Abstract
The mucosal glycocalyx of the ocular surface constitutes the point of interaction between the tear film and the apical epithelial cells. Membrane-associated mucins (MAMs) are the defining molecules of the glycocalyx in all mucosal epithelia. Long recognized for their biophysical properties of hydration, lubrication, anti-adhesion and repulsion, MAMs maintain the wet ocular surface, lubricate the blink, stabilize the tear film and create a physical barrier to the outside world. However, it is increasingly appreciated that MAMs also function as cell surface receptors that transduce information from the outside to the inside of the cell. A number of excellent review articles have provided perspective on the field as it has progressed since 1987, when molecular cloning of the first MAM was reported. The current article provides an update for the ocular surface, placing it into the broad context of findings made in other organ systems, and including new genes, new protein functions and new biological roles. We discuss the epithelial tissue-equivalent with mucosal differentiation, the key model system making these advances possible. In addition, we make the first systematic comparison of MAMs in human and mouse, establishing the basis for using knockout mice for investigations with the complexity of an in vivo system. Lastly, we discuss findings from human genetics/genomics, which are providing clues to new MAM roles previously unimagined. Taken together, this information allows us to generate hypotheses for the next stage of investigation to expand our knowledge of MAM function in intracellular signaling and roles unique to the ocular surface.
Keywords: Ocular surface, glycocalyx, membrane-associated mucin, signal transduction, epithelial tissue-equivalent, knockout mouse
I. Introduction
The wet ocular surface comprises the stratified squamous mucosal epithelia of the cornea/conjunctiva and the overlying tear film (Gipson, 2007). These cells are continually renewed in a process whereby daughter cells generated by division of basal cells at the basement membrane are displaced upward in the cell layers, become increasingly flattened, and undergo terminal differentiation. At the ocular surface, tight junctions form to seal the space between adjacent apical cells, creating a paracellular barrier to entry of noxious substances from the environment. In addition, the plasma membranes of apical cells develop folds called microplicae, that project outward into the tear film, and from which the mucosal glycocalyx is elaborated.
The mucosal glycocalyx constitutes the point of interaction between the tear film and the apical cells of the ocular surface epithelia. It forms a transcellular barrier to the outside world, defending and protecting, while also allowing selective penetration. Its water-holding properties maintain the wet surface and it lubricates the blink. Its interaction with the preocular tear film is stabilizing and facilitates spreading, thus, maintaining a smooth and refractive surface of high optical quality.
The word “mucin” is usually taken to mean an extracellular secretion of goblet cells, or other secretory gland cells, that forms a gel, with the primary role to coat, lubricate, and protect the epithelial surfaces of the body. However, the epithelial membrane-associated mucins (MAMs1), expressed by most glandular and ductal epithelial cells, form a distinct mucin subgroup. MAMs serve as the major component molecules of the mucosal glycocalyx of the ocular surface and other tissues, and are the focus of this article. Both secreted and membrane-associated subgroups are encoded by members of the MUC gene family.
MUC family mucins are characterized by a series of tandem amino acid repeats of identical or highly similar sequence, rich in serine, threonine and proline residues. The serine and threonine residues serve as the site for O-linked glycosylation, and O-linked oligosaccharide chains account for 50–90% of the mass of the molecule. Tandem repeats are also found in other mucins, but the sites for O-linked glycosylation are not as densely spaced, meaning that glycans comprises less of the total mass. Biophysical properties of mucins are largely determined by the extent and nature of the O-linked glycosylation rather than to the polypeptide sequence itself (with the exception of the serines/threonines that are modified) (Argueso and Gipson, 2001). The multiplicity of the mucin tandem amino acid repeats amplifies the properties of mucins dependent on these structures, in both secreted mucins and MAMs (Hollingsworth and Swanson, 2004).
Much of the early interest in MAMs was driven by their pathological roles in cancers. Attempts to develop antibodies recognizing tumor-associated antigens in the 1980s led to identification of high molecular weight glycoproteins with the properties of mucins. Biochemical preparations proved to be heterogenous in composition, thus, determination of mucin amino acid sequences purified by conventional biochemical purification methods of the time proved difficult. The development of antibodies reactive with specific core protein epitopes provided the means for purification of individual mucins by affinity chromatography, enabling cloning of their cDNAs and genes. This provided the probes needed for study of mucin roles in cancer, as well as other mucosal tissues.
Molecular cloning of the first MUC gene was reported in 1987 (Gendler et al., 1987). In 1990, an international workshop was held in San Francisco, California with the goal to sort out the numerous antibodies that had been generated (Taylor-Papadimitriou, 1991). At about that time, the naming convention for the MUC gene family became established. In the 2007 Friedenwald Award Lecture, Dr. Ilene Gipson describes the process of characterizing MAMs of the ocular surface mucosal glycocalyx, first using a monoclonal antibody developed in her lab, then using probes from other labs, as they became available (Gipson, 2007).
A number of review articles have provided perspective on the field over the years, with regard to cancers and various organ systems (Apostolopoulos and McKenzie, 1994; Apostolopoulos and McKenzie, 2017; Bafna et al., 2010; Bhavanandan, 1991; Carraway et al., 2007; Carraway et al., 2003; Gendler and Spicer, 1995; Gendler et al., 1991; Gum, 1992; Hattrup and Gendler, 2008; Hilkens et al., 1992; Hollingsworth and Swanson, 2004; Kim, 2012; Moniaux et al., 2001; Rose, 1992; Seregni et al., 1997; Singh and Hollingsworth, 2006; Strous and Dekker, 1992; van Putten and Strijbis, 2017; Xing et al., 2000; Xing et al., 2001). This includes the ocular surface (Ablamowicz and Nichols, 2016; Argueso, 2013; Argueso and Gipson, 2001; Baudouin et al., 2018; Gipson, 2004, 2007; Gipson and Argueso, 2003; Gipson et al., 2004; Gipson and Inatomi, 1998; Govindarajan and Gipson, 2010; Guzman-Aranguez and Argueso, 2010; Jentoft, 1990; Mantelli and Argueso, 2008; Mantelli et al., 2013).
The current article provides an update for the ocular surface, placing it into the broad context of findings made in other organ systems, and including new genes, new protein functions, and new biological roles. We discuss the epithelial tissue-equivalent with mucosal differentiation, the key model system that have made these advances possible. In addition, we make the first systematic comparison of the MAMs in human and mouse, establishing the basis for using transgenic and knockout mice for the next phase of discovery. We conclude with a section on findings from human genetics, which have generated the proposal of intriguing new MAM roles that were previously unimagined.
II. Genes, Gene Expression, Protein Localization
A. General
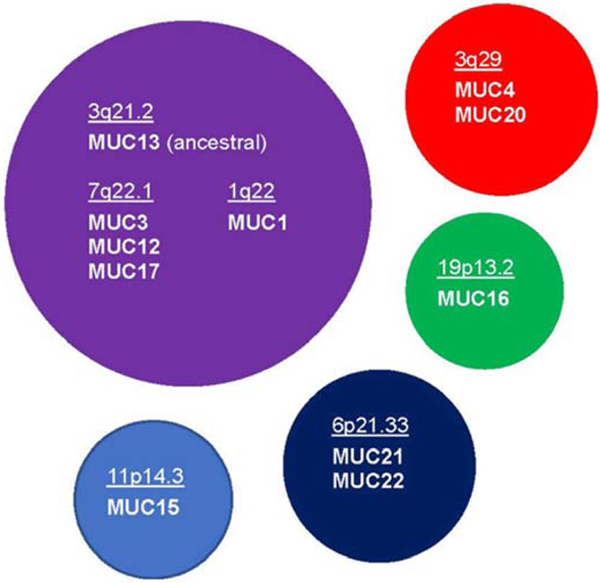
Table 1 lists the currently recognized twenty-one genes of the human Mucin (MUC) gene group, as defined by the HUGO Gene Nomenclature Committee (HGNC). There is no MUC18 in this series because it is a well-published alias for the unrelated gene MCAM. It should be noted that the existence of a separate MUC3A and MUC3B gene in the human genome is still under investigation (personal communication, Dr. Eric Cox, National Center for Biotechnology Information (NCBI)). We continue to list both genes here (as does the HGNC website), but discuss only MUC3A going forward in this article.
Table 1: Human MUC Gene Family.
Ordered by chromosomal location
| Gene Symbol | Cytogenetic Band | Expressing Tissue(s) at the Ocular Surface; Protein Presence in Tears |
|---|---|---|
| Secreted Mucins (gel-forming) | ||
| MUC2 | 11p15.5 | conjunctiva (RT-PCR only) (1,2); tears (low level) (3) |
| MUC5AC | 11p15.5 | Conjunctiva (1,2,4,5); goblet cells (3,5); lacrimal duct goblet cells (6); tears (3) |
| MUC5B | 11p15.5 | lacrimal gland (RT-PCR only) (7); not in tears (3) |
| MUC6 | 11p15.5 | |
| Secreted Mucins (soluble) | ||
| OVGP1 (MUC9) | 1p13.2 | |
| MUC7 | 4q13.3 | conjunctival epithelia (2,4,7); lacrimal gland (7); not in tears (3) |
| MUC19 | 12q12 | |
| MUC8 | 12q24.33 | |
| Membrane-Associated Mucins | ||
| MUC1 | 1q22 | corneal & conjunctival epithelia (2,4,8); lacrimal gland (6,7); tears (3) |
| MUC13 | 3q21.2 | conjunctival epithelium (RT-PCR only) (2,4) |
| MUC4 | 3q29 | conjunctiva, much less in corneal epithelium (2,4,5,9); lacrimal gland (7); tears (3) |
| MUC20 | 3q29 | corneal & conjunctival epithelia (4); not in tears (4) |
| EMCN (MUC14) | 4q24 | |
| MUC21 | 6p21.33 | corneal epithelium and lacrimal gland (this paper) |
| MUC22 | 6p21.33 | corneal epithelium and lacrimal gland (this paper) |
| MUC3B | 7q22 | |
| MUC3A | 7q22.1 | |
| MUC12 | 7q22.1 | |
| MUC17 | 7q22.1 | conjunctival epithelium (RT-PCR only) (2) |
| MUC15 | 11p14.3 | conjunctival epithelium (RT-PCR only) (2,4) |
| MUC16 | 19p13.2 | corneal & conjunctival epithelia (2,4,10); mucin granules of conjunctival goblet cells (11); lacrimal gland (12); tears (3) |
Citations
(McKenzie et al., 2000)
(Corrales et al., 2009)
The genes in Table 1 are ordered by chromosomal location, and are subdivided into those that encode secretory mucins (gel-forming and soluble), and those that encode membrane-associated mucins (MAMs).
Eight of the genes listed in Table 1 encode secretory mucins. The products of four of these genes form extremely large oligomeric gels through linkage of protein monomers via disulfide bonds. There are also four secretory mucins that do not form oligomeric gels. The gel-forming mucin OVGP1, is primarily expressed by oviduct epithelial cells. The others are expressed by many different mucous epithelia. Protein products of MUC5AC, MUC2, MUC5B and MUC7 have been detected in the ocular surface epithelia and/or in the lacrimal gland (reviewed in (Gipson, 2004)). However, only MUC5AC, and very low levels of MUC2, are found in the tears (Gipson, 2004).
Thirteen of the genes listed in Table 1 encode MAMs. EMCN is unusual in that it is an “endothelial mucin”, expressed primarily by vascular endothelial cells. The other twelve genes encode “epithelial mucins”, expressed by mucosal epithelia. Detection at the human ocular surface of RNA transcripts and protein products encoded by MUC1, MUC4, MUC16 and MUC20 has been reported in published articles in the scientific literature.
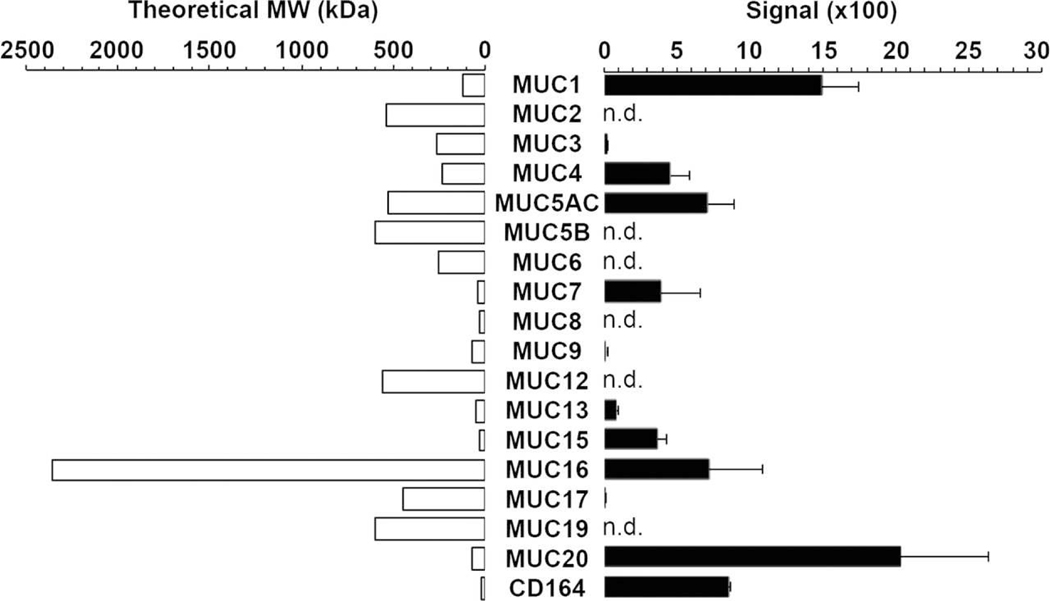
Figure 1 shows a direct comparison by RT-PCR of MAM mRNAs accumulated at the apical surface of human conjunctival epithelium as sampled by impression cytology, a technique in which a supportive filter is pressed on the ocular surface and then removed along with adherent material. It was determined that MUC20 is the gene most highly expressed in this location (Woodward and Argueso, 2014).
Figure 1. Mucin gene expression in human conjunctival epithelium.
Microarray analysis of impression cytology samples indicates that MUC20 is the most highly expressed mucin gene in human conjunctiva. n.d.: not detected. CD164 was previously designated as MUC24. MUC21 and MUC22 are not included in this analysis.
From (Woodward and Argueso, 2014), with permission.
We report here, for the first time, that RNA transcripts and proteins encoded by the more recently characterized genes MUC21 (Itoh et al., 2008) and MUC22 (Hijikata et al., 2011) are also expressed at the human ocular surface (see below).
B. MUC1, MUC4, MUC16
MUC1 was originally called the polymorphic epithelial mucin or episialin, a glycoprotein identified at the cell surface of human mammary carcinomas (Gendler et al., 1990; Ligtenberg et al., 1990). High expression levels in tumors correlate with a poor patient prognosis due to its ability to promote cell growth and survival (Xu et al., 2015). The National Cancer Institute ranks MUC1 as #2 in priority on a list of antigens for development as cancer vaccine targets, where major criteria are immunogenicity, oncogenicity and therapeutic function (Cheever et al., 2009).
MUC4 is the human homologue of rat sialomucin complex (SMC) (Moniaux et al., 1999), a high molecular weight glycoprotein heterodimer, originally discovered on the cell surfaces of the highly metastatic 13762 rat mammary adenocarcinoma (Carraway et al., 2000; McNeer et al., 1997). MUC16 corresponds to the CA125 antigen (O’Brien et al., 2001; Yin and Lloyd, 2001), a well-studied human ovarian cancer marker (Bast et al., 1983; Meyer and Rustin, 2000).
Human genes for MUC1, MUC4 and MUC16 are located on chromosomes 1, 3 and 9, respectively (Table 1). These genes are expressed in epithelial cells that line the mucosal surfaces of many different tissues. In general, it was found that RNA is expressed throughout the epithelial layers, but the translated protein accumulates only in the most apical layer (discussed (Lomako et al., 2010)).
The difference between RNA expression and protein accumulation of MUC1, MUC4 and MUC16 has been clearly documented at the ocular surface. Thus, in situ hybridization has revealed MUC1 mRNA in all cells of both corneal and conjunctival epithelia of humans (Gipson, 2000). However, immunoreactive MUC1 protein can be detected only in apical cells of the corneal epithelium, and in apical and sub-apical cells of the conjunctival epithelium (Inatomi et al., 1995).
The available antibodies for rat Muc4 reacted against carbohydrate epitopes, and were non-specific in humans, so the localization in the epithelial layers has still not been determined in humans. However, the rat Muc4 antibody 15H10, stained only the superficial epithelial layers of rat corneal and conjunctival epithelia (Pflugfelder et al., 2000; Swan et al., 2002). MUC16 protein was demonstrated in apical cells of corneal epithelia and in apical and sub-apical cells of conjunctival epithelia (Argueso et al., 2003).
Because many of the early antibodies used for immunolocalization were reactive only to MAM carbohydrate moieties, it has been conjectured that the apparently more restricted pattern of MAM protein localization as compared to mRNA expression might actually represent restricted glycosylation, and thus, restricted epitope expression. However, this explanation has not held up with newer studies using antibodies against MAM protein epitopes. A more likely explanation is regulation at the level of protein accumulation. In rat mammary gland epithelium, it was shown that Muc4 protein accumulation is restricted to the apical cell layer via a novel post-translational mechanism (Price-Schiavi et al., 2000). This was first defined in rat tumor cells, where it was shown that the proteosome degrades Muc4 (Swan et al., 2002). In stratified cultures of rat corneal epithelial cells, Muc4 protein levels are also regulated via the proteosome, which is apparently less active in the apical cell layer (Lomako et al., 2010).
The relative expression patterns of MUC1, MUC4, and MUC16 mRNAs differ across the ocular surface epithelia. MUC1 and MUC16 mRNAs are homogeneously expressed across the corneal and conjunctival epithelia (Argueso et al., 2003; Gipson, 2000). However, MUC4 mRNA is most abundant in conjunctival epithelium with an apparent diminution toward central corneal epithelium (Inatomi et al., 1996; Pflugfelder et al., 2000).
Of significance for inflammatory and autoimmune diseases of the ocular surface, MUC1 is also expressed by immune cells that are resident in the ocular surface epithelial, or that infiltrate due to inflammatory or immunological events, including B cells, T cells, monocytes, macrophages and dendritic cells (Agrawal et al., 1998; Brugger et al., 1999; Leong et al., 2003; Wykes et al., 2002).
In addition to apical epithelial cells of the ocular surface, MUC16 was immunolocalized to goblet cells of the conjunctiva, associated with the goblet cell mucin granule membrane (Gipson et al., 2016). A similar localization to goblet cell mucin granules is seen in the respiratory epithelium, as well as mucus cells in the submucosal gland (Davies et al., 2007; Kesimer et al., 2013).
MUC1, MUC4 and MUC16 are also expressed in the lacrimal gland (Jager et al., 2007; Jumblatt et al., 2003; Paulsen et al., 2004). A particularly detailed study of MUC16 was performed (Jager et al., 2007). MUC16 immunoreactivity was associated with the plasma membrane in accessory lacrimal glands. In the main lacrimal gland, as well as acinar cells and columnar cells of the nasolacrimal ducts, MUC16 immunoreactivity was also detected in intracytoplasmic vesicles. Subepithelial serous glands of the nasolacrimal ducts were also stained. Reactivity was further visible in secretion products within the lumen of serous acini and the nasolacrimal passage.
Considering that MAMs are membrane-tethered, the location of MUC16 within secreted lacrimal gland fluids seems contradictory at first. However, many of the MAMs can be immunodetected as soluble forms in the various extracellular fluids of the body (Moniaux et al., 2001). Early papers conjecture about whether this is due to their secretion. However, it is now known that part of the extracellular component of the MAM is shed from mucosal epithelial surfaces into extracellular fluids (discussed more in Section III). Consistent with this, immunoreactivity for MUC1, MUC4 and MUC16 has been detected in human tear fluid (Spurr-Michaud et al., 2007). Thus, the mucin component of tears is primarily a mixture of the secreted mucin MUC5AC and the soluble shed subunits of MUC1, MUC4, and MUC16.
In tracheobronchial, gastrointestinal and reproductive tracts, gel-forming mucins are secreted from goblet cells to create a viscous mucous layer which spreads over the epithelial glycocalyx. Imaging studies of fixed tissues have suggested that a distinct mucous layer is also associated with the epithelial glycocalyx. This is overlain by the aqueous component of tears, which is surfaced by lipid (Holly and Lemp, 1977; Johnson and Murphy, 2004; Nichols et al., 1985). However, a study utilizing biophysical methodologies of unfixed tissues has questioned the idea of distinct layers (Hodson and Earlam, 1994). In mice, studies employing electron microscopy following in vivo cryofixation with freeze substitution revealed a homogenous, fine network-like structure throughout the tear film, consistent with a model of mucins suspended in the aqueous phase (Tran et al., 2003). It is now generally accepted that the aqueous and mucin components of the tears combine to create a single layer of mucoaqueous gel (Willcox et al., 2017).
The single-phase model of the tear film makes functional sense, as a distinct layer of thick, light-scattering mucus on the surface of the cornea would obscure the central visual axis. Further to this idea, MUC5AC in tear fluid was shown to have an increased electrophoretic mobility compared to MUC5AC isolated from conjunctival tissue (Berry et al., 2004; Spurr-Michaud et al., 2007) and there is evidence that MUC5AC can be cleaved (Lidell and Hansson, 2006). A smaller size mucin molecule might facilitate the mixing of the aqueous and fluid components of tears.
With their complement of highly glycosylated mucins, the tears are hydrophilic and hydroscopic, maintaining fluid on the ocular surface, resisting drainage and contributing to lubrication of epithelial surfaces to limit frictional damage (Mantelli and Argueso, 2008). The tears move easily between the lid and over the glycocalyx because the mucins of both have anionic character that creates repulsive forces between them (Gipson, 2004). The tear mucins are also believed to trap and remove surface debris through movement over the ocular surface (Gipson and Inatomi, 1998).
C. MUC20
MUC20 was identified in a screen for genes with altered expression in renal tissues of patients with immunoglobulin A nephropathy (Higuchi et al., 2004b). It is localized at human cytogenetic locus 3q29, just upstream of MUC4. The two genes lie “head-to-head”, being transcribed in opposite directions. Directly upstream of MUC20 is MUC20P1, a gene fragment related to MUC20 by sequence. Classified as a pseudogene, it seems likely that MUC20P1 arose through a duplication of a portion of MUC20.
In human eyes, immunoreactive MUC20 was detected throughout the entire ocular surface epithelia, but predominantly within the plasma membrane region of intermediate cell layers. MUC20 also was observed in the cytoplasm of apical cells within the stratified squamous epithelium of the conjunctiva, but not in goblet cells (Woodward and Argueso, 2014). It was not found in tears. Thus, MUC20 exhibits a different localization pattern as compared to the other expressed MAMs.
D. MUC21 and MUC22
MUC21 and MUC22 came to our attention during a genome-wide association study to identify single nucleotide polymorphisms (SNPs) associated with steroid-induced ocular hypertension (Jeong et al., 2015). Figure 2 shows the position of the two genes, located adjacent to one another at human chromosomal locus 6p21.33, both transcribed in the same direction. This genomic region lies within the major histocompatibility complex (MHC) on chromosome 6. MUC21 was identified by homology search using a cDNA fragment encoding mouse epiglycanin, a cell surface glycoprotein expressed by a subline of TA3 mouse mammary carcinoma cells. Analysis using tissue cDNA libraries indicated that the gene is expressed in numerous mucosal tissues, including lung, large intestine, thymus and testis (Itoh et al., 2008).
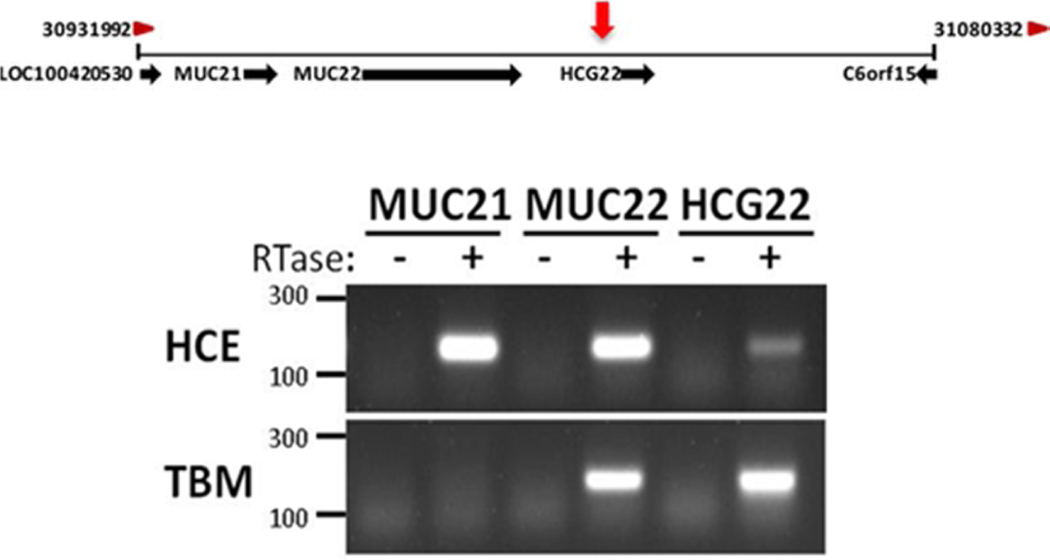
Figure 2. Location of Genes for MUC 21 and MUC22 at Chromosomal Region 6p21.32–33 and Expression in the Corneal Epithelium.
Top: Schematic of chromosomal region 6p21.32–33 from NCBI Gene depicting annotated genes surrounding an identified quantitative trait locus (QTL) for steroid-induced ocular hypertension (red arrow) in the transcriptional promotor region of HCG22.
Bottom: Total RNA was purified from cultured primary human corneal epithelial cells (HCE) and cells of the trabecular meshwork (TBM) cell line TM-1, and used for cDNA synthesis. RT-PCR using the cDNA was performed using specific primers from MUC21, MUC22, and HCG22; the products were resolved on a 1.5% agarose gel. Primers were designed to detect only the coding transcript. Similar results were obtained using three primary TBM cell lines (not shown).
RTase: reverse transcriptase; HCE: primary corneal epithelial cells obtained from corneal rims.
From (Jeong et al., 2015) with permission.
MUC22 was identified as part of a study on diffuse panbronchiolitis (DPB), a rare complex genetic disease of the respiratory system. An HLA-associated major susceptibility gene for DPB was located within the 200 kb in the class I region 300 kb telomeric of the HLA-B locus on chromosome 6. Within this candidate region, a novel mucin gene was identified, located adjacent to MUC21. MUC22 expression was examined by PCR screening of a commercial human multiple tissue cDNA panel. Expression was detected in the mucosal tissues of lung, placenta and testis (Hijikata et al., 2011). MUC22 mRNA expression was also detected in a tissue-equivalent model of primary human bronchial epithelial cells. Immunolocalization analysis in lung identified MUC22 antibody staining within the cytoplasm of serous cells of the submucosal gland. The submucosal glands are responsible for secretion of the periciliary liquid, which is analogous to the tear fluid secreted by the lacrimal gland. It is important for mucous clearance in the airways (Sharma et al., 1998).
