Abstract
Opioid use disorder (OUD) is defined as the chronic use or misuse of prescribed or illicitly obtained opioids and is characterized by clinically significant impairment. The etiology of OUD is multifactorial as it is influenced by genetics, environmental factors, stress response and behavior. Given the profound role of the gut microbiome in health and disease states, in recent years there has been a growing interest to explore interactions between the gut microbiome and the central nervous system as a causal link and potential therapeutic source for OUD. This review describes the role of the gut microbiome and opioid-induced immunopathological disturbances at the gut epithelial surface, which collectively contribute to OUD and perpetuate the vicious cycle of addiction and relapse.
Keywords: Opioids, OUD, Microbiome, Gut-brain axis, Enteric nervous system
Introduction
According to the 2019 National Survey on Drug Use and Health, non-medical opioid use in the Unites States is estimated at 2.3% among adolescents (age 12–17), 5.3% among young adults (age 18–25), and 3.6% among adults (age ≥ 26) with the rates of opioid use being significantly higher than that of any other illicit drug (SAMSHA 2020). Consequently, this has resulted in the rise of OUD and a concomitant increase in drug-related overdoses, with increased morbidity and mortality among patients. Additionally, the opioid crisis poses a huge economic burden, partly attributable to higher direct medical costs for patients (Ghate et al. 2010) and indirect costs due to loss of productivity (Reinhart et al. 2018).
OUD is generally a chronic and relapsing illness, involving opioid dependence and addiction as well as withdrawal symptoms when opioids are discontinued. Morbidity and mortality related to OUDs have increased significantly in the past two decades (Vallersnes et al. 2019; Strain 2021). OUDs have substantial negative consequences on mental and physical health, work performance, and social function making OUDs a leading cause of disability in the USA. The disorder also imposes a financial burden; a national study reported healthcare costs were approximately triple among Medicaid patients who misused opioids, compared with Medicaid patients who did not misuse opioids (McAdam-Marx et al. 2010). Although medication for OUD treatment, such as methadone and buprenorphine has been demonstrated to lessen both opioid-associated inpatient costs and overall outpatient visits, compared with healthcare use among untreated patients with OUD (Baser et al. 2011), however, these medications come with their inherent deficiencies leading to higher relapse rates following abstinence. Thus, there is a clear need for effective maintenance and interventional therapies and diagnostics to treat OUD.
Understanding the mechanisms that drive the co-morbidities associated with opioid use are important for battling opioid addiction and for improving patient outcomes. Interestingly, both human and animal studies implicate a crucial role of the gut microbiome in regulating brain development and function, as well as in contributing to behavioral abnormalities associated with opioid use (Collins and Bercik 2009; Heijtz et al. 2011; Clarke et al. 2013; Selkrig et al. 2014; Cussotto et al. 2018; Ren and Lotfipour 2020). This review will summarize recent literature on how alterations in the gut microbiome and opioid-induced immunopathological changes at the gut epithelial surface may influence the central nervous system (CNS) functions, which may contribute to OUD.
Impact of Opioid Exposure on the Gut Microbiome: a Comparison of Human and Pre-Clinical Models
The gut microbiome encompasses trillions of microorganisms (bacteria, archaea, fungi, protists and viruses) residing within the human gastrointestinal tract along with their genes and metabolites. In recent years, there has been a growing interest to explore how opioid use affects the gut microbiome. This is unsurprising as alterations in the gut microbiome have widely been implicated in health and disease states, and the gut microbiome can be significantly altered by dietary and environmental factors (Phillips 2009; De Filippo et al. 2010; Leeming et al. 2019; Scepanovic et al. 2019). Numerous clinical studies have also shown that alterations in the gut microbiome are associated with metabolic disease, inflammation, drug tolerance, dependence, and withdrawal, sharing many commonalities with the adverse consequences of opioid use (Bravo et al. 2011; Le Chatelier et al. 2013; Taylor et al. 2016; Lee et al. 2018; Xiao et al. 2018). Thus, there have been increasing efforts to correlate adverse effects of opioid use with gut microbial changes for therapeutic targeting. This is particularly important in light of the observation that one of the major physiological consequences of opioid use is severe constipation, which is speculatively implicated for morphine induced gut pathologies including barrier compromise and bacterial translocation. In a recent publication however, we show that constipation resulting from non-opioid inducers, e.g. low fiber diet, does not result in gut barrier disruption/bacterial translocation or alteration in the gut microbiome as is observed with chronic opioid use (Banerjee et al. 2016). Given the complexity and confounders of polydrug use and dietary patterns in humans, animal models are commonly used for phylogenetic and metagenomic analyses to examine mechanisms driving host-gut microbiome interactions and their physiological consequences. Among these animal models, rodents, particularly mice and rats, have commonly been used for economic reasons, with non-human primates (NHPs) also gaining popularity due to their genetic and physiological closeness to humans (Nagpal et al. 2018).
These human and preclinical models have shown considerable variability in describing the microbial composition with opioid use, but altogether consistently point towards microbial dysbiosis (Table 1). Clinical studies of chronic opioid users have shown reduction in the family Bacteroidacea or the genus Bacteroides from the phylum Bacteroidetes (Acharya et al. 2017; Xu et al. 2017). However, the abundance of Prevotella has varied between studies (Xu et al. 2017; Barengolts et al. 2018). In the phylum, Firmicutes, whereas Clostridiales, particularly Ruminococcacea was decreased in abundance (Acharya et al. 2017), increases in Ruminococcus (Xu et al. 2017) and Lactobacillales have also been reported. Additionally, increased abundance of Bifidobacterium from phylum Actinobacteria has also been reported (Barengolts et al. 2018). Investigations utilizing NHPs to explore microbial changes with opioid use are few. In one study utilizing NHPs, opioid use primarily resulted in an increase in Methanobacteriaceae, and a decrease in abundance of both Streptococcaceae and Ruminococcaceae from the phylum Firmicutes. Notably, the decrease in Ruminococcaceae was consistent with Acharya et al. (2017), though increases in Ruminococcus have been found in other human studies (Xu et al. 2017). The vast majority of animal models of opioid exposure have been conducted in rodents, in particular mice. Changes in microbial communities were most consistent between studies based on experimental treatment. For instance, with subcutaneous 25 mg slow release morphine pellets, decreased abundance of Bacteroidetes (Banerjee et al. 2016) and Bacteriodales (Kang et al. 2017) from the phylum Bacteroidetes was observed. In contrast, there was increased abundance in the entire phylum Firmicutes (Banerjee et al. 2016) or specifically the genus Staphylococcus and Enterococcus (Meng et al. 2015). On the other hand, with a 75 mg slow release pellet, decreased abundance of Clostridiales and Lactobacillales in the phylum Firmicutes, and increased abundance of Enterobacteriales from phylum Proteobacteria was reported (Kang et al. 2017). Intraperitoneal injections consistently resulted in a decrease in Lactobacillus or Lactobacillaceae (Lee et al. 2018; Zhang et al. 2019) from phylum Firmicutes, but an increased abundance of Ruminococcus (Lee et al. 2018). Hydromorphone exposure in mice paralleled findings of opioid exposure in rats, with decreased abundance of Firmicutes and increased abundance of Verrucomicrobia (O’Sullivan et al. 2019; Sharma et al. 2020; Simpson et al. 2020) and Proteobacteria (Sharma et al. 2020; Simpson et al. 2020), though the abundance of Bacteroidetes varied in these studies (O’Sullivan et al. 2019; Simpson et al. 2020).
Table 1.
Summary of studies indicating opioid use associated alteration in gut microbiota and its phenotypic response
| Substance used | Species | Treatment/stage | Change in Microbiome | Phenotypic response | Reference |
|---|---|---|---|---|---|
|
| |||||
| Multi-opioids | Human | Chronic user outpatients and Inpatients | ↓ Bacteroidaceae ↓ Clostridiales XI ↓ Ruminococcaceae |
Cirrhosis and readmissions | (Acharya et al. 2017) |
| Multi-opioids | Human | Chronic user outpatients | ↑ Bifidobacterium ↓ Prevotella copri ↑ Lactobacillales |
Type 2 diabetes (T2D) and psychiatric conditions | (Barengolts et al. 2018) |
| Multi-opioids | Human | Chronic user | ↓ Bacteroides ↑ Prevotella ↑ Ruminococcus |
Substance use addiction | (Xu et al. 2017) |
| Morphine | NHP | Intramuscular Injections (21d) |
↑ Methanobacteriaceae ↓ Streptococcaceae ↓ Ruminococcaceae |
Increase inflammation | (Sindberg et al. 2019) |
| Morphine | Mouse | Subcutaneous slow release pellet (25 mg, 3d) | ↑ Staphylococcus ↑ Enterococcus |
Inflammation and sepsis | (Meng et al. 2015) |
| Morphine | Mouse | Intraperitoneal injections (10 mg/kg, every 12 h, 4 d) | ↑ Ruminococcus ↓ Lactobacillus |
Hyperalgesia, and impaired reward response | (Lee et al. 2018) |
| Morphine | Mouse | Subcutaneous slow release pellet (75 mg, 5d) | ↓ Clostridiales ↓ Lactobacillales ↓ Bacteriodales ↑ Enterobacteriales |
Morphine antinociceptive tolerance | (Kang et al. 2017) |
| Morphine | Mouse | Subcutaneous slow release pellet (25 mg, 3d) | ↑ Enterococcus faecalis | Augment analgesic tolerance | (Wang et al. 2018) |
| Morphine | Mouse | Intraperitoneal injections (15 mg/kg, 8d) | ↓ Bifidobacteria ↓ Lactobacillaceae |
Develop morphine analgesic tolerance | (Zhang et al. 2019) |
| Morphine | Mouse | Subcutaneous slow release pellet (25 mg, 2d) | ↑ Firmicutes ↓ Bacteroidetes |
Gut barrier disruption and systemic inflammation | (Banerjee et al. 2016) |
| Morphine | Rat | Subcutaneous slow release pellet (75 mg) | ↓ Firmicutes ↑ Bacteroidetes ↓ Actinobacteria ↑ Verrucomicrobia |
Opioid withdrawal | (O’Sullivan et al. 2019) |
| Hydromorphone | Mouse | Intraperitoneal injections (7.5 mg/kg, every 12 h, 7 d) | ↓ Firmicutes ↑ Proteobacteria ↑ Verrucomicrobia |
Increase gut and systemic inflammation | (Sharma et al. 2020) |
| Oxycodone | Rat | Subcutaneous injections (2 mg/ kg, every 12 h for 5 d) | ↓ Bacteroidetes ↓ Firmicutes ↑ Cyanobacteria ↑ Proteobacteria ↑ Verrucomicrobia |
Opioid dependence | (Simpson et al. 2020) |
Which preclinical models lend themselves most relevant to clinical translation of how opioid use affects the human microbiota still remains unclear. Species comparisons and extrapolation to humans is difficult to conduct due to the confounding effects of sampling locations, providers, rearing facilities, genetic backgrounds, geographical settings, experimental protocols, and sequencing analysis approaches (Hugenholtz and de Vos 2018; Nagpal et al. 2018; Park and Im 2020). This is compounded by the high inter-individual variability in human gut microbial signatures due to dietary behavior and lifestyles. At the phylum level, clinical studies have shown alterations in Bacteroidetes, Firmicutes, and Actinobacteria with opioid use, consistent with rodent studies. In particular, there seems to be a consistently decreased abundance from the phylum Bacteroidetes in both human and rodent studies. Though the overall β-diversity of gut microbiota signatures suggest that the gut microbiota in humans is closer to NHPs than to mice and rats, with mice closer to humans than rats, the limited number of studies utilizing NHP prevented seeing this close association (Nagpal et al. 2018). In contrast, several other studies claim rat microbiome being more closer to human than mice and therefore gut microbiome of humanized rat models provide better representation of human donor than mouse model (Wos-Oxley et al. 2012; Nguyen et al. 2015). Thus, a comprehensive investigation of how opioid use affects the intestinal bacterial diversity and composition in NHPs, along with humans and rodents, is much warranted.
