Abstract
Introduction
The availability and affordability of safe, effective, accessible, and high-quality essential medicines is a critical benchmark for achieving the right to good health, and it is also one of the goals of the global health development agenda. To that end, it is critical to conduct rigorous studies to identify the major challenges confronting developing countries, particularly those in Africa.
Objective
The purpose of this review was to identify the major challenges that Africans face in obtaining reasonably priced and readily available essential medicines.
Methods
Generally the Boolean operators “AND” and “OR” were employed. Making progress also involves using duplicate checks, field definitions, and comparisons of articles and criteria. The analysis included all English-language papers published in any African country between 2005 and 2022, depending on the year of publication. The technique searches electronic databases for key phrases related to essential medication availability and affordability, such as PubMed, Web of Science, Scopus, Science Direct, Plos Medicine, and Google Scholar.
Results
A total of 91 articles; by using search engines and handpicking including duplicates, were primarily searched. The electronic database search earned 78 articles while only eleven studies met the criteria for review and were reviewed of which 5 (50%) were from East African countries. Inadequate human resources, financial constraints, high cost of available medications on the market, poor inventory management, manual consumption forecasting, inefficiencies in drug registration, and trade-related aspects of intellectual property rights agreement regulations are all obstacles to the availability of essential medicines in African nations.
Conclusion
This review revealed that in Africa, the availability and affordability of essential medicines face numerous challenges. The primary challenge, according to the review research, is a lack of adequate financing to pay for an appropriate set of essential medications, which account for a significant portion of household spending.
Keywords: African countries, availability, affordability, essential medicines, challenges
Introduction
Curative, preventative, promotional, and rehabilitative health treatments are all provided in healthcare facilities with the aid of available and affordable medicines.1 Unaffordable prices and non-availability of medicines have become one of the most pressing concerns for patients and healthcare systems in high-, middle- and low-income countries.2,3 Efforts to ensure access to medicines are mainly driven by an ethical imperative: people should not be denied access to life-saving or health-promoting interventions for unfair reasons, including economic or social causes.4 Within the African context, certain limitations exist concerning their access to basic medicines. Many people living in Africa have a problem with accessing medicines and this undoubtedly contributes to the poor health metrics of most countries in the region.4 The availability and cost of medications are considered two of the most crucial factors for providing healthcare users with quality healthcare, along with other necessary pharmaceutical drugs and medical types of equipment, in health facilities.5 Lack of access to medications is a major barrier to society receiving higher-quality healthcare services.1
The management of factors that affect the availability and affordable pricing of critical medicines is necessarily addressed to increase access to them in healthcare institutions.4 Making access to a better supply, more responsible drug use, and high-quality, cost-effective care should be the goal of drug selection.6 To be available and affordable drugs should be used in a suitable, safe, cost-effective, secure, and accessible way.7
Given that from 2001 to 2007, just 30% of all both private and public health facilities in African countries had access to vital medicines, more than 70% of the population in Africa was impacted.1 Drug purchasing costs deeply consume the budget for healthcare spending in developed countries which will hit 50% to 90% of non-personal costs.8
What is Access to Medicine?
Due to the complexity of the word “access”; “access to essential medicines” has no single; widely-accepted definition; access is defined as the ability to obtain needed medications both physically and affordably.9 In this review; the term Access to Medicine (ATM) is conceptualized on the notion that a “percentage of the population who have access to at least a list of 20 EMs always available and affordable in a health care facility or medicine outlet, within an hour’s walk from the home of the patient to the service area”.4,9
The availability and cost of important medications depend on their availability and affordability in the health delivery systems, from Regional Medical Stores to Private pharmacies, and their affordability to the general public through the national health insurance program or pocket payments.10 It may be constrained by the lack of medications at service delivery locations, the inability to pay for it because the client is not insured, or the inability to pay out-of-pocket.11
A top objective for worldwide public health issues is to provide access to effective treatment.12 Poverty is the most important issue, meaning that neither the poor nor their governments can afford to purchase essential medicines or ensure their rational use in well-run health systems. Availability and affordability are the two core issues placed at the center of debates about medicine use in international health coverage,11,13 these dimensions are; geographical and accessibility, availability, acceptability, financial affordability quality otherwise known as the 4As which are first suggested by Penchansky in 1981, but later also adopted and popularized by the WHO years later.
