Abstract
Four children with cystic fibrosis, ranging in age from 10 to 40 months, were admitted to a specialized pediatric unit for evaluation and treatment of malnutrition. All were below the fifth percentile for weight despite appropriate pancreatic enzyme replacement and outpatient nutritional counseling. Dietary evaluation revealed oral intake of 48% to 62% of that required for growth. Standardized nursing and psychological assessments of feeding behaviors during meals indicated a low acceptance rate of foods and a high rate of maladaptive feeding behaviors. Treatment consisted of behavioral management using positive reinforcement of food acceptance, extinction of negative behaviors, and parent training. Mean percentage of caloric intake increased from 54% to 92% for the four patients. At long-term follow-up, the patients who continued the program demonstrated substantial and persistent catch-up growth. Behavioral feeding disorders may contribute to failure to thrive in patients with cystic fibrosis and must be considered when growth failure occurs despite correct medical management and apparently mild pulmonary and gastrointestinal involvement.
Keywords: feeding, failure to thrive, cystic fibrosis, behavioral modification
Growth failure with undernutrition is common in infants and young children with cystic fibrosis (CF) and is frequently the presenting symptom.1 Cystic fibrosis is the most common lethal genetic syndrome in white infants. However, improved diagnostic and medical management procedures have increased life expectancy from early childhood to young adulthood for most patients. Malnutrition is a significant problem for many children with CF. Thus, close monitoring of nutritional status and nutritional rehabilitation programs are important components of medical management. Nutritional recommendations for children with CF include intake of 150% to 200% of calories recommended for healthy peers to maximize growth and minimize risk of infection.2 Some studies suggest that children with CF consumed far less than these recommendations, making maintenance of adequate oral intake a continuing parental and medical concern.2,3 Underlying physiological, disease-related abnormalities in the gastrointestinal and respiratory systems contribute to the need for bolstered caloric intake. For example, malabsorption of fats due to pancreatic insufficiency characteristic of CF and the increased work of breathing secondary to chronic lung infections are illness-related problems affecting the need for greater oral intake.4 At the same time, disease-associated abdominal pain, secondary taste aversions, and fatigue may interfere with appetite and adequate food intake.
Little attention has been paid to the contribution of behavioral variables to growth and development in CF. Chronic lung infections, hospitalizations, and vomiting with coughing paroxysms may contribute to anorexia and behavioral feeding problems, particularly in younger patients. Infant and maternal behavior, attachment, and interaction patterns, especially around feeding, also may be altered, given parental need to be unusually concerned about child health and caloric consumption.5–7 Because of the potential for medical and psychological factors to interact in the expression of eating disorders and malnutrition in children with CF, we describe the behavioral management of four young children with CF who were referred to a specialized pediatric inpatient unit for evaluation of food refusal, inadequate oral intake, and growth failure, despite stable disease and optimal medical management.
Patients and Methods
All patients were hospitalized on the Medical-Behavioral Center (MBC), a 15-bed pediatric unit designed for diagnosis and intervention of complex medical problems in which combined medical and psychological management are necessary, i.e., psychophysiological disorders, complicated failure to thrive, and chronic illnesses in which psychological factors adversely affect medical treatment. Staffing includes a pediatrician and clinical psychologist as co-directors, psychologists, behavioral pediatricians, a dietitian, nurse clinician, and specially trained pediatric nursing staff.
All four children (three girls, one boy) with cystic fibrosis (CF) under 5 years old referred to the unit for evaluation of feeding and growth problems over a 3-year period are reported here. The diagnosis of CF for all children was made before they were a year old by a pediatric pulmonologist, based on elevated sweat chloride levels and characteristic clinical findings or a family history of CF. Additional medical diagnoses, age at referral, growth status, and description of feeding problems for the patients are shown in Table 1. None were mentally retarded, had other developmental disabilities, motor or sensory deficits, or ever had received parenteral or enteral feedings. Despite aggressive medical treatment, feeding problems and poor growth had been present for at least several months prior to referral, and no improvement in oral intake or growth had occurred during an initial hospitalization of 3 to 30 days on a regular pediatric unit. Parents were interviewed to obtain a history of the child’s feeding behavior, with special emphasis on age of onset of problems; circumstances perceived as related to the feeding problems; previous methods used to increase child’s intake; usual place, timing, and duration of meals; preferred and refused foods; and behaviors at meals. Psychology and nursing staff observed a minimum of three baseline meals, noting the child’s and parent’s mealtime behaviors, including the child’s self-feeding, acceptance and refusal of offered food, gagging, crying, tantrums, and emesis, and parental responses to these behaviors. Particularly noted were parental response behaviors of force feeding, coaxing the child to eat, threatening or bribing, inconsistency in verbalizations, and allowing the child to leave or avoid the meal.