Figure 2 shows expression analysis of MUC21 and MUC22 at the mRNA level by RT-PCR in three different cell types of the anterior segment: 1) a trabecular meshwork cell line, 2) primary cultures of trabecular meshwork cells, and 3) primary cultures of human corneal epithelial cells in monolayer culture. Both genes were expressed by the corneal epithelial cells. This figure was previously published (Jeong et al., 2015).
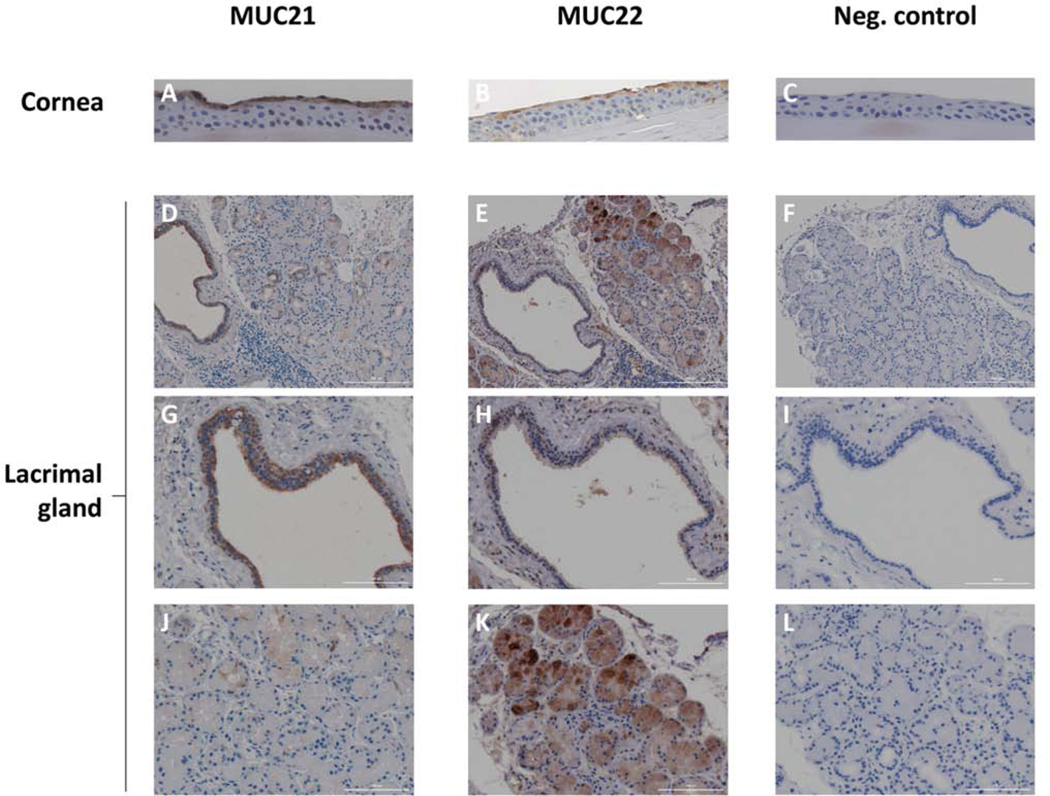
Figure 3 depicts previously unpublished results of MUC21 and MUC22 immunolocalization in the human corneal epithelium and lacrimal gland. Formalin-fixed, paraffin-embedded tissues were cross-sectioned, then the sections were processed and indirectly immunostained as described (Itakura et al., 2019). A 3,3′-diaminobenzidine (DAB) chromogen kit was used to detect secondary antibody binding. The affinity-purified MUC21 primary antibody was derived from a rabbit polyclonal antisera raised against a peptide from the human MUC21 cytoplasmic tail. The affinity-purified MUC22 antibody was characterized in one of our labs (Hijikata et al., 2011). It was derived from a rabbit polyclonal antisera raised against a peptide from the human MUC22 extracellular domain.
Figure 3. Immunolocalization of MUC21 and MUC22 in the Human Corneal Epithelium and the Human Lacrimal Gland.
An anterior segment isolated from a human donor eye was formalin-fixed within 24-hours post-mortem and paraffin-embedded. A formalin-fixed human lacrimal gland embedded in paraffin was obtained from the Ophthalmic Pathology Laboratory of Tufts Medical Center. Tissues cross-sections were prepared, then processed and indirectly immunostained for MUC21 or MUC22 as described (Itakura et al., 2019). The human MUC21 primary antibody was purchased from Sigma-Aldrich Corp. (St. Louis, MO). It is derived from a rabbit polyclonal antisera raised against a peptide from the human MUC21 cytoplasmic tail (561-CVRNSLSLRN TFNTAVYHPH GLNHGLGPGP GGNHGAPHRP RWSPNWFWRR PVSSIAMEMS GRNS-624), then affinity-purified. The human MUC22 primary antibody was characterized in one of our labs, as described (Hijikata et al., 2011). A rabbit polyclonal antisera produced by GENENET (Fukuoka, Japan) was raised against a peptide (TPTNVIKPSGYLQP) from the human MUC22 stem region located just before the transmembrane domain, then affinity-purified. A 3,3′-diaminobenzidine (DAB) chromogen kit was used to detect secondary antibody binding (Vector Laboratories, Burlingame, CA). The negative control (Neg. control) omitted the primary antibody. Sections were counterstained with hematoxylin. A-C) Cross-sections through the anterior segment focusing on immunostaining results (brown color) in the cornea epithelium. The hematoxylin counterstain is dark blue. Magnification = 40X. D-L) Cross-sections through the lacrimal showing immunostaining results (brown color). The hematoxylin counterstain is dark blue. D-F) Low magnification view (10X); G-I) Higher magnification (40X) focusing on a lacrimal duct; J-L) Higher magnification focusing on serous acini. These experimental findings have not been previously published.
Both MUC21 and MUC22 antibodies stained cells of the apical layer of human corneal epithelia. Both also stained specific cells in the lacrimal gland, but the pattern for each was different.
Muc21 antibody staining of lacrimal gland was intense in the epithelial cells lining the lacrimal ducts. Staining appeared to be both membranous and cytoplasmic. Only the occasional acinus was stained; again, staining was both membranous and cytoplasmic.
In contrast, MUC22 antibody primarily stained the serous acini. Staining was cytoplasmic (much as seen in the serous cells of the lung submucosal gland) and was concentrated within intracytoplasmic vesicles. Epithelial cells lining some lacrimal ducts were also stained with the MUC22 antibody, although fewer of these than for MUC21, and staining was both cytoplasmic and membranous. In some cases, immunoreactive material was observed within a duct.
This analysis identifies, for the first time, two new genes expressed at the ocular surface, as assessed by the dual criteria of RT-PCR (mRNA) and immunolocalization (protein). The protein products of both genes are also localized to the lacrimal gland.
III. Structure/Function
A. General
1. Length and Conformation
The MAMs are the largest of the membrane-associated glycoproteins. Table 2 ranks human MAMs by the length of their polypeptide chain. The longest MAM, MUC16, is close to 15,000 amino acids. The clustering of O-linked oligosaccharide chains within the tandem repeats creates steric interactions between carbohydrate and peptide, inducing the peptide core to adopt a stiff and extended conformation. This results in projection of the MAM well above the cell surface, far beyond other membrane-associated proteins (Jentoft, 1990). MAMs would, therefore, be the first molecules encountered by invading pathogens, and are thus positioned to shield and protect the cell surface.
Table 2:
Human Epithelial MAMs Ordered by Polypeptide Length
| Symbol | Amino acids | Predicted backbone mass | NCBI Protein database accession number | Isoforms |
|---|---|---|---|---|
| MUC16 | 14,507 | 1,519 kDa | NP_078966.2 | 14 |
| MUC4 | 7,418 | 734 kDa | NP_001309397 | 4 |
| MUC12 | 5,335 | 543 kDa | NP_00157934.1 | 1 |
| MUC17 | 4,493 | 452 kDa | NP_001035194.1 | 2 |
| MUC3A | 3,323 | 345 kDa | NP_005951.1 | 6 |
| MUC22 | 1,786 | 175 kDa | NP_001309398.1 | 3 |
| MUC20 | 709 | 72 kDa | NP_001269435.1 | 4 |
| MUC21 | 626 | 60 kDa | NP_001309300.2 | 3 |
| MUC13 | 512 | 55 kDa | NP_149038.3 | 1 |
| MUC1 | 484* | 50 kDa | NP_001191215.1 | 20 |
| MUC15 | 361 | 39 kDa | NP_001128563.1 | 3 |
Expression of MAMs in darker grey (MUC12 and MUC3A) has not been detected at the ocular surface; expression of MAMs in lighter gray (MUC17, MUC13) are documented only by RT-PCR
Protein data derived from the NCBI Protein database; listed here is the amino acid number of the longest isoform identified, with its accession number; a longer isoform of MUC1 (1255 amino acids) is listed in the UniProtKB database
Estimated molecular weight of the protein backbone mass was computed using: https://web.expasy.org/compute_pi/
The canonical MUC1 protein listed in the UniProt database is much longer, at 1255 amino acids (discussed more in the text)
Table 2 gives a count for the number of isoforms of each MAM listed in the NCBI Gene database. This includes splice variants as well as variants with insertions and deletions. It is important to note that genomic information such as this, is a work in progress. For example, while gathering information for the table, we observed a large discrepancy between the length of the MUC1 protein isoforms currently represented by NCBI (which top out at 484 amino acids) and the canonical isoform P15941–1 of 1255 amino acids in length, as represented by UniProt (https://www.uniprot.org/help/about). This was perplexing, as published articles describe the longer form (Bafna et al., 2010).
In consulting with NCBI staff, we learned that P15941–1 is based on a mRNA, J05582.1, that was cloned from a pancreatic tumor (Lan et al., 1990). NCBI does not usually list variants from cancer tissues, as they may be unique to the specific tumor. In fact, J05582.1 aligns poorly to the current human genome assembly, suggesting this is the case. However, it was noted that alignment of other mucin genes in the mouse and human genomes has also been difficult due to their high sequence repeat content, suggesting that it is equally possible the alignment problem lies on the genome side. This is being reported to the Genome Reference Consortium so that it might be revisited in the next human assembly update (personal communication, Dr. Eric Cox, NCBI).
2. Biophysical Properties
As noted in the Introduction to this article, the tandem amino acid repeat unit, densely modified by O-linked glycan chains, is the distinguishing feature of MUC family mucins. The number of tandem repeats can vary considerably among individuals within a population, leading to the designation of “VNTR” for variable number of tandem repeats (Gendler and Spicer, 1995). This variation accounts for some of the isoforms of a given MAM listed in Table 2; many more have been identified in cancer cells.
The extensive glycosylation of the numerous serine and threonine residues within tandem repeat regions, confers a hydrated, hydrophilic character (Argueso and Gipson, 2001). The densely-packed glycan chains also confer an antiadhesive character to cell surfaces. In cultured cancer cells, overexpression of MAMs stimulates cell detachment from their substratum, which is more pronounced the greater the number of tandem repeats (Berry et al., 2001). MAMs were also shown to confer a disadhesive character to the apical surface of corneal epithelial cells, suggesting that they provide boundary lubrication and prevent adhesion of facing cell surfaces (i.e. corneal epithelium and tarsal conjunctiva) during blinking or sleeping (Sumiyoshi et al., 2008).
The glycan moieties may vary depending on the mucin type, the site of mucin expression, and the physiological or pathological conditions (Chaturvedi et al., 2008). Abnormalities in MAM O-glycosylation have been identified in many disorders where the stability of the tears is compromised, such as contact lens wear and dry eye (e.g., (Gipson et al., 2004); reviewed in (Guzman-Aranguez and Argueso, 2010)). When the glycocalyx is altered pathologically, wetting of the cornea becomes imperfect and tear stability is compromised.
3. Cell Surface Receptor
In addition to the functions conferred by the O-linked glycan chains, it is increasingly appreciated that MAMs also serve as cell surface receptors that sense the extracellular environment and transduce signals intracellularly. This has been studied primarily using monolayer cultures of various types of cancer cells. In these cells, MAMs have been shown to activate or inhibit intracellular signaling cascades that regulate inflammation, cell-cell interactions, differentiation and apoptosis (Constantinou, 2011; Hollingsworth and Swanson, 2004; van Putten and Strijbis, 2017).
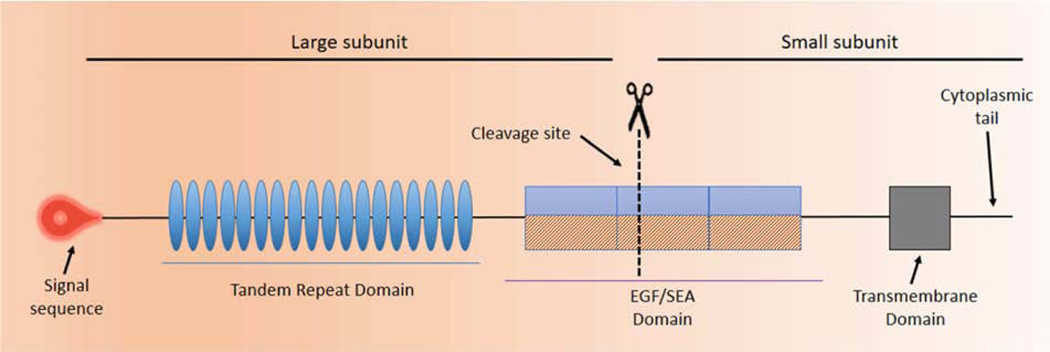
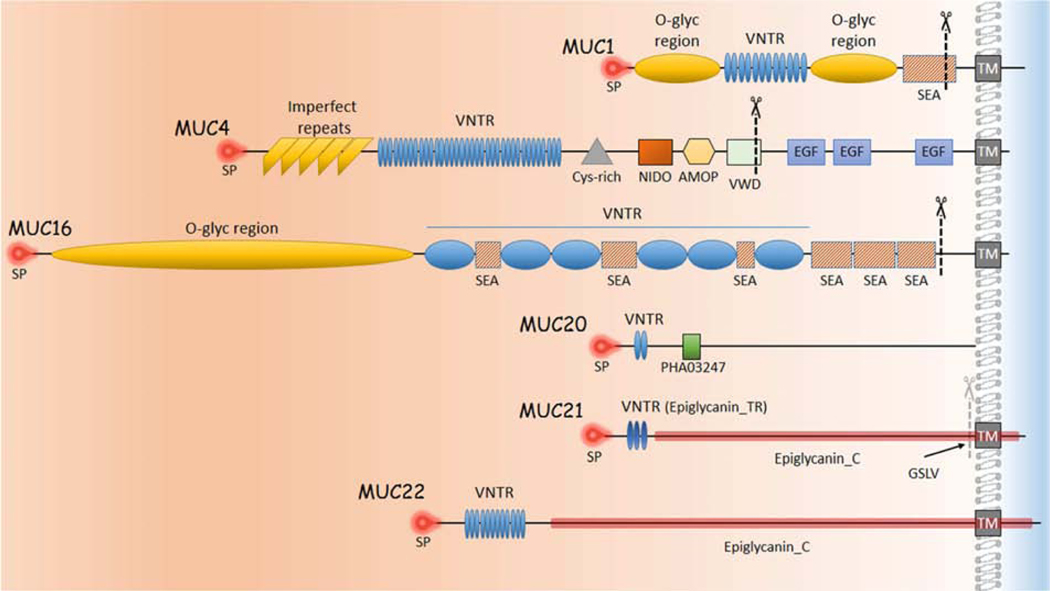
Figure 4 depicts a prototypical MAM, the structure of which is similar to a classic, single-pass transmembrane immune receptor. A signal peptide motif is found at the N-terminal of the precursor polypeptide chain to enable its membrane insertion; it may be retained in the mature protein (1). The mature protein is composed of two subunits that self-associate, arising from intracellular cleavage. The large subunit is entirely extracellular and contains the VNTR. The small subunit consists of a short extracellular region, a single-pass transmembrane domain and a cytoplasmic tail (CT).
Figure 4. Prototype of a Membrane Associated Mucin (MAM).
The graphic depicts a prototypical MAM, the structure of which is similar to a classic, single-pass transmembrane immune receptor. A signal peptide motif is found at the N-terminal of the precursor polypeptide chain to enable its membrane insertion; it may be retained in the mature protein (1). The mature protein is composed of two subunits that self-associate, arising from intracellular cleavage. The large subunit is entirely extracellular and contains the VNTR. The small subunit consists of a short extracellular region, a single-pass transmembrane domain, and a cytoplasmic tail (CT). The large subunit of the MAM, together with the extracellular portion of the small subunit, comprise the extracellular domain (ED). The ED also contains conserved sequence motifs as modular elements such as the Sperm protein, Enterokinase and Agrin module (SEA) and EGF-like modules.
The large subunit of the MAM, together with the extracellular portion of the small subunit, comprise the extracellular domain (ED). Besides the VNTR, with its sites for O-linked glycosylation. a number of sites for N-linked glycosylation are interspersed across the ED. The ED also contains conserved sequence motifs as modular elements that are mixed and matched in each MAM.
The Sperm protein, Enterokinase and Agrin module (SEA) and EGF-like modules are found in multiple MAMs and are shown on the MAM prototype in Figure 4. Other conserved sequence modules specific to individual MAMs are listed in Table 3. Most are located in the ED. These modules participate in signal transduction, as discussed in the next subsection.
Table 3:
Conserved Motifs Found in Human MAMs Expressed at the Ocular Surface
| Motif | Definition | MAMs with the Motif |
|---|---|---|
| SEA | Sperm protein, Enterokinase and Agrin module. Regulates or binds carbohydrate side chains | MUC1, MUC13, MUC16, MUC17 |
| KdpC | K+-transporting ATPase, c chain module; interacts with KdpA subunit to assemble and stabilize the Kdp complex |
MUC1 |
| AMOP | Adhesion-associated domain | MUC4 |
| NIDO | Extracellular domain of unknown function in nidogen (entactin) and hypothetical proteins | MUC4 |
| VWD | Von Willebrand factor type D domain | MUC4 |
| EGF-like | Cysteine-rich EGF-like modules | MUC3, MUC4, MUC12, MUC13, MUC17 |
| PHA03247 | Large tegument protein UL36, found in Herpes simplex virus, provisional | MUC20 |
| Epiglycanin_TR | Tandem-repeating region of mucin, epiglycanin-like | MUC21 |
| Epiglycanin_C | Non-tandem repeat portion of ED, including cleavage site, transmembrane domain and CT | MUC21, MUC22 |
All except the EGF-like motif are identified in the individual gene profiles in the NCBI Gene database
As mentioned briefly in Section II of this article, many of the MAMs can be immunodetected as soluble forms in the various extracellular fluids of the body, and are also found in the extracellular media of cultured cells (Moniaux et al., 2001). This is the result of “shedding” of the large subunit of the MAM as it projects from the cell surface. Shedding may occur spontaneously, but can be stimulated by binding of the large subunit to bacteria and other ligands. A number of biologically important proteins bind the carbohydrate side-chains of MAMs, including galectins, selectins and siglecs (sialic acid-binding immunoglobulin-type lectins)(Bochner and Zimmermann, 2015). Cytokines and extracellular proteinases also promote shedding, as do a variety of physical conditions such as mechanical force or changes in pH, ionic concentration or degree of hydration (Albertsmeyer et al., 2010; Hollingsworth and Swanson, 2004). Shedding may be one stimulus initiating signal transduction.
Table 4 lists CTs in epithelial MAMs, ranked by length. The CTs are quite short in comparison to the EDs. Strikingly (but of unknown significance), the two longest MAMs (MUC16 and MUC4) have considerable shorter CTs than the others. The CTs of individual MAMs are dissimilar in sequence and length and do not contain conserved domains (except for MUC21 and MUC22). Ligand binding, shedding of the large subunit, or other external stimuli leads to engagement of receptor tyrosine kinases or other protein kinases and phosphorylation at specific tyrosine or serine/threonine residues in the CT. Phosphorylation initiates signal transduction cascades that regulate inflammation, cell-cell interactions, differentiation and apoptosis (Bafna et al., 2010; Kato et al., 2012).
Table 4:
Human Epithelial MAMs Ordered by Length of the CT
| Symbol | Amino acid number |
|---|---|
| MUC22 | 92 |
| MUC17 | 89 |
| MUC15 | 74 |
| MUC12 | 74 |
| MUC3A | 73 |
| MUC1 | 72 |
| MUC13 | 69 |
| MUC21 | 66 |
| MUC16 | 31 |
| MUC4 | 22 |
| MUC20 | N/A |
Transmembrane domain predicted by use of the TMPred tool (Hofmann and Stoffel, 1993) on the ExPASy Bioinformatics Resource Portal (Artimo et al., 2012)
4. Evolution
Other than the serine and threonine residues needed for O-linked glycosylation, the amino acid sequences and length of the tandem repeats differ among the different MAMs. The lack of sequence similarity suggests that the MAMs independently evolved the VNTR. In evolutionary biology, organisms not closely related, can independently evolve similar traits as a result of having to adapt to similar environments or ecological niches. This process is called convergent evolution.
On the other hand, evaluation of the shared modules has revealed evolutionary relationships among the MAMs (Dekker et al., 2002). For example, MUC1 has no sequence similarity with the other MAMs except for the presence of the SEA module. This module originated from HSPG2 (perlecan). The MUC1 SEA module is most closely related to those found in MUC3, MUC12 and MUC17 (all grouped at chromosomal locus 7q22.1) as well as MUC13 (at 3q21.2). MUC13 appears to be the ancestral gene, with the others likely the result of duplicative events in evolution. MUC1 (at 1q22) may have lost the two EGF-like modules found in the other subfamily members after its duplication (Duraisamy et al., 2006).
In contrast, MUC16 SEA modules are most-closely related to the SEA module found in the chicken AGRN (agrin) gene. The prototype of this SEA module appears to have evolved before the divergence of birds and mammals (Duraisamy et al., 2006).
MUC4 has a number of modules not found in other MAMs. The NIDO module evolved from an ancestor common to the NIDO (nidogen) protein, and the AMOP and VWD modules originated from an ancestor common to the Sushi-domain containing proteins (Duraisamy et al., 2006).
MUC20, which lies adjacent to MUC4 at chromosomal locus 3q29, also lacks these modules. However, analysis using the EMBL-EBL multiple sequence alignment tool, Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) indicates significant sequence similarity between the MUC4 VNTR and the entire length of MUC20 (S. Jeong, previously unpublished data). This strongly suggests that MUC20 arose by duplication of the MUC4 VNTR followed by sequence divergence.
The most recently discovered MUC21 and MUC22, clustered together at chromosomal locus 6p21.33, also do not share motifs in common with the other MAMs. The VNTR of MUC21 is recognized as a conserved motif called Epiglycanin_TR (Table 3) that is shared by genes of two lower species. The VNTR of MUC22 is not part of this family. However, MUC21 and MUC22 share sequence similarity with one another through the Epiglycanin_C domain, which covers the region downstream of the VNTR, including a putative cleavage site, the transmembrane domain and the CT (Table 3). The presence of this domain provides evidence that MUC21 and MUC22 are the result of an evolutionary duplication event.
Figure 5 depicts the suggested evolutionary relationships among the epithelial MAMs. This analysis supports the concept that MAMs arose largely through a process of convergent evolution, but reveals that they can be grouped into evolutionarily-related subgroups based on their genetic backgrounds. Within a group, evolutionary duplicative events appear to have led to divergent evolution, the process whereby groups from the same common ancestor accumulate differences to serve specific purposes.
Figure 5. Proposed Evolutionary Subgroupings of Epithelial Membrane Associated Mucins (MAMs).
The best evidence is that MAMs arose largely through a process of convergent evolution, but they can be grouped into evolutionarily-related subgroups based on their genetic backgrounds, as shown in the graphic. The rationale for the groupings is discussed in the text.
Some of the information in this graphic is summarized from (Duraisamy et al., 2006).
The complete analysis shown here has not been previously published.
B. MUC1, MUC4 and MUC16
1. The Extracellular Domain
Figure 6 depicts the modular architecture of the EDs of MUC1, MUC4 and MUC16 (as well as MUC20, MUC21 and MUC22, to be discussed later).
Figure 6. Modular Architecture of Ocular Surface Membrane Associated Mucins (MAMs).
Shown are the extended conformations of MAM proteins prior to intracellular processing, but with the final relationship to the plasma membrane depicted. The extracellular domain of each protein is to the left of the plasma membrane. MAMs could not be drawn to scale because of extreme size differences, but an effort was made to depict relative differences in overall size, and relative location and sizes of the modular units. The signal peptides are located at the amino-terminus of each protein. The approximate intracellular cleavage sites of each mucin are indicated by scissors. MUC20 has been experimentally determined to associate with the plasma membrane, but no transmembrane domain has been identified.
SP: signal peptide; TM: transmembrane domain; VNTR: Variable Number Tandem Repeats; conserved modular domains as in Table 3. GSLV: proposed cleavage site for MUC21.
The tandem repeats in the VNTR of MUC1 are 20 amino acids in length, with 25 to 125 repetitions. In MUC4 they are 16 amino acids in length, with 145 to 395 repetitions. MUC1 contains one SEA module, located just proximal to the transmembrane domain. MUC4 lacks a SEA module, but has modules for conserved motifs AMOP, NIDO, and VWD, clustered together, distal to the VNTR. Flanking the VNTR in MUC1 are two regions of unique sequence that are serine and threonine rich and heavily O-glycosylated, like the VNTR. Similarly, MUC4 has a region of imperfect tandem repeats proximal to the tandem repeat unit, also heavily-glycosylated.
The VNTR of MUC16 is unusual in that it contains long, only partially conserved tandem repeat units of 156 amino acids. Variants of this gene encode proteins with 10 to 60 repeats (NCBI Gene); the variant listed in Table 2 has a shorter VNTR than reported in (O’Brien et al., 2001; O’Brien et al., 2002). Proximal to the VNTR is a long region of unique sequence (12,070 amino acids) that is serine and threonine rich and (like the VNTR) is heavily O-glycosylated. Interspersed in the VNTR, and distal to it are a total of 56 SEA modules. The VNTR also contains interspersed leucine-rich repeats and ankyrin repeats (not shown). Each of these features contribute to the very long ED of MUC16 (Perez and Gipson, 2008).
It has been estimated that an extended, O-glycosylated polypeptide of 20 amino acid residues is approximately 5 nm long (Jentoft, 1990). This would mean that human MUC1 extends about 200 – 500 nm above the cell surface. MUC4 would extend at least 2 microns and MUC16 could be twice that, at 4 microns.
During its biosynthesis, MUC1 is cleaved within the SEA module (Palmai-Pallag et al., 2005), while MUC4 is cleaved within the juxtamembrane VWD module (Rossi et al., 1996). This processing occurs in the endoplasmic reticulum after N-glycosylation (Ligtenberg et al., 1992). The complex then migrates to the Golgi apparatus, where it is O-glycosylated, and finally moves to the cell surface, where the two subunits remain strongly associated via non-covalent interactions. It was long speculated that MUC16 harbors single sites for proteolysis in each of the two SEA modules adjacent to the plasma membrane, one being analogous to the MUC1 site. However, it was shown recently that actual cleavage takes place in the juxtamembrane ectodomain stretch of twelve amino acids, and occurs within the Golgi/post-Golgi cellular compartment (Das et al., 2015).
