Abstract
Cranial microsurgery is an essential procedure for accessing the brain through the skull to introduce neural probes that measure and manipulate neural activity. Neuroscientists have typically used tools such as high-speed drills adapted from dentistry to perform these procedures. As the number of technologies available for neuroscientists has increased, the corresponding cranial microsurgery procedures to deploy them have become more complex. A robotic tool that automatically performs these procedures would standardize cranial microsurgeries across neuroscience laboratories and democratize the more challenging procedures. We have recently engineered a robotic surgery platform that utilizes principles of computer numerical control (CNC) machining to perform a wide variety of automated cranial procedures. Here we describe steps to adapt, configure, and use an inexpensive desktop CNC mill equipped with a custom-built surface profiler for performing CNC-guided microsurgery on mice. Detailed instructions are provided to utilize this ‘Craniobot’ for performing circular craniotomies for coverslip implantation, large craniotomies for implanting transparent polymer skulls for cortex wide imaging access and skull thinning for intact skull imaging. The Craniobot can be set up in less than 2 weeks using parts that cost < $1500 and we anticipate that the Craniobot can be easily adapted for use in other small animals.
INTRODUCTION
Cranial microsurgery is the first procedure performed in many neuroscience experiments. It is essential for accessing the brain through the skull, for the introduction of probes for measuring or manipulating neural activity, or implantation of cranial windows for long-term imaging access. Advances in miniaturized neurotechnologies designed for high-density neural recording1–5, optogenetic stimulation6,7, and pharmacological manipulation8,9 have provided neuroscientists with enhanced capabilities to interface with the brain. Increasingly, experiments using these neurotechnologies involve simultaneously interrogating multiple regions spread across the brain10,11. These experiments require performing precisely spaced craniotomies to introduce multiple neural probes. In parallel, large-scale imaging technologies such as ultra-wide-field imaging systems12–14 have necessitated the implantation of cranial windows covering the entire hemisphere of the cortex or curved glass cranial windows across the whole dorsal cortex15. As the complexity and scale of these experiments has increased, so too has the need to perform increasingly challenging microsurgical procedures. In mice, these delicate procedures need to be done on sub-millimeter thick skull without injuring the underlying brain or dura. The precision and quality of the surgical preparation can significantly impact the outcome of the experiment and engender experimental variability between researchers. Automating cranial microsurgeries may be a means to not only reduce this experimental variability, but to also enhance the neuroscience community’s ability to design increasingly complex experiments that are difficult to conduct manually.
To this end, several efforts have been made to automate cranial microsurgery using various types of feedback, including force or impedance16–18 measurements. Along these lines, we have recently developed the ‘Craniobot’, a fully open-source cranial microsurgery platform19. The Craniobot uses computer numerical control (CNC) machining to perform cranial microsurgery on mice. A desktop CNC mill is adapted to incorporate a custom-built stereotax to head-fix an anesthetized rodent for automated cranial surgery. The Craniobot utilizes a custom-engineered surface profiler to measure the skull surface topology along the perimeter of a desired craniotomy. This profile is used to interpolate a 3D milling path traced by the CNC mill. The milling depth can be iteratively increased to thin the skull along the craniotomy perimeter until it can be extracted via fracturing. This allows very precise control of the bone removal process (Fig. 1a). We have demonstrated the ability of the Craniobot to perform various-sized craniotomies for glass coverslip and transparent polymer skull implantation, skull thinning, and pilot hole drilling for implanting bone anchor screws.
Figure 1. Overview: (a) Steps of the automated cranial surgery using the Craniobot:
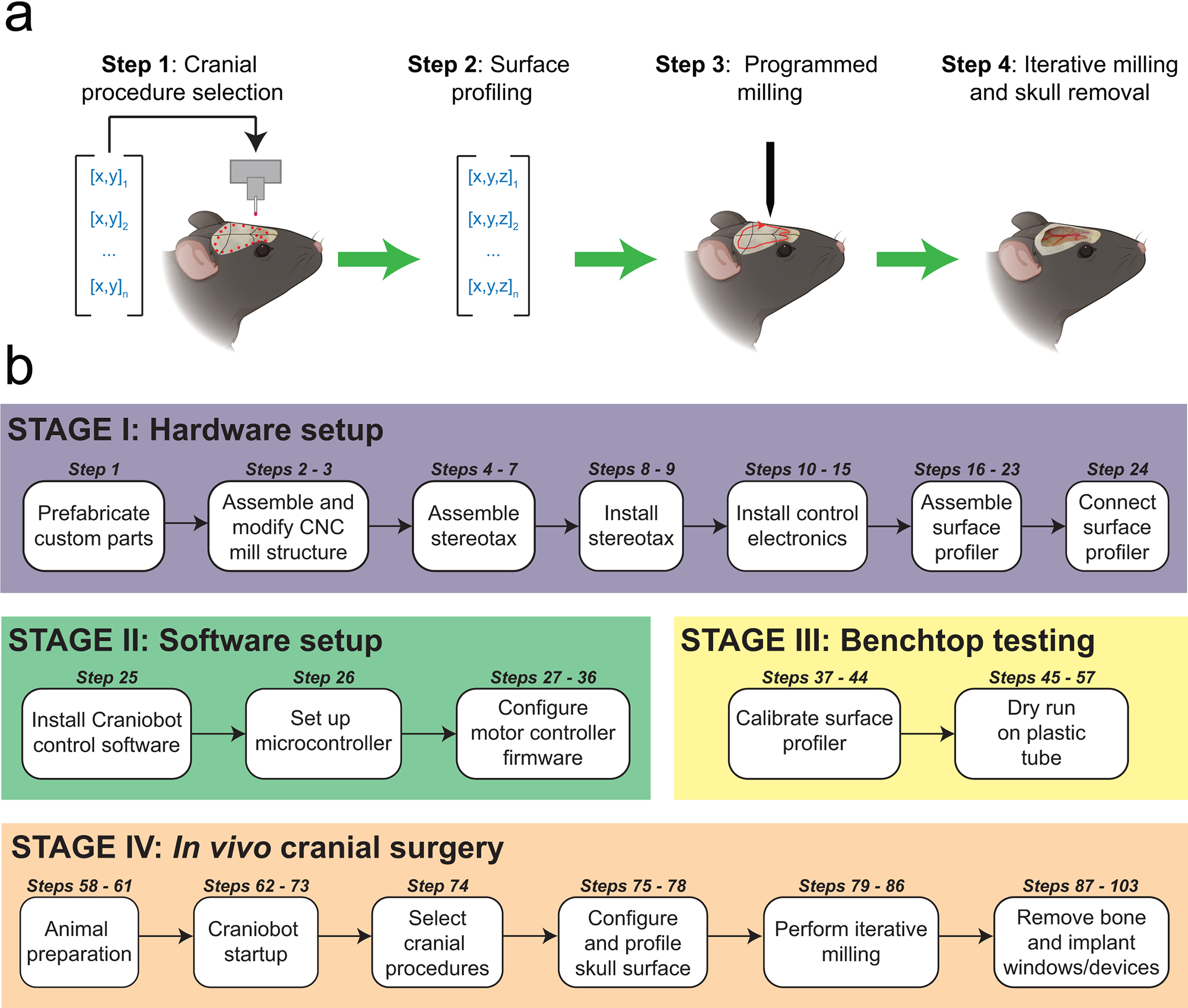
Step 1: A set of n xy-coordinate of pilot points defining a selected cranial procedure is used to create a 2D path on the mouse skull. Step 2: A surface profiler is used to register the z-coordinate at each of those points, creating a set of n 3D points that can be interpolated to generate a continuous path mapped onto the skull surface. Step 3: A z-offset is applied to the measured path to generate skull milling commands. Step 4: Computer numerical controlled (CNC) milling is performed with the depth of milling increased iteratively until the desired depth is achieved for a given cranial procedure. (b) A summary of the protocol for adapting a desktop CNC milling machine for automated cranial microsurgeries in mice is given.
This protocol provides instructions to assemble, test, and operate the Craniobot for performing automated cranial microsurgery. An overview of the protocol illustrating the different stages is shown in Figure 1b. In Stage I of the protocol (Steps 1 – 24), instructions are given for assembling the hardware, including the customization of a desktop CNC mill and assembly of a low-surface profiler (Fig. 2). Stage II of the protocol instructs the user on installing the software for controlling the Craniobot hardware and performing initial configuration of the control electronics (Steps 25 – 36). Stage III of the protocol describes benchtop calibration of the surface profiler (Steps 37 – 43) followed by instructions to perform a test run on a plastic tube or equivalent as a proxy for the skull (Steps 45 – 57). This will allow the user to get familiar with the Craniobot hardware and software, fine tune the hardware, and pre-emptively troubleshoot any hardware issues. Once these steps are complete, the Craniobot can be used for cranial microsurgeries, described in Stage IV of the protocol (Steps 58 – 103).
Figure 2. Craniobot hardware:
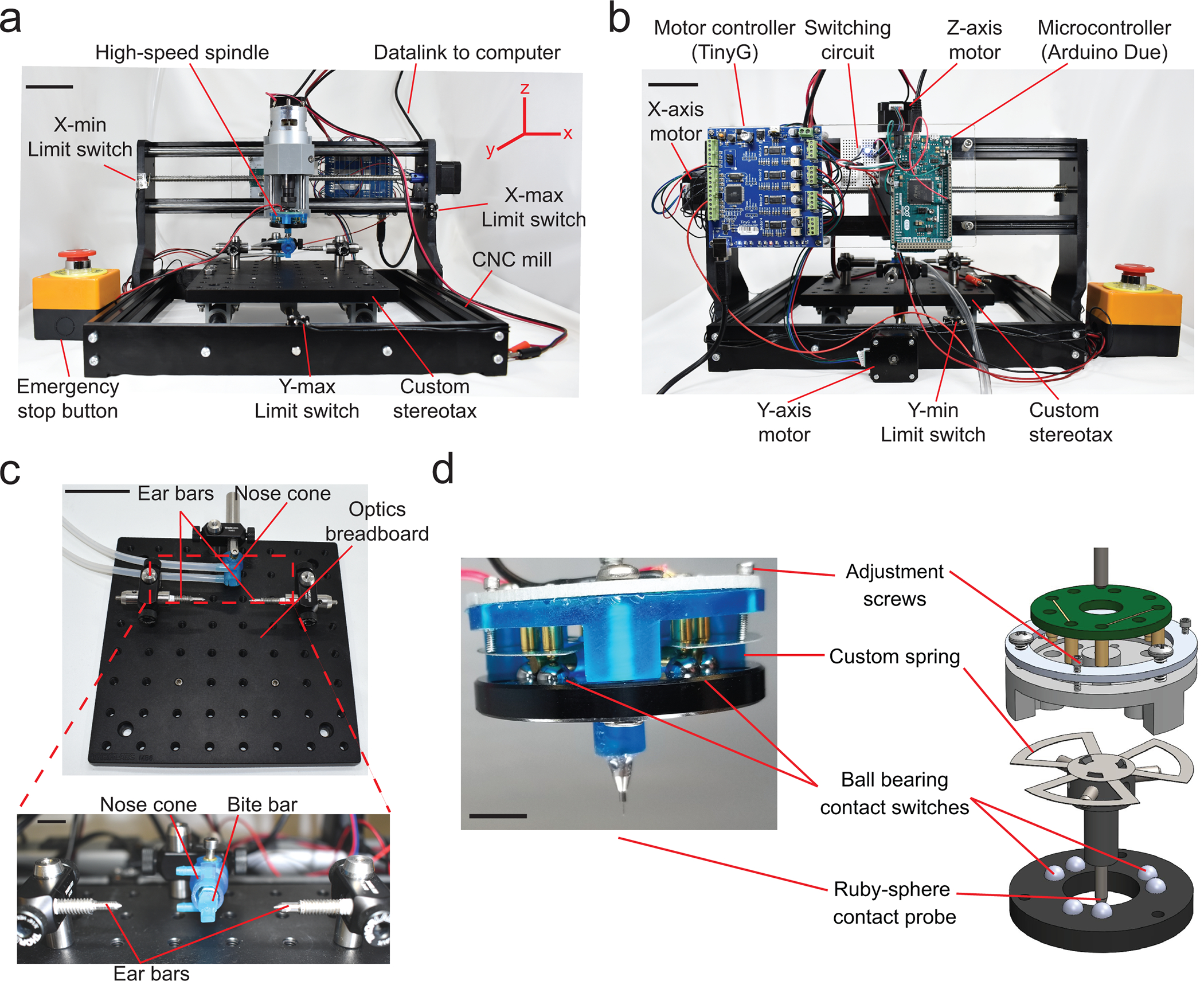
(a) Front view of the Craniobot which consists of a three-axis CNC mill, a custom built stereotax, and a high-speed spindle. Scale bar, 5 cm. (b) Back view of the Craniobot showing three stepper motors and control electronics including a motor controller, a microcontroller, and a switching circuit. Scale bar, 5 cm. (c) Photographs of the custom built stereotax. Detailed step-by-step instructions on assembling the CNC mill and adapting it for automated surgeries is shown in Supplementary Figure 1. Scale bars in c and bottom inset, 5 cm and 1 cm, respectively. (d) Photograph and computer-aided design (CAD) schematic of the custom-built surface profiler indicating the key parts. Scale bar, 10 mm. Detailed step-by-step instructions on assembling the surface profiler is shown in Supplementary Figure 2.
We have written this protocol under the assumption that the user has some familiarity with mouse cranial microsurgery. We provide instructions for automatically performing four types of cranial procedures: circular craniotomies of a desired diameter and location, craniotomies of arbitrary shapes as specified by the user, surface milling for skull thinning, and drilling pilot holes for bone anchor screw implantation. Combinations of these procedures can be queued and performed in the same surgery. Instructions are provided for subsequent chronic implantation of planar glass coverslips, implantation of See-Shells, or transparent polymer skulls20 and reinforcing thinned skull for intact skull imaging.
In the course of writing this protocol, we made a few improvements to the Craniobot described previously19. First, we have changed the CNC mill that is adapted for automated cranial surgery from a 2020 CNC mill to a 3018 CNC mill. The four digits represent the lateral positioning range of the CNC mill (i.e., 20 cm x 20 cm and 30 cm by 18 cm respectively). The change allowed us to streamline and simplify multiple hardware assembly steps that otherwise required time-consuming fabrication of custom parts. In principle, any CNC mill that has a travel range greater than 15 cm in the x and y directions and greater than 18 cm in the z direction, with the spindle located ~3 cm above the base of the CNC mill, can be adapted as a Craniobot. Such CNC mills are readily available at several e-commerce websites and are relatively inexpensive (< $500). We have also developed a user-friendly graphical user interface (GUI) to control the Craniobot (Supplementary Software 1). The GUI allows the user to first perform hardware configurations and then guides the user through the different stages of the protocol. This is a significant improvement over the previous study, in which the Craniobot was controlled by command line prompts in the Python programming environment. We also note that a MATLAB-based GUI is available at our GitHub repository (www.github.com/bsbrl) where we will deposit future versions of the software.
Comparison with manual procedures:
There are two fine sensorimotor tasks critical for performing cranial microsurgeries: the precise, iterative removal of bone tissue using a hand-held dental drill, and tactile assessment of readiness of the bone to be fractured and extracted. Humans have highly developed tactile sensation with the ability to discern minute changes in bone integrity and structure and they are adept at ascertaining the readiness of the bone to be removed. There is, however, considerable variability in performing repeated motor tasks required to remove sub-millimeter thick bone. The Craniobot, as well as other automated craniotomy approaches16,17, automates this task and makes bone removal a controlled and reproducible process. In our previous study19, we performed craniotomies across the whole dorsal cortex (as illustrated in Figure 8b), noting the final depth of automated milling. We next measured the thickness of the excised bone in each of these experiments at various points along the perimeter of the excised bone and found that, in all cases (n = 19 surgeries), the final depth of milling was less than the minimum thickness of the skull. Further, immunohistology experiments confirmed that automated drilling did not cause any acute injury to the underlying brain tissue19. Such precise control during manual removal of sub-millimeter thick bone requires significant practice and training. Thus, the Craniobot can significantly reduce the entry barrier for new surgeons or laboratories exploring new microsurgery procedures.
