all current relevant studies in this field. Only articles that followed a standardized structure of introduction, method, results, and discussion (IMRAD) were included. The search was narrowed to only include academic articles published from January 2000 to January 2022 (the time of executing this review). Moreover, the studies included in this review must have specifically stated that the plant materials studied have been cultivated and collected from Jordan. Articles included in this review were then critically scrutinized and qualitatively classified into emerging themes with the support of NVivo software V10.
3. Results and discussion
The search initially yielded 3000 articles, which were sorted according to the inclusion criteria mentioned earlier. Articles were then screened by the phytochemical researcher to determine relevance (148 articles). The final selection of articles was concluded by two independent researchers and validated by the research team. The full-text 124 relevant articles that met all inclusion criteria were then analyzed, as shown in Fig. 1.
Fig. 1.
Flowchart of search strategy and study selection based on PRISMA guidelines.
3.1. Medicinal plants with anticancer activity
Cancer is the leading cause of death [18]. Flowing after heart disease, cancer is the major cause of mortality among the Jordanian population (https://www.cdc.gov/globalhealth/countries/jordan/default.htm). Cancer is a complex disease characterized by the uncontrolled proliferation of cells. Conventional treatments have several side effects and limitations. Therefore researchers have screened a number of medicinal plants for anticancer activities [19,20].
Cancer is one of the top diseases leading to death after cardiovascular diseases. Plants - derived natural products are constantly take the attention as a promising source to develop new anticancer therapies with more selectivity with no or few serious adverse effects. Cancer hallmarks are biological capabilities acquired by cancer cells during neoplastic transformation. Targeting multiple cancer hallmarks is a promising strategy to treat cancer. The majority of Jordanian plants have a capacity to target the hallmarks of cancerous cells [21].
As shown in Table 2, 41% of tested plant extracts were from leaves, 31% were from aerial parts, and 8% were fruits. Ethanol was the most common extraction solvent (47%), followed by water (13%). It was also found that the human breast adenocarcinoma cell line (MCF-7 cell) was the most targeted by several extracts. The principal method used as an indicator of cell viability, proliferation, and cytotoxicity was the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay (MTT assay) followed by the brine shrimp lethality test and sulphorhodamine B assay [4,22].
Table 2.
Medicinal plants with anticancer activity.
| Plant species | Family | Vernacular name | Part used | Method/Solvent of extraction | Study design | Result | Reference |
|---|---|---|---|---|---|---|---|
| Achillea santolina L. | Asteraceae | Kaisoom | Aerial parts | Hydrodistillation/Ethanol | In vitro model (MCF-7) | IC50 : 24.12 (MCF-7) | [38] |
| Chrysanthemum coronarium L. | Asteraceae | Besbas or Bassoum | Aerial parts | Maceration/Methanol | In vitro model (T47D) | IC50: 22.6 (T47D) | [39] |
| It might be a potential source of natural compounds that can be developed into new antineoplastic agents | |||||||
| Ducrosia flabellifolia Boiss. | Apiaceae | AlHaza | Aerial parts | Maceration/Ethanol | In vitro model (MCF-7, Hep-2, Vero) | IC50: 25.34 (MCF-7), 98.0 (Hep-2), and 87.5 (Vero) | [13,40] |
| Elaeagnus angustifolia | Elaeagnaceae | NM | Leaf | Maceration/Ethylacetate | In vitro model (T47D) | IC50: 23.05 (T47D) | [41] |
| Eucalyptus camaldulensis Dehnh | Myrtaceae | NM | Leaf | Percolation/Ethanol | In vivo model (mice), skin cancer induced by 7,12-dimethylbenz(a) anthracene | It reduced 8-OHdG in cultured human lymphocytes in a dose-response manner, chromosomal damage, and the incidence number of tumors per animal and delayed the onset of tumors. | [42] |
| Gynandriris sisyrinchium L.Parl | Iridaceae | Sawsan | Leaf & bulb | Isolated compound | In vitro model (HL-60) | The cytotoxic activity against the human promyelocytic leukemia HL-60 cells revealed that 3′-Methyl tenuifone (refer to Fig. 2) was the most active (40 μM). The results indicate that the cytotoxicity is mediated by apoptosis. | [30,43] |
| Inula graveolens L. | Asteraceae | NM | Aerial parts | Decoction/Ethanol | In vitro model (MCF-7, T47D) | IC50: 3.8 (MCF-7), 11.0 (T47D). | [25] |
| Inula graveolens L. | Asteraceae | NM | Aerial parts | Hydrodistillation/Volatile oil | In vitro model (MCF-7, T47D) | IC50: 51.5 (MCF-7), 57.2 (T47D) | [44] |
| Inula viscosa | Asteraceae | Taioon | Not mentioned | Isolated compounds | In vitro model (MCF-7, Hep-2, Vero cell) | Nepetin, IC50 : 5.9 (MCF-7), 11.3 (Hep-2), 103.5 (Vero cell) | [9,27] |
| Hispidulin, IC50 : 10.4 (MCF-7), 19.5 (Hep-2), 105.5 (Vero cell) | |||||||
| Inula viscosa | Asteraceae | Taioon | Flowers | Methanol | In vitro model (MCF-7) | IC50:15.8 (MCF-7) | [9,45] |
| Laurus nobilis L. | Lauraceae | Bay leaf | Fruits | Hydrodistillation/Ethanol | In vitro model (T47D, MCF-7) | IC50 : 28 (MCF-7), 12.3 (T47D) | [46] |
| Laurus nobilis L. | Lauraceae | Bay leaf | Leaf | Ethanol | In vitro model (MCF-7) | IC50 : 24.49 (MCF-7) | [47] |
| Mandragora autumnalis Bertol | Solanaceae | Tuffah el melanin, Yabroh | Leaf | Maceration/Ethanol, hexane | In vitro model (MCF-7) | MCF-7, IC50: 100 (ethanol), 400 (hexane) | [48,49] |
| Origanum syriacum | Lamiaceae | Zaatar, Mardakoosh | Leaf | Reflux/Ethanol | In vitro model (MCF-7) | IC50 : 6.4 (MCF-7) | [9,47] |
| Ocimum basilicum | Lamiaceae | Habaq | Leaf | Hydrodistillation/Volatile oil | In vitro model (MDA-MB-231, MCF-7, U-87 MG) | IC50: 432.3 (MDA-MB-231), 320.4 (MCF-7), 431.2 (U-87 MG) | [9,50] |
| Ononis hirta | Fabaceae | Showk AL-Jamal | Aerial parts | Maceration/Methanol | In vitro model (MCF-7) | IC50 : 28.0 (MCF-7) | [45] |
| Origanum dayi | Lamiaceae | Zaatar | Aerial parts | Reflux/Ethanol | In vitro model (MCF-7, T47D) | IC50: 99.4 (MCF-7), 250 (T47D) | [43,51] |
| Pistacia atlantica | Anacardiaceae | Bottom | Leaf, galls | Hydrodistillation/Volatile oil | In vitro model (MCF-7, T47D) | Leaves oil, IC50 : 12.9 (MCF-7), 15.7 (T47D) | [52] |
| Gall's oil, IC50:17.5 (T47D) | |||||||
| Pistacia palaestina Boiss. | Anacardiaceae | Bottom | Fruit | Hydrodistillation/Volatile oil | In vitro model (renal cell adenocarcinoma, human amelanotic melanoma cells) | IC50: 204.70 (renal cell adenocarcinoma), 356.98 (human amelanotic melanoma cells) | [36] |
| Salvia dominica L. | Lamiaceae | Meriamia | Leaf | Reflux/Ethanol | In vitro model (MCF-7, T47D, BT-474, vero) | IC50: 5.8 (MCF-7), 12.9 (T47D), 14.0 (BT-474), 5.2 (Vero) | [16] |
| Salvia dominica L. | Lamiaceae | Meriamia | Aerial parts | Decoction/Ethanol | In vitro model (MCF-7, T47D) | IC50: 5.8 (MCF-7), 12.83 (T47D) | [26] |
| Salvia fruticosa | Lamiaceae | Meriamia | Leaf | Reflux/Ethanol | In vitro model (MCF-7, ZR-75-1, BT-474) | IC50: 25.6 (MCF-7), 18.4 (ZR-75-1), 17.4 (BT-474) | [16,53] |
| Salvia horminum | Lamiaceae | Meriamia | Leaf | Reflux/Ethanol | In vitro model (T-47D, BT-474) | IC50: 25.4 (T-47D), 20.5 (BT-474) | [16,43] |
| Salvia triloba L.f. | Lamiaceae | Meriamia | Aerial parts | Reflux/Ethanol | In vitro model (MCF-7, T47D) | IC50 : 29.9 (MCF-7) 38.9 (T47D) | [26,49] |
| The antiproliferative activities of ethanolic extracts from two Salvia spp were found to be due to both apoptosis and necrosis | |||||||
| Salvia ceratophylla | Lamiaceae | Lessan Alhia | Leaf | Reflux/Water | In vitro model (HCT-116, Caco2) | IC50: 28.0 (HCT-116), 9.2 (Caco2) | [43,54] |
| Salvia eigii | Lamiaceae | NF | Leaf | Reflux/Water | In vitro model (HCT-116) | IC50: 30.3 (HCT-116) | [54] |
| Salvia greggii A. | Lamiaceae | Autumn sage | Aerial parts | Hydrodistillation/Volatile oil | In vitro model (MCF-7, HCT116) | IC50: 35.4 (MCF-7), 23.6 (HCT116) | [35] |
| Salvia syriaca | Lamiaceae | Lessan Althor | Leaf | Reflux/Ethanol | In vitro model (T-47D, BT-474) | IC50: 24.1 (MCF-7), 23.9 (BT-474) | [16,43] |
| Salvia triloba | Lamiaceae | Meriamia | Leaf | Reflux/Ethanol | In vitro model (MCF-7) | IC50 25.25 (MCF-7) | [47] |
| Schinus molle | Anacardiaceae | Pepper tree, pink pepper, or Brazilian pepper | Leaf | Hydrodistillation/Volatile oil | In vitro model (HCT116, Caco 2, MCF-7, T47D) | IC50: 43.3 (HCT116), 21.1 (Caco 2), 31.0 (MCF-7), 36.8 (T47D) | [55] |
| Schinus molle | Anacardiaceae | Pepper tree, pink pepper, or Brazilian pepper | Leaf | Infusion/Ethanol | In vitro model (HCT116, Caco 2, MCF-7, T47D) | IC50: 32.8 (HCT116), 47.9 (Caco 2), 28.0 (MCF-7), 42.3 (T47D) | [55] |
| Silybum marianum L. | Asteraceae | Shok Aljmal, Khurfaish Aljmal | Leaf | Percolation/Ethanol | In vivo model (mice), skin cancer induced by 7,12-dimethylbenz(a) anthracene | It reduced 8-OHdG in cultured human lymphocytes in a dose-response manner, chromosomal damage, tumor incidence, and papilloma frequency and delayed the onset of tumors. | [9,42] |
| Tamarix aphylla | Tamaricaceae | Athel | Aerial parts | Maceration/Ethanol, water | In vitro model (MCF-7) | IC50 2.2 (water), 26.7 (ethanol), (MCF-7) | [24,43] |
| Viscum cruciatum Sieb. | Santalaceae | Dabaq | Leaf | Maceration/Methanol | In vitro model (Burkitt’s lymphoma cells) | IC50: 14.21 mg/mL (Burkitt’s lymphoma cells) | [33,43] |
| Varthemia iphionoides | Asteraceae | Ktaile | Aerial parts | Soxhlet/Dichlorome-thane | In vitro (EMT6, MCF 7,T47D), in vivo | IC50: 120 (EMT6), 270 (MCF-7), 160 (T47D) | [9,56] |
| Withania obtusifolia | Solanaceae | NM | Leaf | Isolated compounds | In vitro model (MIDA-MB-435, SW-620) | IC50: 1.7 (MIDA-MB-435), 0.3(SW-620) mM | [28] |
BSLT* (brine shrimp lethality test): results are expressed as LC50 values (mg/mL; concentration to kill 50% of the brine shrimp). EC50: is a measure of the concentration of a drug, antibody or toxicant which induces a response halfway between the baseline and maximum after a specified exposure time. Green Monkey Kidney fibroblast. MDA-MB-435 human melanoma, SW-620 human colon cancer. HL-60 human promyelocytic leukemia. Caco2 Colorectal adenocarcinoma. HRT18 Rectum adenocarcinoma. A375.S2 Malignant melanoma (epithelial-like), WM1361A Malignant melanoma (melanocyte). * Lethality concentration, EC50 concentration to inhibit growth by 50%, LC50 concentration to kill 50% of the brine shrimp. IC50: is a measurement representing the halfway point in which a compound of interest produces complete inhibition of a biological or biochemical function. NM: no mentioned.