Cleavage of MUC1 within the SEA module also can occur extracellularly via the proteolytic action of ADAM17 (Thathiah et al., 2003) or MT1MMP (Thathiah and Carson, 2004). MUC16 is cleaved extracellularly by proteases such as MMP7, ELNE (neutrophil elastase) and bacterial metalloprotease (ZmpC), although the exact site(s) is not known (Blalock et al., 2008; Govindarajan et al., 2012). Extracellular cleavage results in enhanced shedding of the large subunit from the cell surface.
The ED of MUC4 (but not MUC1 or MUC16) has three EGF-like modules located distal to the cleavage site (Hanson and Hollingsworth, 2016). The one closest to the transmembrane domain is similar in sequence to the EGF-like domain found in ERBB3, a receptor tyrosine kinase of the EGFR family. Rat Muc4 was shown to interact via this EGF-like module with ERBB2, another member of the family. The protein-protein interaction induced specific phosphorylation of ERBB2 and led to downstream signaling (Jepson et al., 2002). Complex formation also potentiated activity of ERBB3 stimulated by binding to NRG1 (neuregulin). This implicated Muc4 in regulation of epithelial cell proliferation in rat carcinoma.
2. The Cytoplasmic Tail
Figure 7 depicts an alignment the CTs of human, mouse and rat MUC1, MUC4 and MUC16, delineating, in red, experimentally confirmed serine, threonine or tyrosine phosphorylations, as curated by the public database PhosphoSitePlus® (Hornbeck et al., 2019). Some of the known MUC1 CT interacting proteins are indicated in red above their recognition sequences. Sites predicted to be phosphorylated by analysis using the NetPhos 3.1 Server (Blom et al., 2004) are delineated in blue, along with predicted protein kinase effector (M.E. Fini, previously unpublished). Polybasic amino acid stretches previously identified in human are highlighted in yellow for all three species.
Figure 7. Sequence Analysis of Membrane-Associated Mucin (MAM) Cytoplasmic Tails (CTs).
Amino acid sequences of the human MAM CTs, as determined by conceptual translation of the mRNA sequence, are shown. At the end of each sequence, the amino acid count is indicated. If there is an orthologue in mouse and rat, this is also shown and conserved amino acids are identified with a line between the two sequences.
Lower case letters in red indicate serine, threonine or tyrosine residues confirmed experimentally to be phosphorylated on the PhosphoSitePlus website. Lower case letters in blue indicate serine, threonine or tyrosine residues predicted by the NetPhos 3.1 Server.
Some of the many confirmed MUC1 interacting proteins are indicated in red above the recognition sequence: serine-threonine kinase GSK3B (SXXXS); tyrosine kinase PIK3R1 (regulatory subunit; Y20HPM); receptor tyrosine kinase EGFR (Y46EKV/Y46EEV); phospholipase PLCG1 (Y35VPP); adherens junction component beta-catenin CTNNB1 (SXXXXXSSL); adaptor protein GRB2 (Y60TNP); tyrosine kinase SRC (Y46EK/EV).
Predicted phosphorylating kinase are indicated in blue above the predicted phosphorylation site. If there are additional predictions for the mouse/rat sequences, these are indicated as well. Predicted phosphorylating kinases: gsk: glycogen synthase kinase-3 isoform; pka: protein kinase A isoforms; pkc: protein kinase C isoforms. Others designated by HUGO nomenclature. The proposed N-terminal pamitoylation site in MUC1 and adjacent polybasic amino acid stretches in MUC1 and MUC16 are in blue text and underlined. Regions predicted to have disordered protein binding properties in human MUC21 and MUC22 are in underlined black text.
This compilation, with its new analyses, has not been previously published.
The CT of MUC1 is the best studied by far. It has been observed that the amino acid sequence is highly conserved across species (Spicer et al., 1991; Vos et al., 1991), as demonstrated here for human, mouse and rat (Figure 7). Seven tyrosines and eleven serines/threonines have been experimentally confirmed to be phosphorylated under various conditions. Four of the confirmed tyrosine phosphorylations are located within sequences that constitute signaling protein binding motifs: Y20HPM (phosphatidylinositol 3-kinase regulatory subunit PIK3R1); Y35VPP (phospholipase PLCG1); Y46EK/EV (SRC family kinases); Y60TNP (GRB2) (Zrihan-Licht et al., 1994). Molecular and biological effects have been determined for all four of these tyrosines as well as two of the serine/threonines.
In just one example, EGFR, a receptor tyrosine kinase of the same family as ERBB2, associates constitutively with the MUC1 CT in human breast carcinoma cells. Active EGFR binds the MUC1 CT at Y46EK/EV and phosphorylates the tyrosine residue (Schroeder et al., 2001). This phosphorylation stimulates SRC binding (Li et al., 2001). SRC strengthens the binding of CTNNB1 (beta-catenin) to the sequence motif SAGNGGSSL by phosphorylating a different tyrosine residue located near the CTNNB1 binding site (Li and Kufe, 2001; Li et al., 2001). Conversely, binding of CTNNB1 is weakened by the activity of GSK3B, which binds the SXXXS motif located proximal to the CTNNB1 binding site, phosphorylating the final serine (Li et al., 1998). These effects on CTNNB1 binding strength, influence the ability of CDH1 (E-cadherin) of the adherens junction (Huang et al., 2005) to compete for binding to CTNNB1. This competition affects epithelial cell-cell adhesion (Quin and McGuckin, 2000; Yamamoto et al., 1997).
Much less is known about the MUC16 CT however, it appears that EGFR-mediated phosphorylation may occur here as well. MUC16 contains two tyrosine residues and one serine residue confirmed experimentally to be phosphorylated (Figure 7). An analysis conducted using the NetPhos 3.1 Server (Blom et al., 2004) predicts that EGFR phosphorylates the proximal tyrosine residue (M.E. Fini, previously unpublished). Large subunit shedding stimulates CT phosphorylation and this is enhanced when cells are treated with EGF (Fendrick et al., 1997).
Also like MUC1, MUC16 binds to CTNNB1-CDH1 complexes (Comamala et al., 2011). MUC16 lacks a canonical CTNNB1 binding site, but pull-down experiments suggest that a polybasic amino acid stretch at the proximal end of the CT interacts with ezrin/radixin/moesin (ERM) actin-binding proteins that then interact with the adherens junction (Blalock et al., 2007). NetPhos 3.1 Server also predicts that MUC16 is phosphorylated by CDK1. In complex with CCNA2 (cyclin A2), CDK1 promotes adhesion complex and actin cytoskeleton organization during interphase and mediates a large increase in adhesion complex area as cells transition from G1 into S (Jones et al., 2018).
The CT of many cell surface receptors migrates to the nucleus to perform additional functions, often initiated by shedding of the large subunit. This has been shown to occur in the case of both MUC1 and MUC16. MUC1 traffics to the nucleus in complex with CTNNB1, raising the possibility that MUC1 might directly influence the transcriptional co-activator activity of CTNNB1 (Li and Kufe, 2001; Ren et al., 2002). Nuclear translocation appears to involve endocytosis of MUC1 from the cell surface as a first step, requiring phosphorylation of Y60TNP and binding of GRB2 (Kinlough et al., 2004).
Essentially nothing is known about function of the MUC4 CT. The amino acid sequence is poorly conserved between humans and mouse/rat, with only three amino acids conserved among the three species (Figure 7). An analysis of the human sequence conducted using the NetPhos 3.1 Server predicts phosphorylation at three different serine residues by protein kinase A and protein kinase C isoforms (M.E. Fini, previously unpublished). Interestingly, two of these serines are conserved in the CT of mouse and rat and are also predicted to be phosphorylated. The CT of mouse/rat are slightly longer than human, and possesses an additional predicted site for phosphorylation site by the catalytic subunit of casein kinase II, CSNK2A1. Interestingly, it has been reported that CSNK2A1 interacts with the adherens junction and modulates intracellular adhesion (Lickert et al., 2000).
C. MUC20
Structural architecture of the MUC20 ED is depicted in Figure 6. The predicted human MUC20 isoform NP_001269435.1 is a polypeptide of 709 amino acids with a signal peptide of 22 amino acids. All four isoforms currently listed in NCBI’s database have predicted signal peptides (a previous report discussed a variant lacking a signal peptide (Higuchi et al., 2004b)). Isoform NP_001269435.1 has 12 tandem repeat units of 19 amino acids each. The other isoforms have three or four tandem repeats. Extensive O-linked glycosylation of the tandem repeats is predicted by sequence analysis using the NetOGlyc 4.0 Server (Steentoft et al., 2013) (M.E. Fini, previously unpublished). MUC20 lacks SEA or EGF-like modules. However, it contains one copy of a conserved domain, PHA03247, which is also found in the large tegument protein of Herpes simplex virus type I (Table 3). This module follows the tandem repeats.
Sequence analysis of human MUC20 identified several hydrophobic regions consistent with plasma membrane association, but no alpha-helical transmembrane domain was recognized (Higuchi et al., 2004b). Application of the TMPred tool (Hofmann and Stoffel, 1993) on the ExPASy Bioinformatics Resource Portal (Artimo et al., 2012) did not identify a transmembrane domain (M.E. Fini, previously unpublished). Nevertheless, when MDCK cells harboring a human MUC20 expression construct were biochemically fractionated, MUC20 protein was identified in the membrane fraction, which includes plasma membrane, endoplasmic reticulum and golgi. Immunoelectron microscopic analysis of whole cells demonstrated localization to the plasma membrane (Higuchi et al., 2004b).
In a second study from the same authors, MUC20 immunoreactivity was observed in the basal membranes of proximal tubular epithelia of the human kidney (Higuchi et al., 2004a). In human ocular surface epithelia, immunoreactive MUC20 was detected predominantly in the cell membrane area of intermediate cell layers (Woodward and Argueso, 2014). Biotin labeling of the surface of corneal epithelial-equivalent cultures revealed only low levels of MUC20 protein on apical glycocalyces.
Thus, current evidence suggests MUC20 is a non-secreted protein retained at the plasma membrane, but possibly extrinsically rather than transmembrane. For this reason, only the MUC20 ED is depicted in Figure 6, and not the distal regions. In the ocular surface study discussed above, MUC20 was not detected in the media of epithelial tissue-equivalent cultures or in human tears, consistent with the idea that it is neither secreted nor shed (Woodward and Argueso, 2014).
Because of uncertainty about its structure, MUC20 is not included in the analysis of CT sequences shown in Figure 6. Analysis, using the NetPhos 3.1 Server, of the short amino acid sequence following a predicted alpha-helical region near the C-terminus of MUC20 predicted no potential phosphorylation sites of statistical significance (M.E. Fini, previously unpublished). Recombinantly-expressed human MUC20 was shown to associate via its C-terminal domain with MET, a receptor tyrosine kinase activated by the extracellular ligand HGF. The interaction prevented GRB2 recruitment to MET, attenuating HGF-induced activation and intracellular signaling (Higuchi et al., 2004a).
D. MUC21 and MUC22
The modular architecture of MUC21 and MUC22 is depicted in Figure 6.
The human MUC21 protein predicted by NCBI Protein entry NP_001309299.1 is the longest of three variant isoforms listed in the NCBI Gene database, and its analysis has not previously been described. The NCBI Protein profile of the variant predicts a signal peptide of 24 amino acids, followed closely by a series of 32 imperfect tandem repeat units of 15 amino acids each. Extensive O-linked glycosylation within the tandem repeat units was predicted for another variant (Itoh et al., 2008) by sequence analysis using the NetOGlyc 4.0 Server (Steentoft et al., 2013). The TMPred tool (Hofmann and Stoffel, 1993) on the ExPASy Bioinformatics Resource Portal (Artimo et al., 2012), predicts an alpha-helical transmembrane region of 21 amino acids, followed by a CT of 59 amino acids (M.E. Fini, previously unpublished).
Analysis of the human MUC22 protein predicted by NCBI Protein NP_001185744.1 has previously been reported (Hijikata et al., 2011). It has an N-terminal signal peptide of 26 amino acids. The ED contains 124 non-identical tandem repeats of 10 amino acids each. Extensive O-linked glycosylation of the tandem repeats is predicted by the NetOGlyc 4.0 Server (Steentoft et al., 2013) (S. Jeong, previously unpublished). Following this is an alpha-helical transmembrane domain of 21 amino acids and an CT of 92 amino acids.
It is not known whether MUC21 or MUC22 are cleaved during their biosynthesis. Both MUC21 and MUC22 lack SEA modules, however, a sequence (GSLV) similar to the putative cleavage site associated with the SEA module in MUC1 is present immediately upstream of the putative transmembrane domain in MUC21 (Itoh et al., 2008). This potential cleavage site is included in the conserved motif Epiglycanin_C, domain shared by MUC22, but the specific sequence is not conserved.
Function of the MUC21 tandem repeats in cell adhesion to the substratum was investigated by transient transfection analysis (Yi et al., 2010). When HEK 293T cells were transfected with a mouse Muc21 expression construct harboring a cDNA containing 84 tandem repeat units, cells were significantly less adherent to each other and to extracellular matrix components than control cells. The anti-adhesion effect was weaker when constructs with smaller numbers of tandem repeats were used, suggesting that the tandem repeat domain plays a crucial role. Antibody binding to the cell surface integrin subunits ITGA5, ITGA6, and ITGB1 was reduced in MUC21 transfectants in a tandem repeat-dependent manner, whereas equal amounts of proteins were detected by Western blot. MUC21 was expressed as a large glycoprotein that was highly glycosylated with O-glycans at the cell surface, as detected by flow cytometry, Western blotting, and lectin blotting. Although at least a portion of Muc21 was glycosylated with sialylated glycans, removal of sialic acid did not influence the ant-adhesive effect.
The MUC21 and MUC22 CTs are depicted in Figure 7. MUC21’s CT is of moderate length as compared to the other MAMs. At 92 amino acids, MUC22’s CT is the longest of all the MAMs expressed at the ocular surface. According to the PSIPRED Workbench (Buchan et al., 2013) tool for predicting protein secondary structure (Jones, 1999), each CT likely contains specific regions of disordered structure, providing elements for binding of other proteins (S. Jeong, previously unpublished).
MUC21’s CT is characterized by the presence of seven proline residues. The proline-rich regions are known to preferentially adopt a polyproline type II helical conformation, an extended structure that facilitates transient intermolecular interactions important to intracellular signaling (Srinivasan and Dunker, 2012). For example, many adapter proteins possess specific protein domains such as the Src homology 3 (SH3) domains and the WW domains that selectively recognize proline-rich regions in their interacting partners (Mansiaux et al., 2011; Peterson and Volkman, 2009).
MUC22’s CT is rich in glycine, which comprises more than 1/4th of the total amino acid residues (26/92). Glycine is unique in that the side chain consists of only a single hydrogen atom, providing for flexible conformation. There is also an enrichment for His, which is unusual in having a PKa of 6.5, ~physiological pH. This means it exists simultaneously in protonated/deprotonated forms, a feature that could activate binding proteins. Two cysteine residues are located at the proximal aspect of the CT as it emerges from the plasma membrane, providing for possible internal disulfide coupling, or coupling with other proteins.
As will be discussed more is Section IV, neither a laboratory rat orthologue to human MUC21, nor mouse or rat orthologues to human MUC22 has been identified. The amino acid sequence of the MUC21 CT is poorly conserved between human and mouse. One site for tyrosine phosphorylation and three sites for serine/threonine phosphorylation have been experimentally confirmed in the human MUC21 CT. Significantly, all four sites are conserved in mouse. A single tyrosine and three serine phosphorylation sites in the MUC22 CT are predicted by the NetPhos 3.1 Server (Figure 7).
EGFR is predicted to phosphorylate the tyrosines in the CTs of both MUC21 and MUC22. The serine/threonine sites are predicted to be phosphorylated by a member of the protein kinase A family and CDK1/CDK5. Activated by cAMP, PKA lies downstream of G protein-coupled receptors (GPCRs) that couple with G’s. Thus, any ligand that activates these GPCRs should also activate MUC21 and MUC22. As noted with regard to MUC16, CDK1 promotes adhesion complex and actin cytoskeleton organization during interphase (Jones et al., 2018). Similarly, CDK5 has been shown to promote the stability of corneal epithelial cell junctions (Arpitha et al., 2013).
IV. Model Systems and Biological Roles
Human studies of the ocular surface are limited to non-invasive techniques such as tear collection and analysis, staining of the ocular surface with vital dyes, and impression cytology for collection of apical cells of the conjunctiva. Biopsies routinely taken in other organs such as skin are not done in cornea because of the resulting pain and disruption of vision. Human cadaver corneas obtained from a local eye bank or from the National Disease Research Interchange (Philadelphia, PA) can be placed in organ culture for study and are amenable to genetic manipulation. This has been an effective model for wound healing studies (e.g., (Castro et al., 2019; Kramerov et al., 2016)), however, the ocular surface glycocalyx is easily damaged in the Optisol storage medium (Chiron Vision, Claremont, CA) that is typically used by the eye and tissue banks.
Human tissue-equivalent and mouse models enable genetic manipulation, and have successfully substituted for investigation of MAM functional roles in the ocular surface mucosal glycocalyx. We discuss these models here. We go on to discuss key findings made using each. Individually and together these models have led to significant advances in our understanding of MAM roles at the ocular surface in health and disease, and hold much promise for new discovery.
A. Human Tissue-Equivalent Model
As described in the first paragraph of our Introduction, cells at the ocular surface are very different from the basal cells of the multilayered epithelia from which they arise. Significantly, while monolayer cultures of corneal or conjunctival epithelial cells express MAMs at the mRNA level, MAM proteins do not accumulate to detectable levels in these cultures. However, MAM proteins accumulate in a polarized manner at the surface of three-dimensional mucosal epithelial tissue-equivalents.
Human tissue-equivalents represent the human ocular surface glycocalyx with substantial fidelity. This model lacks in vivo complexity, for example, there is no immune system contribution. However, its reductionist nature provides an advantage for the study of molecular pathway(s), as well as for isolating the contributions of individual tissues to complex biological responses.
Epithelial tissue-equivalent technology was developed first for skin. A technique for successful serial cultivation of epidermal keratinocytes was reported in 1975 by Rheinwald and Green (Rheinwald and Green, 1975). This involved plating dissociated cells on a feeder layer of mouse 3T3 fibroblasts that had been previously irradiated to preclude their proliferation. Cells of the feeder layer secrete soluble factors into the culture medium, and also deposit extracellular matrix on the culturing surface, facilitating keratinocyte cell attachment and growth, and enabling the clonogenic expansion of individual cells (Green et al., 1977). Stratification is then induced by increasing the calcium concentration and by “airlifting”, i.e., reducing the volume of culture medium so that the keratinocytes were located to the air–medium interface. Under these conditions, proliferating basal cells remain in close proximity to the gradient of nutrients provided by diffusion (Bernstam et al., 1986; Prunieras et al., 1983).
In recent years, defined media such as Keratinocyte Growth Medium® (Lonza, Walkersville, MD) and Epilife® (Invitrogen, Carlsbad, CA) have become commercially-available for serial culture and differentiation to an epidermal- or corneal epithelial-equivalent without the use of feeder layers (Argueso and Gipson, 2012; Rasmussen et al., 2013).
Human corneal epithelial-tissue equivalents typically made use of primary cells isolated from corneal–limbal rims discarded at the time of corneal transplantation. These usually contain tissue-specific stem cells. However, the finite replicative lifespan of the amplified progeny cells makes them impractical for research approaches requiring stable genetic transfection or genome modification. For such experiments, immortalized epithelial cell lines that retain differentiation characteristics have become widely used.
Corneal cell lines have been developed by immortalization with viral oncogenes, including adenovirus E1A, the SV40 large T antigen, and HPV16-E6/E7, but their effectiveness as research models has been hampered by both genetic instability, as well as a lack of normal growth and differentiation. This is likely because expression of oncogenes perturbs cell differentiation programs (Weinberg, 1998). For example, cell lines immortalized with SV40 large T antigen were found to stratify and make proteins that distinguish differentiated corneal epithelia, but they did not synthesize glycosylated MAMs (Gipson et al., 2003).
Newer cell lines utilizing an active version of the TERT gene for immortalization, have been more successful. TERT encodes the catalytic subunit of telomerase, an enzyme that repairs telomeres damaged during chromosome replication. Located at the ends of chromosomes, telomeres have been compared to the metal clips at the ends of shoelaces, the “caps” that prevent the shoelace from unraveling. A natural constraint on the ability to completely replicate chromosome ends leads to a shortening of telomeres, with each cell replication. At some point telomeres change from a “capped” state to an “uncapped” state, signaling cell senescence. Telomerase is active in development, but is silenced in almost all organ systems from the embryo onwards, except germ cells (and stem cells, to some extent). Adding an active copy of TERT compensates for erosion of chromosome ends during the process of replication and makes the cell line carrying the gene functionally immortal.
Gipson and colleagues (Gipson et al., 2003) created a corneal epithelial cell line, HCLE, using a combination of strategies. Heeding reports that knockdown of CDKN2A (p16) and/or TP53 (p53) tumor suppressor pathways is necessary to immortalize human epithelial cells (Kiyono et al., 1998; Rheinwald et al., 2002; Weinberg, 1998), they first transduced primary cultures of human corneal–limbal and conjunctival epithelial cells with mutant CDK4 and dominant-negative TP53-expressing constructs. Then they added a TERT-expressing construct. When grown in high-calcium medium on plastic and type I collagen, cells of both lines stratified and differentiated. HCLE cells expressed corneal epithelial–specific keratins K3 and K12, and both HCLE and HCjE cells expressed K19. As in native tissue, both cell lines expressed MUC1, MUC4, and MUC16 and immunoreactive MUC1 and MUC16 proteins were localized to the apical cell layers of the stratified cultures. Importantly, both cell lines produced glycosylated mucins.
A second immortalized corneal epithelial cell line, hTCEpi, was developed from primary cultures of human corneal epithelial cells (Robertson et al., 2005). Heeding reports that CDKN2A induction can be bypassed under appropriate culture conditions, only a TERT-expressing construct was used. Indeed, it was observed that CDKN2A activity was gradually downregulated with increasing passaging of TERT-immortalized cells and did not require direct abrogation. Air-lifting produced a well stratified epithelium (five to seven cell layers) with apical ZO1-stained tight junctions. Submersed culture demonstrated increasing expression of stratification markers (keratins K5/K14) with K3-corneal keratin marker expression in long-term, air-lifted culture.
During limbal epithelial cell expansion in vitro, air-lifting has been shown to increase cellular stratification, enlarge surface cells, trigger cellular differentiation, and increase barrier function (Chen et al., 2017b). However, airlifting also appears to mimic some of the changes described in severe dry eye and squamous metaplasia, with reduced expression of mucosal markers (Li et al., 2008; Lin et al., 2014). A recent study examined mucosal marker expression in the hTCEpi epithelial equivalents created without airlifting (Yanez-Soto et al., 2015). Cells expressed MUC1, MUC4 and MUC16 mRNA and proteins, with a maximum between days 1 and 3 of the stratification process. Taken together, these studies suggest that airlifting should not be employed when mucosal differentiation of epithelial-equivalents is required.
At the same time as they created the HCLE corneal epithelial cell line, Gipson and colleagues developed a conjunctival cell line, HCjE using the same approach (Gipson et al., 2003). Other conjunctival cell lines have been created by other groups (Garcia-Posadas et al., 2017; Li et al., 2008; Lin et al., 2014). Conjunctival epithelial cell lines are especially useful, because there is not the tissue source that discarded donor corneal–limbal rims provide. They have been used as a model for dry eye and ocular surface inflammation.
1. Rose Bengal Exclusion
The most commonly used method for tracking damage to the ocular surface is staining with water soluble “vital” dyes (Abelson and Ingerman, 2005). This includes damage due to a variety of ocular surface diseases, including dry eye, (keratoconjunctivitis sicca), a desiccating condition of the ocular surface affecting 20% or more of the population in North America, Europe, and Asia (Craig et al., 2017). Vital dye staining is also observed after exposure to contact lenses soaked in certain multipurpose contact lens cleaning solutions (MPS), a phenomenon that has been called solution-induced corneal staining (SICS) (Maldonado-Codina et al., 2013). Similarly, the most frequently used preservative in topical eye drops, benzalkonium chloride, causes damage to the ocular surface and vital dye staining (Baudouin et al., 2010).
Fluorescein dye was first used clinically in 1882 for evaluation of corneal epithelial defects (Pflüger, 1882). Rose bengal dye became popular in the 1930s for dry eye diagnosis because of the distinctive “punctate” staining pattern observed at the ocular surface of patients (Sjögren, 1933). Rose bengal is now infrequently used in clinical practice, because of patient discomfort (Bron et al., 2015), but fluorescein continues to be used, and is the standard endpoint for clinical trials of investigational new drugs for dry eye (e.g., (Holland et al., 2017)).
Studies published in the early 1990s reported that healthy, living cells in monolayer culture, but not dead cells, take up rose bengal (Feenstra and Tseng, 1992b) and that uptake is blocked by addition of tear components such as mucins. Fluorescein is the parent compound from which rose bengal was derived; thus, the two dyes are closely related but differ somewhat in uptake properties (Kim, 2000). Living corneal epithelial cells in monolayer culture take up fluorescein in the same way as rose bengal, but at a lower level, requiring visualization under epifluorescent illumination (Feenstra and Tseng, 1992a). Unlike rose bengal staining, fluorescein uptake did not appear to be blocked by mucins added to monolayer cell cultures (Feenstra and Tseng, 1992a).
Later it was shown that human corneal epithelial cells in culture exclude rose bengal autonomously if they differentiate and elaborate a mucosal glycocalyx, i.e., develop into an epithelial tissue-equivalent with mucosal differentiation (Argueso et al., 2006). Inhibition of O-glycosylation by knockdown of T-synthase, a galactosyltransferase required for synthesis of core1 O-glycans, decreased surface O-glycosylation and increased dye penetrance. This demonstrated the importance of the O-glycans in forming a transcellular barrier to dye entry (Argueso et al., 2009). This also was the first indication that monolayer cell cultures, because they do not elaborate a mucosal glycocalyx, are not a valid model of the ocular surface. In contrast, the mucosal glycocalyx associated with the cell surface does not appear to be a significant barrier to fluorescein penetrance (unpublished observations).
Molecular knockdown experiments using the HCLE tissue-equivalent model have shown that MUC16 is essential for the exclusion of rose bengal dye (Blalock et al., 2007). MUC16 appears to be the major MAM component in promoting transcellular barrier to vital dye penetration. In fact, knockdown of MUC1 decreased dye penetrance (Gipson et al., 2014). The lectin LGALS3 (galectin-3), is required to cooperate in the exclusion of rose bengal, as inhibition of LGALS3 binding to MAMs resulted in increased rose bengal staining (Argueso et al., 2009). LGALS3 contains a conserved carbohydrate-binding domain with affinity towards beta-galactosides and forms multimers via intermolecular interactions via its N-terminal domain. It can therefore mediate crosslinking of glycoproteins such as MAMs.
Thus, the transcellular barrier to dye uptake is comprised of extended, heavily glycosylated MAM EDs, pulled together into an organized lattice-like structure via LGALS3. The dominance of MUC16 over MUC1 may be due, not only to the exceptional length of its ED, but also its the heavy O-glycosylation, providing a substantial hydrophilic surface. MUC16 would provide more surface for glycan-galectin interactions to hold the molecules in a tight conformation. Abrogation of MUC1 with its shorter ED, would mean a more uniform glycocalyx, potentially resulting in a more substantial barrier. When the MAM repertoire is mixed, several levels of MAM-galectin association may be present with MUC16 EDs extending further from the cell membrane than MUC1 EDs. This uneven, mixed-length lattice could create spaces for dyes to reach the cell surface (Gipson et al., 2014).
