Abstract
Honey is a valuable source of nutrients, minerals and phenolic compounds. Phenolic acids and flavonoids are associated with health benefits of honey and can serve as markers for distinguishing honey types. This study aimed at determining the phenolic profile of four Hungarian unifloral honeys that were not analyzed previously. After verifying their botanical origin with melissopalynological analysis, total reducing capacity was determined with Folin-Ciocalteau method, and phenolic composition was analyzed with HPLC-DAD-MS. From the 25 phenolic substances examined, pinobanksin was the most abundant, followed by chrysin, p-hydroxybenzoic acid and galangin. Quercetin and p-syringaldehyde were detected only in acacia honey, which contained higher levels of chrysin and hesperetin compared to the other three honeys. Milkweed and linden honeys displayed higher levels of caffeic, chlorogenic, ferulic and p-coumaric acids compared to acacia and goldenrod honeys. Taxifolin may serve as a unique marker compound of milkweed honey. Goldenrod honey contained the highest level of syringic acid. Principal component analysis supported the indicator role of polyphenols in honey identification, discriminating clearly the four unifloral honeys. Our results suggest that phenolic profiles may be useful to find markers of honey's floral origin, but geographical origin can strongly influence the composition of characteristic compounds.
Keywords: Phenolic profile, HPLC, Melissopalynology, Flavonoid, Marker, Principal component analysis
Graphical abstract
Highlights
-
•
20 polyphenolic compounds were identified and quantified in 4 Hungarian honeys.
-
•
Pinobanksin was the most abundant flavonoid component in Hungarian honeys.
-
•
Quercetin and p-syringaldehyde may be used as marker compounds of acacia honey.
-
•
Polyphenol amounts were similar in low TRC milkweed and high TRC goldenrod honey.
-
•
PCA supported that polyphenols can aid identification and differentiation of honeys.
1. Introduction
Honey, as a food product of natural origin, provides both nutritional and health benefits, being a good source of carbohydrates, amino acids, organic acids and minerals (Ranneh et al., 2021). The beneficial effects of honey, including its antioxidant, antimicrobial and anti-inflammatory potential can be associated with phenolic compounds (Cianciosi et al., 2018; Ranneh et al., 2021; Feknous and Boumendjel 2022), which at the same time can serve as marker compounds for distinguishing various honey types (Cheung et al., 2019; Becerril-Sánchez et al., 2021).
The phenolic profiles of acacia and linden honeys, which are commonly available in Europe, were determined in samples from France, Germany, Italy, Norway, Poland, Romania, Serbia and Sweden (Tomás-Barberán et al., 2001; Kečkeš et al., 2013; Salonen et al., 2017; Di Marco et al., 2018; Bobiş et al., 2021; Kędzierska-Matysek et al., 2021). The phenolic compounds and antioxidant activity of goldenrod honey were investigated in Serbia and Poland (Kečkeš et al., 2013; Jasicka-Misiak et al., 2018; Halagarda et al., 2020; Dżugan et al., 2022). Hungary is known to produce several types of high quality honeys, being an important honey supplier in the European market. In the past decade various research groups characterized the physicochemical properties (Czipa et al., 2019), mineral composition (Czipa et al., 2015; Ördög et al., 2017; Sajtos et al., 2019; Varga et al., 2020; Bodó et al., 2021; Farkas et al., 2022; Kocsis et al., 2022), total phenolic content (TPC) or total reducing capacity (TRC) and antioxidant activity (Bodor et al., 2018; Czipa et al., 2019; Kiss et al., 2019; Bodó et al., 2020, 2021; Czigle et al., 2022; Farkas et al., 2022; Kocsis et al., 2022) of Hungarian acacia, linden, goldenrod and milkweed honeys. However, data are lacking regarding their phenolic compound profile. The present study aimed at assessing the polyphenol content and composition of the above four unifloral honey types from Hungary, which could contribute to their proper identification and the appropriate evaluation of their quality.
