Abstract
Background
The capsaicin (vanilloid) receptor, VR1, is an agonist-activated ion channel expressed by sensory neurons that serves as a detector of chemical and thermal noxious stimuli.
Results
In the present study we investigated the properties of VR1 ion channels expressed in Xenopus oocytes. A VR1 subunit with a FLAG epitope tag at the C-terminus was constructed. When examined for size on an SDS gel, VR1-expressing oocytes produced a doublet corresponding to the size of the monomer and a band at about twice the molecular weight of the monomer. A consensus site for N-linked glycosylation was identified in the primary sequence at position 604. In channels in which the putative glycosylation site was mutated from asparagine to serine (N604S), the larger of the two monomer bands could no longer be detected on the gel. Electrophysiological experiments showed these unglycosylated channels to be functional. The high molecular weight band observed on the gel could represent either a dimer or a monomer conjugated to an unknown factor. To distinguish between these possibilities, we coexpressed a truncated VR1 subunit with full-length VR1. A band of intermediate molecular weight (composed of one full-length and one truncated subunit) was observed. This dimer persisted under strongly reducing conditions, was not affected by capsaicin or calcium, and was refractory to treatment with transglutaminase inhibitors.
Conclusions
The persistence of this dimer even under harsh denaturing and reducing conditions indicates a strong interaction among pairs of subunits. This biochemical dimerization is particularly intriguing given that functional channels are almost certainly tetramers.
Background
Nociceptors are specialized primary afferent neurons and the first cells in the series of neurons that lead to the sensation of pain [1-8]. The receptors in these cells can be activated by different noxious chemical or physical stimuli [9-11]. The essential functions of nociceptors include the transduction of noxious stimuli into depolarizations that trigger action potentials, conduction of action potentials from peripheral sensory sites to synapses in the central nervous system, and conversion of action potentials into neurotransmitter release at presynaptic terminals, all of which depend on ion channels [6,12-16]. Recent expression cloning has led to the identification of the first pain sensory receptor. The cloned receptor is called VR1 (vanilloid receptor subtype 1) [9,10]. The nucleotide sequence of VR1 predicts a protein of 838 amino acids with a molecular mass of 95 kDa. The predicted topological organization consists of six transmembrane domains with a hydrophobic loop between the fifth and sixth domain which lines the ion conducting pore [17]. VR1 has been expressed heterologously in several cell lines and has intrinsic sensitivity to thermal stimuli and to capsaicin (a pungent extract of the Capsicum pepper family) [18]. VR1 does not discriminate among monovalent cations [19]; however, it exhibits a notable preference for divalent cations with a permeability sequence of Ca2+ > Mg2+ > Na+ ≈ K+ ≈ Cs+[9]. Ca2+ is especially important to VR1 function, as extracellular Ca2+ mediates desensitization [20,21], a process which enables a neuron to adapt to specific stimuli by diminishing its overall response to a particular chemical or physical signal. Although not activated by voltage alone, VR1 currents show outward rectification and a region of negative resistance in the current-voltage relation.
The VR1 channel is a member of the superfamily of ion channels with six membrane-spanning domains, with highest homology to the trp family of ion channels. For those ion channels within this superfamily for which stoichiometry has been directly examined, all have been shown to be composed of four six-transmembrane domain subunits or pseudosubunits, with auxiliary subunits sometimes present as well [22]. An initial characterization of VR1 channels expressed in Cos and CHO cells has recently revealed that, under certain conditions, they run as multimers on pseudo-native (PFO) gels, with tetramers being one of the primary bands observed [23]. Thus, like other six membrane spanning domain channels, VR1 almost certainly forms as a tetramer; whether it combines with homologous subunits to form heteromeric channels remains to be determined.
In this study we have examined the electrophysiological and biochemical properties of VR1 expressed in Xenopus oocytes. We found that its apparent affinity for the ligand capsaicin is comparable to that observed by others. When examined for size on denaturing gels, we found that the monomer appeared to be a doublet and that there was a band that corresponded to roughly twice the molecular weight of the monomer bands. Through site-directed mutagenesis, we determined that the doublet represented unglycosylated and glycosylated forms of the VR1 subunit monomer and identified the glycosylation site as N604. Next, using a VR1 subunit engineered to be of different size, we show that the larger band on the gel represented dimerized subunits. Several mechanisms underlying dimerization were examined and ruled out. Since VR1 likely forms as a tetramer, the strong interaction we observed between pairs of subunits raises the question of whether this subunit interaction is involved in VR1 function.