On the other hand, the most active plant against breast cancer cell line MCF-7 was Tamarix aphylla with an IC50 of 2.2 μg/mL. Inula graveolens (IC50 of 3.80 μg/mL), Salvia dominica (IC50 of 5.80 μg/mL) and Origanum syriacum (IC50 of 6.4 μg/mL) exhibited also potent and promising antiproliferative activities on a breast cancer cell line (MCF-7).
Tamarix aphylla is s known ‘Athel’ in Jordan, belongs to family Tamaricaceae. Different parts of the plants are used traditionally for wounds, abscess, headache, fever, skin diseases and for rheumatism and joint pain. Several phytochemicals had been identified; alkaloids, flavonoids, phenolics, tannins and terpenes [23]. In the study of Alhourani both the aqueous and ethanol extracts showed promising cytotoxic activity against MCF-7 cells comparable to cisplatin and with safety even higher than cisplatin against fibroblast (aqueous extract IC50: 80 μg/mL, cisplatin: 9.1 μg/mL) [24].
Inula gravelones is known as stinkwort within the sunflower family. The ethanol extract induced a highly pronounced augmentation of MCF7-caspase-8. The antiproliferative is related to its phytocomponents quercetin and luteolin [25].
Salvia sp is used widely in the Jordanian traditional medicine for common cold, infectious disease and stomach disorders. Salvia dominica ethanol extract was biologically active against MCF7 via proapoptotic cytotoxic mechanisms specifically regulated by p21 [26].
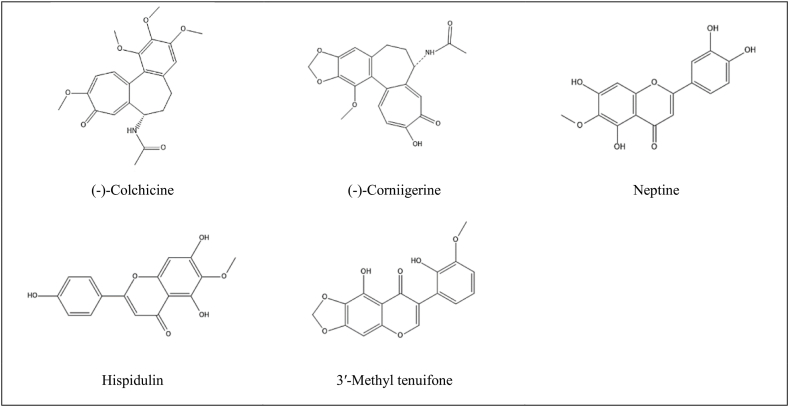
The following researches studied the anticancer activity of an isolated compound. In one study, two Jordanian Colchicum species of Colchicum hierosolymitanum and Colchicum tunicatum were fractionated, and several compounds were isolated guided by the brine shrimp lethality test. The colchicine type, the 3-demethyl -colchicine type, and the cornigerine compound were cytotoxic on the same magnitude as the positive control, camptothecin (IC50 values of 3.7–7.7 mg/mL) against MCF-7 human breast carcinoma, NCI–H460 human large cell lung carcinoma, and SF-268 human astrocytoma [4]. Refer to Fig. 2 for the structure of colchicine and cornigerine.
Fig. 2.
Bioactive compounds isolated from different medicinal plants of Jordan with anticancer activity.
Four flavonoids isolated from Jordanian Inula viscosa exhibited high antiproliferative activity against different cell lines (MCF-7 human breast carcinoma, NCI–H460 human large cell lung carcinoma, and SF-268 human astrocytoma). These flavonoids are hispidulin, 3-O-methylquercetin, 3,3′-di-O-methylquercetin, and nepetin [27]. Refer to Fig. 2 for the structure of hispidulin and neptine.
Seven withanolides were isolated from the ethanolic extract of the leaves of Withania obtusifolia, namely obtusifonolide, sitoindoside IX, 6a-chloro-5b-hydroxy withaferin A, isowithanone, 2,3-dihydro-3-ethoxywithaferin A, daturataturin A, and withaferin A. The latter compound had the most potent IC50 against the MDA-MB-435 (melanoma) and SW-620 (colon) cell lines [28,29]. Finally, only one isoflavonoid compound isolated from Gynandriris sisyrinchium was cytotoxic against the human promyelocytic leukemia HL-60 cells (40 μM) [30]. It is well known that plant extracts contain several secondary metabolites of different classes, like; alkaloids, flavonoids, terpenes, saponins, and volatile oil, that act synergistically and in combination to promote anticancer activity [13].
Different mechanistic pathways discuss the extract’s anticancer activity, which includes activation of natural killer cells (NK), which play an essential role in the immune defense against cancer cells, inducing apoptosis as revealed by DNA fragmentation, nuclear condensation, and the formation of apoptotic bodies in treated cancer cells [13,31]. Ability to enhance the secretion of TNF-α (tumor necrosis factor-alpha) and IL-1β (interleukin 1β) and inhibit the release of IL-8 [32,33]. Direct and indirect antiangiogenic properties may have chemotherapeutic and/or chemoprevention potentials. The direct antiangiogenic activity was recognized using the rat aortic assay, anti-HUVEC (human umbilical vein endothelial cells) proliferation, migration, and CAM assay (Chorioallantoic Membrane Assay). The indirect antiangiogenic activity was verified by assessing the effect on VEGF (Vascular endothelial growth factor) and HIF-1α (Hypoxia-inducible factor 1-alpha) mRNA (messenger RNA) and VEGF protein expression under normoxic and hypoxic conditions [34,35]. An anti-migratory effect by reducing CXCL8 (C-X-C Motif Chemokine Ligand 8) levels, a chemokine that contributes to CRC (colorectal cancer) cell proliferation and metastasis [36]. Lastly, several plants have decreased DNA oxidative damage and own radical scavenging properties [37].
3.2. Medicinal plants with antimicrobial activity
Resistance to antimicrobial medications is a global threat [49]. However, plants always fuel us with various bioactive compounds that can be used to develop new medicines as antibiotics [49]. The antimicrobial activity of Jordanian plant extracts and volatile oils have been investigated. Many researches exhibited promising antimicrobial activities of these plants in pharmaceutical and food preservation systems. A total of 131 plant species were investigated for their antimicrobial activities as shown in Table 3, Table 4. These plant species were found to belong to 49 plant families. After scrutinizing the literature, it was determined that Asteraceae (n = 40), Lamiaceae (n = 32), and Fabaceae (n = 12) were the prominent three families according to the number of species studies conducted in this domain of research. Varthemia iphionoides (n = 6), Teucrium polium (n = 5), Achillea fragrantissima (n = 4), Gundelia tournefortii (n = 4), Inula viscosa (n = 4), for example, were the five most studied species which are also common herbs in the traditional medical system. Therefore, they can be utilized as food or are easily accessible targets for scientific research.
Table 3.
Asteraceae antimicrobial activity against Gram-positive bacteria.
| Scientific name | Method of extraction/Part used | The concentration of plant extract | Type of bacteria | Antimicrobial effect (DZI, MIC) | Reference |
|---|---|---|---|---|---|
| Achillea fragrantissima | Soxhlet, Aerial parts | 400 μg/mL | S. aureus | MIC (mg/mL): 2.9 (80% methanol) | [63] |
| Maceration, whole plant | 0.97–250 mg/mL | S. aureus, MRSA | DZI (mm): 7,7 (methanol) | [67] | |
| Maceration, whole plant | 200 mg/mL | S. aureus | DZI (mm): 19.5 (95% ethanol) | [84] | |
| Decoction, aerial parts | 5–100 mg/mL | S. aureus | MIC (mg/mL): 25 (water), 6.25 (alcohol) | [81] | |
| MRSA | MIC (mg/mL): 12.5 (alcohol) | ||||
| S. pneumoniae | DZI (mm): 12.5 (water), 3.12 (alcohol) | ||||
| B. cereus | MIC (mg/mL): 1.56 (alcohol) | ||||
| E. faecalis | MIC (mg/mL): 12.5 (alcohol) | ||||
| Maceration, aerial parts | 2 mg/disc | S. aureus, B. subtilis, M. luteus | DZI (mm): 24, 8.5, 9.16 (80% methanol) | [87] | |
| Achillea biebersteinni | Maceration, aerial parts | 5–100 mg/mL | S. aureus | DZI (mm): 17.2 (95% ethanol) | [84] |
| Reflux, whole plant | 5–100 mg/mL | S. aureus | MIC(mg/mL): 6.25 (hydro-alcoholic), 25 (water) | [83] | |
| MRSA | MIC (mg/mL): 3.12 (hydro-alcoholic), 25 (water) | ||||
| S. pneumoniae | MIC (mg/mL): 1.56 (hydro-alcoholic) | ||||
| B. cereus | MIC (mg/mL): 1.56 (hydro-alcoholic) | ||||
| E. faecalis | MIC (mg/mL): 12.5 (hydro-alcoholic | ||||
| Maceration, flowers | 100 μg/mL | P. acne | MIC(mg/mL): 10 (methanol) | [91] | |
| Achillea falcata | Reflux, aerial parts | 5–100 mg/mL | S. aureus | MIC (mg/mL): 50 (water) | [82] |
| Reflux, aerial parts | 5–100 mg/mL | S. aureus | MIC (mg/mL): 6.25 (ethanol 70%) | ||
| Reflux, aerial parts | 5–100 mg/mL | MRSA | MIC (mg/mL): 12.5 mg/mL (ethanol 70%) | ||
| Reflux, aerial parts | 5–100 mg/mL | E. faecalis | MIC (mg/mL): 12.5 mg/mL (ethanol 70%) | ||
| Achillea millefolium | Maceration, plantlet | Ethanol 40 mg/100 μl and water 30 mg/100 μl | S. aureus | DZI (mm): 19.0 (water), 20.8 (ethanol) | [94] |
| Achillea membranacea | Maceration, leaves | 500 mg/mL | S. aureus | DZI (mm): 19 (ethanol), 12.3 (methanol) | [57] |
| Maceration, leaves | 500 mg/mL | S. aureus | DZI (mm): 16.0 (water), 13.7 (ethanol), 30.3 (methanol), 12.7 (acetone) | ||
| MRSA | DZI (mm): 26.3 (water), 20.7 (water), 14.3 (methanol), 19.7 (acetone) | ||||
| Achillea santolina | Maceration, NMa | 200 mg/mL | S. aureus | DZI (mm): 11.2 | [84] |
| Achillea tomentosa | Steam distillation, aerial parts | 100 μL of the diluted Volatile oil per each agar well. | S. aureus, B. subtilis | (RIZD 103 ± 4.73%), (RIZD 80%) | [95] |
NM: not mentioned. S. aureus (Staphylococcus aureus), B. subtilis (Bacillus subtilis), M. luteus (Micoroccocus luteus), S. pneumoniae (Streptococcus pneumoniae), K. pneumoniae (Klebsiella pneumoniae), B. cereus (Bacillus cereus), E. faecalis (Enterococcus faecalis), P. acne (Propionibacterium acne).
Table 4.
