Abstract
Background
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a novel coronavirus that caused an ongoing pandemic of a pathology termed Coronavirus Disease 19 (COVID-19). Several studies reported that both COVID-19 and RTEL1 variants are associated with shorter telomere length, but a direct association between the two is not generally acknowledged. Here we demonstrate that up to 8.6% of severe COVID-19 patients bear RTEL1 ultra-rare variants, and show how this subgroup can be recognized.
Methods
A cohort of 2246 SARS-CoV-2-positive subjects, collected within the GEN-COVID Multicenter study, was used in this work. Whole exome sequencing analysis was performed using the NovaSeq6000 System, and machine learning methods were used for candidate gene selection of severity. A nested study, comparing severely affected patients bearing or not variants in the selected gene, was used for the characterisation of specific clinical features connected to variants in both acute and post-acute phases.
Results
Our GEN-COVID cohort revealed a total of 151 patients carrying at least one RTEL1 ultra-rare variant, which was selected as a specific acute severity feature. From a clinical point of view, these patients showed higher liver function indices, as well as increased CRP and inflammatory markers, such as IL-6. Moreover, compared to control subjects, they present autoimmune disorders more frequently. Finally, their decreased diffusion lung capacity for carbon monoxide after six months of COVID-19 suggests that RTEL1 variants can contribute to the development of SARS-CoV-2-elicited lung fibrosis.
Conclusion
RTEL1 ultra-rare variants can be considered as a predictive marker of COVID-19 severity, as well as a marker of pathological evolution in pulmonary fibrosis in the post-COVID phase. This notion can be used for a rapid screening in hospitalized infected people, for vaccine prioritization, and appropriate follow-up assessment for subjects at risk.
Trial Registration NCT04549831 (www.clinicaltrial.org)
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-023-02458-7.
Keywords: COVID-19, Pulmonary fibrosis, RTEL1, Long COVID
Background
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a new coronavirus that became pandemic in 2019. The disease caused by this virus was named Coronavirus Disease 2019 (COVID-19) [1]. The course of SARS-CoV-2 infection is unpredictable, with symptoms ranging from absent to severe, sometimes even with a lethal outcome [2].
In addition to demographic risk factors, such as old age and/or male sex, the neutrophil to lymphocyte ratio (NLR) has been shown to have the greatest predictive value for poor outcomes in patients with COVID-19. Genetic markers of severity and susceptibility to infection were also considered [3, 4]. In particular, telomere shortening is associated with a higher risk of developing severe COVID-19 [5]. Different studies reported that COVID-19 associates with shorter telomere length, revealing that severe COVID-19 survivors have shorter telomeres compared with patients recovered from milder COVID-19 [5, 6]. The critical shortness of telomeres results from permanent DNA damage, with the induction of cell senescence and apoptosis [7]. Several human pathologies are characterized by telomere shortening. Fibrosis in the lung, liver, or kidney is often associated with dysfunction in telomere-binding proteins and generally with pathogenic variants in genes relevant to the homeostasis of telomeres, such as RTEL1 [8].
Pathogenic variants in RTEL1 gene, encoding for a helicase that regulates telomere elongation, have been identified in rare interstitial pneumoniae, called Idiopathic Pulmonary Fibrosis (IPF) [9]. Moreover, RTEL1-mutated pulmonary fibrosis families display a precocious onset of pulmonary disease, concomitant liver pathologies, and in some cases early reversible neutropenia [10]. Some of these patients also present autoimmune conditions, suggesting that, in heterozygous carriers of RTEL1 aberrations, fibrosis results from the combination of such monogenic defects with environmental factors and autoimmune diseases [11, 12]. Cellular and molecular pathways, including TGF-beta and IL-6 over-production [13, 14], are shared between IPF and COVID-19. From a genetic point of view, GWAS studies identified some tens of quantitative loci involved in COVID-19 severity/susceptibility [3]. Common, low-frequency, rare, and ultra-rare coding variants were also found to contribute to COVID-19 severity [15]. Limited data are now available regarding telomere length and COVID-19 progression [16, 17]; RTEL1 variants, however, have never been investigated as a possible mechanistic connection between the two. This study aims to describe the clinical characteristics of an Italian cohort of COVID-19 patients, either bearing ultra-rare variants of RTEL1 or not.
Materials and methods
Study design and populations
A cohort of 2246 SARS-CoV-2-positive subjects, collected within the GEN-COVID Multicenter study (https://sites.google.com/dbm.unisi.it/gen-covid), was used in this work. The application of the post-Mendelian model allowed us to extract the genetic features contributing to the COVID-19 phenotype [15]. Patients were classified using a modified version of the World Health Organization COVID-19 outcome scale [18]. The following six categories of severity were identified: (1) death; (2) hospitalized, receiving invasive mechanical ventilation; (3) hospitalized, receiving continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) ventilation; (4) hospitalized, receiving low-flow supplemental oxygen; (5) hospitalized, not receiving supplemental oxygen; and (6) not hospitalized. In order to obtain a clinical classification as independent as possible from age and sex, that allowed us to define a cohort in which the genetic features were more relevant to determine the severe/mild phenotype, we performed an adjustment starting from the clinical categories. We applied two ordered logistic regression, separately for males and females cohort, and cases who received a treatment higher than expected by age were classified as severe, while patients who received a treatment less severe than expected by age were considered not severe; subjects matching the expected treatment outcomes according to age were excluded from the model [20]. A subset of 512 COVID-19 patients, for which all clinical and laboratory parameters were available, was selected. This subset of patients is stratified based on RTEL1 genotype: a case group of 151 mutated patients (126 hospitalized patients and 25 not-hospitalized patients) is defined, composed of 92 males and 59 females from various regions of Italy; 361 non-mutated patients became our control group, subdivided among 222 males and 139 females and monitored at the COVID-19 wards of the Siena University Hospital from March 1st, 2020 to July 1st, 2021. SARS-CoV-2 positivity was confirmed by a nasopharyngeal antigenic swab, performed upon admission. All data were collected prospectively at the time of hospitalization and gathered in an electronic database in anonymous form. For each patient clinical, radiological, immunological, laboratory, and survival information has been collected. Functional data, including the percentages of forced vital capacity (FVC) and the diffusing capacity of the lung for carbon monoxide (DLCO), were also collected at 6 (± 1) months of follow-up, monitored at Siena University Hospital. This longitudinal study was conducted in a sub-subset of the 512 cohort, namely, RTEL1 mutated patients attending Siena hospital (12) and 18 non-mutated patients matched for age and sex. The GEN-COVID Multicenter study was performed in accordance with all relevant international, European, Italian, and institutional guidelines, and approved in advance by the University Hospital (Azienda Ospedaliero-Universitaria Senese) Ethical Review Board, Siena, Italy (Prot n. 16917, dated March 16th, 2020).
Whole exome sequencing (WES) analysis
WES was performed using the NovaSeq6000 System (Illumina, San Diego, CA, USA) with at least 97% coverage at 20×, as previously described [19]. Data were represented in a binary mode on a gene-by-gene basis [15, 19, 20].