All key aspects of health care delivery entail the use of medications, however, evidence suggests that the availability of these drugs in Africa considerably hinders the lack of access to vital medications. More than one-third of the world’s population lacks consistent access to essential medicines, undermining the goals of efficiency, equity, and health system development. Medicines are the most significant tools society uses for disease alleviation, cure, adjuvant therapy, diagnosis, and prevention. The problem is made worse by the fact that 50% to 60% of the populace in many underdeveloped African countries lacks such access.11
The World Health Organization (WHO) has pushed for the continuous availability of a group of medicines dubbed Essential Medicines;14 these drugs are chosen to meet the most important healthcare needs that lead to better healthcare, better drug management, and better use of financial resources, all of which improve access to care.15 The WHO has established an 80% benchmark as an acceptable limit for the availability of critical medicines in member countries and has a model list from which countries have constructed their National Essential Medicines Lists.16
Concept of Essential Medicine Availability
The Essential medicine idea, a major advancement in health care began in 1977 when the first essential medicines list was released by the World Health Organization (WHO) as one of the most cost-effective aspects of modern health care in the proper use of Ems;5 intending to give prioritize to a limited list of vital and essential life-saving medicines that are safe, effective, good quality and have an affordable price for treating the priority healthcare needs of the majority of the population.17 The query to have access to safe and effective medicines is so vital that it has been anticipated as a basic human right by the World Health Organization.4 Thus availability is the percentage of medicine or pharmaceutical outlets where medicines were found on the day of the survey. The selection process for them has evolved from expert assessment to selection based on evidence.12
Many new medications are continually being released by companies. But not all medicines are essential for preserving human life, and as a result, not all of them are equally beneficial to the healthcare system.18 While studies show that there is less than ideal accessibility of critical medications in underdeveloped countries, the WHO recommends that at least 80% of the available necessary medicines in a well-functioning health system be made available. For example, a 2014 study on the accessibility of 15 essential medications in public and private healthcare facilities in 36 low- and middle-income countries (LMIC) found that general generic medications were not adequately accessible in both the public and private healthcare sectors, with median availability of 38% and 64%, respectively.19
Measuring Medicines Affordability
Medicine affordability is the cost of medicines or treatment with medicines about lowest paid government employees income2,14 that would be required to pay to purchase from the private sector a one-month course of medicine at the standard or common dose.20 The number of days’ income that the lowest-paid government employee would have to fork over to cover treatment is used as a metric for affordability.2 It can be assessed by comparing the cost of pharmaceuticals to international reference prices (IRPs),20 which are average rates offered to developing countries and not for the benefit of pharmaceutical companies.21 The World Health Organization and Health Action International recommend using the LPGW’s income as a benchmark for comparing the cost of medication care to the availability of medications.2,20 Since the current global minimum salary is US $ per day, the lowest-paid unskilled government employee’s daily wage will be used to determine whether or not medicines are affordable.11
The percentage of tracer drugs that were usable at the time of the survey was used to compare facilities in the same industry5 in the case of an inter-sectoral comparison, availability is determined by the percentage of facilities in a certain sector that has a specific tracer drug.11 The following categories have been identified in this literature study to describe the availability of vital medicines: 50% is quite low; 51–65% is low; 66–80% is a rather high percentage, and > 80% is considered to be high. The availability comparisons were evaluated if the survey areas were based on the same WHO regions and the WHO essential medicine list was used.11
Context of African Countries’ Medicine Availability
Over 30% of the world’s population does not have regular access to essential medicines. Over half of Africa’s population lacks access. 2011, WHO study found that poor medicine accessibility, particularly in the public sector, is a significant barrier to access to essential medicines.1 According to a WHO study on essential medicines in African regions, the median availability of essential medicines was 61.5%, which was significantly higher than the availability of non-essential medicines, which was 27.3%. The median availability of critical drugs in the public sector was 40% and 78.1% in the private sector, respectively, compared to 6.6% and 57.1% for non-essential medicines.1,16
Throughout the years, the African region has witnessed an alarming shortage of vital medications in the public sector, forcing patients to pay higher costs in the private sector or forego medicines to treat their symptoms and diseases.9 As revealed during the workshop, the cause was that African countries lacked acceptable, weak, or no methods for monitoring and assessing critical medicine accessibility.13,22
Materials and Methods
Literature Searching Strategy
For this scoping review, comprehensive literature searches were undertaken in the electronic databases PubMed, Web of Science, Scopus, Science Direct, Plos Medicine, and Google Scholar.
To find relevant papers addressing barriers to access of “Essential medicines” studies conducted in any African country between 2005 and 2023. The search method consists of search terms for essential medicines or drug access, affordability, and availability analysis methods, and a drug accessibility search description filter. Boolean operator words (“AND” and “OR”) were used to combine them. Field specifications (Title, Abstract, All fields), duplication checks, and a comparison between articles and criteria are also used as a technique for making progress. The search terms used were: “Access”, OR “availability”, OR “pricing”, OR “cost”, OR “affordability”, AND “essential medicines”, OR “medicines”, OR “drugs”, OR “therapies”, OR “key drugs”, AND “challenges”, OR “hindering factors”, OR “factors affecting”, “OR” “barriers” “obstacles”, OR “crisis” OR “problems”, “OR” “bottlenecks”, “AND” “Africa”, “AND” “African countries”, OR “Sub-Saharan Africa”. After the searches, duplicated studies were removed and further screened for inclusion using the article text content, title, and abstract and search results from each database were saved in the individual electronic databases and exported into medley referencing software.
Choosing an Article
The evaluation covered studies that intended to analyze challenges influencing the availability and affordability of vital medicines in Africa/African countries. Papers written in English, open access in portable document formats, and all study designs were considered, but studies published exclusively as dissertations, abstracts, editorials, or clinical views before 2005 and outside of Africa were omitted. Articles were first selected based on their titles and abstracts, and full-text articles were examined for those potentially fulfilling the inclusion criteria.
Evaluation of Methodological Quality
Before including selected articles, methodological quality assessment was carried out to ensure that data extraction met the quality criteria, using the preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram and guidance provided by the Center for Reviews and Dissemination. Furthermore, each of the eleven included articles was evaluated using evidence-based librarianship (EBL) critical article appraisal criteria23 adapted for studies reporting cross-sectional such as population, data collection, study design, and results applied in the articles by using yes (Y), no (N), unclear(U), and not applicable (NA) words for each assessment questions. The calculation for overall validity was: (Y+N+U=T); If Y/T ≥75% or if N+U/T ≤ 25% then it can safely conclude that the study is valid.23 A score of less than 75% was considered low quality, 75% to 90% was considered moderate quality, and more than 90% was considered high quality.23 Three reviewers participated in this evaluation and separately assessed the complete text of each article, yielding kappa statistics of excellent inter-observer agreement of 85.7%. Any disagreements were settled through conversation.
Data Abstraction
Using an abstraction form, the author, year, study design, target population, overview, sample size, number of items assessed, country, the institution of study, data collection techniques, and factors impacting medicine availability were retrieved from each included study article. We used a comprehensive method to synthesize the evidence from the included studies and additional data sources.