Asteraceae antimicrobial activity against Gram-negative bacteria.
| Scientific name | Part used | The concentration of plant extract | Type of bacteria | Antimicrobial effect (DZI, MIC) | Reference |
|---|---|---|---|---|---|
| Achillea fragrantissima | Maceration, whole plant | 200 mg/mL | K. pneumonia | DZI: 10.2 mm (ethanol) | [84] |
| Achillea fragrantissima | Maceration, aerial parts | 2 mg/disc | K. pneumonia, E. coli | DZI: 9, 9 mm (methanol) | [87] |
| Achillea fragrantissima | Soxhlet, aerial parts | 400 μg/mL | E. coli, P. aeruginosa, E. cloacae, P. mirabilis | DZI: 23, 18, 18, 17 mm (methanol) | [63] |
| Achillea fragrantissima | Decoction, aerial parts | 5–100 mg/mL | K. pneumoniae | MIC (mg/ml): 12.5 (hydro-alcohol) | [81] |
| Achillea biebersteinni | Maceration, whole plant | 200 mg/mL | K. pneumonia, E. coli, E. cloacae | DZI: 8.5 mm, 8.5 mm, 9 mm (ethanol) | [84] |
| Achillea biebersteinii | Whole plant | 5–100 mg/mL | K. pneumoniae | MIC: 25 mg/mL (water), 3.12 mg/mL (hydro-alcoholic). | [83] |
| Achillea membranacea | Maceration, leaves | 500 mg/mL | S. typhimurium, P. aeruginosa, E. coli | 20 mm (ethanol), 25 mm (ethanol), 17.3 mm (ethanol), 16.3 mm (methanol). | [57] |
| Achillea millefolium | Maceration, plantlet | 30 mg/100 μl | S. paratyphi, P. vulgaris, K. oxytoca, Proteus mirabilis | DZI: 21.2, 19.6, 14.4, 14.5 mm (water) | [94] |
| Achillea millefolium | Maceration, plantlet | 40 mg/100 μl | S. paratyphi, P. vulgaris, K. oxytoca, P. mirabilis | DZI: 20.2, 19.8, 10.33, 10.33 mm (ethanol) | |
| Achillea falcata | Reflux, aerial parts | 5–100 mg/mL | K. pneumoniae | MIC: 12.5 mg/mL (70% ethanol) | [82] |
| Achillea santolina | Maceration, whole plant | 200 mg/mL | K. pneumonia, E. cloacae | DZI: 9 mm, 12 mm (ethanol) | [84] |
| Achillea tomentosa | Steam distillation, aerial parts | 100 μL of the diluted volatile oil | P. aeruginosa | RIZD 90% (Volatile oil) | [95] |
K. pneumoniae (Klebsiella pneumoniae), B. cereus (Bacillus cereus), E. faecalis (Enterococcus faecalis), P. acne (Propionibacterium acne), E. coli (Escherichia coli), P. aeruginosa (Pseudomonas aeruginosa), P. mirabilis (Proteus mirabilis), E. cloacae (Enterobacter cloacae), S. typhimurium (Salmonella typhimurium), S. paratyphi (Salmonella paratyphi), P. vulgaris (Proteus vulgaris) K. oxytoca (Klebsiella oxytoca).
3.2.1. Plant tissue, extract and bacteria types
This review revealed that 47% of the plant extracts explored in the 60 papers were extracted from leaves, and more than 30% were from aerial parts. The subsequent most common part was the flowers (26%). The crude ethanolic extracts were the most common (37%), followed by the methanolic extracts (32%), aqueous extracts (16%), ethylacetate extracts (6%), hexane extracts (4%), and chloroform extracts (3%). It was also found that Staphylococcus aureus was the most targeted Gram-positive bacteria (69%) including methicillin-resistant staphylococcus aureus (MRSA), followed by Bacillus subtilis (17%), Bacillus cereus (1%), Streptococcus pneumonia (2%), Enterococcus faecalis (1.5%), Staphylococcus epidermidis (1%), Micoroccocus luteus (2%) and Propionibacterium acne (6%). On the other hand, Escherichia coli was the most targeted Gram-negative bacteria (35%), followed by Pseudomonas aeruginosa (27%). The other strains were Enterobacter aerogenes (1.7%), Enterobacter cloacae (3.4%), Proteus mirabilis (1.7%), Klebsiella pneumonia (14%) Salmonella paratyphi (1.6%), Proteus vulgaris (2.1%), Proteus mirabilis (2%), Erwinia carotovora (1.2%), Helicobacter pylori (6.7%), and Chromobacterium violaceum (3.3%). Fig. 3, Fig. 4, Fig. 5 provide further details on the topic as follows.
Fig. 3.
(A) Plant tissues extracted to test against Gram-negative bacteria, (B) Method of extraction used to create the extract.
Fig. 4.
(A) Plant tissues extracted to test against Gram-positive bacteria, (B) Method of extraction used to create the extract.
Fig. 5.
Mean and standard deviation of diameter zone of inhibition (DZI) of extracts in the top six families, with significant differences in DZI values (mm) for (A) Gram-negative bacteria and (B) Gram-positive bacteria reported. *: p < 0.05.
The majority of studies conducted illustrated that the Araceae family had the highest overall mean diameter zone of inhibition (DZI) against Gram-negative and positive bacteria. The DZI for Gram – negative and positive respectively were (22.30 mm, 22.63 mm) [[57], [58], [59], [60], [61]]. The other families that were studied against Gram-negative for their antimicrobial activities were (Apiaceae, Asteraceae, Fabaceae, Rosaceae, Lamiaceae) with DZI values ranging from 15.7 to 38 mm [[62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72]]. Among solvents used for extraction, acetone showed the best (highest) mean DZI (17.4 mm), while hexane extracts were the lowest (7.4 mm). On the other hand, ethyl acetate extracts were the most potent (MIC 0.3 mg/mL), while methanol extract was the least potent (MIC 8.8 mg/mL). Seeds extract had the highest mean diameter zone of inhibition (18.4 mm), while flowers had a low MIC value (0.86 mg/mL). Helicobacter pylori is the most affected bacteria species in comparison to other bacteria species.
On the other hand, several solvents were used to investigate the antimicrobial activity against Gram-positive bacteria, among which acetone showed the best (highest) mean DZI (17.57 mm), while chloroform extracts were the lowest (6.5 mm). On the other hand, ethyl acetate showed the best (lowest) mean MIC (0.41 mg/mL), while water showed the highest MIC (14.2 mg/mL). Flowers extract has the highest mean DZI (18.26 mm). While leaves have the lowest MIC values (1.3 mg/mL).
3.2.2. Plant families antimicrobial activity
3.2.2.1. Araceae
Over 800 species of Araceae are of economic importance in ornamental, edible and medicinal uses [73]. This family is represented in Jordan by three genera and five species, namely Arum palestinum, Arum dioscoridis, Arum hygrophilum, Biarum angustatum and Eminium spiculatum (Blume) Kuntze [74]. Many species are used in traditional medicine, such as Arum discoridis and Arum hygrophillum, collectively called Louf, which is used for treating circulatory system problems and internal bacterial infection and as a spice and cooked [57,58].
Several studies evaluated the antibacterial activity of Arum discoridis and Arum hygrophillum leaves aqueous extract and revealed broad spectrum of inhibition. Additionally, the ethanol and water extracts had broad-spectrum antimicrobial activity against MRSA, Staphylococcus aureus, Bacillus subtilis, Staphylococcus epidermidis, Salmonella typhimurium, Listeria monocytogenes, Pseudomonas aeruginosa and Escherichia coli, with a DZI ranges from 20 mm to 36 mm [[57], [58], [59]]. Literature also revealed that Eminium spiculatum aqueous stem extract -the only representative of the genus Eminium grown wild in Jordan-had potential antibacterial activity against MRSA (DZI: 21.7 mm) and Pseudomonas aeruginosa. Moreover, the aqueous leaf extract shows inhibitory activity against Escherichia coli [74]. Arum palestinum showed activity towards Propionibacterium acne with MIC of 0.125 mg/mL [61].
3.2.2.2. Apiaceae
The Apiaceae (Umbelliferae) family comprises many genera used as culinary herbs and in folk medicine [75]. In addition, many plant species were used to improve the antimicrobial activity of conventional antibiotics, including Pimpinella anisum and Lepidium sativum [76].
Ammi visnaga (known as Khella) extract showed a significant inhibitory effect on Escherichia coli at (MIC: 800 ppm), possessing activity against Pseudomonas aeruginosa (MIC 3000 ppm), Staphylococcus aureus (MIC: 4000 ppm) and Candidca albicans (MIC: 2000 ppm) [77]. Using the volatile oils extracted from Foeniculum vulgare, Anethum graveolens, Coriandrum sativum, and Apium graveolens against Bacillus subtilis. It was also established that Apium gravelones (MIC: 1.25 mg/mL) had the best antibacterial activity amongst the family [75].
Studies highlighted that Pimpinella anisum and Ducrosia flabellifolia exhibited moderate antimicrobial activity against Staphylococcus aureus and weak activity against Escherichia coli [64,78]. The volatile oils of Foeniculum vulgare leaves showed growth inhibitory activity against Bacillus subtilis with MICs 10 mg/mL. In addition, the fennel seed oil had similar activity against Gram positive and Gram negative bacteria [75].
3.2.2.3. Asteraceae
The Asteraceae (Compositae) family comprises over 1600 genera and 2500 species and is the most prominent flowering plant family. For centuries, it has been used in diet and medicine [79]. Members of this family share similar chemical composition and demonstrate several pharmacological effects such as antioxidant, anti-inflammatory, diuretic, wound healing and antimicrobial activities [79], (Table 3, Table 4).
The genus Achillea (commonly referred to as yarrow) is mainly distributed in the northern hemisphere, most indigenous to Europe and the Middle East. Achillea species are widely used in popular medicine of several cultures due to numerous pharmacological properties, such as analgesic, healing, anti-inflammatory, antioxidant, antispasmodic, antihemorrhoidal, stomachic, antiseptic and emmenagogue [80,81]. The genus Achillea has been studied in several researches [63,67,76,82,83]. Traditionally, it has been used to treat severe illnesses since ancient times and known as Qaisoum [84]. The best results were revealed by Achillea biebersteinii extracts which illustrated activity against several bacterial types except for Pseudomonas aeruginosa. The most susceptible of the Gram-positive bacteria was Streptococcus pneumoniae, B. cereus and Staphylococcus aureus, and of the Gram-negative was Klebsiella pneumoniae. The results differ by the type of extract and the part of the plant used [[83], [84], [85]].
Inula viscosa is widely distributed and used to treat several medical conditions, including wounds, intestinal worms, respiratory tract disorders, bone, blood pressure, and diabetes [27]. The crude extracts of Inula viscosa were active against bacterial strains Staphylococcus aureus, Bacillus subtilis, Propionibacterium acne, MRSA and Pseudomonas aeruginosa, inactive against Escherichia coli, Candidca albicans and Aspergillus niger [61,[86], [87], [88]]. Inula viscosa is used widely in treating gastroduodenal disorders. In one study, the ethanolic crude extract showed intense activity against different isolates of Helicobacter pylori with an MIC of 83 mg/mL and MBC of 104 mg/mL [89].
Centaurea damascena is another plant studied for its antimicrobial activity. Traditionally, it is used to treat gastritis and as a condiment for hot tea. Several Gram-positive and Gram-negative bacteria were susceptible to Centaurea damascena methanol extract. The MIC ranges from 0.123 mg/ml to 1.1 mg/mL. For example, the MIC against Staphylococcus aureus, Bacillus subtilis and Enterobacter aerogenes was 0.123 mg/mL [90].
Other plants as Cichorium pumilum and Chrysanthemum coronarium had moderate antimicrobial activity against Propionibacterium acnes (MIC 2.5 mg/mL), Staphylococcus aureus (DZI: 20 mm), Bacillus subtilis (DZI: 19 mm) and S. epidermidis (DZI: 18 mm) [85,91]. Also, Sonchus oleraceus exhibited prominent antiquorum sensing activity (18 mm) [92]. Alqudah et al. studied Geropogon hybridus’s antibacterial activity against both Gram-positive and Gram-negative bacteria and showed the highest potency (IC50 0.83 mg/mL) against Escherichia coli [62]. Gundelia tournefortii leaves extract also showed antibacterial activity against Gram-positive as Staphylococcus aureus, Salmonella typhimurium and MRSA (DZI: 15.7–16.7 mm). And Gram-negative pathogens as Pseudomonas aeruginosa and Escherichia coli (DZI: 14.3–10.7 mm) [60].