Statistical analysis
The LASSO logistic regression machine learning approach used in the post-Mendelian model allow us to extract relevant genetic features associated with COVID-19 clinical outcome, as already described [15, 20].
In this study, we consider only ultra-rare (Minor Allele Frequency < 0.001) autosomal dominant gene variants (presence of at least one variant) as Boolean features.
Clinical data were stored in Microsoft Excel. Results were expressed as means plus/minus a standard deviation (M ± SD), or medians and quartiles (25th and 75th percentiles) for continuous variables as necessary. The Shapiro–Wilk test was applied to evaluate the normal distribution of data. Chi-square tests or Fisher exact tests were used for categorical variables as appropriate. Comparisons between control and patient groups were conducted by Student’s t-test or Mann–Whitney U test, while for multiple comparisons a one-way ANOVA or non-parametric tests (Kruskal–Wallis test and Dunn test) were performed. Statistical analysis and graphic representation of the data were performed using dedicated software, namely GraphPad Prism 9.4.2 (Graphpad Holdings, LLC, San Diego, CA, USA) and Jamovi (version 1.8.1) [Computer Software] (Retrieved from https://www.jamovi.orgc). For all tests, p-values of less than 0.05 were considered statistically significant.
Results
RTEL1 ultra-rare variants associate with severity in COVID-19
The LASSO logistic regression extracted RTEL1 ultra-rare variants as one of the most important features associated with severity [15]. Exome analysis of 2246 SARS-CoV-2 infected subjects of different severity, belonging to GEN-COVID cohort, stratified by sex and adjusted by age, shows an association between RTEL1 ultra-rare variants and severity with an OR = 1.63 (95% CI 1.04 to 2.58; p-value = 0.03), (Table 1). In the total cohort, 151 patients carried one RTEL1 ultra-rare variant, 126 being hospitalized, and 25 being not. The specific variants are illustrated in Additional file 1: Table S1a, b.
Table 1.
Chi Square test in the GEN-COVID cohort
| Phenotype | Ultra-rare variants (%) | Wild type (%) | Total |
|---|---|---|---|
| Severe | 59 (66.3) | 931 (54.7) | 990 |
| Not severe | 30 (33.7) | 772 (45.3) | 882 |
| Total | 89 | 1703 | 1792 |
OR = 1.63, (95% CI 1.04–2.58), p-value = 0.03
RTEL1 mutated patients are younger and require more respiratory support and duration of hospitalization
Demographic, clinical and survival data are reported in Table 2. No differences in terms of gender distribution, in the frequencies of bilateral pneumonia on chest X-ray, and in survival rate are identified relating to RTEL1 genotype. RTEL1 mutated patients, on the other hand, result to be younger than other patients, with fewer duration of hospitalization. Similarly, the percentage of patients that required respiratory support with CPAP during hospitalization is significantly higher among wildtype (WT) patients than the cohort of patients with RTEL1 ultra-rare variants, because the latter underwent more often intubation.
Table 2.
Demographic, Clinical and survival data
| RTEL1 mutation | RTEL1 WT | p values | |
|---|---|---|---|
| Gender (M/F) (% of male) | 92/59 (61) | 222/139 (61) | ns |
| Age (M ± S.D.) | 59.11 ± 16.32 | 65.5 ± 14.22 | < 0.0001 |
| Days of Hospitalization (M ± S.D.) | 22.15 ± 16.08 | 27.28 ± 34.48 | 0.048 |
| Bilateral pneumoniae (yes/no) (%yes) | 51 (34) | 137 (38) | ns |
| Oxygen Administration (yes/no) (%yes) | 104 (69) | 332 (92) | 0.0023 |
| Type of oxygen Therapy | |||
| Nasal cannula (%) | 72 (48) | 166(46) | 0.0117 |
| CPAP/High Flows (%) | 46 (31) | 148 (41) | |
| Intubation (%) | 33 (21) | 46 (13) | |
| Survival (Death) (%death) | 12 (8%) | 22 (6%) | ns |
| Number of comorbidities | ns | ||
| No comorbidities | 59 | 46 | |
| 1 | 18 | 28 | |
| 2 | 12 | 13 | |
| 3 | 10 | 9 | |
| > 4 | 1 | 4 |
RTEL1-mutated patients have more autoimmune diseases as comorbidity
The number and type of comorbidities in RTEL1-mutated versus WT individuals are reported in Table 2. The number of comorbidities affecting each patient is similar for the two groups. However, RTEL1-mutated patients are more affected by autoimmune diseases (12% in mutated patients and 6% in WT patients) and hypertension (40% in mutated patients and 29% in WT patients) compared to other patients. Diabetes, lung disease, cancer, dyslipidemia, and hypothyroidism show similar incidences in the two cohorts.
Impaired liver function and NLR
Concerning laboratory findings, total bilirubin, Alanine aminotransferase (ALT), and IL-6 levels in the blood are significantly higher in the population with RTEL1 variants. The concentrations of Aspartate aminotransferase (AST) showed a similar trend, although without reaching statistical significance (Table 3).
Table 3.
Laboratories parameters of analyzed cohort
| RTEL1 mutation | RTEL1 WT | p values | |
|---|---|---|---|
| Total bilirubin (mg/dl) | 20.91 ± 42.17 | 15.62 ± 3.076 | < 0.0001 |
| AST (U/L) | 53.99 ± 61.09 | 48.15 ± 82.9 | 0.0555 |
| ALT (U/L) | 63.11 ± 55.61 | 54.61 ± 74.70 | 0.0084 |
| Creatinine (mg/dl) | 2.2 ± 8.8 | 1.26 ± 3.34 | 0.0512 |
| D-Dimer (ng/dl) | 1888 ± 2774 | 2722 ± 7040 | 0.0534 |
| Fibrinogen (mg/dl) | 485.3 ± 203.7 | 597.6 ± 160.9 | 0.0007 |
| IL-6 (pg/ml) | 17.35 ± 33.29 | 6.46 ± 16.69 | 0.0159 |
| Platelets (103/mm3) | 208.3 ± 82.64 | 222.6 ± 101.7 | ns |
| CRP (mg/dl) | 51.35 ± 131 | 83.9 ± 276.3 | < 0.0001 |
| LDH (U/L) | 428.4 ± 329.2 | 348.1 ± 370.2 | ns |
| Lypase (U/L) | 56.37 ± 52.32 | 65.14 ± 18.5 | ns |
| Pancreatic Amylase (U/L) | 46.65 ± 22.16 | 56.65 ± 83.64 | ns |
| Gamma Glutamin Transferase (U/L) | 86.29 ± 103.8 | 70.69 ± 133.4 | ns |
With respect to controls, carriers of RTEL1 ultra-rare variants also showed a significant decrease in creatinine, D-dimer, fibrinogen, and c-reactive protein (CRP), (Table 3).