Figure 8. Chronic implantation using the Craniobot:
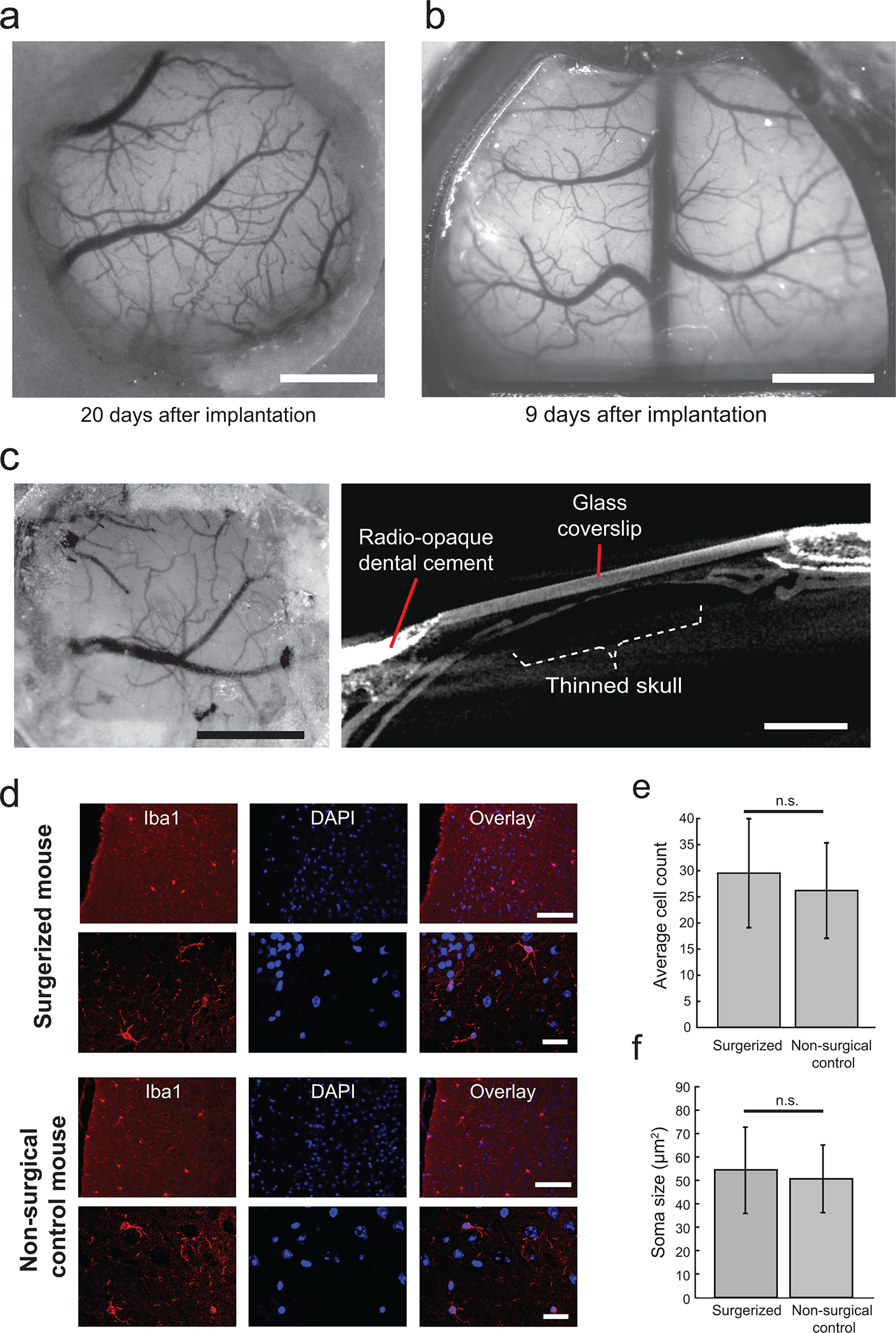
(a) Photograph taken from a Thy1-GCaMP6f mouse with a chronically implanted circular glass coverslip. Scale bar, 1 mm. (b) Photograph of a Thy1-GCaMP6f mouse taken with a chronically implanted See-Shell. Scale bar, 2 mm. (c) Photograph taken from an adult male C57BL/6J mouse skull after a thinning operation using the Craniobot reinforced with clear dental cement and a glass coverslip. Surface milling was performed to a depth of 70 μm in a 2 mm × 2 mm square. Scale bar, 1 mm. (d) Representative images of stained microglia via Iba1 (Wako Cat# 019–19741, RRID:AB_839504) staining and DAPI shown in both mice that underwent automated surface milling and non-surgical control mice. Images were taken at 20X magnification (top row) and 40X magnification (bottom row). Scale bars, 100 μm (top row) and 25 μm (bottom row). (e) Bar graph of average count of microglia in 891 × 662 μm of cortical tissue directly underneath the bone tissue milled using the Craniobot compared with microglia count from same brain region in non-surgerized mice. n. s indicates not significant (n= 6 mice in each group, p = 0.08, Student’s t-test). (f) Bar graph of average soma size of microglia in cortical tissue directly underneath the bone tissue milled using the Craniobot compared with average soma size of microglia from same brain region in non-surgerized mice. n. s indicates not significant (n = 10 cells assessed in each group, p = 0.61, Student’s t-test) (a), (c), (d – top row), and (e) are adapted from ref: Ghanbari et al Sci Rep 2018. All experiments were approved by the University of Minnesota’s Institutional Animal Care and Use Committee (IACUC).
Qualitatively, we observe higher bleeding from the vasculature in the skull when compared to manual drilling with a high-speed drill. We hypothesize that when using the pneumatic air drill, the higher rotational speed of the cutting tool, which rotates at ~30,000 revolutions per minutes (rpm), as compared to the rotational speed of the CNC mill, ~5000 rpm, results in tissue cauterization during bone removal. One limitation of the Craniobot as compared to manual surgeries is that it relies on the initial surface profiling step to compute the craniotomy path. While this is advantageous for large craniotomies and skull thinning procedures, the surface profiling step requires additional time and therefore may not significantly enhance the speed of the procedure overall.
Comparison with other automated craniotomy methods
The key difference between various robotic surgery systems is the sensing scheme used to determine the surface profile of the skull. The Craniobot utilizes the contact-force-based surface profiler to sense the skull surface as compared to previous automated surgery systems, wherein impedance sensing16 or a force-sensing drill bit17 has been utilized. Force-feedback-based drills typically require large actuation forces and it is unclear if such a system can be effectively used on small animal models where significant deformation of the skull can occur at such actuation forces. Given that the current Craniobot is designed to work with mice, we will limit the discussion here to compare the Craniobot with impedance sensing based automated craniotomy systems.
The Craniobot surface profiler constructs a virtual 3D path of the skull surface defining the desired craniotomy perimeter. Once the profiling is performed, iterative milling is employed to incrementally thin the skull down along the craniotomy path until it is ready to be extracted via fracturing. In contrast, the impedance sensing based ‘autodriller’16 utilizes the change in impedance when the drill bit penetrates through the skull to determine the exact skull thickness in each subject. As repeated measurements of impedance are required as the drill progresses through the bone, the process is slower than a single contact force based surface profile. Another drawback of the impedance sensing technique is that it is sensitive to moisture. Vasculature in the skull, which is abundant, particularly in the lateral areas of the dorsal cortex, may trigger false positives resulting in erroneous readout of skull thickness. The autodriller has previously been used to create precisely spaced arrays of small craniotomies for introduction of multiple intracellular recording electrodes21 or a combination of intracellular electrodes and high-density extracellular probes22. In contrast, the Craniobot is useful when performing large craniotomies19, particularly in regions of the skull where there may be vasculature and also for skull thinning operations.
Additionally, we note that we had explored the possibility of using laser scanning methods to perform the surface profiling. Laser scanning, could potentially drastically speed up the process of profiling the skull. However, the profiles obtained with inexpensive lasers are resolution limited (> 50 μm) This is not sufficient for our purpose of milling the skull with is often less than 150 μm thick. An added advantage of the using a profiling mounted on the milling spindle is that it is a relatively quick process to co-register the profiler coordinate system to the milling coordinate system. This would be hard to do for a laser scanning approach.
Previous automated craniotomy robots have typically integrated components for robotic actuation into existing stereotaxes16. In contrast, the Craniobot incorporates a stereotax into a CNC mill. This provides several advantages as well as a few limitations. First, this allows the Craniobot to harness the G-code programming environment – a high level programming language that has vast libraries of functions developed for precision fabrication purposes. While we utilize a small fraction of these capabilities, future versions of the Craniobot can take advantage of these advanced CNC machining capabilities. Second, desktop CNC machines are relatively inexpensive. With the use of open-source control electronics and off-the-shelf optomechanical components for building the stereotax, the entire Craniobot can be built for a fraction of the cost of a commercial manual stereotax. A drawback of this approach however is that fine adjustments to the relative heights and position of the bite bar and nose cone with respect to ear bars is tedious as there is no adjustment screw mechanism. This could be fixed in future versions of the stereotax by incorporating micrometer drives to facilitate such adjustments. Finally, the Craniobot has been used for surgeries on adult C57BL6 mice (#000664, Jackson Laboratories), Thy1-GCaMP6f mice (#024276, Jackson Laboratories) and Thy1-YFP mice (#003782, Jackson Laboratories) of ages 8–16 weeks. In principle, automated microsurgery can be performed on mice of other ages as well as other small animal models such as rats.
REAGENTS
Mice and surgery
Isoflurane (Piramal Critical Care Inc., Bethlehem, PA)
Slow-releasing buprenorphine (Buprenorphine SR LAB, Zoopharm, Windsor, CO)
Meloxicam (Loxicom, Norbrook)
1% Lidocaine (Auromedics Pharma LLC)
Cyanoacrylate glue (VetBond, 3 M Inc.)
1 mL syringe with 25G x 5/8” needle (8881501160, Covidien)
1 mL syringe with 28G x 5/8” needle (1180128012, Covidien)
Dental cement (S380, C&B Metabond, Parkell Inc.)
0.9% sodium chloride injection, USP (Hospira)
Ophthalmic eye ointment (Puralube, Dechra Vetrinary Products)
Betadine (10% Povidone Iodine, Purdue Products)
Cotton-tipped applicators (896-WC, Puritan)
Cold light source for surgery station (L2, Leica)
Self-tapping skull screws (F000CE094, Morris Precision Screws and Parts)
Hair trimmer (8786, Wahl)
#50 drill bit (P86–02-38, Pearson Dental)
Stereomicroscope for surgery station (M60, Leica)
Animal temperature controller (TCAT-2LV, Physitemp)
Micro-curette (10080–05, Fine Science Tools)
Scissors (14060–09, Fine Science Tools)
200 μm square end mill (13908, Harvey Tool Inc.)
300 μm ball end mill (24512, Harvey Tool Inc.)
Needle tipped stylus (II M2 030 03 015, ITP Styli LLC, St Louis, MO)
Ruby sphere tipped stylus (TH M2 003 03 010, ITP Styli LLC, St Louis, MO)
4 mm diameter glass coverslip (64–0724, Warner Instruments Inc.)
Quick-setting silicone sealant (KWIK-SIL, World Precision Instruments)
Blunt forceps (11253–20, Fine Science Tools)
Animals: C57BL/6Jmice, 8- to 12-week-old mice (000664, Jackson Laboratories), male or female, Thy1-YFP (003709, Jackson Laboratories), Thy1-GCaMP6f mice (024276, Jackson Laboratories) although other strains of mice can also be used.
! CAUTION: Ensure all animal experiments are approved by the appropriate institutional animal care and use committee.
70% Ethanol (97064–708, VWR Inc.)
Ringer’s Solution (7953–03, Hospira)
Gauze pads (NON25443, Medline)
Trinocular/eye piece camera (SS View camera, Science Supply Inc.)
Post-operative recovery heating pad (72–0492, Harvard Apparatus)
Benchtop autoclave (Bioclave Mini, Benchmark Scientific)
Equipment and tools
3018 CNC mill (ASIN: B07DXMFY38, Amazon)
DC power supply (B07DT62KL8, Amazon Inc. or equivalent)
Switching power supply (EXPSFD006582, MYSWEETY)
Motor controller (TinyG, Synthetos Inc.)
Microcontroller (Arduino Due, Adafruit Inc.)
Emergency stop button (a14041700ux0692, Uxcell)
Touch probe (SPU-40, Tormach Inc.)
Small breadboard (Tiny Breadboard, Adafruit Inc.)
Limit switch (Z5065-ND, Digikey)
Red 24 AWG wires (KSW24R-0100, Jonard Tools)
Black 24 AWG wires (R30BLK-0100, Jonard Tools)
Banana plug connectors (#3694, Adafruit Inc.)
M3 screws (90592A085, McMaster-Carr Co.)
4–40 screws (91772A112, McMaster-Carr Co.)
2–56 socket head screws (92196A079, McMaster-Carr Co.)
3/16” 0–80 Philips flat head screws (91771A054, McMaster-Carr Co.)
3/32” 0–80 Philips flat head screws (91771A848, McMaster-Carr Co.)
4–40 nylon unthreaded spacers (93657A061, McMaster-Carr Co.)
4–40 nylon threaded standoffs (92319A877, McMaster-Carr Co.)
Aluminum hex standoff (91115A198, McMaster-Carr Co.)
¼”−20 threaded set screws (SS25S10, Thorlabs Inc.)
6–32 1” set screw (91375A153, McMaster-Carr Co.)
0.125” brass rods (2575T1, McMaster-Carr Co.)
M3 threaded brass insert (HBN-250, Hilitchi)
Optics breadboard (MB8, Thorlabs Inc.)
2” long 0.5” diameter optics post (TR2, Thorlabs Inc.)
Right-angle clamps (RA90, Thorlabs Inc.)
1.5” long 0.5” diameter optics post (TR1.5, Thorlabs Inc.)
1/8” inner diameter polyethylene tubing (Tygon AJK00007, Saint-Gobain S.A.)
4–40 tap (26955A24, McMaster-Carr Co.)
2–56 tap (26955A21, McMaster-Carr Co.)
M2 tap (8305A78, McMaster-Carr Co.)
0–80 tap (15J611, Grainger)
Hacksaw (6927A12, McMaster-Carr Co.)
Precision knife (X3201, X-ACTO)
Epoxy glue (ScotchWeld, 3M Inc.)
Teflon tape (TaegaSeal, TaegaTech)
50 ml Plastic Tubes (352070, Corning)
Stage I: Hardware setup
Overview:
The Craniobot consists of a three-axis CNC mill and custom-built stereotax, which enables the performance of automated surgeries on anesthetized mice (Fig. 2). The control electronics are mounted on the back of the CNC mill (Fig. 2b). The stereotax consists of an optics breadboard onto which posts and clamps are fixed for mounting and adjusting the ear bars and bite bar (Fig. 2c). The electronics used to control the Craniobot are schematically illustrated in Figure 3. One power supply provides power to the CNC mill via the motor controller and a second power supply powers the spindle. The spindle is used to fit either the surface profiler or end mill. The factory electronics that come with the CNC mill were replaced with an open-source motor controller, a microcontroller, and a breadboard on which a switching circuit was installed. The Craniobot uses four limit switches, two each for the x and y axes, to ensure the motors do not exceed the maximum travel range. One of the limit switch circuits in the z-direction is connected to an emergency stop button, and the other limit switch circuit is connected to the surface profiler via a switching circuit. The surface profiler was modified from a commercially available touch sensor, which requires a custom-built pull-up resistor circuit to be incorporated in the switching circuit. A complete list of parts required for assembling the Craniobot and the surface profiler is included in the Bill of Materials (Supplementary File 1).