Methods of Reporting
The Preferred Reporting Items for Systematic Reviews and Metaanalyses (PRISMA) guideline was used to report the result of this systematic review.24
Inclusion Criteria
Articles published between 2005–2022
Articles of studies conducted in African countries
Articles focus on challenges in medicine availability, affordability, and accessibility
Articles that title, abstract, and full text addressing issues of medicine access barriers
Exclusion Criteria
Publications published in non-English languages and journals,
Publications that prejudice incomplete discussions,
Publications of research not conducted in the African continent,
Articles that are not listed completely (abstract only),
Articles that do not focus and discuss on challenges in medical accessibility.
Results
The Results of a Literature Search
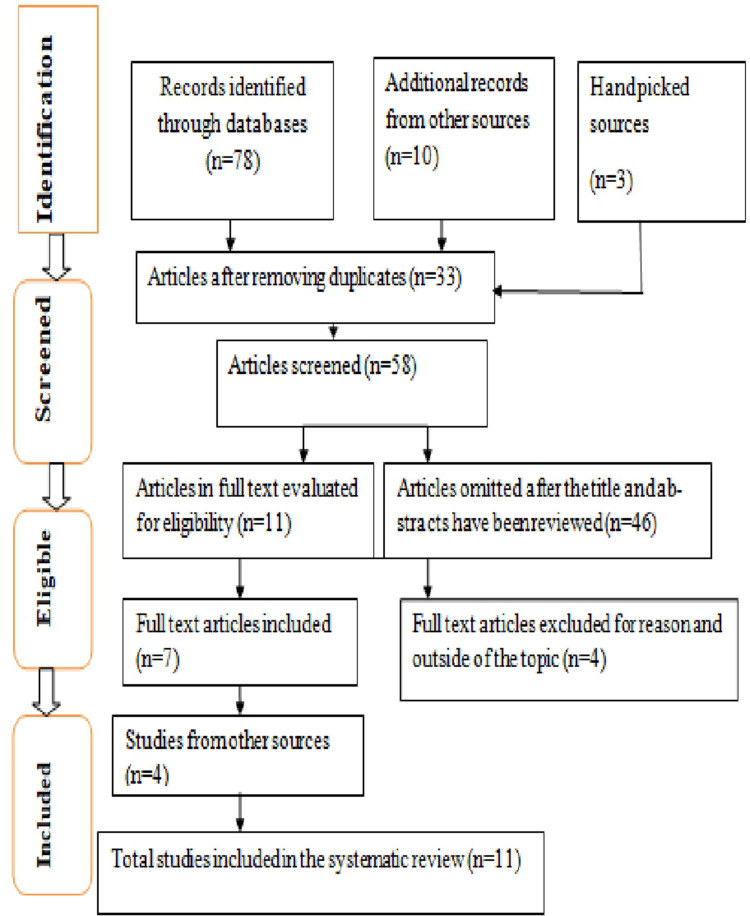
Figure 1 shows that the preliminary information retrieval system across all databases resulted in 88 studies (68 from Pub Med). After removing duplicates and irrelevant research, eleven studies were reviewed.
Figure 1.
A diagram of literature searching using the PRISMA checklist.
The Listed Studies’ Methodological Quality
When the EBL assessment checklist was used to verify the accuracy of the reporting, the results revealed that the majority of studies were of medium reliability, with 2 (20%) being of excellent quality.
Study Characteristics
The study characteristics are summarized in Table 1 and Table 2 below. Both tables show how the 11 included articles differ significantly in terms of sample size and location, and response. 5 of the studies were qualitative, 4 were cross-sectional, 2 were mixed, and they were almost all conducted in hospitals.
Table 1.
Quality Assessment of Eligible Studies Using the EBL Critical Appraisal Tool
| The Lead Author and Year | Study Design | Quality Evidence of % Score |
|---|---|---|
| (Abiye et al 2013)17 | Cross-sectional | 82.1% |
| (Fredrick and Muturi 2016) 8 | Mixed | 75% |
| (Zuma SM and Modiba LM2019) 16 | Qualitative descriptive | 92.9% |
| (Modisakeng et al 2020)25 | Qualitative descriptive | 85.7% |
| (Bagonza et al 2015)26 | Cross-sectional | 89.3% |
| (Chandani et al 2012)27 | Mixed | 78.5% |
| Kanda and M. A. Iravo 201528 | Qualitative case study | 92.9% |
| (Brhlikova et al 2020)29 | Qualitative | 78.6% |
| (Irene et al 2016)18 | Cross-sectional | 89.3% |
| (Droti et al 2019)30 | Qualitative | 75% |
| (Oridanigo et al 2021)2 | Cross-sectional | 77.5% |
Table 2.