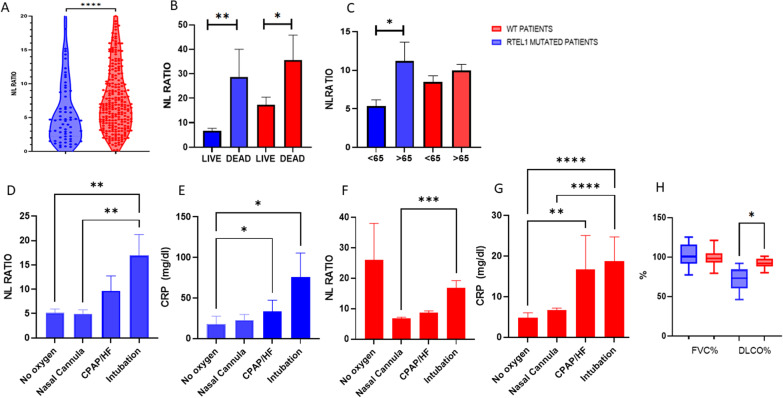
As Fig. 1A shows, NLR is significantly higher in the unmutated population. Particularly after cohort stratification by survival rate, higher NLRsreported in dead patients irrespective of their RTEL1 genotype (Fig. 1B). Interestingly, grouping by age highlighted that patients older than 65 years and bearing an ultra-rare RTEL1 variant had higher values of these parameters (Fig. 1C).
Fig. 1.
A Values of N/L ratio in WT (red) and RTEL1-mutant (blue). B Values of N/L ratio after stratification for survival rate (dead/live) and C for age (< 65 years and > 65 years) in WT (red) and RTEL1-mutant (blue). D The values of N/L ratio and E CRP in RTEL1-mutant after the stratification based on ventilatory support. F The values of N/L ratio and G CRP in WT patients after the stratification based on ventilatory support. H The percentages of FVC and DLCO in RTEL1-mutant versus WT individuals after 6 months of follow-up. NL neutrophil to lymphocytes, HF high flows. *p < 0.05 **p < 0.01 ***p < 0.001 ****p < 0.0001
NLR and CRP levels were also analyzed based on ventilatory support. Among RTEL1-mutated patients, NLR is significantly higher in intubated, compared to those who did not require oxygen or used nasal cannulas; in the same way, CRP is relevantly more concentrated in patients requiring either CPAP ventilation or intubation (Fig. 1D, E).
NLR did not correlate with ventilatory support in the WT population, while CRP showed the same trend reported for RTEL1-mutated, namely showing higher values for intubated and CPAP-treated patients (Fig. 1F, G).
Decreased diffuse lung capacity in RTEL1-mutated patients at six months post-SARS-CoV-2 infection
To understand the role of RTEL1 on fibrotic development, post-COVID-19 pulmonary function tests were performed in a group of twelve patients with ultra-rare RTEL1 variants. Exams included FVC, DLCO, and laboratory analyses. The cohort was matched for age and sex to 18 patients with WT RTEL1 variants. Comparison analyses unveiled a relevant DLCO decrease for RTEL1 mutants (71.8 ± 14.4% versus 92.12 ± 5.6, respectively; p = 0.02). No differences are found for other parameters under consideration.
Discussion
The current COVID-19 pandemic represents a major public health concern, with more than 600 million cases worldwide at the time of writing (https://covid19.who.int/). The number of survivors improved in the last period, due to the development and deployment of vaccinations and other treatments [21]. Follow-up pneumological evaluations on people surviving severe COVID-19 evidenced a 40% chance of developing pulmonary sequelae with the potential for a neat decrease in life quality [22].
The development of pulmonary fibrosis following COVID-19 remains an open challenge for research. Little information is present in the literature, and no clear correlation emerged between the severity of COVID-19 and the development of fibrosis within the first year of post-infection monitoring [23]. Known shared molecular pathways between pulmonary fibrosis and COVID-19 are almost limited to those pertaining aberrant inflammation in association with dysregulated repair mechanisms and fibrogenesis [14]. Regarding genetic alterations, some similarities emerged between pulmonary fibrosis and altered lung functions following COVID-19. MUC5B and SFTPD are considered interesting loci associated with COVID-19 severity, and both genes are strongly correlated with pulmonary fibrosis onset [24]. Shorter telomeres are linked to worse COVID-19 symptoms, among which appears a delayed resolution of radiographic lung abnormalities [6, 25]. Telomere shortening is consistently observed in older adults, and therefore it is considered a reliable marker of aging associated with an increased risk of developing cardiovascular diseases and other disorders [26], including pulmonary fibrosis [27]. In COVID-19, shorter telomeres in peripheral blood cells are associated with less favorable prognosis [5], while this aspect does not seem to be influenced by age in post-COVID-19 analyses, possibly indicating that SARS-CoV-2 infection reduces telomere length directly [6].
In this paper, we demonstrated a relationship between RTEL1 genotype and COVID-19 phenotype, both in the acute and post-acute phase of the disease. Genetic alterations on RTEL1 may account for up to 8.6% of hospitalized patients. Recent but sound evidence identifies the gene as a major driver of interstitial lung disease (ILD) and heterozygous variants have been reported in about 5–9% of familial ILD [12, 28]. Our interpretation of these data is that SARS-CoV-2 infection triggers the underneath genetic susceptibility due to RTEL1 variants, leading to both a need for higher respiratory support in the acute phase, as well as to a chronic fibrotic process, which may eventually result in open ILD depending on specific (often private) variant (see Additional file 1: Table S1).
Our analyses found that RTEL1-mutated patients are younger, although with comparatively prolonged hospitalization and more frequent need for invasive ventilation. We believe this all makes RTEL1 a valid prognostic marker for COVID-19. Moreover, the mutated cohort more often presented impaired liver function and undesirable NLR in peripheral blood. Borie et al., described liver involvement for ILD subjects with RTEL1 alterations. Interestingly, these patients are seemingly less prone to develop pathological extrapulmonary phenotypes [29].
Both neutropenia and lymphocytosis can result in an NLR decrease. Previous studies do not generally mention neutropenia and lymphocytosis as a manifestation of COVID-19 infection. To the best of our knowledge, the phenomenon is only described in a few case reports [30, 31], or in patients with a concomitant hematological malignancy or solid tumor [32–34]. Kannengiesser et al. describe an early reversible neutropenia after cyclophosphamide treatment in pulmonary fibrosis patients with RTEL1 variants [10]. Among hematological abnormalities, the incidence of lymphopenia ranged from 40 to 80%, with increased NLR [35] associated with higher mortality rates, especially for severe cases of COVID-19 [36, 37]. We hypothesize that NLR alterations stem from the invasiveness of ventilation procedures. Exclusively for RTEL1 mutated, NLR trends reflected ventilation treatments better than CRP, which is currently the most used prognostic biomarker for COVID-19 [38–40].
Our RTEL1-mutated patients showed a higher prevalence of autoimmune COVID-19 comorbidities. This is in line with results from another cohort of individuals concomitantly presenting interstitial pneumoniae, autoimmune diseases, and RTEL1 variants in heterozygosity [41]: similarly to COVID-19 population, such patients also showed clinical manifestation related to a telomere syndrome, such as hematological abnormalities (i.e., neutrophil and lymphocyte alterations) and liver pathologies, along with an earlier manifestation of the pulmonary disease [42, 43].