The Gut Microbiome, Metabolome and the CNS in Opioid Use Disorder
Alterations in the gut microbiome have been implicated in various neuropsychiatric disorders, including OUD (Bercik et al. 2011; Kiraly et al. 2016; Han et al. 2018; Meckel and Kiraly 2019; Zhuang et al. 2020). A recent study showed differential alteration in gut microbial communities during acquisition, extinction and reinstatement of morphine conditioned-place preference (CPP) (Zhang et al. 2021). In this study, the gut bacterial community richness in mice was shown to increase following morphine CPP, whereas it decreases with prolonged abstinence. In addition, an increased abundance of Verrucomicrobia and decreased Bacteriodes abundance was observed during the acquisition stage. Interestingly, this shift in bacterial abundance showed a trend to recovery during the extinction stage. Consistent with these findings, another study found a correlation between the gut microbial composition of rats to their sensitivity to morphine CPP (Zhang et al. 2020a) where the relative abundance of certain taxa such as Alloprevotella and Romboutsia correlated to the morphine CPP scores. More recently, Thomaz et al. showed an attenuation in morphine withdrawal behavior in mice following antibiotic treatment or fecal microbiota transplant (Thomaz et al. 2021). Together, these studies indicate that the gut microbiome impacts the development of physical dependence to opioids, and that therapeutic interventions aimed at manipulating the gut microbiome may reduce somatic symptoms of opioid withdrawal.
Growing evidence suggest a strong correlation between gut dysbiosis (particularly a decreased Firmicutes to Bacteroides ratio) and neuroinflammation observed in opioid withdrawal (O’Sullivan et al. 2019). In addition, Burma et al. demonstrated that microglial cells mediate opioid withdrawal by activating P2X7 receptors, which triggers the release of ATP through the PANX1 channel and postsynaptic facilitation in the spinal dorsal horn (Burma et al. 2017). In this study, pharmacological blockade of PANX1 channels using probenecid or mefloquine suppressed ATP release and reduced withdrawal severity. The involvement of microglial cells in opioid dependence was further strengthened by a recent study by Reiss et al. who showed a crucial role for the microglial μ- opioid receptors (MORs) in morphine analgesic tolerance, hyperalgesia and withdrawal in mice (Reiss et al. 2020). In this study, morphine analgesic tolerance in the hot plate assay was delayed in male and female mice lacking MORs in microglia. Interestingly, they also found a sex-dependent contribution of microglial MOR in opioid-induced hyperalgesia and physical dependence.
An increasing body of evidence supports a critical connection between gut microbiota and the development and function of microglia. The importance of this association is evidenced by the global microglial defects observed in mice with a compromised microbiome, including germ-free (GF) mice (Erny et al. 2015), antibiotic-treated mice, and mice exposed to morphine (Lee et al. 2018). GF mice fail to develop microglia with a mature phenotype, indicated by prominent expression of surface molecules such as CSF1R, F4/80, and CD31 that are downregulated over the course of maturity (Erny et al. 2015). Upon histopathological examination, the microglia of GF mice show elongated processes, increases in cell volume and number, and intrusions into neighboring cell territories, among other morphological irregularities (Erny et al. 2015). Analysis of microglial-mRNA expression reveals downregulation of genes associated with cell activation, transcription activation, and pathogen recognition along with upregulation of genes involved with inhibition of transcription and apoptosis, cell cycle stimulation, and cell proliferation (Erny et al. 2015). The consequences of gut dysbiosis on microglia are not limited to development. Perturbations to the gut microbiome caused by chronic treatment of adult mice with antibiotics produce morphological impairments in microglia that are comparable to those seen in GF mice (Erny et al. 2015), suggesting that a healthy and complex gut microbiome is integral not just for the development, but also the homeostasis of microglia. Intermittent treatment with morphine similarly induces a state of gut dysbiosis that is associated with an altered microglial phenotype and behavioral deficits that mirror those observed in antibiotic-treated mice (Lee et al. 2018). The physiological impact of these collective defects in microglia manifests notably in diminished immune responsiveness. GF mice administered with an lipopolysaccharide (LPS) challenge, either intracerebrally or intraperitoneally, show reduced induction of pro-inflammatory cytokines and chemokines as compared to mice with a more complex gut microbiome (Erny et al. 2015). A viral challenge to GF mice similarly results in microglial morphological malformations accompanied by an impaired immune response of pro-inflammatory cytokines (Erny et al. 2015). Collectively, these results suggest that a depleted gut microbiome not only impairs microglial development, but also attenuates resistance to bacterial or viral infection. Remarkably, these impairments are reversible by several manipulations. Microglial defects in mice with a disrupted gut microbiome are at least partly restored by recolonization with a complex microbiome (by microbiota transplant or cohousing with treatment naïve mice), normalizing cell morphology, mRNA levels, and downstream behavioral effects (Erny et al. 2015; Lee et al. 2018). Alternatively, defects in microglial morphology and gene expression can be largely restored with diet supplementation with short chain fatty acids (SCFAs) (Erny et al. 2015), microbial metabolites that are vital to colonic immune cell regulation. The possibility of rescuing microglial deficits by microbe recolonization or SCFA supplementation suggests a potential for therapeutic intervention of microglial pathology in various disease states.
Although the exact mechanisms underlying the gut-microglial axis remain ambiguous, several pathways of cross-talk are recognized. Principally, gut bacteria and their neuroactive metabolites can interact with the vagus nerve and affect microglial-induced inflammation in the CNS (Forsythe et al. 2014). When an LPS challenge is combined with vagal nerve stimulation, microglial production of pro-inflammatory cytokines is attenuated as compared to an LPS challenge alone (Meneses et al. 2016). This effect is abolished following vagotomy (Meneses et al. 2016). Gut microbiota can also modulate intestinal barrier permeability, enabling the entry of immune-stimulating substances into systematic circulation (Karczewski et al. 2010). Additionally, microbiota can directly stimulate immune cells in the gut, altering peripheral inflammation (Fung et al. 2017; Abdel-Haq et al. 2019). Metabolites of gut microbiota, including neurotransmitters like serotonin (Yano et al. 2015) and acetylcholine, and SCFAs (Conn et al. 1983) can enter circulation and pass the blood brain barrier, altering levels of inflammation in the CNS (Glebov et al. 2015). The relative contributions of gut microbes in influencing pathways involved in microglial activation remain undetermined.
While the relationship between the gut microbiome and microglia is not fully understood, alterations in gut microbiota are strongly implicated in the various stages of OUD. For instance, elevated microglial activation has been observed with drug use and has been shown to play a role in tolerance, anxiety, and withdrawal (Reiss et al. 2020). Accordingly, as gut microbiota can modulate microglial activation, the microbiome may be a mechanism underlying elevated microglia activation both during and after drug use, though this remains to be studied.
Emerging data from research on gut microbiome and its influence on CNS focuses on the role of microbial metabolites in modulating nervous health and behavior (Table 2). Microbial metabolites are absorbed in gut and enters systemic circulation and reach distant organs including brain to exert its effects and acts as a messenger for the “gut-brain-axis”. Opioid treatment has also been reported to change level of bacterial metabolites mainly bile acids (BAs) and SCFA. Primary BA are synthesized from cholesterol in hepatocytes; intestinal bacteria transform primary BA into secondary BA through the use of the bacterial enzymes bile salt hydrolase (BSH) and hydroxysteroid dehydrogenase (HSDH). Firmicutes, Bacteroides, Eubacterium and Clostridium express the BSH enzyme which is required to deconjugate taurine and glycine conjugated primary and secondary BAs. Enteric bacteria expressing the HSDH enzyme required for the conversion of primary to secondary BA includes Bacteroides, Eubacterium, Clostridium, Lactobacillus and Escherichia. Bile acid has been shown to modulate tight junction and altered levels of BAs can lead to blood brain barrier (BBB) permeabilization (Raimondi et al. 2008; Quinn et al. 2014). In rodents, morphine treatment decreases primary and secondary BA levels in the fecal content (Wang et al. 2018). Whereas non-human primate studies consistently show a decrease in primary BA including cholate and glycocholate. In contrast, increases in several secondary BAs have also been reported (Sindberg et al. 2019). Decrease in primary BA after morphine treatment can be a direct host response to morphine treatment. However, since gut bacteria play crucial role for conversion of primary BAs to secondary BAs, therefore, difference in bacterial community changes observed after morphine treatment can account for the contrasting secondary BA profile in both species.
Table 2.
Summary of studies showing role of bacterial metabolites on nervous health and behavior
| Bacterial metabolite | Class | Study Model | Host response | Reference |
|---|---|---|---|---|
|
| ||||
| CDCA and DCA | Bile Acid | BA injection in Sprague Dawley rats | Increased BBB permeability | (Quinn et al. 2014) |
| Obeticholic acid (OCA) | Bile Acid | Oral gavage of BA in HFHS (high fat, high sugar) fed mice | Improve metabolic disorder driven Anxiety | (Wu et al. 2021) |
| Sodium propionate, sodium butyrate and sodium acetate | SCFA | Added to drinking water in mice | Increased microglial maturation and function | (Erny et al. 2015) |
| Sodium butyrate | SCFA | BTBR autism mouse model | Attenuates social behavior deficits | (Kratsman et al. 2016) |
| Sodium butyrate | SCFA | Chronic mild stress model in Wistar rats | Anti-depressant and anti-manic properties | (Resende et al. 2013) |
| Propionate | SCFA | Experimental autoimmune encephalomyelitis | Ameliorate experimental autoimmune encephalomyelitis (EAE) disease course | (Haghikia et al. 2015) |
| P-cresol | Aromatic acid metabolite | BTBR autism mouse model | Triggers anxiety and hyperactivity at lower doses and exacerbate autism like behavior at higher dose | (Pascucci et al. 2020) |
| TMAO | Amine oxide | C57Bl/6 mice on low-choline diet supplemented with TMAO | Induced neuroinflammation and decline in cognitive function | (Brunt et al. 2021) |
| Tryptophan | Amino acid | EAE mouse model of multiple sclerosis | Decreased neuroinflammation and decreased EAE score | (Rothhammer et al. 2016) |
SCFA are bacterial fermentation products; the main SCFA producing bacteria in the human gut belong to the family Ruminococcaceae and family Lachnospiraceae and in mice, Muribaculaceae, Lachnospiraceae, and Ruminococcaceae. Studies have established the role of SCFA in modulating gastrointestinal function, neuro-immune regulation, host metabolism, BBB integrity (Table 2). In stool samples of individuals on methadone maintenance treatment, reduced levels of SCFAs along with a depletion of SCFA producing bacteria Bifidobacteria and Akkermensia municiphila have been reported (Cruz-Lebron et al. 2021). Additionally, recent studies further demonstrate the potential role of SCFA in mediating morphine reward pathways (Hofford et al. 2021). Interestingly, antinociceptive tolerance in morphine treated mice has shown to be prevented with oral butyrate treatment, highlighting the impact of SCFA reconstitution following morphine treatment (Akbarali and Dewey 2019; Meng et al. 2019).
Studies exploring the role of other bacterial metabolites including tryptophan and indole derivatives, trimethylamine N-oxide (TMAO), and amino acids are sparse. Additionally, how opioid treatment modulates SCFAs and other bacterial metabolites also remains understudied.
Opioids, Microbiome and Blood Brain Barrier
CNS is protected by the BBB which keeps it safe from different pathogens and toxic molecules. Anatomically, the BBB is formed by the cerebrovascular endothelial cells sealed by tight junctions, pericytes and foot processes of astrocytes. The tight junctions are impermeable for most of the substances and specialized transporters are required for the movement of molecules into the brain (Pardridge 2007). Opioids can cross BBB to exert it analgesic effects mostly by binding to MOR in CNS. P glycoprotein (P-gp) is one of the efflux transporters at BBB and morphine and other opioids act as substrate for P-gp (Bostrom et al. 2008). Long term exposure to morphine can up-regulate expression of P-gp transporter which may contribute towards increased BBB permeability and morphine tolerance (Mahajan et al. 2008; Chaves et al. 2017). An in-vitro study showing morphine treatment reduced the expression of tight junction protein (TJP) zona occludens-1 (ZO-1) in human brain microvascular endothelial cells through platelet derived growth factor (PDGF) and MOR provides mechanistic insight into the role of morphine in BBB damage (Wen et al. 2011). On the contrary, study on Sprague–Dawley rats showed that morphine treatment did not alter BBB permeability during dependence stage, however, morphine withdrawal causes significant breakdown in BBB resulting in neuronal damage seen in animals undergoing withdrawal (Sharma and Ali 2006). BBB structure and function can also be impacted by gut microbial composition and metabolic profile (Braniste et al. 2014). Therefore, changes in gut microbial composition may affect the CNS function through BBB permeability. However, research on the role of dysbiotic community on BBB structure and function is sparse.