Results
Electrophysiological and biochemical properties of VR1
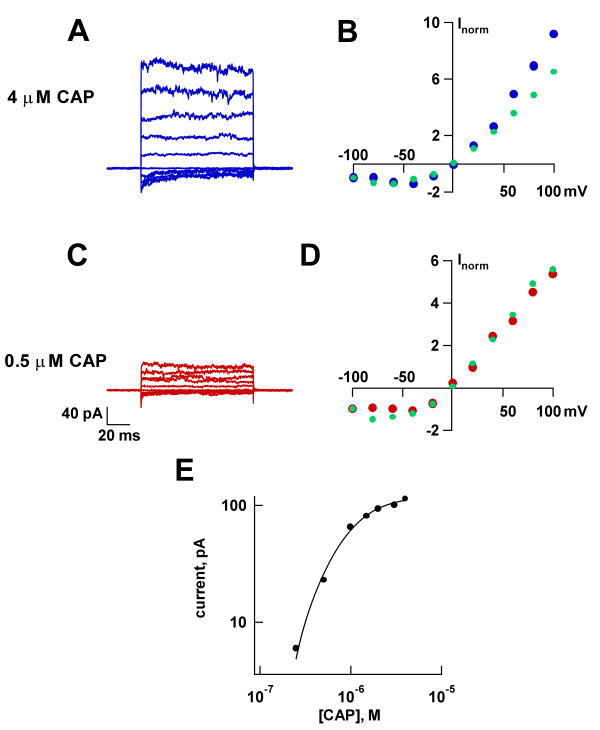
We expressed VR1 channels in Xenopus oocytes in order to characterize their electrophysiological and biochemical properties. Using outside-out patch-clamp recordings, we studied the responses of the channels to capsaicin. Figure 1A depicts a current family obtained using a saturating (4 μM) capsaicin concentration when the voltage was stepped from a holding potential of 0 mV to from -100 to +100 mV. A current-voltage relation measured from these currents (Figure 1B) shows the pronounced outward rectification exhibited by VR1. This rectification has been shown to be independent of external divalent cations [24], and is likely due to a combination of rectification in unitary conductance [9] and voltage-dependent gating [24]. Application of a lower concentration of capsaicin (0.5 μM) activated a smaller fraction of the channels in this patch, giving a smaller overall current (Figure 1C). By plotting the normalized currents in the presence of 4 μM and 0.5 μM capsaicin on the same graph (Figure 1D), we show that the voltage dependence remains unaltered under both experimental conditions. Figure 1E shows a dose-response relation for activation of VR1 channels by capsaicin for the same patch. Fits of dose-response relations with the Hill equation (see Experimental Procedures) yielded values of n = 1.8 ± 0.06 and a K½ = 614 ± 110 nM (mean of 5 patches). These values are similar to those previously reported [20,24]. These data indicate that in our system VR1 RNA is expressed as a functional protein with characteristics similar to those reported by other groups.
Figure 1.
VR1 forms functional channels in Xenopus oocytes. (A) Current family activated by a saturating capsaicin concentration (4 μM) in an outside-out patch obtained from stepping the voltage from a holding potential of 0 mV to between -100 and +100 mV. For scale bar see (C). (B) Current-voltage relation for currents activated by 4 μM capsaicin. Data were normalized to the value of the current at -100 mV. (C) Current family activated by a sub-saturating capsaicin concentration (0.5 μM). (D) Current-voltage relation for currents activated by 0.5 μM capsaicin (red) with data from currents obtained with 4 μM capsaicin shown also (blue). Data were normalized to the value of the current at -100 mV. (E) Dose-response relation for activation by capsaicin plotted on a double-log scale. The smooth curves are fits with the Hill equation with K½ = 440 nM, Imax = 567 pA, and n = 2.2. Filled circles represent actual data values. All data in this figure are from the same patch.
To examine the size of VR1 with SDS/PAGE, we constructed a VR1 subunit with a FLAG epitope tag on its extreme C-terminal end (Figure 2A). By using both this C-terminal FLAG epitope and an N-terminal epitope for which a commercial antibody is available, we could determine whether the band(s) we observed corresponded to full-length VR1 subunits. When oocytes injected with VR1 RNA were examined with SDS/PAGE and Western blot, three bands were apparent (Figures 2B,2C,2D). Two of these bands were seen as a doublet at about 80 kDa and 84 kDa (best seen in Figure 2D). A third band of about 200 kDa was also observed. The size of these bands was the same for blots probed with the N-terminal antibody (Figure 2B) or the FLAG antibody (Figure 2C), indicating that they represent full-length VR1 subunits. For the remainder of this study we used the FLAG antibody due to the lower background it produced.
Figure 2.