Summary of Reviewed Studies
| No | Author/Year | Country | Target Population | Overview | Study Design | Challenges of Drug Availability and Affordability |
|---|---|---|---|---|---|---|
| 1 | (Abiye et al 2013)17 | Ethiopia | Interviewing 384 patients visiting the public, red cross and private pharmacy retail outlets | Among 230 EMs checked for availability based on the list of drugs, only 128 (55.65%) drugs were available, and of these drugs only 18.63% were affordable, 33.54% fairly affordable and 47.83% were unaffordable. | Facility based cross-sectional | Non-availability, high price, inaccessibility, and ineffectiveness (quality problems) of drugs prescribed from facilities. |
| 2 | (Fredrick and Muturi 2016)8 | Kenya | The target population of the study was 351 administrative staff of public health facilities. | Public health facilities lack Ems because there is no manipulation of ICT to predict consumer trends and no adequately qualified staff. | Mixed With the stratified sampling method |
An uncoordinated medicine supply chain, there is no price regulation, leading to an acute shortage of EMs. Poor and manual forecasting, insufficiently trained human resources, poor health system leadership, and poor coordination between healthcare systems leadership and processes |
| 3 | Zuma SM and Modiba LMl 2019)16 | South Africa, Ethiopia, Nigeria, Uganda, Kenya, Zimbabwe, Malawi | 15 Pharmacist and individual interviews from private and public health facilities, literature review, | Consistent supply and provisioning of essential medicines which is the second most expensive item after human resource expenditure is key in the treatment and management of prevailing diseases | Qualitative descriptive study | Medicine theft, lack of pharmacy personnel, poor stock management system, limited production supply, and production capacity, poor delivery, procurement failure, inadequate budget allocation, centralized procurement |
| 4 | (Modisakeng et al 2020)25 | South Africa | 32 people, 27 pharmacists from the four levels of care (district, regional, tertiary, and central), and 5 drug controllers/ assistant managers | In the public sector, the availability median of brands and generic drugs at the lowest price is 10% and 75%. In the private sector, the median availability of each product is 28.6%. In the public sector, most products are generic. | Qualitative descriptive study | The procurement process included the non-payment of suppliers, poor supplier performance, a lengthy buy-out process, and a shortage of APIs. Poor pharmaceutical supply chain management |
| 5 | (Bagonza et al 2015)26 | Uganda | Include 303 pharmacy participants with the administration of a structured questionnaire and interview | The article focuses on 5 tracer drugs used in high-priority clinical areas in the public sector to assess their availability, finding that the average availability of tracer drugs in public pharmacies is 63.3%, while the rest are not. | Facility based cross-sectional | Staff inadequacy, poor inventory management, manual procurement, fluctuations in price, demand and supply uncertainty, inadequate funding, bureaucracy/unnecessary delays, and inadequate infrastructures. |
| 6 | Chandani Y et al 2012 27 | Ethiopia, Malawi, and Rwanda | Qualitative interviews of 694 community health workers; 235, 139, and 320 from Ethiopia, Malawi, and Rwanda respectively, and direct observation and available document review | Percentage of community health workers using various transport means to collect supplies, in Ethiopia (n, 235) (by foot 54, bicycle<1, public transport 23 other ways 22).In Malawi (n, 139) (foot 11, bicycle 79, public transport 9, other 1) and in Rwanda (n, 320) (foot 88, bicycle 10, public transport <1 other 1) | Qualitative and quantitative surveys | Transportation problems, long lead time, lack of remuneration, traditional way of transportation which is not fast, unable to carry heavy/bulky items, availability of service delivery stations at last miles of the supply chain, presence of still developing logistic system, national level procurement occurs without sufficient data to define the actual community level need. |
| 7 | Kanda MK and Iravo MA28 | Kenya | Focused on 60 employees of healthcare workers selected by the non-probability purposive sampling procedure. | Several problems are noted as poor design/malfunctioning supply chain management; most suppliers do not use and even are not familiar with the latest technologies, failure by the government to allocate sufficient budget to public health is reflecting the persisting failure to attain the 15% public health spending level |
Qualitative case study design, Qualitative interview | Inefficient procurement, distribution system, supply chain inefficiency, lack of technology use in the supply chain, lack of staff competencies, disease burden, incomplete tender registers and poorly defined roles of key players, and lack of transportation infrastructure. |
| 8 | Brhlikova P et al 2020 29 | Uganda | Semi-structured interviews with 20 key informants recruited from the government bodies, professional councils, pharmaceutical producers and distributors, and international organizations | Of the 3130 brands of human medicines and vaccines registered on the NDR, 880 (28%) medicines and vaccines are listed on the Ugandan EML. Of 135, 143, and 129 EML-listed VEN medicines only 42, 60, and 64 are registered respectively | Facility-based qualitative interview | Regulatory constraints in the registration of Ems, insufficient local production, donor-based procurement policies, limited budget for procurement, no incentives for production and registration of EMs |
| 9 | (Irene et al 2016)18 | Nigeria | 92 pharmacy professionals working in public and private health institutions | About 56.5% of the respondent felt the high price of drugs, 52.1% and 66.3% of the respondents believed that the unavailability of all the prescribed drugs and unavailability of the drugs, in general, constituted a major barrier to access | Facility based cross-sectional | High cost of drugs, insufficient health facilities and staff, poor medicine supply, low fund investment for health and lack of access to prescribed drugs, unavailability of drugs, centralized medical stores, prescriptions out of EML, no timely delivery, Poor funding, lack of staff training on logistics/inventory management and forecasting, lack of human resource. |
| 10 | Droti B et al 2019 30 | Benin, Burkina Faso, democratic republic Congo, Mauritania, Sierra Leone, Togo, Uganda, and Zimbabwe. | Survey a total of 3353 hospitals and primary health care facilities in 8 countries found in rural and urban areas, interviewing and administering a structured questionnaire to health care workers and direct observation | Of 12 EMs selected mean availability was particularly low ranging from 22% to 40% in 8 countries. In almost all countries, the mean availability of EMs was higher in hospitals than in primary care facilities in urban facilities than in rural ones | The facility-based qualitative and quantitative method | Central supply store location, regulatory issues, few public facilities, and trust in these facilities is a low, limited budget for medicine procurement, waiting from aids/ donations |