Finally, the decreased DLCO after six months of COVID-19 suggests that RTEL1 variants can contribute to the development of lung fibrosis following COVID-19. However, this data needs to be confirming in a largest cohort also considering HRCT and other clinical and functional parameters. It is well demonstrated that in the majority of patients, DLCO and respiratory symptoms tend to normalize or improve one year after hospitalization [44]. There are, however, about 33% cases in which respiratory dysfunction persists, requiring prolonged follow-up. RTEL1 variants are reportedly the first genetic risk factor for the prediction of lung impairment after COVID-19. A wider cohort is needed for an accurate early identification of these patients.
Conclusion
In conclusion, our findings establish shared clinical risk factors between COVID-19 and pulmonary fibrosis. RTEL1 ultra-rare variants can be considered as a predictive marker of COVID-19 severity, as well as a marker of pathological evolution for pulmonary fibrosis in the post-COVID phase. This notion can be exploited for rapid screening in hospitalized infected people, for vaccine prioritization, and for appropriate follow-up assessment in subjects at risk.
Supplementary Information
Additional file 1. Table S1a. RTEL1 ultra-rare variants in hospitalized COVID-19 patients. Table S1b. RTEL1 ultra-rare variants in not-hospitalized COVID-19 patients.
Acknowledgements
This study is part of the GEN-COVID Multicenter study, https://sites.google.com/dbm.unisi.it/gen-covid, the Italian multicenter study aimed at identifying the COVID-19 host genetic bases. Specimens were provided by the COVID-19 Biobank of Siena, which is part of the Genetic Biobank of Siena, member of BBMRI-IT, Telethon Network of Genetic Biobanks (project no. GTB18001), EuroBioBank, and RD-Connect. All authors of this paper are members of the European Reference Network on rare respiratory diseases (ERN-LUNG). We thank the CINECA consortium for providing computational resources, and the Network for Italian Genomes (NIG; http://www.nig.cineca.it) for its support.
GEN-COVID Multicenter study (https://sites.google.com/dbm.unisi.it/gen-covid)
Francesca Mari1,2,3, Sergio Daga1,2, Ilaria Meloni1,2, Mirella Bruttini1,2,3, Susanna Croci1,2, Mirjam Lista1,2, Debora Maffeo1,2, Elena Pasquinelli1,2, Viola Bianca Serio1,2, Enrica Antolini1,2, Simona Letizia Basso1,2, Samantha Minetto1,2, Rossella Tita3, Maria Antonietta Mencarelli3, Caterina Lo Rizzo3, Anna Maria Pinto3, Francesca Ariani1,2,3, Francesca Montagnani2,4, Mario Tumbarello2,4, Ilaria Rancan2,4, Massimiliano Fabbiani4, Paolo Cameli5, David Bennett5, Federico Anedda6, Simona Marcantonio6, Sabino Scolletta6, Federico Franchi6, Maria Antonietta Mazzei7, Susanna Guerrini7, Edoardo Conticini8, Luca Cantarini8, Bruno Frediani8, Danilo Tacconi9, Chiara Spertilli Raffaelli9, Arianna Emiliozzi9, Marco Feri10, Alice Donati10, Raffaele Scala11, Luca Guidelli11, Genni Spargi12, Marta Corridi12, Cesira Nencioni13, Leonardo Croci13, Gian Piero Caldarelli14, Davide Romani15, Paolo Piacentini15, Maria Bandini15, Elena Desanctis15, Silvia Cappelli15, Anna Canaccini16, Agnese Verzuri16, Valentina Anemoli16, Manola Pisani16, Agostino Ognibene17, Maria Lorubbio17, Alessandro Pancrazzi17, Massimo Vaghi18, Antonella D'Arminio Monforte19, Federica Gaia Miraglia19, Mario U. Mondelli20,21, Stefania Mantovani20, Raffaele Bruno20,22, Marco Vecchia20, Marcello Maffezzoni22, Enrico Martinelli23, Massimo Girardis24, Stefano Busani24, Sophie Venturelli24, Andrea Cossarizza25, Andrea Antinori26, Alessandra Vergori26, Stefano Rusconi27,28, Matteo Siano28, Arianna Gabrieli28, Agostino Riva27,28, Daniela Francisci29, Elisabetta Schiaroli29, Carlo Pallotto29, Saverio Giuseppe Parisi30, Monica Basso30, Sandro Panese31, Stefano Baratti31, Pier Giorgio Scotton32, Francesca Andretta32, Mario Giobbia32, Renzo Scaggiante33, Francesca Gatti33, Francesco Castelli34, Eugenia Quiros-Roldan34, Melania Degli Antoni34, Isabella Zanella35,36, Matteo della Monica37, Carmelo Piscopo37, Mario Capasso38,39, Roberta Russo38,39, Immacolata Andolfo38,39, Achille Iolascon38,39, Giuseppe Fiorentino40, Massimo Carella41, Marco Castori41, Giuseppe Merla38,42, Gabriella Maria Squeo42, Filippo Aucella43, Pamela Raggi44, Rita Perna44, Matteo Bassetti45,46, Antonio Di Biagio45,46, Maurizio Sanguinetti47,48, Luca Masucci47,48, Alessandra Guarnaccia47, Serafina Valente49, Alex Di Florio49, Marco Mandalà50, Alessia Giorli50, Lorenzo Salerni50, Patrizia Zucchi51, Pierpaolo Parravicini51, Elisabetta Menatti52, Tullio Trotta53, Ferdinando Giannattasio53, Gabriella Coiro53, Fabio Lena54, Gianluca Lacerenza54, Cristina Mussini55, Luisa Tavecchia56, Lia Crotti57,58,59,60,61, Gianfranco Parati57,58, Roberto Menè57,58, Maurizio Sanarico62, Marco Gori63,64, Francesco Raimondi65, Alessandra Stella65, Filippo Biscarini66, Tiziana Bachetti67, Maria Teresa La Rovere68, Maurizio Bussotti69, Serena Ludovisi70, Katia Capitani71, Simona Dei72, Sabrina Ravaglia73, Annarita Giliberti74, Giulia Gori74, Rosangela Artuso74, Elena Andreucci74, Angelica Pagliazzi74, Erika Fiorentini74, Antonio Perrella75, Francesco Bianchi2,75, Paola Bergomi76, Emanuele Catena76, Riccardo Colombo76, Sauro Luchi77, Giovanna Morelli77, Paola Petrocelli77, Sarah Iacopini77, Sara Modica77, Silvia Baroni78, Giulia Micheli79, Marco Falcone80, Donato Urso80, Giusy Tiseo80, Tommaso Matucci80, Davide Grassi81, Claudio Ferri81, Franco Marinangeli82, Francesco Brancati83,84, Antonella Vincenti85, Valentina Borgo85, Stefania Lombardi85, Mirco Lenzi85, Massimo Antonio Di Pietro86, Francesca Vichi86, Benedetta Romanin86, Letizia Attala86, Cecilia Costa86, Andrea Gabbuti86, Alessio Bellucci86, Marta Colaneri22, Patrizia Casprini87, Cristoforo Pomara88, Massimiliano Esposito88, Roberto Leoncini89, Michele Cirianni89, Lucrezia Galasso89, Marco Antonio Bellini90, Chiara Gabbi91, Nicola Picchiotti63, Simone Furini2,92