The gut microbiome plays key role in maintaining BBB integrity as is indicated in studies where GF mice have been shown to have increased BBB permeability compared to pathogen-free mice with normal gut flora. Braniste et al. have demonstrated that TJPs, occludin and claudin-5 levels were significantly lower in several brain regions of GF mice compared to control mice which is the contributing factor towards BBB permeability (Braniste et al. 2014). Furthermore, they showed that administration of normal gut flora from pathogen-free mice or oral treatment with the bacterial metabolite sodium butyrate to GF adult mice induced an increase in the expression of occludin (Braniste et al. 2014). Altogether, these observations suggest that the BBB integrity and the expression of tight junction protein occludin in the cerebrovascular endothelial cells are sensitive to changes in the intestinal microflora.
Microbial metabolites, such as SCFAs, and neurotransmitters that enter the systemic circulation and influence different organs including brain. SCFAs can cross the BBB via monocarboxylate transporters located on endothelial cells and influence BBB integrity by upregulating the expression of TJPs (Silva et al. 2020). γ-Aminobutyric acid (GABA) is a very well know inhibitory neurotransmitter in the central nervous system. GABA transporters (GAT2/BGT-1) is present at the BBB and is involved in GABA efflux transport from the brain into the circulating blood across the BBB (Takanaga et al. 2001). However, whether microbial-derived GABA can cross BBB and influx into the brain remains a matter of dispute (Boonstra et al. 2015).
Toll like receptor (TLR) that play a crucial role in recognizing molecules derived from viruses, fungi, bacteria, and protozoa has also been shown to regulate BBB permeability (Nagyoszi et al. 2010; Li et al. 2013; Johnson et al. 2018). Johnson et al. investigated the global responsiveness of human cerebral microvascular endothelial cells (hCMVECs) to a panel of commercial TLR ligands. They observed responses to the TLR3 ligand Poly(I:C), TLR4 ligand LPS and to the TLR7 ligand Imiquimod, but not to any of the other TLR ligands (Johnson et al. 2018). In the same study they measured endothelial barrier function using Electric-cell Substrate Impedance Sensing (ECIS) and demonstrated that Poly(I:C) and LPS both caused an acute reduction in barrier strength, whereas Imiquimod caused an immediate and sustained strengthening of the barrier. Nagyőszi et al. demonstrated the expression of TLR2, TLR3, TLR4 and TLR6 on rat and human cerebral endothelial cells. Oxidative stress significantly upregulated the expression of these receptors whereas tumor necrosis factor-α (TNF-α) upregulated the expression of TLR2 and TLR3. Furthermore, they found, that activation of TLR2/6 leads to an increased permeability which is accompanied by a downregulation of occludin and claudin-5 expression and disappearance of these TJPs from the cell membrane (Nagyoszi et al. 2010). Since morphine treatment has been shown to change expression of TLRs in intestinal tissue, future research should focus on studying how opioids change TLR expression in BBB which can have direct impact on its permeability.
Transport of blood-borne cytokines across the BBB is now known to be an operational pathway by which immune system can directly affect CNS functions (Banks 2005, 2015). Astrocytes through their end-feet provides structural support to BBB and maintain homeostasis in brain (Abbott et al. 2006). In-vitro study using NT2 cell line showed that activation of astrocytes by inflammatory mediators such as TNFα and interleukin-1β (IL-1β) lead to transient increase in release of several pro-inflammatory cytokines as well as several leukocytes chemoattractant which lead to astrocyte cell death, compromising BBB integrity (Burkert et al. 2012; van Kralingen et al. 2013). Cellular response of endothelial cell using human cerebral microvascular endothelial cells (hCMVECs) to the pro-inflammatory mediators TNFα and IL1β has also been studied. O’Carroll et al. demonstrated that expression of leukocyte adhesion molecules ICAM-1 and VCAM-1 is up-regulated in endothelial cells after TNFα and IL-1β treatment (O’Carroll et al. 2015). Along with that they also observed an increased secretion of several leukocyte recruitment chemokines after stimulation with TNFα or IL-1β.
Opioid-Induced-Gut-Dysbiosis in Epithelial Damage (Tight Junctions), Gut Permeability, Systemic Translocation and Regeneration
On a microscopic level, opioid induced gut dysbiosis has also been shown to contribute to tolerance and withdrawal behaviors, originating from structural changes to the gut epithelium itself. Multiple studies have shown that opioid treatment compromises intestinal barrier function and promotes inflammatory responses in cecal ligation, puncture-induced sepsis, and dextran sulfate sodium (DSS)-induced colitis models (Meng et al. 2015; Sharma et al. 2020). Interactions between the gut microbiota and the gut epithelial barrier play an important role in host homeostasis; compromised gut barrier integrity allow bacteria and their toxic products to enter systemic circulation, triggering a systemic inflammatory response and neuroinflammation. Such neuroinflammation has been shown to contribute to the development of withdrawal and tolerance associated with chronic opioid use (Taylor et al. 2015; Lee et al. 2018; Zhang et al. 2019).
While the effects of opioids on the integrity of intestinal barrier have not been fully elucidated, abundant evidence implicates intracellular cross talk between MOR signaling and tight junction dynamics through TLR signaling (Fig. 1) (Meng et al. 2013; Zhang et al. 2020b). Intestinal barrier integrity is regulated by multiple factors including the organization of TJPs and the renewal of the epithelium by intestinal stem cells. TJPs between intestinal epithelial cells selectively allow the transport of nutrients, electrolytes, water, and some macromolecules, while excluding potentially harmful substances (Camilleri et al. 2012; Buckley and Turner 2018). TJPs include transmembrane and paracellular proteins. The transmembrane molecules such as the claudin family members and the occludin are responsible for sealing the paracellular pathway between the epithelial cells. The scaffold proteins such as ZO-1 andZO-2 cross-link and anchor the transmembrane proteins (Peterson and Artis 2014). Disruption of TJP organization compromises the intestinal barrier function and is associated with a variety of infectious and inflammatory diseases such as Crohn’s disease, ulcerative colitis and celiac disease (Schulzke et al. 2009). Of note, TLR signaling is well known to regulate intestinal permeability by modulating intestinal TJP organization through direct and indirect mechanisms. For example, activation of TLR4 by LPS increases the permeability of the intestinal cell monolayer by disrupting the complex consisting of TJPs, F-actins and myosin in a myosin light chain kinase (MLCK) dependent manner (Forsythe et al. 2002). TLR signaling is also involved in gut barrier modulation indirectly by regulating the expression levels of different proinflammatory cytokines such as TNF-α, IL-1β, interferon-γ (IFN-γ), and interleukin-6 (IL-6), which leads to increased permeability by altering the expression and localization of TJPs (Desai et al. 2002; Bruewer et al. 2003; Tazuke et al. 2003; Utech et al. 2005; Suzuki et al. 2011). Interestingly, previous studies have shown that morphine can disrupt intestinal barrier function and damage TJP organization via modulation of MLCK in a TLR dependent manner (Meng et al. 2013).
Fig. 1.
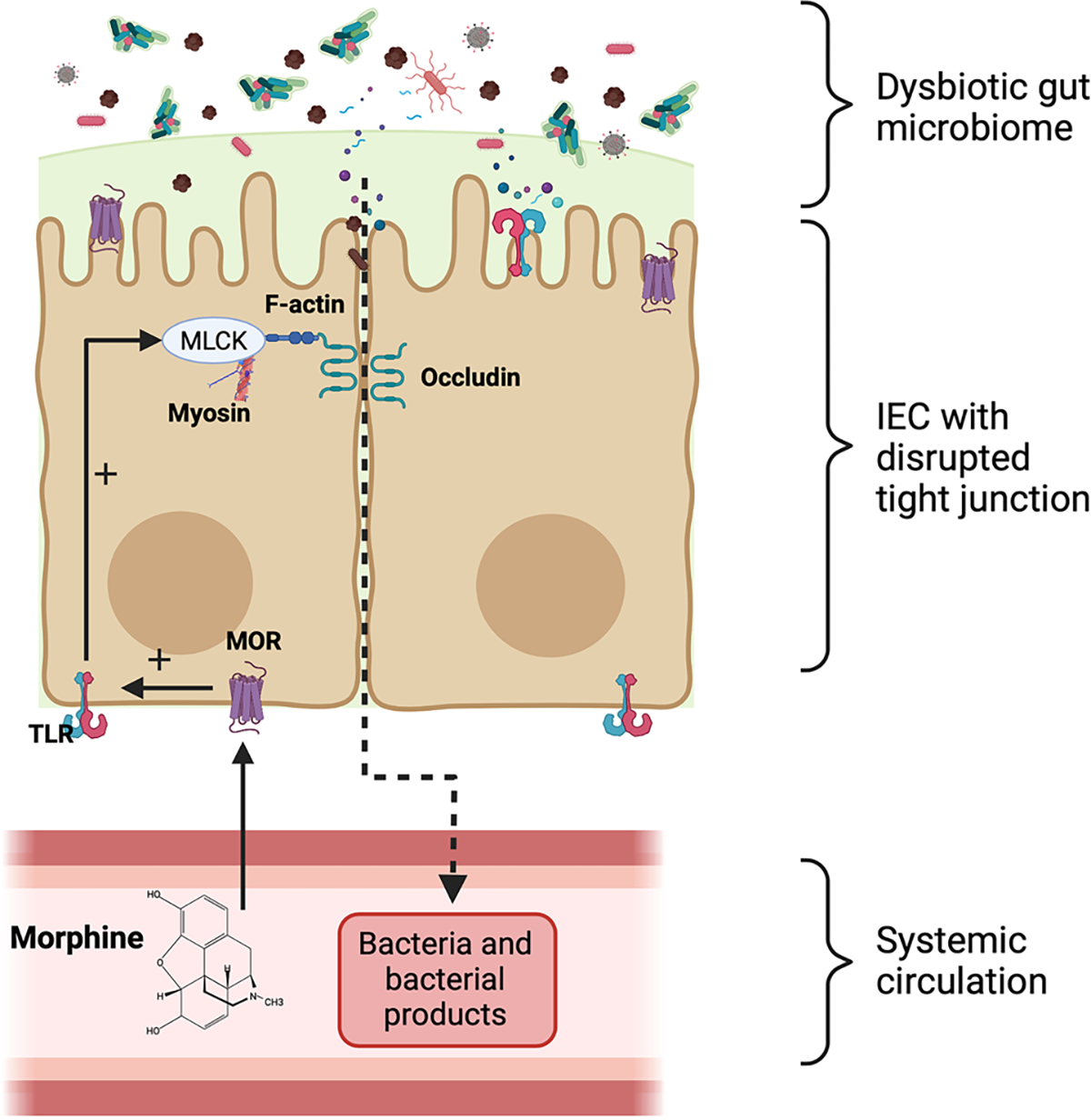
Morphine treatment upregulates TLR expression in intestinal tissue. Increased TLR signaling in intestinal epithelial cells leads to MLCK induced tight junction redistribution and increase intestinal permeability. (Adapted and modified from Meng et al. 2013). (IEC, Intestinal epithelial cell)
Increased epithelial cell death and deficient crypt tissue repair also contribute to disruption of the normal intestinal barrier. The role of opioid agonists in intestinal tissue recovery are complex. In humanized bone marrow-liver-thymus (HuBLT) mice, morphine treatment inhibits the proliferation of crypt stem cells by inhibiting Notch signaling (Meng et al. 2019). In contrast, MOR-specific agonist [D-Arg2,Lys4] dermorphin-(1,4)-amide (DALDA) has also been shown to attenuate intestinal injury in DSS-induced colitis and ischemia reperfusion models (Goldsmith et al. 2013). Such different outcomes might be explained by pathway-selective signaling of the opioid receptor. In line, many studies have shown that different opioid agonists can differentially activate various signaling pathways, although all opioid agonists bind to the MOR (Zheng et al. 2010). Therefore, further studies are needed to understand how microbiota modulate different opioid signaling pathways and specifically, the consequence to intestinal physiology.