VR1 is glycosylated at N604. (A) Cartoon (not to scale) of proposed subunit topology for a single VR1 subunit. Shown are the epitopes for the N-terminal and FLAG antibodies used in Western blot experiments, and the consensus sequence for glycosylation, located just distal to the fifth transmembrane domain at position 604. The red circle depicts the approximate localization of this consensus sequence. The line parting from the circle points to the sequence of this N-glycosylation site and the yellow box shows the asparagine which was substituted for a serine in order to produce the glycosylation mutant (N604S). (B) Western blot of oocytes expressing VR1 probed with the N-terminal antibody. Uninjected oocytes and oocytes expressing VR1 were prepared as described in Experimental Procedures. The monomer was observed as a doublet at 80 kDa and at 84 kDa in VR1-injected oocytes and a third band of 200 kDa was also observed in these oocytes. No bands were observed in the uninjected oocytes. (C) Western blot of oocytes expressing VR1 probed with the FLAG antibody. Bands are as in (B). (D) Western blot of oocytes expressing VR1 or N604S. Uninjected oocytes and oocytes expressing VR1 or N604S were prepared as described in Experimental Procedures. The monomer doublet in the VR1 is not present in the N604S mutant; only the 80 kDa band is observed. No bands were observed in the uninjected oocytes. This blot was probed with the FLAG antibody.
Identification of the glycosylation site in VR1
A doublet of monomers has been previously observed for VR1 expressed in CHO cells [23]. In that case, treatment of cells with peptide-N-glycosidase F eliminated the larger monomer band, suggesting that it represented a glycosylated form of VR1. Although the difference in apparent size in that study was 19 kDa and we see a difference of only 4 kDa (Figure 2C), we wondered whether glycosylation might explain our doublet as well. We therefore examined the predicted amino acid sequence of VR1 in order to identify potential glycosylation sites (see Experimental Procedures). Figure 2A depicts the proposed topology of the six transmembrane domains of VR1. A consensus sequence for N-linked glycosylation is located just distal to the fifth transmembrane domain at position 604. We introduced a point mutation to change the asparagine at position 604 into a serine (N604S). If indeed N604 is a glycosylation site, channels expressed from this N604S construct would be expected to lack glycosylation. Figure 2D shows that in N604S channels the upper band of the doublet has been eliminated. These data indicate that wild-type VR1 channels expressed in Xenopus oocytes are present in both unglycosylated and glycosylated forms and that the N-linked glycosylation occurs at position 604.
Once we determined that the N604S mutation efficiently removed the glycosylated form of the monomer, we tested to be certain that N604S channels were present in the plasma membrane and had electrophysiological characteristics similar to those observed in wild-type channels. We performed patch-clamp recordings on oocytes injected with N604S RNA. The results of these experiments are shown in Figure 3. Like for wild-type VR1 channels, capsaicin activated N604S channels in a concentration-dependent manner (Figures 3A, 3C, and 3E). Current voltage relations at both a saturating (Figure 3B) and a subsaturating (Figure 3D) concentration of capsaicin show that the voltage dependence of N604S channels is similar to that of wild-type VR1 channels (in Figure 3 compare data from N604S channels [blue and red] to data from wild-type VR1 channels [green]): the ratio of the current at +100 mV to that at -100 mV is not statistically different from wild-type VR1. Furthermore, the dose-response relation in Figure 3E shows that the apparent affinity of N604S channels for capsaicin is not significantly different from wild-type channels (t-test, p > 0.05), with n = 2.3 ± 0.3 and K½ = 780 ± 71 nM (mean of 5 patches). These data indicate that eliminating the N-glycosylation site of the VR1 channel gives rise to a functional protein electrophysiologically similar to wild-type VR1.
Figure 3.
N604S forms functional channels, with properties like that of wild type. (A) Current family activated by a saturating capsaicin concentration (4 μM) in an outside-out patch obtained from stepping the voltage from a holding potential of 0 mV to between -100 and +100 mV. (B) Current-voltage relation for currents activated by 4 μM capsaicin for N604S (blue) and wild-type VR1 channels (green). Data were normalized to the value of the current at-100 mV. (C) Current family activated by a sub-saturating capsaicin concentration (0.5 μM). (D) Current-voltage relation for currents activated by 0.5 μM capsaicin for N604S (red) and wild-type VR1 (green) channels. Data were normalized to the value of the current at -100 mV. (E) Dose-response relations for activation by capsaicin plotted on a double-log scale. The smooth curves are fits with the Hill equation with K½ = 905 nM, Imax = 114 pA, and n = 1.8. Filled circles represent actual data values, plotted on a double-log axis. All data in this figure is from the same patch.