TABLE 1.
Behavioral Treatment of Feeding Disorders in Cystic Fibrosis
| Patient | Medical Diagnosis | Age Referred (Mo) | Feeding Problems | Weight for length (Percentiles) |
|---|---|---|---|---|
| 1 | Hyponatremia Hypochloremic dehydration Failure to thrive |
10 | Refusal Tantrums |
<1st |
| 2 | Pneumonia Malnutrition Failure to thrive |
42 | Refusal Gags |
<5th |
| 3 | Bronchiolitis Otitis media Failure to thrive |
13 | Refusal Emesis Selectivity |
<5th |
| 4 | Reactive airway disease Seizures Failure to thrive |
20 | Refusal Gags Emesis |
<5th |
A registered dietitian evaluated the child’s growth status, noting weight/age, length or height/age; percentage of desirable body weight for height/length based on National Center for Health Statistics growth standards;8 calculation of nutrient needs for optimal repletion and catch-up growth; diet prior to referral; previous use of supplements; and adherence to enzyme and vitamin recommendations. Daily caloric intake was determined based on nurses’ completion of menu records for each meal and snack.
Treatment consisted of developing a hierarchy of steps and criteria for success, allowing the child access to food and drinks only at specified mealtimes, the use of reinforcers of socialization, praise, stickers, access to toys, music, claping, and preferred foods immediately after appropriate eating, and the use of time-out as a consequence for non-eating.9–13 (Time-out is a commonly used consequence for negative behaviors that involves removal of the child from a stimulating place of interaction.) For the children in this study, it usually involved the caregiver’s removing toys or social interaction after the food refusal. Parents also were taught basic principles of contingency management including shaping (rewarding successive approximations of the targeted eating behavior); positive reinforcement (rewarding the child with socialization, access to toys after completion of the desired behavior); and ignoring (inattention to inappropriate behaviors). Treatment procedures initially were implemented by nursing and psychology staff. At first, all children were fed either in a private feeding room or a screened-off area in the patient dining room to minimize distraction. After consistent oral intake was established, parents were taught to carry out the procedures.
CASE REPORTS
Patient 1
A 10-month-old black girl diagnosed with CF at 3 months of age, was admitted to a pediatric unit for her third hospitalization for hyponatremia, hypochloremic dehydration, and 600 g weight loss. She had a history of poor oral intake and poor weight gain since birth. At admission, her weight of 5.7 kg was below the fifth percentile for age and weight/length was below the third percentile. Although her dehydration stabilized rapidly, intake continued to be erratic and inadequate. On observation, she demonstrated many food refusal behaviors including head turning, tantrums, and batting the spoon away. Some staff observed that she ate less and demonstrated more behavioral problems when her mother, rather than nursing staff, was feeding her. It was thought she would benefit from close observation of feeding and assessment of mother-child interactions, and transfer to the MBC was effected.
At transfer, her admission weight was 6.38 kg, less than the first percentile and her caloric intake was only 48% of that necessary for optimal repletion and catch-up growth. She did not demonstrate refusal behaviors but was easily distracted and extremely active. She tired quickly during meals, working increasingly harder to breathe, which interfered with her eating. A behavioral feeding plan was initiated with the objective of providing as many calories as possible without exhausting her. The plan included changing medication and aerosol schedules to increase her ability and willingness to accept food. She was initially bottle fed a 27-calorie per ounce formula in a nondistracting environment. Limited amounts of solids were then added. The nipple hole was enlarged to reduce the work of sucking. Later, liquid during the meal was provided in a Tippy cup that required less energy to drink than did the bottle.