Globally, opioid-induced gut epithelial damage has been associated with lowered host defenses to various diseases. For instance, opioid treatment in mice has been shown to disrupt the gut epithelial barrier resulting in systemic bacterial translocation to the spleen, liver and peritoneum (Hilburger et al. 1997; Meng et al. 2015). In line, multiple clinical studies have also reported that opioid exposure is associated with intestinal barrier dysfunction, increased intestinal permeability, and increased morbidity when exposed to various infectious diseases. For example, a retrospective analysis indicated that opioid-treated patients hospitalized with a diagnosis of sepsis had a significantly higher risk of death (Zhang et al. 2018). Animal studies have further shown that both morphine treatment and morphine withdrawal lower host defense to enteric bacteria including Salmonella enterica, Pseudomonas aeruginosa, and Citrobacter rodentium (a murine mucosal pathogen that shares the similar virulence factors with the related human pathogens enteropathogenic Escherichia coli and enterohemorrhagic E. coli) (Feng et al. 2006; Breslow et al. 2010; Babrowski et al. 2012; Wang et al. 2020a). Interestingly, opioid antagonist naltrexone has been shown to block acute endotoxic shock by inhibiting the production of proinflammatory cytokine TNF-α (Greeneltch et al. 2004). Thus, exploring mechanisms underlying disrupted epithelial barrier function caused by morphine treatment is of great interest.
Opioid Modulation of the Gut Microbiome and its Impact on Immune System
Opioids are well known to affect the innate and adaptive immune system through direct interactions with immune cells via opioid receptors or indirect interactions via bacterial metabolites. Immune cells have been reported to express μ-, κ- and σ- opioid receptors along with non-classical opioid like receptors, allowing them to mount varied responses in immune cell types. Additionally, human peripheral blood mononuclear cells (PBMCs) and platelets have been shown to have morphine binding mu opioid receptors (Mehrishi and Mills 1983). In the past few decades, there has been increasing efforts to elucidate mucosal immune responses to opioids.
Such investigations have shown that opioids can directly affect cells principally involved in innate immunity. For instance, opioids can alter chemokine production in macrophages, with studies showing increased IL-6, IL-12 and TNFα production after morphine treatment (Roy et al. 1998; Peng et al. 2000). MOR agonist treatment in human PBMCs also show a significant increase in MCP-1, RANTES, and IP-10 chemokine RNA and protein levels (Wetzel et al. 2000). Recent studies using methadone, a widely used opioid receptor agonist, show reduced expression of interferons and interferon stimulated anti-HIV genes in macrophages (Wang et al. 2020b). Signaling through μ-, κ- and σ- opioid receptors has also been shown to exert varied immune responses in dendritic cells (DCs) (Makarenkova et al. 2001; Kirst et al. 2002). κ-opioid receptors agonists dynorphin A and U50,488H suppress the ability of DCs to induce T-cell proliferation in a concentration dependent manner (Kirst et al. 2002). However, antigen uptake and maturation of DCs remained unchanged. Recent studies have further shown that morphine treatment exerts immuno-stimulatory effects on DCs and enhanced T cell stimulatory capacity of LPS matured DCs, further showing significant modulation of innate immune cells by opioids (Messmer et al. 2006).
Additionally, there is great consensus that acute and chronic opioid treatment significantly affects adaptive immunity. For instance, chronic morphine treatment leads to an increase in circulating Treg cells and the functional activity of Th17 cells (Cornwell et al. 2013). Prolonged opioid treatment has long been associated with impaired T-cell function, increased T-cell apoptosis, modified T cell differentiation and altered cytokine response (Bryant and Roudebush 1990; Singhal et al. 1999, 2001; Sacerdote et al. 2000; Han et al. 2020). Contrary to the previously believed immunosuppressive role of opioids, recent research indicates a dual effect of opioids. Activation of different opioid receptors on immune cells might demonstrate contrasting and inverse effects on immune cells. Furthermore, different opioid drugs have been shown to have differential immunomodulatory properties. For example, MOR signaling in T cell lymphocytes by fentanyl, methadone and beta endorphins results in strong induction of IL4, whereas treatment with morphine and buprenorphine leads to significantly lowered levels of IL4 mRNA and protein, which suggests agonist biased MOR signaling in T cells (Borner et al. 2013). Opioids have also been shown to affect B lymphocytes, which are major contributors of humoral immunity. In rodent models, morphine pelleting or intraperitoneal morphine treatment causes reduced mitogenic response to bacterial LPS in B cells (Bryant et al. 1988; Bhargava et al. 1994). Furthermore, in regards to B cell function, opioids have been reported to have variable effects on immunoglobulin production, which may be dependent on specific opioid receptor agonists. In an in-vivo study, μ-opioid receptor agonist treatment resulted in an increase in IgM and IgG; on the contrary treatment with an σ-opioid receptor agonist lead to immunosuppression with reduced IgG and IgM production (Cheido et al. 2014). Rodent studies have also shown that morphine treatment reduces major histocompatibility complex II (MHCII) expression levels on B lymphocytes by 33% and that this effect is mediated by the hypothalamic–pituitary–adrenal (HPA) axis (Nugent et al. 2011). Since MHCII is an important cell communicator for immune cells, morphine mediated decrease in MHCII expression may contribute to the immunosuppressive effects of morphine.
Apart from direct interactions of opioids on immune cells through opioid receptors, opioids can also influence immune cells indirectly through bacterial metabolites. Most attention has been paid to the role of BAs and SCFAs in modulating immune cell response. BAs directly participate in gut mucosal defense due to their bactericidal properties (Watanabe et al. 2017). The role of BA signaling in regulating intestinal homeostasis is further confirmed by the development of severe colitis in BA receptor knock out TGR5−/− as well as FXR−/− mouse models (Renga et al. 2013; Biagioli et al. 2017).
Other bacterial metabolites such as SCFA also have essential roles in regulating mucosal immune homeostasis. SCFA are transported across intestinal epithelial cells via monocarboxylate transporter (MCT1) and slc58a (SMCT) to enter systemic circulation (Iwanaga et al. 2006). SCFA receptors including G-protein coupled receptors (GPCR) known as free fatty acid receptors (FFAR 2 or GPCR 43), FFAR 3 (GPCR 41) GPR 109A and olfactory receptor 78 (OLF 78) have been shown to be present on intestinal L-cells and innate as well as adaptive immune cells (Kimura et al. 2011; Tolhurst et al. 2012; Singh et al. 2014; Luu et al. 2018). SCFA not only acts as a major source of energy for intestinal epithelial cell, but they also promote intestinal epithelial barrier function (Kelly et al. 2015; Park et al. 2016). SCFAs, particularly butyrate also have been shown to have several immunomodulatory functions that have been extensively studied and reviewed (Cox et al. 2009; Arpaia et al. 2013; Chang et al. 2014; Singh et al. 2014; Nastasi et al. 2015; Deleu et al. 2021; Simpson et al. 2021) and fecal SCFA levels in inflammatory bowel disease (IBD) samples have shown to be reduced (Kaczmarczyk et al. 2021).
So far, opioid treatment has been established to alter levels of SCFA and BAs but the impact of opioids on other bacterial metabolites is still understudied. Additionally, the impact of metabolic environment on host immune response further needs to be explored. With current gaps in knowledge, future research should focus on identifying key metabolites markers changing after opioid use which can be targeted therapeutically for OUD.
Opioids, Gut Microbiota and the Enteric Nervous System (ENS)
Opioid use and the gut microbiota may also influence ENS functions at the gut epithelium. The gastrointestinal (GI) tract is innervated by a complex network of neurons and glial cells, which are bundled together in the submucosal (Meissner) and the myenteric (Auerbach) plexus (Laranjeira and Pachnis 2009; Furness 2012). These neurons and glial cells of the ENS are derived from the neural crest cells that colonize the embryonic gut at E9-E9.5 in mice (Rothman et al. 1986; Heanue and Pachnis 2007; Coelho-Aguiar et al. 2015). In the ENS, the differentiation of enteric neurons starts prior to the start of enteric glial cells (EGCs). However, EGCs outnumber neurons, and the ratio of enteric neurons to EGC in the submucosal and myenteric plexus varies from 1:4 to 1:10 depending on the species (Savidge et al. 2007; Hoff et al. 2008; Esposito et al. 2016). Both enteric neurons and EGCs play a crucial role in the regulation of intestinal epithelial barrier functions including nutrient absorption and protection from gut microbial pathogens.
A number of studies have verified the expression of μ-, κ- and σ- opioid receptors in myenteric and submucosal plexus neurons (Bagnol et al. 1997; Sternini et al. 2004; Poole et al. 2011; Lay et al. 2016; DiCello et al. 2020) suggesting a potential direct effect of opioids on the enteric neuronal functions. The myenteric plexus neurons regulate GI motor activity and peristalsis, whereas neurons in the submucosal plexus, which are in close proximity to the epithelial cells, regulate blood flow, immune cell migration, and various mucosal functions in the epithelium (Bertrand et al. 1998; Furness et al. 1998). In addition, the submucosal plexus consists of two types of secretomotor neurons, the ascending cholinergic neurons (immunoreactive for neuropeptide Y; NPY) and the descending non-cholinergic neurons, which through the release of Acetylcholine (ACh) and vasoactive intestinal polypeptide (VIP), respectively, control epithelial cell secretion (Cooke and Carey 1985; Keast et al. 1985a, b). These neuronal mediators also regulate epithelial barrier integrity. For example, ACh and NPY have been shown to increase epithelial permeability (Neunlist et al. 1998; Gareau et al. 2006; Overman et al. 2012). Consistently, increased NPY levels have been reported in mouse models of colitis, whereas NPY knockout mice show improved barrier function and developed less severe colitis (Chandrasekharan et al. 2008, 2013). In contrast, VIP decreases intestinal permeability, promotes epithelial cell proliferation, stimulates mucus production, regulates ZO-1 tight-junction protein expression and induces production of interleukin-8 (a chemokine that attracts immune cells) in epithelial cells, and thereby contributes to both maintenance and immune surveillance of the epithelial barrier (Farack et al. 1987; Neunlist et al. 1998, 2003; Toumi et al. 2003, 2004; Foong et al. 2010; De Quelen et al. 2011). Opioid receptor activation at the enteric circuitry has been shown to suppress neuronal excitability, decrease GI secretion, promote water and electrolyte absorption and cause impairments in peristalsis. These effects of opioids are partly mediated through inhibition of the release of neuronal mediators including VIP, ACh, substance P and nitric oxide (De Luca and Coupar 1996; Iwata et al. 2007; Brock et al. 2012).
The protective effects of EGCs at the gut epithelium may be impaired by chronic opioid use. EGCs like astrocytes in the CNS, are crucial for the development and survival of enteric neurons (Abdo et al. 2010, 2012; De Giorgio et al. 2012), and therefore may also regulate neuron-controlled epithelial barrier function. Regulation by EGCs of the intestinal epithelium was demonstrated in a study by Neunlist et al. that showed a significant increase in crypt hyperplasia after ablation of EGCs (Neunlist et al. 2007). In another study, ablation of EGCs by targeting the glial fibrillary acidic protein (GFAP) caused fatal jejuno-ileitis in mice (Bush et al. 1998; Cornet et al. 2001). Together, these studies suggest that EGCs could directly or indirectly control epithelial cell proliferation and self-renewal. Consistently, EGCs produce various trophic factors including glia derived neurotropic factor (GDNF), which through the induction of ZO-1 protein may promote intestinal barrier function (Xiao et al. 2014; Meir et al. 2015; Bauman et al. 2017). Other EGC-derived factors that promotes epithelial barrier integrity include proEGF (Van Landeghem et al. 2011), TGF-β1 (Neunlist et al. 2007), S-nitrosoglutathione (Savidge et al. 2007; Flamant et al. 2011; Li et al. 2016), 15-hydroxyeicosatetraenoic acid (Pochard et al. 2016) and 15-deoxy-delta-12,14-prostaglandin J2 (Abdo et al. 2012; Coquenlorge et al. 2016).