| 11 | (Oridanigo et al 2021)2 | Ethiopia | Face-to-face patient exit interview, 626 patients in six health facilities and prescription paper review | Of 15 EMs selected based on cost/price; 31.1% and 43.6% of patients were not incurred, the price in health centers and hospitals respectively, and farmers have not incurred the price of EMS as employees and merchants. | Facility-based cross-sectional with exit interviewing and document review | Occupational and income status were important predictors of the affordability of essential medicines. Unenrolled in community-based health insurance schemes. Cost of medicines was not affordable for two-thirds of the patients, family income, residence, and several economically dependent family members influence the perceived affordability of essential medicines. |
Discussion
Challenges of Essential Medicine Availability
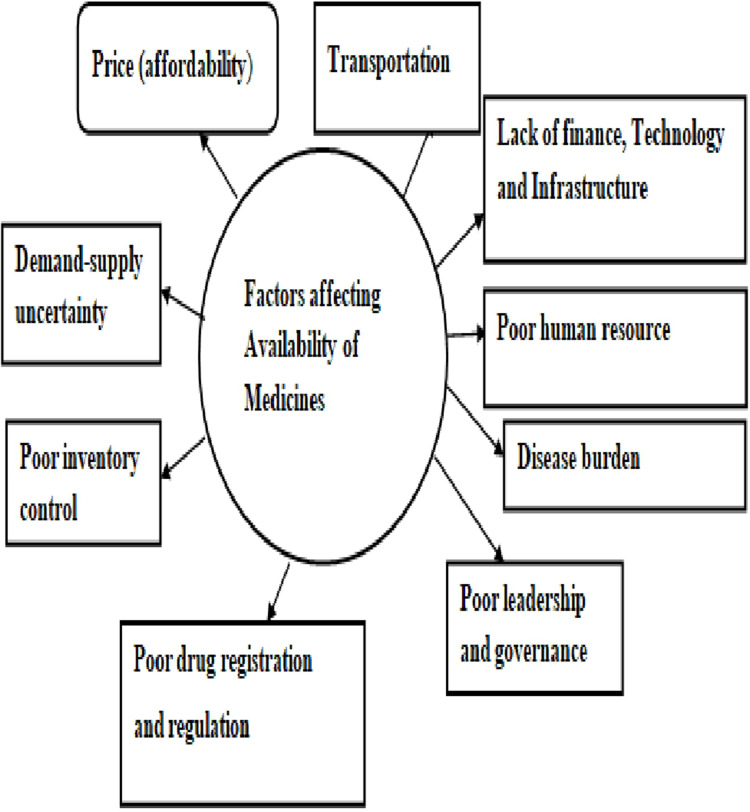
The conceptual framework of factors influencing the availability of essential medicines is illustrated in Figure 2.
Figure 2.
A conceptual framework of factors affecting essential medicine availability.
According to the World Bank, approximately 80% of the African continent’s population, primarily those in the middle and lower income brackets, rely on public health facilities to access health care.25 Several factors have been linked to affecting the accessibility of essential medicines in Africa’s public and private health facilities. However, empirical research on the humanitarian supply of medicines does not provide a clear picture of the nature of associations.7
Price (Affordability)
The poor are paying more.31 One of the most significant barriers to accessing essential medicines is cost.20 This happens when there is a mismatch between the available resources to fund needed medicines and the total cost of the medicine. Medicines consume up to 20–60% of health expenditure in developed and transitional countries, and in developing African nations, nearly 90% of the population buys medicines out of pocket, making medicines the second-largest item of family spending after food.32
Over 50 price and affordability surveys were conducted around the world in 2007, revealing that high medicines prices in the private sector were around 80 times higher than the international reference price in most low- and middle-income countries.33 The study found that price has the same impact on the availability of essential medicines in Africa as it did in Asian poor countries.6
In the case of high-cost EMs, access issues are exacerbated, and treatments are also prohibitively expensive, requiring the lowest-paid government employee to work for 15 days to pay for a one-month supply of the required medicines.33 Every year, over 10 million people die needlessly because of the lack of availability to existence medicines, primarily in LMICs (Africa), and the cost of medicine is one of the most significant barriers to access.32 In 2015, approximately 1.6 million Africans died from malaria, tuberculosis, and HIV-related illnesses. These diseases can be prevented or treated if the timely availability of appropriate and affordable medicines, vaccines, and other health services is provided.1
According to the study, in Ethiopia, 45.6%, 45.7%, and 8.7% of those who did not buy drugs from public health institutions stated that drugs were not available, drugs are expensive, and drugs are ineffective as their reasons for not purchasing, respectively, and 11.8% of patients forgive treatment due to cost.17 Consequently, overwhelmingly high prices for essential medicines in comparison to obtainable income contribute significantly to morbidity and mortality, especially in economically disadvantaged communities.11
Because of the shortage and lack of access to medicines in public health facilities, most poor residents must cover medical services and transportation to private medical centers out of pocket.34 High retail prices for medicines in low-income country settlements are probably driving the community further into poor health. According to the study, branded medicines were 13.8 times more expensive than generic medicines.7
The least generic medicines as well as brand names were purchased at 2.9 and 32.6 more than the average internationally accepted price levels of correlating medicines, respectively. Assuming a completely disposable income, this would hold 0.03 to 1.33 days’ salaries for the lowest-paid public servant to charge for treatment courses of selected single-prescription medications. This was ascertained in a study conducted in Ethiopia (p <0.005) price of medicine purchasing power is significantly associated with income.17 To increase the supply of medications, decrease costs, and improve affordability; unless these factors are likely to impact negatively healthcare access to essential medicines.35
Drug Registration and Regulation
It is mandatory to register medicines to list essential national medicines to enact laws on prescribing and dispensing, labeling, and generic medicines to regulate availability and marketing. In response to pharmaceutical-related crises, drug regulation has evolved over the last 50 years (Moran et al 2011). The early regulatory standards were primarily focused on guaranteeing the pharmaceutical quality of medications, with later developments in the early 1960s focusing on the development of standards for testing the efficacy and safety of new medicines as well.29
Even though drug regulation standards have been in place for at least 50 years, there are numerous issues regarding the safety and quality of medicines in both developed and emerging nations. The primary goal of drug regulation is to keep the public healthy.36 Because medicines are not ordinary goods, they must fulfill essential health needs, and access to essential medicines is a fundamental human right, according to the WHO.37
Aside from safety, quality, and efficacy prerequisites, there is a debate about regulating the drug companies more widely and attempting to control what they supply; thus, the demand for safe, effective, and affordable medicines is largely dependent on imported medicines. It is assumed that approximately 79% of all pharmaceuticals consumed in Africa are imported.38 This significantly raises healthcare costs and puts people at risk of medication shortages (Narsai et al 2015). Medicine regulation in developing countries is unable to respond effectively to the medicines registration system, owing to a lack of effective legislation, quality manufacturing capacity, adequate human resources, and insufficient time for medicine registration.16