Although the mother-child interaction was generally appropriate, the patient’s mother benefited from learning feeding skills to increase oral intake when the child was not accepting food as readily. Skills taught the mother included allowing her child to rest and breathe between bites, then pacing the meal more slowly, immediate reinforcement of food acceptance through social reward, and systematically ignoring avoidance behaviors. After 13 days of treatment, patient 1 was discharged from the unit weighing 6.8 kg and consuming 100% of recommended calories. At 18 months of age, follow-up data indicated that feeding behaviors continued to improve after unit discharge and that she had demonstrated good growth and weight gain, with weight for length increasing to the fourth percentile. By 29 months of age, her weight for length had increased to the tenth percentile.
Patient 2
A 3.5-year-old white girl diagnosed at 1 year old had a long history of poor weight gain and growth. On her first admission for congestion, coughing, decreased energy, and intermittent low-grade temperatures, she weighed 11.6 kg (<fifth percentile), with weight/height in the fifth percentile. Her mother reported the child had few favored foods and ate only small amounts of these. Her mother stated that her child ate better at preschool and that she, herself, had difficulty setting limits due to her guilt and fears related to CF. She stated directly that she feared her daughter “might die.” She also reported substantial marital discord. The patient demonstrated poor eating on the medical ward, refusing her formula, and eating only 56% of required calories.
By transfer to the MBC on the eighth day of hospitalization, her weight had decreased to 11.4 kg. A behavioral feeding program was instituted that included three stuctured meals and two snacks, toy play, social reinforcement, and a sticker program for appropriate eating with time-outs for inappropriate behavior. Sharp verbal “No(s)!” were used when she attempted to gag. Her mother observed nurses and subsequently was taught the above program. Gradually, her mother began supervising meals by herself and at discharge had improved considerably in her ability to implement the behavioral program. At discharge, patient 2 was eating 67% of required calories and had reached 11.5 kg. Because the family lived out of state, discharge was somewhat premature, with optimal caloric intake not attained in hospital. Moreover, the family was not able to be maintained in regular follow-up. When seen again at 53 months of age, her weight/height had slowly increased to the fifth percentile. However, when seen at 66 months, her growth had again fallen below the fifth percentile. Her parents reported considerable psychological stress in the home, including a marital separation that they said affected their child’s sleeping, eating, and school performance. They had not re-engaged in behavioral or other psychological intervention since discharge.
Patient 3
A 13-month-old boy, diagnosed at 3 months of age, initially grew well but developed feeding refusal behaviors between 6 and 12 months of age. When offered solids or new foods, he averted his head, screamed, kicked, and frequently gagged to induce emesis. His mother responded by not feeding him these foods and by removing him from the highchair when he began to cry. Outpatient treatment had been attempted with no improvement. His mother explained, “Maybe he feels my anxiety—I’m so afraid he will aspirate.” During admission history, his mother said she had been told of a child in the hospital who had been admitted with history of aspiration and was in a vegetative state.
He was admitted first to a medical floor for aggressive pulmonary therapy. He weighed 8.4 kg, less than the fifth percentile for age. A gastroenterologist was consulted and agreed with the initial impression of a behavioral feeding disorder. He was transferred on day 3 to the MBC for evaluation and treatment.
Nutritional assessment indicated he had reached the fifth percentile for length and weight/age at age 6 months but subsequently had been falling off the growth curve. Although patient 3 was at an appropriate weight for his length, his length was well below the fifth percentile, indicating stunting and chronic undernutrition. At transfer, his weight (8.1 kg) was well below the fifth percentile. Dietary evaluation indicated he was eating 50% of the calories recommended for growth and weight gain. His diet had become severely restricted, as he ate only yogurt. A behavioral feeding program was initiated that included using bites of yogurt, social praise, and toy play as reinforcers for acceptance of bites of non-preferred food. In addition, sharp “No(s)!” or distraction followed gags. Time-outs were used for refusal behaviors. The last 10 to 15 minutes of the meal were used to develop age-appropriate feeding skills (e.g., self-feeding, use of Tippy cup).
His mother observed meals initially, then was instructed in the feeding program and developed adequate ability to feed him. At discharge, patient 3 was eating 100% of his required calories, eating a wide variety of age-appropriate foods, was able to drink from a cup and to finger feed. His weight had increased to 9.15 kg (eighth percentile). Follow-up at 18 months of age indicated his weight to be at the twenty-fifth pecentile for age. By 33 months of age, his weight was at the fiftieth percentile. His length for age had increased to the eighth percentile.