In a recent study, we demonstrated that morphine treatment reduces the barrier-enhancing effects of EGCs on intestinal epithelial cells in vitro, and that this effect of morphine was associated with a decrease in GDNF mRNA and protein levels in EGCs (Bauman et al. 2017). In addition, accumulating evidence implicates a central role for EGCs in the development of abdominal pain, mainly through production of immunoregulatory signals and modulation of nociceptors within the myenteric plexus (Morales-Soto and Gulbransen 2019). EGCs, like glial cells of the CNS, may regulate opioid-induced hyperalgesia through altered purinergic signaling (Watkins et al. 2005; Morales-Soto and Gulbransen 2019). Consistently, long-term exposure of mice to morphine increases purinergic P2X receptor activity in EGCs (Bhave et al. 2017). Of note, under inflammatory conditions of the gut, EGCs may acquire a reactive phenotype, exhibit properties of antigen presenting cells (by expressing MHC class II), recruit immune cells, and contribute to the epithelial damage through production and release of S100β, nitric oxide, IL-6 and IL-1β (Esposito et al. 2006, 2007; Cirillo et al. 2009, 2011; Xiao et al. 2011). Additionally, we have previously reported that chronic exposure of mice to morphine increases the expression of IL-6, IL-1β and TNFα in the small intestine (Meng et al. 2013; Zhang et al. 2019). The morphine-treated mice also showed severe disruption of the gut epithelium and subsequent translocation of gut bacteria into mesenteric lymph node and liver (Meng et al. 2013). Whether or not these detrimental effects of morphine at the gut epithelium are a consequence of a phenotypic switch (from trophic to reactive) in the EGCs remains unclear. In a recent study, the reactive phenotype of EGCs in a mouse model of Gulf War illness was associated with TLR4 activation (Kimono et al. 2019). In this study, EGC reactivity in Gulf War chemical exposed mice was reduced following antibiotic treatment, suggesting a direct modulation of EGC function through TLR4 by the gut microbiota. In line, interestingly, morphine-induced gut epithelial damage is significantly reduced in TLR4 deficient mice (Meng et al. 2013). In addition, TLR4-deficient mice did not develop opioid-induced bowel dysfunction (Beckett et al. 2018). Collectively, these studies suggest that EGCs under physiological conditions confer protection to both enteric neurons and the gut epithelium, and that a perturbation of the gut epithelial homeostasis (that is, microbial dysbiosis and TLR4 activation) following chronic opioid use may trigger EGCs to cause detrimental effects in the ENS.
Emerging evidence indicates an important role of the gut microbiota in ENS development. For example, De Vadder et al. showed that the gut microbiota regulates maturation of the ENS through a mechanism involving serotonin (5-HT) receptor activation (De Vadder et al. 2018). In this study, there was an increase in proliferation of enteric neuronal progenitors when GF mice were colonized with microbiota from conventionally raised mice. Another study by Kabouridis et al. showed that the gut microbiota is essential for postnatal development of EGCs of the intestinal mucosa (Kabouridis and Pachnis 2015). Microbial metabolites may be directly responsible for migration of EGCs to lamina propria and maturation of enteric neurons in the ENS. This is further supported with the observation that microbiota plays a role in mediating opioid-induced damage and inflammation at the gut epithelium as well as in antinociceptive tolerance (Zhang et al. 2019). Taken together, understanding the relationship between the gut microbiota and the ENS is crucial to identifying novel therapeutic targets in opioid-induced bowel dysfunction (Camilleri and Boeckxstaens 2017).
Summary and Conclusion
A substantial amount of research has been conducted in past few decades oriented towards understanding how the gut microbiome influences the gut-brain-axis under health and disease states. Environmental factors, including the use of illicit drugs, have been shown to cause dramatic changes in gut microbial composition; specifically, they are known to shift the gut microbiome towards a more pathogenic state. Accumulating evidence has further shown that changes in the gut microbiome often coincide with changes in microbial metabolites. Importantly, molecular information in the form of microbial metabolites play an important role in maintaining central as well as enteric nervous system health. For instance, opioid-mediated alterations in microbial metabolites such as SCFAs and BAs may have direct effects on CNS and ENS maturation and function (Fig. 2). This altered metabolic profile also contributes towards dysregulated immune homeostasis. Collectively, these changes may contribute towards neurological disorders as well as opioid induced co-morbidities.
Fig. 2.
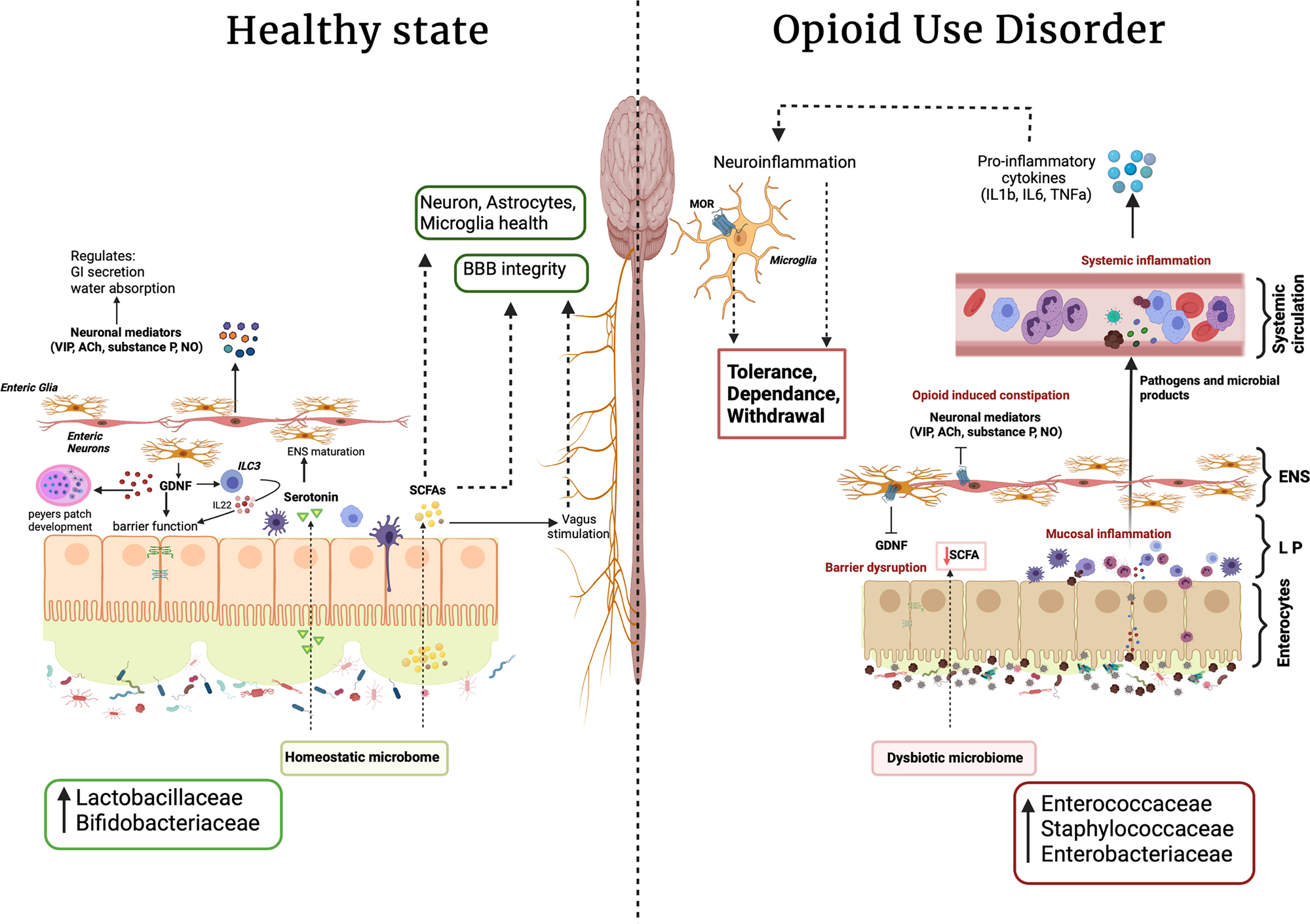
Opioid use and gut-brain axis: opioid induced gut dysbiosis leads to mucosal, systemic, and neuroinflammation which contributes to opioid associated comorbidities such as tolerance, dependence and withdrawal. (GDNF, glia derived neurotropic factor; SCFA, short-chain fatty acid; BA, bile acid; ACh, Acetylcholine; VIP, vasoactive intestinal polypeptide; NO, nitric oxide; ENS, enteric nervous system; MOR, μ opioid receptor; LP, lamina propria; ILC3, innate lymphoid cells 3; BBB, blood brain barrier)
Recent studies also identify contributions of gut microbiota in ENS maturation through modulation of neurotransmitters. The bi-directional facet of the gut-brain axis is also reflected by the fact that secretory factors such as GDNF by enteric glia also regulate microbial homeostasis through mucosal immune system maturation as well as by strengthening epithelial tight junction functions. Opioid mediated decreases in the expression of GDNF are also associated with increased intestinal permeability and altered immune surveillance at the intestinal mucosal surface. Altogether, these factors contribute to maintaining the pro-inflammatory environment observed with opioid treatment, leading to downstream opioid-associated comorbidites such as opioid tolerance, dependence, and withdrawal (Fig. 2). Furthermore, opioid-inhibited-release of neuronal mediators (e.g., VIPs, ACh, NO) from enteric neurons cause decreased GI secretion, increased water and electrolyte absorption, and decreased peristalsis, leading to opioid-induced constipation which further contributes to microbial dysbiosis following opioid use (Fig. 2). In conclusion, while pharmacological treatments for OUDs are available, they are not effective for all patients. It is therefore important that we improve our understanding of the role of the microbiome in OUDs, as new discoveries may elucidate novel targets for future therapeutics. These therapies could target pathways involved in microbial signaling within the gut-brain axis either by manipulating the composition of the gut microbiome or by manipulating its products via drugs designed to alter and exploit microbial metabolism. In particular, while no clinical trials exist of microbiome modulation in opioid-dependent patients, murine studies using fecal microbial transplant (FMT) or probiotics have shown much promise. For instance, FMT from naïve mice donors to antibiotic treated mice was shown to restore normal reward behavior, microglia morphology, sensitivity to pain, and to attenuate naloxone-precipitated opioid withdrawal in morphine-dependent mice (Lee et al 2018; Thomaz et al 2021). Additionally, others have shown that the VSL#3 probiotic cocktail attenuated morphine analgesic tolerance and hyperalgesia in opioid dependent mice, suggesting the beneficial effects of probiotic pretreatment in prolonging the analgesic properties of morphine (Zhang et al 2019). Still, much work needs to be done in this field, together with elucidating how prebiotics or synbiotics may improve GI and neuropsychiatric pathology in opioid-dependent patients. Indeed, while these potential therapeutics may not prove a panacea, they have enormous potential to be developed as or supplement existing therapies (Fig. 2).
Acknowledgements
This work was supported by the National Institutes of Health Grants (R01DA044582, R01DA043252, R01DA050542, R01DK117576, and T32DA045734) awarded to SR, by the Miami Center for AIDS Research (CFAR) via a pilot grant (P30AI073961) awarded to SM, and by the University of Miami Scientific Awards Committee (UM SAC) pilot grant (PG013459) and the Miami CFAR pilot grant (GR009434) to US.
Footnotes
Conflict of Interest All authors declare that they have no conflict of interest in this manuscript.