2. Materials and methods
2.1. Reagents
The phenolic compounds apigenin, caffeic acid, chlorogenic acid, chrysin, eriodictyol, ferulic acid, galangin, gallic acid, gentisic acid, hesperetin, isorhamnetin, kaempferol, luteolin, naringenin, naringin, p-coumaric acid, p-hydroxybenzoic acid, p-syringaldehyde, pinobanksin, protocatechualdehyde, quercetin, quercitrin, syringic acid, taxifolin and vanillic acid, as well as the Folin-Ciocalteau reagent and anhydrous sodium carbonate which were used to measure total reducing capacity, were purchased from Merck Life Science Ltd., Darmstadt, Germany. The LC-MS grade solvents water, acetonitrile, methanol and acetic acid (≥99.7%) were bought from Honeywell International Ltd., Budapest, Hungary.
2.2. Honey samples
The honey samples were purchased from three local apiaries in Hungary; acacia (Robinia pseudoacacia), linden (Tilia spp.) and goldenrod (Solidago gigantea) honeys were harvested in the Southwest Transdanubium area, while milkweed (Asclepias syriaca) honey originated from the Southern Great Plain area. They were stored at room temperature (20–21 °C) in the dark for a maximum of three weeks. For each honey type (Table 1), measurements were carried out on 3 parallel samples; altogether, 12 honey samples were analyzed.
Table 1.
Sensory characteristics, color and TRC of analyzed honey samples.
| Nr. | Honey Type, Plant Name | Dominant pollen type (%) | Sensory Characteristics (Odor and Consistency) | ABS450-720 (mAU) | TRC (mg GAE kg-1) |
|---|---|---|---|---|---|
| 1 | Acacia, Robinia pseudoacacia | Robinia 59.9% | Pale, yellowish green, weak odor, liquid, viscous | 100 ± 8a | 149.9 ± 35a |
| 2 | Milkweed, Asclepias syriaca | Robinia 76.3% Brassica 23.7% | Light yellowish amber, intense flower-like odor, viscous | 215 ± 3b | 187.6 ± 62ac |
| 3 | Linden, Tilia spp. | Tilia 54.9% | Light amber, strong odor, fine granulated, semisolid | 303 ± 5c | 312.3 ± 33b |
| 4 | Goldenrod, Solidago gigantea | Solidago 42.7% | Amber, moderately intense odor, semisolid, fine granulated | 415 ± 4d | 280.2 ± 62bc |
ABS450-720: absorbance of diluted honey samples referring to their color; TRC: Total Reducing Capacity. Each code number in the first column represents three biological replicates (n = 3) of honey samples. Data in the same column with different superscripted letters mean significant differences among various honeys according to Student's t-test (p < 0.01 (ABS) and p < 0.5 (TRC)).
2.3. Sensory evaluation of honeys
From each honey type, 3 separate jars were evaluated, characterizing their color, odor, consistency and degree of crystallization. Color was described with terms ranging from pale yellow to dark amber. Odor was characterized as weak, moderate or intense, in some cases adding specific characters such as flower-like. Consistency was described as liquid, viscous or semisolid. If crystallization was observed, its degree and the size of crystals was mentioned, e.g. fine granulated.
2.4. Melissopalynological analysis
The floral sources of honey samples were determined with microscopic pollen analysis, based on the method of (Von Der Ohe et al., 2004). Pollen preparations were examined with a Nikon Eclipse E200 light microscope equipped with Michrome 20 MP CMOS digital camera (Auro-Science Consulting Ltd., Budapest, Hungary), using Capture 1.2 software. From each honey sample, a minimum of 500 pollen grains were identified at the level of plant species, genus or family. The relative frequency of pollen types was calculated as the percentage of total pollen grain number.
2.5. Determination of color intensity (ABS450)
Color intensity was determined following the protocol of (Beretta et al., 2005). Honey solutions (50% w/v) were prepared with 45–50 °C water, sonicated for 5 min, then filtered (0.45 μm pore size, Agilent Technologies, Milan, Italy). Absorbance was measured at 450 and 720 nm with a Shimadzu UV-1800 spectrophotometer (Shimadzu Schweiz GmbH, Reinach, Switzerland). Color intensity was calculated as the difference between absorbance at 450 and 720 nm, and results were expressed as milli-absorbance unit (mAU).