Identification of the nature of the VR1 high molecular size band
At 200 kDa, the third band observed in Figure 2 was approximately twice the molecular weight of the monomer. We therefore suspected that it might represent a dimer of VR1 subunits. However, it could also arise from a monomer in combination with another cellular factor. To distinguish between these possibilities, we engineered a VR1 mutant in which amino acids 2–52 in the N-terminal were deleted (termed Δ2–52). This construct gave rise to a high molecular weight band of 180 kDa, 20 kDa smaller than that observed for wild-type VR1 (Figure 4). When coexpressed with VR1, heteromeric channels composed of both types of subunits are expected to form. If the higher molecular weight band does represent a dimer of subunits, the heteromeric VR1/Δ2–52 ought to be intermediate in size between the homomeric dimers. When equal amounts of VR1 and Δ2–52 RNA's were coinjected into oocytes, three bands were observed: 200, 190 and 180 kDa (Figure 4). The 200 kDa band represents the homomeric VR1 dimer whereas the 180 kDa band represent the homomeric Δ2–52 dimer. The middle band, at 190 kDa, is indeed intermediate in size. Our interpretation is that this 190 kDa band is composed of one wild-type VR1 subunit and one Δ2–52 subunit, indicating that the high molecular weight band represents subunit dimers. Furthermore, quantification of the intensity of the bands on the gel revealed a 1:2:1 ratio of the three bands. This ratio of the three ways in which the two types of subunit can assemble is what would be expected if assembly between the different types of subunits were random.
Figure 4.
The 200 kDa band represents a dimer of VR1 subunits. Western blot of oocytes expressing wild-type VR1, Δ2–52, or wild-type VR1 + Δ2–52. Oocytes were prepared as described in Experimental Procedures. The band observed for VR1-express ing oocytes was at 200 kDa, the band observed for the Δ2–52-expressing oocytes was at 180 kDa, while oocytes injected with a 1:1 ratio of VR1 and Δ2–52 RNA yield three bands of 200 kDa, 180 kDa, and 190 kDa. This blot was probed with the FLAG antibody.
Next, as with N604S, we wanted to be certain that Δ2–52 expressed as a functional channel in Xenopus oocytes. The results of electrophysiological experiments are shown in Figure 5. We found Δ2–52 to form functional, capsaicin-activated channels in Xenopus oocytes. Although an increase in rectification was consistently observed with these channels, compared to wild-type VR1 (Figure 5B), the ratio of the current at +100 mV to that at -100 mV is not statistically different from wild-type VR1. Fits of dose-response relations (Figure 5E) with the Hill equation yielded a mean n value of 2.1 ± 0.26, and a mean K½ value of 1.98 ± 0.8 μM; neither n nor K½ were significantly different from VR1 (t-test, p > 0.05, for 5 patches).
Figure 5.
Δ2–52 forms functional, capsaicin-activated channels. (A) Current family activated by a saturating capsaicin concentration (4 μM) in an outside-out patch obtained from stepping the voltage from a holding potential of 0 mV to between -100 and +100 mV. (B) Current-voltage relation for currents activated by 4 μM capsaicin for Δ2–52 (blue) and wild-type VR1 (green) channels. Data were normalized to the value of the current at -100 mV. (C) Current family activated by a sub-saturating capsaicin concentration (0.5 μM). (D) Current-voltage relation for currents activated by 0.5 μM capsaicin for Δ2–52 (blue) and wild-type VR1 (green) channels. Data were normalized to the value of the current at -100 mV. (E) Dose-response relations for activation by capsaicin plotted on a double-log scale. The smooth curves are fits with the Hill equation with K½= 390 nM, Imax = 1630 pA, and n = 1.5. Filled circles represent actual data values. All data in this figure are from the same patch.
Examination of putative cross-linking factors
A previous study has reported that the presence of capsaicin and chemical cross-linkers influence the formation of multimers in VR1 [23]. Moreover, this study reported that cross-linking could be a Ca2+ mediated process, through the activation of endogenous transglutaminases. In our expression system, the presence of a dimer was seen even in the absence of capsaicin. Thus, we set out to study the factors that could be involved in the formation of this complex. Our first approach was to determine whether this dimer could be due to the presence of a disulfide bond between subunits. Although we have β-mercaptoethanol in the sample buffer for all experiments, it is possible that a disulfide bond refractory to reduction by this reagent was present. We therefore used the stronger reducing agents DTT and TCEP in the biochemical assays, and used them in various steps of the purification (see Materials and methods). Figure 6A shows the results for this experiment. The control lane represents the results obtained from oocytes processed under control conditions (with β-mercaptoethanol as the only reducing agent). The next two lanes shown are those of oocytes exposed to the reducing agents DTT (100 mM) and TCEP (20 mM) both during processing and in the sample buffer. We quantified the ratio of intensity of the monomer band to the dimer band. Neither DTT nor TCEP treatment produced a difference in this ratio compared to the control condition (t-test, p > 0.05 for 3 independent experiments). These data indicate that the presence of the VR1 dimer is likely not due to an intersubunit disulfide bond.
Figure 6.