We have been making use of HCLE corneal epithelial-equivalents with mucosal differentiation to investigate mechanisms of rose bengal staining due to damaging stress. Figure 8 shows the effect of oxidative stress on rose bengal dye uptake in the HCLE corneal epithelial-equivalents with mucosal differentiation. This effect is highly reproducible and quantifiable. One of our groups showed that benzalkonium chloride or MPS treatment of HCLE epithelial-equivalents with mucosal differentiation causes an increase in rose bengal staining, and we provided evidence for the associated shedding of a portion of the MUC16 large subunit (Gordon et al., 2011). More recently, we showed that oxidative stress causes shedding of LGALS3 into the culture medium (Webster et al., 2018). These results suggest that rose bengal staining of the ocular surface under conditions of stress is caused by disruption of the mucosal glycocalyx.
Fig 8. Application of Oxidative Stress to HCLE Epithelial-Equivalents with Mucosal Differentiation.
Islands of cells with mucosal differentiation at the surface of HCLE epithelial-equivalents exclude rose bengal (A and B). When oxidative stress is applied (10 mM tBHP in DMEM/F12 medium for 2 hours, as previously described (Webster et al., 2018)), many of these cells lose their transcellular barrier function and rose bengal penetrates (C and D). Rose Bengal staining was performed by incubating the cells 5 minutes in 0.1% Rose Bengal as previously described (Argueso et al., 2006). Magnification: A and C are 4x; B and D are 10x.
This example has not been previously published, but is similar to findings published in (Webster et al., 2018)
2. Desquamation
In the Introduction to this article, we discussed how the ocular surface epithelia are continually renewed in a process whereby daughter cells generated by division of basal cells at the basement membrane are displaced upward in the cell layers, become increasingly flattened and undergoing mucosal differentiation. Once differentiated, the apical layer of mucosal epithelial cells do not remain static, but are shed fairly rapidly, in a process called desquamation.
Scanning electron microscopy (SEM) of the ocular surface of various mammals has revealed a contiguous mosaic of polygonal cell shapes with a range of sizes, each having a light, medium, or dark appearance, the dark reflex cells being predominantly the largest (Doughty, 2016; Pfister, 1973). The dark reflex is due to a reduction in the size and number of microplicae and microvillae (Hazlett et al., 1980; Pfister, 1973). Within a short time, the most mature cells are shed from the cell surface and are replaced by the cells in the epithelial cell layer beneath. It has been estimated that new basal epithelial cells move upwards and are lost from the surface in a period of 3.5 to 7 days in mouse, rat, guinea pig, and dog (Hanna and O’Brien, 1960). Turnover time of the human corneal epithelium has been estimated to be on the order of 1 week (Hanna et al., 1961).
The molecular mechanisms controlling desquamation at the ocular surface are essentially unknown. Much more is known about the process in the epiidermis (Milstone, 2004), however, with its complex, stratified architecture, the epidermis may not be the best comparison. In the granular layer, epidermal keratinocytes undergo the last steps of their terminal differentiation program resulting in cornification. The coordinated conversion of living keratinocytes into corneocytes, the building blocks of the cornified layer, represents a unique form of programmed cell death (Eckhart et al., 2013). Keratinocytes activate anti-pyroptosis pathways to prevent inflammation and premature cell death during terminal differentiation (Eckhart and Tschachler, 2018).
In contrast to corneocytes, essentially all cells in the apical layer of the ocular surface epithelia remain alive, as evidenced by uptake of calcein dye (an indicator of cell viability) (Ren and Wilson, 1996). Some earlier references in the literature suggest that cell death during desquamation occurs by apoptosis, pointing to observations of a small percentage of ANXA5-binding or TUNEL-positive cells at the apical layer of human corneal epithelial tissue-equivalent culture (Jester et al., 2003), and at the apical layer of the normal corneal epithelium of mice (Strong et al., 2005; Yeh et al., 2003). However, other studies reported that the desquamation process at the ocular surface did not involve bleb formation or nuclear DNA laddering characteristic of apoptosis (Lomako et al., 2005; Ren and Wilson, 1996). The number of apoptotic cells increases considerably when the ocular surface is under desiccating stress (e.g., (Yeh et al., 2003{Bauskar, 2015 #1494), suggesting that apoptosis is primarily a mode of cell death following damage.
Thus, a specialized form of cell death may be utilized at the ocular surface, as in the epidermis. In the adult rabbit, apical cells display ring-shaped depressions (“craters”) or full-thickness holes. The microvilli of the underlying cell can be seen at the base of a hole, having parameters consistent with a light cell (Pfister, 1973). The ring-shaped features are smallest on light cells, slightly larger on medium cells, and largest on dark cells (Doughty, 2006). These findings have suggested an orderly sequence of events leading to desquamation, which starts when a cell reaches the ocular surface. At that time, a hole forms and then expands in a controlled manner as the cell matures from a light cell into a dark cell. Hole formation exposes the underlying, less mature light cell. As the overlying mature cell is shed, the underlying light cell reaches the surface and the sequence of events begins again. Because hole formation exposes the well-formed microvillae on the underlying cells, it may represent a unique process for cell turnover that functions to maintain tear film stability (Pfister, 1973). Once shed, desquamated corneal epithelial cells no longer take up calcein; instead their nuclei stain with ethidium (an indicator of non-viability) (Ren and Wilson, 1996).
Corneocytes in the epidermis and epithelial cells in the apical layer at the ocular surface must actively dissociate themselves from neighboring cells to desquamate. In the epidermis, a critical event in this process includes the rearrangement of junctional desmosome complexes and their ultimate enzymatic digestion, regulated by the interplay between multiple proteases and their inhibitors (Has, 2018). In Xenopus frogs, ocular surface desquamation follows a circadian cycle and the proteinase MMP2 is located in the right place, at the right time, to disrupt intracellular junctional proteins (Wiechmann et al., 2014). Whether MMP2 is functionally involved in the process of desquamation remains to be determined.
A surface view of a human cornea, immunostained with an antibody to MUC1 or MUC16, reveals a mosaic of light, medium, and dark cells very similar to the ocular surface imaged by SEM (Gipson, 2014). The intensity of immunostaining correlates indirectly to the cell surface area, the largest (and presumably most mature) dark cells staining the least. Thus, as apical cells mature, membrane folds are reduced and cell area increases, MUC1/ and MUC16 are corresponding lost from the cell surface.
Two studies have implicated MAMs in desquamation of the ocular surface epithelia. The first employed a rat corneal epithelial-equivalent model with mucosal differentiation. Cells that appeared to be desquamating in culture exhibited a high level of Muc4 accumulation. Since Muc4 has been shown to be a potent anti-adhesive and a repressor of apoptosis in cancer cells, it was proposed that it might play a role in the non-apoptotic desquamation process in normal cells (Lomako et al., 2005).
A study using the HCLE tissue-equivalent model with mucosal differentiation implicated MUC16 in desquamation. Knockdown of MUC16 decreases transepithelial resistance, a measure of paracellular barrier integrity (Gipson et al., 2014). This was associated with decreased expression of ZO1 and OCLN, which encode components of tight junctions. Knockdown of MUC16 also resulted in disruption of the actin cytoskeleton associated with tight junctions and reduced surface microplicae leading to greater apical surface cell area (Gipson et al., 2014). It is suggested that the ezrin binding domain in the ICD of MUC16 may mediate this effect, by interaction with the actin cytoskeleton of the microplicae. Loss of microplicae and the ‘lubricating’ membrane-associated mucin MUC16 may cause the cells to stick to secreted mucins in the tear film and facilitate their removal during desquamation (Gipson, 2004).
It will be important to follow up on these findings to define the process of desquamation at the ocular surface.
3. Barrier to Infection
MUC1 provides a substantial barrier to infection in various mucosal organ systems (reviewed in (Dhar and McAuley, 2019)). Two mechanisms for this protection have been described.
First, MUC1 serves as an adhesion receptor for various pathogenic bacteria and viruses (e.g. (Boll et al., 2017; Dhar et al., 2017; Lillehoj et al., 2015; Lillehoj et al., 2002; Linden et al., 2009; McAuley et al., 2017)). The MUC1 ED contains the glycan antigens Lewisb, sialyl Lewisa, and sialyl Lewisx, which can serve as binding sites for the bacterial adhesins (Linden et al., 2009). MUC1 binds the respiratory pathogen P. aeruginosa via bacterial flagellin (Lillehoj et al., 2002). Rather than a way for pathogens to access cells however, binding to MUC1 appears to inhibit infection by triggering large subunit shedding (Lillehoj et al., 2015; Linden et al., 2009). In this way, the MUC1 large subunit serves as a releasable decoy, promoting bacterial and viral clearance.
MUC1 expressed on the surface of macrophages also binds pathogens. However, in this case, binding inhibits infection in a different way. Phagocytosis of P. aeruginosa by alveolar macrophages contributes to its clearance from the lungs (Dhar et al., 2017). Binding to MUC1 on the surface of macrophages appears to be the first step in phagocytosis, as MUC1-deficient macrophages are inefficient at phagocytosing pneumococci.
If MUC1 binding of pathogens limits infection, then increasing the amount of MUC1 on the cell surface should be beneficial. P. aeruginosa stimulates alveolar macrophages to release TNFA, which induces MUC1 protein levels in airway epithelial cells (Dhar et al., 2017). Overexpression of MUC1 by epithelial cells or the addition of sialylated synthetic MUC1 constructs, reduced Influenza A viral infection in vitro. Respiratory Syncytial Virus and human metapneumovirus also stimulate MUC1 expression, consistent with a role in protection against infection (Banos-Lara Mdel et al., 2015; Li et al., 2010).
Respiratory Syncytial Virus is also a strong inducer of MUC21 and MUC22 expression (Banos-Lara Mdel et al., 2015). MUC21 is one of the most upregulated RNAs in bronchoalveolar lavage fluid of children with pneumonia due to severe infections of the atypical bacteria Mycoplasma pneumoniae (Wang et al., 2017). These findings suggest that MUC21 and MUC22 are also involved in defense against infection.
Staphylococcus aureus is among the most common bacterial pathogens involved in ocular surface infection. S. aureus adheres to the surface of many cell types via the bacterial-encoded fibronectin-binding protein, and this also appears to be the case for corneal epithelial cells (Jett and Gilmore, 2002). The current model for fibronectin-binding protein-mediated adhesion and invasion proposes a fibronectin-dependent bridging between S. aureus fibronectin-binding proteins and host cell alpha5beta1 integrin (Massey et al., 2001). However, this model was developed from the findings of studies performed in monolayer cultures; other factors may be at play in mucosal epithelia. In fact, S. aureus does not adhere to apical cell surfaces in the presence of an intact glycocalyx (Govindarajan et al., 2012; Jett and Gilmore, 2002; Spurr-Michaud et al., 1988).
In the HCLE tissue-equivalent with mucosal differentiation, knockdown of MUC16 increased cell surface adherence of Staphylococcus aureus (Blalock et al., 2007; Gipson et al., 2014), but knockdown of MUC1 has the opposite effect (Gipson et al., 2014). This suggested to the authors that the barrier to pathogens is improved in the absence of MUC1, much like the barrier to rose bengal dye. Alternatively, MUC1 may be essential for bacterial adherence, but MUC16 interferes with bacterial access to MUC1. More work is needed to understand these alternatives better.
4. Dampening of the Innate Immune Response
Pathogens that penetrate the first line-of-defense at the glycocalyx are recognized by Toll-like receptors, a family of innate immune receptors expressed by epithelial cells and resident leukocytes. Toll-like receptors sense danger signals and pathogen-associated molecular patterns intrinsic to microorganisms and initiate an innate immune response (Basu and Fenton, 2004). For example, TLR5 recognizes bacterial flagellin, the major protein constituent of the flagella. This leads to NF-kappaB activation and induced expression and secretion of proinflammatory cytokines such as TNFA and IL8, thus stimulating inflammation.
Recent studies using primary human bronchial epithelial cells in culture have demonstrated that MUC1 coordinates with Toll-like receptors to control the resolution of acute inflammation essential to the prevention of chronic inflammatory disease. Thus, TGFA also activates EGFR, resulting in phosphorylation of the MUC1 CT. The activated MUC1 CT then associates with TLR3 and TLR5, inhibiting recruitment of TRIF and MYD88, suppressing NF-kappaB activation and thereby dampening the innate immune response (Kato et al., 2016). MUC1 also suppresses NF-kappaB activation in response to TLR3, 4, 7, and 9 agonists, suggesting that it may be a universal regulator of TLR signaling (Ueno et al., 2008).
This finding was recently confirmed and extended in the HCLE tissue-equivalent model with mucosal differentiation (Menon et al., 2015). It was found that knockdown of either MUC1 or MUC16 released dampened expression of the proinflammatory cytokines TNFA, IL6 and IL8 in response to ligand-activated TLR2 and TLR5 (activated with heat-killed Listeria monocytogenes and flagellin, respectively).
B. Mouse as a Model System
The mouse has become the premier mammalian model for disease research because of its small size, ease of genetic manipulation and relatively short generation time in comparison to other mammals (Justice and Dhillon, 2016). While humans and mice branched from a common ancestor approximately 80 million years ago, there are close physiological similarities between the two species. Nevertheless, humans and mice have adapted to different environments and so they have also evolved many differences in how they use molecules and molecular pathways that may not be as easily apparent (Perlman, 2016).
For many years, the rabbit was the preferred animal model for ocular surface research (Prince, 1964). This has changed; the mouse is now the “go to” model for investigation of ocular surface biology and disease mechanisms, including epithelial repair (Fini and Stramer, 2005; Saika et al., 2002) and dry eye (Barabino and Dana, 2004; Schrader et al., 2008; Stern and Pflugfelder, 2017), and for efficacy studies on investigational therapeutics (e.g., (Bauskar et al., 2015)). With regard to the biological role of MAMs, transgenic mice provide an important complement to cell culture models, as they enable a more comprehensive understanding in the full context of the organism. However, as we detail in this Subsection, there are molecular differences between the mucosal glycocalyx of humans and mice that must be taken into consideration. We believe this is the first time these differences have been comprehensively compared.
1. Ocular Surface System
The ocular surface system includes the corneal and conjunctival epithelia, the lacrimal glands, and the innervation connecting them. This has been called the “lacrimal functional unit” (Stern et al., 1998). The ocular surface system also includes the tear film, the eyelids, the Meibomian glands and accessory glands, the nasolacrimal duct and the integrative functions of the endocrine, immune, and vascular systems (Gipson, 2007).
The anatomy and physiology of the human and mouse ocular surface system is very similar, but not identical; the implications for function must be taken into consideration when using mouse as a model. Figure 9 is a schematic of the mouse eye and ocular surface system. The size and position of the glands with respect to the eye are approximately to scale.
Figure 9. Schematic of the Mouse Ocular Surface System.
A) Eye in the mouse showing positioning of the glands; B) Larger and side view of A; C) Larger view of the isolated eye cross section.
ELG: Extraorbital Lacrimal Gland; ILG: Intraorbital Lacrimal Gland; HG: Harderian Gland
The mucosal ocular surface is kept continually wet by the tear film. Measurement of the tear film electrical profile in mice produced an average thickness of 7 um (Tran et al., 2003). This is at the upper end of the range of measurements in humans (King-Smith et al., 2000; Wang et al., 2003). The tear film is composed of water, glucose, salts, and lipids, and proteins (including mucins). A small number of highly abundant proteins are estimated to comprise more than 90% of the total human tear protein by weight, including LYZ (lysozyme), LTF (lactoferrin), LCN1 (tear lipocalin) and LACRT (lacritin) (Zhou and Beuerman, 2012). The remaining 10% is highly complex; in the most comprehensive mass spectrometry list, 1543 tear proteins were identified (Zhou et al., 2012). At ~30 ug/mL, abundance of the molecular chaperone CLU is substantially lower than that of the major tear proteins (e.g., ~50 fold less than LCN1 and ~10-fold less than LACRT), but near the upper end of abundance for the other proteins. A recent proteomics analysis of mouse tears identified 139 different proteins (Karn and Laukaitis, 2015). Members of three large protein families were identified that have no counterparts in humans: androgen-binding proteins, exocrine secreted peptides and major urinary proteins. The last group are members of the lipocalin family that mediate female recognition of potential mates. Not surprisingly, CLU is found in mouse tears as in human tears, with a concentration estimated at ~5 ug/mL (Bauskar et al., 2015).
Plasma membrane ridge-like folds or pillar like projections, called microplicae or microvillae, project into the tear film from the surface of the apical epithelial cells at the ocular surface in all vertebrate species examined (around 150 nm high in guinea pig) (Nichols et al., 1983). Viewed coronally, these projections form different patterns in different species (e.g., (Doughty, 1990, 2004, 2016; Pfister, 1973)). The pattern of microplicae projecting from the surface of apical epithelial cells of the mouse ocular surface appears very similar to human (Danjo et al., 2000). The microprojections increase cell surface area, enhancing the stability of the tear film. In turn, the tear film smooths the ocular surface, neutralizing negative optical effects (Johnson and Murphy, 2004). Various staining techniques reveal a well-developed glycocalyx in both human and mouse e.g., (Gipson, 2007; Wells and Hazlett, 1984).
The corneal and conjunctival epithelia in mouse and human have a similar stratified squamous structure. However, the corneal epithelium of mouse has an average of 13 cell layers, which is about twice the number found in human (Henriksson et al., 2009). This is a result of an increase in squamous cell layers. Other epithelial features, such as desmosomal junctions, hemidesmosomes, and basement membrane are similar to human. The epithelium contributes ~30% percent of the total corneal thickness in mouse, but only ~10% of the total corneal thickness in humans (Li et al., 1997). Mouse corneal epithelial cells do not express the keratin K3, while humans and other mammals express the K3/K12 keratin pair (Chaloin-Dufau et al., 1993).
Goblet cells residing in the conjunctiva are secretory cells comprising the primary source of soluble and gel-forming mucins (Gipson and Inatomi, 1998). In humans, the conjunctival goblet cells secrete MUC5AC; in mice, Muc5b is also secreted at lower levels (Gupta et al., 2011). Dendritic cells of the conjunctiva are located in close proximity to goblet cells, and evidence suggests they modulate one another’s function (Contreras-Ruiz and Masli, 2015).
The submucosal glands supporting the ocular surface epithelia in humans include the lacrimal glands located in the anterior and lateral region of the roof of the orbit, and the accessory lacrimal glands located in the fornix of the conjunctiva and at the edge of the upper tarsus (Gipson, 2004). The lacrimal glands are the main source of the aqueous and serous (protein) components of the tears, although they produce some mucins and lipids too. In mammals, there are two types: 1) a superior lacrimal gland, with multiple ducts that open in the lateral half of the upper conjunctival sac and 2) inferior lacrimal glands, with only one duct that opens into the lateral canthus. Humans have only the superior lacrimal gland composed of a larger orbital lobe and a smaller palpebral lobe. Rodents have only the inferior gland, which is divided into an intra-orbital and extra-orbital portion, the latter located below the ear next to the parotid glands.
Two additional accessory glands also contribute to the aqueous component of the tears in humans: the Krause and Wolfring glands, whose ducts open in the conjunctiva. These glands have a mixed population of both serous and mucus cell types (Seifert et al., 1994). They are not found in mice (Sakai, 1989).
Transcriptomic analysis of lacrimal gland revealed that the most highly expressed genes differ between humans and mice (Ozyildirim et al., 2005). In humans, cDNAs encoding LYZ, LCN1, LTF, LACRT, PRR4 and PROL1 were most abundant. However, the top five transcripts in mouse corresponded to major urinary protein family member Obp1a; androgen-binding protein family member C2c (now called Scgb2b20); a novel hypothetical protein that was named lacrein, which bears some similarities to LACRT, and may be the mouse counterpart; Sptl1, a key enzyme in sphingolipid metabolism; and a putative hydrolytic enzyme similar to Lipf, also involved in lipid metabolism. These differences mirror the differences in the respective tear proteomes of human and mouse.
Found in both humans and mice are the Meibomian glands, named in honor of Heinrich Meibom, who was the first to describe them in detail. They are sebaceous glands that produce the lipid portion of the tear film, helping to prevent evaporation. The Meibomian glands are located side by side in a row, aligned perpendicular to the edge of both upper and lower eyelids. They are cluster-shaped, with multiple acini that lead into a central duct. The release of their content is induced by blinking (Ross and Pawlina, 2015). Acylated omega-hydroxy fatty acids (OAHFA) are the major amphiphilic component of meibum in both humans and mice (Butovich et al., 2012).
Also found in both humans and mice are the Zeiss and Moll’s glands located next to the eyelashes. The first are sebaceous glands, associated with the hair follicle. Moll’s glands are a type of modified sweat gland found at the base of the eyelashes. Both contribute to the lipid portion of the tear (Ross and Pawlina, 2015).
The nasolacrimal duct is a channel located in the medial canthus of the eye, which communicates with the nasal cavity. Its function is to absorb the components of the tears, in this way helping to regulate the tear level. The eyelids are important, not only for being the place where glands are lodged, but also for their ability to move in the blink, covering the entire surface of the eye when closed. They constitute an additional defense barrier and distribute the content of the tear film, as well as help to renew them during the blink (Murube, 2009). The tear film must remain continuous between blinks in order to fulfill its function. Mice have a much slower blink rate than humans, reflective of the fact that mouse tear stability is markedly greater (Duke-Elder and Gloster, 1968).
Most tetrapods, including rodents, have a “third eyelid” or nictitating membrane (from Latin nictare, to blink); however, this structure is vestigial in humans (Heralgi et al., 2017). The transparent or translucent nictitans can be drawn across the eye from the medial canthus. It protects the ocular surface against trauma and maintains moisture, while also allowing for vision. The third eyelid is also believed to help keep the surface of the eye moist by holding the tear film against the cornea better than the eyelids do by themselves. Loss of the third eyelid through trauma or in the treatment of neoplasia frequently results in chronic irritation of the cornea and remaining conjunctiva (Murube, 2009). Attached to the nictitans is the Harderian gland, which can secret mucous, serous fluid or lipid. In some animals, the Harderian gland may produce up to 50% of the tear film (Chieffi et al., 1996). Its disposition varies depending on the animal group; in rodents it is found around the back of the eyeball (Sakai, 1989). Like the nictitans, the Harderian gland is absent in humans. The greater tear film stability in mice as compared to humans may be due in part to the additional lipid secreted by the Harderian gland.
Like other mucosal epithelia, the conjunctiva contains conjunctiva-associated lymphoid tissue (CALT) (Knop and Knop, 2000; Reinoso et al., 2012). The component cells are thought to be a key location for the generation of adaptive immune mechanisms of the ocular surface. Dendritic cells in the human conjunctiva are detected in organized follicles of CALT and diffusely distributed through the stroma along with intraepithelial lymphocytes (Gandhi et al., 2013; Reinoso et al., 2012). Postnatal development and ultrastructure of CALT in the mouse is similar to humans (Siebelmann et al., 2013). Topical stimulation with C. trachomatis or ovalbumin/cholera toxin B led to CALT generation exclusively in the nictitating membrane (Steven et al., 2008). Electron microscopy showed intraepithelial lymphocytes and follicles consisting of lymphocytes, dendritic cells, and macrophages.
2. Genes
With publication in 2002 of the mouse chromosome 16 draft DNA sequence, and a full sequence comparison of human chromosome 19 with related mouse sequences, it became possible to draw preliminary conclusions about similarity of the mouse and human genomes for the first time (Copeland et al., 2002). While chromosome number and size differ between human and mouse, their genomes appeared remarkably similar, not only in how the genes are organized on the chromosomes, but also at the level of individual genes and their DNA sequences. However, that once the human and mouse genomes were fully sequenced and published in 2008 and 2009, respectively, a more complete story emerged (Church et al., 2009). Despite the striking synteny between homologous DNA segments, one-fifth of mouse genes are new copies that have emerged in the last 90 million years of mouse evolution. This helps to explain many of the differences that distinguish human and mouse biology.
Table 5 compares all human epithelial MAM genes with the corresponding mouse ortholog. The mouse genes are in their expected location based on chromosomal synteny between the two species. The exon count for individual genes is well-conserved.
Table 5:
Comparison of Human and Mouse Epithelial MAM Genes Ordered by Human Chromosomal Locus
| Human | Mouse | ||||
|---|---|---|---|---|---|
| Symbol | Locus | Exons | Symbol | Locus | Exons |
| MUC1 | 1q22 | 5 | Muc1 | 3 F1 | 7 |
| MUC13 | 3q21.2 | 12 | Muc13 | 16 B3 | 13 |
| MUC20 | 3q22 | 4 | Muc20 | 16 B3 | 5 |
| MUC4 | 3q22 | 25 | Muc4 | 16 B3 | 25 |
| MUC21 | 6p21.33 | 3 | Muc21 | 17 B1 | 3 |
| MUC22 | 6p21.33 | 5 | Muc22 | N/A | N/A |
| MUC3 | 7q22.1 | 13 | Muc3 | 5 G2 | 14 |
| MUC12 | 7q22.1 | 12 | Muc12 | N/A | N/A |
| MUC17 | 7q22.1 | 13 | Muc17 | N/A | N/A |
| MUC15 | 11p14.2 | 5 | Muc15 | 2 E3 | 4 |
| MUC16 | 19p13.2 | 88 | Muc16 | 9 A2 | 89 |
Muc20 and Muc4 lie adjacent to one another in mice, as in humans. However, in the mouse genomic region syntenic with the human MUC3, MUC13 and MUC17 gene cluster, only a single gene Muc3 has been fully established. This may be because there are several gaps in the current mouse genomic assembly that make annotation challenging. Several genes upstream of Muc3: Gm31160 and Gm40349, have been annotated, and they may represent additional Muc family genes. The Genome Reference Consortium is tentatively planning to release an updated mouse assembly this year, and the mouse genome will be reannotated soon after that becomes publicly available (Dr. Eric Cox, NCBI, personal communication).
The gene cluster of MUC21 and MUC22 at human cytogenetic locus 6p21.33 may legitimately be more streamlined in mice. When we examined the NCBI Gene database for mouse orthologs, we identified a predicted gene with similarity to both MUC21 and MUC22. This was located just downstream of MUCL3, and just upstream of HCG22, similar to human MUC21 and MUC22. The structure of the ambiguous gene, with 3 exons, was similar to human MUC21, but the coding sequence length was more like MUC22. A BLAST analysis revealed coding sequence similarity across the entire length of MUC21, while the similarity to MUC22 was localized to the conserved Epiglycanin_C domain.
According to the NCBI Gene database, 92 organisms have a MUC21 ortholog, while only 30 organisms have a MUC22 ortholog. In addition to mouse, other rodents, including shrew mouse, Ryukyu mouse, Alpine marmot, golden hamster and naked mole-rat, all have Muc21, but appear to be missing Muc22. This preliminary analysis suggests that human MUC22, likely the result of a tandem gene duplication during evolution, is conserved in numerous related organisms, but is absent in mouse and other rodents.