-
1
Pre-fabricate custom parts: The Craniobot and surface profiler are assembled using a combination of off-the-shelf parts and custom-fabricated components. We used a 3D printer, laser cutter, water-jet cutter, machine shop lathe and desktop grinder to fabricate the custom parts. All computer-aided design files of these custom components can be found in Supplementary File 2, which is a compressed file archive. Table 1 lists each of these components, along with instructions on how to fabricate them or, alternately, procure them from a commercial fabrication service. Follow the instructions in Table 1 to fabricate the custom parts that are needed. Steps below involving use of these components are flagged with a ‘PREFABRICATION REQUIRED’ note.
Figure 3. Craniobot control electronics:
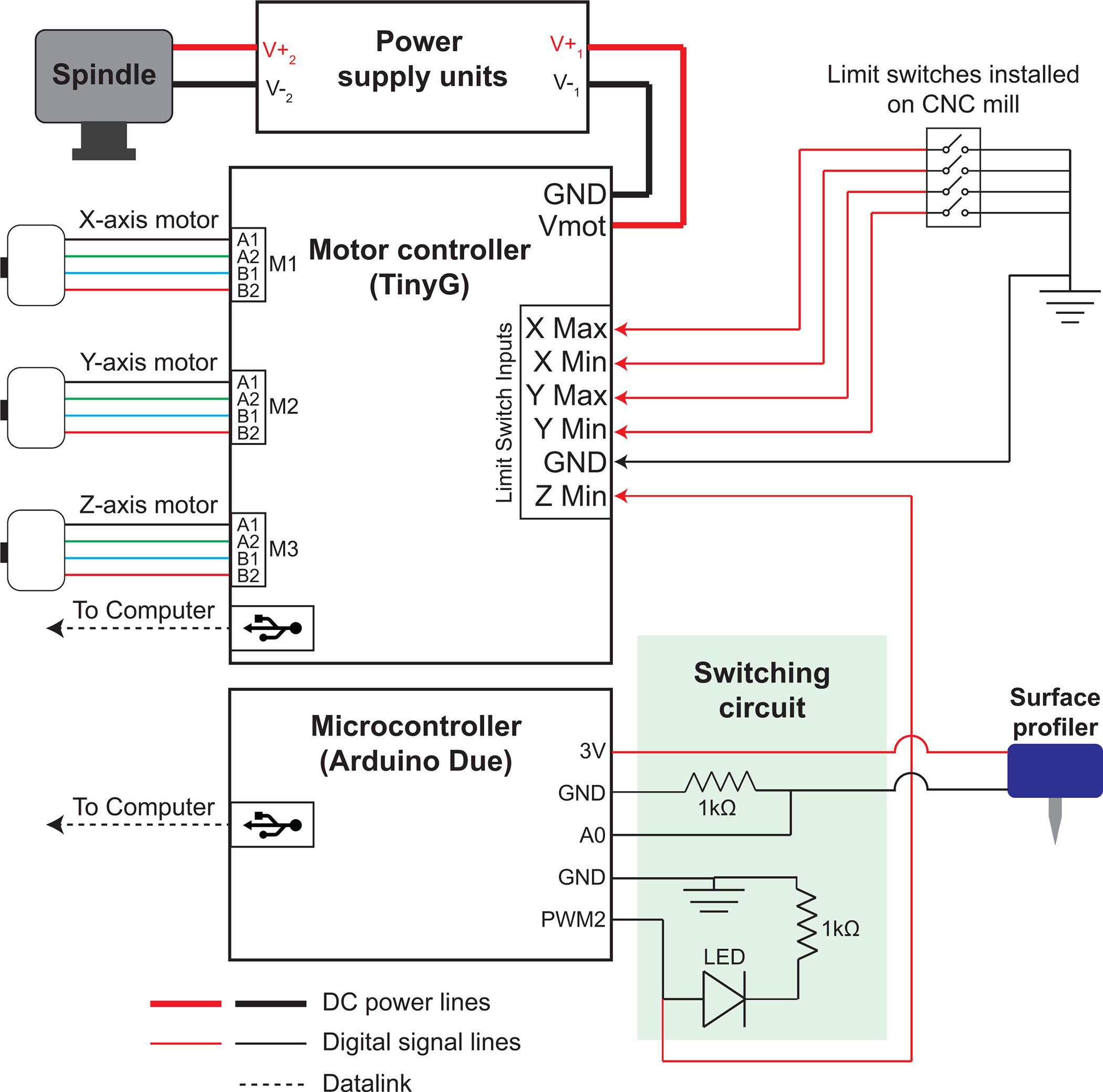
DC power supplies provide power to the CNC mill and the high-speed spindle. An open-source motor controller is used to drive the stepper motors in the x, y, and z directions. It connects to the computer via universal serial bus (USB) interface. Four limit switches, two each for the x and y axes to ensure the motors do not exceed the maximum travel range, are connected to the limit switch input terminals in the motor controller. One of the limit switch circuits in the z-direction is connected to an emergency stop button, and the other limit switch circuit is connected to the surface profiler via a switching circuit. A microcontroller is used to connect the surface profiler to the motor controller via the switching circuit.
TABLE 1:
List of custom-built parts required for Craniobot and surface profiler assembly and instructions for fabricating or procuring them
| PART | PROTOCOL STEP # | FABRICATION INSTRUCTIONS |
|---|---|---|
| Stereotax | 2 | Use Craniobot Base Plate Adaptor.dxf in Supplementary File 2 to laser cut the stereotax mounting plate from ¼” acrylic sheet. Alternately: The CAD file can be used to procure the sheet from a commercial fabrication vendor. |
| Circuit board holder | 3 | Use circuit_board_holder.dxf in Supplementary File 2 to laser cut the circuit board holder from ¼” acrylic sheet. Alternately: The CAD file can be used to procure the sheet from a commercial fabrication vendor. |
| Bite bar | 5 | Use bite_bar_hex.stl in Supplementary File 2 to 3D print the bite bar from PMMA resin using a stereolithgraphy printer (Form 2, Formlabs Inc.). Alternately: The CAD file can be used to procure the sheet from a commercial fabrication vendor. |
| Nose cone | 6 | Use Anesthesia_mask_hex.stl in Supplementary File 2 to 3D-print the nose cone from PMMA resin using a stereolithgraphy printer (Form 2, Formlabs Inc.). Alternately: The CAD file can be used to procure the sheet from a commercial fabrication vendor. |
| Stylus holder | 17 | Use Tip_holder.stl in Supplementary File 2 to 3D print the stylus holder from PMMA resin using a stereolithgraphy printer (Form 2, Formlabs Inc.). Alternately: The CAD file can be used to procure the sheet from a commercial fabrication vendor. |
| Custom spring | 18 | Use Stainless_spring.dxf in Supplementary File 2 to waterjet cut the custom spring from 0.0045” thick 300 series stainless steel. Alternately: The CAD file can be used to procure the sheet from a commercial fabrication vendor. |
| Housing | 19 | Use Case_main.stl in Supplementary File 2 to 3D print the housing from PMMA resin using a stereolithgraphy printer (Form 2, Formlabs Inc.). Alternately: The CAD file can be used to procure the sheet from a commercial fabrication vendor. |
| Aluminum ring | 22 | Use WaterJetCut_Aluminum ring.dxf in Supplementary File 2 to waterjet cut the aluminum ring from 0.0625” thick 6062 aluminum. Alternately: The CAD file can be used to procure the sheet from a commercial fabrication vendor. |
CNC mill assembly and modification
-
2
Build and modify CNC mill: The CNC mill used in this protocol comes with an assembly manual. Follow the instructions as pictorially depicted in Supplementary Figure 1 to assemble the CNC mill. Do not attach the CNC mill base and the control electronics included with the original assembly package. Instead of the CNC mill base, install the stereotax mounting plate. An assembled CNC mill at this stage is shown in Supplementary Figure 1h.
PREFABRICATION REQUIRED See Table 1 for instructions on fabricating the stereotax mounting plate.
-
3
Install circuit board holder: Mount the circuit board holder to the back of the CNC mill using 4–40 screws and 4–40 unthreaded nylon standoffs.
PREFABRICATION REQUIRED See Table 1 for instructions on fabricating the circuit board holder.
Stereotax assembly and integration into CNC mill
-
4
Fabricate ear bars: Grind down two 1” long, ¼ - 20 set screws using a bench grinder or lathe. Apply Teflon tape to the distal ends and thread the ground ear bars into the 2” long, 0.5” diameter optics post.
-
5
Assemble bite bar: Insert the 3D-printed bite bar into the aluminum hex standoff and fix it using epoxy glue. Fasten a 2” long, 0.5” diameter optics post to the other end of the hex standoff.
PREFABRICATION REQUIRED See Table 1 for instructions on 3D printing the bite bar.
-
6
Assemble nose cone: Press fit an M3 brass insert into the top hole of the nose cone by heating the hole with a solder iron. Reinforce with epoxy glue.
PREFABRICATION REQUIRED See Table 1 for instructions on 3D printing the nose cone.
-
7
Assemble stereotax: Fasten 1.5” long, 0.5” diameter optics posts into the holes as shown in Figure 2c. Mount the right-angle clamps to the top of each optics post and secure by tightening the thumbscrews. Insert the posts connected to the bite and ear bars into the horizontal slots of the right-angle clamps.
-
8
Connect to anesthesia machine: Use 1/8” inner diameter polyethylene tubing to connect the nose cone to the isoflurane anesthesia dispenser.
-
9
Install stereotax in CNC mill: Use 0.5” long, 1/4”−20 set screws to mount the stereotax in the CNC mill.
Control electronics installation
-
10
Mount electronics on circuit board holder: Use 4–40 screws and threaded nylon 4–40 standoffs to mount the motor controller and microcontroller to the circuit board holder. Use a hot glue gun to glue a small breadboard to the circuit board holder between the motor controller and microcontroller as shown in Figure 2b.
-
11
Install limit switches: Solder 2’ long, 24 AWG wires to the contacts labeled “NO” and “C” on each limit switch. Use a #50 drill bit to drill two holes 1 cm apart on the side faces of the CNC mill (Fig. 2a). Tap these holes with a 2–56 tap and use 2–56 socket head screws to fasten the limit switches at each of these locations. Connect the “NO” wire from the limit switch on the left of the CNC mill to the terminal denoted as Xmin on the motor controller, that from the limit switch on the right of the CNC mill to Xmax on the motor controller, that from the limit switch at the back of the CNC mill to Ymin on the motor controller, and that from the limit switch at the front of the CNC mill to Ymax on the motor controller. Connect all “C” wires to the ground terminals indicated as GND on the motor controller. Clean up wiring using zip-ties.
-
12
Install emergency stop button: Connect the positive terminal of the emergency stop button to the Zmax terminal on the motor controller. Connect the negative terminal of the emergency stop button to the GND terminal.
-
13
Install switching circuit: Install components of the circuitry contained within the green box labeled ‘Switching circuit’ in Figure 3 on the breadboard. Alternatively, this circuit can be put together in a more robust, dedicated PCB.
-
14
Connect stepper motors: Connect each of the stepper motors to the motor controller by attaching the four colored wires on each motor as follows: black to A1, green to A2, blue to B1, and red to B2.
-
15
Connect power: Connect the spindle of the CNC mill to the DC power supply with a pair of banana plug connectors. Connect the switching power supply to the motor controller.
Surface profiler assembly
Overview:
The surface profiler (Fig. 2d) was built by modifying a commercially available touch probe. The touch probe profiles hard metallic substrates and requires an actuation force of ~5.5 N to detect contact with a surface. We found that, at this actuation force, the touch probe exerts significant force on the skull resulting in noticeable skull deformation. Thus, the stock compression spring was removed and was replaced with a custom-designed stainless-steel spring enclosed in a modified housing (Supplementary Fig. 2a). The rest of the surface profiler electronics, which includes three normally closed switches connected in series, is retained from the original sensor. Each switch is made of two spherical stainless-steel contacts mounted on the original case cover. The spherical contacts are electrically bridged by a brass cylindrical contact when the switch is closed. Three such cylindrical contacts are radially arranged in the tip holder of the surface profiler. The tip holder and custom spring are sandwiched between the case cover and a custom-built housing such that the three brass pins are pressed against the spherical contacts on the case cover to create a normally closed switch. Upon contact with the skull, one of the radial cylindrical contacts lifts off the spherical contacts and triggers the surface profiler. This modification resulted in a surface profiler requiring an actuation force between 48 mN and 98 mN, allowing us to profile the skull surface without deforming the skull.
Lowering the contact force increased the contact resistance within the surface profiler circuitry, and it could therefore no longer be used as a passive switch circuit. To reduce the voltage across the surface profiler, a 1 kΩ resistance was connected in series. The signals from the voltage divider are measured using the analog input on the microcontroller. An analog voltage threshold is then used to determine if the touch probe has been actuated and this information is sent out to the motor controller via a digital channel on the microcontroller (Fig. 3).
-
16
Disassemble touch probe: Disassemble the touch probe by unscrewing the socket head fasteners and removing the case cover, stylus holder, brass pins, and spring from the assembly. Use a precision knife to cut off the outer black wire casing such that the PCB and inner wiring can be removed. Retrieve the PCB with its wiring attached and the case cover. These two components serve as the main contact switch circuit (Supplementary Fig. 2b).
-
17
Assemble stylus holder: Use a hacksaw to cut three 1/2” long pins from 0.125” brass rods. Press fit the brass pins them into the three horizontal holes in the stylus holder. Thread the bottom center hole of the stylus holder using an M2 tap. The stylus can be threaded into the bottom center hole once the surface profiler is fully assembled (Supplementary Fig. 2c).
PREFABRICATION REQUIRED See Table 1 for instructions on 3D printing the stylus holder.
-
18
Fit the custom spring on top of the stylus holder (Supplementary Fig. 2d).
PREFABRICATION REQUIRED See Table 1 for instructions on waterjet cutting the custom spring.
-
19
Thread the three larger holes in the outer circle of the housing using a 4–40 tap. Thread the three smaller holes in the outer circle using a 0–80 tap. Thread the three inner holes with a 4–40 tap (Supplementary Fig. 2e).
PREFABRICATION REQUIRED See Table 1 for instructions on 3D printing the housing.
-
20
Mount and secure the case cover retrieved from the touch probe onto the housing with 4–40 screws (Supplementary Fig. 2f).
-
21
Attach the wired PCB retrieved from the touch probe onto the housing using 4–40 screws (Supplementary Fig. 2g).
-
22
Fasten the aluminum ring onto the housing using 4–40 screws (Supplementary Fig. 2h). Screw 0–80 screws through the aluminum ring and housing until all three screw tips evenly contact the custom spring (Supplementary Fig. 2h).
PREFABRICATION REQUIRED See Table 1 for instructions on waterjet cutting the aluminum ring.
-
23
Insert a 1” long, 6–32 set screw through the housing center hole. (Supplementary Fig. 2i). Use epoxy to bond the set screw to the housing.
!! CRITICAL STEP: The 6–32 set screw is used to mount the surface profiler into the spindle during surface profiling and should fit tightly into center hole. Do not force the screw into the center hole if it does not fit. Further, do not insert the screw if there is a clearance between the set screw and the center hole as it may cause the stylus tip to be offset from the spindle axis, resulting in inaccurate surface profiling.
-
24
Connect the surface profiler to the switching circuit as shown in the circuit diagram (Fig. 3).
! CAUTION: As the stylus is fragile and easily damaged, dismount the stylus and store it in its container when not in use.
This completes the hardware setup.
STAGE II: Software setup
Overview
Once the hardware is fully assembled, configuration steps and benchtop calibration tests must be completed prior to using the Craniobot for automated cranial surgery. First, the motor controller must be configured according to the CNC machine mechanical properties and parameters. Second, the surface profiler interfaces with a microcontroller which must be configured, followed by calibration to ensure that the right amount of force actuates the surface profiler. Third, the Craniobot control software needs to be installed. We provide a software program ‘Craniobot.exe’ written in Python 3 programming language (Supplementary Software 1). This protocol describes the GUI written in Python 3 and works in the Window operating system. A MATLAB based program along with detailed instructions on installation and use can be found at the GitHub repository (www.github.com/bsbrl).