1 Medical Genetics, University of Siena, Siena, 53100, Italy
2 Med Biotech Hub and Competence Center, Department of Medical Biotechnologies, University of Siena, Siena, 53100, Italy
3 Genetica Medica, Azienda Ospedaliero-Universitaria Senese, Siena, 53100, Italy
4 Department of Medical Sciences, Infectious and Tropical Diseases Unit, Azienda Ospedaliera Universitaria Senese, Siena, 53100, Italy
5 Unit of Respiratory Diseases and Lung Transplantation, Department of Internal and Specialist Medicine, University of Siena, Siena, 53100, Italy
6 Dept of Emergency and Urgency, Medicine, Surgery and Neurosciences, Unit of Intensive Care Medicine, Siena University Hospital, Siena, 53100, Italy
7 Department of Medical, Surgical and Neuro Sciences and Radiological Sciences, Unit of Diagnostic Imaging, University of Siena, 53100, Italy
8 Rheumatology Unit, Department of Medicine, Surgery and Neurosciences, University of Siena, Policlinico Le Scotte, Siena 53100, Italy
9 Department of Specialized and Internal Medicine, Infectious Diseases Unit, San Donato Hospital Arezzo 52100, Italy
10 Department of Emergency, Anesthesia Unit, San Donato Hospital, Arezzo, Italy
11 Department of Specialized and Internal Medicine, Pneumology Unit and UTIP, San Donato Hospital, Arezzo, 52100, Italy
12 Department of Emergency, Anesthesia Unit, Misericordia Hospital, Grosseto, 58100 Italy
13 Department of Specialized and Internal Medicine, Infectious Diseases Unit, Misericordia Hospital, Grosseto, 58100 Italy
14 Clinical Chemical Analysis Laboratory, Misericordia Hospital, Grosseto, 58100, Italy
15 Dipartimento di Prevenzione, Azienda USL Toscana Sud Est, 53100 Italy
16 Dipartimento Tecnico-Scientifico Territoriale, Azienda USL Toscana Sud Est, 53100, Italy
17 UOC Laboratorio Analisi Chimico Cliniche, Arezzo, 52100, Italy
18 Chirurgia Vascolare, Ospedale Maggiore di Crema, 26013 Italy
19 Department of Health Sciences, Clinic of Infectious Diseases, ASST Santi Paolo e Carlo, University of Milan, Milan, 20142, Italy
20 Division of Clinical Immunology—Infectious Diseases, Department of Medicine, Fondazione IRCCS Policlinico San Matteo, Pavia, 27100, Italy
21 Department of Internal Medicine and Therapeutics, University of Pavia, 27100 Italy
22 University of Pavia, Pavia, 27100 Italy
23 Department of Respiratory Diseases, Azienda Ospedaliera di Cremona, Cremona, 26100, Italy
24 Department of Anesthesia and Intensive Care, University of Modena and Reggio Emilia, Modena, 41121, Italy
25 Department of Medical and Surgical Sciences for Children and Adults, University of Modena and Reggio Emilia, Modena, 41121, Italy
26 HIV/AIDS Department, National Institute for Infectious Diseases, IRCCS, Lazzaro Spallanzani, Rome, 00161, Italy
27 III Infectious Diseases Unit, ASST-FBF-Sacco, Milan, 20146, Italy
28 Department of Biomedical and Clinical Sciences Luigi Sacco, University of Milan, Milan, 20146, Italy
29 Infectious Diseases Clinic, “Santa Maria della Misericordia” Hospital, University of Perugia, Perugia, 06100, Italy
30 Department of Molecular Medicine, University of Padova, Italy
31 Clinical Infectious Diseases, Mestre Hospital, Venezia, Italy.
32 Department of Infectious Diseases, Treviso Hospital, Local Health Unit 2 Marca Trevigiana, Treviso, Italy
33 Infectious Diseases Clinic, ULSS1, Belluno, Italy
34 Department of Infectious and Tropical Diseases, University of Brescia and ASST Spedali Civili Hospital, Brescia, Italy
35 Department of Molecular and Translational Medicine, University of Brescia, Italy;
36 Clinical Chemistry Laboratory, Cytogenetics and Molecular Genetics Section, Diagnostic Department, ASST Spedali Civili di Brescia, Italy
37 Medical Genetics and Laboratory of Medical Genetics Unit, A.O.R.N. "Antonio Cardarelli", Naples, Italy
38 Department of Molecular Medicine and Medical Biotechnology, University of Naples Federico II, Naples, Italy
39 CEINGE Biotecnologie Avanzate, Naples, Italy
40 Unit of Respiratory Physiopathology, AORN dei Colli, Monaldi Hospital, Naples, Italy
41 Division of Medical Genetics, Fondazione IRCCS Casa Sollievo della Sofferenza Hospital, San Giovanni Rotondo, Italy
42 Laboratory of Regulatory and Functional Genomics, Fondazione IRCCS Casa Sollievo della Sofferenza, Foggia, Italia
43 Department of Medical Sciences, Fondazione IRCCS Casa Sollievo della Sofferenza Hospital, San Giovanni Rotondo, Italy
44 Clinical Trial Office, Fondazione IRCCS Casa Sollievo della Sofferenza Hospital, San Giovanni Rotondo, Italy
45 Department of Health Sciences, University of Genova, Genova, Italy
46 Infectious Diseases Clinic, Policlinico San Martino Hospital, IRCCS for Cancer Research Genova, Italy
47 Microbiology, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Catholic University of Medicine, Rome, Italy
48 Department of Laboratory Sciences and Infectious Diseases, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
49 Department of Cardiovascular Diseases, University of Siena, Siena, Italy
50 Otolaryngology Unit, University of Siena, Italy
51 Department of Internal Medicine, ASST Valtellina e Alto Lario, Sondrio, Italy
52 Study Coordinator Oncologia Medica e Ufficio Flussi Sondrio, Italy
53 First Aid Department, Luigi Curto Hospital, Polla, Salerno, Italy
54 Department of Pharmaceutical Medicine, Misericordia Hospital, Grosseto, Italy.
55 Infectious Diseases Clinics, University of Modena and Reggio Emilia, Modena, Italy
56 U.O.C. Medicina, ASST Nord Milano, Ospedale Bassini, Cinisello Balsamo (MI), Italy
57 Istituto Auxologico Italiano, IRCCS, Department of Cardiovascular, Neural and Metabolic Sciences, San Luca Hospital, Milan, Italy