We communicated this to NCBI in an effort to resolve the gene identity. The annotation of the gene related to both human MUC21 and MUC22 was changed in the NCBI database to identify it as Muc21 (Dr. Eric Cox, NCBI, personal communication). It should be noted that MUC22 was not found in human by automated algorithms, but instead required application of focused analysis techniques (Hijikata et al., 2011). Thus, it is possible that a separate Muc22 gene may still be discovered in the mouse genome.
3. Proteins
Table 6 compares the polypeptide length of all human MAM proteins with their corresponding mouse orthologs, using data mined from NCBI databases.
Table 6:
Comparison of Human and Mouse Epithelial MAMs Ordered by Human Protein Backbone Length
| Human | Mouse | |||||
|---|---|---|---|---|---|---|
| Symbol | Amino Acids | Symbol | Amino Acids | Accession number | Isoforms | |
| MUC16 | 14,507 | Muc16 | 8,817 | XP_911240936.1 | 3 | |
| MUC4 | 7,418 | Muc4 | 3,470 | NP_536705.3 | 1 | |
| MUC12 | 5,335 | Muc12 | N/A | N/A | N/A | |
| MUC17 | 4,493 | Muc17 | N/A | N/A | N/A | |
| MUC3 | 3,313 | Muc3 | 1,802 | XP_006504604.2 | 2 | |
| MUC22 | 1,786 | Muc22 | N/A | N/A | 1 | |
| MUC20 | 709 | Muc20 | 688 | NP_001139346.1 | 2 | |
| MUC21 | 626 | Muc21 | 1,606 | NP_001231583.1 | N/A | |
| MUC13 | 512 | Muc13 | 573 | NP_034869 | 3 | |
| MUC1* | 484 | Muc1 | 631 | NP_038633.1 | 1 | |
| MUC15 | 361 | Muc15 | 331 | NP_766567.1 | 2 | |
Listed here is the amino acid count of the longest isoform identified in the NCBI Gene database for each MAM gene
The canonical MUC1 protein listed in the UniProt database is much longer, at 1255 amino acids (discussed more in the text)
One of the immediately obvious differences between human and mouse orthologs is the substantially shorter protein backbone length of the long MAMs. The length differences are 0.6-fold, 0.5-fold, and 0.5-fold for Muc16/MUC16, Muc4/MUC4 and Muc3/MUC3, respectively, due to contraction of the VNTR. By contrast, the small MAMs show conservation of protein backbone length between mouse and human. This suggests that there is selective evolutionary pressure to maintain the VNTR length below a certain threshold in mice, and that there is pressure to exceed that threshold in humans.
The longer MUC backbones of humans contain more sites for O-linked glycosylation. Thus, it might be hypothesized that a longer backbone is more effective in maintaining ocular surface moisture and could be important in humans, which lack the third eyelid and associated Harderian gland present in most other mammals.
To test this hypothesis, we examined results from a classic study characterized the nictitating membrane in 22 species of subhuman primates (Arao and Perkins, 1968). Subhuman primates are transitional in evolution with respect to the third eyelid and Harderian gland. Table 7 lists the findings on nictitans coverage of the ocular surface from this study, comparing to the MUC16 protein backbone length, as mined from the NCBI database. The species covered by the two datasets did not match perfectly by species, but genus comparisons are included.
Table 7.
Comparison of MUC16 Length and Nictitans Coverage of the Ocular Surface across Primate Species Ranked by Evolutionary Distance from Human
| Genus and Species | Family | Exon Count | Amino Acid Count | Isoforms | Nictitans Coverage |
|---|---|---|---|---|---|
| Human (Homo sapiens) | Great Apes | 88 | 14,507 | 14 | vestigial |
| Chimpanzee (Pan satyrus) | 110 | 14,498 | 2 | vestigial | |
| Western Gorilla (Gorilla gorilla) | 70 | 14,076 | 1 | 1/10 | |
| Crab-Eating Macaque (Macaca fascicularis) | Old World Monkey | 75 | 14,346 | 1 | 1/8 |
| Pig-Tailed Macaque (Macaca nemestrina) | 90 | 14,035 | 1 | 1/8 | |
| Green Monkey (Cercopithecus aethiops) | 84 | 14,188 | 1 | 1/5 | |
| White Tufted-Ear Marmoset (Callithrix jacchus) | New World Monkey | 59 | 5,635 | 1 | 1/3.5 |
| Bolivian Squirrel Monkey (Saimiri boliviensis) | 100 | 4,002 | 1 | ||
| Common Squirrel Monkey (Saimiri sciureus) | 1/3 | ||||
| Chinese Tree Shrew (Tupaia chinensis) | Treeshrew | 75 | 11,696 | 1 | |
| Tree Shrew (Tupaia glis) | 1/2 | ||||
| Grey Mouse Lemur (Microcebus murinus) | Lemur | 101 | 8,928 | 1 | |
| Mongoose Lemur (Lemur mongoz) | 1/2 | ||||
| Small-Eared Galago (Otolemur garnettii) | Bush baby | 52 | 3,345 | 1 | |
| Potto (Perodicticus potto) | Loris | 1 |
Listed here is the amino acid count of the longest isoform identified in the NCBI Gene database.
The relative sizes of the nictitans are expressed as the ratios of total eye surface area to nictitans coverage when the membrane was extended laterally with the aid of dissecting forceps.
The nictitans eye coverage was found to vary widely among the different primate species, ranging from vestigial (human and chimpanzee) to the entire eye (Potto). The eye coverage increased consistently with increasing evolutionary distance from humans. However, the relationship between evolutionary distance and MUC16 length was not so clear cut. Great Apes and Old World Monkeys all have very similar MUC16 lengths – the longest of the primate families at ~14,000 amino acids – and nictitans coverage ranges from vestigial to 1/5th of the eye surface. New World Monkeys, the next lower evolutionary group, have much shorter MUC16 lengths (~4,000 to 5,500) and more substantial eye coverage by the nictitans (~1/3rd). However, Treeshrews. Lemurs and Bushbabies had widely variable MUC16 lengths, despite nictitans coverage of ~half the eye. We conclude that the reasons for the large variability of MUC16 length across species cannot be due simply to nictitans membrane and Harderian gland differences.
4. Gene Expression, Protein Localization
The three ocular surface MAMs documented in humans and mice localize differently at the ocular surface. Each is found primarily at the apical aspect of the ocular surface in both species. However, their distribution between corneal epithelium and conjunctival epithelia differs substantially. Figure 10 diagrams the distribution of these three MAMs at the ocular surface of human and mouse.
Figure 10. Comparison of MUC1, MUC4, and MUC16 Localization at the Human and Mouse Ocular Surface.
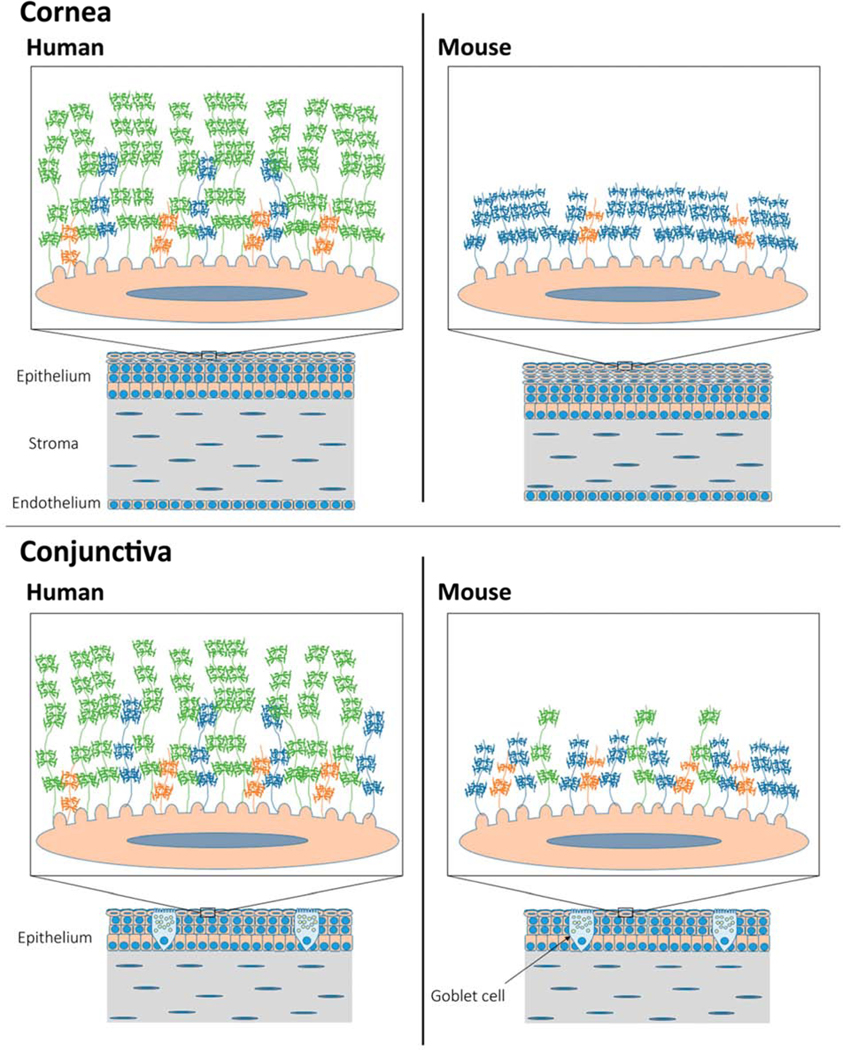
The graphics compare the corneal epithelium (top) and conjunctiva (bottom) from human (left) and mouse (right). As depicted, the corneal epithelium in mouse has more cell layers than human; the human corneal stroma is thicker than that of mouse.
The expanded insets depict a single apical epithelia cell from the corneal or conjunctival epithelium of mouse or human, showing the surface microplicae. With the EDs of MAMs MUC1, MUC4, and MUC16 projecting outward into the tear film. The EDs of the two longest MAMs, MUC16 and MUC4, are substantially shorter in mouse than human. MUC4 appears to substitute for MUC16 on the corneal epithelium of mouse, which further reduces the overall length of MAM EDs on the corneal epithelium.
MUC1: orange; MUC4: blue; MUC16: green.
Unlike in humans, where MUC1 localizes uniformly across the surface of the corneal and conjunctival epithelia, very little Muc1 mRNA was detected in the corneal epithelium of mouse (Kardon et al., 1999). However, Muc1 mRNA was easily detectable in the mouse conjunctival epithelium, as well as the epithelium of the Harderian gland. The amount of Muc1 mRNA was about 5.5 times greater in the Harderian gland when compared to the conjunctiva. Similarly, an antibody to the Muc1 CT gave only a weak reaction in corneal epithelium of mouse, but reacted strongly with conjunctival epithelium (Kardon et al., 1999). The strongest staining was seen at the luminal aspects of the Harderian gland epithelium.
The stratified epithelia of the human conjunctiva and peripheral cornea express MUC4, but the amount of the protein attenuates with progression towards the central cornea (Inatomi et al., 1996). In mouse, mRNA extracted from cornea or conjunctiva was positive for Muc4 (Danjo et al., 2000; Lange et al., 2003). Likewise, Muc4 expression in the central and peripheral cornea, as well as the conjunctiva, was demonstrated by in situ hybridization. Muc4 antibody bound in a linear pattern along apical cells throughout the corneal and conjunctival epithelium (Lange et al., 2003). In rat, a similar localization is found in the ocular surface epithelia (Price-Schiavi et al., 1998; Swan et al., 2002).
The biggest difference between mice and humans is seen for MUC16. In humans, MUC16 is distributed uniformly across corneal and conjunctival epithelia, and in goblet cells. In mice, Muc16 has been identified only at the apical aspect of the conjunctival epithelium (Shirai et al., 2014) and on goblet cells (Wang et al., 2008). In contrast, the corneal epithelium appears to lack MUC16.
Thus, Muc4, appears to substitute for Muc16 at the surface of the mouse cornea. This means the MAM length at the corneal epithelial surface is effectively about 4-fold shorter in mice than in humans.
In order to make full use of the mouse model for functional studies, it will be important to extend the characterization of MAM gene expression to Muc21.
5. Knockout Mouse Findings
To date, knockout mice lacking the MAMs Muc1, Muc4, Muc13 and Muc16 have been generated. Homozygous lines are viable and fertile, with no apparent anatomical defects. Reviews have been published describing the effects of Muc1 and Muc16 deficiency in cancers and gastrointestinal tract inflammation (Joshi et al., 2015), and at the ocular surface (Shirai and Saika, 2015). Reported phenotypes are listed in Table 8. This list is not comprehensive for Muc1.
Table 8.
MAM Knockout Mouse Phenotypes
| Gene | System | Phenotype | Citation |
|---|---|---|---|
| Muc1 | Cancer | Reduced breast tumor growth, increased tumor cell resistance to chemotherapy drugs | (Spicer et al., 1995) |
| Reduced pancreatic tumor progression and metastasis | (Besmer et al., 2011) | ||
| Reduced pancreatic tumor resistance to chemotherapy | (Nath et al., 2013) | ||
| Increased lung adenocarcinoma multiplicity; increased EREG production that activates the EGFR pathway for lung carcinogenesis | (Xu et al., 2017) | ||
| Myeloid | Increased differentiation of bone marrow progenitors into myeloid-derived suppressor cells | (Nagaraj et al., 2009) | |
| Gastrointestinal | Increased intestinal susceptibility to bacterial infection due to local deficiency of Muc1 | (McAuley et al., 2007) | |
| Increased systemic spread of orally delivered Campylobacter jejuni | (McAuley et al., 2007) | ||
| Lethality of H. Pylori infection | (McGuckin et al., 2007) (Ng et al., 2016; Ng and Sutton, 2016) |
||
| increased inflammatory response to pathogen exposure, NOD1 and Toll-like receptor ligands; increased chemokine secretion in response to TNFA | (Sheng et al., 2013) | ||
| Respiratory | Dexamethasone resistance in a model for chronic obstructive pulmonary disease | (Milara et al., 2018) | |
| Increased morbidity and mortality due to influenza A viral infection | (McAuley et al., 2017) | ||
| Worsening of inflammation and fibrosis in a model for interstitial lung disease | (Kato et al., 2017) | ||
| Kidney | Worsening of kidney damage and failed recovery associated with reduced transcription factor HIF1A activation in an ischemia-reperfusion injury model; at later time points, this effect is reversed | (Pastor-Soler et al., 2015) (Gibier et al., 2017) |
|
| Ocular Surface | Spontaneous ocular surface infection | (Kardon et al., 1999) | |
| No ocular surface phenotype | (Danjo et al., 2000) | ||
| Muc4 | Gastrointestinal | Reduced susceptibility to colon inflammation; reduced infiltration by F4/80(+) macrophages; reduced IL1B and TNFA expression | (Das et al., 2016) |
| Muc13 | Gastrointestinal | Mild focal neutrophilic inflammation in intestines of aged mice; increased susceptibility to inflammation in a colon inflammatory mode; increased infiltration by F4/80(+) macrophages; increased IL1B and TNFA expression | (Sheng et al., 2011) |
| Decreased chemokine secretion in response to TNFA in intestine; anti-inflammatory response to pathogen exposure, NOD1 and Toll-like receptor ligands | (Sheng et al., 2013) | ||
| Muc16 | Reproductive | Increased male reproductive efficiency | (Cheon et al., 2009) |
| Gastrointestinal | |||
| Ocular Surface | Spontaneous ocular surface inflammation; altered epithelial cell dynamics; increased rate of ocular surface epithelial regeneration | (Shirai et al., 2014) |
Phenotypes that are opposite of expected are in blue text
An examination of results listed in Table 8 leads to the general conclusion that loss of a specific MAM, no latter which one, leads to common phenotypes: inhibition of cancer growth and progression in the case of cancer models, promotion of infection and inflammation in infection models, and increased inflammation and fibrosis in the unperturbed state or in injury models. Perplexingly however, just the opposite is observed in a small number of these studies. These anomalies are underlined in blue in Table 8. Four different explanations have been offered.
The first explanation is raised in findings using the Muc1 knockout mouse in cancer models. Muc1 is overexpressed and aberrantly glycosylated in adenocarcinomas and in hematological malignancies. Epithelial tumors in Muc1 knockout mice exhibit reduced growth when compared to congenic controls (Besmer et al., 2011). This is consistent with other studies supporting a role for MUC1 as an oncogene promoting tumor development, progression, metastasis and resistance to chemotherapeutics. However, a surprising lung tumor-suppressing effect was observed when the Muc1 knockout mouse was bred into the NNK A/J mouse lung cancer model (Xu et al., 2017). In studies to address mechanism, human MUC1 was found to suppress EREG production in both normal fibroblasts and malignant cells. The authors favor an explanation in which Muc1 exhibits distinct functions in epithelial, stromal and cancer cells in the tumor microenvironment, and that the sum of these effects results in tumor suppression.
A second reason for anomalous results may be due to compensatory mechanisms, whereby loss of one mucin is compensated by changes in expression of another. This phenomenon may mask the significance of a given MAM in tissue homeostasis when studied using knockout mouse models. For example, homozygous Muc1 knockout mice exhibit a compensatory increase in Muc4 in mammary tissue (Spicer et al., 1995), while homozygous Muc16 knockout mice demonstrate reduced Muc1 expression in the uterus and lung (Cheon et al., 2009). Knockout of multiple MAMs in the same mouse could potentially circumvent such compensatory mechanisms and provide further insights into their regulatory interplay.
A third factor that complicates analysis occurs when myeloid-derived immune cells contribute to the process under observation, since these cells also express Muc1. Studies show that loss of Muc1 can affect the immune cell repertoire that develops from the bone marrow (Poh et al., 2009). Thus, phenotypes in Muc1 knockout mice could be due either to loss of Muc1 locally from the organ being examined, or loss of Muc1 from infiltrating immune cells. The relative role of Muc1 expressed locally in a tissue versus infiltrating immune cells can be examined experimentally by creating chimeric mice. This is done by transplanting a knockout or normal mouse with normal or knockout bone marrow, respectively. Studies in two gastrointestinal infection models that used the chimeric mouse approach confirmed the importance of making this assessment (McAuley et al., 2007; McGuckin et al., 2007; Ng et al., 2016; Ng and Sutton, 2016).
The last factor is mouse genetic background, as differences in the immune response among different inbred mouse strains are well-documented (Sellers, 2017). Results of two different studies suggest that genetic background may be a factor affecting the ocular surface phenotype of Muc1 knockout mice. In the first study, Muc1 knockout mice were found to be predisposed to developing eye inflammation when compared to normal littermates (Kardon et al., 1999). However, a different lab found no differences between Muc1 knockout mice and littermate controls (Danjo et al., 2000). Ocular surface epithelia of knockout mice had a normal appearance of surface microplicae, a well-developed glycocalyx on the apical cell membrane, and a normal appearance of goblet cell mucin packets. There was no convincing evidence that bacterial adherence on the cornea was increased. Muc4 expression was not upregulated in Muc1 knockout mice compared with control. No ocular surface infections were observed in Muc1 knockout mice, which were housed in the animal facility over a period of 26 months.
There is only one other published study on the ocular surface phenotype of MAM knockout mice, and this utilized the Muc16 knockout. A careful examination of the ocular surface revealed no gross defects (Shirai et al., 2014). However, tissue analysis revealed basal-like cells in the suprabasal layer of the corneal epithelium, with an increase in cell proliferation. The loss of Muc16 accelerated regeneration of an experimentally-created corneal epithelial defect. In the conjunctiva, transcription factors STAT3 and JUNB were activated, and the cytokine IL6 was upregulated. The incidence of myofibroblast appearance and macrophage invasion were more marked in knockout stroma than in wild-type stroma after epithelial repair. Thus, the loss of Muc16, which is expressed only in the conjunctiva, still affects homeostasis of the corneal epithelium and stroma.
C. Insight from Human Genetics
While we cannot experimentally manipulate the genome of humans as we do in mice or cultured cells, we can study the effects of naturally-occurring mutations and polymorphisms to obtain insight into function of the associated gene. As in mouse transgenic and knockout models, human genetic findings are hypothesis-generating. For the most part, these hypotheses still need to be addressed, and thus provide a good way to conclude this article. In this section, we discuss evidence for function of MAMs expressed at the ocular surface from two different types of inherited disorders: 1) monogenic diseases and 2) complex diseases.
Monogenic diseases are caused by alterations in a single gene, and they segregate in families according to classic Mendelian principles of inheritance. As a rule, genetic diseases with Mendelian inheritance patterns are caused by mutations in the coding region of the gene. Dominant inheritance patterns manifest a disease phenotype when only a single copy of the mutant gene is inherited. So-called “gain-of-function” mutations usually result in toxicity of the mutant protein, providing little biological insight. On the other hand, “dominant-negative” mutations, which interfere with the function of the protein encoded by the wild-type gene copy, may give insight into biological role.
Recessive inheritance patterns require that both copies of the gene are mutated for the disease to manifest, due to “loss-of-function”. Recessive mutations are much like knockdown in cell culture or in mouse knockout models, often being informative of the gene’s biological role. Dominantly inherited phenotypes resulting from a reduction in functional gene dosage may similarly provide functional clues.
The vast majority of human diseases with a genetic contribution are multifactorial, also referred to as complex diseases. Examples include cardiovascular disease, cancer, diabetes, psychiatric disorders and glaucoma. Complex diseases are caused by variation in many genes. The variants can be single nucleotide polymorphisms (SNPs) or short deletions/insertions, duplications, and inversions. Each gene variant associated with a complex disease confers a degree of risk, but the presence of the variant does not necessarily mean the disease will be manifest.
Complex disease genetics is investigated through genome-wide association studies (GWAS). Genomic microarrays are used to analyze millions of variants at one time and investigate their statistical association to a disease phenotype or “quantitative trait”. Unlike genetic diseases with Mendelian inheritance patterns, about 88% of GWAS hits are intergenic or intronic (Hindorff et al., 2009). These are typically located in gene regulatory regions and control the expression level of the gene and its protein product.
It should be noted that, since microarrays do not contain every possible SNP (or other variant), significant SNPs discovered by GWAS usually are only a “tag” of the causative polymorphism. That having been said, current genomic microarrays sample SNPs very densely across the genome and analysis of SNPs not included in the microarray, located physically close to the tag SNP and in linkage disequilibrium (i.e., inherited along with the tag SNP), can often identify likely causative SNP(s) and provide functional insight (e.g., (Jeong et al., 2015)).
1. MUC1 and Ion Channel Stability
As noted in Section III, the number of tandem repeats within the VNTR of a given MAM can vary considerably among individuals within a population (Gendler and Spicer, 1995). As an example, in a study examining the MUC1 gene of 69 northern Europeans, the number of tandem repeat units varied from 21 to 125 (Gendler et al., 1990). In numerous studies, MUC1 VNTR polymorphisms have been linked to susceptibility for both H. pylori-induced gastritis and gastric cancer. Individuals with short MUC1 alleles are at a higher risk (Carvalho et al., 1997; Silva et al., 2001; Vinall et al., 2002). Homozygotes for small MUC1 VNTR alleles were significantly associated with gastric carcinoma as well as with chronic atrophic gastritis and incomplete intestinal metaplasia, the two well-established precursor lesions of gastric carcinoma, suggesting that MUC1 genotypes may define different susceptibility backgrounds in the gastric carcinogenesis pathway (Silva et al., 2001). At this time, we are not aware of any MAM VNTR polymorphisms associated with susceptibility to ocular surface disease, but it seems likely they will be discovered.
A monogenic disease caused by a VNTR mutation is medullary cystic kidney disease, an autosomal dominant kidney disorder leading to end stage renal disease. In a study employing DNA sequencing combined with other molecular techniques (Kirby et al., 2013), it was found that each of six affected families harbored an equivalent, but apparently independently arising, mutation in the MUC1 gene: the insertion of a single C in one copy (but a different copy in each family) of the tandem repeat unit within the VNTR domain. In all cases, the insertion was predicted to cause a translational reading frame shift. This creates a new stop codon that terminates translation prior to large subunit cleavage site.
How the MUC1 mutation causes disease pathology is not known, however, the cumulative effects of mild toxicity due to gain-of-function could explain some of the symptoms, e.g., fibrosis. As these patients rarely have cysts, the disease has been renamed autosomal dominant tubule-interstitial kidney disease due to MUC1 mutations, abbreviated as Mucin 1 Kidney Disease (Al-Bataineh et al., 2017). Affected patients affected exhibit only renal disease, despite the presence of mutant MUC1 protein in the epithelial cells of multiple organs, including at the ocular surface (Al-Bataineh et al., 2017). This points to the likely mild nature of mutant protein toxicity. It is important to remember that affected patients exhibit one wild-type copy of MUC1 and this may be sufficient for function in most organs and sub-organs, including the ocular surface.
A clue to the natural role of MUC1 in the kidney was provided by identification of another gene implicated in medullary cystic kidney disease, UMOD. The shed ED of UMOD, also known as Uromodulin or Tamm-Horsfall protein, is the most abundant protein in urine. Mice expressing UMOD with the disease-causing human mutations have less urinary UMOD, and also exhibit hypercalciura and renal calcium crystals corresponding with reduced immunostaining for the renal calcium channel TRPV5 (Nie et al., 2016). In transiently transfected HEK293 cells, co-expression with UMOD or addition of exogenous UMOD increased TRPV5 surface currents, reduced TRPV5 endocytosis and increased TRPV5 cell surface expression, consistent with a role of the shed UMOD ED in stabilization of TRPV5.
Because of similarities between the two proteins, similar studies were carried out with MUC1, with similar results. Interestingly, urinary MUC1 is also reduced in patients with calcium nephrolithiasis, a common type of kidney stone. Cell culture studies revealed that TRPV5 surface expression is also enhanced by binding LGALS3 (galectin-3). The MUC1 enhancement of TRPV5 surface expression proceeds by LGALS3-dependent crosslinking of O-glycans on MUC1 with the N-glycan on TRPV5.
Thus, LGALS3 crosslinking of MUC1 with the TRPV5 ion transport channel at the surface of epithelial cells appears to provide a novel mechanism for regulation of their function. It seems likely that the MUC1 ED might more broadly enhance surface expression of transient receptor potential (TRP) family ion transport channels by a similar mechanism of crosslinking and maintenance at the cell surface. For example, a large genome-wide association study focused on serum concentrations of cations revealed that the highest association with low serum magnesium levels (hypomagnesemia) was a very common genetic variant of MUC1 (rs4072037) that adds nine amino acids to the extracellular N-terminus of the protein (Meyer et al., 2010).
A SNP in the magnesium transporter TRPM6 was also associated with low serum magnesium but to a lesser extent than the MUC1 variant. Interestingly, the MUC1 SNP was associated with higher bone mineral density and lower fasting glucose levels which could proceed by a direct interaction of either the transmembrane MUC1 or shed MUC1 with transporters within the kidney tubule.
The findings in kidney suggest possible parallels to the ocular surface. TRP channels have been identified in the corneal epithelium (TRPV1, TRPV3, TRPV4, TRPM8, and TRPC4), in the conjunctiva (TRPV1, TRPV2, and TRPV4), and in the eyelid (TRPM8) (Reinach et al., 2015). These channels are expressed by the epithelial cells. TRPA1, TRPV1 and TRPM8 are also expressed on corneal afferent nerve endings of the ophthalmic branch of the trigeminal nerve (Reinach et al., 2015). TRPM8 is a cold-sensing receptor activated in ocular surface nerves after evaporation of the tear film (which results in cooling), thus regulating wetness of the ocular surface (Parra et al., 2010). Nerve TRPV1 is activated by hypertonic challenge, which in turn leads to an increased release of pro-inflammatory cytokines (Pan et al., 2011). Dysfunction of these ion channels has been suggested as a possible pathophysiological mechanism in dry eye disease (Belmonte et al., 2017). Whether MUC1 can regulate TRP channel activity at the ocular surface will be an interesting question to investigate.