References
- Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53 [DOI] [PubMed] [Google Scholar]
- Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK (2019) Microbiome-microglia connections via the gut-brain axis. J Exp Med 216:41–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdo H, Mahe MM, Derkinderen P, Bach-Ngohou K, Neunlist M, Lardeux B (2012) The omega-6 fatty acid derivative 15-deoxy-Delta(1)(2), (1)(4)-prostaglandin J2 is involved in neuroprotection by enteric glial cells against oxidative stress. J Physiol 590:2739–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdo H, Derkinderen P, Gomes P, Chevalier J, Aubert P, Masson D, Galmiche JP, Berghe PV, Neunlist M, Lardeux B (2010) Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J 24:1082–1094 [DOI] [PubMed] [Google Scholar]
- Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, Ganapathy D, Fagan A, Sikaroodi M, Bajaj JS (2017) Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther 45:319–331 [DOI] [PubMed] [Google Scholar]
- Akbarali HI, Dewey WL (2019) Gastrointestinal motility, dysbiosis and opioid-induced tolerance: is there a link? Nat Rev Gastroenterol Hepatol 16:323–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, Poroyko V, Liu DC, Zaborina O, Alverdy JC (2012) Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg 255:386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnol D, Mansour A, Akil H, Watson SJ (1997) Cellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tract. Neuroscience 81:579–591 [DOI] [PubMed] [Google Scholar]
- Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, Dauer P, Chen C, Dalluge J, Johnson T, Roy S (2016) Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 9:1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA (2005) Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des 11:973–984 [DOI] [PubMed] [Google Scholar]
- Banks WA (2015) The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav Immun 44:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barengolts E, Green SJ, Eisenberg Y, Akbar A, Reddivari B, Layden BT, Dugas L, Chlipala GJPO (2018) Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. 13:e0194171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baser O, Chalk M, Fiellin DA, Gastfriend DR (2011) Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care 17(Suppl 8):S235–248 [PubMed] [Google Scholar]
- Bauman BD, Meng J, Zhang L, Louiselle A, Zheng E, Banerjee S, Roy S, Segura BJ (2017) Enteric glial-mediated enhancement of intestinal barrier integrity is compromised by morphine. J Surg Res 219:214–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett EAH, Staikopoulos V, Hutchinson MR (2018) Differential effect of morphine on gastrointestinal transit, colonic contractions and nerve-evoked relaxations in Toll-Like Receptor deficient mice. Sci Rep 8:5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM (2011) The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141:599–609, 609 e591–593 [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB (1998) Electrical mapping of the projections of intrinsic primary afferent neurones to the mucosa of the guinea-pig small intestine. Neurogastroenterol Motil 10:533–541 [DOI] [PubMed] [Google Scholar]
- Bhargava HN, Thomas PT, Thorat S, House RV (1994) Effects of morphine tolerance and abstinence on cellular immune function. Brain Res 642:1–10 [DOI] [PubMed] [Google Scholar]
- Bhave S, Gade A, Kang M, Hauser KF, Dewey WL, Akbarali HI (2017) Connexin-purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation. FASEB J 31:2649–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioli M, Carino A, Cipriani S, Francisci D, Marchiano S, Scarpelli P, Sorcini D, Zampella A, Fiorucci S (2017) The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis. J Immunol 199:718–733 [DOI] [PubMed] [Google Scholar]
- Boonstra E, de Kleijn R, Colzato LS, Alkemade A, Forstmann BU, Nieuwenhuis S (2015) Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Front Psychol 6:1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner C, Lanciotti S, Koch T, Hollt V, Kraus J (2013) mu opioid receptor agonist-selective regulation of interleukin-4 in T lymphocytes. J Neuroimmunol 263:35–42 [DOI] [PubMed] [Google Scholar]
- Bostrom E, Hammarlund-Udenaes M, Simonsson US (2008) Blood-brain barrier transport helps to explain discrepancies in in vivo potency between oxycodone and morphine. Anesthesiology 108:495–505 [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyas B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108:16050–16055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow JM, Feng P, Meissler JJ, Pintar JE, Gaughan J, Adler MW, Eisenstein TK (2010) Potentiating effect of morphine on oral Salmonella enterica serovar Typhimurium infection is mu-opioid receptor-dependent. Microb Pathog 49:330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C, Olesen SS, Olesen AE, Frokjaer JB, Andresen T, Drewes AM (2012) Opioid-induced bowel dysfunction: pathophysiology and management. Drugs 72:1847–1865 [DOI] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A (2003) Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 171:6164–6172 [DOI] [PubMed] [Google Scholar]
- Brunt VE, LaRocca TJ, Bazzoni AE, Sapinsley ZJ, Miyamoto-Ditmon J, Gioscia-Ryan RA, Neilson AP, Link CD, Seals DR (2021) The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. Geroscience 43:377–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant HU, Roudebush RE (1990) Suppressive effects of morphine pellet implants on in vivo parameters of immune function. J Pharmacol Exp Ther 255:410–414 [PubMed] [Google Scholar]
- Bryant HU, Bernton EW, Holaday JW (1988) Morphine pellet-induced immunomodulation in mice: Temporal relationships. J Pharmacol Exp Ther 245:913–920 [PubMed] [Google Scholar]
- Buckley A, Turner JR (2018) Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkert K, Moodley K, Angel CE, Brooks A, Graham ES (2012) Detailed analysis of inflammatory and neuromodulatory cytokine secretion from human NT2 astrocytes using multiplex bead array. Neurochem Int 60:573–580 [DOI] [PubMed] [Google Scholar]
- Burma NE, Leduc-Pessah H, Trang T (2017) Genetic deletion of microglial Panx1 attenuates morphine withdrawal, but not analgesic tolerance or hyperalgesia in mice. Channels (austin) 11:487–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV (1998) Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93:189–201 [DOI] [PubMed] [Google Scholar]
- Camilleri M, Boeckxstaens G (2017) Dietary and pharmacological treatment of abdominal pain in IBS. Gut 66:966–974 [DOI] [PubMed] [Google Scholar]
- Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN (2012) Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil 24:503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan B, Bala V, Kolachala VL, Vijay-Kumar M, Jones D, Gewirtz AT, Sitaraman SV, Srinivasan S (2008) Targeted deletion of neuropeptide Y (NPY) modulates experimental colitis. PLoS One 3:e3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan B, Jeppsson S, Pienkowski S, Belsham DD, Sitaraman SV, Merlin D, Kokkotou E, Nusrat A, Tansey MG, Srinivasan S (2013) Tumor necrosis factor-neuropeptide Y cross talk regulates inflammation, epithelial barrier functions, and colonic motility. Inflamm Bowel Dis 19:2535–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PV, Hao L, Offermanns S, Medzhitov R (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 111:2247–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves C, Remiao F, Cisternino S, Decleves X (2017) Opioids and the blood-brain barrier: a dynamic interaction with consequences on drug disposition in brain. Curr Neuropharmacol 15:1156–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheido MA, Gevorgyan MM, Zhukova EN (2014) Comparative evaluation of opioid-induced changes in immune reactivity of CBA mice. Bull Exp Biol Med 156:363–365 [DOI] [PubMed] [Google Scholar]
- Cirillo C, Sarnelli G, Turco F, Mango A, Grosso M, Aprea G, Masone S, Cuomo R (2011) Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol Motil 23:e372–382 [DOI] [PubMed] [Google Scholar]
- Cirillo C, Sarnelli G, Esposito G, Grosso M, Petruzzelli R, Izzo P, Cali G, D’Armiento FP, Rocco A, Nardone G, Iuvone T, Steardo L, Cuomo R (2009) Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol Motil 21:1209–e1112 [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF (2013) The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18:666–673 [DOI] [PubMed] [Google Scholar]
- Coelho-Aguiar JDM, Bon-Frauches AC, Gomes AL, Verissimo CP, Aguiar DP, Matias D, Thomasi BB, Gomes AS, Brito GA, Moura-Neto V (2015) The enteric glia: Identity and functions. Glia 63:921–935 [DOI] [PubMed] [Google Scholar]
- Collins SM, Bercik P (2009) The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 136:2003–2014 [DOI] [PubMed] [Google Scholar]
- Conn AR, Fell DI, Steele RD (1983) Characterization of alpha-keto acid transport across blood-brain barrier in rats. Am J Physiol 245:E253–260 [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Carey HV (1985) Pharmacological analysis of 5-hydroxytryptamine actions on guinea-pig ileal mucosa. Eur J Pharmacol 111:329–337 [DOI] [PubMed] [Google Scholar]
- Coquenlorge S, Van Landeghem L, Jaulin J, Cenac N, Vergnolle N, Duchalais E, Neunlist M, Rolli-Derkinderen M (2016) The arachidonic acid metabolite 11beta-ProstaglandinF2alpha controls intestinal epithelial healing: deficiency in patients with Crohn’s disease. Sci Rep 6:25203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet A, Savidge TC, Cabarrocas J, Deng WL, Colombel JF, Lassmann H, Desreumaux P, Liblau RS (2001) Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn’s disease? Proc Natl Acad Sci U S A 98:13306–13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell WD, Lewis MG, Fan X, Rappaport J, Rogers TJ (2013) Effect of chronic morphine administration on circulating T cell population dynamics in rhesus macaques. J Neuroimmunol 265:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, Monsma F, Vassileva G, Maguire M, Gustafson E, Bayne M, Chou CC, Lundell D, Jenh CH (2009) Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol 15:5549–5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Lebron A, Johnson R, Mazahery C, Troyer Z, Joussef-Pina S, Quinones-Mateu ME, Strauch CM, Hazen SL, Levine AD (2021) Chronic opioid use modulates human enteric microbiota and intestinal barrier integrity. Gut Microbes 13:1946368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF (2018) The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol 51:80–101 [DOI] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107:14691–14696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgio R, Giancola F, Boschetti E, Abdo H, Lardeux B, Neunlist M (2012) Enteric glia and neuroprotection: Basic and clinical aspects. Am J Physiol Gastrointest Liver Physiol 303:G887–893 [DOI] [PubMed] [Google Scholar]
- De Luca A, Coupar IM (1996) Insights into opioid action in the intestinal tract. Pharmacol Ther 69:103–115 [DOI] [PubMed] [Google Scholar]
- De Quelen F, Chevalier J, Rolli-Derkinderen M, Mourot J, Neunlist M, Boudry G (2011) n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J Physiol 589:4341–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder F, Grasset E, Holm LM, Karsenty G, Macpherson AJ, Olofsson LE, Backhed F (2018) Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci USA 115:6458–6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S (2021) Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine 66:103293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai TR, Leeper NJ, Hynes KL, Gewertz BL (2002) Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res 104:118–123 [DOI] [PubMed] [Google Scholar]
- DiCello JJ, Carbone SE, Saito A, Rajasekhar P, Ceredig RA, Pham V, Valant C, Christopoulos A, Veldhuis NA, Canals M, Massotte D, Poole DP (2020) Mu and delta opioid receptors are coexpressed and functionally interact in the enteric nervous system of the mouse colon. Cell Mol Gastroenterol Hepatol 9:465–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, de Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 18:965–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, De Filippis D, Cirillo C, Sarnelli G, Cuomo R, Iuvone T (2006) The astroglial-derived S100beta protein stimulates the expression of nitric oxide synthase in rodent macrophages through p38 MAP kinase activation. Life Sci 78:2707–2715 [DOI] [PubMed] [Google Scholar]
- Esposito G, Sarnelli G, Capoccia E, Cirillo C, Pesce M, Lu J, Cali G, Cuomo R, Steardo L (2016) Autologous transplantation of intestine-isolated glia cells improves neuropathology and restores cognitive deficits in beta amyloid-induced neurodegeneration. Sci Rep 6:22605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Cirillo C, Sarnelli G, De Filippis D, D’Armiento FP, Rocco A, Nardone G, Petruzzelli R, Grosso M, Izzo P, Iuvone T, Cuomo R (2007) Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology 133:918–925 [DOI] [PubMed] [Google Scholar]
- Farack UM, Reiter J, Gross M, Moroder L, Wunsch E, Loeschke K (1987) Influence of vasoactive intestinal peptide, secretin, and Ala4, Val5-secretin on the net movements of electrolytes, fluid, and mucus in the rat colon in vivo. Scand J Gastroenterol Suppl 139:32–36 [DOI] [PubMed] [Google Scholar]
- Feng P, Truant AL, Meissler JJ Jr, Gaughan JP, Adler MW, Eisenstein TK (2006) Morphine withdrawal lowers host defense to enteric bacteria:Spontaneous sepsis and increased sensitivity to oral Salmonella enterica serovar Typhimurium infection. Infect Immun 74:5221–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahe MM, Meurette G, Marteyn B, Savidge T, Galmiche JP, Sansonetti PJ, Neunlist M (2011) Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut 60:473–484 [DOI] [PubMed] [Google Scholar]