Moreover, two species of Onopordum were investigated, namely, Onopordum blancheanum and Onopordum jordanicolum. Generally, Onopordum jordanicolum exhibited antibacterial activity against Staphylococcus aureus, MRSA, K. pneumonia, and P. mirabillis. The ethanol extract of flowers displayed significant antibacterial activity against Staphylococcus aureus with the best MIC and MMC (minimum microbial concentration) values. In addition, the acetone extract displayed significant antifungal activities against Aspergillus brasiliensis and Candidca albicans with the best MIC and MMC values. On the other hand, the flower extracts of Onopordum blancheanum produced antibacterial and antifungal activities against Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, and Proteus mirabillis, Aspergillus brasiliensis and Candida albicans [93].
Different Artemisia species as Artemisia herba–alba, Artemisia sieberi, and Artemisia inculata were investigated against several bacteria species. A. herba-alba extract was found to possess a high inhibitory effect on Staphylococcus aureus at MIC 3000 ppm, (DZI: 14–16 mm), Pseudomonas aeruginosa at MIC 4000 ppm, and Escherichia coli at MIC 4000 ppm (DZI 17 mm) [77]. Conversely, Artemisia sieberi exhibited weak inhibitory activity against Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, and Enterobacter cloacae [70]. Moderate antimicrobial activity against Helicobacter pylori was observed when Artemisia inculata ethanol extract was tested [89].
3.2.2.4. Fabaceae
The Fabaceae (Leguminosae) is one of the most prominent flowering plant families. These plants have been the main part of meals in arid and semiarid regions since 6000 BCE due to their protein richness [74]. There is also proof of humans’ use of these plants in Asia, Europe, and North America for medicinal purposes. Recently, species of this family have been found naturally or are cultivated everywhere around the globe except the poles [96,97]. Phytochemical metabolites of Fabaceae have industrial and medicinal importance. This family is a rich source of phytochemicals, including alkaloids, flavonoids, lectins, saponins, carotenoids, and phenolic acids, which have anticancer properties, and the use of such phytochemicals is increasing over time [97].
It was found that Alhgi graecorum, for example, exhibited antimicrobial activity in a dose-dependent manner against several bacterial isolates. The MIC was between 1.1 and 1.8 mg/mL [63].
Lupinus varius was also found to display a broad spectrum of antimicrobial activity. The seed extract exhibited high antibacterial activity against Pseudomonas aeruginosa with a mean inhibition zone of 28.7 ± 1.5 mm and antifungal activity against Candida albicans with a mean inhibition zone of 31.3 ± 0.6 mm. Surprisingly, it was highlighted that MRSA and Escherichia coli were susceptible to all parts of Lucius varius, both aqueous and alcohol extracts of leaf and alcohol extracts of the flower. They showed inhibition zone values ranging from 15.0 ± 1.0 to 24.0 ± 2.0 mm for MRSA and from 9.3 ± 1.5 mm to 19.0 ± 1.0 mm for Escherichia coli [57,60].
Furthermore, Lathyrus hirticarpus leaves extract exhibited broad antibacterial activity spectrum. It showed an inhibition zone of 17.3 mm for S. pyogenes using the methanol extract, 21.7 mm and 19.3 for Staphylococcus aureus and Klebsiella pneumonia, respectively using acetone extract, 20.67, 30.3 for Pseudomonas aeruginosa and Proteus mirabilis respectively using the aqueous extract, 22.33 mm for Escherichia coli using the methanol extract [93]. ]. In addition, the ethanol extract exhibited antifungal activity against Aspergillus brasiliensis and Candida albicans [93]. Moderate activity was observed using Ononis natrix against Gram-positive Bacillus subtilis and Brevibacillus brevis, and the Gram-negative Escherichia coli [72]. It is also noteworthy that the n-hexane extracts of the aerial parts of Ononis hirta showed enjoyable activity against Pseudomonas aeruginosa at MIC of 0.125 g/mL [88].
3.2.2.5. Lamiaceae
Lamiaceae, or the mint family, is known for its economic value. It is reputation comes from the aromatic scene within its leaves and flowers. Salvia is the most well-known genus of the Lamiaceae family. Its antimicrobial activity has been established in the literature [98]. Different studies demonstrated a weak to moderate antimicrobial activity against Gram-positive bacteria as Staphylococcus aureus, MRSA, Bacillus subtilis, and Brevibacillus brevis, with inhibition zone values ranging from 9 to 16.2 mm. It also demonstrated weak activity against Escherichia coli and Klebsiella penumonia with an inhibition zone of 5–10 mm, refer to Table 3, Table 4.
Teucrium polium extracts exhibited a different degree of antimicrobial activity. The most interesting activity was for n-hexane extract of the aerial parts against Pseudomonas aeruginosa with an MIC value of 125 μg/mL [88]. Moreover, the MIC of Rosmarinus officinalis ranges between 1.0 and 1.2 mg/ml against Staphylococcus aureus, MRSA (MIC: 0.5 1.5 mg/ml), and Propionibacterium acne (0.5 mg/ml) [67,70,92]. The Gram-negative bacteria are more susceptible to Rosmarinus officinalis extracts, especially Escherichia coli (0.9 mg/ml) [63].
Thymus is another well-known species. The leaves of Thymus capitatus possess stronger antimicrobial activities than stem extracts against the bacterium Staphylococcus aureus and the bacterium Pseudomonas aeruginosa [65]. In another study, Thymus capitatus leaves extract exhibited broad-spectrum activity as it successfully inhibited the growth of several tested microorganisms with varying degrees. The results of the disc diffusion method indicated that Pseudomonas aeruginosa was the most sensitive strain (22 mm), followed by Staphylococcus aureus, Enterobacter aerogenes and Escherichia coli [65,99]. It was also found that the volatile oils of Thymus serpyllum and Thymus vulgaris produced inhibition zones of 8–20 mm and 5–20 mm, respectively [64].
Origanum jordanicum methanol extract had growth inhibitory activity against Staphylococcus epidermidis at 0.781 mg/mL MIC [100]. On the other hand, the volatile oils of O. syriacum had significant antimicrobial activity against Propionibacterium acne (MIC 1 mg/ml) and moderate activity against H. pylori [89,91]. Additionally, Mentha rotundifolia and Origanum syriacum were tested against Propionibacterium acne; the MIC was 2 and 1 mg/mL, respectively [91].
Lavandula angustifolia had moderate inhibitory activity against Gram-positive bacteria with a DZI range from 9.5 to 15 mm and weak antibacterial activity against Gram-negative bacteria 7–10.5 mm [92]. Lavandula officinalis showed good activity against Helicobacter pylori isolate I and II with diameter zone of inhibition 32 (mm) and 35 mm, respectively [89]. Origanum jordanicum was used by the Bedouin of Petra for ages for food and medicine. The methanol extract demonstrated paramount results against Staphylococcus epidermidis at 0.781 mg/mL MIC, and it was effective against Candida albicans [69]
3.2.2.6. Rosaceae
The extracts of Sarcopoterium spinosum were found to be effective against several tested bacterial strains. At relatively low concentrations, it exhibited that it can completely inhibit the growth of Gram-positive bacteria (Staphylococcus aureus 0.5 mg/ml, Bacillus subtilis 1 g/ml, Micrococcus luteus 1 mg/ml). Nonetheless, Gram-negative bacteria were inhibited at higher concentrations (Escherichia coli 1 mg/ml, Klebsiella penumonia 2 mg/ml) [87]. One study illustrated that the methanolic and ethanolic leaf and fruit extracts of Rubus sanguineus have significant inhibitory activity against Gram-positive bacteria (DZI: 18–22 mm) and Candida. Yet, they have not shown any significant activity against Gram-negative bacteria [101].
3.3. Medicinal plants with antiplatelet activity
The leaves and flowering tops of Varthemia iphionoides were used to assess the antiplatelet activity. In vitro antiplatelet activity was investigated for the aqueous and alcoholic extracts of the plant, for the isolated volatile oil, and four different isolated flavonoids, including xanthomicrol, kumatokenin, jaceidin and 3, 3V-di-O-methyl quercetin (refer to Fig. 6). The aqueous extract, xanthomicrol, and kumatokenin showed antiplatelet activity, while the volatile oil and the alcoholic extracts did not. The antiplatelet activity of the aqueous extract has been shown to follow a concentration-dependent manner against both collagen and adenosine diphosphate (ADP); concentrations of 30 and 20 Ag/ml exhibited complete inhibition [102].
Fig. 6.
Isolated flavonoids with antiplatelet activity. (A) Xanthomicrol, (B) Kumatokenin.
Rheum palaestinum, traditionally used in Jordan, was evaluated for its antiplatelet activity by using the aerial parts of the plant. The antiplatelet activity was investigated for two stilbene derivatives that were isolated and identified. These structures were detected via spectroscopic data, including MS and NMR. The two stilbenes were trans-resveratrol-3-O-b-d-glucopyranosid (Piceid) and rhapontigenin-3-O-b-d-glucopyranoside (rhaponticin), refer to Fig. 7. Upon examination, the two stilbenes showed antiplatelet activity on human platelet-rich plasma (PRP) aggregation induced by collagen and ADP [103].
Fig. 7.
Isolated stilbenes with antiplatelet activity. (A) Resveratrol 3-O-d-glucopyranoside, (B) Rhapontigenin 3-O-d-glucopyronoside.
The antiplatelet activity of Gundelia tournefortii was also evaluated using the aerial parts of the plant, and five compounds were isolated, including scopoletin, isoscopoletin, esculin, and a mixture of b-sitosterol and stigmasterol. A Hydrodistillation technique was used to obtain the volatile oil. The antiplatelet activity of the plant was tested in vitro, relying on adenosine-50-diphosphate (ADP) and arachidonic acid (AA) as agonists. Only the chloroform extract has expressed mild inhibitory activity on platelet aggregation induced by ADP and arachidonic acid (AA) [104].
Full antiplatelet aggregation activity was observed by methanolic extracts of aerial and root parts of Aristolochia maurorum, the acidic fractions, aristolochic acid standard, the aristolochic acid I, II, and their combination. This antiplatelet activity was observed to be variable between the two phases of platelet aggregation based on concentration [105]. Furthermore, the platelet aggregation Inhibitors derived from aerial Parts Grown in Jordan were also assessed, and multiple compounds were isolated from the plant, including a flavonoid glycoside (rutin) and several minor compounds.
The antiplatelet activity for the crude methanolic and ethylacetate extracts of Ruta chalepensis and three major isolated compounds was measured by the aggrometric method for measuring aggregation. Both ethylacetate and methanol extracts exhibited inhibitory activity against ADP- induced platelet aggregation (ADP-IA). However, only ethylacetate extract showed activity on collagen-induced platelet aggregation (Co-IA). In addition, Bergapten exhibited more inhibitory activity against ADP-IA than chalepensin, which expressed more activity against Co-IA than bergapten [106].
The volatile oil obtained from air-dried aerial flowering parts of Achillea bieberstienii showed dose-dependent solid inhibition of platelet aggregation caused by ADP (10 μM) and collagen (2 μg/mL). Furthermore, it was noticed that at lower concentrations (10 and 20 μg/mL), the essential oil showed higher antiplatelet activity against ADP-induced aggregation compared to collagen-induced aggregation. And, at higher concentrations (50 and 60 μg/mL), complete inhibition of aggregation induced by ADP and collagen was achieved [107]. In addition, both α-terpinene and p-cymene showed a concentration-dependent increase in the inhibitory activity [107]. This review also found that several concentrations of Achillea biebersteinii extracts showed a non-dose-dependent enhancement of platelet aggregation induced by adenosine diphosphate (ADP) and collagen [83].