The GUI (Fig. 4) is divided into two panels, guiding the user through the sequence of steps shown in Figure 1a. Panel 1 changes according to different modes through which the program sequentially progresses. Panel 2 is static and accessible throughout the Craniobot operation. Panel 2 displays the position of the Craniobot spindle with respect to the current origin and has control buttons to set the jogging speed, jog the motors, and send custom G-code commands. Initially, Panel 1 of the GUI is in ‘Select Procedures’ mode. Here, the GUI allows the user to select one of four procedures (Fig. 4a). Selecting any of the four procedures in this window opens a secondary dialog prompting the user to select parameters defining the profile of the desired cranial procedure (Fig. 4b). Once procedures are selected, Panel 1 changes to ‘Surface Profile Configuration’ mode (Fig. 4b) during surface profiling, followed by ‘Milling Configuration’ mode (Fig. 4c) during milling. Detailed descriptions of each of the GUI functions are provided in Table 2.
Figure 4. Craniobot software graphic user interface (GUI):
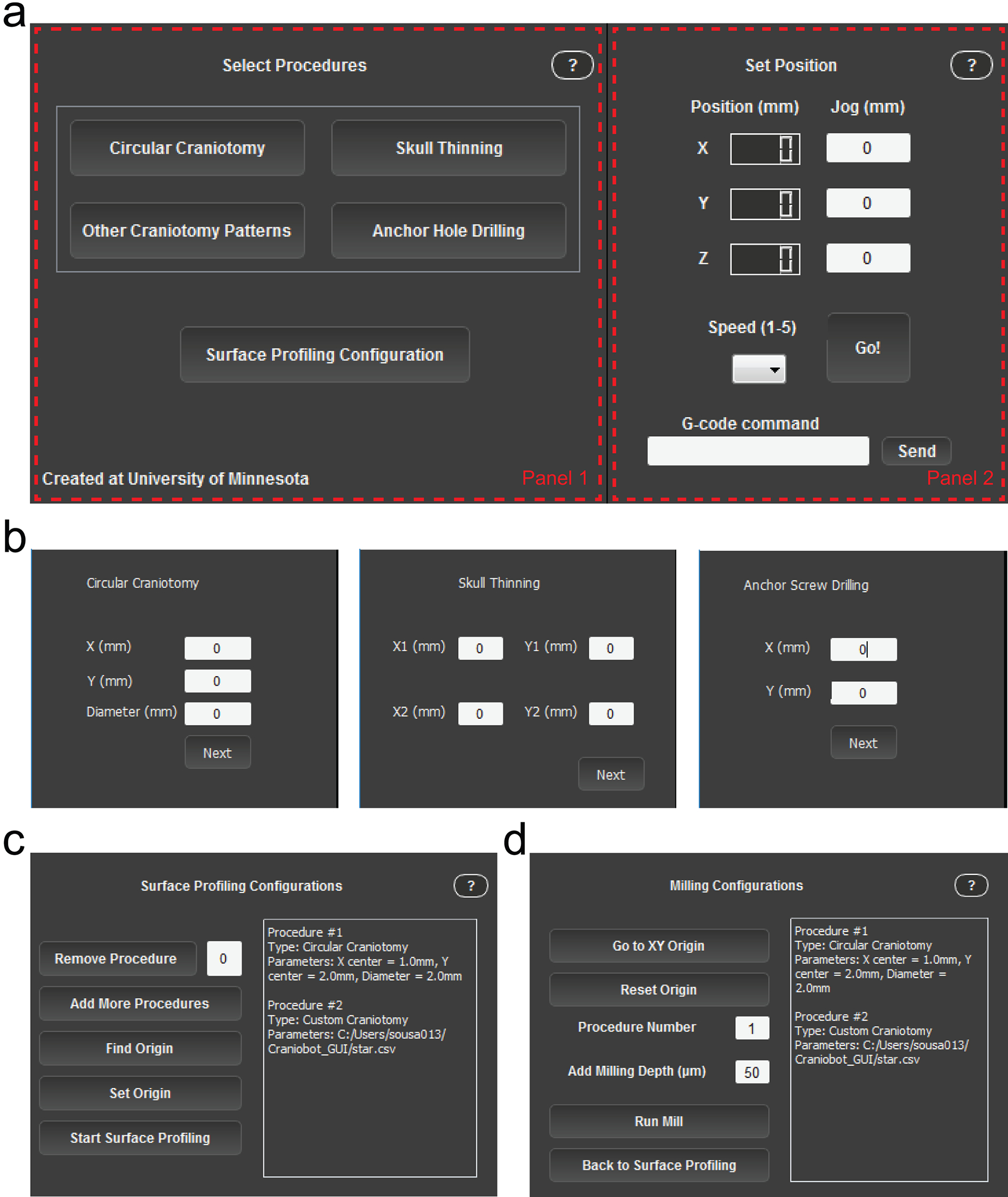
(a) Screenshot of the GUI during ‘Select Procedures’ mode. Panel 1 changes as the Craniobot progresses through the different stages of the program. Panel 2 is fixed, allowing the user to track spindle position, jog the CNC machine and send custom G-code commands (b) Panel 1 when selecting the parameters that define a circular craniotomy (left), skull thinning procedure (middle), and anchor hole drilling (right). (c) Panel 1 during surface profiling. (d) Panel 1 during iterative milling. Table 2 provides a list of user interface elements in the GUI and describes their functions.
TABLE 2.
List of buttons available in the Craniobot GUI and their functions
| BUTTON | FUNCTION |
|---|---|
| Speed |
Ranges from a value of 1 – 5 and defines the speed of the jogging commands as the number selected times 100 mm/min. |
| Go |
Executes the jog command based on the entered values on the x, y, and z text boxes. Small jog steps can also be executed using the up/down arrows (y), left/right arrows (x) and PgUp/PgDn buttons (z). |
| Send |
Sends a serial communication G-code command to the motor controller with the text entered in the box on the left. |
| Circular Craniotomy |
Opens a secondary window and prompts the user to select the xy-center of the circle offset from Bregma as well as the diameter of it. Creates a G-code sequence to probe points along the circle with a 0.35 μm gap and displays the 2D map of such points. |
| Skull Thinning |
Opens a secondary window and prompts the user to select the xy-coordinates of two points that define a rectangle. Creates a G-code sequence to probe points within the rectangle in a rastering pattern with a 0.15 μm gap and displays the 2D map of such points. |
| Other Craniotomy Patterns |
Opens a file search dialog that prompts the user to select a user-defined .csv file in any location of the hard drive in which every line defines a point in the format “Xi,Yi”. Creates a G-code sequence to probe the points sequentially as defined by the line order of the file itself and displays the 2D map of such points. |
| Pilot Hole Drilling |
Opens a secondary window and prompts the user to select the xy-position to drill a hole with the diameter of the end mill. Creates a G-code command defined by a single point at the position defined. |
| Surface Profiling Configuration |
Closes the “Select Procedures” window and opens the ‘Surface Profiling Configuration’ window. Generates a queue with the desired cranial procedures in the order selected. |
| Remove Procedure |
Removes a procedure from the queue based on the procedure number entered on the text box immediately next to this button. The associated numbers are displayed on the text box on the right of the window, along with the procedure type and parameters. |
| Add More Procedures |
Brings the user back to the ‘Select Cranial Procedures’ window in case the user wants to add other procedures to the queue. The previously selected procedures are still going to be queued unless they have been removed previously by the ‘Remove Procedure’ button. |
| Find Origin |
Probes down in the direction of the bed of the machine until contact is detected by the surface profiler and stops. |
| Set Origin |
Redefines the origin of the machine. Mostly used to position the machine with respect to Bregma. |
| Start Surface Profiling |
Executes the surface profiling routine for all procedures in the queue. The software will be frozen until all procedures have been profiled. A 3D plot of the profiled points is shown after execution is completed. |
| Go to XY Origin |
Brings the machine back to the (0, 0, Zc) position in which Zc is the current z position of the machine. |
| Reset Origin |
Redefined the origin of the machine. Mostly used to reset the origin after the surface profiler has been replaced for the end mill. |
| Run Mill |
Creates a 3D interpolated path based on the surface profiling step and runs the milling routine for the procedure entered above according to the number shown in the queue on the right side of the window. The ‘Milling Depth’ value defines the milling depth. This value defines the negative z-offset from the surface measurement that will be performed in the next milling pass. |
| Back to Surface Profiling |
Brings the user back to the surface profiling configuration window. If this button is pressed, all previously gathered surface profiling data will be lost. |
-
25
Install software: Download ‘Craniobot.zip’ (Supplementary Software 1) and extract the files from the file archive. This software bundle is a stand-alone application and includes Python installation file as well as all the libraries required to run the Craniobot software. Launch the installer for the software by double clicking to open Craniobot_1.0.exe. Choose the destination folder and finish the installation. Once the installation is complete, a start menu shortcut will be automatically created. In order to find it, simply search for ‘Craniobot’ on the Windows start menu. It should also be listed under ‘All Programs’ in the Windows start menu.
-
26
Set up microcontroller: Install the Arduino software and open it (Supplementary Software 2). Click on Tools/Board and ensure that the ‘Arduino Due’ board is recognized by the software. If the board model is not shown under this menu, the boards needs a software installation under ‘Boards Manager’. More information on how to install board packages can be found online (https://www.arduino.cc/en/Guide/HomePage). Next, ensure that the correct port is selected under Tools/Port. Typically, this is indicated as ‘COM1’. Open the ‘.ino’ file downloaded as Supplementary File 3 and run it in the Arduino software. The relevant pins are defined in the start of the code. Check that they are the same as those wired in the board and, if not, change accordingly.
Motor controller configuration
Overview:
The Craniobot utilizes an open-source motor controller to control the CNC mill. Prior to operation, it is necessary to input and configure parameters specific to the CNC mill hardware for proper operation. Steps 27 - 36 guide the user through this stage that needs to be performed only in the first instance that the Craniobot is used. Of note, these instructions are specific to the CNC mill used in this study. If a different CNC mill is used, the configuration steps may need to be changed. For generic instructions on configuring the motor driver to any CNC mill hardware, refer to: www.github.com/synthetos/TinyG/wiki.
-
27
Initialize software: Open ‘Craniobot.exe’ and press ‘Start’. The GUI will change to ‘Select Procedures’ mode. Do not select any procedure in Panel 1 of the GUI. Use the jog commands in Panel 2 of the GUI or the key board shortcuts to move to the motors in each direction to ensure that serial communication is established. Steps 28 – 36 can be performed by inputting G-code commands into the text input box in Panel 2 of the GUI and clicking ‘Send’.
? TROUBLESHOOTING
-
28
Set units: Enter ‘g21’ in the command line and click ‘Send’ to set the programming units to millimeters.
-
29
Configure Cartesian coordinate system: Enter ‘$1ma=0’ in the command line and click ‘Send’ to assign Motor 1 to the x-axis. Enter ‘$2ma=1’ in the command line and click ‘Send’ to assign Motor 2 to the y-axis. Enter ‘$3ma=2’ in the command line and click ‘Send’ to assign Motor 3 to the z-axis.
-
30
Configure motor rotation per step: Enter ‘$1sa=4’ in the command line and click ‘Send’ to configure the x-axis motor step angle. Enter ‘$2sa=4’ and click ‘Send’ to configure the y-axis motor step angle. Enter ‘$3sa=4’ and click ‘Send’ to configure the z-axis motor step angle. This ensures that the motor controller is configured to rotate the motors by 4 degrees in each step.
-
31
Configure number of microsteps: Enter ‘$1mi=8’ in the command line and click ‘Send’ to configure the x-axis motor to have eight microsteps. Enter ‘$2mi=8’ and click ‘Send’ to configure the y-axis motor to have eight microsteps. Enter ‘$3mi=8’ and click ‘Send’ to configure the z-axis motor to have eight microsteps.
-
32
Configure travel per leadscrew revolution: Enter ‘$1tr=1.75’ in the command line and click ‘Send’ to configure the x-axis motor travel per revolution. Enter ‘$2tr=1.75’ and click ‘Send’ to configure the y-axis motor travel per revolution. Enter ‘$3tr=1.75’ and click ‘Send’ to configure the z-axis motor travel per revolution. The motors are now coupled to leadscrews that translate 1.75 mm per revolution of the motor.
-
33
Configure motor polarity: Enter ‘$1po=0’ in the command line and click ‘Send’ to configure the x-axis motor polarity. Enter ‘$2po=1’ and click ‘Send’ to configure the y-axis motor polarity. Enter ‘$3po=0’ and click ‘Send’ to configure the z-axis motor polarity. Motor polarity determines the positive direction of motion. In the Craniobot, motion in the x and z axes are achieved by moving the spindle whereas motion in the y-axis is achieved by moving the stereotax relative to the spindle. Furthermore, the direction of movement of the spindle is defined with respect to the fixed CNC frame. Thus, for the tool to follow a Cartesian axis orientation, it is necessary to invert the polarity of the y-axis for positive tool motion to occur from left to right (x-axis), bottom to top (z-axis) and front to back (y-axis).
-
34
Configure potentiometers: Turn the potentiometers corresponding to each motor on the motor controller all the way counterclockwise. Turn the Motor 1 potentiometer by 45° clockwise to set the x-axis motor. Turn the Motor 2 potentiometer by 90° clockwise to set the y-axis motor. Turn the Motor 3 potentiometer by 90° clockwise to set the z-axis motor. The potentiometers regulate the current supplied to the motors and this provides a starting point for tuning the current. Use the jog commands to move each motor by 2 – 3 mm. If the motor hums but doesn’t move when a jog command is sent it is indicative of lack of current supply to the motor. In this case, turn the potentiometer clockwise by 1 – 5° and re-jog the motor. If the motor makes excessive noise when moving, stops mid-way through the movement, or makes the board excessively hot, the current may be too high. In this case, decrease the current supply by turning the potentiometer counterclockwise by 1 – 5 degrees. Repeat this process until each of the motors moves smoothly without humming. Do not exceed the potentiometers’ range of motion, approximately −135° to 135°.
? TROUBLESHOOTING
-
35
Configure maximum velocities: Enter ‘$xvm=600’ in the command line and click ‘Send’ to set the maximum velocity for the x-axis at 600 mm/min. Enter ‘$yvm=600’ and click ‘Send’ to set the maximum velocity for the y-axis at 600 mm/min. Enter ‘$zvm=800’ and click ‘Send’ to set the maximum velocity for the z-axis at 800 mm/min. Each motor has a limit on its maximum velocity, which depends on the hardware. To operate in a safe range, it is important to limit the maximum velocity that each motor is commanded by the motor controller.
? TROUBLESHOOTING
-
36
Configure limit switches: Enter ‘$st=0’ in the command line and click ‘Send’ to set all the limit switches to ‘normally open’ type. Enter ‘$xsn=2’ and click ‘Send’ to set the Xmin limit switch to ‘limit only’. Enter ‘$xsx=2’ and click ‘Send’ to set the Xmax limit switch to ‘limit only’. Enter ‘$ysn=2’ and click ‘Send’ to set the Ymin limit switch to ‘limit only’. Enter ‘$ysx=2’ and click ‘Send’ to set the Ymax limit switch to ‘limit only’. Enter ‘$zsn=2’ and click ‘Send’ to set the Zmin limit switch to ‘limit only’. Enter ‘$zsx=2’ and click ‘Send’ to set the Zmax limit switch to ‘limit only’. The Craniobot uses six limit switch channels on the motor controller. Two pairs of channels are used for setting the minimum and maximum travel range of the x and y axes. One pair of channels is connected to the surface profiler and the emergency stop button. This step ensures all limit switches are ‘normally open’ type and the Craniobot uses them in the ‘limit only’ configuration.