MUC1 has also been linked to another ion channel, CFTR, the cystic fibrosis transmembrane conductance regulator. CFTR is an ABC transporter-class ion channel protein that plays a critical role in the transmembrane transport of chloride. It is the driving force of fluid transport in various epithelial cells. Mutations in CFTR cause cystic fibrosis, a disease with a recessive inheritance pattern – homozygosity for a specific CFTR mutation – or compound heterozygosity for two different mutations. The disease manifests as disruption of exocrine function of the pancreas, intestinal glands, biliary tree, bronchial glands and sweat glands. Cystic fibrosis has long been established as a disease involving excessive mucus accumulation. A major symptom is the buildup of thick, sticky mucus in the lungs, which leads to life-threatening lung infections.
Surprisingly, Cftr knockout mice had lower levels of RNA expression and similar levels of protein for secreted mucins Muc2 and Muc5ac, as well as Muc3. However, there was a six-fold increase in Muc1 RNA expression in the colon of the Cftr KO mouse and a moderate increase in Muc1 protein. Breeding of the Cftr knockout mouse onto a Muc1 null background, resulted in mice with a significant reduction in intestinal mucus accumulation (Parmley and Gendler, 1998). It was proposed that Muc1 predominantly contributes to mucinous obstruction of the gastrointestinal tract during cystic fibrosis.
The role of Cftr in lacrimal gland function has only recently received attention. Studies in rabbit demonstrated that Cftr is localized in both acinar and ductal cells, with its predominant presence in the ducts, suggesting it may play a key role in lacrimal ductal fluid secretion (Lu and Ding, 2012). Indeed, clinical studies have reported dry eye symptoms in cystic fibrosis patients (e.g., (Sheppard et al., 1989)). Moreover, a significant reduction in tear secretion is observed in Cftr KO mice, which also develop ocular surface disease, as evidenced by vital dye staining (Berczeli et al., 2018). It will be interesting to learn whether MAMs play a role in stabilization of the Cftr ion channel in the lacrimal gland.
2. MUC21 and MUC22
As noted in a prior section, MUC21 and MUC22 are located within the major histocompatibility complex (MHC) on chromosome 6. It encodes over 160 proteins of diverse function, at least half of which are directly involved in immune responses, including genes for major histocompatibility complexes HLA-A, HLA-B, and HLA-C. This is significant in view of the accumulating evidence for MAM roles in dampening of the immune response.
The MHC is the most polymorphic part of the human genome and MUC22 exhibits more coding sequence alleles than even most HLA class I or II genes (Norman et al., 2017). Several polymorphisms link MUC21 and MUC22 to ocular surface diseases with an immune component.
A SNP in the intragenic region upstream of MUC21 (rs2844682) (Yang et al., 2014) has been associated with predisposition to Stevens-Johnson syndrome, a hypersensitivity complex affecting skin and mucous membranes, which manifests at the ocular surface as severe, mucous-deficient dry eye. Mechanisms of this disease are poorly understood, however other genes associated with this disease suggest defects in pathways of adaptive and innate immune responses, sensing/processing of microbial and danger signals, and inflammation.
A SNP in MUC22 (rs17190071) was identified associating with Behcet’s disease, another genetically complex condition, characterized by recurrent inflammatory attacks affecting the orogenital mucosa, eyes and skin (Remmers et al., 2010). SNPs linked with systemic lupus erythematosis (Fernando et al., 2012; International Consortium for Systemic Lupus Erythematosus et al., 2008) and psoriasis (Feng et al., 2009) were associated with both MUC21 (rs886403, rs9295938) and MUC22 (e.g. rs3871466, rs9366764, rs13191258); these autoimmune diseases also have ocular surface involvement.
MUC21 and MUC22 have also been linked to respiratory disease. Polymorphisms in the MUC21-MUC22 region were associated with asthma by admixture mapping and GWAS in the Latino population (Galanter et al., 2014). Polymorphisms in the MUC22-HCG22 region were associated with Japanese late onset asthma (Yatagai et al., 2016) and childhood asthma in the Chinese population (Chen et al., 2017a).
Clues to MUC22 function are provided by its association with diffuse panbronchiolitis (DPB), a rare, complex genetic disease of the respiratory system. DPB mainly occurs among the Japanese but has been reported in other (mostly east Asian) populations. A SNP in intron 2 of MUC22 was positively associated with DPB (higher disease risk). In addition, a VNTR polymorphism in exon 3, which greatly reduces the size of the molecule (1,890-base pair deletion), was negatively associated with DPB (lower disease risk) (Hijikata et al., 2011).
DPB causes nodule-like lesions of respiratory bronchioles, chronic sinusitis, and intense coughing with large amounts of sputum production. The term diffuse signifies that lesions appear throughout both lungs, while panbronchiolitis refers to inflammation found in all layers of the respiratory bronchioles. Symptoms occur from the second to the fifth decade of life and are slowly progressive, ultimately resulting in respiratory failure if untreated.
Infection the bronchioles by bacteria such as Haemophilus influenzae or Pseudomonas aeruginosa can cause their infiltration by inflammatory cells; thus, treatment of DPB involves long-term use of macrolide antibiotics. However, when infection is resolved, inflammation often continues, for unknown reasons. Inflammation can be so severe that nodules containing inflammatory cells form in the walls of the bronchioles. Inflammation and infection also result in the production of excess mucus. The combination of inflammation, nodule development, infection, mucus, and frequent cough contributes to the breathing difficulties of patients.
The genetic study mentioned above (Hijikata et al., 2011) investigated MUC22 expression in a tissue-equivalent model of primary human bronchial epithelial cells. Significantly, expression of MUC22 mRNA was increased more than 100-fold by treatment with polyinosine-polycytidylic acid (double-stranded RNA) or lipopolysaccaride, which mimic viral or bacterial infection, respectively. Moreover, immunostaining with MUC22 antibody was much more intense in the cytoplasm of serous cells of the lung submucosal gland from patients with DPB as compared to normal.
These intriguing results suggest two hypotheses about the pathogenesis of DPB and the role of MUC22 in the normal lung. First, the SNP associated with disease in MUC22’s second intron might be a regulatory polymorphism that results in excessive MUC22 production and large subunit shedding into the bronchioles. Second, MUC22 could be directly involved in regulation of periciliary fluid secretion and mucous clearance in the bronchioles following infection.
Of interest with relation to our hypotheses, a connection to cystic fibrosis has also been considered in the search for a cause of DPB. Much like DPB, cystic fibrosis shows a genetic predominance among one geographic group to the rarity of others. Thus, while DPB dominates among East Asians, cystic fibrosis mainly affects individuals of European descent and is the most common genetic disease of this group (Elborn, 2016) A common polymorphism in this gene occurs in Asians not necessarily affected by either disease. Whether CFTR could contribute to DPB is still under investigation.
How might these findings relate to the ocular surface? Lacrimal glands secrete the serous component of the tears and are thus analogous to the serous cells of the lung’s submucosal glands. We provide new data in Section II demonstrating that MUC22 is robustly expressed in the serous acini of the lacrimal gland.
Could genetic polymorphisms that cause DPB also cause lacrimal gland disease? Currently we have no information about this point (Naoto Keicho and Minako Hijikata, personal communication). DPB is managed by pulmonary physicians, to whom patients may not relate their eye symptoms. Considering the findings on MUC22 expression in the lacrimal gland, a possible relationship between DPB and lacrimal gland disease due to pathogen infection should be considered.
VI. Conclusions and Future Directions
MAMs are the defining molecules of the mucosal epithelial glycocalyx. A comprehensive structure/function characterization of MAMs at the ocular surface is extremely important to development of strategies for manipulating the glycocalyx to therapeutic advantage. While much progress has been made, much more is needed. In this article we update, including with previously unpublished data, the list of MAMs expressed by, and localized to, the ocular surface and lacrimal gland. They may now be recognized as MUC1, MUC4, MUC16, MUC21 and MUC22. In addition, MUC20 is expressed by the corneal/conjunctival epithelia, but is localized to deeper cellular layers. We then go on to update what is currently known about the structure/function of these MAMs, compiling known information with new, previously unpublished sequence analyses. The compiled information is then considered in relation to biological roles. Taken together, this information allows us to arrive at some conclusions about the MAMs expressed at the ocular surface as a group, and to generate hypotheses for the next stage of investigation.
As discussed herein, it is increasingly appreciated that MAMs play an important role as cell surface receptors that sense the extracellular environment and transduce signals intracellularly. It has been stated that the next big frontier in the MAM field is to expand our knowledge of their function in intracellular signaling (van Putten and Strijbis, 2017). With regard to the ocular surface, this frontier is vast. In cancer cells, MAM activation or inhibition of intracellular signaling cascades has been shown to regulate the biological processes of inflammation, cell-cell interactions, differentiation and apoptosis (Constantinou, 2011; Hollingsworth and Swanson, 2004; van Putten and Strijbis, 2017). But how activation or inhibition of intracellular signaling translates to roles at the ocular surface, with its non-proliferative, specialized squamous epithelial cells, is still largely unknown.
Our compilation of structure/function data and further analysis in Section III indicates that interaction with EGFR receptor tyrosine kinase family members is a common theme for all MAMs expressed at the ocular surface. A tyrosine residue in the MUC1 CT is phosphorylated by EGFR family members and it is predicted that a tyrosine residue in the MUC16 CT is also phosphorylated by EGFR family members. Moreover, MUC21 and MUC22 each has a tyrosine in their CT predicted to be phosphorylated by EGFR family members. Thus, even though the amino acid sequences of their CTs differ, it appears that these MAMs may converge functionally in this way.
EGFR phosphorylation of the MUC1 CT affects the binding to CTNNB1, which modulates the strength of the adherens junction connecting individual epithelial cells. This may be another common signaling theme among the MAMs. Like MUC1, the MUC16 CT binds to CTNNB1-CDH1 complexes and evidence suggests that this is through ezrin/radixin/moesin (ERM) actin-binding proteins, as discussed in Section IV. We also identified possible phosphorylation of some of the other MAM CTs by protein kinases that regulate intracellular adhesion. In cancer cells, changes in intracellular adhesion promote malignancy. In the apical epithelial cells of the ocular surface, the most logical link is to desquamation. As so little is known about desquamation at the ocular surface, this will be a significant area for future investigation.
As discussed in Section III of this article, MUC1 translocation has also been linked to the regulation of intracellular adhesion. These studies were made possible by the development of antibody reagents specific for the MUC1 CT. MUC16 also translocates to the nucleus, but nothing is known about the function. New, specific antibodies generated against the MUC16 CT have been recently reported (Aithal et al., 2018; Gipson et al., 2017). These will be invaluable for advancing this area of investigation. Similar reagents are needed for MUC21 and MUC22.
Besides EGF, the inflammatory cytokine TGFA can activate EGFR family receptors. As also discussed in Section IV, EGFR phosphorylation of the MUC1 CT initiates a signaling cascade that dampens the innate immune response, through association with toll-like receptors. This finding was confirmed in the ocular surface model and extended by demonstration that MUC16 also participates in this pathway. As noted above, the newer ocular surface MAMs, MUC21 and MUC22, have the potential for EGFR phosphorylation of the CT and genetic evidence presented in this article suggest that might also play a role in the innate immune response.
Many (if not most) ocular surface diseases are thought to have an autoimmune component (although the autoimmune antigen often has not been identified). For example, in dry eye, Inflammatory mediators and exposure of autoantigens at the ocular surface due to desiccating stress leads to an auto-immune-like adaptive T cell-mediated response. With amplification, this becomes a “vicious cycle of inflammation”, driving disease pathology (Pflugfelder and de Paiva, 2017). Individual TLRs are specific for different pathogens and danger signals (Moresco et al., 2011). Thus, the specific TLR with which a MAM interacts could provide for a unique functional response. This idea must still be addressed.
Indeed, results of expression studies discussed in Section IVC3 suggest that individual MAMs respond selectively to different pathogens. Our sequence analysis of the MAM CTs predicted sites for protein kinase A phosphorylation as a common theme. Protein kinase A lies downstream of a subgroup of G protein-coupled receptors (GPCRs) that couple with the heterotrimeric Galpha(s) protein. GPCRs respond to a diverse array of extracellular signals. Galpha(s) activates adenyl cyclase, which elevates intracellular cAMP levels, activating protein kinase A. Elevation of cAMP is used by the cell in myriad ways to modulate innate immune functions (McDonough and Rodriguez, 2011); pathogens utilize mechanisms to reduce intracellular cAMP to suppress these functions (Serezani et al., 2008). Perhaps then, protein kinase A phosphorylation of MAM CTs is also involved in dampening the innate immune response. This idea, while entirely conjectural, can be approached experimentally.
MUC20 is something of an enigma. As presented in Section III, sequence analysis suggests that the MUC20 gene arose from duplication of a portion of the MUC4 VNTR domain, then underwent divergent evolution. It is the MAM mRNA most highly expressed in the apical layer of the human conjunctiva. However, the protein is found predominantly in intermediate cell layers of the corneal epithelium, and it is not detectable in tears, suggesting it plays a different role than the other ocular surface MAMs. MUC20 clearly associates with the plasma membrane, but does not appear to have a transmembrane domain. Nevertheless, recombinantly-expressed human MUC20 was shown to associate via its C-terminal domain with MET, a receptor tyrosine kinase activated by the extracellular ligand HGF. Interaction with MUC20 attenuated HGF-induced activities in kidney epithelial cells. In cornea, HGF is known to facilitate the migration and proliferation of epithelial cells, and to inhibit apoptosis. How this might relate to the role of MUC20 in ocular surface epithelia is, at this time, unknown. However, it may be speculated that MUC20 plays a role in regulating epithelial cell dynamics in the multi-layered epithelia.
When compared to the other MAMs localized to the ocular surface, MUC4 presents some intriguing differences. This MAM has the special capacity to directly activate EGFR family receptors adjacent to it in the plasma membrane via an EGF-like domain on its ED. However, it seems unlikely that activated EGFR family members phosphorylate the very short MUC4 CT, which has only a single tyrosine residue that is not predicted to be a phosphorylation site.
MUC4 appears to substitute for MUC16 in the corneal epithelium of mice and rats, but there are no obvious binding sites for proteins in the CT that might participate in regulation of the adherens junction. A difference between the CT of mouse/rat and human may be significant however; the mouse/rat CT is slightly longer than human, creating a predicted site for serine phosphorylation by casein kinase II. Intriguingly, casein kinase II phosphorylation of CDH1 increases its interaction with CTNNB1, strengthening the adherens junction and intracellular adhesion (Lickert et al., 2000). Perhaps MUC4 competition for casein kinase II binding regulates adherens junction strength, an idea that can be experimentally tested. However, while MUC16 levels decrease as cells age and knockdown studies suggest that this may lead to changes that precede desquamation, MUC4 levels are reported to be higher in desquamating cells. Thus, if these two MAMs substitute for one another in regulating desquamation, it seems likely that they do so via evolutionarily convergent mechanisms.
Encouragingly, analysis of Muc16 knockout mice revealed effects on epithelial dynamics, as discussed in Section IV.B.5. Basal-like cells were observed in the suprabasal layer of the corneal epithelium, with an increase in cell proliferation. The loss of Muc16 accelerated regeneration of an experimentally-created corneal epithelial defect. The recent availability of the Muc4 knockout mouse now provides the opportunity for more significant results. We predict phenotypes related to epithelial cell dynamics, desquamation, rose bengal exclusion and dry eye disease.
Drugs used to treat ocular surface disease and glaucoma are applied topically to the ocular surface. Corneal and conjunctival epithelia are the key tissues in absorption of these drugs, however, the role of the mucosal barrier in drug delivery to the ocular surface is still essentially undefined (Ruponen and Urtti, 2015) and will be a very important area for future research. MAMs may decrease or increase ocular bioavailability depending on the magnitude of their role as barrier or retention sites, respectively. In every way examined to date (e.g., (Bauskar et al., 2015; Mauris et al., 2013; Pflugfelder et al., 2005)), ocular surface barrier function in mice is equivalent to humans, despite substitution of Muc4 for MUC16, and despite the fact that the mouse Muc4 ED is shorter than the ED of human MUC4. Thus, the mouse can provide an important model for studies of factors determining drug absorption at the ocular surface, and it will be important to fully characterize structure/function of the mucosal glycocalyx.
We end this Section with a final, very conjectural, but intriguing hypothesis about a new MAM mechanism. In Section V.A.1, we discuss genetic evidence for the role of MUC1 in enhancing the functionality of a TRP family ion channel involved in calcium resorption in the kidney. MUC1 stabilizes the ion channel at the surface of the cell, inhibiting its endocytosis. MAM stabilization of ion channels is a novel mechanism, not previously considered. It is hard to imagine that the TRP ion channel is the only example. As we discussed, TRP ion channels have been linked to ocular surface disease. In Section V.A.2, we conjecture that MUC22 might similarly stabilize another type of ion channel, CFTR, thus regulating periciliary liquid secretion and mucous clearance in the bronchioles of the lung following infection, and possibly also the lacrimal gland. MAM regulation of ion channel function would be of great importance to ocular surface biology and disease, and is an area that should be a priority for investigation.
Highlights.
MUC1, −4, −16, −20, −21 and −22 are expressed at the ocular surface
Their glycoprotein products provide barrier function and act as cell surface receptors
Biological roles are being defined in the human epithelial tissue-equivalent model
Knockout mouse models add in vivo complexity
Human genetics/genomics offers functional clues
Publication Statement
This article has not been published previously (except in the form of an abstract, a published lecture or academic thesis) and it is not under consideration for publication elsewhere. Publication is approved by all authors and individuals acknowledged, and tacitly or explicitly by the responsible authorities where the work was carried out. If accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
VI. Acknowledgements and Funding
The authors dedicate this review to Dr. Ilene Gipson (Harvard Medical School). Many of the advances described herein come from her extensive body of work. Together with her staff colleagues and trainees, Dr. Gipson has raised our understanding of ocular surface mucins to the molecular level. We gratefully acknowledge her thoughtful commentary on a draft of this article. The authors also thank Dr. Eric Cox (National Center for Biotechnology Information) for his expert consultation in compilation and comparison of human and mouse MAM genes. The USC Department of Pathology’s Immunohistochemistry Laboratory is gratefully acknowledged for technical assistance with the previously unpublished immunohistochemical studies.
Supported by NIH grant R01EY026479 and a Schaffer Innovative Glaucoma Research Grant from the Glaucoma Research Foundation to MEF. Additional support was provided by the Massachusetts Lions Eye Research Fund to the Department of Ophthalmology at Tufts, and by unrestricted and challenge grants from Research to Prevent Blindness, Inc., New York, NY, to the Departments of Ophthalmology at USC and Tufts, respectively. The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Declaration of Interest
MEF and SJ are named as inventors on an issued United States patent entitled “Structure/Function of Clusterin Pharmaceuticals” and a pending patent application entitled “Method to Protect and Seal the Ocular Surface” (United States application 16/103,741, filed Aug 14, 2018), assigned to the University of Southern California and related to work mentioned herein. MEF is a co-founder and serves as Chief Scientific Officer for Proteris Biotech, Inc., a company focused on developing pharmaceuticals for treating eye disease. The other authors have no commercial or proprietary interest in any concept or product described in this article.