Other challenges in the medicine registration process include noncompliance with medicine registration guidelines in different countries, prolonged medicine registration time or dossier rejection, poor medicine dossier assessment, cost of current good manufacturing practice inspection, and quality testing procedures that did not keep pace with the increasing demand of pharmaceutical industries for registration of their medicine in an African country.36,38
The balance between monitoring pharmaceuticals in the interests of public health and encouraging pharmaceutical industry development has shifted in favor of the inventive industry over the last 10 to 15 years. Furthermore, the current political climate encourages multinational corporations to maintain their supply monopolies via free-trade pacts, political lobbying, patent legislation, political lobbying, and legal pressures.11
Transportation
Transportation is widely acknowledged as a challenge in obtaining pharmaceutical care due to road closures, poor road construction, and transportation shortages; however, it is underexplored in terms of the clarity needed for tackling more immediate health and transportation policy interventions.27,28 Outlined that transportation infrastructure affects the total supply chain performance of pharmaceuticals due to poor infrastructure construction, development, heavy traffic overcrowding in large cities, and poor availability in rural areas of Africa.
According to Chandani et al, transportation has been recognized as one of the critical obstacles to health care in several large-scale studies focusing on underserved populations in three African countries.39 Okoro et al examined data from the 2002 psychosocial risk factor monitoring program and discovered that 9% of older adults (65 and older) did not receive required healthcare because of transport issues, implying that they may reside in rural regions, no longer drive, or reliant on private or mass transit.28
According to Ahmed et al, in a home-to-home survey of the non-elderly urban poor, 30% of informants had a barrier to transportation to health care, with those living in poverty being disproportionately affected. In most developing African countries, distribution is difficult due to poor road networks, which, combined with the limited distribution of wholesalers’ cars, trucks, and vans, jeopardizes the supply of essential medicines.8,33,40
Finance, Technology, and Infrastructure
According to Wirtz et al, the first major challenge is a lack of adequate financing to pay for the acquisition of essential medications. The findings support the notion that the majority of suppliers do not use and are even unfamiliar with the most recent technologies and that the level of technology use affects the efficiency of medical drug supply in Kenyan health facilities.28
According to Fredrick & Muturi’s analysis in Kenya; to establish the professional qualifications of the personnel charged with the responsibility of managing medicine supplies in public health facilities only 29% were trained in Pharmaceutical technology, 5% were trained in Supply management whereas 6% were trained on other courses and 8% on Administration of pharmaceuticals.
Reduced scale, expensive asset bases coupled with older technology, higher financing costs, a lack of integration with active pharmaceutical ingredient suppliers, and unreliable supporting infrastructure such as electricity, water, and transportation are all constraints for regional and domestic manufacturers.28
In the literature review, both global and regional sources confirmed that pharmaceutical manufacturers operating in the SADC and EAC regions generally produce at a higher cost than larger international generic manufacturers.1
Low investment in essential medicines for health, a lack of comprehensive health financing policies and strategic plans, extensive out-of-pocket payments, a lack of social safety nets to protect the poor, poor financial management, inefficient resource allocation and use, and weak mechanisms for coordinating partner support constrain health financing in the African region.30,41
Human Resource
In national efforts to increase access to essential medicines and primary healthcare service delivery, the incapability of competent dispensary personnel at the medical center delivering services level to handle medication and the supply chain has become a critical bottleneck.41 Extreme shortages of highly qualified health professionals, rooted in African countries, have been exacerbated by disparities in labor distribution and brain drain in this crisis, jeopardizing the delivery of effective public health interventions to those in need, particularly in remote rural areas.16,41
Professional human capitals are the building block of every nation’s health care system. Lack of this for health is probably the biggest challenge facing implementation, ensuring uninterrupted supply and scaling up of the needed pharmaceutical programs in developing and low-resource countries, according to various writers.41
Addressing human resource constraints at the health facility level can improve drug warehouse and logistics management, knowledge transfer, and supply chain efficiency, resulting in increased medicine availability in health facilities. MSH, 2012 and USAID Deliver Project, 2013 determined this with a p-value of = 0.00, indicating that a skilled and inspired human resource is a critical part of successful supply chain management in an efficient health system.1
Uncertainty in Demand and Supply
The most difficult hurdle in logistics activities is uncertainty. Overall, there will be no indication or assurance that a catastrophic event will occur, how many people will be affected, what infrastructure will remain intact, which suppliers will donate, how much, how many, and what types of EMs will be required, or what other challenges may arise.28 An effective logistics system makes goods available to end users when they are needed.39 It includes all operations related to the movement and conversion of goods from the raw material stage to the end-user stage.33 When, where, where from, which one, how much, and how many times; in short, the fundamental parameters required for a successful supply chain setup are highly uncertain.30,39
A workshop by WHO (2006) outlines the difficulties of the medicine supply in African countries; the main barriers are poor information, communication, and consumption data, inadequate storage facilities and temperature control systems, and a lack of quality assurance procedures.30 Another critical feature of the supply chain system is the limitation in the delivery of drugs from various factories to service points where patients can obtain them.39
Disease Burden
Africa shoulders the world’s burden of disease. It is the epicenter of the global resurgence of infectious epidemics and pandemic diseases.1 Africans are stroked by the trouble of communicable diseases like- HIV, diarrhea, measles, cholera, and tuberculosis that have long been overcome elsewhere with the help of modern medicine and efficient public health systems.11 Disease outbreaks are the most common news heard in Africa preceded by conflict and political instability.42