This completes the Craniobot software setup. For more details on setting up the TinyG, as well as additional parameters that can be tuned, refer to: www.github.com/synthetos/TinyG/wiki.
STAGE III: Benchtop testing
Surface profiler calibration
The surface profiler is designed such that the custom spring is pre-loaded. This ensures that the contact switch circuitry is normally closed and is an open circuit when a force of <98 mN is applied during surface profiling. The pre-load is controlled by the adjustment screws. Prior to using the surface profiler, it must be calibrated so that an appropriate actuation force triggers it into an open state during surface profiling. Low actuation force may result in false positives and high force may result in skull deformation.
-
37
Install the stylus on the surface profiler.
-
38
Screw three 0–80 adjustment screws into the threaded adjustments holes so that they are at the lowest position.
-
39
Install the surface profiler in the spindle.
-
40
Place a weighing scale under the surface profiler. If needed, remove the stereotax from the machine so that the surface profiler is at least 1 mm above the scale.
-
41
Open ‘Craniobot.exe’ and click ‘Start’. The GUI will change to ‘Select Procedures’ mode (Fig. 4). Do not select any procedure in Panel 1 of the GUI. Press ‘Surface Profiling configuration’ to change the GUI to the ‘Surface Profile Configuration’ mode (Fig. 4b).
? TROUBLESHOOTING
-
42
Use the jog commands in Panel 2 of the GUI (or the keyboard functions) to bring the tip of the surface profiler stylus ~2 mm above the center of the weighing scale platform. Click ‘Find Origin’ and the Craniobot will lower the surface profiler at a speed of 5 mm/min until one of the contact switches opens. The measurement observed on the weighing scale denotes the force required to open the switch. Note the weight on the scale.
? TROUBLESHOOTING
-
43
Use jog commands to retract the surface profiler up by 1 – 2 mm. Repeat Step 42 a few times (e.g., n = 6) to take multiple measurements and compute the average force needed to trigger the contact switch of the surface profiler.
-
44
Carefully turn each of the three adjustment screws to adjust the actuation force. The actuation force required to trigger the surface profiler reduces by ~200 mN (20 gram-force) for each turn of the screw. Ensure all three screws are equally turned to balance the actuation force. For ideal performance the surface profiler needs to be triggered by an actuation force of 49 mN - 98 mN (5 – 10 gram-force, Fig. 5a). Repeat Step 44 until the surface profiler is actuated at the desired actuation force range.
? TROUBLESHOOTING
Figure 5. Surface profiler performance.
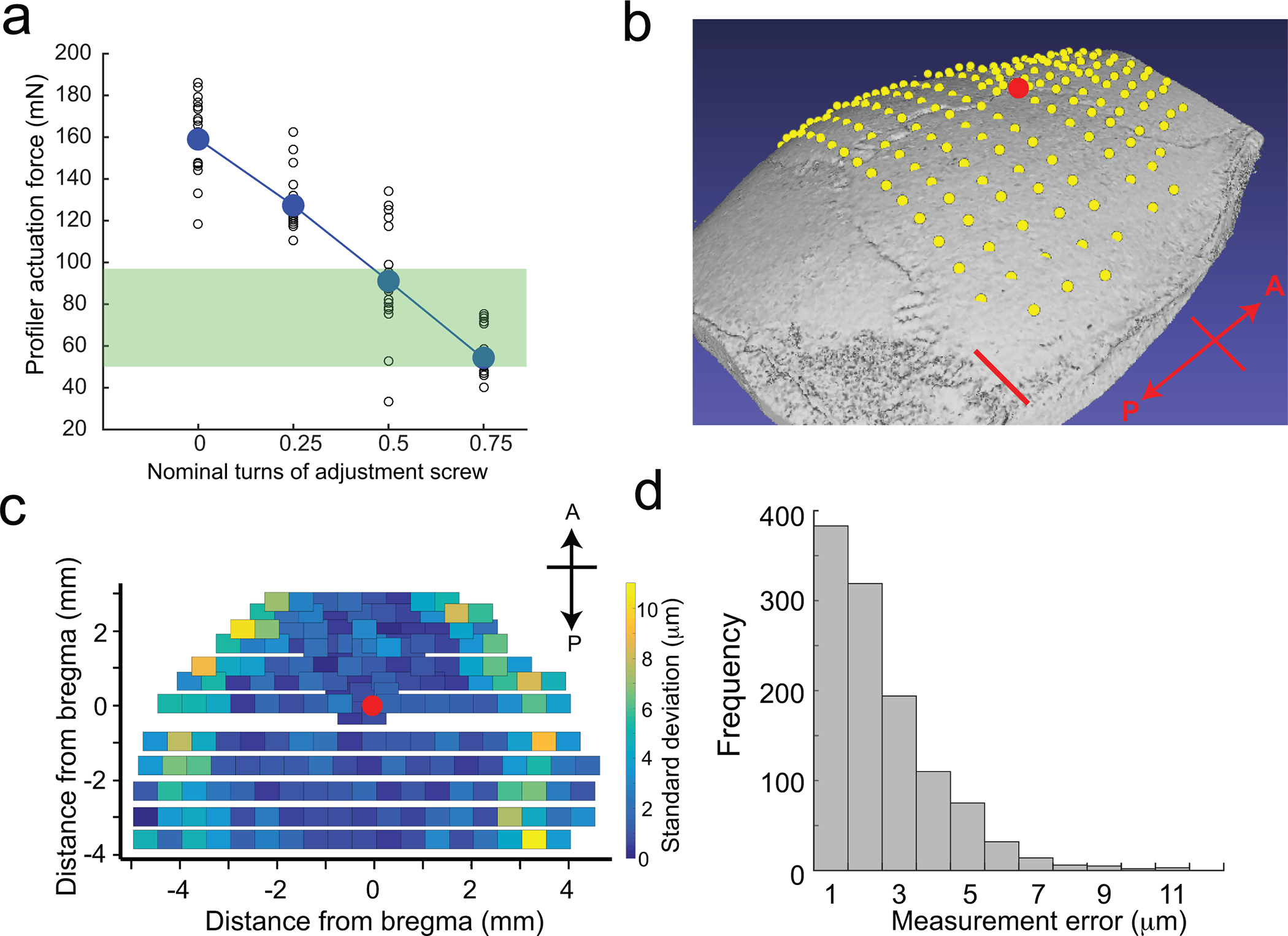
(a) Calibration curve indicating the change in actuation force required to trigger the surface profiler as a function of nominal turns of the adjustment screws. The green bar indicates the range of actuation forces in which the surface profiler can be used for profiling the skull surface. (b) Average point cloud computed after profiling 192 points across the dorsal cortex of a C57BL/6J mouse skull superimposed on a micro-CT scan of the same subject. Scale bar, 1 mm. The red dot indicates Bregma. (c) Standard deviations of repeated measurements taken across the same points in (b) showing the spatial variation in measurement error. The red dot indicates Bregma. (d) Histogram of measurement errors at 192 points each in six mice. Figure 5 adapted from ref: Ghanbari*, Rynes* et al Sci Rep 2019. All experiments were approved by the University of Minnesota’s Institutional Animal Care and Use Committee (IACUC).
This completes the calibration of the surface profiler.
Dry-run of Craniobot on a plastic tube
Steps 58 - 103 provide instructions for using the Craniobot for automated microsurgery in mice. We highly recommend the user follows the alternate protocol detailed in Steps 45 to 57 to perform a dry-run of a circular craniotomy on a plastic test tube (e.g., 50 mL conical tube). This will allow the user to become familiar with the Craniobot operation, and troubleshoot any hardware and software issues prior to experimenting on live animals.
-
45
Run ‘Craniobot.exe’ to execute the Craniobot GUI and click ‘Start’. The Craniobot GUI will shift to ‘Select Procedures’ mode (Fig. 4). A list of four choices are available to the user in Panel 1 of the GUI: (i) circular craniotomy, (ii) other craniotomy patterns, (iii) skull thinning, and (iv) anchor hole drilling. Select the circular craniotomy option. Panel 1 of the GUI will change as illustrated in Figure 4b. Enter the location of the center of the craniotomy (x and y coordinates relative to the reference point) and the diameter of the desired craniotomy, then click ‘Next’.
? TROUBLESHOOTING
-
46
Create holes in a 3 cm diameter plastic tube and securely install it in the stereotax (Fig. 6a).
-
47
Jog the end mill at least 15 cm away from the surgical area in the x and y directions using the jog keys or the jog buttons on the GUI (Fig. 4a). Fit the surface profiler into the spindle.
? TROUBLESHOOTING
-
48
Observe the LED in the switching circuit on the back of the Craniobot. If the LED is ON, the switch on the surface profiler is closed. Proceed to Step 49. If the LED is OFF, the switch on the surface profiler is open. Gently push the stylus up and release it to reset the switching circuit.
? TROUBLESHOOTING
-
49
Use a fine permanent marker to mark a reference point on the plastic tube which serves as an ‘origin’ point for the CNC mill (Fig. 6b).
-
50
Click ‘Next’ to return to ‘Select Procedures’ mode. Press ‘Surface Profiling Configuration’. (Fig 4a). The GUI will now move to ‘Surface Profiling Configuration’ mode (Fig. 4b). Use the jog commands in Panel 2 of the GUI to guide the surface profiling stylus tip to ~1–2 mm above the reference point marked in Step 49. This is a good time to acquaint yourself with the moveable components of the CNC mill. As the tip approaches the reference point, exercise caution while using jog commands to prevent accidentally hitting the plastic tube with the surface profiler stylus tip. Decrease the jogging speed to 1 (50 mm/min) using the ‘Speed’ control button. Alternately, decrease the jogging distance of each step to 1 mm or lower.
-
51
Click ‘Find Origin’ (Fig. 4b). The Craniobot will lower the stylus tip at a rate of 5 mm/min until it touches the skull surface (Fig. 6b, top left).
-
52
Verify that the stylus tip is at the reference point by visualizing it under a stereomicroscope. If it is not, raise it ~1 mm in the z direction using jog commands, then make fine lateral adjustments in the x-y direction to correct its position. Repeat Step 51. Once the stylus tip stops at the reference point, click ‘Set Origin’. The numerical indicators in Panel 2 of the GUI will now indicate ‘0’ for all axes. Use the trinocular or eye piece camera on the microscope to photograph the stylus tip position on the skull surface. This photo will be useful as a reference for setting the origin of the end mill in Step 54.
-
53
Click ‘Start Surface Profiling’ to begin surface profiling. Once the profiling is complete, the software will automatically open an .html window showing a 3D plot with the measured points for each of the selected procedures.
? TROUBLESHOOTING
-
54
Move the spindle away from the plastic tube using the jog commands. Replace the surface profiler with the end mill. Click ‘Go to XY origin’ in Panel 1 to bring the tool directly above the reference point. Then, using the z-axis jog command, slowly lower the end mill until it touches the reference point. Click ‘Reset Origin’ (Fig 4c). Use the reference image taken in Step 52 to ensure that the end mill is in the same location. If there is a lateral offset, use the jog commands to make fine lateral adjustment to realign with the reference point.
-
55
The text window on the right of Panel 1 will show the configuration of the selected procedures and the current drilling depth. Enter the drilling depth for the first milling pass. A reasonable number to start with is 40 – 50 μm.
-
56
Turn on the DC power source connected to the spindle. The spindle should start rotating.
-
57
Click ‘Run Mill’ to start milling. An .html window will open to display the interpolated 3D milling path. After the milling pass is complete, the spindle moves to 2 mm above the reference point. Figure 6b (bottom) illustrates a 50 μm deep circular craniotomy milled on the plastic tube.
Figure 6: Benchtop testing of Craniobot function on plastic tube and key steps during surface profiling:
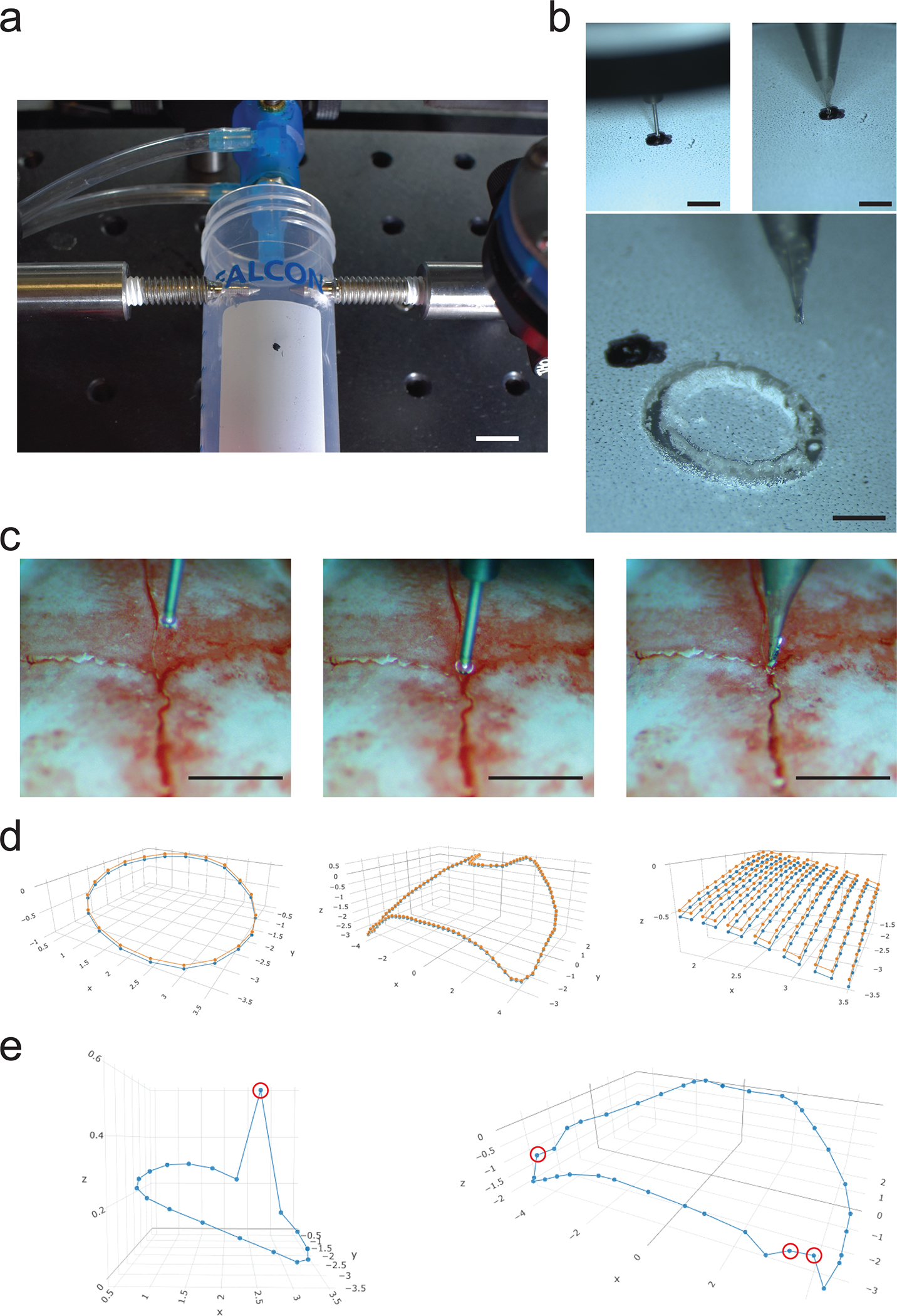
(a) Photograph illustrating a plastic tube adapted and secured in the Craniobot stereotax for mock circular craniotomy procedure. Scale bar, 10 mm. (b) Top left: Photograph illustrating the surface profiler stylus tip at the marked ‘origin’ on the surface of the plastic tube. Top right: Photograph illustrating the end mill at the marked ‘origin’ on the surface of the plastic tube. Bottom: Photograph of end result of milling a circular trench of depth 50 μm using the Craniobot. Scale bars, 1 mm. Surface profiling: (c) Left: Photograph of the stylus prior to starting ‘Find Origin’. Middle: Stylus after stopping at Bregma. Right: Photograph of the end mill positioned at Bregma prior to CNC milling. Scale bars, 1 mm. (d) Left: Result of surface profile displayed after profiling a circular craniotomy of diameter 3 mm centered at 2 mm to the right and 2 mm posterior to Bregma. Center: Result of surface profile displayed after profiling a large craniotomy covering both hemispheres of the dorsal cortex. Right: Result of surface profiles displayed after profiling a rectangular area with non-adjacent corners located at (−1.5 mm ML, −1.5 mm AP) and (−3.5 mm ML, −3.5 mm AP) for skull thinning. Circular craniotomy and whole dorsal cortex craniotomy surface profiles were performed on a male, 12-week-old C57BL/6J mouse. Profiling for skull thinning procedure was performed on a male, 16-week-old C57BL/6J mouse. Orange dots indicate pilot points at which the skulls were profiled, blue dots indicate points offset by a depth of 50 μm. Orange lines simulate interpolated path taken by the end mill at the skull surface. Blue lines simulate the linearly interpolated path taken by the end mill if offset by a depth of 50 μm. (e) Erroneous surface profiles resulting from false positive measurements: Left: when profiling for a circular craniotomy and Right: when profiling for a craniotomy over the dorsal cortex. False positive measurements are indicated by red circles.