2.6. Determination of total reducing capacity (TRC)
TRC was determined using the Folin–Ciocalteau method (Singleton et al., 1999) with minor modifications. Honey solutions were prepared by adding 1 mL distilled water to 0.1 g of the honey sample. From this solution 0.5 mL was taken, and we added 100 μL 10% (v/v) Folin–Ciocalteau reagent, 300 μL distilled water and 400 μL 6% (w/v) Na2CO3 solution. After 20 min incubation, absorbance was measured at 760 nm with a Shimadzu UV-1800 spectrophotometer. The results were expressed as mg of gallic acid per kg of honey (mg GAE kg−1). Gallic acid solutions in the range of 50–200 μg mL−1 were used as standard to establish the calibration curve.
2.7. Sample preparation for HPLC-DAD-MS measurement
From each honey sample 10 g was diluted with 10 mL of 2% (w/v) sodium chloride solution, and was extracted five times with 10 mL of ethyl acetate. Organic fractions were collected and ethyl acetate was evaporated with a Rotavapor R-3 rotary evaporator equipped with V-700 vacuum pump (Büchi, Donau Lab Ltd, Budapest, Hungary) at 40 °C. Afterwards, concentrated samples were dissolved in the mixture of methanol and 2.5 mM acetic acid (50/50, v/v) by vortex homogenization, then centrifuged at 18000 rpm for 5 min at room temperature (20–21 °C) with an Eppendorf Centrifuge 5430 R. 5 μL of the supernatant was analyzed by HPLC-DAD-MS.
2.8. Quantification of phenolic compounds using HPLC-DAD-MS
Phenolic compounds of the extracts were analyzed with high performance liquid chromatography (Waters Alliance 2695) coupled with a photodiode array detector (Waters 2996 PDA) and a single quadrupole detector (Waters SQD).
The chromatographic separation was performed at 40 °C on a Kinetex F5 (Phenomenex) analytical column (2.5 μm, 3.0 × 100 mm) using gradient elution. The gradient consisted of solvent A (2.0 mM acetic acid) and solvent B (2.0 mM acetic acid/acetonitrile, 25/75, v/v) applied at a flow rate of 0.4 mL/min as follows: from 99% A at 0 min to 20% A at 25 min in a linear gradient; from 20% A at 25 min to 0% A at 25.5 min in a linear gradient; from 0% A at 25.5 min to 99% A at 26 min in a linear gradient. The injected volume was 5 μL.
The photodiode array scan range was set from 210 to 600 nm. The mass spectrometer scan range was set from 30 to 800 m/z both in negative and positive ion modes. Compounds were identified by comparing their retention times, UV spectra and mass spectra with those of standards and were quantified using external standard calibration curves.
2.9. Statistical analysis
Statistical analyses were carried out using Excel® (Microsoft Corp., Redmond, WA, USA) and the PAST software package version 3.11 (Hammer et al., 2001) at a 5% or 0.1% significance level (p < 0.05, p < 0.001), after normality checking with the Shapiro–Wilk test. Data were expressed as means ± standard deviations (SD). Pairwise comparisons were performed with Student's t-tests. To describe relatedness among honey types, a centered and standardized principal component analysis (PCA) was performed.
3. Results and discussion
3.1. Sensory characteristics, color, TRC and pollen analysis of honey samples
The sensory characteristics of each honey sample corresponded to the descriptions of the given honey type (Oddo et al., 2004). The lightest colored honey was acacia, whereas the darkest was goldenrod honey, reflected by the increasing order of color intensity acacia < milkweed < linden < goldenrod (Table 1). The criterion of the dominant pollen type being Robinia, Tilia, and Solidago was met by acacia, linden and goldenrod honeys, respectively. Based on the above, these three honey samples were declared as true unifloral honeys. In milkweed honey, as expected, no Asclepias pollinia were observed, but Robinia pollen grains were present in high percentage. However, based on sensory characters and the beekeeper's declaration that bee hives were kept in the vicinity of Asclepias stands, milkweed honey was also treated as unifloral honey. Total reducing capacity (TRC), which is frequently referred to as total polyphenol content (TPC), is often associated with the color of honey (Combarros-Fuertes et al., 2018; Halagarda et al., 2020; Bodó et al., 2021; Hunter et al., 2021). This was true for our light colored acacia and milkweed honeys, but the TRC of linden honey with lower color intensity value was higher than that of goldenrod honey with higher ABS value.