The pattern of infectious disease in Africa’s population is one of a double burden, with chronic noncommunicable diseases on the rise as a result of poverty.30 For some African countries, HIV/AIDS, other disease pandemics, and an increased incidence of injuries could result in the pattern being described as a quadruple burden.1
The burden of disease has a significant impact on how many people have the necessary medication at any given moment. The term “responsibility” refers to the act of determining whether or not a person is responsible for his or her actions. The term “electronic commerce” refers to the sale of goods and services over the Internet.11 The initial emphasis placed on the treatment of communicable diseases, the majority of which are acute, could explain the trend, which is similar to other low-income parts of the world. According to various studies, countries must urgently adapt and adapt their essential drugs. All priority medicines were listed and made available as a component of attempts to optimize patient care.43
Governance and Leadership
According to Kirigia and Barry,41 in Africa, there are serious leadership and governance challenges that include fragile public health leadership and management; inadequate health-related legislation and enforcement. This effect can be seen in Ethiopia and Malawi, where a lack of cooperation between government and non-governmental organizations in providing access to essential medicines has a significant impact on accessibility.6
To a large extent, the value of management and administration in a country’s health service clarifies the quality of health services delivered by the country’s health system.16 The delivery of health care is the center of the health system and is consistent with policies leadership and engagement with the community and other acts.5,33
From a lack of coordination medicines distribution chain to a lack of price regulation, societal inequalities in the medical facility, employees, and drug delivery, a lack of collaboration between the three levels of the government and the private and public sectors, to adherence to standard treatment guidelines and rational prescribing and use of medicines.5 The formation of an essential medicines ranking does not guarantee access to such medications, but it is important to develop policies and allocate funds to make them available.16 All of these issues point to poor governance and a lack of coordination between health system leadership and operations.10
According to Bate, governance issues are a barrier to local pharmaceutical production. Due to the government’s ability to direct aid to specific producers, local production supported by foreign aid to local public sector producers can distort the market by protecting a specific local producer against another more efficient and competent producer. Politicians may use aid funds to award production contracts to political allies.5 The authors advocate for legislative and regulatory mechanisms, as well as stronger governance policies, as critical to the long-term growth and establishment of local pharmaceutical production.5
Inventory Control/Management
Management systems for inventory or forms are required to collect information such as consumption data to identify successes and efficiency constraints.27 Inventory management consists of information about actual consumption, demand, stock levels, adjustments, and losses and is necessary for resupply planning.28 Poor inventory control in African countries is best described by incorrect inventory records, an absence of systematic stock monitoring, and unspecified purchasing quantity and frequency procedures, which are all connected to a relative paucity of understanding of the meaning of managing inventory, as well as inefficient and ineffective management.16,39
Public health facilities suffer acute to chronic lack of EMs, and many patients die of easily curable diseases.16 Even after some successes over the last three decades, in which African governments have undertaken several initiatives to improve the supply chain for medicines, stockouts at primary healthcare facilities remain an undeniable threat to the health of the people of Africa.9 The poor and rural communities that rely on public facilities for health care are typically the most vulnerable to the effects of stockouts. When these remote facilities run out of supplies, the consequences are far-reaching: Patients frequently make costly trips to medical facilities to keep up with their prescriptions.25
Inventory management challenges in African nations are influenced by the presence of variable regulatory and institutional laws, and operational limitations in forecasting supply and demand to perform quantification and procurement planning Because of differing consumption habits and limited information sources, appropriate safety stocks have been required to deal with demand and supply ambiguity, resulting in high inventory holding costs.16,19
Study’s Limitation
The current study has limitations in the following areas: the review focuses not on one influencing factor in detail and not on all influencing factors but analyzes the factors that affect the drug’s availability in most conditions and are mentioned frequently in most of the reviewed articles, and the review did not attempt to assess the challenges that can exist in the accessibility of essential medicines across the continent as a whole, ignoring national economies, policies, and regulations that have a significant impact on the availability and affordability of essential medicines.
Conclusion and Recommendation
Medicine availability, which is the second most expensive after human resource costs and accounts for 20 to 60% of some countries’ national budgets, is still significantly below the WHO-defined goal in Africa, and according to the reviewed articles, availability does not exceed 65% and purchasing costs are unaffordable. Healthcare provision is ineffective in the absence of consistent availability and affordability of medical systems, and a well-run healthcare system and the well-being of the health seeker cannot be established. It has been demonstrated that access to availability and affordability of medicines in African countries present numerous challenges, and it is critical for the continent to address these issues as it works to strengthen its health systems to achieve universal health coverage. We urge African continent country governments, respective stakeholders, and national health institutions to devote focused attention and unreserved prioritized efforts, as well as policy initiatives, to strengthen reliable price affordability and availability of essential medicines through customized means and countries’ health policy-compatible strategies.