STAGE IV: In vivo cranial surgery
! CAUTION: Ensure all animal experiments are approved by the appropriate institutional animal care and use committee.
Animal preparation
-
58
Place all autoclavable surgical tools and the end mill in an autoclavable container or sterilization wrap and autoclave using a benchtop autoclave.
-
59
Sterilize the workspace and non-electrical equipment including the table, CNC mill, stereotax, and surgical tools. using 70% ethanol. While handling electrical equipment (e.g. microscope, computer), ensure that your gloves have recently been sprayed with 70% ethanol before proceeding with the surgery.
-
60
Sterilize the implants by soaking in 70% ethanol for 2 – 3 min followed by rinsing in sterile saline.
-
61
To perform a craniotomy of arbitrary shape, generate a comma separated values (.csv) file in a spreadsheet software containing the x-y coordinates of the pilot points relative to Bregma for the desired cranial procedure (see Supplementary File 4 for examples).
! CAUTION: Avoid designing craniotomy profiles with pilot points near skull sutures and sinuses, since these regions are susceptible to deformation even at low forces applied by the contact sensor.
Craniobot startup
-
62
Sterilize the stylus tip on the surface profiler using a medical grade sterilizing agent (preferably 70% ethanol or iso-propyl alcohol). Rinse with sterile saline briefly. Attach the stylus tip to the surface profiler.
-
63
Jog the end mill at least 15 cm away in the x and y directions from the surgical area using the jog keys or the jog buttons on the GUI (Fig. 4a). Fit the surface profiler into the spindle.
-
64
Observe the LED in the switching circuit on the back of the Craniobot. If the LED is ON, the switch on the surface profiler is closed. Proceed to Step 65. If the LED is OFF, the switch on the surface profiler is open. Gently push the stylus up and release it to reset the switching circuit.
? TROUBLESHOOTING
-
65
Transfer the mouse from the home cage to the anesthesia induction chamber. Anesthetize the mouse using approximately 4–5% isoflurane in medical grade oxygen with a flowrate of 0.5 – 0.6 mL/min. Note that the percent of isoflurane to be used may vary from one isoflurane station to another.
!! CRITICAL STEP: Ensure that the mouse is under deep anesthesia and unresponsive to toe pinch stimuli prior to surgery.
-
66
Shave the head using a hair trimmer. Remove hair clippings using cotton-tipped applicators.
-
67
Cover the eyes with ophthalmic eye ointment.
-
68
Administer appropriate analgesics and anti-inflammatory drugs (optional): slow-release buprenorphine subcutaneously at 1 mg/kg body weight and meloxicam subcutaneously at 2 mg/kg. Slow-release buprenorphine needs to be injected 2 – 3 hours prior to the first incision.
-
69
Transfer and head-fix the mouse on the stereotax. Ensure the animal temperature controller is set to regulate the body temperature at 37.5°C. Set the isoflurane concentration to ~1.5%.
!! CRITICAL STEP: Ensure that the mouse is rigidly fixed in the stereotax by tapping on the head with a micro-curette and checking that the head does not move. If the mouse is loosely fixed, it may result in erroneous surface profiling measurements or lead to errors in later steps of the protocol.
-
70
Periodically (every 5 – 10 minutes) monitor the breathing rate and toe pinch response of the mouse. The mouse should breathe at ~1 breath/s. If it breathes slower, decrease the isoflurane concentration by %0.1–0.3. If it breathes faster, increase the isoflurane concentration by %0.1–0.3, and pause the experiment until the breathing rate reaches ~1 breath/s. Ensure that the mouse does not respond to toe pinch stimuli. Inject 1 ml/kg body weight lactated Ringer’s solution at ~37.5°C subcutaneously every 2–3 hours during long procedures to keep the mouse hydrated.
-
71
Sterilize the scalp area by alternately scrubbing three times with betadine and 70% ethanol using cotton-tipped applicators. Ensure that all hairs are removed during this procedure.
-
72
Subcutaneously inject a local anesthetic (1% Lidocaine) at the incision site. Tent up the scalp using blunt forceps to separate it from skull and use scissors to cut the skin and expose the skull over the region of interest.
-
73
Use scissors and a micro-curette to scrape the skull surface and remove fascia before cranial procedure.
PAUSE POINT: The following section describes the use of the Craniobot. The emergency stop button can be used at any point of operation to stop the Craniobot operation. If the emergency stop button is used, all motors will stop instantly, and the communications between the motor controller and the computer will cease, causing the GUI to shut down. The surgery cannot be resumed from this point if using the GUI. It is therefore recommended to use the emergency stop button in only case of an emergency.
Selecting cranial procedures
-
74Select procedures: Run ‘Craniobot.exe’ to execute the Craniobot GUI and click ‘Start’. The GUI will shift to ‘Select Procedures’ mode (Fig. 4). Four choices are available to the user in Panel 1: (i) circular craniotomy, (ii) other craniotomy patterns, (iii) skull thinning, and (iv) anchor hole drilling. Select a cranial procedure from this list. Multiple procedures can be selected if needed.
- If ‘Circular Craniotomy’ was selected, Panel 1 provides the user with options to specify the location and dimensions of the circular craniotomy (Fig. 4b). Enter the x and y coordinates of the center of the craniotomy (relative to Bregma) and the diameter of the desired craniotomy. Click ‘Next’ when done.
-
If ‘Other Craniotomy Patterns’ was selected, the GUI will prompt the user to upload a comma-separated values (.csv) file with x and y coordinates of pilot points (relative to Bregma) where surface profiling needs to be performed. An example code for creating a 4 mm x 4 mm square craniotomy and a craniotomy spanning the whole dorsal cortex are included as Supplementary File 4. Upload the .csv file specifying the desired craniotomy profile. Click ‘Next’ when done.NOTE: The interpolated machining path will be more accurate if the number of pilot points specified is higher, but also increases the profiling time (7 s/point). If designing custom craniotomy path, ensure the pilot points for profiling are ~0.5 mm.
- If ‘Skull Thinning’ was selected, Panel 1 provides the user with options to specify the location and dimensions of the rectangular area for skull thinning (Fig. 4b). Specify the x and y coordinates of two non-adjacent vertices of the rectangle. The Craniobot will generate a raster pattern with points separated by 0.15 mm covering the rectangular area. Click ‘Next’ when done.
- If ‘Anchor Hole Drilling’ option was selected, the GUI will prompt a window asking for the x and y coordinates where bone screws need to be implanted (Fig. 4b). Enter the desired coordinates and click ‘Next’.
Configuring and profiling skull surface
-
75
Clicking ‘Next’ in the previous step results in the GUI returning to ‘Select Procedures’ mode. If more than one procedure is desired, repeat Step 74. After choosing and configuring all the desired cranial procedures, click ‘Surface Profiling Configuration’. (Fig 4a). The GUI will now move to ‘Surface Profiling Configurations’ mode (Fig. 4c). Use the jog commands in Panel 2 to guide the stylus tip to a location 1 – 2 mm above Bregma (Fig. 6c left, Supplementary Video 1).
! CAUTION: As the tip approaches Bregma, exercise caution while using jog commands to prevent accidentally hitting the skull with the surface profiler stylus tip. Decrease the jogging speed to 1 (50 mm/min) using the ‘Speed’ control button. Alternately, decrease the jogging distance of each step to 1 mm or lower.
-
76
Click ‘Find Origin’ (Fig. 4c). The Craniobot will lower the stylus tip at a rate of 5 mm/min until it touches the skull surface (Fig. 6c middle).
? TROUBLESHOOTING
-
77
Verify that the stylus tip is at Bregma by visualizing it under a stereomicroscope. If it is not, raise it ~1 mm in the z direction using jog commands, then make fine adjustments to position it above Bregma. Repeat Step 76. Once satisfied that the stylus tip is right on Bregma, click ‘Set Origin’.
Tip: Use the trinocular or eye piece camera on the microscope to take a photograph of the position of the stylus tip on the surface of the skull (Fig. 7a). This will be useful as a reference setting the origin of the end mill during Step 81 (Fig. 6c right).
-
78
Click ‘Start Surface Profiling’. The Craniobot will begin surface profiling. Once the profiling is completed, the software will automatically open an .html window of a 3D plot with the measured points for each of the procedures selected. Figure 6d illustrates 3D plots of surface profiles generated for a 3.3 mm diameter circular craniotomy at 2 mm to the right and 2 mm posterior from Bregma, a user-specified craniotomy across the whole dorsal cortex and a 3D surface profile for skull thinning a rectangular area 1.5 mm posterior and lateral to Bregma. Carefully observe the surface profiler during this operation. Occasionally, false positives may occur due to vibrations wherein the profiler stops in the air prior to contacting the brain before proceeding to the next step. Figure 6e illustrates two such instances in which erroneous profiles are registered. If this occurs, repeat this step after eliminating sources of vibration.
? TROUBLESHOOTING
Figure 7. Surface-profile-guided machining:
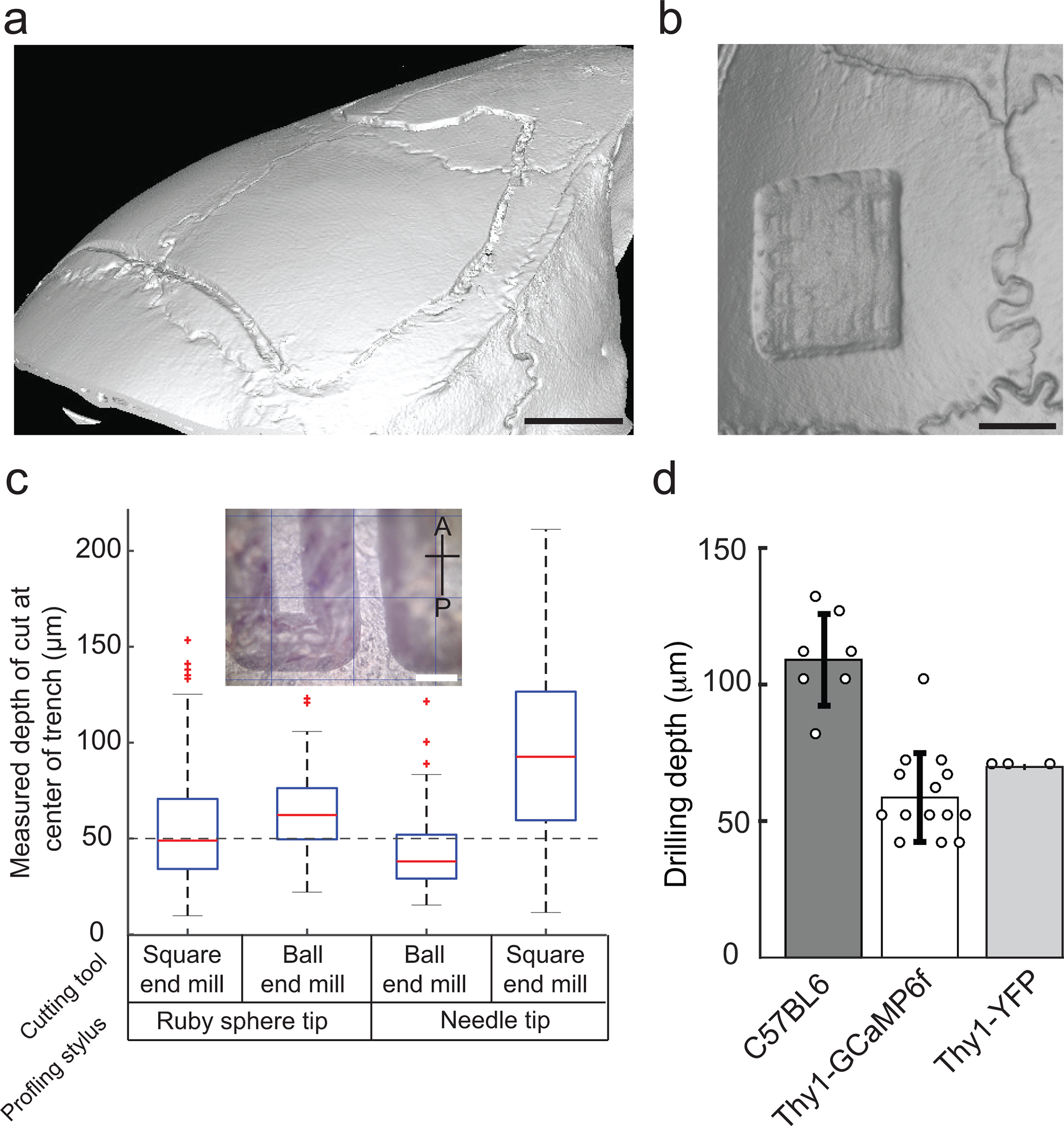
(a) Boxplot of the measured trench depth for four possible combinations of surface profiling and end mills for 50 μm depth of cut. Inset: Photomicrographs of trenches milled in the anterior posterior direction by the Craniobot. Scale bar, 250 μm (b) Bar plots of the final depth of milling used for performing craniotomies over the whole dorsal cortex in three strains of mice of ages ranging from 8 to 20 weeks. (c) Micro-CT scan of a mouse skull after 50 μm deep trench milled for a craniotomy across the whole dorsal cortex. Scale bar, 1 mm. (d) Micro-CT scan of a mouse skull after surface milling down to a depth of 70 μm for a skull thinning procedure. Scale bar, 1 mm. (a), (c), and (d) are adapted from ref: Ghanbari*, Rynes* et al Sci Rep 2019. All experiments were approved by the University of Minnesota’s Institutional Animal Care and Use Committee (IACUC).
Iteratively milling
-
79
Ensure there is no skin or fat tissue on the milling path. If there is, gently cut or push it away using a micro-curette and cotton-tipped applicators.
-
80
Use the jog commands to move the spindle away from the skull for tool exchange. Replace the surface profiler with the end mill. For craniotomies, a 200 μm square end mill works best, whereas for skull thinning a 300 μm ball end mill works best.
-
81
Click ‘Go to XY origin’ in Panel 1 to bring the end mill directly above Bregma. Then, using the z-axis jog command, slowly lower the end mill until it meets Bregma. Once the tool is just touching the skull surface at Bregma (Fig. 6c right), click ‘Reset Origin’ (Fig 4d).
Tip: The reference image taken in Step 77 is useful for ensuring the end mill is at the same Bregma location.
!! CRITICAL STEP: Ensure the end mill is registered to the same position, else, the craniotomy will be laterally offset. If there is a lateral offset, use the ‘x,y’ jog commands to realign with Bregma.