- Foong JP, Parry LJ, Gwynne RM, Bornstein JC (2010) 5-HT(1A), SST(1), and SST(2) receptors mediate inhibitory postsynaptic potentials in the submucous plexus of the guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 298:G384–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe P, Bienenstock J, Kunze WA (2014) Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol 817:115–133 [DOI] [PubMed] [Google Scholar]
- Forsythe RM, Xu DZ, Lu Q, Deitch EA (2002) Lipopolysaccharide-induced enterocyte-derived nitric oxide induces intestinal monolayer permeability in an autocrine fashion. Shock 17:180–184 [DOI] [PubMed] [Google Scholar]
- Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9:286–294 [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC (1998) Intrinsic primary afferent neurons of the intestine. Prog Neurobiol 54:1–18 [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH (2006) Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res 59:83–88 [DOI] [PubMed] [Google Scholar]
- Ghate SR, Haroutiunian S, Winslow R, McAdam-Marx C (2010) Cost and comorbidities associated with opioid abuse in managed care and Medicaid patients in the United Stated: a comparison of two recently published studies. J Pain Palliat Care Pharmacother 24:251–258 [DOI] [PubMed] [Google Scholar]
- Glebov K, Lochner M, Jabs R, Lau T, Merkel O, Schloss P, Steinhauser C, Walter J (2015) Serotonin stimulates secretion of exosomes from microglia cells. Glia 63:626–634 [DOI] [PubMed] [Google Scholar]
- Goldsmith JR, Perez-Chanona E, Yadav PN, Whistler J, Roth B, Jobin C (2013) Intestinal epithelial cell-derived mu-opioid signaling protects against ischemia reperfusion injury through PI3K signaling. Am J Pathol 182:776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeneltch KM, Haudenschild CC, Keegan AD, Shi Y (2004) The opioid antagonist naltrexone blocks acute endotoxic shock by inhibiting tumor necrosis factor-alpha production. Brain Behav Immun 18:476–484 [DOI] [PubMed] [Google Scholar]
- Haghikia A et al. (2015) Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43:817–829 [DOI] [PubMed] [Google Scholar]
- Han C, Lei D, Liu L, Xie S, He L, Wen S, Zhou H, Ma T, Li S (2020) Morphine induces the differentiation of T helper cells to Th2 effector cells via the PKC-theta-GATA3 pathway. Int Immunopharmacol 80:106133. [DOI] [PubMed] [Google Scholar]
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, Kaelberer MM, Bohorquez DV, Shammah-Lagnado SJ, de Lartigue G, de Araujo IE (2018) A neural circuit for gut-induced reward. Cell 175:665–678 e623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V (2007) Enteric nervous system development and Hirschsprung’s disease: Advances in genetic and stem cell studies. Nat Rev Neurosci 8:466–479 [DOI] [PubMed] [Google Scholar]
- Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108:3047–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilburger ME, Adler MW, Truant AL, Meissler JJ Jr, Satishchandran V, Rogers TJ, Eisenstein TK (1997) Morphine induces sepsis in mice. J Infect Dis 176:183–188 [DOI] [PubMed] [Google Scholar]
- Hoff S, Zeller F, von Weyhern CW, Wegner M, Schemann M, Michel K, Ruhl A (2008) Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J Comp Neurol 509:356–371 [DOI] [PubMed] [Google Scholar]
- Hofford RS, Mervosh NL, Euston TJ, Meckel KR, Orr AT, Kiraly DD (2021) Alterations in microbiome composition and metabolic byproducts drive behavioral and transcriptional responses to morphine. Neuropsychopharmacology 46:2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz F, de Vos WM (2018) Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci 75:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga T, Takebe K, Kato I, Karaki S, Kuwahara A (2006) Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed Res 27:243–254 [DOI] [PubMed] [Google Scholar]
- Iwata H, Tsuchiya S, Nakamura T, Yano S (2007) Morphine leads to contraction of the ileal circular muscle via inhibition of the nitrergic pathway in mice. Eur J Pharmacol 574:66–70 [DOI] [PubMed] [Google Scholar]
- Johnson RH, Kho DT, O’Carroll SJ, Angel CE, Graham ES (2018) The functional and inflammatory response of brain endothelial cells to Toll-Like Receptor agonists. Sci Rep 8:10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabouridis PS, Pachnis V (2015) Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J Clin Invest 125:956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarczyk O, Dabek-Drobny A, Wozniakiewicz M, Pasko P, Dobrowolska-Iwanek J, Wozniakiewicz A, Piatek-Guziewicz A, Zagrodzki P, Mach T, Zwolinska-Wcislo M (2021) Fecal levels of lactic, succinic and short-chain fatty acids in patients with ulcerative colitis and Crohn disease: a pilot study. J Clin Med 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Mischel RA, Bhave S, Komla E, Cho A, Huang C, Dewey WL, Akbarali HI (2017) The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci Rep 7:42658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM (2010) Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 298:G851–859 [DOI] [PubMed] [Google Scholar]
- Keast JR, Furness JB, Costa M (1985a) Distribution of certain peptide-containing nerve fibres and endocrine cells in the gastrointestinal mucosa in five mammalian species. J Comp Neurol 236:403–422 [DOI] [PubMed] [Google Scholar]
- Keast JR, Furness JB, Costa M (1985b) Different substance P receptors are found on mucosal epithelial cells and submucous neurons of the guinea-pig small intestine. Naunyn Schmiedebergs Arch Pharmacol 329:382–387 [DOI] [PubMed] [Google Scholar]
- Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP (2015) Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17:662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimono D, Sarkar S, Albadrani M, Seth R, Bose D, Mondal A, Li Y, Kar AN, Nagarkatti M, Nagarkatti P, Sullivan K, Janulewicz P, Lasley S, Horner R, Klimas N, Chatterjee S (2019) Dysbiosis-associated enteric glial cell immune-activation and redox imbalance modulate tight junction protein expression in Gulf War illness pathology. Front Physiol 10:1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108:8030–8035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, Ribeiro EA, Russo SJ, Nestler EJ (2016) Alterations of the hst microbiome affect behavioral responses to cocaine. Sci Rep 6:35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst A, Wack C, Lutz WK, Eggert A, Kampgen E, Fischer WH (2002) Expression of functional kappa-opioid receptors on murine dendritic cells. Immunol Lett 84:41–48 [DOI] [PubMed] [Google Scholar]
- Kratsman N, Getselter D, Elliott E (2016) Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology 102:136–145 [DOI] [PubMed] [Google Scholar]
- Laranjeira C, Pachnis V (2009) Enteric nervous system development: Recent progress and future challenges. Auton Neurosci 151:61–69 [DOI] [PubMed] [Google Scholar]
- Lay J, Carbone SE, DiCello JJ, Bunnett NW, Canals M, Poole DP (2016) Distribution and trafficking of the mu-opioid receptor in enteric neurons of the guinea pig. Am J Physiol Gastrointest Liver Physiol 311:G252–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Chatelier E et al. (2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500:541–546 [DOI] [PubMed] [Google Scholar]
- Lee K, Vuong HE, Nusbaum DJ, Hsiao EY, Evans CJ, Taylor AMW (2018) The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology 43:2606–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeming ER, Johnson AJ, Spector TD, Le Roy CI (2019) Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang Y, Wang X, Ye L, Zhou Y, Persidsky Y, Ho W (2013) Immune activation of human brain microvascular endothelial cells inhibits HIV replication in macrophages. Blood 121:2934–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang X, Zhou H, Liu W, Li J (2016) Exogenous S-nitrosoglutathione attenuates inflammatory response and intestinal epithelial barrier injury in endotoxemic rats. J Trauma Acute Care Surg 80:977–984 [DOI] [PubMed] [Google Scholar]
- Luu M, Weigand K, Wedi F, Breidenbend C, Leister H, Pautz S, Adhikary T, Visekruna A (2018) Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci Rep 8:14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan SD, Aalinkeel R, Sykes DE, Reynolds JL, Bindukumar B, Fernandez SF, Chawda R, Shanahan TC, Schwartz SA (2008) Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol 28:528–541 [DOI] [PubMed] [Google Scholar]
- Makarenkova VP, Esche C, Kost NV, Shurin GV, Rabin BS, Zozulya AA, Shurin MR (2001) Identification of delta- and mu-type opioid receptors on human and murine dendritic cells. J Neuroimmunol 117:68–77 [DOI] [PubMed] [Google Scholar]
- McAdam-Marx C, Roland CL, Cleveland J, Oderda GM (2010) Costs of opioid abuse and misuse determined from a Medicaid database. J Pain Palliat Care Pharmacother 24:5–18 [DOI] [PubMed] [Google Scholar]
- Meckel KR, Kiraly DD (2019) A potential role for the gut microbiome in substance use disorders. Psychopharmacology 236:1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrishi JN, Mills IH (1983) Opiate receptors on lymphocytes and platelets in man. Clin Immunol Immunopathol 27:240–249 [DOI] [PubMed] [Google Scholar]
- Meir M, Flemming S, Burkard N, Bergauer L, Metzger M, Germer CT, Schlegel N (2015) Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro. Am J Physiol Gastrointest Liver Physiol 309:G613–624 [DOI] [PubMed] [Google Scholar]
- Meneses G, Bautista M, Florentino A, Diaz G, Acero G, Besedovsky H, Meneses D, Fleury A, Del Rey A, Gevorkian G, Fragoso G, Sciutto E (2016) Electric stimulation of the vagus nerve reduced mouse neuroinflammation induced by lipopolysaccharide. J Inflamm (lond) 13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Banerjee S, Li D, Sindberg GM, Wang F, Ma J, Roy S (2015) Opioid exacerbation of gram-positive sepsis, induced by gut microbial modulation, is rescued by IL-17A neutralization. Sci Rep 5:10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S (2013) Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 8:e54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Banerjee S, Zhang L, Sindberg G, Moidunny S, Li B, Robbins DJ, Girotra M, Segura B, Ramakrishnan S, Roy S (2019) Opioids impair intestinal epithelial repair in HIV-infected humanized mice. Front Immunol 10:2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer D, Hatsukari I, Hitosugi N, Schmidt-Wolf IG, Singhal PC (2006) Morphine reciprocally regulates IL-10 and IL-12 production by monocyte-derived human dendritic cells and enhances T cell activation. Mol Med 12:284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Soto W, Gulbransen BD (2019) Enteric glia: a new player in abdominal pain. Cell Mol Gastroenterol Hepatol 7:433–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R, Wang S, Solberg Woods LC, Seshie O, Chung ST, Shively CA, Register TC, Craft S, McClain DA, Yadav H (2018) Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front Microbiol 9:2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagyoszi P, Wilhelm I, Farkas AE, Fazakas C, Dung NT, Hasko J, Krizbai IA (2010) Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem Int 57:556–564 [DOI] [PubMed] [Google Scholar]
- Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, Biagi E, Andersen MH, Brigidi P, Odum N, Litman T, Woetmann A (2015) The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep 5:16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Frieling T, Rupprecht C, Schemann M (1998) Polarized enteric submucosal circuits involved in secretory responses of the guinea-pig proximal colon. J Physiol 506(Pt 2):539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A (2003) Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285:G1028–1036 [DOI] [PubMed] [Google Scholar]
- Neunlist M, Aubert P, Bonnaud S, Van Landeghem L, Coron E, Wedel T, Naveilhan P, Ruhl A, Lardeux B, Savidge T, Paris F, Galmiche JP (2007) Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Gastrointest Liver Physiol 292:G231–241 [DOI] [PubMed] [Google Scholar]
- Nguyen TL, Vieira-Silva S, Liston A, Raes J (2015) How informative is the mouse for human gut microbiota research? Dis Model Mech 8:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AL, Houghtling RA, Bayer BM (2011) Morphine suppresses MHC-II expression on circulating B lymphocytes via activation of the HPA. J Neuroimmune Pharmacol 6:130–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll SJ, Kho DT, Wiltshire R, Nelson V, Rotimi O, Johnson R, Angel CE, Graham ES (2015) Pro-inflammatory TNFalpha and IL-1beta differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J Neuroinflammation 12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SJ, Malahias E, Park J, Srivastava A, Reyes BAS, Gorky J, Vadigepalli R, Van Bockstaele EJ, Schwaber JS (2019) Single-cell glia and neuron gene expression in the Central Amygdala in opioid withdrawal suggests inflammation with correlated gut dysbiosis. Front Neurosci 13:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman EL, Rivier JE, Moeser AJ (2012) CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One 7:e39935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM (2007) Blood-brain barrier delivery. Drug Discov Today 12:54–61 [DOI] [PubMed] [Google Scholar]
- Park JC, Im SH (2020) Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med 52:1383–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Kotani T, Konno T, Setiawan J, Kitamura Y, Imada S, Usui Y, Hatano N, Shinohara M, Saito Y, Murata Y, Matozaki T (2016) Promotion of intestinal epithelial cell turnover by commensal bacteria: Role of short-chain fatty acids. PLoS One 11:e0156334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci T, Colamartino M, Fiori E, Sacco R, Coviello A, Ventura R, Puglisi-Allegra S, Turriziani L, Persico AM (2020) P-cresol alters brain dopamine metabolism and exacerbates autism-like behaviors in the BTBR mouse. Brain Sci 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Mosser DM, Adler MW, Rogers TJ, Meissler JJ Jr, Eisenstein TK (2000) Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. J Leukoc Biol 68:723–728 [PubMed] [Google Scholar]