3.2. Phenolic compound profiles and principal component analysis of honey samples
From the 25 phenolic substances examined, each honey sample contained apigenin, caffeic acid, chrysin, ferulic acid, galangin, hesperetin, kaempferol, naringenin, p-coumaric acid, p-hydroxybenzoic acid, pinobanksin, protocatechualdehyde, syringic acid and vanillic acid; whereas eriodictyol, gallic acid, isorhamnetin, luteolin and naringin was not detected in any of the samples. Chlorogenic acid, gentisic acid, p-syringaldehyde, quercetin, quercitrin and taxifolin were present only in some of the honey samples (Table 2). The most abundant component in our samples was pinobanksin, followed by chrysin, p-hydroxybenzoic acid and galangin.
Table 2.
Phenolic components in honey samples (μg/g).
| Phenolic compound | Acacia | Milkweed | Linden | Goldenrod |
|---|---|---|---|---|
| apigenin | 1.43 ± 0.10 a | 2.82 ± 0.40 b | 1.21 ± 0.21 a | 1.29 ± 0.22 a |
| caffeic acid | 0.64 ± 0,16 a | 5.79 ± 0.81 b | 6.81 ± 1.28 b | 1.69 ± 0.42 c |
| chlorogenic acid | – | 1.08 ± 0.10 a | 0.36 ± 0.14 b | – |
| chrysin | 10.58 ± 0.19 a | 8.76 ± 0.73 b | 8.13 ± 0.26 b | 7.12 ± 0.17 c |
| ferulic acid | 0.58 ± 0.09 a | 1.72 ± 0.19 b | 3.80 ± 0.33 c | 0.73 ± 0.09 a |
| galangin | 4.25 ± 0.45 ab | 3.95 ± 0.13 a | 3.38 ± 0.32 bc | 2.88 ± 0.24 c |
| gentisic acid | – | 0.64 ± 0.41 a | 0.12 | 0.31 ± 0.08 b |
| hesperetin | 1.69 ± 0.10 a | 0.98 ± 0.05 b | 0.92 ± 0.32 b | 0.34 ± 0.06 c |
| kaempferol | 0.88 ± 0.09 a | 2.03 ± 0.23 bc | 1.66 ± 0.19 b | 2.36 ± 0.23 c |
| naringenin | 0.33 ± 0.02 ab | 0.39 ± 0.04 a | 0.42 ± 0.13 ab | 0.28 ± 0.03 b |
| p-coumaric acid | 1.68 ± 0.42 a | 6.14 ± 0.58 b | 7.30 ± 1.18 b | 2.74 ± 0.30 c |
| p-hydroxybenzoic acid | 5.41 ± 0.28 a | 9.70 ± 0.28 b | 7.19 ± 0.43 c | 8.43 ± 1.31 bc |
| p-syringaldehyde | 0.54 ± 0.07 | – | – | – |
| pinobanksin | 13.76 ± 0.40 a | 14.14 ± 2.14 ab | 10.90 ± 1.17 bc | 9.93 ± 1.33 c |
| protocatechu-aldehyde | 0.60 ± 0.18 a | 1.12 ± 0.35 a | 0.81 ± 0.15 a | 0.72 ± 0.29 a |
| quercetin | 2.71 ± 0.82 | – | – | – |
| quercitrin | – | 0.11 | 0.19 ± 0.03 | – |
| syringic acid | 0.44 ± 0.12 a | 0.24 ± 0.14 a | 0.23 ± 0.15 a | 0.55 ± 0.08 b |
| taxifolin | – | 0.21 ± 0.11 | – | – |
| vanillic acid | 0.40 ± 0.04 a | 0.95 ± 0.18 b | 0.83 ± 0.24 b | 0.63 ± 0.19 ab |
| Total | 52.7 ± 3.5a | 66.5 ± 3.7b | 59.3 ± 3.2ab | 64.2 ± 5.2b |
Data are means ± standard deviations of three independent determinations (n = 3). Mean values of phenolic content with different superscripted letters (a-c) in the same row mean significant differences among various honeys according to Student's t-test (p < 0.01).