VR1 dimerization is not affected by reducing agents, capsaicin, Ca2+ or transglutaminase inhibitors. (A) Western Blot of the effect of reducing agents on the VR1 dimer. The addition of DTT (100 mM) and TCEP (20 mM) did not modify the ratio of monomer to dimer in VR1-expressing oocytes (p > 0.05, for 3 independent experiments). (B) Effect of Ca2+ on dimer formation in VR1. The addition of Ca2+ to the biochemical assays (in the presence or the absence of capsaicin, lanes 1 and 2) did not modify the ratio of monomer to dimer (p > 0.05, for 3 independent experiment). Addition of EGTA (2 mM) to the assays (lanes 3 and 4) did not modify the monomer to dimer ratio either (p > 0.05, for 3 independent experiments). For the Ca2+-free condition, the expected free Ca2+ concentration was 0.4 nM, calculated using WebMax C version 2.1 http://www.stanford.edu/~cpatton/webmaxc2.htm. and assuming a contaminant level of 5 μM Ca2+ in our water. For the condition in which Ca2+ was present, we added 1.8 mM Ca2+ and no chelator to the solution (see Materials and Methods). (C) Effects of transglutaminase inhibitors on dimerization of VR1. The addition of cysteamine (20 mM) and MDC (250 μM) to oocytes did not alter the amount of dimer in relation to monomer when compared to the control lane which did not receive any treatment (p > 0.05, for 3 independent experiments).
We next addressed whether Ca2+ could affect the monomer:dimer ratio in our expression system. Oocytes were processed for biochemical assays under the various experimental conditions depicted in Figure 6B. In the first lane we show protein obtained from oocytes exposed to Ca2+ (1.8 mM in frog Ringer's solution) but not to capsaicin; both monomer and dimer bands can be observed. When capsaicin and Ca2+ were added together, the ratio of monomer: dimer remained unchanged in comparison to the previous experiment (p > 0.05, for 3 independent experiments). We then tested whether eliminating Ca2+ from the oocyte media would affect this ratio. As seen in the last two lanes of this gel, the presence of EGTA (2 mM) did not alter the formation of the VR1 dimer, whether capsaicin was or was not present in the assay – the monomer:dimer ratio did not differ from control conditions (p > 0.05, for 3 independent experiments under the same conditions). Our data indicate that under our experimental conditions Ca2+ does not play a pivotal role in VR1 dimerization.
Finally, we tested whether the transglutaminase inhibitors cysteamine and monodansylcadaverine (MDC) could affect this process. As shown in Figure 6C (second and third lanes), the addition of these compounds for 1 hr to the solution bathing the oocytes and to the homogenization solution did not modify the monomer:dimer ratio when compared to the control lane (p > 0.05, for 3 independent experiments). The concentrations of cysteamine and MDC used here are identical to those previously shown to disrupt transglutaminase-induced cross-linking of VR1 channels in other cell types (Kedei et al., 2001). This result comes as no surprise since transglutaminases are known to be Ca2+ dependent [25], and our previous experiment demonstrates that Ca2+ has no effect on the dimerization we observed.
Discussion
In this study we have examined the properties of VR1 in a Xenopus oocyte heterologous expression system. We report the following findings: (1) Full-length VR1 runs as a doublet of monomers on SDS gels, with apparent molecular weights of 80 and 84 kDa. The smaller band represents unglycosylated subunits and the larger band represents subunits glycosylated at N604. (2) Channels engineered to lack glycosylation (N604S mutants) are correctly folded and targeted to the plasma membrane, with functional properties similar to wild-type channels. (3) A 200 kDa band is also apparent on VR1 SDS gels. By coexpressing full-length and truncated subunits we show that this band represents a dimer of subunits. (4) The interaction between the pair of subunits in the dimer band was quite strong; the intensity of the dimer band relative to monomer was not affected by capsaicin or calcium, and remained unchanged after treatment with reducing agents and transglutaminase inhibitors.
Unglycosylated and glycosylated forms of the VR1 monomer
We show that, in Xenopus oocytes, VR1 is expressed in both glycosylated and unglycosylated forms, with ~4 kDa difference in their apparent molecular weights. The complete disappearance of the upper band of the monomer doublet for the N604S mutation strongly suggests that the extracellular linker between the fifth transmembrane domain and the P-loop of VR1 is subject to N-linked glycosylation. Moreover, since channel function did not appear to be affected by the absence of glycosylation (Figure 3), it appears that glycosylation at this site is not essential for correct folding and targeting of the protein to the plasma membrane.
The position of the glycosylation site in the structure of the channels is in a region of known importance in channel function. Lying between the fifth transmembrane domain and the P-loop, this region contributes to the extracellular vestibule of the ion-conducting pore [26]. The vestibules of ion channels are thought to influence the permeation of ions [27,28] and in CNG channels it has been reported that elimination of the N-glycosylation site (which is in an analogous position between S5 and the P-loop) can induce changes in the apparent half-blocking constants for extracellular and intracellular Mg2+[29].