- Peterson LW, Artis D (2014) Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol 14:141–153 [DOI] [PubMed] [Google Scholar]
- Phillips ML (2009) Gut reaction: Environmental effects on the human microbiota. Environ Health Perspect 117:A198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochard C, Coquenlorge S, Jaulin J, Cenac N, Vergnolle N, Meurette G, Freyssinet M, Neunlist M, Rolli-Derkinderen M (2016) Defects in 15-HETE production and control of epithelial permeability by human enteric glial cells from patients with Crohn’s disease. Gastroenterology 150:168–180 [DOI] [PubMed] [Google Scholar]
- Poole DP, Pelayo JC, Scherrer G, Evans CJ, Kieffer BL, Bunnett NW (2011) Localization and regulation of fluorescently labeled delta opioid receptor, expressed in enteric neurons of mice. Gastroenterology 141:982–991 e918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S (2014) Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis 46:527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, Apicella C, Capasso L, Paludetto R (2008) Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol 294:G906–913 [DOI] [PubMed] [Google Scholar]
- Reinhart M, Scarpati LM, Kirson NY, Patton C, Shak N, Erensen JG (2018) The economic burden of abuse of prescription opioids: a systematic literature review from 2012 to 2017. Appl Health Econ Health Policy 16:609–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Maduna T, Maurin H, Audouard E, Gaveriaux-Ruff C (2020) Mu opioid receptor in microglia contributes to morphine analgesic tolerance, hyperalgesia, and withdrawal in mice. J Neurosci Res [DOI] [PubMed] [Google Scholar]
- Ren M, Lotfipour S (2020) The role of the gut microbiome in opioid use. Behav Pharmacol 31:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renga B, Mencarelli A, Cipriani S, D’Amore C, Carino A, Bruno A, Francisci D, Zampella A, Distrutti E, Fiorucci S (2013) The bile acid sensor FXR is required for immune-regulatory activities of TLR-9 in intestinal inflammation. PLoS One 8:e54472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende WR, Valvassori SS, Reus GZ, Varela RB, Arent CO, Ribeiro KF, Bavaresco DV, Andersen ML, Zugno AI, Quevedo J (2013) Effects of sodium butyrate in animal models of mania and depression: implications as a new mood stabilizer. Behav Pharmacol 24:569–579 [DOI] [PubMed] [Google Scholar]
- Rothhammer V et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman TP, Tennyson VM, Gershon MD (1986) Colonization of the bowel by the precursors of enteric glia: Studies of normal and congenitally aganglionic mutant mice. J Comp Neurol 252:493–506 [DOI] [PubMed] [Google Scholar]
- Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA (1998) Morphine modulates NF kappa B activation in macrophages. Biochem Biophys Res Commun 245:392–396 [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Manfredi B, Gaspani L, Panerai AE (2000) The opioid antagonist naloxone induces a shift from type 2 to type 1 cytokine pattern in BALB/cJ mice. Blood 95:2031–2036 [PubMed] [Google Scholar]
- SAMSHA (2020) Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. HHS Publication No. PEP20–07–01–001, NSDUH Series H-55 [Google Scholar]
- Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV (2007) Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132:1344–1358 [DOI] [PubMed] [Google Scholar]
- Scepanovic P, Hodel F, Mondot S, Partula V, Byrd A, Hammer C, Alanio C, Bergstedt J, Patin E, Touvier M, Lantz O, Albert ML, Duffy D, Quintana-Murci L, Fellay J, Milieu Interieur C (2019) A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome 7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulzke JD, Ploeger S, Amasheh M, Fromm A, Zeissig S, Troeger H, Richter J, Bojarski C, Schumann M, Fromm M (2009) Epithelial tight junctions in intestinal inflammation. Ann NY Acad Sci 1165:294–300 [DOI] [PubMed] [Google Scholar]
- Selkrig J, Wong P, Zhang X, Pettersson S (2014) Metabolic tinkering by the gut microbiome: Implications for brain development and function. Gut Microbes 5:369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma HS, Ali SF (2006) Alterations in blood-brain barrier function by morphine and methamphetamine. Ann NY Acad Sci 1074:198–224 [DOI] [PubMed] [Google Scholar]
- Sharma U, Olson RK, Erhart FN, Zhang L, Meng J, Segura B, Banerjee S, Sharma M, Saluja AK, Ramakrishnan S, Abreu MT, Roy S (2020) Prescription opioids induce gut dysbiosis and exacerbate colitis in a murine model of inflammatory Bowel disease. J Crohns Colitis 14:801–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva YP, Bernardi A, Frozza RL (2020) The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (lausanne) 11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S, McLellan R, Wellmeyer E, Matalon F, George O (2021) Drugs and bugs: the gut-brain axis and substance use disorders. J Neuroimmune Pharmacol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson S, Kimbrough A, Boomhower B, McLellan R, Hughes M, Shankar K, de Guglielmo G, George O (2020) Depletion of the microbiome alters the recruitment of neuronal ensembles of oxycodone intoxication and withdrawal. eNeuro 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindberg GM, Callen SE, Banerjee S, Meng J, Hale VL, Hegde R, Cheney PD, Villinger F, Roy S, Buch S (2019) Morphine potentiates dysbiotic microbial and metabolic shifts in acute SIV infection. J Neuroimmune Pharmacol 14:200–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V (2014) Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal P, Kapasi A, Reddy K, Franki N (2001) Opiates promote T cell apoptosis through JNK and caspase pathway. Adv Exp Med Biol 493:127–135 [DOI] [PubMed] [Google Scholar]
- Singhal PC, Kapasi AA, Reddy K, Franki N, Gibbons N, Ding G (1999) Morphine promotes apoptosis in Jurkat cells. J Leukoc Biol 66:650–658 [DOI] [PubMed] [Google Scholar]
- Sternini C, Patierno S, Selmer IS, Kirchgessner A (2004) The opioid system in the gastrointestinal tract. Neurogastroenterol Motil 16(Suppl 2):3–16 [DOI] [PubMed] [Google Scholar]
- Strain EC (2021) Meaning and purpose in the context of opioid overdose deaths. Drug Alcohol Depend 219:108528. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yoshinaga N, Tanabe S (2011) Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 286:31263–31271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaga H, Ohtsuki S, Hosoya K, Terasaki T (2001) GAT2/BGT-1 as a system responsible for the transport of gamma-aminobutyric acid at the mouse blood-brain barrier. J Cereb Blood Flow Metab 21:1232–1239 [DOI] [PubMed] [Google Scholar]
- Taylor AM, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, Xue L, Olmstead MC, De Koninck Y, Evans CJ, Cahill CM (2015) Microglia disrupt mesolimbic reward circuitry in chronic pain. J Neurosci 35:8442–8450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Castonguay A, Ghogha A, Vayssiere P, Pradhan AA, Xue L, Mehrabani S, Wu J, Levitt P, Olmstead MC, De Koninck Y, Evans CJ, Cahill CM (2016) Neuroimmune regulation of GABAergic neurons within the ventral tegmental area during withdrawal from chronic morphine. Neuropsychopharmacology 41:949–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazuke Y, Drongowski RA, Teitelbaum DH, Coran AG (2003) Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr Surg Int 19:321–325 [DOI] [PubMed] [Google Scholar]
- Thomaz AC, Iyer V, Woodward TJ, Hohmann AG (2021) Fecal microbiota transplantation and antibiotic treatment attenuate naloxone-precipitated opioid withdrawal in morphine-dependent mice. Exp Neurol 343:113787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61:364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toumi F, Neunlist M, Cassagnau E, Parois S, Laboisse CL, Galmiche JP, Jarry A (2003) Human submucosal neurones regulate intestinal epithelial cell proliferation: Evidence from a novel co-culture model. Neurogastroenterol Motil 15:239–242 [DOI] [PubMed] [Google Scholar]
- Toumi F, Neunlist M, Denis MG, Oreshkova T, Laboisse CL, Galmiche JP, Jarry A (2004) Vasoactive intestinal peptide induces IL-8 production in human colonic epithelial cells via MAP kinase-dependent and PKA-independent pathways. Biochem Biophys Res Commun 317:187–191 [DOI] [PubMed] [Google Scholar]
- Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A (2005) Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: Myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 16:5040–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallersnes OM, Jacobsen D, Ekeberg O, Brekke M (2019) Mortality, morbidity and follow-up after acute poisoning by substances of abuse: a prospective observational cohort study. Scand J Public Health 47:452–461 [DOI] [PubMed] [Google Scholar]
- van Kralingen C, Kho DT, Costa J, Angel CE, Graham ES (2013) Exposure to inflammatory cytokines IL-1beta and TNFalpha induces compromise and death of astrocytes; implications for chronic neuroinflammation. PLoS One 8:e84269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Landeghem L, Chevalier J, Mahe MM, Wedel T, Urvil P, Derkinderen P, Savidge T, Neunlist M (2011) Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am J Physiol Gastrointest Liver Physiol 300:G976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Meng J, Zhang L, Roy S (2020a) Opioid use potentiates the virulence of hospital-acquired infection, increases systemic bacterial dissemination and exacerbates gut dysbiosis in a murine model of Citrobacter rodentium infection. Gut Microbes 11:172–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S (2018) Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 8:3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MR, Wu DD, Luo F, Zhong CJ, Wang X, Zhu N, Wu YJ, Hu HT, Feng Y, Wang X, Xiong HR, Hou W (2020b) Methadone inhibits viral restriction factors and facilitates HIV infection in macrophages. Front Immunol 11:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Fukiya S, Yokota A (2017) Comprehensive evaluation of the bactericidal activities of free bile acids in the large intestine of humans and rodents. J Lipid Res 58:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF (2005) Glia: novel counter-regulators of opioid analgesia. Trends Neurosci 28:661–669 [DOI] [PubMed] [Google Scholar]
- Wen H, Lu Y, Yao H, Buch S (2011) Morphine induces expression of platelet-derived growth factor in human brain microvascular endothelial cells: implication for vascular permeability. PLoS One 6:e21707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel MA, Steele AD, Eisenstein TK, Adler MW, Henderson EE, Rogers TJ (2000) Mu-opioid induction of monocyte chemoattractant protein-1, RANTES, and IFN-gamma-inducible protein-10 expression in human peripheral blood mononuclear cells. J Immunol 165:6519–6524 [DOI] [PubMed] [Google Scholar]
- Wos-Oxley M, Bleich A, Oxley AP, Kahl S, Janus LM, Smoczek A, Nahrstedt H, Pils MC, Taudien S, Platzer M, Hedrich HJ, Medina E, Pieper DH (2012) Comparative evaluation of establishing a human gut microbial community within rodent models. Gut Microbes 3:234–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Han Y, Zheng Z, Zhu S, Chen J, Yao Y, Yue S, Teufel A, Weng H, Li L, Wang B (2021) Obeticholic acid inhibits anxiety via alleviating gut microbiota-mediated microglia accumulation in the brain of high-fat high-sugar diet mice. Nutrients 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HW, Ge C, Feng GX, Li Y, Luo D, Dong JL, Li H, Wang H, Cui M, Fan SJ (2018) Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol Lett 287:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Wang W, Chen W, Sun L, Li X, Zhang C, Yang H (2014) GDNF is involved in the barrier-inducing effect of enteric glial cells on intestinal epithelial cells under acute ischemia reperfusion stimulation. Mol Neurobiol 50:274–289 [DOI] [PubMed] [Google Scholar]
- Xiao WD, Chen W, Sun LH, Wang WS, Zhou SW, Yang H (2011) The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation. Mol Cell Neurosci 46:527–534 [DOI] [PubMed] [Google Scholar]
- Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, Chen X, Wu Q, Li X, Wang K (2017) Bacterial diversity of intestinal microbiota in patients with substance use disorders revealed by 16S rRNA gene deep sequencing. Sci Rep 7:3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Deji C, Fan J, Chang L, Miao X, Xiao Y, Zhu Y, Li S (2021) Differential alteration in gut microbiome profiles during acquisition, extinction and reinstatement of morphine-induced CPP. Prog Neuropsychopharmacol Biol Psychiatry 104:110058. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang J, Yang C, Chen T, Wang Z, Li J, Qin F, Deng Q, Zhang X (2020a) Sensitivity to morphine reward associates with gut dysbiosis in rats with morphine-induced conditioned place preference. Front Psychiatry 11:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Meng J, Ban Y, Jalodia R, Chupikova I, Fernandez I, Brito N, Sharma U, Abreu MT, Ramakrishnan S (2019) Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. JPotNAoS 116:13523–13532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S (2020b) Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front Immunol 11:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Meng J, Lian Q, Chen X, Bauman B, Chu H, Segura B, Roy S (2018) Prescription opioids are associated with higher mortality in patients diagnosed with sepsis: a retrospective cohort study using electronic health records. PLoS One 13:e0190362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zeng Y, Zhang X, Chu J, Loh HH, Law PY (2010) mu-Opioid receptor agonists differentially regulate the expression of miR-190 and NeuroD. Mol Pharmacol 77:102–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Yang R, Wang W, Qi L, Huang T (2020) Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J Neuroinflammation 17:288. [DOI] [PMC free article] [PubMed] [Google Scholar]