Patient 4
A 21-month-old white female, diagnosed with CF at 10 weeks of age, presented after several previous admissions to another hospital for wheezing and poor nutrition with failure to gain weight. At admission, her weight of 8.5 kg was below the fifth percentile for age, and she had lost 0.5 kg in 3 months. In the hospital, she demonstrated poor intake, refusing to eat anything except juices and popsicles and consuming only 62% of calories required for repletion and catch-up growth. She was transferred to the MBC on the eighth hospital day of admission at weight of 8.4 kg.
Parental history indicated that she had been a poor feeder since birth but had a decrease in appetite with the transition from baby to table foods. Her parents reported trying numerous interventions on an inconsistent basis, including rigidly structuring mealtimes, attempting to feed throughout the day, and force-feeding. When force-fed, patient 4 would accept the bite, then gag and vomit what she had swallowed. She would begin meals by saying “No!” and refusing offered bites. She cried and screamed until she was removed from the chair and put on her mother’s lap. Her mother readily admitted that she was too “lenient” and could not deal with “fighting with her.” She tended to give in and “spoil her” because she didn’t know “how long she would live.” The behavioral treatment program included praising for accepted bites and ignoring all refusal behaviors. Her mother easily learned the feeding program after observation and instruction. At discharge, after 11 days’ treatment, her intake had increased to 100% of recommended calories and weight had increased to 9.86 kg (>10th percentile for age). The mother was also referred for counseling because of issues regarding her child’s diagnosis with CF, which she had raised during the hospitalization. Longitudinal follow-up to 4 years of age indicated continued growth with weight for height at the twenty-fifth percentile.
RESULTS
Table 2 summarizes the changes in caloric intake for each patient during the treatment period, as well as longer-term follow-up of growth status after discharge. With individual behavioral therapy, patients increased their daily caloric intake from a mean of 54% of requirements at the initiation of the behavioral program to a mean of 92% at discharge. All patients were seen for follow-up at least twice, ranging from as early as 7 months to as late as 24 months postdischarge. Immediate postdischarge follow-up regarding maintenance of the behavioral management plan was implemented through weekly outpatient visit or by phone consultation, as needed. Patient 2 was unable to be maintained on a program because of parental discontinuance but did return for medical checkup. Three of the four patients demonstrated continued catch-up growth with percentiles of weight for age and height at follow-up ranging from the tenth to the fiftieth percentiles (Figs. 1–4).
TABLE 2.
Food Intake and Follow-up
| Patient | Percent Recommended Calories Ingested at Admission | Percent Recommended Calories Ingested at Discharge | No. Days of Treatment | Weight/Length Percentiles (Mo Age at Follow-up) |
|---|---|---|---|---|
| 1 | 48 | >100 | 13 | >10th (29) |
| 2 | 56 | 67 | 8 | <5th (66) |
| 3 | 50 | 100 | 33 | 50th (33) |
| 4 | 62 | >100 | 18 | 25th (50) |
| Mean | 54 | 92 |
FIGURE 1.