Whereas two monomer bands had been previously observed for VR1 expressed in CHO cells [23], a major difference between this study and ours is the difference in size between the two monomer bands (19 kDa – about five times larger than we observe). On the other hand, Kedei et al. [23] show a doublet in channels purified from DRG cells, which express VR1 endogenously, that is similar to the one we observe. Further, unlike in CHO cells, no additional high molecular weight glycosylated bands were observed.
Dimerization of VR1
As a member of the six-transmembrane domain superfamily of ion channels, VR1 most likely assembles into tetrameric complexes. Evidence that VR1 is capable of forming multimers has been previously reported when studied under pseudo-native conditions [23]. In this previous study, tetramers were the major band observed, although larger and smaller bands were also seen. Interestingly, there is precedent for an ion channel to retain some intersubunit interactions even on denaturing gels like those used here. The bacterial K+ channel KcsA, whose X-ray crystal structure has been solved and is definitively a tetramer [30], runs as a tetramer on SDS gels [31]. Furthermore, mutations that disrupt a known intersubunit interface at the level of the pore disrupt this biochemical tetramerization. The structural interactions that underlie tetramerization of KcsA on gels are disrupted only by heating the sample and by pH 12 [31], treatments that had no effect on the dimerization of VR1 we observed (data not shown). Although it is tempting to conclude that the pH- and heat-resistent dimerization we observe with VR1 results from a covalent interaction, we cannot rule out other explanations such as strong hydrophobic interactions.
What does a dimer observed on a gel mean given that the channels are almost certainly tetramers? Precedent for dimerization of limited domains of ion channels abounds. Cyclic nucleotide-gated channels, for example, appear to exhibit functional dimerization of their cyclic nucleotide-binding domains [32]. The "RCK" domain of BK channels has a dimerization domain even though BK channels, too, are tetrameric at the level of the pore [33]. Finally, the GluR2 ligand-binding core has a dimerization interface in the crystal structure [34]. Evidence, including the clustering of residues involved in receptor desensitization at this interface, suggests that the dimerization is not just crystallographic but functional. The dimerization observed in the above examples all involve ligand-binding sites. Given that VR1 is also a ligand-activated ion channel it is tempting to speculate that it may too contain such a dimer interface. Recent work identifying amino acid residues that likely comprise part of the capsaicin-binding site [35] suggest that capsaicin binds at the interface between transmembrane segment 3 and the cytoplasm. Could this be a point of intersubunit contact? Although the dimerization we observe may represent a native intersubunit interaction, other possibilities must be considered. For example, hydrophobic interactions can cause membrane proteins to aggregate during purification. Alternatively, a covalent interaction may link pairs of subunits. This possibility will be investigated in the future.
Materials and methods
Heterologous expression of channels in Xenopus oocytes
Segments of ovary were removed from anesthetized Xenopus laevis. After gross mechanical isolation, individual oocytes were defolliculated by incubation with collagenase 1A (1 mg/mL) in Ca2+-free OR2 medium (82.5 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.6) for 1.5–3 hours. The cells were then rinsed and stored in frog Ringer's solution (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH 7.6) at 14°C. Oocytes were injected with 50 nL mRNA solution within two days of harvest.
Electrophysiology
Electrophysiological recordings and/or biochemistry were performed 4–8 days after injection. After brief exposure to a hypertonic medium, the vitelline membrane was stripped from each oocyte with forceps. Outside-out patch-clamp recordings were made using symmetrical NaCl/HEPES/EDTA solutions consisting of 130 mM NaCl, 10 mM HEPES, 1 mM EDTA and 10 mM EGTA (pH 7.2). Capsaicin was prepared as a 4 mM stock in dry ethanol and was added to the extracellular solution only. The solution bathing the extracellular surface of the patch was changed using a RSC-200 rapid solution changer (Molecular Kinetics, Pullman, WA). Unless otherwise indicated, all chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Pipettes were polished to a resistance of 0.3–1 MΩ and immediately before use were dipped in a seal glue composed of one part light mineral oil, one part heavy mineral oil, and 10% parafilm beads to promote formation of high-resistance seals [36]. Currents were low pass-filtered at 2 kHz and sampled at a 10 kHz with an Axopatch 200B (Axon Instruments, Union City, CA). Data were acquired and analyzed with the PULSE data acquisition software (Instrutech, Elmont, NY) and were plotted and fit using Igor Pro (Wavemetrics Inc., Lake Oswego, OR). All currents shown are difference currents in which the current in the patch in the absence of capsaicin has been subtracted. All dose-response curves were measured at +100 mV at room temperature. Smooth curves shown in dose-response relations are fits with the Hill equation:
![]()
where I is the current at a given concentration of capsaicin, Imax is the maximal current, K½ is the concentration of half-maximal activation and n is the Hill coefficient. Current-voltage relations were plotted using the data obtained from voltage jumps from -100 to +100 mV for 100 ms from a holding potential of 0 mV. Data was normalized by dividing the values of the current at different voltages by the value of the current at -100 mV. When pooled data are discussed in the text, they represent the mean ± standard error of the mean (SEM) A Student's t-test (two-tailed) was performed on some data, as discussed in the text. The significance level was set at p > 0.05.