Acacia honey contained higher levels of chrysin and hesperetin compared to the other three honey types. From the set of four Hungarian honeys, quercetin and p-syringaldehyde were detected only in acacia honey. Quercetin was measured also in Polish acacia honey (Kędzierska-Matysek et al., 2021), but it was not detected in Romanian and Serbian acacia honeys, rather it was proposed as a phenolic marker of sunflower honey (Tomás-Barberán et al., 2001; Kečkeš et al., 2013; Di Marco et al., 2018; Bobiş et al., 2021). Similar to our findings, kaempferol was one of the characteristic compounds of German, Polish, Serbian and Italian acacia honey. Italian samples examined by (Di Marco et al., 2018) did not contain apigenin, caffeic acid and p-coumaric acid, in contrast to Hungarian and Serbian acacia honey. It has to be noted that a different set of Italian acacia honeys, along with German and French samples, contained caffeic acid, ferulic acid, p-coumaric acid, chrysin, kaempferol, pinobanksin and quercetin (Tomás-Barberán et al., 2001), similar to our findings. Polish acacia honeys were characterized by similar phenolic acid composition as Hungarian samples, except for the absence of caffeic acid (Kędzierska-Matysek et al., 2021). Chlorogenic acid was not detected in either Hungarian, nor Italian acacia samples (Tomás-Barberán et al., 2001; Kečkeš et al., 2013; Di Marco et al., 2018).
Our milkweed and linden honey samples displayed significantly higher levels of caffeic, chlorogenic, ferulic and p-coumaric acids compared to acacia and goldenrod honeys; ferulic acid reaching its highest concentration in linden honey from the four honey types studied. In contrast to Hungarian and Serbian linden honeys, Italian linden honey did not contain caffeic acid, chlorogenic acid and p-coumaric acid, all of which reached high concentrations in our linden honey sample. Caffeic, chlorogenic and vanillic acid were missing also from Norwegian and Swedish linden honeys, but they contained ferulic and p-coumaric acid, similarly to our samples. Apigenin was detected in Hungarian, Italian, Nordic and Serbian linden samples, but not in Polish samples; while kaempferol was detected in Hungarian, Italian and Polish linden samples, but not in Nordic and Serbian ones. Chrysin and galangin were measured in both Hungarian and Serbian linden samples. Quercetin was present only in the Italian sample (Kečkeš et al., 2013; Salonen et al., 2017; Di Marco et al., 2018; Kędzierska-Matysek et al., 2021).
The polyphenol composition of milkweed honey was analyzed for the first time in our study. It was characterized by higher levels of apigenin, chlorogenic acid, kaempferol and p-hydroxybenzoic acid compared to the other three honey samples. Taxifolin, which was present only in milkweed honey, may serve as a unique marker compound of this honey type.
Hungarian, Polish and Serbian goldenrod honeys each contained the phenolic acids caffeic acid and p-hydroxybenzoic acid; and the flavonoids apigenin, chrysin, galangin and kaempferol (Kečkeš et al., 2013; Jasicka-Misiak et al., 2018; Halagarda et al., 2020). In addition, ferulic acid, p-coumaric acid, vanillic acid and naringenin were present in Hungarian and Polish samples (Jasicka-Misiak et al., 2018; Halagarda et al., 2020). In contrast, p-coumaric acid was not detected in Serbian goldenrod honey (Kečkeš et al., 2013). Quercetin was measured in Polish and Serbian goldenrod samples (Kečkeš et al., 2013; Jasicka-Misiak et al., 2018; Halagarda et al., 2020), but not in Hungarian ones. The phenolic acids chlorogenic acid, cinnamic acid, ellagic acid, gallic acid, 3,4-dihydroxybenzoic acid and trans-ferulic acid; and the flavonoids epicatechin, myricetin, genistein and pinocembrin were detected in goldenrod honeys from Poland (Jasicka-Misiak et al., 2018; Halagarda et al., 2020).