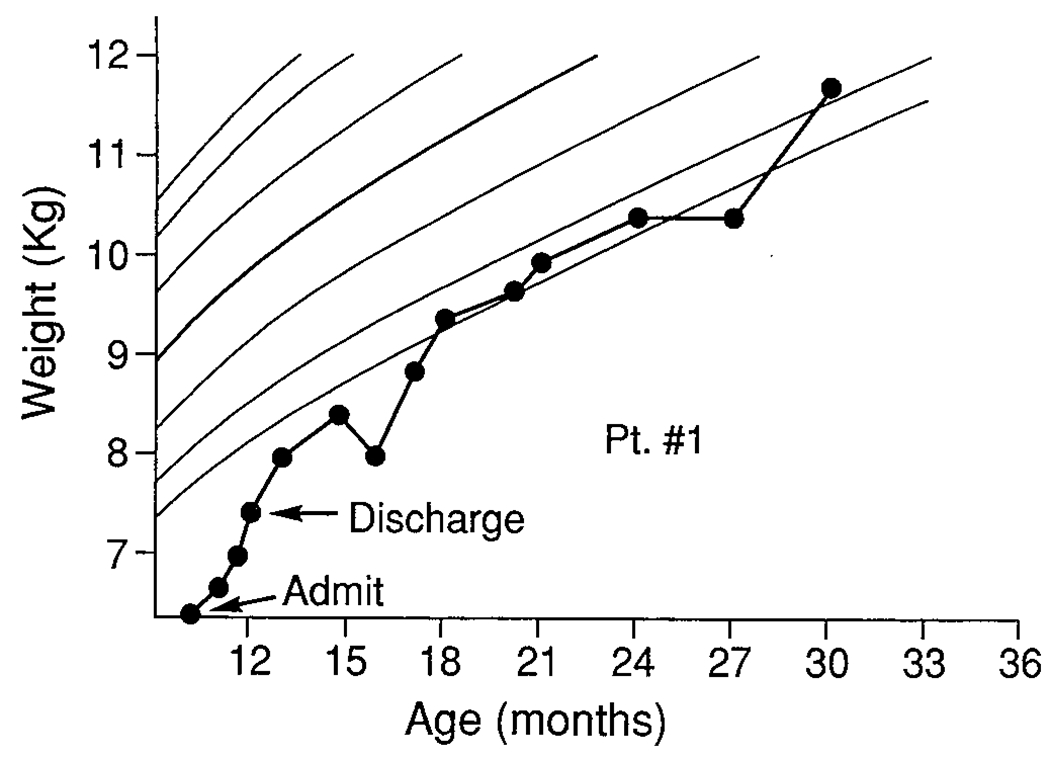
Patient 1.
FIGURE 4.
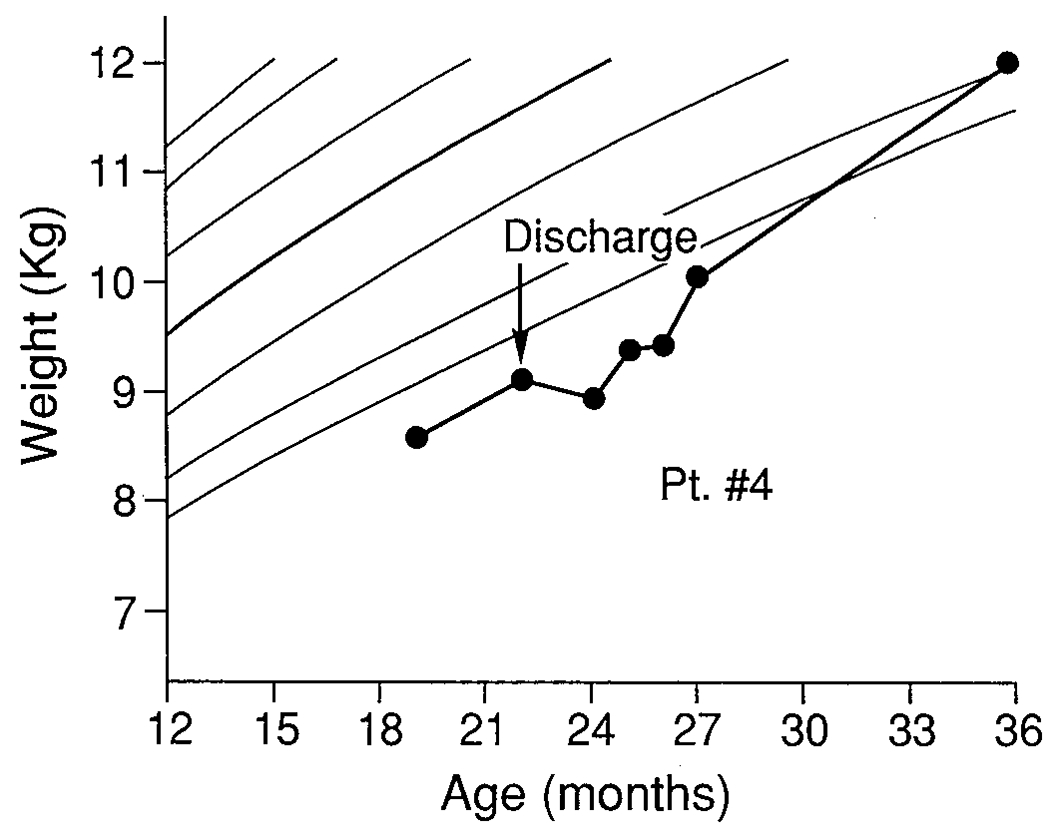
Patient 4.