Permissions
Permission is in the process of being obtained for use of copyrighted material from other sources (including the Internet)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: HUGO nomenclature used for genes and their products. CT: cytoplasmic tail; ED: extracellular domain GRC: Genome Reference Consortium; HGNC: HUGO Gene Nomenclature Committee; MAM: membrane-associated mucin; MPS: multipurpose contact lens cleaning solution; NCBI: National Center for Biotechnology Information; RT-PCR: reverse transcription-polymerase chain reaction; SEM: scanning electron microscopy; TEM: transmission electron microscopy; VNTR: variable number tandem repeats
VII. References
- Abelson MB, Ingerman A, 2005. The Dye-namics of Dry-Eye Diagnosis. Review of Ophthalmology https://www.reviewofophthalmology.com/article/the-dye-namics-of-dry-eye-diagnosis. [Google Scholar]
- Ablamowicz AF, Nichols JJ, 2016. Ocular Surface Membrane-Associated Mucins. The ocular surface 14, 331–341. [DOI] [PubMed] [Google Scholar]
- Agrawal B, Krantz MJ, Parker J, Longenecker BM, 1998. Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer research 58, 4079–4081. [PubMed] [Google Scholar]
- Aithal A, Junker WM, Kshirsagar P, Das S, Kaur S, Orzechowski C, Gautam SK, Jahan R, Sheinin YM, Lakshmanan I, Ponnusamy MP, Batra SK, Jain M, 2018. Development and characterization of carboxy-terminus specific monoclonal antibodies for understanding MUC16 cleavage in human ovarian cancer. PloS one 13, e0193907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bataineh MM, Sutton TA, Hughey RP, 2017. Novel roles for mucin 1 in the kidney. Curr Opin Nephrol Hypertens 26, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsmeyer AC, Kakkassery V, Spurr-Michaud S, Beeks O, Gipson IK, 2010. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Experimental eye research 90, 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolopoulos V, McKenzie IF, 1994. Cellular mucins: targets for immunotherapy. Crit Rev Immunol 14, 293–309. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos V, McKenzie IFC, 2017. Cellular Mucins: Targets for Immunotherapy. Crit Rev Immunol 37, 421–437. [DOI] [PubMed] [Google Scholar]
- Arao T, Perkins E, 1968. The nictitating membranes of primates. Anat Rec 162, 53–70. [DOI] [PubMed] [Google Scholar]
- Argueso P, 2013. Glycobiology of the ocular surface: mucins and lectins. Jpn J Ophthalmol 57, 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, Gipson IK, 2001. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Experimental eye research 73, 281–289. [DOI] [PubMed] [Google Scholar]
- Argueso P, Gipson IK, 2012. Assessing mucin expression and function in human ocular surface epithelia in vivo and in vitro. Methods in molecular biology 842, 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N, 2009. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. The Journal of biological chemistry 284, 23037–23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK, 2003. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Investigative ophthalmology & visual science 44, 2487–2495. [DOI] [PubMed] [Google Scholar]
- Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK, 2006. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Investigative ophthalmology & visual science 47, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpitha P, Gao CY, Tripathi BK, Saravanamuthu S, Zelenka P, 2013. Cyclin-dependent kinase 5 promotes the stability of corneal epithelial cell junctions. Molecular vision 19, 319–332. [PMC free article] [PubMed] [Google Scholar]
- Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H, 2012. ExPASy: SIB bioinformatics resource portal. Nucleic acids research 40, W597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafna S, Kaur S, Batra SK, 2010. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 29, 2893–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banos-Lara Mdel R, Piao B, Guerrero-Plata A, 2015. Differential mucin expression by respiratory syncytial virus and human metapneumovirus infection in human epithelial cells. Mediators Inflamm 2015, 347292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabino S, Dana MR, 2004. Animal models of dry eye: a critical assessment of opportunities and limitations. Investigative ophthalmology & visual science 45, 1641–1646. [DOI] [PubMed] [Google Scholar]
- Bast RC Jr., Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L, Zurawski VR Jr., Knapp RC, 1983. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. The New England journal of medicine 309, 883–887. [DOI] [PubMed] [Google Scholar]
- Basu S, Fenton MJ, 2004. Toll-like receptors: function and roles in lung disease. American journal of physiology. Lung cellular and molecular physiology 286, L887–892. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F, 2010. Preservatives in eyedrops: the good, the bad and the ugly. Progress in retinal and eye research 29, 312–334. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Rolando M, Benitez Del Castillo JM, Messmer EM, Figueiredo FC, Irkec M, Van Setten G, Labetoulle M, 2018. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Progress in retinal and eye research. [DOI] [PubMed] [Google Scholar]
- Bauskar A, Mack WJ, Mauris J, Argueso P, Heur M, Nagel BA, Kolar GR, Gleave ME, Nakamura T, Kinoshita S, Moradian-Oldak J, Panjwani N, Pflugfelder SC, Wilson MR, Fini ME, Jeong S, 2015. Clusterin Seals the Ocular Surface Barrier in Mouse Dry Eye. PloS one 10, e0138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, Dartt DA, Galor A, Hamrah P, Ivanusic JJ, Jacobs DS, McNamara NA, Rosenblatt MI, Stapleton F, Wolffsohn JS, 2017. TFOS DEWS II pain and sensation report. The ocular surface 15, 404–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berczeli O, Vizvari E, Katona M, Torok D, Szalay L, Rarosi F, Nemeth I, Rakonczay Z, Hegyi P, Ding C, Toth-Molnar E, 2018. Novel Insight Into the Role of CFTR in Lacrimal Gland Duct Function in Mice. Investigative ophthalmology & visual science 59, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstam LI, Vaughan FL, Bernstein IA, 1986. Keratinocytes grown at the air-liquid interface. In Vitro Cell Dev Biol 22, 695–705. [DOI] [PubMed] [Google Scholar]
- Berry M, Ellingham RB, Corfield AP, 2004. Human preocular mucins reflect changes in surface physiology. The British journal of ophthalmology 88, 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, McMaster TJ, Corfield AP, Miles MJ, 2001. Exploring the molecular adhesion of ocular mucins. Biomacromolecules 2, 498–503. [DOI] [PubMed] [Google Scholar]
- Besmer DM, Curry JM, Roy LD, Tinder TL, Sahraei M, Schettini J, Hwang SI, Lee YY, Gendler SJ, Mukherjee P, 2011. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer research 71, 4432–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhavanandan VP, 1991. Cancer-associated mucins and mucin-type glycoproteins. Glycobiology 1, 493–503. [DOI] [PubMed] [Google Scholar]
- Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK, 2008. Release of membrane-associated mucins from ocular surface epithelia. Investigative ophthalmology & visual science 49, 1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, Gipson IK, 2007. Functions of MUC16 in corneal epithelial cells. Investigative ophthalmology & visual science 48, 4509–4518. [DOI] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S, 2004. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4, 1633–1649. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Zimmermann N, 2015. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. The Journal of allergy and clinical immunology 135, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll EJ, Ayala-Lujan J, Szabady RL, Louissaint C, Smith RZ, Krogfelt KA, Nataro JP, Ruiz-Perez F, McCormick BA, 2017. Enteroaggregative Escherichia coli Adherence Fimbriae Drive Inflammatory Cell Recruitment via Interactions with Epithelial MUC1. MBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, Argueso P, Irkec M, Bright FV, 2015. Clinical staining of the ocular surface: mechanisms and interpretations. Progress in retinal and eye research 44, 36–61. [DOI] [PubMed] [Google Scholar]
- Brugger W, Buhring HJ, Grunebach F, Vogel W, Kaul S, Muller R, Brummendorf TH, Ziegler BL, Rappold I, Brossart P, Scheding S, Kanz L, 1999. Expression of MUC-1 epitopes on normal bone marrow: implications for the detection of micrometastatic tumor cells. J Clin Oncol 17, 1535–1544. [DOI] [PubMed] [Google Scholar]
- Buchan DW, Minneci F, Nugent TC, Bryson K, Jones DT, 2013. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic acids research 41, W349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Lu H, McMahon A, Eule JC, 2012. Toward an animal model of the human tear film: biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Investigative ophthalmology & visual science 53, 6881–6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway KL 3rd, Funes M, Workman HC, Sweeney C, 2007. Contribution of membrane mucins to tumor progression through modulation of cellular growth signaling pathways. Curr Top Dev Biol 78, 1–22. [DOI] [PubMed] [Google Scholar]
- Carraway KL, Price-Schiavi SA, Komatsu M, Idris N, Perez A, Li P, Jepson S, Zhu X, Carvajal ME, Carraway CA, 2000. Multiple facets of sialomucin complex/MUC4, a membrane mucin and erbb2 ligand, in tumors and tissues (Y2K update). Frontiers in bioscience : a journal and virtual library 5, D95–D107. [DOI] [PubMed] [Google Scholar]
- Carraway KL, Ramsauer VP, Haq B, Carothers Carraway CA, 2003. Cell signaling through membrane mucins. BioEssays : news and reviews in molecular, cellular and developmental biology 25, 66–71. [DOI] [PubMed] [Google Scholar]
- Carvalho F, Seruca R, David L, Amorim A, Seixas M, Bennett E, Clausen H, Sobrinho-Simoes M, 1997. MUC1 gene polymorphism and gastric cancer--an epidemiological study. Glycoconjugate journal 14, 107–111. [DOI] [PubMed] [Google Scholar]
- Castro N, Gillespie SR, Bernstein AM, 2019. Ex Vivo Corneal Organ Culture Model for Wound Healing Studies. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloin-Dufau C, Pavitt I, Delorme P, Dhouailly D, 1993. Identification of keratins 3 and 12 in corneal epithelium of vertebrates. Epithelial Cell Biol 2, 120–125. [PubMed] [Google Scholar]
- Chaturvedi P, Singh AP, Batra SK, 2008. Structure, evolution, and biology of the MUC4 mucin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22, 966–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM, 2009. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clinical cancer research : an official journal of the American Association for Cancer Research 15, 5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JB, Zhang J, Hu HZ, Xue M, Jin YJ, 2017a. Polymorphisms of TGFB1, TLE4 and MUC22 are associated with childhood asthma in Chinese population. Allergol Immunopathol (Madr) 45, 432–438. [DOI] [PubMed] [Google Scholar]
- Chen LW, Chen YM, Lu CJ, Chen MY, Lin SY, Hu FR, Chen WL, 2017b. Effect of air-lifting on the stemness, junctional protein formation, and cytokeratin expression of in vitro cultivated limbal epithelial cell sheets. Taiwan J Ophthalmol 7, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon DJ, Wang Y, Deng JM, Lu Z, Xiao L, Chen CM, Bast RC, Behringer RR, 2009. CA125/MUC16 is dispensable for mouse development and reproduction. PloS one 4, e4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieffi G, Baccari GC, Di Matteo L, d’Istria M, Minucci S, Varriale B, 1996. Cell biology of the harderian gland. Int Rev Cytol 168, 1–80. [DOI] [PubMed] [Google Scholar]
- Church DM, Goodstadt L, Hillier LW, Zody MC, Goldstein S, She X, Bult CJ, Agarwala R, Cherry JL, DiCuccio M, Hlavina W, Kapustin Y, Meric P, Maglott D, Birtle Z, Marques AC, Graves T, Zhou S, Teague B, Potamousis K, Churas C, Place M, Herschleb J, Runnheim R, Forrest D, Amos-Landgraf J, Schwartz DC, Cheng Z, Lindblad-Toh K, Eichler EE, Ponting CP, Mouse Genome Sequencing C, 2009. Lineage-specific biology revealed by a finished genome assembly of the mouse. PLoS Biol 7, e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comamala M, Pinard M, Theriault C, Matte I, Albert A, Boivin M, Beaudin J, Piche A, Rancourt C, 2011. Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. Br J Cancer 104, 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou PE, Danysh BP; Dharmaraj N; Carson DD, 2011. Transmembrane mucins as novel therapeutic targets. . Expert Rev Endocrinol Metab. 6, 835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Ruiz L, Masli S, 2015. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PloS one 10, e0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland NG, Jenkins NA, O’Brien SJ, 2002. Genomics. Mmu 16--comparative genomic highlights. Science 296, 1617–1618. [DOI] [PubMed] [Google Scholar]
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, Stapleton F, 2017. TFOS DEWS II Definition and Classification Report. The ocular surface 15, 276–283. [DOI] [PubMed] [Google Scholar]
- Danjo Y, Hazlett LD, Gipson IK, 2000. C57BL/6 mice lacking Muc1 show no ocular surface phenotype. Investigative ophthalmology & visual science 41, 4080–4084. [PubMed] [Google Scholar]
- Das S, Majhi PD, Al-Mugotir MH, Rachagani S, Sorgen P, Batra SK, 2015. Membrane proximal ectodomain cleavage of MUC16 occurs in the acidifying Golgi/post-Golgi compartments. Scientific reports 5, 9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JR, Kirkham S, Svitacheva N, Thornton DJ, Carlstedt I, 2007. MUC16 is produced in tracheal surface epithelium and submucosal glands and is present in secretions from normal human airway and cultured bronchial epithelial cells. The international journal of biochemistry & cell biology 39, 1943–1954. [DOI] [PubMed] [Google Scholar]
- Dekker J, Rossen JW, Buller HA, Einerhand AW, 2002. The MUC family: an obituary. Trends in biochemical sciences 27, 126–131. [DOI] [PubMed] [Google Scholar]
- Dhar P, McAuley J, 2019. The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation. Front Cell Infect Microbiol 9, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar P, Ng GZ, Dunne EM, Sutton P, 2017. Mucin 1 protects against severe Streptococcus pneumoniae infection. Virulence 8, 1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty MJ, 1990. Morphometric analysis of the surface cells of rabbit corneal epithelium by scanning electron microscopy. Am J Anat 189, 316–328. [DOI] [PubMed] [Google Scholar]
- Doughty MJ, 2004. Further assessment of the size, shape and surface features of superficial cells of the bovine corneal epithelium, using scanning electron microscopy. Current eye research 28, 203–214. [DOI] [PubMed] [Google Scholar]
- Doughty MJ, 2006. Quantitative analysis of ring-shaped (crater-like) features at the tear film-epithelial interface of the rabbit cornea as assessed by scanning electron microscopy. Current eye research 31, 999–1010. [DOI] [PubMed] [Google Scholar]
- Doughty MJ, 2016. Corneal Surface and Superficial Cells as Viewed by Scanning Electron Microscopy and Impression Cytology Sampling. Cornea 35, 243–248. [DOI] [PubMed] [Google Scholar]
- Duke-Elder SS, Gloster J, 1968. The protective mechanism. The movements of the eyelids. Henry Kimpton, London. [Google Scholar]
- Duraisamy S, Ramasamy S, Kharbanda S, Kufe D, 2006. Distinct evolution of the human carcinoma-associated transmembrane mucins, MUC1, MUC4 AND MUC16. Gene 373, 28–34. [DOI] [PubMed] [Google Scholar]
- Eckhart L, Lippens S, Tschachler E, Declercq W, 2013. Cell death by cornification. Biochimica et biophysica acta 1833, 3471–3480. [DOI] [PubMed] [Google Scholar]
- Eckhart L, Tschachler E, 2018. Control of cell death-associated danger signals during cornification prevents autoinflammation of the skin. Exp Dermatol 27, 884–891. [DOI] [PubMed] [Google Scholar]
- Elborn JS, 2016. Cystic fibrosis. Lancet 388, 2519–2531. [DOI] [PubMed] [Google Scholar]
- Feenstra RP, Tseng SC, 1992a. Comparison of fluorescein and rose bengal staining. Ophthalmology 99, 605–617. [DOI] [PubMed] [Google Scholar]
- Feenstra RP, Tseng SC, 1992b. What is actually stained by rose bengal? Archives of ophthalmology 110, 984–993. [DOI] [PubMed] [Google Scholar]
- Fendrick JL, Konishi I, Geary SM, Parmley TH, Quirk JG Jr., O’Brien TJ, 1997. CA125 phosphorylation is associated with its secretion from the WISH human amnion cell line. Tumour Biol 18, 278–289. [DOI] [PubMed] [Google Scholar]
- Feng BJ, Sun LD, Soltani-Arabshahi R, Bowcock AM, Nair RP, Stuart P, Elder JT, Schrodi SJ, Begovich AB, Abecasis GR, Zhang XJ, Callis-Duffin KP, Krueger GG, Goldgar DE, 2009. Multiple Loci within the major histocompatibility complex confer risk of psoriasis. PLoS genetics 5, e1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando MM, Freudenberg J, Lee A, Morris DL, Boteva L, Rhodes B, Gonzalez-Escribano MF, Lopez-Nevot MA, Navarra SV, Gregersen PK, Martin J, Imagen, Vyse TJ, 2012. Transancestral mapping of the MHC region in systemic lupus erythematosus identifies new independent and interacting loci at MSH5, HLA-DPB1 and HLA-G. Ann Rheum Dis 71, 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini ME, Stramer BM, 2005. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea 24, S2–S11. [DOI] [PubMed] [Google Scholar]
- Galanter JM, Gignoux CR, Torgerson DG, Roth LA, Eng C, Oh SS, Nguyen EA, Drake KA, Huntsman S, Hu D, Sen S, Davis A, Farber HJ, Avila PC, Brigino-Buenaventura E, LeNoir MA, Meade K, Serebrisky D, Borrell LN, Rodriguez-Cintron W, Estrada AM, Mendoza KS, Winkler CA, Klitz W, Romieu I, London SJ, Gilliland F, Martinez F, Bustamante C, Williams LK, Kumar R, Rodriguez-Santana JR, Burchard EG, 2014. Genome-wide association study and admixture mapping identify different asthma-associated loci in Latinos: the Genes-environments & Admixture in Latino Americans study. The Journal of allergy and clinical immunology 134, 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NB, Su Z, Zhang X, Volpe EA, Pelegrino FS, Rahman SA, Li DQ, Pflugfelder SC, de Paiva CS, 2013. Dendritic cell-derived thrombospondin-1 is critical for the generation of the ocular surface Th17 response to desiccating stress. Journal of leukocyte biology 94, 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Posadas L, Soriano-Romani L, Lopez-Garcia A, Diebold Y, 2017. An engineered human conjunctival-like tissue to study ocular surface inflammatory diseases. PloS one 12, e0171099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler SJ, Burchell JM, Duhig T, Lamport D, White R, Parker M, Taylor-Papadimitriou J, 1987. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proceedings of the National Academy of Sciences of the United States of America 84, 6060–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D, 1990. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. The Journal of biological chemistry 265, 15286–15293. [PubMed] [Google Scholar]
- Gendler SJ, Spicer AP, 1995. Epithelial mucin genes. Annual review of physiology 57, 607–634. [DOI] [PubMed] [Google Scholar]
- Gendler SJ, Spicer AP, Lalani EN, Duhig T, Peat N, Burchell J, Pemberton L, Boshell M, Taylor-Papadimitriou J, 1991. Structure and biology of a carcinoma-associated mucin, MUC1. Am Rev Respir Dis 144, S42–47. [DOI] [PubMed] [Google Scholar]
- Gipson IK, 2000. In situ hybridization techniques for localizing mucin mRNA. Methods in molecular biology 125, 323–336. [DOI] [PubMed] [Google Scholar]
- Gipson IK, 2004. Distribution of mucins at the ocular surface. Experimental eye research 78, 379–388. [DOI] [PubMed] [Google Scholar]
- Gipson IK, 2007. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Investigative ophthalmology & visual science 48, 4390; 4391–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, 2014. Comparison of the Transmembrane Mucins MUC1 and MUC16 in Human Corneal Epithelial Barrier Function, International Conference on Eye Research, 21st Biennial Meeting, Hyatt Regency San Francisco at Embarcadero, San Francisco, California, USA. [Google Scholar]
- Gipson IK, Argueso P, 2003. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol 231, 1–49. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Hori Y, Argueso P, 2004. Character of ocular surface mucins and their alteration in dry eye disease. The ocular surface 2, 131–148. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Inatomi T, 1998. Cellular origin of mucins of the ocular surface tear film. Advances in experimental medicine and biology 438, 221–227. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Mandel U, Menon B, Michaud S, Tisdale A, Campos D, Clausen H, 2017. Generation and characterization of a monoclonal antibody to the cytoplasmic tail of MUC16. Glycobiology 27, 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL, 2003. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Investigative ophthalmology & visual science 44, 2496–2506. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, 2016. Human conjunctival goblet cells express the membrane associated mucin MUC16: Localization to mucin granules. Experimental eye research 145, 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB, 2014. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PloS one 9, e100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GM, Moradshahi N, Jeong S, Lane C, Fini ME, 2011. A novel mechanism of increased infections in contact lens wearers. Investigative ophthalmology & visual science 52, 9188–9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan B, Gipson IK, 2010. Membrane-tethered mucins have multiple functions on the ocular surface. Experimental eye research 90, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan B, Menon BB, Spurr-Michaud S, Rastogi K, Gilmore MS, Argueso P, Gipson IK, 2012. A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PloS one 7, e32418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H, Rheinwald JG, Sun TT, 1977. Properties of an epithelial cell type in culture: the epidermal keratinocyte and its dependence on products of the fibroblast. Prog Clin Biol Res 17, 493–500. [PubMed] [Google Scholar]
- Gum JR Jr., 1992. Mucin genes and the proteins they encode: structure, diversity, and regulation. American journal of respiratory cell and molecular biology 7, 557–564. [DOI] [PubMed] [Google Scholar]
- Gupta D, Harvey SA, Kaminski N, Swamynathan SK, 2011. Mouse conjunctival forniceal gene expression during postnatal development and its regulation by Kruppel-like factor 4. Investigative ophthalmology & visual science 52, 4951–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman-Aranguez A, Argueso P, 2010. Structure and biological roles of mucin-type O-glycans at the ocular surface. The ocular surface 8, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna C, Bicknell DS, O’Brien JE, 1961. Cell turnover in the adult human eye. Archives of ophthalmology 65, 695–698. [DOI] [PubMed] [Google Scholar]
- Hanna C, O’Brien JE, 1960. Cell production and migration in the epithelial layer of the cornea. Archives of ophthalmology 64, 536–539. [DOI] [PubMed] [Google Scholar]
- Hanson RL, Hollingsworth MA, 2016. Functional Consequences of Differential O-glycosylation of MUC1, MUC4, and MUC16 (Downstream Effects on Signaling). Biomolecules 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Has C, 2018. Peeling Skin Disorders: A Paradigm for Skin Desquamation. The Journal of investigative dermatology 138, 1689–1691. [DOI] [PubMed] [Google Scholar]
- Hattrup CL, Gendler SJ, 2008. Structure and function of the cell surface (tethered) mucins. Annual review of physiology 70, 431–457. [DOI] [PubMed] [Google Scholar]
- Hazlett LD, Spann B, Wells P, Berk RS, 1980. Desquamation of the corneal epithelium in the immature mouse: a scanning and transmission microscopy study. Experimental eye research 31, 21–30. [DOI] [PubMed] [Google Scholar]
- Henriksson JT, McDermott AM, Bergmanson JP, 2009. Dimensions and morphology of the cornea in three strains of mice. Investigative ophthalmology & visual science 50, 3648–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heralgi M, Thallangady A, Venkatachalam K, Vokuda H, 2017. Persistent unilateral nictitating membrane in a 9-year-old girl: A rare case report. Indian J Ophthalmol 65, 253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T, Orita T, Katsuya K, Yamasaki Y, Akiyama K, Li H, Yamamoto T, Saito Y, Nakamura M, 2004a. MUC20 suppresses the hepatocyte growth factor-induced Grb2-Ras pathway by binding to a multifunctional docking site of met. Molecular and cellular biology 24, 7456–7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, Waga I, Nanayama T, Yamamoto Y, Munger W, Sun HW, Falk RJ, Jennette JC, Alcorta DA, Li H, Yamamoto T, Saito Y, Nakamura M, 2004b. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. The Journal of biological chemistry 279, 1968–1979. [DOI] [PubMed] [Google Scholar]
- Hijikata M, Matsushita I, Tanaka G, Tsuchiya T, Ito H, Tokunaga K, Ohashi J, Homma S, Kobashi Y, Taguchi Y, Azuma A, Kudoh S, Keicho N, 2011. Molecular cloning of two novel mucin-like genes in the disease-susceptibility locus for diffuse panbronchiolitis. Human genetics 129, 117–128. [DOI] [PubMed] [Google Scholar]
- Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV, 1992. Cell membrane-associated mucins and their adhesion-modulating property. Trends in biochemical sciences 17, 359–363. [DOI] [PubMed] [Google Scholar]
- Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, Manolio TA, 2009. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proceedings of the National Academy of Sciences of the United States of America 106, 9362–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson S, Earlam R, 1994. Of an extracellular matrix in human pre-corneal tear film. J Theor Biol 168, 395–398. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Stoffel W, 1993. TMbase - A database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 374, 166. [Google Scholar]
- Holland EJ, Luchs J, Karpecki PM, Nichols KK, Jackson MA, Sall K, Tauber J, Roy M, Raychaudhuri A, Shojaei A, 2017. Lifitegrast for the Treatment of Dry Eye Disease: Results of a Phase III, Randomized, Double-Masked, Placebo-Controlled Trial (OPUS-3). Ophthalmology 124, 53–60. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ, 2004. Mucins in cancer: protection and control of the cell surface. Nature reviews. Cancer 4, 45–60. [DOI] [PubMed] [Google Scholar]
- Holly FJ, Lemp MA, 1977. Tear physiology and dry eyes. Survey of ophthalmology 22, 69–87. [DOI] [PubMed] [Google Scholar]
- Hornbeck PV, Kornhauser JM, Latham V, Murray B, Nandhikonda V, Nord A, Skrzypek E, Wheeler T, Zhang B, Gnad F, 2019. 15 years of PhosphoSitePlus(R): integrating post-translationally modified sites, disease variants and isoforms. Nucleic acids research 47, D433–D441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D, 2005. MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer research 65, 10413–10422. [DOI] [PubMed] [Google Scholar]
- Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK, 1995. Human corneal and conjunctival epithelia express MUC1 mucin. Investigative ophthalmology & visual science 36, 1818–1827. [PubMed] [Google Scholar]
- Inatomi T, Spurr-Michaud S, Tisdale AS, Zhan Q, Feldman ST, Gipson IK, 1996. Expression of secretory mucin genes by human conjunctival epithelia. Investigative ophthalmology & visual science 37, 1684–1692. [PubMed] [Google Scholar]
- International Consortium for Systemic Lupus Erythematosus, G., Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA, 2008. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nature genetics 40, 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura T, Webster A, Chintala SK, Wang Y, Gonzalez JM Jr., Tan JC, Vranka JA, Acott T, Craft CM, Sibug Saber ME, Jeong S, Stamer WD, Martemyanov KA, Fini ME, 2019. GPR158 in the Visual System: Homeostatic Role in Regulation of Intraocular Pressure. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 35, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Kamata-Sakurai M, Denda-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M, Irimura T, 2008. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology 18, 74–83. [DOI] [PubMed] [Google Scholar]
- Jager K, Wu G, Sel S, Garreis F, Brauer L, Paulsen FP, 2007. MUC16 in the lacrimal apparatus. Histochemistry and cell biology 127, 433–438. [DOI] [PubMed] [Google Scholar]
- Jentoft N, 1990. Why are proteins O-glycosylated? Trends in biochemical sciences 15, 291–294. [DOI] [PubMed] [Google Scholar]
- Jeong S, Patel N, Edlund CK, Hartiala J, Hazelett DJ, Itakura T, Wu PC, Avery RL, Davis JL, Flynn HW, Lalwani G, Puliafito CA, Wafapoor H, Hijikata M, Keicho N, Gao X, Argueso P, Allayee H, Coetzee GA, Pletcher MT, Conti DV, Schwartz SG, Eaton AM, Fini ME, 2015. Identification of a Novel Mucin Gene HCG22 Associated With Steroid-Induced Ocular Hypertension. Investigative ophthalmology & visual science 56, 2737–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson S, Komatsu M, Haq B, Arango ME, Huang D, Carraway CA, Carraway KL, 2002. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene 21, 7524–7532. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Fisher S, Spiekerman J, Chang JH, Wright WE, Shay JW, 2003. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life human corneal fibroblasts. Investigative ophthalmology & visual science 44, 1850–1858. [DOI] [PubMed] [Google Scholar]
- Jett BD, Gilmore MS, 2002. Internalization of Staphylococcus aureus by human corneal epithelial cells: role of bacterial fibronectin-binding protein and host cell factors. Infect Immun 70, 4697–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ME, Murphy PJ, 2004. Changes in the tear film and ocular surface from dry eye syndrome. Progress in retinal and eye research 23, 449–474. [DOI] [PubMed] [Google Scholar]
- Jones DT, 1999. Protein secondary structure prediction based on position-specific scoring matrices. Journal of molecular biology 292, 195–202. [DOI] [PubMed] [Google Scholar]
- Jones MC, Askari JA, Humphries JD, Humphries MJ, 2018. Cell adhesion is regulated by CDK1 during the cell cycle. The Journal of cell biology 217, 3203–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Kumar S, Bafna S, Rachagani S, Wagner KU, Jain M, Batra SK, 2015. Genetically engineered mucin mouse models for inflammation and cancer. Cancer Metastasis Rev 34, 593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE, 2003. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea 22, 41–45. [DOI] [PubMed] [Google Scholar]
- Justice MJ, Dhillon P, 2016. Using the mouse to model human disease: increasing validity and reproducibility. Dis Model Mech 9, 101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon R, Price RE, Julian J, Lagow E, Tseng SC, Gendler SJ, Carson DD, 1999. Bacterial conjunctivitis in Muc1 null mice. Investigative ophthalmology & visual science 40, 1328–1335. [PubMed] [Google Scholar]
- Karn RC, Laukaitis CM, 2015. Comparative Proteomics of Mouse Tears and Saliva: Evidence from Large Protein Families for Functional Adaptation. Proteomes 3, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Lillehoj EP, Kim KC, 2016. Pseudomonas aeruginosa stimulates tyrosine phosphorylation of and TLR5 association with the MUC1 cytoplasmic tail through EGFR activation. Inflamm Res 65, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Lillehoj EP, Park YS, Umehara T, Hoffman NE, Madesh M, Kim KC, 2012. Membrane-tethered MUC1 mucin is phosphorylated by epidermal growth factor receptor in airway epithelial cells and associates with TLR5 to inhibit recruitment of MyD88. Journal of immunology 188, 2014–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ, 2013. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol 6, 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, 2000. The use of vital dyes in corneal disease. Current opinion in ophthalmology 11, 241–247. [DOI] [PubMed] [Google Scholar]