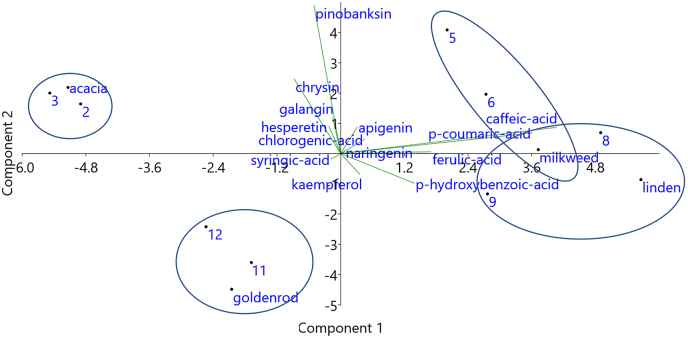
Principal component analysis interpreted the indicator role of the studied polyphenolic components without the specific ones (p-syringaldehide and quercetin in acacia honey and taxifolin in milkweed honey) in identification and differentiation of honey types and demonstrate well the above findings (Fig. 1). The first two main components explained 83.6% of the total variance, with the first component (PC1) 59.5% and the second one (PC2) 24.1%. The biplot gave the clear discrimination of the four unifloral honeys studied. Milkweed and linden honeys were located close to each other, on the positive PC1 based on their relatively high caffeic, chlorogenic, ferulic and p-coumaric acid content. Chrysin and hesperetin were useful in clustering acacia honey on the negative PC1, while goldenrod was characterized by negative PC1 and PC2 values of the plot. A number of attempts have been made to separate honey types from each other using PCA of various parameters besides polyphenolic compounds with more or less success (Di Marco et al., 2018; Nascimento et al., 2018; Halagarda et al., 2020). In our case the polyphenolic fingerprint of the studied honeys offered valuable information about the role of bioactive components in distinguishing honey types.
Fig. 1.
Principal component analysis based on the phenolic composition of Hungarian acacia, milkweed, linden and goldenrod honeys.
Our analysis of the phenolic profile of Hungarian unifloral honeys revealed that the phenolic acid and flavonoid composition of acacia, linden and goldenrod honeys was only partially overlapping with results obtained for the same honey types in different European countries. In several instances, the floral markers suggested by other research groups in different countries were not confirmed in our study; while in other cases we measured phenolic compounds that could not be detected in other European samples of the same honey type. In lack of other European data, the polyphenol composition of Hungarian milkweed honey could not be compared with that of samples from different countries.
Our study highlighted that further studies are necessary to examine the phenolic profile of the same honey type from different countries and years of collection. (Jasicka-Misiak et al., 2018) and (Escriche et al., 2014) reported that the year in which the honey was harvested can significantly influence the level of some phenolic compounds, e.g. gallic acid, p-hydroxybenzoic acid, p-coumaric acid and cinnamic acid, and the concentration of these compounds decreased with the age of honeys. However (Escriche et al., 2014), pointed out that the year of collection did not alter the phenolic composition to such extent that would interfere with discriminating honeys of diverse botanical origin.
Phenolic compounds are frequently associated with health benefits of honey (Ranneh et al., 2021; Feknous and Boumendjel 2022). For example, caffeic, cinnamic, phenyllactic and syringic acid, and the flavonoids chrysin, galangin, pinocembrin and pinobanksin are thought to be responsible for the antibacterial and/or antioxidant activity of manuka honey (Weston et al., 1999; Gheldof et al., 2002). Thus, different levels of particular components may explain differences in the biological activity of various honey types. Further studies are necessary to determine which phenolic compounds are responsible for the various health benefits of acacia, milkweed, linden and goldenrod honeys.
4. Conclusions
Our results suggest that phenolic profiles of honeys may be useful to find markers of their floral origin, but the country or region of origin can strongly influence the presence or absence of characteristic compounds. The unique combination of phenolic substances can be suitable for authenticating certain honey types, but this is not true for all kinds of honey. The variation in the composition of phenolic substances may contribute to differences between various honey types regarding their health benefits.
Funding
Financial support of the study was provided by a grant from the National Research, Development and Innovation Office; NKFIH K 132044.