3.4. Medicinal plants with antidiabetic activity
Using crude plant extracts to treat diabetes is seen as a practice in traditional medicine in Jordan. It was documented around 70 species indigenous to Jordan are used traditionally to treat diabetes [108]. Therefore, several plants were evaluated for their in vivo/in vitro hypoglycemic activities [108]. Some of these plants exhibited dual or mono alpha-amylase and glucosidase activity, like Aloe vera, Arum dioscoridis, Arum palaestinum, Arum hygrophilum, Crataegus aronia, Adiantum capillus-veneris, Origanum syriacum, Geranium graveolens, Varthemia iphionoides, Paronychia argentea, Rosmarinus officinalis and Olea europaea [[109], [110], [111], [112], [113], [114], [115], [116]].
Both Achilla santolina and Eryngium creticum exhibited a dose-dependent pancreatic β-cell proliferation [117]. ]. In addition, Geranium graveolens and Varthemia iphionoides augmented β-cell mass expansion [118]. On the other hand, Pistacia atlantica augmented acute β cell insulin secretory activity and the integrity of cells [117]. Also, Sarcopoterium spinosum showed insulinotropic and proliferative effects in the pancreas [118].
The blood glucose data obtained indicate that aqueous extract from Alkanna strigosa produced hypoglycemic effects in alloxan-induced diabetic rats similar to a standard antidiabetic drug, metformin, by enhancing insulin production and decreasing glucagon production [119].
Collectively, using different assay techniques for in vivo and in vitro models the antidiabetic activity for Jordanian plants and herbs was confirmed by majority of research, presented their proposed mechanism of actions. Similar to conventional antidiabetic drugs, the Jordanian herbs exhibited secretagogue activity, inhibition of hepatic gluconeogenesis and intestinal carbohydrate absorption, and enhancement of glucose uptake by adipose and muscle tissues [120].
3.5. Medicinal plants with anti-hyperlipidaemia activity
The pharmacological inhibition of dietary lipid digestion and absorption can induce favorable amelioration of dyslipidemia, atherosclerosis and obesity. Adiantum capillus-veneris, for instance, exerted marked triacylglycerol-reducing capacities at levels higher than atorvastatin [109].
In a dose-dependent manner, Arum species, Anthemis palaestina, Lavandula angustifolia, Origaum syriacum, Salvia triloba and spinosa, Ononis natrix, Fagonia arabica, Majorana syriaca, Malva nicaeensis, Rosmarinus officinalis, Hypericum triquetrifolium, Chrysanthemum coronarium, Paronychia argentea were reported to inhibit the gastric absorption of triglycerides by the inhibition of pancreatic lipase [111,114,[121], [122], [123], [124]]. Additionally, one study highlighted that the administration of the aerial parts extract of Alkanna strigosa resulted in a significant reduction in the mean values of serum cholesterol, triglyceride, and LDL and an increase in the mean values of HDL [119].
3.6. Medicinal plants to treat gastrointestinal tract disorder
Many herbal extracts are used in folk medicine to treat digestive disorders, including peptic ulcers. Helicobacter pylori is one of the most prevalent human infections and have been implicated as a predisposing factor for peptic, duodenal ulcer, chronic active gastritis, gastric cancer and lymphoma [89,125]. The Peels extract of Punica granatum displayed a significant inhibitory effect on Helicobacter pylori growth and urease enzyme activity. In addition, it showed a synergistic and a reduction in the MIC of metronidazole four-fold [126].
3.7. Medicinal plants of Jordan: miscellaneous
3.7.1. Female antifertility and abortifacient effects
Inula iscosa leaf extract exhibited the anti-implantation and mid-term abortifacient effects in rats. The aqueous extract administered in the first days of gestation caused diminished fetal implantation and reduced the number of corpora lutea and blood progesterone levels. Moreover, its administration on mid-term gestation caused abortion [127]. It was also underlined that Artemisia monosperma leaves are taken in folk medicine by certain women of Jordan for abortion induction. The plant outcome in pregnant rats was assessed using the ethanolic leaves extract. The intraperitoneal administration of 150 mg/kg or 300 mg/kg of the plant ethanolic extract at different gestation times causes severe harm to rat pregnancy outcomes [128,129].
3.7.2. Anti-gout effect
The methanol extract of Salvia spinose had a potent inhibition of xanthine oxide in vitro. However, a further biological investigation is needed to verify the reported inhibitory activity using an animal model [130].
3.7.3. Anti-inflammatory
The methanolic extracts of Urtica pilulifera leaves had a significant anti-arthritic effect. It also exhibited an inhibitory effect by inhibiting paw swelling, skin lesions, and articular deformity [131]. The proanthocyanidins fraction of Cistus incanus inhibited TPA-induced edema, and the pure isolated compounds inhibited COX 1 and COX 2 with high selectivity toward COX 2 In addition, Eucalyptus globulus and Arum palaestinum revealed a remarkable inhibitory activity against IL-1a proinflammatory cytokines [61].
3.7.4. Anticholinesterase activity (antialzheimer’s activity)
The anticholinesterase activity of Achillea fragrantissima was assessed in vitro. Depending on the solvent type, the ethyl acetate fraction showed the highest inhibitory effect on acetylcholinesterase (AChE). In contrast, the hydro-alcoholic fraction expressed the highest inhibitory activity on butyryl-cholinesterase (BChE). The study findings highlighted possible applications of A. fragrantissima flower extracts as adjuvant therapy in Alzheimer’s disease management [132].
The volatile oil and the aqueous, ethyl acetate, and chloroform extracts of Schinus molle were assayed for their inhibitory effect against acetylcholinesterase (AChE) and butyryl-cholinesterase (BChE). Most of the extracts revealed moderate to strong inhibitory activities, and the chloroform extract showed the strongest inhibitory activity against both enzymes compared to Tacrine. The leaves essential oil and the ethyl acetate extract exhibited moderate inhibitory activity, but the fruit essential oil was considered inactive against BChE [133].
3.7.5. Wound healing
The wound-healing activity of fresh crude aerial parts of Portulaca oleracea (50 mg) was studied using Mus musculus JVI-1. The results showed that Portulaca oleracea accelerated the wound-healing process by decreasing the surface area of the wound and increasing the tensile strength [134]. It signifies the maturity and strength of the collagen fibers formed in the area of the incision wound [135]. The hexane extract of Alkanna strigos was also found to possess wound-healing properties by improving the tensile strength of incision wounds [135].
It was also demonstrated that the aqueous extracts from the dried aerial parts of Inula viscosa supported wound healing, accelerated the repair, and organized the epidermis in the presence of mature scar tissue in the dermis. The capacity of wound healing with the Inula viscosa could be explained based on the plants' anti-inflammatory effects, which are well documented in the literature [136]. Another study, Antirrhinum majushis, revealed innovative healing power. The flowers’ extract ointment (5% w/w) was superior compared to the leaves extract (5% w/w) and the positive-control ointments (MEBO) (1.5% w/w) Moreover, Schinus molle aqueous extract was also reported to enhance the fibrosis, neovascularization and re-epithelialization of the epidermis and sub-epidermal cells in the regenerated tissue. In addition, it increased the tensile strength of the skin in vivo model [137].
3.7.6. Anti-ulcer
One study reported that the combination of Punica granatum extract and metronidazole exhibited a synergistic activity against H. pylori with MIC of 0.156 mg/ml [126]. The protective effect of Anchus strigosa was also reported using ether and chloroform soluble fraction in ethanol-induced ulcer model in rats [138].
3.7.7. Xanthine oxidase inhibitory activity
Many tested species of Jordanian plants were reported to have good activity in inhibiting xanthine oxidase enzyme, namely Salvia spinosa (IC50 = 0.053 mg/ml), Anthemis palestina (0.168 mg/ml), Chrysanthemum coronarium (0.199 mg/ml), Achillea biebersteinii (0.36 mg/ml), and Rosmarinus officinalis (0.65 mg/ml) [130].
3.7.8. Sleep deprivation-induced memory impairments
Chronic sleep deprivation is well known to impair short- and long-term memory formation. Treatment of animals with Arbutus andrachne fruit extract was reported to prevent long-term and short-term memory impairment at specific doses. Moreover, Arbutus andrachne fruit extract normalized the reduction in the hippocampus GSH/GSSG ratio and activity of GPx, and catalase. This effect is probably due to normalizing oxidative stress in the hippocampus [139].
4. Limitations
The findings of this study gave an indicative impression of the Jordanian medicinal flora. However, this review included only 124 studies that met the inclusion criteria. Which merely comprises a limited number of the 2543 Jordan’s medicinal plant. Furthermore, despite efforts made to include all related articles in the literature. Non-English articles could not be included in this review.
5. Conclusions and perspectives
The current review gathered the data from several research articles that clearly showed the important of medicinal herbs and plants in the treatment of several diseases including cancer, diabetes, and infectious disease. The review provided an account of the studies conducted for plants growing and used traditionally for treating several diseases in Jordan using in vitro and in vivo models emphasizing the therapeutic potentials of these species. Medicinal plants are the only natural resource for developing effective, safe, quality drugs.