- Kim KC, 2012. Role of epithelial mucins during airway infection. Pulm Pharmacol Ther 25, 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith PE, Fink BA, Fogt N, Nichols KK, Hill RM, Wilson GS, 2000. The thickness of the human precorneal tear film: evidence from reflection spectra. Investigative ophthalmology & visual science 41, 3348–3359. [PubMed] [Google Scholar]
- Kinlough CL, Poland PA, Bruns JB, Harkleroad KL, Hughey RP, 2004. MUC1 membrane trafficking is modulated by multiple interactions. The Journal of biological chemistry 279, 53071–53077. [DOI] [PubMed] [Google Scholar]
- Kirby A, Gnirke A, Jaffe DB, Baresova V, Pochet N, Blumenstiel B, Ye C, Aird D, Stevens C, Robinson JT, Cabili MN, Gat-Viks I, Kelliher E, Daza R, DeFelice M, Hulkova H, Sovova J, Vylet’al P, Antignac C, Guttman M, Handsaker RE, Perrin D, Steelman S, Sigurdsson S, Scheinman SJ, Sougnez C, Cibulskis K, Parkin M, Green T, Rossin E, Zody MC, Xavier RJ, Pollak MR, Alper SL, Lindblad-Toh K, Gabriel S, Hart PS, Regev A, Nusbaum C, Kmoch S, Bleyer AJ, Lander ES, Daly MJ, 2013. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nature genetics 45, 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ, 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396, 84–88. [DOI] [PubMed] [Google Scholar]
- Knop N, Knop E, 2000. Conjunctiva-associated lymphoid tissue in the human eye. Investigative ophthalmology & visual science 41, 1270–1279. [PubMed] [Google Scholar]
- Kramerov AA, Saghizadeh M, Ljubimov AV, 2016. Adenoviral Gene Therapy for Diabetic Keratopathy: Effects on Wound Healing and Stem Cell Marker Expression in Human Organ-cultured Corneas and Limbal Epithelial Cells. Journal of visualized experiments : JoVE, e54058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA, 1990. Cloning and sequencing of a human pancreatic tumor mucin cDNA. The Journal of biological chemistry 265, 15294–15299. [PubMed] [Google Scholar]
- Lange C, Fernandez J, Shim D, Spurr-Michaud S, Tisdale A, Gipson IK, 2003. Mucin gene expression is not regulated by estrogen and/or progesterone in the ocular surface epithelia of mice. Experimental eye research 77, 59–68. [DOI] [PubMed] [Google Scholar]
- Leong CF, Raudhawati O, Cheong SK, Sivagengei K, Noor Hamidah H, 2003. Epithelial membrane antigen (EMA) or MUC1 expression in monocytes and monoblasts. Pathology 35, 422–427. [DOI] [PubMed] [Google Scholar]
- Li HF, Petroll WM, Moller-Pedersen T, Maurer JK, Cavanagh HD, Jester JV, 1997. Epithelial and corneal thickness measurements by in vivo confocal microscopy through focusing (CMTF). Current eye research 16, 214–221. [DOI] [PubMed] [Google Scholar]
- Li W, Hayashida Y, Chen YT, He H, Tseng DY, Alonso M, Chen SY, Xi X, Tseng SC, 2008. Air exposure induced squamous metaplasia of human limbal epithelium. Investigative ophthalmology & visual science 49, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bharti A, Chen D, Gong J, Kufe D, 1998. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Molecular and cellular biology 18, 7216–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC, 2010. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. American journal of physiology. Lung cellular and molecular physiology 298, L558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kufe D, 2001. The Human DF3/MUC1 carcinoma-associated antigen signals nuclear localization of the catenin p120(ctn). Biochemical and biophysical research communications 281, 440–443. [DOI] [PubMed] [Google Scholar]
- Li Y, Ren J, Yu W, Li Q, Kuwahara H, Yin L, Carraway KL 3rd, Kufe D, 2001. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. The Journal of biological chemistry 276, 35239–35242. [DOI] [PubMed] [Google Scholar]
- Lickert H, Bauer A, Kemler R, Stappert J, 2000. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. The Journal of biological chemistry 275, 5090–5095. [DOI] [PubMed] [Google Scholar]
- Lidell ME, Hansson GC, 2006. Cleavage in the GDPH sequence of the C-terminal cysteine-rich part of the human MUC5AC mucin. The Biochemical journal 399, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtenberg MJ, Kruijshaar L, Buijs F, van Meijer M, Litvinov SV, Hilkens J, 1992. Cell-associated episialin is a complex containing two proteins derived from a common precursor. The Journal of biological chemistry 267, 6171–6177. [PubMed] [Google Scholar]
- Ligtenberg MJ, Vos HL, Gennissen AM, Hilkens J, 1990. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. The Journal of biological chemistry 265, 5573–5578. [PubMed] [Google Scholar]
- Lillehoj EP, Hyun SW, Liu A, Guang W, Verceles AC, Luzina IG, Atamas SP, Kim KC, Goldblum SE, 2015. NEU1 Sialidase Regulates Membrane-tethered Mucin (MUC1) Ectodomain Adhesiveness for Pseudomonas aeruginosa and Decoy Receptor Release. The Journal of biological chemistry 290, 18316–18331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj EP, Kim BT, Kim KC, 2002. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. American journal of physiology. Lung cellular and molecular physiology 282, L751–756. [DOI] [PubMed] [Google Scholar]
- Lin H, Qu Y, Geng Z, Li C, Wu H, Dong N, Liu Z, Li W, 2014. Air exposure induced characteristics of dry eye in conjunctival tissue culture. PloS one 9, e87368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin TH, Sutton P, McGuckin MA, 2009. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog 5, e1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomako J, Lomako WM, Carothers Carraway CA, Carraway KL, 2010. Regulation of the membrane mucin Muc4 in corneal epithelial cells by proteosomal degradation and TGF-beta. Journal of cellular physiology 223, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomako J, Lomako WM, Decker SJ, Carraway CA, Carraway KL, 2005. Non-apoptotic desquamation of cells from corneal epithelium: putative role for Muc4/sialomucin complex in cell release and survival. Journal of cellular physiology 202, 115–124. [DOI] [PubMed] [Google Scholar]
- Lu M, Ding C, 2012. CFTR-mediated Cl(−) transport in the acinar and duct cells of rabbit lacrimal gland. Current eye research 37, 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Codina C, Read ML, Efron N, Dobson CB, Morgan PB, 2013. Observation of solution-induced corneal staining with fluorescein, rose bengal and lissamine green. Contact lens & anterior eye : the journal of the British Contact Lens Association 36, 267–270. [DOI] [PubMed] [Google Scholar]
- Mansiaux Y, Joseph AP, Gelly JC, de Brevern AG, 2011. Assignment of PolyProline II conformation and analysis of sequence--structure relationship. PloS one 6, e18401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, Argueso P, 2008. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol 8, 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelli F, Mauris J, Argueso P, 2013. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol 13, 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey RC, Kantzanou MN, Fowler T, Day NP, Schofield K, Wann ER, Berendt AR, Hook M, Peacock SJ, 2001. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell Microbiol 3, 839–851. [DOI] [PubMed] [Google Scholar]
- Mauris J, Mantelli F, Woodward AM, Cao Z, Bertozzi CR, Panjwani N, Godula K, Argueso P, 2013. Modulation of ocular surface glycocalyx barrier function by a galectin-3 N-terminal deletion mutant and membrane-anchored synthetic glycopolymers. PloS one 8, e72304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley JL, Corcilius L, Tan HX, Payne RJ, McGuckin MA, Brown LE, 2017. The cell surface mucin MUC1 limits the severity of influenza A virus infection. Mucosal Immunol 10, 1581–1593. [DOI] [PubMed] [Google Scholar]
- McAuley JL, Linden SK, Png CW, King RM, Pennington HL, Gendler SJ, Florin TH, Hill GR, Korolik V, McGuckin MA, 2007. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. The Journal of clinical investigation 117, 2313–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough KA, Rodriguez A, 2011. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol 10, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin MA, Every AL, Skene CD, Linden SK, Chionh YT, Swierczak A, McAuley J, Harbour S, Kaparakis M, Ferrero R, Sutton P, 2007. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133, 1210–1218. [DOI] [PubMed] [Google Scholar]
- McNeer RR, Price-Schiavi SA, Komatsu M, Fregien N, Carraway CAC, Carraway KL, 1997. Sialomucin complex in tumors and tissues. Frontiers in bioscience : a journal and virtual library 2, d449–459. [DOI] [PubMed] [Google Scholar]
- Menon BB, Kaiser-Marko C, Spurr-Michaud S, Tisdale AS, Gipson IK, 2015. Suppression of Toll-like receptor-mediated innate immune responses at the ocular surface by the membrane-associated mucins MUC1 and MUC16. Mucosal Immunol 8, 1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Rustin GJ, 2000. Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer 82, 1535–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, Ehret GB, Boerwinkle E, Felix JF, Leak TS, Harris TB, Yang Q, Dehghan A, Aspelund T, Katz R, Homuth G, Kocher T, Rettig R, Ried JS, Gieger C, Prucha H, Pfeufer A, Meitinger T, Coresh J, Hofman A, Sarnak MJ, Chen YD, Uitterlinden AG, Chakravarti A, Psaty BM, van Duijn CM, Kao WH, Witteman JC, Gudnason V, Siscovick DS, Fox CS, Kottgen A, Genetic Factors for Osteoporosis, C., Meta Analysis of, G., Insulin Related Traits, C., 2010. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS genetics 6. Milstone, L.M., 2004. Epidermal desquamation. J Dermatol Sci 36, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK, 2001. Structural organization and classification of the human mucin genes. Frontiers in bioscience : a journal and virtual library 6, D1192–1206. [DOI] [PubMed] [Google Scholar]
- Moniaux N, Nollet S, Porchet N, Degand P, Laine A, Aubert JP, 1999. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. The Biochemical journal 338 ( Pt 2), 325–333. [PMC free article] [PubMed] [Google Scholar]
- Moresco EM, LaVine D, Beutler B, 2011. Toll-like receptors. Curr Biol 21, R488–493. [DOI] [PubMed] [Google Scholar]
- Murube J, 2009. Basal, reflex, and psycho-emotional tears. The ocular surface 7, 60–66. [DOI] [PubMed] [Google Scholar]
- Ng GZ, Menheniott TR, Every AL, Stent A, Judd LM, Chionh YT, Dhar P, Komen JC, Giraud AS, Wang TC, McGuckin MA, Sutton P, 2016. The MUC1 mucin protects against Helicobacter pylori pathogenesis in mice by regulation of the NLRP3 inflammasome. Gut 65, 1087–1099. [DOI] [PubMed] [Google Scholar]
- Ng GZ, Sutton P, 2016. The MUC1 mucin specifically inhibits activation of the NLRP3 inflammasome. Genes Immun 17, 203–206. [DOI] [PubMed] [Google Scholar]
- Nichols B, Dawson CR, Togni B, 1983. Surface features of the conjunctiva and cornea. Investigative ophthalmology & visual science 24, 570–576. [PubMed] [Google Scholar]
- Nichols BA, Chiappino ML, Dawson CR, 1985. Demonstration of the mucous layer of the tear film by electron microscopy. Investigative ophthalmology & visual science 26, 464–473. [PubMed] [Google Scholar]
- Nie M, Bal MS, Yang Z, Liu J, Rivera C, Wenzel A, Beck BB, Sakhaee K, Marciano DK, Wolf MT, 2016. Mucin-1 Increases Renal TRPV5 Activity In Vitro, and Urinary Level Associates with Calcium Nephrolithiasis in Patients. Journal of the American Society of Nephrology : JASN 27, 3447–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman PJ, Norberg SJ, Guethlein LA, Nemat-Gorgani N, Royce T, Wroblewski EE, Dunn T, Mann T, Alicata C, Hollenbach JA, Chang W, Shults Won M, Gunderson KL, Abi-Rached L, Ronaghi M, Parham P, 2017. Sequences of 95 human MHC haplotypes reveal extreme coding variation in genes other than highly polymorphic HLA class I and II. Genome research 27, 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien TJ, Beard JB, Underwood LJ, Dennis RA, Santin AD, York L, 2001. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour Biol 22, 348–366. [DOI] [PubMed] [Google Scholar]
- O’Brien TJ, Beard JB, Underwood LJ, Shigemasa K, 2002. The CA 125 gene: a newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol 23, 154–169. [DOI] [PubMed] [Google Scholar]
- Ozyildirim AM, Wistow GJ, Gao J, Wang J, Dickinson DP, Frierson HF Jr., Laurie GW, 2005. The lacrimal gland transcriptome is an unusually rich source of rare and poorly characterized gene transcripts. Investigative ophthalmology & visual science 46, 1572–1580. [DOI] [PubMed] [Google Scholar]
- Palmai-Pallag T, Khodabukus N, Kinarsky L, Leir SH, Sherman S, Hollingsworth MA, Harris A, 2005. The role of the SEA (sea urchin sperm protein, enterokinase and agrin) module in cleavage of membrane-tethered mucins. The FEBS journal 272, 2901–2911. [DOI] [PubMed] [Google Scholar]
- Pan Z, Wang Z, Yang H, Zhang F, Reinach PS, 2011. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Investigative ophthalmology & visual science 52, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley RR, Gendler SJ, 1998. Cystic fibrosis mice lacking Muc1 have reduced amounts of intestinal mucus. The Journal of clinical investigation 102, 1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C, 2010. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nature medicine 16, 1396–1399. [DOI] [PubMed] [Google Scholar]
- Paulsen F, Langer G, Hoffmann W, Berry M, 2004. Human lacrimal gland mucins. Cell and tissue research 316, 167–177. [DOI] [PubMed] [Google Scholar]
- Perez BH, Gipson IK, 2008. Focus on Molecules: human mucin MUC16. Experimental eye research 87, 400–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman RL, 2016. Mouse models of human disease: An evolutionary perspective. Evol Med Public Health 2016, 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson FC, Volkman BF, 2009. Diversity of polyproline recognition by EVH1 domains. Front Biosci (Landmark Ed) 14, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister RR, 1973. The normal surface of corneal epithelium: a scanning electron microscopic study. Investigative ophthalmology 12, 654–668. [PubMed] [Google Scholar]
- Pflüger N, 1882. Zur Ernährung der cornea. Klin Monatsbl Augenheilkd 20, 69–81. [Google Scholar]
- Pflugfelder SC, de Paiva CS, 2017. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 124, S4–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, Olmos LC, Li DQ, Fini ME, 2005. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. The American journal of pathology 166, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Liu Z, Monroy D, Li DQ, Carvajal ME, Price-Schiavi SA, Idris N, Solomon A, Perez A, Carraway KL, 2000. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Investigative ophthalmology & visual science 41, 1316–1326. [PubMed] [Google Scholar]
- Poh TW, Bradley JM, Mukherjee P, Gendler SJ, 2009. Lack of Muc1-regulated beta-catenin stability results in aberrant expansion of CD11b+Gr1+ myeloid-derived suppressor cells from the bone marrow. Cancer research 69, 3554–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Schiavi SA, Meller D, Jing X, Merritt J, Carvajal ME, Tseng SC, Carraway KL, 1998. Sialomucin complex at the rat ocular surface: a new model for ocular surface protection. The Biochemical journal 335 ( Pt 2), 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price-Schiavi SA, Zhu X, Aquinin R, Carraway KL, 2000. Sialomucin complex (rat Muc4) is regulated by transforming growth factor beta in mammary gland by a novel post-translational mechanism. The Journal of biological chemistry 275, 17800–17807. [DOI] [PubMed] [Google Scholar]
- Prince JH, 1964. The Rabbit in Eye Research. Thomas, Springfield, IL. [Google Scholar]
- Prunieras M, Regnier M, Woodley D, 1983. Methods for cultivation of keratinocytes with an air-liquid interface. The Journal of investigative dermatology 81, 28s–33s. [DOI] [PubMed] [Google Scholar]
- Quin RJ, McGuckin MA, 2000. Phosphorylation of the cytoplasmic domain of the MUC1 mucin correlates with changes in cell-cell adhesion. International journal of cancer. Journal international du cancer 87, 499–506. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Thomas-Virnig C, Allen-Hoffmann BL, 2013. Classical human epidermal keratinocyte cell culture. Methods in molecular biology 945, 161–175. [DOI] [PubMed] [Google Scholar]
- Reinach PS, Mergler S, Okada Y, Saika S, 2015. Ocular transient receptor potential channel function in health and disease. BMC Ophthalmol 15 Suppl 1, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso R, Martin-Sanz R, Martino M, Mateo ME, Blanco-Salado R, Calonge M, Corell A, 2012. Topographical distribution and characterization of epithelial cells and intraepithelial lymphocytes in the human ocular mucosa. Mucosal Immunol 5, 455–467. [DOI] [PubMed] [Google Scholar]
- Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, Le JM, Yang B, Korman BD, Cakiris A, Aglar O, Emrence Z, Azakli H, Ustek D, Tugal-Tutkun I, Akman-Demir G, Chen W, Amos CI, Dizon MB, Kose AA, Azizlerli G, Erer B, Brand OJ, Kaklamani VG, Kaklamanis P, Ben-Chetrit E, Stanford M, Fortune F, Ghabra M, Ollier WE, Cho YH, Bang D, O’Shea J, Wallace GR, Gadina M, Kastner DL, Gul A, 2010. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet’s disease. Nature genetics 42, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Wilson G, 1996. Apoptosis in the corneal epithelium. Investigative ophthalmology & visual science 37, 1017–1025. [PubMed] [Google Scholar]
- Ren J, Li Y, Kufe D, 2002. Protein kinase C delta regulates function of the DF3/MUC1 carcinoma antigen in beta-catenin signaling. The Journal of biological chemistry 277, 17616–17622. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H, 1975. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6, 331–343. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricala C, O’Toole KM, 2002. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Molecular and cellular biology 22, 5157–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV, 2005. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investigative ophthalmology & visual science 46, 470–478. [DOI] [PubMed] [Google Scholar]
- Rose MC, 1992. Mucins: structure, function, and role in pulmonary diseases. The American journal of physiology 263, L413–429. [DOI] [PubMed] [Google Scholar]
- Ross M, Pawlina W, 2015. Histology: A Text and Atlas, with Correlated Cell and Molecular Biology, 7th ed. [Google Scholar]
- Rossi EA, McNeer RR, Price-Schiavi SA, Van den Brande JM, Komatsu M, Thompson JF, Carraway CA, Fregien NL, Carraway KL, 1996. Sialomucin complex, a heterodimeric glycoprotein complex. Expression as a soluble, secretable form in lactating mammary gland and colon. The Journal of biological chemistry 271, 33476–33485. [DOI] [PubMed] [Google Scholar]
- Ruponen M, Urtti A, 2015. Undefined role of mucus as a barrier in ocular drug delivery. Eur J Pharm Biopharm 96, 442–446. [DOI] [PubMed] [Google Scholar]
- Saika S, Ohnishi Y, Ooshima A, Liu CY, Kao WW, 2002. Epithelial repair: roles of extracellular matrix. Cornea 21, S23–29. [DOI] [PubMed] [Google Scholar]
- Sakai T, 1989. Major ocular glands (harderian gland and lacrimal gland) of the musk shrew (Suncus murinus) with a review on the comparative anatomy and histology of the mammalian lacrimal glands. J Morphol 201, 39–57. [DOI] [PubMed] [Google Scholar]
- Schrader S, Mircheff AK, Geerliing G, 2008. Animal Models of Dry Eye. Dev. Ophthalmol. 41, 298–312. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Thompson MC, Gardner MM, Gendler SJ, 2001. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. The Journal of biological chemistry 276, 13057–13064. [DOI] [PubMed] [Google Scholar]
- Seifert P, Spitznas M, Koch F, Cusumano A, 1994. Light and electron microscopic morphology of accessory lacrimal glands. Advances in experimental medicine and biology 350, 19–23. [DOI] [PubMed] [Google Scholar]
- Sellers RS, 2017. Translating Mouse Models. Toxicol Pathol 45, 134–145. [DOI] [PubMed] [Google Scholar]
- Seregni E, Botti C, Massaron S, Lombardo C, Capobianco A, Bogni A, Bombardieri E, 1997. Structure, function and gene expression of epithelial mucins. Tumori 83, 625–632. [DOI] [PubMed] [Google Scholar]
- Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M, 2008. Cyclic AMP: master regulator of innate immune cell function. American journal of respiratory cell and molecular biology 39, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Dudus L, Nielsen PA, Clausen H, Yankaskas JR, Hollingsworth MA, Engelhardt JF, 1998. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. American journal of respiratory cell and molecular biology 19, 30–37. [DOI] [PubMed] [Google Scholar]
- Sheppard JD, Orenstein DM, Chao CC, Butala S, Kowalski RP, 1989. The ocular surface in cystic fibrosis. Ophthalmology 96, 1624–1630. [DOI] [PubMed] [Google Scholar]
- Shirai K, Okada Y, Cheon DJ, Miyajima M, Behringer RR, Yamanaka O, Saika S, 2014. Effects of the loss of conjunctival Muc16 on corneal epithelium and stroma in mice. Investigative ophthalmology & visual science 55, 3626–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai K, Saika S, 2015. Ocular surface mucins and local inflammation--studies in genetically modified mouse lines. BMC Ophthalmol 15 Suppl 1, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebelmann S, Gehlsen U, Huttmann G, Koop N, Bolke T, Gebert A, Stern ME, Niederkorn JY, Steven P, 2013. Development, alteration and real time dynamics of conjunctiva-associated lymphoid tissue. PloS one 8, e82355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva F, Carvalho F, Peixoto A, Seixas M, Almeida R, Carneiro F, Mesquita P, Figueiredo C, Nogueira C, Swallow DM, Amorim A, David L, 2001. MUC1 gene polymorphism in the gastric carcinogenesis pathway. European journal of human genetics : EJHG 9, 548–552. [DOI] [PubMed] [Google Scholar]
- Singh PK, Hollingsworth MA, 2006. Cell surface-associated mucins in signal transduction. Trends Cell Biol 16, 467–476. [DOI] [PubMed] [Google Scholar]
- Sjögren H, 1933. Zur kenntnis der keratoconjunctivitis sicca. Acta ophthalmologica. Supplement 2. [DOI] [PubMed] [Google Scholar]
- Spicer AP, Parry G, Patton S, Gendler SJ, 1991. Molecular cloning and analysis of the mouse homologue of the tumor-associated mucin, MUC1, reveals conservation of potential O-glycosylation sites, transmembrane, and cytoplasmic domains and a loss of minisatellite-like polymorphism. The Journal of biological chemistry 266, 15099–15109. [PubMed] [Google Scholar]
- Spicer AP, Rowse GJ, Lidner TK, Gendler SJ, 1995. Delayed mammary tumor progression in Muc-1 null mice. The Journal of biological chemistry 270, 30093–30101. [DOI] [PubMed] [Google Scholar]
- Spurr-Michaud S, Argueso P, Gipson I, 2007. Assay of mucins in human tear fluid. Experimental eye research 84, 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr-Michaud SJ, Barza M, Gipson IK, 1988. An organ culture system for study of adherence of Pseudomonas aeruginosa to normal and wounded corneas. Investigative ophthalmology & visual science 29, 379–386. [PubMed] [Google Scholar]
- Srinivasan M, Dunker AK, 2012. Proline rich motifs as drug targets in immune mediated disorders. Int J Pept 2012, 634769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Bennett EP, Mandel U, Brunak S, Wandall HH, Levery SB, Clausen H, 2013. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J 32, 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC, 1998. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 17, 584–589. [DOI] [PubMed] [Google Scholar]
- Stern ME, Pflugfelder SC, 2017. What We Have Learned From Animal Models of Dry Eye. Int Ophthalmol Clin 57, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven P, Rupp J, Huttmann G, Koop N, Lensing C, Laqua H, Gebert A, 2008. Experimental induction and three-dimensional two-photon imaging of conjunctiva-associated lymphoid tissue. Investigative ophthalmology & visual science 49, 1512–1517. [DOI] [PubMed] [Google Scholar]
- Strong B, Farley W, Stern ME, Pflugfelder SC, 2005. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea 24, 80–85. [DOI] [PubMed] [Google Scholar]
- Strous GJ, Dekker J, 1992. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol 27, 57–92. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi M, Ricciuto J, Tisdale A, Gipson IK, Mantelli F, Argueso P, 2008. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Investigative ophthalmology & visual science 49, 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan JS, Arango ME, Carothers Carraway CA, Carraway KL, 2002. An ErbB2-Muc4 complex in rat ocular surface epithelia. Current eye research 24, 397–402. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, 1991. Report on the first international workshop on carcinoma-associated mucins. International journal of cancer. Journal international du cancer 49, 1–5. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Blobel CP, Carson DD, 2003. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. The Journal of biological chemistry 278, 3386–3394. [DOI] [PubMed] [Google Scholar]
- Thathiah A, Carson DD, 2004. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. The Biochemical journal 382, 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CH, Routledge C, Miller J, Miller F, Hodson SA, 2003. Examination of murine tear film. Investigative ophthalmology & visual science 44, 3520–3525. [DOI] [PubMed] [Google Scholar]
- Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, Kim KC, 2008. MUC1 mucin is a negative regulator of toll-like receptor signaling. American journal of respiratory cell and molecular biology 38, 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten JPM, Strijbis K, 2017. Transmembrane Mucins: Signaling Receptors at the Intersection of Inflammation and Cancer. J Innate Immun 9, 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinall LE, King M, Novelli M, Green CA, Daniels G, Hilkens J, Sarner M, Swallow DM, 2002. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology 123, 41–49. [DOI] [PubMed] [Google Scholar]
- Vos HL, de Vries Y, Hilkens J, 1991. The mouse episialin (Muc1) gene and its promoter: rapid evolution of the repetitive domain in the protein. Biochemical and biophysical research communications 181, 121–130. [DOI] [PubMed] [Google Scholar]
- Wang J, Fonn D, Simpson TL, Jones L, 2003. Precorneal and pre- and postlens tear film thickness measured indirectly with optical coherence tomography. Investigative ophthalmology & visual science 44, 2524–2528. [DOI] [PubMed] [Google Scholar]
- Wang K, Gao M, Yang M, Meng F, Li D, Lu R, Wang Y, Zhuang H, Li M, Cheng G, Wang X, 2017. Transcriptome analysis of bronchoalveolar lavage fluid from children with severe Mycoplasma pneumoniae pneumonia reveals novel gene expression and immunodeficiency. Hum Genomics 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheon DJ, Lu Z, Cunningham SL, Chen CM, Luo RZ, Xing D, Orsulic S, Bast RC Jr., Behringer RR, 2008. MUC16 expression during embryogenesis, in adult tissues, and ovarian cancer in the mouse. Differentiation; research in biological diversity 76, 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A, Chintala SK, Kim J, Ngan M, Itakura T, Panjwani N, Argueso P, Barr JT, Jeong S, Fini ME, 2018. Dynasore protects the ocular surface against damaging oxidative stress. PloS one 13, e0204288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg RA, 1998. Telomeres. Bumps on the road to immortality. Nature 396, 23–24. [DOI] [PubMed] [Google Scholar]
- Wells PA, Hazlett LD, 1984. Complex carbohydrates at the ocular surface of the mouse: an ultrastructural and cytochemical analysis. Experimental eye research 39, 19–35. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Ceresa BP, Howard EW, 2014. Diurnal variation of tight junction integrity associates inversely with matrix metalloproteinase expression in Xenopus laevis corneal epithelium: implications for circadian regulation of homeostatic surface cell desquamation. PloS one 9, e113810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox MDP, Argueso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, Papas EB, Rolland JP, Schmidt TA, Stahl U, Suarez T, Subbaraman LN, Ucakhan OO, Jones L, 2017. TFOS DEWS II Tear Film Report. The ocular surface 15, 366–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AM, Argueso P, 2014. Expression Analysis of the Transmembrane Mucin MUC20 in Human Corneal and Conjunctival Epithelia. Investigative ophthalmology & visual science 55, 6132–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes M, MacDonald KP, Tran M, Quin RJ, Xing PX, Gendler SJ, Hart DN, McGuckin MA, 2002. MUC1 epithelial mucin (CD227) is expressed by activated dendritic cells. Journal of leukocyte biology 72, 692–701. [PubMed] [Google Scholar]
- Xing PX, Apostolopoulos V, Karkaloutsos J, McKenzie IF, 2000. Monoclonal antibodies to mucin VNTR peptides. Methods in molecular biology 125, 369–381. [DOI] [PubMed] [Google Scholar]
- Xing PX, Apostolopoulos V, Pietersz G, McKenzie IF, 2001. Anti-mucin monoclonal antibodies. Frontiers in bioscience : a journal and virtual library 6, D1284–1295. [DOI] [PubMed] [Google Scholar]
- Xu F, Liu F, Zhao H, An G, Feng G, 2015. Prognostic Significance of Mucin Antigen MUC1 in Various Human Epithelial Cancers: A Meta-Analysis. Medicine (Baltimore) 94, e2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chen W, Leng S, Padilla MT, Saxton B, Hutt J, Tessema M, Kato K, Kim KC, Belinsky SA, Lin Y, 2017. Muc1 knockout potentiates murine lung carcinogenesis involving an epiregulin-mediated EGFR activation feedback loop. Carcinogenesis 38, 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Bharti A, Li Y, Kufe D, 1997. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. The Journal of biological chemistry 272, 12492–12494. [DOI] [PubMed] [Google Scholar]
- Yanez-Soto B, Leonard BC, Raghunathan VK, Abbott NL, Murphy CJ, 2015. Effect of Stratification on Surface Properties of Corneal Epithelial Cells. Investigative ophthalmology & visual science 56, 8340–8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LP, Zhang AL, Wang DD, Ke HX, Cheng Q, Wang C, 2014. Stevens-Johnson syndrome induced by the cross-reactivity between teicoplanin and vancomycin. J Clin Pharm Ther 39, 442–445. [DOI] [PubMed] [Google Scholar]
- Yatagai Y, Hirota T, Sakamoto T, Yamada H, Masuko H, Kaneko Y, Iijima H, Naito T, Noguchi E, Tamari M, Kubo M, Takahashi A, Konno S, Makita H, Nishimura M, Hijikata M, Keicho N, Homma S, Taguchi Y, Azuma A, Kudoh S, Hizawa N, 2016. Variants near the HLA complex group 22 gene (HCG22) confer increased susceptibility to late-onset asthma in Japanese populations. The Journal of allergy and clinical immunology 138, 281–283 e213. [DOI] [PubMed] [Google Scholar]
- Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC, 2003. Apoptosis of ocular surface cells in experimentally induced dry eye. Investigative ophthalmology & visual science 44, 124–129. [DOI] [PubMed] [Google Scholar]
- Yi Y, Kamata-Sakurai M, Denda-Nagai K, Itoh T, Okada K, Ishii-Schrade K, Iguchi A, Sugiura D, Irimura T, 2010. Mucin 21/epiglycanin modulates cell adhesion. The Journal of biological chemistry 285, 21233–21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin BW, Lloyd KO, 2001. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. The Journal of biological chemistry 276, 27371–27375. [DOI] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, 2012. Tear analysis in ocular surface diseases. Progress in retinal and eye research 31, 527–550. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW, 2012. In-depth analysis of the human tear proteome. Journal of proteomics 75, 3877–3885. [DOI] [PubMed] [Google Scholar]
- Zrihan-Licht S, Baruch A, Elroy-Stein O, Keydar I, Wreschner DH, 1994. Tyrosine phosphorylation of the MUC1 breast cancer membrane proteins. Cytokine receptor-like molecules. FEBS letters 356, 130–136. [DOI] [PubMed] [Google Scholar]