CRediT authorship contribution statement
Ágnes Farkas: Conceptualization, Resources, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition. Györgyi Horváth: Writing – review & editing. Mónika Kuzma: Methodology, Investigation, Validation, Writing – review & editing. Mátyás Mayer: Methodology, Investigation, Validation. Marianna Kocsis: Conceptualization, Formal analysis, Methodology, Visualization, Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Agnes Farkas reports financial support was provided by National Research Development and Innovation Office.
Acknowledgements
This work was supported by a grant from the National Research, Development and Innovation Office; NKFIH K 132044.
Handling Editor: Professor A.G. Marangoni
Contributor Information
Ágnes Farkas, Email: agnes.farkas@aok.pte.hu.
Györgyi Horváth, Email: horvath.gyorgyi@gytk.pte.hu.
Mónika Kuzma, Email: monika.kuzma@aok.pte.hu.
Mátyás Mayer, Email: matyas.mayer@aok.pte.hu.
Marianna Kocsis, Email: kocsis.marianna@pte.hu.
Data availability
Data will be made available on request.
References
- Becerril-Sánchez A.L., Quintero-Salazar B., Dublán-García O., Escalona-Buendía H.B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants. 2021;10:1700. doi: 10.3390/antiox10111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta G., Granata P., Ferrero M., et al. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta. 2005;533:185–191. [Google Scholar]
- Bobiş O., Bonta V., Cornea-Cipcigan M., et al. Bioactive molecules for discriminating Robinia and helianthus honey: high-performance liquid chromatography–electron spray ionization–mass spectrometry polyphenolic profile and physicochemical determinations. Molecules. 2021;26:4433. doi: 10.3390/molecules26154433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodó A., Radványi L., K\Hoszegi T., et al. Melissopalynology, antioxidant activity and multielement analysis of two types of early spring honeys from Hungary. Food Biosci. 2020;35 [Google Scholar]
- Bodó A., Radványi L., Kőszegi T., et al. Quality evaluation of light- and dark-colored Hungarian honeys, focusing on botanical origin, antioxidant capacity and mineral content. Molecules. 2021;26:2825. doi: 10.3390/molecules26092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor Z., Koncz F.A., Rashed M.S., et al. Application of near infrared spectroscopy and classical analytical methods for the evaluation of Hungarian honey. Prog. Agric. Eng. Sci. 2018;14:11–23. [Google Scholar]
- Cheung Y., Meenu M., Yu X., Xu B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019;22:290–308. [Google Scholar]
- Cianciosi D., Forbes-Hernández T.Y., Afrin S., et al. Phenolic compounds in honey and their associated health benefits: a review. Molecules. 2018;23:2322. doi: 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combarros-Fuertes P., Estevinho L.M., Dias L.G., et al. Bioactive components and antioxidant and antibacterial activities of different varieties of honey: a screening prior to clinical application. J. Agric. Food Chem. 2018;67:688–698. doi: 10.1021/acs.jafc.8b05436. [DOI] [PubMed] [Google Scholar]
- Czigle S., Filep R., Balažová E., et al. Antioxidant capacity determination of Hungarian-, Slovak-, and polish-origin goldenrod honeys. Plants. 2022;11:792. doi: 10.3390/plants11060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czipa N., Andrási D., Kovács B. Determination of essential and toxic elements in Hungarian honeys. Food Chem. 2015;175:536–542. doi: 10.1016/j.foodchem.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Czipa N., Phillips C.J., Kovács B. Composition of acacia honeys following processing, storage and adulteration. J. Food Sci. Technol. 2019;56:1245–1255. doi: 10.1007/s13197-019-03587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco G., Gismondi A., Panzanella L., et al. Botanical influence on phenolic profile and antioxidant level of Italian honeys. J. Food Sci. Technol. 2018;55:4042–4050. doi: 10.1007/s13197-018-3330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dżugan M., Mi\lek M., Kielar P., et al. SDS-PAGE protein and HPTLC polyphenols profiling as a promising tool for authentication of goldenrod honey. Foods. 2022;11:2390. doi: 10.3390/foods11162390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriche I., Kadar M., Juan-Borrás M., Domenech E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014;142:135–143. doi: 10.1016/j.foodchem.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Farkas Á., Balázs V.L., Kõszegi T., et al. Antibacterial and biofilm degradation effects of Hungarian honeys linked with botanical origin, antioxidant capacity and mineral content. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.953470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feknous N., Boumendjel M. Natural bioactive compounds of honey and their antimicrobial activity. Czech J. Food Sci. 2022;40:163–178. [Google Scholar]