!! CRITICAL STEP: Ensure the end mill is touching but not exerting force on the skull when registering the origin. This may result in an offset in the z-direction below the plane that surface profiling was performed in, leading to inaccurate drilling depths.
! CAUTION: Ensure the tip of the end mill is above the topmost point of the skull by at least 1 mm before clicking ‘Go to XY origin’.
-
82
Irrigate the skull with sterile saline to keep it moist and cool during the milling procedure without disturbing the end mill. The text window on the right of Panel 1 will show the configuration of the selected procedures and the current drilling depth. Enter the drilling depth for the first milling pass. Since skull thickness is not measured for each mouse before drilling, start with a conservative drilling depth such as 40 – 50 μm.
-
83
Turn on the DC power source connected to the spindle. The spindle should start rotating.
-
84
Click ‘Run Mill’ to start milling. An .html window will open to display the interpolated 3D milling path. After the first milling pass, the spindle is taken back to a point 2 mm above Bregma.
-
85Inspect the skull
- Craniotomy procedure: At the end of each milling pass, use blunt forceps to gently press the grooves created by the end mill. If the milled skull section is sufficiently thinned down to be fractured around the periphery without exerting extra force, it is ready to be removed. The bone at the bottom of the skull, the trabecula, is typically soft when pressed with blunt forceps. Proceed to Step 87. If not, specify an increased drilling depth, as done in Step 82 (increments of 10 μm in each successive mill are recommended). Typically, in craniotomies performed in 8–16 week old C57BL/6J mice over the dorsal cortex, the skull is ready to be removed after drilling down to depth 107.85 ± 16.79 μm from the skull surface (Fig. 7b).
- Skull thinning procedure: At the end of each milling pass, rinse all skull debris by irrigating with sterile saline and cleaning with cotton-tipped applicators. Surface blood vessels in the brain should be clearly visible at this stage. If not, repeat Steps 82 – 84 until the skull is thin and surface blood vessels are clearly seen. Do not force saline or any surgical tool into the milled skull area. Other indicators that the skull is sufficiently thin are seepage of cerebrospinal fluid through the skull or skull deformation in response to gentle tapping with a microcurette. Using a forceps or micro-curette, gently move the tool across the milled skull area to dislodge any trapped skull fragments.
? TROUBLESHOOTING
-
86
Bone anchor screws: Implant a self-tapping screw ~2 mm posterior and ~3 mm mediolateral to Lambda to help with securing the implant.
Bone removal and device implantation
-
87
If performing a skull thinning procedure, proceed to Step 99. For craniotomies, irrigate the skull with sterile saline. Use a pair of blunt forceps to gently press down the milled skull section at several points to fracture it. Anchor one arm of a blunt forceps underneath the skull island at one of these points and gently lift the skull island. Rinse the brain with saline and remove the bone residues. Visually inspect to ensure all the milled skull has been removed. Gently place saline-soaked gauze pads on the brain for 2 – 5 min to absorb any bleeding from the skull and keep the brain moist until the cranial window is implanted.
-
88
Device implantation: Steps 89 – 92 below describe implantation of glass coverslips after skull removal. There are several previously written protocols for implantation of glass coverslips23–25. Below is one procedure specific to our protocol. If the user is performing a large cranial window implantation, proceed to Step 93. If the user is attempting to reinforce a thinned skull for intact skull imaging, proceed to Step 99.
Circular coverslip implantation
-
89
Place the coverslip on top of the brain and align it to the craniotomy outline.
-
90
Gently press the coverslip down onto the brain using a pair of blunt forceps, ensuring the outer boundary of the coverslip contacts the skull. Silicone oil or agarose gel may be applied between the brain and the coverslip to avoid the formation of air bubbles under the coverslip26. Apply a few drops of cyanoacrylate glue using a 28-gauge syringe needle to make a thin and narrow film around the periphery of the coverslip to bond it to the skull.
-
91
Apply dental cement to secure the coverslip to the skull and let it fully cure.
-
92
Cover the glass coverslip with a 1 – 2 mm thick layer of quick-setting silicone sealant to protect the implant. Proceed to Step 101.
See-Shell implantation
-
93
Follow instructions in Supplementary Note 1 and Supplementary Figure 4 to assemble a See-Shell implant. Sterilize the See-Shell implant, without the titanium head-plate, by soaking in 70% ethanol for 3 – 5 minutes followed by rinsing in sterile saline. Remove the gauze pad placed in Step 87 and irrigate the brain surface with saline. Gently place the implant on the exposed brain such that the boundary of the implant coincides with the edge of the craniotomy. Now use cotton-tipped applicators to wick excess saline surrounding the implant.
-
94
Use a pair of forceps to gently press down the implant onto the skull. Apply force directly only on the areas where the implant rests on the bone and not on the flexible PET film. Use a syringe with a 28-gauge needle to apply a few drops of cyanoacrylate glue to glue the implant to the skull. Press the implant down on the skull to ensure a thin layer of glue is applied between the skull and the implant and none of the glue travels under the implant into the brain. Let the glue dry for 5 – 7 minutes.
-
95
Check if the brain area is sealed properly by applying a few drops of saline around the edges of the implant and observing if the saline travels under the implant. Repeat Step 94 to fully seal the implant.
-
96
Apply dental cement along the periphery of the implant to cement it to the skull. Ensure that the bone screws implanted at the base of the implant are fully covered by dental cement. Further, ensure that no dental cement enters the 0–80 threaded holes in the implant. Use the micro-curette to clear the threaded holes of any dental cement if this happens. Allow 5 – 10 minutes for the cement to cure.
-
97
Use three 3/32” long, flat head 0–80 screws to fasten the titanium head-plate to the See-Shell. Prepare new batch of dental cement and apply it around the areas where the titanium head-plate is fastened to the See-Shell.
!! CRITICAL STEP: As the frame of the See-Shell is made of a flexible polymer, it is critical that dental cement is applied generously around the 0–80 screw fasteners so that the titanium headpost is firmly attached to the implant.
-
98
Use two 3/16” long, flat head 0–80 screws to fasten the protective 3D-printed cap onto the titanium head-plate. Proceed to Step 101.
Thinned skull reinforcement
-
99
Clean the polished skull area using sterile saline. Use cotton-tipped applicators to carefully remove any grit particles.
-
100
Apply clear dental cement on the thinned and polished skull. Before the cement cures, gently place a glass coverslip on the cement and press until a thin layer of clear dental cement remains between the thinned skull and the glass coverslip. Wait until it has fully cured to cover the glass coverslip with quick-setting silicone sealant.
Post-operative recovery
-
101
Transfer the mouse to a clean home cage on top of a heating pad and let the mouse wake up and move around comfortably.
-
102
Monitor the mouse for three days post-operation and allow mouse to fully recover prior to further experiments.
-
103
(Optional) administer appropriate post-operation anti-inflammatory drugs and analgesics (e.g. meloxicam, buprenorphine).
TIMING
Step 1: Prefabrication of custom parts: 3 – 4 days, longer lead time if procuring parts from commercial fabrication services
Steps 2 – 3: CNC mill assembly: 2 hours
Steps 4 – 9: Stereotax assembly: 1 hour
Steps 10 – 15: Control electronics installation: 2 hours
Steps 16 – 24: Surface profiler assembly: 2 hours
Steps 25 – 36: Software installation and motor controller configuration: 3 hours
Steps 37 – 44: Calibrating the surface profiler: 2 hours
Steps 45 – 57: Dry run of the Craniobot on plastic tube: 2 hours
Steps 58 – 103: Cranial surgery: Circular coverslip implantations typically take 1 – 2 hours, See-Shell implantation take 2 – 3 hours, and skull thinning operations take 3 – 4 hours
ANTICIPATED RESULTS
CNC mill assembly and performance:
Upon completion of this protocol, the user will have a functioning Craniobot in less than two weeks. The hardware does not require significant maintenance once fully setup. Depending on usage, it is recommended that the user check the actuation force of the surface profiler and re-calibrate it following Steps 37- 44 every three weeks. Since the Craniobot utilizes off-the-shelf kits for building the CNC mill, the exact specifications of the step resolution may vary in each implementation. We were able to achieve a minimum step size of 1 μm with both the 2020 CNC mill19 and the 3018 CNC mill described in this protocol. We did not find any backlash errors that caused improper CNC-guided milling, but this might be an issue if the CNC mill is not assembled in accordance to the assembly instructions. Because the CNC mill consists of movable parts and spindle that causes some vibration, it is recommended that the user periodically checks the fasteners holding up the CNC mill assembly and tightening any loose fasteners.
Surface profiling:
Results obtained using the surface profiler are illustrated in Figure 5. Once the surface profiler is calibrated, it is possible to profile the entire dorsal skull surface with high accuracy and obtain 3D coordinates. In the original study, we performed skull surface profiling on multiple C57BL/6J mice and superimposed them on computer tomography (CT) scans of the same mice to qualitatively assess the accuracy of the surface profiling. One such comparison illustrating the surface profiler capabilities to accurately reproduce the surface topology of the mouse is illustrated in Figure 5b. Examining the spatial variation in measurement error, we found that profiling more lateral areas of the skull resulted in larger measurement errors (Fig. 5c). Across all mice investigated (n=6, 3 male and 3 female) and over 97% of the points profiled, the measurement error was less than 6 μm with a maximum error of 11 μm (Fig. 5d). The average measurement error was 1.7 ± 1.4 μm when surface profiling skulls of female mice (n = 3 mice), and 2.2 ± 1.8 μm when surface profiling skulls of male mice (n = 3 mice)19. Higher measurement errors typically occur at lateral extremes from the midline.
A typical surface profile obtained for performing a circular craniotomy of diameter 3.3 mm centered at +2.2 mm medio-lateral (ML) and −2.1 mm anterior-posterior (AP) is illustrated in Figure 6d (left). Similarly, surface profiles obtained prior to performing a large craniotomy across the whole dorsal cortex is illustrated in Figure 6d (middle). The ability to program arbitrary shapes for the craniotomy was utilized to create an indent at the top of the craniotomy, which allowed the end mill to avoid a major sinus located ~ 1.5 mm anterior to Bregma along the midline. This significantly reduces bleeding during the craniotomy and enhances post-surgery recovery. Finally, a surface profile obtained prior to a skull thinning procedure is illustrated in Figure 6d (right). The surface profiler is occasionally triggered to an open state due to minor vibrations even when the stylus has not contacted skull. Examples of erroneous surface profiles with such false positives are illustrated in Figure 6e. It is very important that the user evaluates the surface profile obtained at the end of Step 65 (See also Table 3: TROUBLESHOOTING).
TABLE 3:
Troubleshooting
| STEP | PROBLEM | POSSIBLE REASON | SOLUTION |
|---|---|---|---|
| 27 | Program does not install properly or fails to open. | Software incompatibility with operating system. | Update the windows version to ensure compatibility with Python 3.6. |
| Program does not start or crash during operation. | User’s windows configuration does not support the Craniobot software version. | Look under the hidden folder username/AppData/Roaming for the file Craniobot.launch.pyw and open it with a text editor. Look at the errors for a list of possible causes. | |
| Program does not progress to Select Procedures mode. | Serial communication not established. | Unplug the motor controller from the power source and from the computer. Reconnect and restart the program. | |
| The motor is not responding to program commands. | The motor controller entered alarm mode. | Use the reset button on the motor controller to recover the system. Close and open the program again. | |
| 34 | The motor hums but doesn’t move when a jog command is sent. | Lack of current supply to the motor. | Turn the potentiometer clockwise by 45 degrees and re-jog the motor. Repeat until the motor moves. Do not exceed the potentiometers' range of motion, approximately −135° to 135°. |
| The motor makes excessive noise when moving, stops mid-way through the movement or makes the board excessively hot. | Excess of current supply to the motor. | Turn the potentiometer counter-clockwise by 45 degrees and re-jog the motor. Repeat until the motor runs smoothly. Do not exceed the potentiometers' range of motion, approximately −135° to 135°. | |
| 35 | The motor stalls or fails to start. | Maximum velocity is too high for the hardware setup. | Lower the maximum velocity issuing the command ‘$xvm=600’. Replace ‘x’ by the desired axis. Decrease the value by 50 until the motor operates normally. |
| 41 | Program does not start or crash during operation. | User’s windows configuration does not support the Craniobot software version. | Look under the hidden folder username/AppData/Roaming for the file Craniobot.launch.pyw and open it with a text editor. Look at the errors for a list of possible causes. |
| Program does not progress to ‘Select Procedures’ mode. | Serial communication not established. | Unplug the motor controller from the power source and from the computer. Reconnect and restart the program. | |
| 42 | The motor is not responding to program commands. | The motor controller entered alarm mode. | Use the reset button on the motor controller to recover the system. Close and open the program again. |
| 44 | Surface profiler keeps moving down even after significant deflection of the stylus. | Incorrect wiring of the switching circuit. | Check wiring and ensure it is per schematic shown in Figure 3 |
| Surface profiler does not move down. | Spring is not pushing down on the stylus holder and the circuit is open. | Gently push the stylus up and release to re-position the stylus holder structure. The LED in the switching circuit should turn on when this is rectified | |
| Oxidation on the ball bearing in switches causes open circuit. | Use microfiber tissue soaked in 100% isopropyl alcohol to gently clean the ball bearings. | ||
| One or more brass pins are not fully in contact with the ball bearings.. | The stylus holder has been improperly assembled. Disassemble the surface profiler and repeat Step 17 taking care to ensure the brass pins are all perpendicular to the central axis of the stylus holder. | ||
| 45 | Program does not progress to Select Procedures mode. | Serial communication not established. | Unplug the motor controller from the power source and from the computer. Reconnect and restart the program. |
| 47 | The motor is not responding to program commands. | The motor controller entered alarm mode. | Use the reset button on the motor controller to recover the system. Close and open the program again. |
| 48 | The LED in the switching circuit is always OFF. | The surface profiler trigger circuit is always open. | See troubleshooting solutions described for Step 44. |
| 53 | The surface profiling results in excessive false positive measurements. | Vibration in the setup are triggering the surface profiler into open circuit mode. | Eliminate sources of vibration. If issue persists, recalibrate the surface profiler. |
| 64 | The LED in the switching circuit is always OFF. | The surface profiler trigger circuit is always open. | See troubleshooting solutions described for Step 44. |
| 76 | Surface profiler does not move down. | See possible reasons and solutions in troubleshooting for Step 44. | |
| 78 | The surface profiling results in excessive false positive measurements. | Vibration in the setup are triggering the surface profiler into open circuit mode. | Eliminate sources of vibration. If issue persists, recalibrate the surface profiler. |
| 85 | Milling does not result in uniform thickness of bone tissue removal throughout the craniotomy path. | Offset between the surface profiler stylus origin and the end mill’s origin. | Repeat Step 81 taking care to ensure that the end mill is registered at the same point as the stylus, as shown in Figure 6c. |
| Skull may have moved after surface profiling. | Mouse improperly fixed in the stereotax. | Ensure that the mouse is fixed rigidly in the stereotax in Step 69. | |
Milling after surface profiling:
Once a surface profile is obtained, the Craniobot computes an interpolated milling path that is offset in the z-direction from this interpolated path by the depth specified by the user (Fig. 6d). Micro-CT scans of a 50-μm deep trench milled for a craniotomy over the dorsal cortex is illustrated in Figure 7a, and a skull thinning operation, via surface milling to a depth of 70 μm, is shown in Figure 7b. In the original study, we programmed the Craniobot to perform milling of 50-μm deep trenches in the anterior posterior direction (Fig. 7c inset) using combinations of surface profiler styli (Ruby sphere tip and needle tip) with end mills (square end mill and ball end mills) with dimensions equivalent to commonly used dental drills and burrs. We found that the combination of the ruby sphere tip stylus and 200-μm square end mill gave an average trench depth of 57.3 ± 6 μm, which was closest to the programmed value.