Mutagenesis
A potential glycosylation site was identified by screening the predicted vanilloid receptor 1 (VR1) channel amino acid sequence [9]. A glycosylation consensus sequence of N-X-T/S was found at positions 604–606 (NNS). A point mutant was constructed as outlined below to replace the asparagine at position 604 with a serine. The mutant construct was designated as N604S.
The point mutation and deletion mutation were constructed by a method involving oligonucleotides synthesized to contain a mutation in combination with wild-type oligonucleotides in PCR amplifications of fragments of the cDNA. The product of the PCR reaction was then cut with two different restriction enzymes to generate a cassette containing the mutation. The cassette was then ligated into the channel cDNA cut with the same two restriction enzymes. After transformation of bacteria with the ligation product, single isolates were selected, and the entire region of the amplified cassettes was sequenced to check for the mutation and insure against second-site mutations. mRNA was synthesized in vitro, using a standard reverse-transcription kit (mMessage mMachine, Ambion, Austin, TX). All constructs, including VR1, were made in a background of a VR1 subunit in which the FLAG epitope (DYKDDDDK) had been spliced on to the C-terminus. The presence of this epitope was found to have no detectable effects on the electrophysiological properties of the channels (data not shown).
SDS/PAGE and Western blot
Oocytes were prepared after the method of Rho et al. [29]. Typically 30 oocytes were lysed by trituration in 200 μL of a solution containing 100 mM Tris-HCl, 100 mM NaCl, 0.5% Triton X-100, 0.05 mg/mL pepstatin, 0.05 mg/mL leupeptin, and 0.05 mg/mL aprotinin (pH 8.0) and the homogenate was incubated for 15 minutes on ice. The homogenate was then centrifuged at 18,400 g for ten minutes at 4°C in a Jouan CR3i centrifuge. The soluble portion of the homogenate was then transferred to a new tube for an additional centrifugation. 10 μL of supernatant was removed, mixed with 20 μL of Laemmli sample buffer containing β-mercaptoethanol (19:1) and incubated at room temperature for five minutes. The samples were then subjected to SDS/PAGE using NuPage 3–8% or 7% Tris-Acetate precast gels (Invitrogen Corp., Carlsbad, CA). Proteins were then transferred to a PVDF membrane and Western blot analysis was performed. For all blots except that shown in Figure 2B, M2 anti-FLAG primary antibody was used, and for the blot shown in Figure 2B a polyclonal antibody raised against the N-terminal sequence of VR1 (amino acids 4–21: RASLDSEESESPPQENSC) was used (Neuromics Inc., Minneapolis, MN). Chemiluminescent detection was then carried out using the SuperSignal West Femto kit (Pierce, Rockford, IL). Chemiluminescent signals were captured with the Flourchem Imager (Alpha Innotech, San Leandro, CA), which has a linear range of 4 O.D. units. Densitometry of bands on Western blots was done with the Flourchem software. For comparison of the ratio of monomer to dimer, we included both monomer bands in the monomer category. Because of the large linear range of detection of our instrument, we could compare this ratio with accuracy even if the amount of total protein varied between gels.
For SDS/PAGE experiments on disulfide bonds, the oocytes were treated as above, except that the lysis buffer contained either 100 mM DTT or 20 mM Tris (2-Carboxyethyl) Phosphine Hydrochloride (TCEP) (Pierce, Rockford, IL). To examine the effect of calcium and capsaicin on VR1 dimer formation, four different conditions were tested. For calcium-free condition, whole oocytes were rinsed three times with calcium-free frog Ringer's solution (96 mM NaCl, 2 mM KCl, 2 mM EGTA, 1 mM MgCl2, 5 mM HEPES, pH 7.6) prior to incubation with or without 10–20 μM capsaicin at room temperature for 30 minutes. For calcium- present condition, whole oocytes were bathed in frog Ringer's solution with or without 10–20 μM capsaicin for the same duration. The oocytes were triturated according to the method described above, except for the capsaicin-present condition, where 10–20 μM capsaicin was included in the lysis buffer. For experiments on transglutaminase inhibitors, the oocytes were bathed for one hour in frog Ringer's solution containing either 20 mM cysteamine or 250 μM MDC. These are expected to be saturating concentrations of the transglutaminase inhibitors. Oocytes were triturated according to the method above, except that either 20 mM cysteamine or 250 μM MDC were included in the lysis buffer.
Acknowledgments
Acknowledgments
We would like to thank Dr. David Julius for his kind gift of the VR1 cDNA clone and Dr. Leon Islas for helpful discussions.