58 Department of Medicine and Surgery, University of Milano-Bicocca, Milan, Italy
59 Istituto Auxologico Italiano, IRCCS, Center for Cardiac Arrhythmias of Genetic Origin, Milan, Italy
60 Istituto Auxologico Italiano, IRCCS, Laboratory of Cardiovascular Genetics, Milan, Italy
61 Member of the European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart-ERN GUARD-Heart
62 Independent Data Scientist, Milan, Italy
63 University of Siena, DIISM-SAILAB, Siena, Italy
64 Maasai, I3S CNRS, Université Côte d'Azur, France
65 Laboratorio di Biologia Bio@SNS, Scuola Normale Superiore, Pisa, Italy
66 CNR-Consiglio Nazionale delle Ricerche, Istituto di Biologia e Biotecnologia Agraria (IBBA), Milano, Italy
67 Direzione Scientifica, Istituti Clinici Scientifici Maugeri IRCCS, Pavia, Italy
68 Istituti Clinici Scientifici Maugeri IRCCS, Department of Cardiology, Institute of Montescano, Pavia, Italy
69 Istituti Clinici Scientifici Maugeri IRCCS, Department of Cardiology, Institute of Milan, Italy
70 Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
71 Core Research Laboratory, ISPRO, Florence, Italy
72 Health Management, Azienda USL Toscana Sud Est, Tuscany, Italy
73 IRCCS C. Mondino Foundation, Pavia, Italy
74 Medical Genetics Unit, Meyer Children's University Hospital, Firenze, Italy
75 Department of Medicine, Pneumology Unit, Misericordia Hospital, Grosseto, Italy.
76 Department of Anesthesia and Intensive Care Unit, ASST Fatebenefratelli Sacco, Luigi Sacco Hospital, Polo Universitario, University of Milan, Milan, Italy
77 Infectious Disease Unit, Hospital of Lucca, Italy
78 Department of Diagnostic and Laboratory Medicine, Institute of Biochemistry and Clinical Biochemistry, Fondazione Policlinico Universitario A. Gemelli IRCCS, Catholic University of the Sacred Heart, Rome, Italy.
79 Clinic of Infectious Diseases, Catholic University of the Sacred Heart, Rome, Italy
80 Department of Clinical and Experimental Medicine, Infectious Diseases Unit, University of Pisa, Pisa, Italy
81 Department of Clinical Medicine, Public Health, Life and Environment Sciences, University of L'Aquila, Italy
82 Anesthesiology and Intensive Care, University of L'Aquila, L'Aquila, Italy
83 Department of Life, Health and Environmental Sciences, University of L'Aquila, 67100, L’Aquila, Italy
84 Human Functional Genomics Laboratory, IRCCS San Raffaele Roma, 00167, Rome, Italy
85 Infectious Disease Unit, Hospital of Massa, Italy
86 Infectious Diseases Unit, Santa Maria Annunziata Hospital, USL Centro, Florence, Italy
87 Laboratory of Clinical Pathology and Immunoallergy, Florence-Prato, Italy
88 Department of Medical, Surgical and Advanced Technologies "G.F. Ingrassia", University of Catania, Catania, Italy
89 Laboratorio Patologia Clinica, Azienda Ospedaliero-Universitaria Senese, Siena, Italy
90 Ambulatory Chronic Polipathology of Siena, Department of Medicine, Surgery and Neurosciences, University of Siena, Siena, Italy
91 Department of Biosciences and Nutrition, Karolinska Institutet, Stockholm, Sweden
92 Bioinformatics, University of Bologna, Italy
Author contributions
LB: study concept and design, drafting of the manuscript. MB: Analysis and interpretation of data, drafting of the manuscript; MdA: analysis and interpretation of data; GB: Statistical analysis, GF: acquisition of data; KZ: statistical analysis, ADI: statistical analysis and drafting the paper; CF: laboratory and statistical analysis and drafting the paper; EB: study supervision, analysis and interpretation of data, critical revision of the manuscript for important intellectual content; AR: study supervision, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
We thank private donors for the support provided to AR (Department of Medical Biotechnologies, University of Siena) for the COVID-19 host genetics research project (D.L n.18 of March 17, 2020). We also thank the COVID-19 Host Genetics Initiative (https://www.covid19hg.org/), MIUR project ‘Dipartimenti di Eccellenza 2018–2020’ to the Department of Medical Biotechnologies University of Siena, Italy, and ‘Bando Ricerca COVID-19 Toscana’ project to Azienda Ospedaliero-Universitaria Senese. We acknowledge Intesa San Paolo for the 2020 charity fund dedicated to the project N B/2020/0119 “Identificazione delle basi genetiche determinanti la variabilità clinica della risposta a COVID-19 nella popolazione italiana”, as well as the Italian Ministry of University and Research for funding within the “Bando FISR 2020” in COVID-19 for the project “Editing dell'RNA contro il SARS-CoV-2: hackerare il virus per identificare bersagli molecolari e attenuare l'infezione—HACKTHECOV” and the Istituto Buddista Italiano Soka Gakkai for funding the project “PAT-COVID: Host genetics and pathogenetic mechanisms of COVID-19” (ID n. 2020-2016_RIC_3). We thank the EU project H2020-SC1-FA-DTS-2018–2020, titled “International consortium for integrative genomics prediction (INTERVENE)”—Grant Agreement No. 101016775. Generous support was also received from private donations by Maurizio Traglio, Enzo Cattaneo and Alberto Borella.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All the enrolled subjects were adults (aged ≥ 18 years), and either they or their legally authorized representatives provided informed consent for participation. The GEN-COVID Multicenter study was approved by the University Hospital (Azienda ospedaliero-universitaria Senese) Ethical Review Board, Siena, Italy (Prot n. 16917, dated March 16, 2020), and by the local internal review boards of all the recruiting hospitals involved.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laura Bergantini and Margherita Baldassarri contributed equally to this work.
Contributor Information
Elena Bargagli, Email: bargagli2@unisi.it.
Alessandra Renieri, Email: alessandra.renieri@unisi.it.