DISCUSSION
These patients illustrate the importance of the evaluation of feeding behaviors, including parent-child interaction during feeding, and the benefits of behavioral treatment in young children with CF and growth failure. In contrast to children previously reported to have feeding difficulties,10–12 these patients were not mentally retarded, developmentally disabled, or fed enterally or parenterally. Psychological and behavioral factors may be overlooked in cases of organic failure-to-thrive, when growth failure may be attributed to the underlying illness, particularly when developmental problems associated with oral-motor dysfunction are not present, as in CF.13,14 Some studies suggest, however, that children with chronic medical illnesses, such as CF, may be more prone to develop behavioral feeding disorders as a result of alterations in both physiological factors and parent-child interactions. McCollum15 noted that feeding problems were reported beyond the first year of life in 57% of a sample of 65 children with CF. Parents of infants with CF also report more stress than do those with a healthy child, primarily with regard to child care, a lower sense of parental competence, and depression.5–9 In addition, young children with CF experience more acute illnesses, hospitalizations, fatigue, and separation from caregivers than do healthy infants. These factors may influence feeding interactions between infants with CF and their mothers, impinging on the growth of the infant.
Food aversions, decreased oral intake, and subsequent poor growth can be maintained in children with CF through several avenues. Acute infections, physical discomfort, environmental disruptions due to hospitalization, and vomiting and gagging associated with coughing spasms may initially contribute to anorexia and food refusal in children with CF. Parental reactions to a child’s refusal to eat vary but can include increased anxiety, forced-feedings, increased attention through coaxing, or removal from the feeding situation, all of which may serve to maintain the child’s avoidance. All these reactions were documented in the hospital chart notes on our patients. Because CF is a fatal disease, maternal anxiety may be heightened, further affecting parental management of the child’s meals. Indeed, several of the mothers in this group spontaneously verbalized that they feared their children would die and stated that this fear affected their interactions with their children during mealtimes.
Our study suggests that behavioral feeding disorders should be considered in young children with CF with growth failure despite optimal medical management. Behavioral assessment of feeding and other mealtime behaviors can lead to the design of interventions that can serve to promote adequate oral intake and improve long-term growth in young children with CF who demonstrate feeding problems. Our primary focus of treatment was on the specific behavioral interactions during the feeding situation that interfered with child feeding and adequate intake. However, other significant psychological issues emerged during the course of assessment and treatment. When parental anxiety, sadness, or marital conflicts were identified, these issues were acknowledged and interpreted as having potential impact on maternal behavior during feedings. Direct recommendations were made to encourage parents to seek counseling for these concerns, but these concerns were not directly treated in the hospital in the three cases in which catch-up growth was maintained. We believe that the lack of success in patient 2 stemmed from several sources, including precipitous, too-early discharge; extreme distance of the family from the CF center, which precluded close follow-up; major family disruption; and chronic psychological stressors that could not be addressed aggressively during the short hospital stay.
All these children were hospitalized as inpatients before their referral for assessment. Although inpatient treatment is costly, these children would have remained in hospital for continued care of their malnutrition, perhaps through nasogastric or other tube feedings, which would not have addressed the negative feeding behaviors. For example, patient 4 had been recommended for gastrostomy tube placement if behavioral treatment proved ineffective. Insurance providers did not question payment for the hospital stays of these children, most likely because the medical conditions were severe. We also have found insurers amenable to continue hospitalization for behavioral treatment when the medical risk is significant, and when a concrete assessment and treatment plan can be outlined, with objective means of assessing progress. We also believe that some inpatient hospitalizations for malnutrition could be avoided if monitoring of health status, dietary analysis, and behaviors during feeding were monitored preventively and treatment was initiated on an outpatient basis. Longitudinal studies of the psychological and behavioral factors associated with malnutrition in infants with CF would enhance our ability to identify parent-infant dyads at risk for impairments in feeding interactions.