Contributor Information
Tamara Rosenbaum, Email: trosenba@u.washington.edu.
Mika Awaya, Email: mika@seanet.com.
Sharona E Gordon, Email: seg@u.washington.edu.
References
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annual Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/S0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Raja SN, Meyer RA, Ringkamp M, Campbell JN. Peripheral neural mechanisms of nociception. In Textbok of pain, ed PD Wall, R Melzack, Edinburgh: Churchill Livingstone. 1999. pp. 11–57.
- Snider WD, MacMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/S0896-6273(00)81003-X. [DOI] [PubMed] [Google Scholar]
- Fields HL. Pain. New York: McGraw-Hill; 1987. p. p354. [Google Scholar]
- Baccaglini PI, Hogan PG. Some rat sensory neurons in culture express characteristics of differentiated pain sensory cells. Proc Natl Acad Sci USA. 1983;80:594–8. doi: 10.1073/pnas.80.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–3. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J Physiol (Paris) 1977;73:251–9. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–43. doi: 10.1016/S0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Cesare P, Moriondo A, Vellani V, McNaughton PA. Ion channels gated by heat. Proc Natl Acad Sci USA. 1999;96:7658–63. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Heyman I, Rang HP. Depolarizing responses to capsaicin in a subpopulation of rat dorsal root ganglion cells. Neurosci Lett. 1985;56:69–75. doi: 10.1016/0304-3940(85)90442-2. [DOI] [PubMed] [Google Scholar]
- Marsh SJ, Stansfeld CE, Brown DA, Davey R, McCarthy D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience. 1987;23:275–89. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Baylor DA. Conductance and kinetics of single cGMP-activated channels in salamander rod outer segments. J Physiol Lond. 1995;483:567–82. doi: 10.1113/jphysiol.1995.sp020607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Zieglgansberger W. The acute effects of capsaicin on rat primary afferents and spinal neurons. Brain Res. 1982;253:125–31. doi: 10.1016/0006-8993(82)90679-5. [DOI] [PubMed] [Google Scholar]
- McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol. 1999;61:835–56. doi: 10.1146/annurev.physiol.61.1.835. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA. 1996;93:15435–9. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh U, Hwang SW, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J Neurosci. 1996;16:1659–67. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 1997;17:3525–37. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J Neurophysiol. 1996;75:1503–14. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–96. doi: 10.1038/35073086. [DOI] [PubMed] [Google Scholar]
- Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem. 2001;276:28613–9. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Harries MH, Prinjha RK, Davis JB, Randall A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1). J Physiol. 2000;525:747–59. doi: 10.1111/j.1469-7793.2000.t01-1-00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand D, Bungay PJ, Elliott BM, Griffin M. Activation of transglutaminase at calcium levels consistent with a role for this enzyme as a calcium receptor protein. Biosci Rep. 1985;5:1079–86. doi: 10.1007/BF01119629. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. New insights into the structure and function of potassium channels. Curr Opin Neurobiol. 1991;1:14–9. doi: 10.1016/0959-4388(91)90005-R. [DOI] [PubMed] [Google Scholar]
- Guidoni L, Torre V, Carloni P. Potassium and sodium binding to the outer mouth of the K+ channel. Biochemistry. 1999;38:8599–604. doi: 10.1021/bi990540c. [DOI] [PubMed] [Google Scholar]
- Hoyles M, Kuyucak S, Chung SH. Energy barrier presented to ions by the vestibule of the biological membrane channel. Biophys J. 1996;70:1628–42. doi: 10.1016/S0006-3495(96)79726-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho S, Lee HM, Lee K, Park C. Effects of mutation at a conserved N-glycosylation site in the bovine retinal cyclic nucleotide-gated ion channel. Febs Lett. 2000;478:246–52. doi: 10.1016/S0014-5793(00)01863-9. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity [see comments]. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Heginbotham L, Odessey E, Miller C. Tetrameric stoichiometry of a prokaryotic K+ channel. Biochemistry. 1997;36:10335–42. doi: 10.1021/bi970988i. [DOI] [PubMed] [Google Scholar]
- Liu DT, Tibbs GR, Paoletti P, Siegelbaum SA. Constraining ligand-binding site stoichiometry suggests that a cyclic nucleotide-gated channel is composed of two functional dimers. Neuron. 1998;21:235–48. doi: 10.1016/S0896-6273(00)80530-9. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Pico A, Cadene M, Chait BT, MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29:593–601. doi: 10.1016/S0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–81. doi: 10.1016/S0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to "hot" chili peppers. Cell. 2002;108:421–30. doi: 10.1016/S0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Lu CC. Giant membrane patches: improvements and applications. Methods Enzymol. 1998;293:267–80. doi: 10.1016/s0076-6879(98)93018-x. [DOI] [PubMed] [Google Scholar]