- Gheldof N., Wang X.-H., Engeseth N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002;50:5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- Halagarda M., Groth S., Popek S., et al. Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland. Antioxidants. 2020;9:44. doi: 10.3390/antiox9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer Ø., Harper D.A., Ryan P.D. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:9. [Google Scholar]
- Hunter M., Ghildyal R., D'Cunha N.M., et al. The bioactive, antioxidant, antibacterial, and physicochemical properties of a range of commercially available Australian honeys. Curr. Res. Food Sci. 2021;4:532–542. doi: 10.1016/j.crfs.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasicka-Misiak I., Makowicz E., Stanek N. Chromatographic fingerprint, antioxidant activity, and colour characteristic of polish goldenrod (Solidago virgaurea L.) honey and flower. Eur. Food Res. Technol. 2018;244:1169–1184. [Google Scholar]
- Kečkeš S., Gašić U., Veličković T.Ć., et al. The determination of phenolic profiles of Serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry. Food Chem. 2013;138:32–40. doi: 10.1016/j.foodchem.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Kędzierska-Matysek M., Stryjecka M., Teter A., et al. Relationships between the content of phenolic compounds and the antioxidant activity of Polish honey varieties as a tool for botanical discrimination. Molecules. 2021;26:1810. doi: 10.3390/molecules26061810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A., Mirmazloum I., Naár Z., Némedi E. Supplementation of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes) extract enhanced the medicinal values and prebiotic index of Hungarian acacia honey. Int. J. Med. Mushrooms. 2019;21 doi: 10.1615/IntJMedMushrooms.2019032897. [DOI] [PubMed] [Google Scholar]
- Kocsis M., Bodó A., K\Hoszegi T., et al. Quality assessment of goldenrod, milkweed and multifloral honeys based on botanical origin, antioxidant capacity and mineral content. Int. J. Mol. Sci. 2022;23:769. doi: 10.3390/ijms23020769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento K.S., Sattler J.A.G., Macedo L.F.L., et al. Phenolic compounds, antioxidant capacity and physicochemical properties of Brazilian Apis mellifera honeys. Lebensm. Wiss. Technol. 2018;91:85–94. [Google Scholar]
- Oddo L.P., Piro R., Bruneau É., et al. Main European unifloral honeys: descriptive sheets. Apidologie. 2004;35:S38–S81. [Google Scholar]
- Ördög A., Tari I., Bátori Z., Poór P. Mineral content analysis of unifloral honeys from the Hungarian Great Plain. Journal of elementology. 2017;22 [Google Scholar]
- Ranneh Y., Akim A.M., Hamid H.A., et al. Honey and its nutritional and anti-inflammatory value. BMC complementary medicine and therapies. 2021;21:1–17. doi: 10.1186/s12906-020-03170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajtos Z., Herman P., Harangi S., Baranyai E. Elemental analysis of Hungarian honey samples and bee products by MP-AES method. Microchem. J. 2019;149 [Google Scholar]
- Salonen A., Virjamo V., Tammela P., et al. Screening bioactivity and bioactive constituents of Nordic unifloral honeys. Food Chem. 2017;237:214–224. doi: 10.1016/j.foodchem.2017.05.085. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Tomás-Barberán F.A., Martos I., Ferreres F., et al. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001;81:485–496. [Google Scholar]
- Varga T., Sajtos Z., Gajdos Z., et al. Honey as an indicator of long-term environmental changes: MP-AES analysis coupled with 14C-based age determination of Hungarian honey samples. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139686. [DOI] [PubMed] [Google Scholar]
- Von Der Ohe W., Oddo L.P., Piana M.L., et al. Harmonized methods of melissopalynology. Apidologie. 2004;35:S18–S25. [Google Scholar]
- Weston R.J., Mitchell K.R., Allen K.L. Antibacterial phenolic components of New Zealand manuka honey. Food Chem. 1999;64:295–301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.