FIGURE 2.
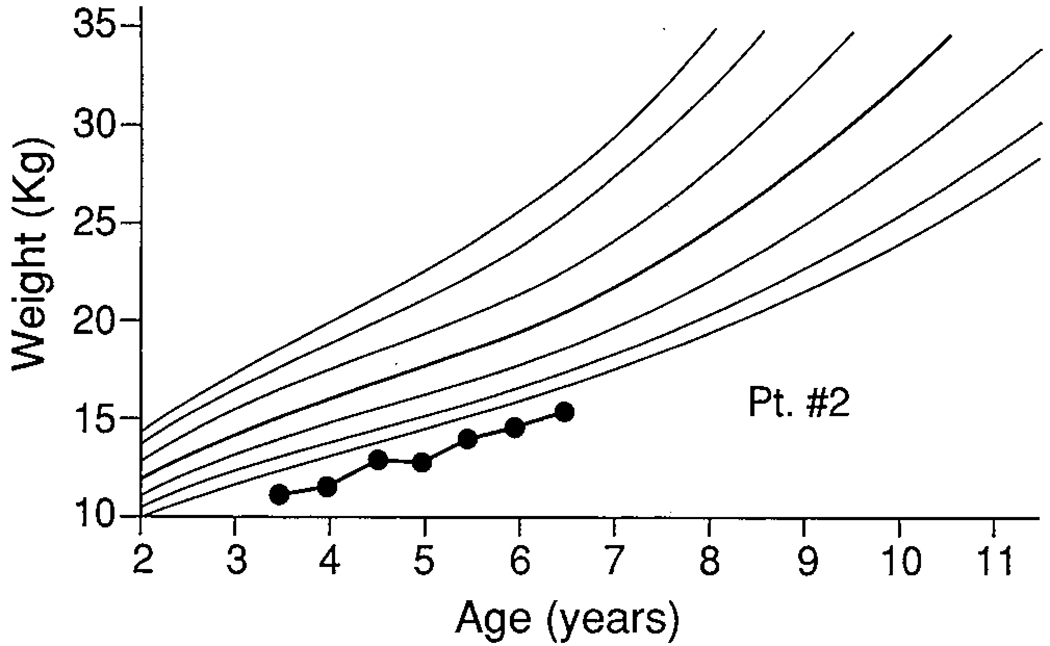
Patient 2.
FIGURE 3.
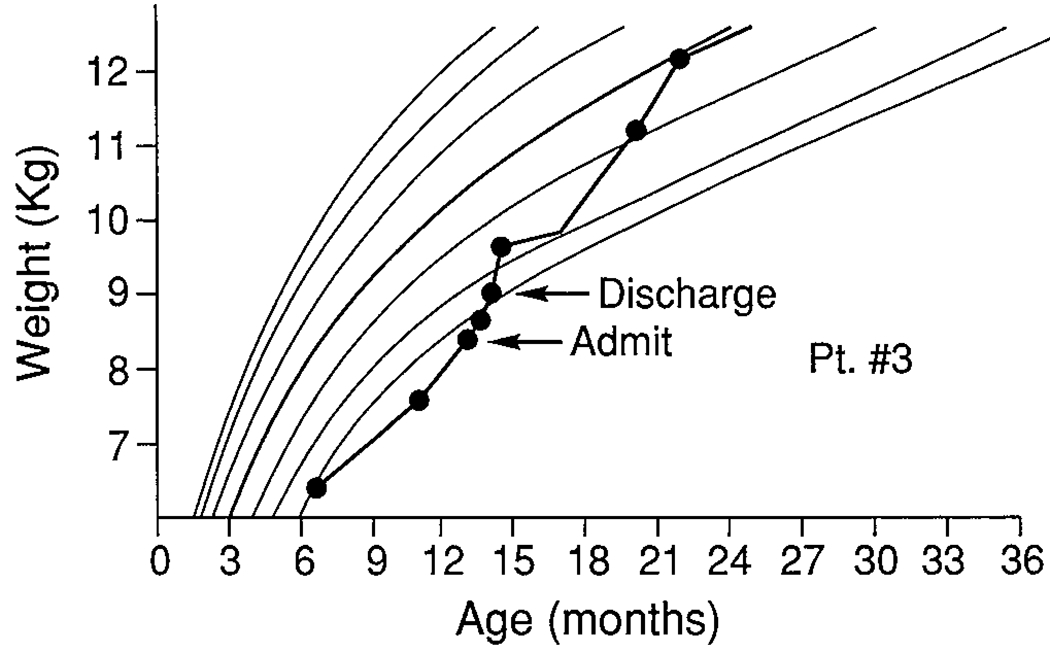
Patient 3.