Therefore, increasing awareness and investigation of phytochemicals of plants of Jordanian flora and the need for further scientific evaluation should be considered as a national priority. This review can give researchers opportunities for using this heritage in exploring new agents to treat several diseases. And help in discovering false medicinal herbs use. In addition, further screening and investigation are required regarding their phytochemicals, mechanism of action and safety profile. Studying active phytochemicals for disease treatment will help develop drugs for safe treatment and cure in the future.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.El-Dahiyat F., Rashrash M., Abuhamdah S., Abu Farha R., Babar Z.-U.-D. Herbal medicines: a cross-sectional study to evaluate the prevalence and predictors of use among Jordanian adults. J. Pharmaceut. Policy Pract. 2020;13:1–9. doi: 10.1186/s40545-019-0200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oran S.A. The status of medicinal plants in Jordan. J. Agric. Sci. Technol. 2014;4(6A) [Google Scholar]
- 3.Zainab L., Hanan T.H.A. An updated assessment on anticancer activity of screened medicinal plants in Jordan: mini review. J. Pharmacogn. Phytochem. 2020;9(5):55–58. [Google Scholar]
- 4.Alali F.Q., Tawaha K., El-Elimat T., Qasaymeh R., Li C., Burgess J., Nakanishi Y., Kroll D.J., Wani M.C., Oberlies N.H. Phytochemical studies and cytotoxicity evaluations of Colchicum tunicatum Feinbr and Colchicum hierosolymitanum Feinbr (Colchicaceae): two native Jordanian meadow saffrons. Nat. Prod. Res. 2006;20(6):558–566. doi: 10.1080/14786410500183381. [DOI] [PubMed] [Google Scholar]
- 5.Poswal F.S., Russell G., Mackonochie M., MacLennan E., Adukwu E.C., Rolfe V. Herbal teas and their health benefits: a scoping review. Plant Foods Hum. Nutr. 2019;74(3):266–276. doi: 10.1007/s11130-019-00750-w. [DOI] [PubMed] [Google Scholar]
- 6.Lafi Z., Aboalhaija N., Afifi F. Ethnopharmacological importance of local flora in the traditional medicine of Jordan: (a mini review) Jordan J. Pharmaceut. Sci. 2022;15(1):132–144. [Google Scholar]
- 7.Basheti I.A., Elayeh E.R., Db D.B.A.N., el Hait S.S. Opinions of pharmacists and herbalists on herbal medicine use and receiving herbal medicine education in Jordan. Trop. J. Pharmaceut. Res. 2017;16(3):689–696. [Google Scholar]
- 8.Abdel-Qader D.H., Albassam A., Ismael N.S., Aljamal M.S., Chen L.-C., Mansoor K., Hamadi S., Al Mazrouei N., Al Meslamani A.Z. Herbal medicine use in the Jordanian population: a nationally representative cross-sectional survey. J. Pharm. Pharmacogn. Res. 2020;8(6):525–536. [Google Scholar]
- 9.Hudaib M., Mohammad M., Bustanji Y., Tayyem R., Yousef M., Abuirjeie M., Aburjai T. Ethnopharmacological survey of medicinal plants in Jordan, Mujib nature reserve and surrounding area. J. Ethnopharmacol. 2008;120(1):63–71. doi: 10.1016/j.jep.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Al-Qura’n S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009;123(1):45–50. doi: 10.1016/j.jep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 11.Abdelhalim A., Aburjai T., Hanrahan J., Abdel-Halim H. Medicinal plants used by traditional healers in Jordan, the Tafila region. Phcog. Mag. 2017;13(Suppl 1):S95. doi: 10.4103/0973-1296.203975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oran S., Al-Eisawi D. Ethnobotanical survey of the medicinal plants in the central mountains (North-South) in Jordan. J. Biodivers. Environ. Sci. (JBES) 2015;6(3):381–400. [Google Scholar]
- 13.Talib W.H., Issa R.A., Kherissat F., Mahasneh A.M. Jordanian Ducrosia flabellifolia inhibits proliferation of breast cancer cells by inducing apoptosis. Br. J. Med. Med. Res. 2013;3:771–783. [Google Scholar]
- 14.Abu-Darwish M.S., Cabral C., Ali Z., Wang M., Khan S.I., Jacob M.R., Jain S.K., Tekwani B.L., Zulfiqar F., Khan I.A. Salvia ceratophylla L. from South of Jordan: new insights on chemical composition and biological activities. Nat. Prod. Bioprosp. 2020;10:307–316. doi: 10.1007/s13659-020-00259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alzweiri M., Al Sarhan A., Mansi K., Hudaib M., Aburjai T. Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 2011;137(1):27–35. doi: 10.1016/j.jep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Dahab R., Afifi F., Kasabri V., Majdalawi L., Naffa R. Comparison of the antiproliferative activity of crude ethanol extracts of nine salvia species grown in Jordan against breast cancer cell line models. Phcog. Mag. 2012;8(32):319. doi: 10.4103/0973-1296.103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abo-Elghiet F., Ibrahim M.H., El Hassab M.A., Bader A., Abdallah Q.M., Temraz A. LC/MS analysis of Viscum cruciatum Sieber ex Boiss. extract with anti-proliferative activity against MCF-7 cell line via G0/G1 cell cycle arrest: an in-silico and in vitro study. J. Ethnopharmacol. 2022;295 doi: 10.1016/j.jep.2022.115439. [DOI] [PubMed] [Google Scholar]
- 18.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 19.Singh G., Passari A.K., Momin M.D., Ravi S., Singh B.P., Kumar N.S. Ethnobotanical survey of medicinal plants used in the management of cancer and diabetes. J. Tradit. Chin. Med. 2020;40(6):1007–1017. doi: 10.19852/j.cnki.jtcm.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. Ca - Cancer J. Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 21.Talib W.H., Daoud S., Mahmod A.I., Hamed R.A., Awajan D., Abuarab S.F., Odeh L.H., Khater S., Al Kury L.T. Plants as a source of anticancer agents: from bench to bedside. Molecules. 2022;27(15):4818. doi: 10.3390/molecules27154818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tawaha K.A. Cytotoxicity evaluation of Jordanian wild plants using brine shrimp lethality test. Jordan J. Appl. Sci. Nat. Sci. 2006;8(1):12. [Google Scholar]
- 23.Suleiman M.H.A. Ethnobotanical, phytochemical, and biological study of Tamarix aphylla and Aerva javanica medicinal plants growing in the Asir region, Saudi Arabia. Trop. Conserv. Sci. 2019;12 [Google Scholar]
- 24.Alhourani N., Kasabri V., Bustanji Y., Abbassi R., Hudaib M. Potential antiproliferative activity and evaluation of essential oil composition of the aerial parts of Tamarix aphylla (L.) H. Karst.: a wild grown medicinal plant in Jordan. Evid. Base Compl. Alternative Med. 2018;2018 doi: 10.1155/2018/9363868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasabri V., Afifi F.U., Abu-Dahab R., Mashallah S. Mitigating efficacy of Inula graveolens (L.) Desf.(asteraceae) in breast adenocarcinoma MCF7 and T47D proliferation: in vitro mechanistic studies of a selected ethnomedicinal plant from Jordan. Romanian Biotechnol. Lett. 2017;22(6) [Google Scholar]
- 26.Abu-Dahab R., Abdallah M.R., Kasabri V., Mhaidat N.M., Afifi F.U. Mechanistic studies of antiproliferative effects of Salvia triloba and Salvia dominica (Lamiaceae) on breast cancer cell lines (MCF7 and T47D) Z. Naturforsch. C Biosci. 2014;69(11-12):443–451. doi: 10.5560/znc.2013-0147. [DOI] [PubMed] [Google Scholar]
- 27.Talib W.H., Zarga M.H.A., Mahasneh A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules. 2012;17(3):3291–3303. doi: 10.3390/molecules17033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alali F.Q., Amrine C.S.M., El-Elimat T., Alkofahi A., Tawaha K., Gharaibah M., Swanson S.M., Falkinham J.O., III, Cabeza M., Sánchez A. Bioactive withanolides from Withania obtusifolia. Phytochem. Lett. 2014;9:96–101. [Google Scholar]
- 29.Uddin Q., Samiulla L., Singh V., Jamil S. Phytochemical and pharmacological profile of Withania somnifera Dunal: a review. J. Appl. Pharmaceut. Sci. 2012:170–175. [Google Scholar]
- 30.Al-Qudah M.A., Saleh A.M., Al-Jaber H.I., Tashtoush H.I., Lahham J.N., Zarga M.H.A., Afifi F.U., Orabi S.T.A. New isoflavones from Gynandriris sisyrinchium and their antioxidant and cytotoxic activities. Fitoterapia. 2015;107:15–21. doi: 10.1016/j.fitote.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Shabsoug B., Khalil R., Abuharfeil N. Enhancement of natural killer cell activity in vitro against human tumor cells by some plants from Jordan. J. Immunot. 2008;5(3):279–285. doi: 10.1080/15376510802312027. [DOI] [PubMed] [Google Scholar]
- 32.Song C., Wei X.-Y., Qiu Z.-D., Gong L., Chen Z.-Y., Ma Y., Shen Y., Zhao Y.-J., Wang W.-h., Yang B. Exploring the resources of the genus Viscum for potential therapeutic applications. J. Ethnopharmacol. 2021;277 doi: 10.1016/j.jep.2021.114233. [DOI] [PubMed] [Google Scholar]
- 33.Assaf A.M., Haddadin R.N., Aldouri N.A., Alabbassi R., Mashallah S., Mohammad M., Bustanji Y. Anti-cancer, anti-inflammatory and anti-microbial activities of plant extracts used against hematological tumors in traditional medicine of Jordan. J. Ethnopharmacol. 2013;145(3):728–736. doi: 10.1016/j.jep.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 34.Zihlif M., Afifi F., Abu-Dahab R., Abdul Majid A.M.S., Somrain H., Saleh M.M., Nassar Z.D., Naffa R. The antiangiogenic activities of ethanolic crude extracts of four Salvia species. BMC Compl. Alternat. Med. 2013;13(1):1–10. doi: 10.1186/1472-6882-13-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abaza I., Aboalhaija N., Alsalman A., Talib W., Afifi F. Aroma profile, chemical composition and antiproliferative activity of the hydrodistilled essential oil of a rare salvia species (Salvia greggii) J. Biol. Active Prod. Nat. 2021;11(2):129–137. [Google Scholar]
- 36.Awwad O., Alabbassi R., Abaza I.F., Coperchini F., Rotondi M., Chiovato L., Afifi F.U. Effect of Pistacia palaestina boiss. essential oil on colorectal cancer cells: inhibition of proliferation and migration. J. Essential Oil Bear. Plants. 2020;23(1):26–37. [Google Scholar]
- 37.Talib W.H. Consumption of garlic and lemon aqueous extracts combination reduces tumor burden by angiogenesis inhibition, apoptosis induction, and immune system modulation. Nutrition. 2017;43:89–97. doi: 10.1016/j.nut.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Al-Jaber H.I., Hammad H.M., Al-Qudah M.A., Abaza I.F., Al-Humaidi J.Y., Khater D.F., Saleh A.M., Barham M., Abu-Zarga M.H., Afifi F.U. Achillea santolina: growth dependent variation in essential oil composition and some in vitro bioactivity studies. Eur. J. Med. Plants. 2016;13(1):1. [Google Scholar]
- 39.Abu-rish E.Y., Kasabri V., Hudaib M.M., Mashalla S.H., AlAlawi L.H., Tawaha K., Mohammad M.K., Mohamed Y.S., Bustanji Y. Evaluation of antiproliferative activity of some traditional anticancer herbal remedies from Jordan. Trop. J. Pharmaceut. Res. 2016;15(3):469–474. [Google Scholar]
- 40.Nawash O., Shudiefat M., Al-Tabini R., Al-Khalidi K. Ethnobotanical study of medicinal plants commonly used by local bedouins in the Badia region of Jordan. J. Ethnopharmacol. 2013;148(3):921–925. doi: 10.1016/j.jep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 41.Ishaqat A., Abu-Dahab R., Hammad H.M., Al-Zihlif M., Abaza I.F., Nassar Z.D., Afifi F.U. Phytochemical analysis and evaluation of anti-angiogenic and antiproliferative activities of the leaves of Elaeagnus angustifolia L. Grown in Jordan. Nat. Prod. Chem. Res. 2018;6:3. [Google Scholar]
- 42.Alzoubi K.H., Khabour O.F., Alkofahi A.S., Mhaidat N.M., Abu-Siniyeh A.A. Anticancer and antimutagenic activity of Silybum marianum L. and Eucalyptus camaldulensis Dehnh. against skin cancer induced by DMBA: in vitro and in vivo models. Pak. J. Pharm. Sci. 2021;34(3) [PubMed] [Google Scholar]
- 43.Taifour H., El-Ohlah A. vol. I. Royal Botanic Garden; Kew: 2014. Jordan Plant Red List. [Google Scholar]