In any surgery, it is difficult to ascertain the final milling depth. It is important not to mill beyond the skull to ensure the brain is not injured during the procedure. In the original study, micro-CT measurements of adult C57BL/6J mice indicated that the skull thickness varies between 100 and 650 μm19. Further, cranial morphometry studies of these mice found that variation in cranial bone shape and size are minimal once the mice reach adulthood27. For instance, the length and width of the frontal bone of C57BL/6J mice were reported to be 7.756 ± 0.073 mm and 5.356 ± 0.101 mm, respectively. The length and width of the parietal bone were reported to be 3.889 ± 0.162 mm and 8.1 ± 0.229 mm, respectively. Further, variance in skull bone size within a strain is on the order of only tens to a couple of hundred micrometers, as confirmed by micro-CT measurements of adult mouse skulls19. Thus, once an experimental heuristic is established for the depth to which skull needs to be iteratively milled, a conservative depth of milling can be used as a starting point. In our experiments, we logged the depths at which drilling was stopped when performing large whole cortex craniotomies in C57BL/6J mice and two strains derived from the C57 strains, Thy1-GCaMP6f mice28 and Thy1-YFP mice29 (Fig. 7d). On average, drilling was stopped at a depth of 106.9 ± 16.9 μm in C57BL/6J mice (n = 13), 63.3 ± 14.7 μm in Thy1-GCaMP6f mice (n = 6 mice) and 50 μm in Thy1-YFP mice (n = 3 mice). Finally, we assessed whether automated drilling causes any acute damage to the underlying brain. In a series of histology experiments, we perfused mice 30 minutes after the Craniobot was used to mill a circular craniotomy to the point where the skull island was ready to be excised. Immunostaining for ionizing calcium-binding adaptor molecule 1 (Iba1, Wako Cat# 019–19741, RRID:AB_839504), found in activated microglia – a marker for acute neural injury, we found no significant difference in number of activated microglia in mice that underwent CNC-guided milling as compared to control mice that did not undergo any procedure (Fig. 8d–e). In the case of injury to the brain, changes in microglia morphology can occur within minutes of an injury and can be evaluated via image analysis30. Within the time period assessed, we do not see any changes in the size of the soma of the microglia (Fig. 8f).
It is possible that some inflammatory response may occur over longer time durations that we did not assess in this study. In most experiments, bone removal will be followed up by implantation of a cranial window or, in the case of skull thinning, reinforcing it with dental cement. There are extensive studies done by several groups looking at the inflammatory response of implantation of planar cranial windows23 and curved glass window31. Furthermore, potential chronic effects of craniotomies done with the Craniobot were addressed in our other paper19. The relative advantages of thinned reinforced skull with respect to implanted planar window has also been well explored24.
Procedures:
The Craniobot has been used in our laboratory for performing 3 mm diameter craniotomies for implantation of glass coverslips (Fig. 8a), craniotomies over the whole dorsal cortex for implantation of See-Shells (Fig. 8b) and skull thinning procedures (Fig. 8c). The time required for surface profiling depends on the number of pilot points at which the z position of the skull is measured. This typically takes 7 seconds per measurement. Thus, for a 3 mm diameter circular craniotomy, the Craniobot takes ~ 150 seconds. For a large craniotomy over the whole dorsal cortex, the skull is profiled at 100 points along the craniotomy path, which takes 5 – 10 minutes. For a skull thinning procedure with a rectangular area of 2 mm x 2 mm, the skull is profiled at 169 points, once in the AP direction and once in the ML direction. This process can take up to 20 minutes.
We have assessed chronical implantation of circular glass coverslips for up to four weeks and polymer skulls that have been implanted after automated surgeries for over 36 weeks (Fig. 8b)20. Skull thinning procedures have been assessed for up to 21 days. In a comparative study between the automated approach and manual surgery for implanted polymer skulls over the whole dorsal cortex, we did not see differences in the eventual viability of the implants. We found that, out of 41 mice that underwent automated craniotomies prior to polymer skull implantation, which was done by three surgeons, over 90% of the mice survived and had clear skulls for up to 340 days.
CONCLUSIONS AND FUTURE DIRECTIONS
We anticipate automated surgical tools will empower the neuroscience community to deploy new neurotechnology in ever increasingly complex procedures. Recent advances in large-scale imaging technologies13,25,32 has prompted several groups to develop preparations for cranial windows covering an entire hemisphere or, more recently, curved glass coverslips15 and See-Shells20 for whole cortex optical access. Automated craniotomies may lower the barrier to entry for new groups trying to establish these techniques in their laboratories. There are also increasing trends towards whole brain activity measurements that require precise placement of high-density recording electrode arrays11, tetrode microdrives33, and intracellular recording electrodes21 to multiple regions of the brain. Fiber photometry devices with precisely shaped arrays for simultaneously monitoring calcium activity from several brain regions34,35 have been developed. Each array requires precise alignment and placement into the brain. Automated surgical devices with micrometer precision have the potential to aid these complicated experiments.
Open source tools have contributed enormously to advances in neuroscience, for instance, accessible tools for electrophysiology36 and software for quantification of behavior37. The field has also seen a significant increase in the use of open-source miniaturized devices for optical imaging of neural activity in freely-behaving mice38,39. Multiple groups have contributed to this ecosystem by rapidly innovating several versions of miniaturized imaging systems for volumetric mapping40, multisite image41, and imaging combined with optogenetic stimulation42.
Along these lines, the Craniobot is engineered to be fully open-source. Our current estimate is that it can be assembled using parts costing less than $1500. Recent studies have shown that motorized surgery platforms can be used to automate the process of aspirating the cortex43, particularly useful for targeting deep neural structures using gradient refractive index (GRIN) lenses in conjunction with miniaturized imaging devices or for widefield imaging of subcortical structures44,45. The G-code programming language used by the Craniobot can be harnessed to incorporate such additional capabilities and perhaps automate the entire surgical procedure. For instance, soft robotic grippers along with motorized scalpels could automate the excision of scalp, vacuum graspers could automate the bone excision process, implant holders could be programmed for automatically positioning the implant and integrated fluidic circuits could be utilized for application of fast curing cement during implantation.
Supplementary Material
Supplementary Video 1: Video demonstrating the use of the Craniobot to perform a 3 mm diameter circular craniotomy centered at 2 mm right and 2 mm posterior to Bregma
Supplementary File 1: A bill of materials in .xlsx spreadsheet format
Supplementary File 2: A compressed file archive consisting of all CAD files of custom fabricated components needed for assembly of the Craniobot and the surface profiler
Supplementary File 3: A file containing the code for the microcontroller to regulate the switching circuit
Supplementary File 4: A compressed file archive consisting .csv files for performing craniotomy over the whole dorsal cortex and a rectangular craniotomy
Supplementary File 5: A compressed file archive consisting of all CAD files required for assembly of the See-Shell implant
Supplementary Software 1: Setup file for installing and using the Craniobot control software
Supplementary Software 2: Setup file for installing and using the Arduino microcontroller
ACKNOWLEDGEMENTS
SBK acknowledges funds from the Mechanical Engineering department, College of Science and Engineering, MnDRIVE RSAM initiative of the University of Minnesota, Minnesota department of higher education, National Institutes of Health (NIH) 1R21NS103098-01, 1R01NS111028, 1R34NS111654, 1R21NS112886 and 1R21 NS111196. LG was supported by the University of Minnesota Informatics Institute’s (UMII) graduate fellowship. We thank Dr. Eric Yttri, Alan Lai and Mark Nicholas of the Yttri lab at Carnegie Mellon University for useful feedback during beta-testing of the Craniobot. We would also like to thank Luiz Bueno, Dr. York Winter of Labmaker.org who provided insights and suggested improvements to streamline hardware assembly. We would also like to thank Dr. Spencer Smith (@labrigger) for discussions on automated technologies for cranial microsurgeries and the strategies for wide-adoption of such technologies, which partially motivated the documentation of this protocol.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
INSTITUTIONAL APPROVAL
All animal experiments described in this paper were approved by the University of Minnesota’s Institutional Animal Care and Use Committee (IACUC)
CODE AVAILABILITY
We have made the control software available with this article as Supplementary Software 1. Further, a MATLAB version of the same is available at our GitHub repository: www.github.com/bsbrl. We will post updated versions of the software at this location.
DATA AVAILABILITY
All data shown here have been sourced and modified from previous articles describing the Craniobot19 and See-Shells20. Full data is available as supplementary datasets accompanying these articles.
REFERENCES
- 1.Jun JJ et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shobe JL, Claar LD, Parhami S, Bakhurin KI & Masmanidis SC Brain activity mapping at multiple scales with silicon microprobes containing 1,024 electrodes. J. Neurophysiol. (2015). doi: 10.1152/jn.00464.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholvin J et al. Close-Packed Silicon Microelectrodes for Scalable Spatially Oversampled Neural Recording. Biomed. Eng. IEEE Trans. 63, 120–130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berényi A et al. Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J. Neurophysiol. (2014). doi: 10.1152/jn.00785.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voigts J, Siegle J, Pritchett DL & Moore CI The flexDrive: An ultra-light implant for optical control and highly parallel chronic recording of neuronal ensembles in freely moving mice. Front. Syst. Neurosci. (2013). doi: 10.3389/fnsys.2013.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wentz CT et al. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. in Journal of Neural Engineering (2011). doi: 10.1088/1741-2560/8/4/046021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim T Il et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science (80-. ). (2013). doi: 10.1126/science.1232437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong JW et al. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell (2015). doi: 10.1016/j.cell.2015.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCall JG et al. Preparation and implementation of optofluidic neural probes for in vivo wireless pharmacology and optogenetics. Nat. Protoc. (2017). doi: 10.1038/nprot.2016.155 [DOI] [PubMed] [Google Scholar]
- 10.Dzirasa K, Fuentes R, Kumar S, Potes JM & Nicolelis MAL Chronic in vivo multi-circuit neurophysiological recordings in mice. J. Neurosci. Methods (2011). doi: 10.1016/j.jneumeth.2010.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stringer C et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science (80-. ). (2019). doi: 10.1126/science.aav7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sofroniew NJ, Flickinger D, King J & Svoboda K A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. Elife (2016). doi: 10.7554/eLife.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stirman JN, Smith IT, Kudenov MW & Smith SL Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain. Nat. Biotechnol. 34, 857–862 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong DC, Tsai PS & Kleinfeld D All-optical osteotomy to create windows for transcranial imaging in mice. Opt. Express 21, 23160 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TH et al. Long-Term Optical Access to an Estimated One Million Neurons in the Live Mouse Cortex. Cell Rep. 17, 3385–3394 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pak N et al. Closed-loop, ultraprecise, automated craniotomies. J. Neurophysiol. 113, 3943–3953 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pohl BM, Schumacher A & Hofmann UG Towards an automated, minimal invasive, precision craniotomy on small animals. in 2011 5th International IEEE/EMBS Conference on Neural Engineering, NER 2011 302–305 (2011). doi: 10.1109/NER.2011.5910547 [DOI] [Google Scholar]
- 18.Loschak P et al. Cranial Drilling Tool with Retracting Drill Bit Upon Skull Penetration. in ASME Design of Medical Devices Conference (2012). [Google Scholar]
- 19.Ghanbari L et al. Craniobot: A computer numerical controlled robot for cranial microsurgeries. Sci. Rep. 9, 1023 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghanbari L et al. Cortex-wide neural interfacing via transparent polymer skulls. Nat. Commun. 10, 1500 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodandaramaiah SB et al. Multi-neuron intracellular recording in vivo via interacting autopatching robots. Elife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen BD et al. Automated in vivo patch-clamp evaluation of extracellular multielectrode array spike recording capability. J. Neurophysiol. (2018). doi: 10.1152/jn.00650.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtmaat A et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drew PJ et al. Chronic imaging and manipulation of cells and vessels through a polished and reinforced thinned-skull. Nat. Methods 7, 981–984 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shih AY, Mateo C, Drew PJ, Tsai PS & Kleinfeld D A Polished and Reinforced Thinned-skull Window for Long-term Imaging of the Mouse Brain. J. Vis. Exp. 3742 (2012). doi: 10.3791/3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alieva M, Ritsma L, Giedt RJ, Weissleder R & van Rheenen J Imaging windows for long-term intravital imaging. IntraVital (2014). doi: 10.4161/intv.29917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawakami M & Yamamura KI Cranial bone morphometric study among mouse strains. BMC Evol. Biol. (2008). doi: 10.1186/1471-2148-8-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dana H et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS One 9, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng G et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron (2000). doi: 10.1016/S0896-6273(00)00084-2 [DOI] [PubMed] [Google Scholar]
- 30.Kozlowski C & Weimer RM An automated method to quantify microglia morphology and application to monitor activation state longitudinally in vivo. PLoS One (2012). doi: 10.1371/journal.pone.0031814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TH et al. Long-Term Optical Access to an Estimated One Million Neurons in the Live Mouse Cortex. Cell Rep. 17, 3385–3394 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sofroniew NJ, Flickinger D, King J & Svoboda K A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hultman R et al. Brain-wide Electrical Spatiotemporal Dynamics Encode Depression Vulnerability. Cell (2018). doi: 10.1016/j.cell.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sych Y, Chernysheva M, Sumanovski LT & Helmchen F High-density multi-fiber photometry for studying large-scale brain circuit dynamics. Nat. Methods (2019). doi: 10.1038/s41592-019-0400-4 [DOI] [PubMed] [Google Scholar]
- 35.Kim CK et al. Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain. Nat. Methods (2016). doi: 10.1038/nmeth.3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegle JH et al. Open Ephys: An open-source, plugin-based platform for multichannel electrophysiology. J. Neural Eng. (2017). doi: 10.1088/1741-2552/aa5eea [DOI] [PubMed] [Google Scholar]
- 37.Mathis A et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. (2018). doi: 10.1038/s41593-018-0209-y [DOI] [PubMed] [Google Scholar]
- 38.Ghosh KK et al. Miniaturized integration of a fluorescence microscope. Nat. Methods (2011). doi: 10.1038/nmeth.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai DJ et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skocek O et al. High-speed volumetric imaging of neuronal activity in freely moving rodents. Nat. Methods (2018). doi: 10.1038/s41592-018-0008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Groot A et al. NINscope: a versatile miniscope for multi-region circuit investigations. bioRxiv (2019). doi: 10.1101/685909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivasan S et al. Miniaturized microscope with flexible light source input for neuronal imaging and manipulation in freely behaving animals. Biochem. Biophys. Res. Commun. (2019). doi: 10.1016/j.bbrc.2019.07.082 [DOI] [PubMed] [Google Scholar]
- 43.Liang B, Zhang L, Moffitt C, Li Y & Lin DT An open-source automated surgical instrument for microendoscope implantation. J. Neurosci. Methods (2019). doi: 10.1016/j.jneumeth.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khaw I et al. Flat-field illumination for quantitative fluorescence imaging. Opt. Express (2018). doi: 10.1364/oe.26.015276 [DOI] [PubMed] [Google Scholar]
- 45.Adam Y et al. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature (2019). doi: 10.1038/s41586-019-1166-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1: Video demonstrating the use of the Craniobot to perform a 3 mm diameter circular craniotomy centered at 2 mm right and 2 mm posterior to Bregma
Supplementary File 1: A bill of materials in .xlsx spreadsheet format
Supplementary File 2: A compressed file archive consisting of all CAD files of custom fabricated components needed for assembly of the Craniobot and the surface profiler
Supplementary File 3: A file containing the code for the microcontroller to regulate the switching circuit
Supplementary File 4: A compressed file archive consisting .csv files for performing craniotomy over the whole dorsal cortex and a rectangular craniotomy
Supplementary File 5: A compressed file archive consisting of all CAD files required for assembly of the See-Shell implant
Supplementary Software 1: Setup file for installing and using the Craniobot control software
Supplementary Software 2: Setup file for installing and using the Arduino microcontroller
Data Availability Statement
All data shown here have been sourced and modified from previous articles describing the Craniobot19 and See-Shells20. Full data is available as supplementary datasets accompanying these articles.