GEN-COVID Multicenter study:
Francesca Mari, Sergio Daga, Ilaria Meloni, Mirella Bruttini, Susanna Croci, Mirjam Lista, Debora Maffeo, Elena Pasquinelli, Viola Bianca Serio, Enrica Antolini, Simona Letizia Basso, Samantha Minetto, Rossella Tita, Maria Antonietta Mencarelli, Caterina Lo Rizzo, Anna Maria Pinto, Francesca Ariani, Francesca Montagnani, Mario Tumbarello, Ilaria Rancan, Massimiliano Fabbiani, Paolo Cameli, David Bennett, Federico Anedda, Simona Marcantonio, Sabino Scolletta, Federico Franchi, Maria Antonietta Mazzei, Susanna Guerrini, Edoardo Conticini, Luca Cantarini, Bruno Frediani, Danilo Tacconi, Chiara Spertilli Raffaelli, Arianna Emiliozzi, Marco Feri, Alice Donati, Raffaele Scala, Luca Guidelli, Genni Spargi, Marta Corridi, Cesira Nencioni, Leonardo Croci, Gian Piero Caldarelli, Davide Romani, Paolo Piacentini, Maria Bandini, Elena Desanctis, Silvia Cappelli, Anna Canaccini, Agnese Verzuri, Valentina Anemoli, Manola Pisani, Agostino Ognibene, Maria Lorubbio, Alessandro Pancrazzi, Massimo Vaghi, Antonella D.’Arminio Monforte, Federica Gaia Miraglia, Mario U. Mondelli, Stefania Mantovani, Raffaele Bruno, Marco Vecchia, Marcello Maffezzoni, Enrico Martinelli, Massimo Girardis, Stefano Busani, Sophie Venturelli, Andrea Cossarizza, Andrea Antinori, Alessandra Vergori, Stefano Rusconi, Matteo Siano, Arianna Gabrieli, Agostino Riva, Daniela Francisci, Elisabetta Schiaroli, Carlo Pallotto, Saverio Giuseppe Parisi, Monica Basso, Sandro Panese, Stefano Baratti, Pier Giorgio Scotton, Francesca Andretta, Mario Giobbia, Renzo Scaggiante, Francesca Gatti, Francesco Castelli, Eugenia Quiros-Roldan, Melania Degli Antoni, Isabella Zanella, Matteo della Monica, Carmelo Piscopo, Mario Capasso, Roberta Russo, Immacolata Andolfo, Achille Iolascon, Giuseppe Fiorentino, Massimo Carella, Marco Castori, Giuseppe Merla, Gabriella Maria Squeo, Filippo Aucella, Pamela Raggi, Rita Perna, Matteo Bassetti, Antonio Di Biagio, Maurizio Sanguinetti, Luca Masucci, Alessandra Guarnaccia, Serafina Valente, Alex Di Florio, Marco Mandalà, Alessia Giorli, Lorenzo Salerni, Patrizia Zucchi, Pierpaolo Parravicini, Elisabetta Menatti, Tullio Trotta, Ferdinando Giannattasio, Gabriella Coiro, Fabio Lena, Gianluca Lacerenza, Cristina Mussini, Luisa Tavecchia, Lia Crotti, Gianfranco Parati, Roberto Menè, Maurizio Sanarico, Marco Gori, Francesco Raimondi, Alessandra Stella, Filippo Biscarini, Tiziana Bachetti, Maria Teresa La Rovere, Maurizio Bussotti, Serena Ludovisi, Katia Capitani, Simona Dei, Sabrina Ravaglia, Annarita Giliberti, Giulia Gori, Rosangela Artuso, Elena Andreucci, Angelica Pagliazzi, Erika Fiorentini, Antonio Perrella, Francesco Bianchi, Paola Bergomi, Emanuele Catena, Riccardo Colombo, Sauro Luchi, Giovanna Morelli, Paola Petrocelli, Sarah Iacopini, Sara Modica, Silvia Baroni, Giulia Micheli, Marco Falcone, Donato Urso, Giusy Tiseo, Tommaso Matucci, Davide Grassi, Claudio Ferri, Franco Marinangeli, Francesco Brancati, Antonella Vincenti, Valentina Borgo, Stefania Lombardi, Mirco Lenzi, Massimo Antonio Di Pietro, Francesca Vichi, Benedetta Romanin, Letizia Attala, Cecilia Costa, Andrea Gabbuti, Alessio Bellucci, Marta Colaneri, Patrizia Casprini, Cristoforo Pomara, Massimiliano Esposito, Roberto Leoncini, Michele Cirianni, Lucrezia Galasso, Marco Antonio Bellini, Chiara Gabbi, Nicola Picchiotti, and Simone Furini
References
- 1.Croci S, Venneri MA, Mantovani S, Fallerini C, Benetti E, Picchiotti N, et al. The polymorphism L412F in TLR3 inhibits autophagy and is a marker of severe COVID-19 in males. Autophagy. 2022;18:1662–1672. doi: 10.1080/15548627.2021.1995152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zecevic M, Kotur N, Ristivojevic B, Gasic V, Skodric-Trifunovic V, Stjepanovic M, et al. Genome-Wide Association Study of COVID-19 Outcomes Reveals Novel Host Genetic Risk Loci in the Serbian Population. Front Genetics [Internet]. 2022 doi: 10.3389/fgene.2022.911010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature. 2021;600:472–7. [DOI] [PMC free article] [PubMed]
- 4.Gelzo M, Cacciapuoti S, Pinchera B, De Rosa A, Cernera G, Scialò F, et al. Prognostic role of neutrophil to lymphocyte ratio in COVID-19 patients: still valid in patients that had started therapy? Front Public Health. 2021 doi: 10.3389/fpubh.2021.664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Vazquez R, Guío-Carrión A, Zapatero-Gaviria A, Martínez P, Blasco MA. Shorter telomere lengths in patients with severe COVID-19 disease. Aging (Albany NY) 2021;13:1–15. doi: 10.18632/aging.202463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mongelli A, Barbi V, Gottardi Zamperla M, Atlante S, Forleo L, Nesta M, et al. Evidence for biological age acceleration and telomere shortening in COVID-19 survivors. Int J Mol Sci [Internet]. 2021;22:6151. doi: 10.3390/ijms22116151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/S0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 8.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet [Internet]. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, et al. Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat Genet [Internet]. 2015;47:512–517. doi: 10.1038/ng.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannengiesser C, Borie R, Ménard C, Réocreux M, Nitschké P, Gazal S, et al. Heterozygous RTEL1 mutations are associated with familial pulmonary fibrosis. Eur Respir J. 2015;46:474–485. doi: 10.1183/09031936.00040115. [DOI] [PubMed] [Google Scholar]
- 11.Juge P-A, Borie R, Kannengiesser C, Gazal S, Revy P, Wemeau-Stervinou L, et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J. 2017;49:1602314. doi: 10.1183/13993003.02314-2016. [DOI] [PubMed] [Google Scholar]
- 12.Cogan JD, Kropski JA, Zhao M, Mitchell DB, Rives L, Markin C, et al. Rare variants in RTEL1 are associated with familial interstitial pneumonia. Am J Respir Crit Care Med. 2015;191:646–655. doi: 10.1164/rccm.201408-1510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Carmona S, Falfán-Valencia R, Verónica-Aguilar A, Buendía-Roldán I, Chávez-Galán L, Hernández-Zenteno RJ, et al. COVID-19 survivor patients carrying the Rs35705950 risk allele in MUC5B have higher plasma levels of mucin 5B. Curr Issues Mol Biol. 2022;44:3283–3290. doi: 10.3390/cimb44080226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergantini L, Mainardi A, d’Alessandro M, Cameli P, Bennett D, Bargagli E, et al. Common molecular pathways between post-COVID19 syndrome and lung fibrosis: a scoping review. Front Pharmacol. 2022;13:748931. doi: 10.3389/fphar.2022.748931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallerini C, Picchiotti N, Baldassarri M, Zguro K, Daga S, Fava F, et al. Common, low-frequency, rare, and ultra-rare coding variants contribute to COVID-19 severity. Hum Genet. 2022;141:147–173. doi: 10.1007/s00439-021-02397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang L, Tang B-S, Guo J-F, Li J-C. Telomere length and COVID-19 outcomes: a two-sample bidirectional Mendelian randomization study. Front Genet. 2022;13:805903. doi: 10.3389/fgene.2022.805903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Victor J, Jordan T, Lamkin E, Ikeh K, March A, Frere J, et al. SARS-CoV-2 hijacks host cell genome instability pathways. Res Sq. 2022;rs.3.rs-1556634.