- 44.Afifi F., Kasabri V., Abaza I. GC-MS composition and antiproliferative activity of Inula graveolens (L.) Desf. essential oil. Arabian J. Med. Aromatic Plants. 2015;1(1):57–66. [Google Scholar]
- 45.Talib W.H., Mahasneh A.M. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci. Pharm. 2010;78(1):33–46. doi: 10.3797/scipharm.0912-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abu-Dahab R., Kasabri V., Afifi F.U. Evaluation of the volatile oil composition and antiproliferative activity of Laurus nobilis L.(Lauraceae) on breast cancer cell line models. Record Nat. Prod. 2014;8(2) [Google Scholar]
- 47.Al-Kalaldeh J.Z., Abu-Dahab R., Afifi F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010;30(4):271–278. doi: 10.1016/j.nutres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Mahmod A.I., Talib W.H. Anticancer activity of Mandragora autumnalis: an in vitro and in vivo study. Pharmacia. 2021;(4):827–836. [Google Scholar]
- 49.Aburjai T., Hudaib M., Tayyem R., Yousef M., Qishawi M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007;110(2):294–304. doi: 10.1016/j.jep.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 50.Aburjai T., Mansi K., Azzam H., Alqudah D.A., Alshaer W., Abuirjei M. Chemical compositions and anticancer potential ofEssential oil from greenhouse-cultivated ocimum basilicum leaves. Indian J. Pharmaceut. Sci. 2020;82(1):179–184. [Google Scholar]
- 51.Yousef I., Oran S., Bustanji Y., Al-Eisawi D., Abu-Irmaileh B. Cytotoxic effect of selected wild medicinal plant species from Jordan on two different breast cancer cell lines, MCF7 and T47D. Biol. Med. 2018;10(4):443. [Google Scholar]
- 52.Awwad O., Abu-Dahab R., Abaza I.F., Alabbassi R., Majdalawi L., Afifi F.U. Effect of the galling aphid of Baizongia pistaciae L. on composition and biological activities of essential oils of Pistacia atlantica Desf. growing wild in Jordan. J. Essential Oil Bear. Plants. 2017;20(3):791–800. [Google Scholar]
- 53.Nawash O., Al-Assaf A., El-oqlah A., Omari M. Floristic features, distribution, and ethnobotany of plants gathered and used by local people from the Mediterranean forest in Northern Jordan. Ethnobot. J. 2014;12:385–396. [Google Scholar]
- 54.Kasabri V., Afifi F.U., Abu-Dahab R., Mhaidat N., Bustanji Y.K., Abaza I., Mashallah S. In vitro modulation of metabolic syndrome enzymes and proliferation of obesity related-colorectal cancer cell line panel by Salvia species from Jordan. Rev. Roum. Chem. 2014;59(59):693–705. [Google Scholar]
- 55.Aboalhaija N.H., Awwad O., Khalil E., Abbassi R., Abaza I.F., Afifi F.U. Chemodiversity and antiproliferative activity of the essential oil of Schinus molle growing in Jordan. Chem. Biodivers. 2019;16(11) doi: 10.1002/cbdv.201900388. [DOI] [PubMed] [Google Scholar]
- 56.Halees R.Y., Talib W.H., Issa R.A. Varthemia iphionoides and Pelargonium graveolens extracts as a treatment of breast cancer implanted in diabetic mice. Phcog. Mag. 2019;15(65):698. [Google Scholar]
- 57.Al-Salt J. Antimicrobial activity of crude extracts of some plant leaves. Res. J. Microbiol. 2012;7:59–67. [Google Scholar]
- 58.Jaber H.M., Al-Hamaideh K.D., Al-Daghistani H.I., Amer N.H., Nassar M.N., Al-Latif A., Saleh M., Al-Nuaimi A.H. Antibacterial activity and chemical composition of Arum hygrophilum boiss crude extracts. Jordan J. Biol. Sci. 2020;13(2) [Google Scholar]
- 59.Al-Daghistani H., Abu-Niaaj L., Bustanji Y., Al-Hamaideh K., Al-Salamat H., Nassar M., Jaber H., Amer N., Abu-Irmaileh B., Al-Nuaimi A. Antibacterial and cytotoxicity evaluation of Arum hygrophilum Bioss. Eur. Rev. Med. Pharmacol. Sci. 2021;25(23):7306–7316. doi: 10.26355/eurrev_202112_27424. [DOI] [PubMed] [Google Scholar]
- 60.Maher O. Antimicrobial activity of some medicinal plants against multidrug resistant skin pathogens. J. Med. Plants Res. 2011;5(16):3856–3860. [Google Scholar]
- 61.Abu-Qatouseh L., Mallah E., Mansour K. Evaluation of anti-propionibacterium acnes and anti-inflammatory effects of polyphenolic extracts of medicinal herbs in Jordan. Biomedical and Pharmacology Journal. 2019;12(1):211–217. [Google Scholar]
- 62.Alqudah A.A., Tarawneh K.A. Antibacterial and antioxidant activities of ethanol extracts of some plants selected from South Jordan. Phcog. J. 2021;13(2) [Google Scholar]
- 63.Tarawneh K., Irshaid F., Jaran A., Ezealarab M., Khleifat K. Evaluation of antibacterial and antioxidant activities of methanolic extracts of some medicinal plants in northern part of Jordan. J. Biol. Sci. 2010;10(4):325–332. [Google Scholar]
- 64.Abu-Darwish M.S., Al-Ramamneh E., Kyslychenko V.S., Karpiuk U.V. The antimicrobial activity of essential oils and extracts of some medicinal plants grown in Ash-shoubak region-South of Jordan. Pak. J. Pharm. Sci. 2012;25(1):239–246. [PubMed] [Google Scholar]
- 65.Qaralleh H.N., Abboud M.M., Khleifat K.M., Tarawneh K.A., Althunibat O.Y. Antibacterial activity in vitro of Thymus capitatus from Jordan. Pak. J. Pharm. Sci. 2009;22(3) [PubMed] [Google Scholar]
- 66.Al-Bakri A.G., Othman G., Afifi F.U. Determination of the antibiofilm, antiadhesive, and anti-MRSA activities of seven Salvia species. Phcog. Mag. 2010;6(24):264. doi: 10.4103/0973-1296.71786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdallah I., Amin A.A., Abdulrahim A. Effect of the methanol extracts of Salvia libanotica, Rosmarinus officinalis, Capparis spinosa and Achillea fragrantissima against two strains of Staphylococcus aureus. Afr. J. Microbiol. Res. 2013;7(29):3750–3753. [Google Scholar]
- 68.Khalil A., Hassawi D.S., Kharma A. Genetic relationship among Salvia species and antimicrobial activity of their crude extract against pathogenic bacteria. Asian J. Plant Sci. 2005;4(5):544–549. [Google Scholar]
- 69.Karak J. Discovering antimicrobial powers of some herbs used by Bedouin in the Jordanian Petra. Ecol. Environ. Conserv. 2020;26(1):433–440. [Google Scholar]
- 70.Irshaid F.I., Tarawneh K.A., Jacob J.H., Alshdefat A.M. Phenol content, antioxidant capacity and antibacterial activity of methanolic extracts derived from four Jordanian medicinal plants. Pakistan J. Biol. Sci.: PJBS. 2014;17(3):372–379. doi: 10.3923/pjbs.2014.372.379. [DOI] [PubMed] [Google Scholar]
- 71.Al-fawwaz A.T., Alsohaili S.A. Antimicrobial activity of Varthemia iphinoides and Majorana syriaca essential oils from Jordan and their potential use as natural food preservatives. J. Nat. Sci. Res. 2015;5(22):155. [Google Scholar]
- 72.Al-Zereini W.A., Al-Rimawi F., Abu-Lafi S., AlakHRAS F., Al-Mazaideh G.M., Salman H.J.A., Jamhour R.M. Identification and antibacterial evaluation of selected Jordanian medicinal plants. Orient. J. Chem. 2018;34(5):2456. [Google Scholar]
- 73.Frausin G., Lima R.B.S., Hidalgo A.d.F., Ming L., Pohlit A.M. Plants of the Araceae family for malaria and related diseases: a review. Rev. Bras. Plantas Med. 2015;17:657–666. [Google Scholar]
- 74.Afifi F.U., Abu-Dahab R. Phytochemical screening and biological activities of Eminium spiculatum (Blume) Kuntze (family Araceae) Nat. Prod. Res. 2012;26(9):878–882. doi: 10.1080/14786419.2011.565558. [DOI] [PubMed] [Google Scholar]
- 75.Alsalman A.-H., Aboalhaija N., Talib W., Abaza I., Afifi F. Evaluation of the single and combined antibacterial efficiency of the leaf essential oils of four common culinary herbs: dill, celery, coriander and fennel grown in Jordan. J. Essential Oil Bear. Plants. 2021;24(2):317–328. [Google Scholar]
- 76.Darwish R.M., Aburjai T., Al-Khalil S., Mahafzah A. Screening of antibiotic resistant inhibitors from local plant materials against two different strains of Staphylococcus aureus. J. Ethnopharmacol. 2002;79(3):359–364. doi: 10.1016/s0378-8741(01)00411-1. [DOI] [PubMed] [Google Scholar]
- 77.Dababneh B.F. Antimicrobial activity of selected Jordanian medicinal plant extracts against pathogenic microorganisms. J. Food Agric. Environ. 2008;6(2):134. [Google Scholar]
- 78.Al-Shudiefat M., Al-Khalidi K., Abaza I., Afifi F.U. Chemical composition analysis and antimicrobial screening of the essential oil of a rare plant from Jordan: Ducrosia flabellifolia. Anal. Lett. 2014;47(3):422–432. [Google Scholar]
- 79.Rolnik A., Olas B. The plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021;22(6):3009. doi: 10.3390/ijms22063009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saeidnia S., Gohari A., Mokhber-Dezfuli N., Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU: J. Facul. Pharm., Tehran Univ. Med. Sci. 2011;19(3):173. [PMC free article] [PubMed] [Google Scholar]
- 81.Hammad H.M., Matar S.A., Litescu S.-C., Abuhamdah S., Al-Jaber H.I., Afifi F.U. Biological activities of the hydro-alcoholic and aqueous extracts of Achillea fragrantissima (Forssk.) grown in Jordan. Nat. Sci. 2014;2014 [Google Scholar]
- 82.Hammad H.M., Litescu S.-C., Matar S.A., Al-Jaber H.I., Afifi F.U. Biological activities of the hydro-alcoholic and aqueous extracts of Achillea falcata L.(Asteraceae) grown in Jordan. Eur. J. Med. Plants. 2014;4(3):259–270. [Google Scholar]
- 83.Hana M.H., Camelia A., Suzan A.M., Simona-Carmen L., Hala I.A.J., Amal S.A., Fatma U.A. Biological activities of the hydro-alchoholic and aqueous extracts of Achillea biebersteinii Afan.(Asteraceae) grown in Jordan. African J. Pharm. Pharmacol. 2013;7(25):1686–1694. [Google Scholar]
- 84.Kharma A., Hassawi D. The antimicrobial activity and the genetic relationship of Achillea species. Biotechnology. 2006;5(4):501–507. [Google Scholar]
- 85.Bardaweel S.K., Hudaib M.M., Tawaha K.A., Bashatwah R.M. Studies on the in vitro antiproliferative, antimicrobial, antioxidant, and acetylcholinesterase inhibition activities associated with Chrysanthemum coronarium essential oil. Evid. base Compl. Alternative Med. 2015;2015 doi: 10.1155/2015/790838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abuhamdah S., Abuhamdah R., Al-Olimat S., Chazot P. Phytochemical investigations and antibacterial activity of selected medicinal plants from Jordan. Eur. J. Med. Plants. 2013;3(3):394–404. [Google Scholar]
- 87.Alfarrayeh I., Tarawneh K., Almajali D., Al-Awaida W. Evaluation of the antibacterial and antioxidant properties of the methanolic extracts of four medicinal plants selected from Wadi Al-Karak/Jordan related to their phenolic contents. Res. J. Pharm. Technol. 2022;15(5):2110–2116. [Google Scholar]
- 88.Talib W.H., Mahasneh A.M. Antimicrobial, cytotoxicity and phytochemical screening of Jordanian plants used in traditional medicine. Molecules. 2010;15(3):1811–1824. doi: 10.3390/molecules15031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Masadeh M.M., Alkofahi A.S., Alzoubi K.H., Tumah H.N., Bani-Hani K. Anti-Helicobactor pylori activity of some Jordanian medicinal plants. Pharmaceut. Biol. 2014;52(5):566–569. doi: 10.3109/13880209.2013.853811. [DOI] [PubMed] [Google Scholar]
- 90.Jaafreh M., Khleifat K., Qaralleh H., Al-limoun M. 2019. Antibacterial and Antioxidant Activities of Centeurea Damascena Methanolic Extract. arXiv preprint arXiv:1911.02243. [Google Scholar]
- 91.Bassam I.A., Khaled T., Mohammad M., Sundus M., Areej M.A. In vitro antimicrobial and anti-inflammatory activity of Jordanian plant extracts: a potential target therapy for acne vulgaris. African J. Pharm. Pharmacol. 2013;7(29):2087–2099. [Google Scholar]