Acknowledgment.
The authors thank the nursing staff of the Medical-Behavioral Center, Rainbow Babies and Childrens Hospital for their help with the feeding programs; also Drs. Robert Stern and Carl Doershuk for their helpful review, and Rose Marie Ashley for typing.
This study was supported in part by NIH Grant RO1-HL38193 and Maternal and Child Health Program Grant MCJ-390592 to Lynn T. Singer and by Core Center DK 27651.
Contributor Information
LYNN T. SINGER, Department of Pediatrics, Case Western Reserve University School of Medicine, Rainbow Babies and Childrens Hospital, Cleveland, Ohio
JANE A. NOFER, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania
LAURA J. BENSON-SZEKELY, Rainbow Babies and Childrens Hospital, Cleveland, Ohio
LEE J. BROOKS, Department of Pediatrics, Case Western Reserve University School of Medicine, Cystic Fibrosis Center, Rainbow Babies and Childrens Hospital, Cleveland, Ohio
REFERENCES
- 1.Matthews L, Drotar D: Cystic fibrosis: A challenging long-term chronic disease. Pediatr Clin North Am 1:133–52, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Bell L, Durie P. Forstner G: What do children with cystic fibrosis eat? J Pediatr Gastroenterol Nutr 3:137–146, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Smith AE, Lloyd-Still JD: Value of computerized dietary analysis in pediatric nutrition: An analysis of 147 patients. J Pediatr 103:820–823, 1983 [DOI] [PubMed] [Google Scholar]
- 4.Hubbard VS: Nutritional considerations in CF. Semin Resp Med 6:308–313, 1985 [Google Scholar]
- 5.Fischer-Fay A, Goldberg S, Simmons RJ, et al. Chronic illness and infant-mother attachment. J Dev Behav Pediatr 9:266–270, 1988 [PubMed] [Google Scholar]
- 6.Breslau N, Staruch K, Mortimer E: Psychological distress in mothers of disabled children. Am J Dis Child 136:682–686, 1982 [DOI] [PubMed] [Google Scholar]
- 7.Goldberg S, Moris P, Simmons RJ, et al. Chronic illness in infancy and parenting stress: A comparison of three groups of mothers. J Pediatr Psychol 15:347–358, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Hammill P, Drizd T, Johnson C, et al. Physical growth: National Center for Health Statistics Percentages. Am J Clin Nutr 32:607–629, 1979 [DOI] [PubMed] [Google Scholar]
- 9.Larson K, Ayllon T, Barnett Q: A behavioral feeding program for failure-to-thrive infants. Behav Res Ther 25:39–47, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Iwata B, Riordan M, Wohl M, et al. Pediatric feeding disorders: Behavioral analysis and treatment, in Accardo P (ed): Failure-to-Thrive in Infancy and Early Childhood: A Multi-disciplinary Team Approach. Baltimore, MD, University Park Press, 1982, pp 297–329 [Google Scholar]
- 11.Handen B, Mandell F, Russo D: Feeding induction in children who refuse to eat. Am J Dis Child 140:52–54, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Hyman S, Porter C, Pate T, et al. Behavioral management of feeding disturbances in urea cycle and organic disorders. J Pediatr 111:558–562, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Singer LT: Long-term hospitalization of nonorganic failure-to-thrive: Hospital course and patient characteristics. J Dev Behav Pediatr 8:25–31, 1987 [PubMed] [Google Scholar]
- 14.Singer LT: Behavioral feeding problems in children with medical illness or developmental disabilities. Contemp Pediatr 7:60–80, 1990 [Google Scholar]
- 15.McCollum A, Gibson L: Family adaptation to the child with cystic fibrosis. J Pediatr 77:57–78, 1970 [DOI] [PubMed] [Google Scholar]