- 18.COVID-19 Therapeutic Trial Synopsis [Internet]. [cited 2023 Jan 14]. Available from: https://www.who.int/publications-detail-redirect/covid-19-therapeutic-trial-synopsis
- 19.Daga S, Fallerini C, Baldassarri M, Fava F, Valentino F, Doddato G, et al. Employing a systematic approach to biobanking and analyzing clinical and genetic data for advancing COVID-19 research. Eur J Hum Genet. 2021;29:745–759. doi: 10.1038/s41431-020-00793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picchiotti N, Benetti E, Fallerini C, Daga S, Baldassarri M, Fava F, et al. Post-Mendelian genetic model in COVID-19. Cardiol Cardiovasc Med. 2021;5(6):673–769. doi: 10.1101/2021.01.27.21250593v1. [DOI] [Google Scholar]
- 21.COVID-19 Host Genetics Initiative. A first update on mapping the human genetic architecture of COVID-19. Nature. 2022;608:E1–10. [DOI] [PMC free article] [PubMed]
- 22.Tarraso J, Safont B, Carbonell-Asins JA, Fernandez-Fabrellas E, Sancho-Chust JN, Naval E, et al. Lung function and radiological findings 1 year after COVID-19: a prospective follow-up. Respir Res. 2022;23:242. doi: 10.1186/s12931-022-02166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hama Amin BJ, Kakamad FH, Ahmed GS, Ahmed SF, Abdulla BA, Mohammed SH, et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann Med Surg (Lond) 2022;77:103590. doi: 10.1016/j.amsu.2022.103590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Moorsel CHM, van der Vis JJ, Duckworth A, Scotton CJ, Benschop C, Ellinghaus D, et al. The MUC5B promoter polymorphism associates with severe COVID-19 in the European population. Front Med [Internet]. 2021 doi: 10.3389/fmed.2021.668024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Retuerto M, Lledó A, Fernandez-Varas B, Guerrero-López R, Usategui A, Lalueza A, et al. Shorter telomere length is associated with COVID-19 hospitalization and with persistence of radiographic lung abnormalities. Immunity Ageing [Internet]. 2022;19:38. doi: 10.1186/s12979-022-00294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng F, Carroll L, Joglekar MV, Januszewski AS, Wong KK, Hardikar AA, et al. Diabetes, metabolic disease, and telomere length. Lancet Diabetes Endocrinol. 2021;9:117–126. doi: 10.1016/S2213-8587(20)30365-X. [DOI] [PubMed] [Google Scholar]
- 27.M’Kacher R, Jaillet M, Colicchio B, Vasarmidi E, Mailleux A, Dieterlen A, et al. Lung fibroblasts from idiopathic pulmonary fibrosis patients harbor short and unstable telomeres leading to chromosomal instability. Biomedicines. 2022;10:310. doi: 10.3390/biomedicines10020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kropski JA, Loyd JE. Telomeres revisited: RTEL1 variants in pulmonary fibrosis. Eur Respir J. 2015;46:312–314. doi: 10.1183/13993003.00710-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borie R, Bouvry D, Cottin V, Gauvain C, Cazes A, Debray M-P, et al. Regulator of telomere length 1 (RTEL1) mutations are associated with heterogeneous pulmonary and extra-pulmonary phenotypes. Eur Respir J. 2019;53:1800508. doi: 10.1183/13993003.00508-2018. [DOI] [PubMed] [Google Scholar]
- 30.Mank VMF, Mank J, Ogle J, Roberts J. Delayed, transient and self-resolving neutropenia following COVID-19 pneumonia. BMJ Case Reports CP [Internet]. 2021;14:e242596. doi: 10.1136/bcr-2021-242596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lerman TT, Sagi M, Shafir Y, Sheena L, Cohen E, Goldberg E, et al. A possible increased risk of metamizole-associated neutropenia among COVID-19 patients. Br J Clin Pharmacol [Internet]. 2021;87:2902–2906. doi: 10.1111/bcp.14703. [DOI] [PubMed] [Google Scholar]
- 32.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.López-Pereira P, Iturrate I, de La Cámara R, Cardeñoso L, Alegre A, Aguado B. Can COVID-19 cause severe neutropenia? Clin Case Rep. 2020;8:3349–3351. doi: 10.1002/ccr3.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taha M, Sharma A, Soubani A. Clinical deterioration during neutropenia recovery after G-CSF therapy in patient with COVID-19. Respir Med Case Rep. 2020;31:101231. doi: 10.1016/j.rmcr.2020.101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh R, Ratre BK, Sirohiya P, Bhatnagar S. Self-limiting severe neutropenia in a patient with COVID-19. BMJ Case Rep. 2021;14:e247057. doi: 10.1136/bcr-2021-247057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tavakolpour S, Rakhshandehroo T, Wei EX, Rashidian M. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parthasarathi A, Padukudru S, Arunachal S, Basavaraj CK, Krishna MT, Ganguly K, et al. The role of neutrophil-to-lymphocyte ratio in risk stratification and prognostication of COVID-19: a systematic review and meta-analysis. Vaccines (Basel) 2022;10:1233. doi: 10.3390/vaccines10081233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rello AP, Martínez AM, Iturbe CV, Escolano-Pueyo A, Bayo EH, Lafarga IA. Assessment of the effectiveness of tocilizumab on mortality and progression to mechanical ventilation or intensive care in patients with COVID-19 admitted to a tertiary hospital. Eur J Hosp Pharm [Internet]. British Medical Journal Publishing Group; 2022 [cited 2023 Jan 14]; Available from: https://ejhp.bmj.com/content/early/2022/09/23/ejhpharm-2022-003366. [DOI] [PMC free article] [PubMed]
- 39.Besutti G, Pellegrini M, Ottone M, Bonelli E, Monelli F, Farì R, et al. Modifications of chest CT body composition parameters at three and six months after severe COVID-19 pneumonia: a retrospective cohort study. Nutrients. 2022;14:3764. doi: 10.3390/nu14183764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zinellu A, Mangoni AA. A systematic review and meta-analysis of the association between the neutrophil, lymphocyte, and platelet count, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio and COVID-19 progression and mortality. Expert Rev Clin Immunol. 2022;18:1187–1202. doi: 10.1080/1744666X.2022.2120472. [DOI] [PubMed] [Google Scholar]
- 41.Yuan Z-Z, Fan L-L, Wang C-Y, Luo H, Liu L. Novel heterozygous mutation of RTEL1 in interstitial pneumonia with autoimmune feature. QJM. 2022;115:253–255. doi: 10.1093/qjmed/hcab315. [DOI] [PubMed] [Google Scholar]
- 42.Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet Med. 2010;12:753–764. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman TW, van der Vis JJ, Biesma DH, Grutters JC, van Moorsel CHM. Extrapulmonary manifestations of a telomere syndrome in patients with idiopathic pulmonary fibrosis are associated with decreased survival. Respirology. 2022;27:959–965. doi: 10.1111/resp.14264. [DOI] [PubMed] [Google Scholar]
- 44.Fortini A, Rosso A, Cecchini P, Torrigiani A, Lo Forte A, Carrai P, et al. One-year evolution of DLCO changes and respiratory symptoms in patients with post COVID-19 respiratory syndrome. Infection. 2022;50:513–517. doi: 10.1007/s15010-022-01755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1a. RTEL1 ultra-rare variants in hospitalized COVID-19 patients. Table S1b. RTEL1 ultra-rare variants in not-hospitalized COVID-19 patients.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.