- 92.Al-Hussaini R., Mahasneh A.M. Microbial growth and quorum sensing antagonist activities of herbal plants extracts. Molecules. 2009;14(9):3425–3435. doi: 10.3390/molecules14093425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maher O. Antimicrobial activities from extracts of seven medicinal plant species against multidrug-resistant bacteria and fungi. J. Pharmacogn. Phytotherapy. 2018;10(3):45–55. [Google Scholar]
- 94.Shatnawi M., Abubaker S., Odat N., Al-Tawaha A.R., Majdalawi M. Antimicrobial activity and micropropagation of some rare and endangered plants of Jordan. J. Ecol. Eng. 2021;22(6) [Google Scholar]
- 95.Alsohaili S.A., Sulaiman S.F. Phytochemical constituents, antioxidant, antibacterial and enzyme inhibition activities of essential oil from the aerial part of Achillea tomentosa L. grown in Jordan. J. Chil. Chem. Soc. 2021;66(1):5093–5097. [Google Scholar]
- 96.Ahmad F., Anwar F., Hira S. Review on medicinal importance of Fabaceae family. Pharmacol. Online. 2016;3:151–157. [Google Scholar]
- 97.Usman M., Khan W.R., Yousaf N., Akram S., Murtaza G., Kudus K.A., Ditta A., Rosli Z., Rajpar M.N., Nazre M. Exploring the phytochemicals and anti-cancer potential of the members of Fabaceae family: a comprehensive review. Molecules. 2022;27(12):3863. doi: 10.3390/molecules27123863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Etsassala N.G., Hussein A.A., Nchu F. Potential application of some Lamiaceae species in the management of diabetes. Plants. 2021;10(2):279. doi: 10.3390/plants10020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Althunibat O.Y., Qaralleh H., Al-Dalin S.Y.A., Abboud M., Khleifat K., Majali I.S., Aldal'in H., Rayyan W.A., Jaafraa A. Effect of thymol and carvacrol, the major components of Thymus capitatus on the growth of Pseudomonas aeruginosa. J. Pure Appl. Microbiol. 2016;10(1):367–374. [Google Scholar]
- 100.Karak J. Discovering antimicrobial powers of some herbs used by Bedouin in the Jordanian Petra. Ecol. Environ. Conserv. 2020;26(1):433–440. [Google Scholar]
- 101.Rana Z., Sawsan O., Khaled K., Suzan M. Antimicrobial activity of leaf and fruit extracts of Jordanian Rubus sanguineus Friv.(Rosaceae) Afr. J. Microbiol. Res. 2013;7(44):5114–5118. [Google Scholar]
- 102.Afifi F.U., Aburjai T. Antiplatelet activity of Varthemia iphionoides. Fitoterapia. 2004;75(7-8):629–633. doi: 10.1016/j.fitote.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 103.Aburjai T.A. Anti-platelet stilbenes from aerial parts of Rheum palaestinum. Phytochemistry. 2000;55(5):407–410. doi: 10.1016/s0031-9422(00)00341-1. [DOI] [PubMed] [Google Scholar]
- 104.Halabi S., Battah A., Aburjai T., Hudaib M. Phytochemical and antiplatelet investigation of Gundelia tournifortii. Pharmaceut. Biol. 2005;43(6):496–500. [Google Scholar]
- 105.Alali F.Q., Tawaha K., Shehadeh M.B., Telfah S. Phytochemical and biological investigation of Aristolochia maurorum L. Z. Naturforsch. C Biosci. 2006;61(9-10):685–691. doi: 10.1515/znc-2006-9-1013. [DOI] [PubMed] [Google Scholar]
- 106.Shehadeh M.B., Afifi F.U., Abu-Hamdah S.M. Platelet aggregation inhibitors from aerial parts of Ruta chalepensis grown in Jordan. Integr. Med. Insights. 2007;2 [Google Scholar]
- 107.Al-Jaber H.I., Hammad H.M., Al-Qudah M.A., Abaza I.F., Al-Humaidi J.Y., Abu-Zarga M.H., Afifi F.U. Volatile oil composition and antiplatelet activity of Jordanian Achillea biebersteinii collected at different growth stages. J. Essential Oil Bear. Plants. 2014;17(4):584–598. [Google Scholar]
- 108.Al-Aboudi A., Afifi F.U. Plants used for the treatment of diabetes in Jordan: a review of scientific evidence. Pharmaceut. Biol. 2011;49(3):221–239. doi: 10.3109/13880209.2010.501802. [DOI] [PubMed] [Google Scholar]
- 109.Kasabri V., Al-Hallaq E.K., Bustanji Y.K., Abdul-Razzak K.K., Abaza I.F., Afifi F.U. Antiobesity and antihyperglycaemic effects of Adiantum capillus-veneris extracts: in vitro and in vivo evaluations. Pharmaceut. Biol. 2017;55(1):164–172. doi: 10.1080/13880209.2016.1233567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Al-Hallaq E.K., Kasabri V., Abdalla S.S., Bustanji Y.K., Afifi F.U. Anti-obesity and antihyperglycemic effects of Crataegus aronia extracts: in vitro and in vivo evaluations. Food Nutr. Sci. 2013;4:972–983. [Google Scholar]
- 111.Afifi F.U., Kasabri V., Litescu S.C., Abaza I.M. In vitro and in vivo comparison of the biological activities of two traditionally and widely used Arum species from Jordan: Arum dioscoridis Sibth & Sm. and Arum palaestinum Boiss. Nat. Prod. Res. 2016;30(16):1777–1786. doi: 10.1080/14786419.2015.1072713. [DOI] [PubMed] [Google Scholar]
- 112.Afifi F.U., Kasabri V., Beltran S., Abuhammad A., Abaza I.F., Ganado O., Al-Gabbiesh A.H. Comparison of different methods in determination of essential oil composition of Origaum syriacum L. from Jordan and its modulation of pancreatic enzymes. Rev. Roum. Chem. 2017;62(1):15–21. [Google Scholar]
- 113.Kasabri V., Afifi F.U., Hamdan I. Evaluation of the acute antihyperglycemic effects of four selected indigenous plants from Jordan used in traditional medicine. Pharmaceut. Biol. 2011;49(7):687–695. doi: 10.3109/13880209.2010.539619. [DOI] [PubMed] [Google Scholar]
- 114.Afifi F.U., Kasabri V., Litescu S., Abaza I.F., Tawaha K. Phytochemical and biological evaluations of Arum hygrophilum boiss.(Araceae) Phcog. Mag. 2017;13(50):275. doi: 10.4103/0973-1296.204551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamdan I.I., Afifi F.U. Screening of Jordanian flora for α-amylase inhibitory activity. Pharmaceut. Biol. 2008;46(10-11):746–750. [Google Scholar]
- 116.Abu Soud R.S., Hamdan L.I., Afifi F.U. Alpha amylase inhibitory activity of some plant extracts with hyporrlycemic activity. Sci. Pharm. 2004;72(1):25–33. [Google Scholar]
- 117.Kasabri V., Abu-Dahab R., Afifi F.U., Naffa R., Majdalawi L. Modulation of pancreatic MIN6 insulin secretion and proliferation and extrapancreatic glucose absorption with Achillea santolina, Eryngium creticum and Pistacia atlantica extracts: in vitro evaluation. J. Exp. Integr. Med. 2012;2:245–254. [Google Scholar]
- 118.Kasabri V., Abu-Dahab R., Afifi F.U., Naffa R., Majdalawi L., Shawash H. In vitro effects of Geranium graveolens, Sarcopoterium spinosum and Varthemia iphionoides extracts on pancreatic MIN6 proliferation and insulin secretion and on extrapancreatic glucose diffusion. Int. J. Diabetes Dev. Ctries. 2013;33(3):170–177. [Google Scholar]
- 119.Mansi K. The hypoglycaemic and hypolipidemic effects of aqueous extract of Alkanna strigosa in alloxan induced diabetic rats. J. Dis. Med. Plants. 2019;5(4):60. [Google Scholar]
- 120.Afifi F.U., Kasabri V. Pharmacological and phytochemical appraisal of selected medicinal plants from Jordan with claimed antidiabetic activities. Sci. Pharm. 2013;81(4):889–932. doi: 10.3797/scipharm.1212-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arabiyat S., Al-Rabi'ee A., Zalloum H., Hudaib M., Mohammad M., Bustanji Y. Antilipolytic and hypotriglyceridemic effects of dietary Salvia triloba Lf (Lamiaceae) in experimental rats. Trop. J. Pharmaceut. Res. 2016;15(4):723–728. [Google Scholar]
- 122.Ala I., Mohammad M., Mohammad H., Khalid T., Talal A.R., Sawsan O., Yasser B. A potential role of Lavandula angustifolia in the management of diabetic dyslipidemia. J. Med. Plants Res. 2011;5(16):3876–3882. [Google Scholar]
- 123.Bustanji Y., Mohammad M., Hudaib M., Tawaha K., Al-Masri I.M., AlKhatib H.S., Issa A., Alali F.Q. Screening of some medicinal plants for their pancreatic lipase inhibitory potential. Jordan J. Pharmaceut. Sci. 2011;4(2):81–88. [Google Scholar]
- 124.Yasser B., Ala I., Mohammad M., Mohammad H., Khalid T., Hatim A., Ihab A., Bashar A.-K. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J. Med. Plants Res. 2010;4(21):2235–2242. [Google Scholar]
- 125.Alkofahi A., Atta A. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J. Ethnopharmacol. 1999;67(3):341–345. doi: 10.1016/s0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 126.Mayyas A., Abu-Sini M., Amr R., Akasheh R.T., Zalloum W., Khdair A., Hamad I., Aburjai T., Darwish R.M., Abu-Qatouseh L. Novel in vitro and in vivo anti-Helicobacter pylori effects of pomegranate peel ethanol extract. Vet. World. 2021;14(1):120. doi: 10.14202/vetworld.2021.120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Al-Dissi N.M., Salhab A.S., Al-Hajj H.A. Effects of Inula viscosa leaf extracts on abortion and implantation in rats. J. Ethnopharmacol. 2001;77(1):117–121. doi: 10.1016/s0378-8741(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 128.Hijazi A.M., Salhab A.S. Effects of Artemisia monosperma ethanolic leaves extract on implantation, mid-term abortion and parturition of pregnant rats. J. Ethnopharmacol. 2010;128(2):446–451. doi: 10.1016/j.jep.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 129.Abu-Odeh A.M., Talib W.H. Middle East medicinal plants in the treatment of diabetes: a review. Molecules. 2021;26(3):742. doi: 10.3390/molecules26030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hudaib M.M., Tawaha K.A., Mohammad M.K., Assaf A.M., Issa A.Y., Alali F.Q., Aburjai T.A., Bustanji Y.K. Xanthine oxidase inhibitory activity of the methanolic extracts of selected Jordanian medicinal plants. Phcog. Mag. 2011;7(28):320. doi: 10.4103/0973-1296.90413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abudoleh S., Disi A., Qunaibi E., Aburjai T. Anti-arthritic activity of the methanolic leaf extract of Urtica pilulifera L. on albino rats. Am. J. Pharmacol. Toxicol. 2011;6:27–32. [Google Scholar]
- 132.Al-Jaber H.I., Abu Zarga M.H., Al-Aboudi A.F., Al-Qudah M.A., Al-Shawabkeh A.F., Abaza I.F., Abuaisheh M.N., Afifi F.U. Essential oil composition and anticholinesterase activity evaluation of Achillea fragrantissima growing wild in Jordan. J. Herbs, Spices, Med. Plants. 2018;24(3):272–281. [Google Scholar]
- 133.Aboalhaija A., Amro R., Abaza I., Khalil E., Al-Aboudi A., Abu-Zarga M., Afifi F. Schinus molle L. Collected from Jordan and Turkey: essential oil composition and anticholinesterase activity. J. Essential Oil Bear. Plants. 2019;22(3):704–716. [Google Scholar]
- 134.Rashed A., Afifi F., Disi A. Simple evaluation of the wound healing activity of a crude extract of Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J. Ethnopharmacol. 2003;88(2-3):131–136. doi: 10.1016/s0378-8741(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 135.Aburjai T., Al-Janabi R., Al-Mamoori F., Azzam H. In vivo wound healing and antimicrobial activity of Alkanna strigose. Wound Med. 2019;25(1) [Google Scholar]
- 136.Khalil E.A., Afifi F.U., Al-Hussaini M. Evaluation of the wound healing effect of some Jordanian traditional medicinal plants formulated in Pluronic F127 using mice (Mus musculus) J. Ethnopharmacol. 2007;109(1):104–112. doi: 10.1016/j.jep.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 137.Aboalhaija N., Afifi F., Al-Hussaini M., Al-Najjar M., Abu-Dahab R., Hasen E., Rashed M., Haq S.A., Khalil E. In vitro and in vivo evaluation of the wound healing potential of the extracts of Schinus molle L.(Anacardiaceae) grown in Jordan. Indian J. Pharmaceut. Sci. 2021;83(2):261–270. [Google Scholar]
- 138.Abbas M., Disi A., Al-Khalil S. Isolation and Identification of anti-ulcer components from Anchusa strigosa root. Jordan J. Pharm. Sci. 2009;2(2) [Google Scholar]
- 139.Alzoubi K.H., Malkawi B.S., Khabour O.F., El-Elimat T., Alali F.Q. Arbutus andrachne L. reverses sleep deprivation-induced memory impairments in rats. Mol. Neurobiol. 2018;55(2):1150–1156. doi: 10.1007/s12035-017-0387-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.