Abbreviations
4As, Availability, Accessibility, Acceptability, Affordability; ATM, Access to Medicines; EAC, East African Community; EBL, Evidence-Based Librarianship; EML, Essential Medicine List; Ems, Essential Medicines; HICs, High Income Countries; IRP, International reference price; LMICs, Low- and Middle-Income Countries; LPGW, Lowest-Paid Government Worker; MDG, Millennium Development Goals; MSH, Management Science for Health; NGOs, Non-Governmental organizations; SADC, South African Development Community; WHO, World Health Organization; WTO, World Trade Organization.
Data Sharing Statement
The data used to support the findings of this study are included in the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors state that there were no commercial or financial relationships that may be considered as a potential conflict of interest during the research.
References
- 1.Wambui MC. Factors influencing availability of essential medicines in public health facilities in Kenya: a case of Embu county; 2017.
- 2.Oridanigo EM, Salgedo WB, Kebene FG. Affordability of essential medicines and associated factors in public health facilities of Jimma Zone, Southwest Ethiopia. Adv Pharmacol Pharm Sci. 2021;2021:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Guideline on Country Pharmaceutical Pricing Policies WHO Guideline on Country Pharmaceutical Pricing Policies. World Health Organization; 2020:1–70. [Google Scholar]
- 4.Adebisi YA, Nwogu IB, Alaran AJ, et al. Revisiting the issue of access to medicines in Africa: challenges and recommendations. Public Heal Challenges. 2022;1:1–13. doi: 10.1002/puh2.9 [DOI] [Google Scholar]
- 5.Bruno O, Nyanchoka OA, Ondieki MC, Nyabayo MJ. Pharmaceutical care & health systems availability of essential medicines and supplies during the dual pull-push system of drugs acquisition in Kaliro District, Uganda. J Pharm Care Heal Syst. 2015;1–5. doi: 10.4172/jpchs.S2-006 [DOI] [Google Scholar]
- 6.Latifah E, Kristina SA, Suryawati S. Overview of drug availability and influencing factors in several low, lower and upper- middle countries: a systematic review search results. Rev Artic. 2019;10:67–72. [Google Scholar]
- 7.Wirtz VJ, Hogerzeil HV, Gray AL, et al. Essential medicines for universal health coverage. Lancet. 2016;389(16). doi: 10.1016/S0140-6736(16)31599-9 [DOI] [PubMed] [Google Scholar]
- 8.Fredrick MW, Muturi W. Factors influencing frequent stock-outs of essential medicines in public health facilities in Kisii County, Kenya. IOSR J Business Manag. 2016;18(10):63–75. doi: 10.9790/487X-1810066375 [DOI] [Google Scholar]
- 9.Obuaku C. Essential medicines in Nigeria: foregrounding access to affordable essential medicines. Afr Soc Rev. 2014;18:42–60. [Google Scholar]
- 10.Obuaku-igwe CC. The Nigerian healthcare system: a study of access to affordable essential medicines and healthcare; 2015.
- 11.Nyanwura EM, Esena RK. Essential medicines availability and affordability: a case study of the top ten registered diseases in Builsa District of Ghana; 2013.
- 12.Bansal D, Purohit VK. Accessibility and use of essential medicines in health care: current progress and challenges in India. J Pharmacol Pharmacother. 2013;4(1):13–19. doi: 10.4103/0976-500X.107642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Victor Z. Organizational practices influencing availability of essential medicines at hospitals in Nairobi county; 2016.
- 14.World Health Organization. The Pharmaceutical Scene in Essential Medicines. World Health Organization; 2008. [Google Scholar]
- 15.Malik M, Hussain A, Shaffiq M, Azmi M, Hassali A, Shafie AA. Pharmaceutical regulatory affairs: open access role of essential drug list in effective management of essential anti- malarial drugs in healthcare system of Pakistan challenges in policy development to practice. Pharmaceut Reg Affairs. 2014;3(2). doi: 10.4172/2167-7689.1000120 [DOI] [Google Scholar]
- 16.Zuma SM, Modiba LM. Challenges associated with provision of essential medicines in the Republic of South Africa and other selected African countries. World J Pharm Res. 2019;8:1532–1547. doi: 10.20959/wjpr20199-15303 [DOI] [Google Scholar]
- 17.Abiye Z, Tesfaye A, Hawaze S. Barriers to access: availability and affordability of essential drugs in a retail outlet of a public health center in south western Ethiopia. J Appl Pharma Sci. 2013;3:101–105. doi: 10.7324/JAPS.2013.31017 [DOI] [Google Scholar]
- 18.Irene C. Improving on the accessibility and availability of essential drugs in Calabar metropolis, cross river state. J Health Med Nurs. 2016;27(2005):45–55. [Google Scholar]
- 19.Victor Z. Organizational practices influencing availability of essential medicines at hospitals in Nairobi county; 2016.
- 20.Mendis S, Fukino K, Cameron A, Laing R, Filipe A, Khatib O. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Lancet. 2007;85(4):279–288. doi: 10.2471/BLT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Measuring affordability and price availability, components. In: Medicine Prices, Measuring Affordability and Price Availability, Components. World Health Organization; 2008:1–310. [Google Scholar]
- 22.Machemedze MMR, Munyuki E. Literature review on bottlenecks to essential medicines production and procurement in East and Southern Africa; 2013:1–23.
- 23.Glynn L, Cleyle S. A critical appraisal tool for library and information research. Library Hi Tech. 2006;24:1–15. doi: 10.1108/07378830610692154 [DOI] [Google Scholar]
- 24.Petticrew M, Shekelle P, Stewart LA, Group P. Preferred reporting items for systematic review and and explanation. BMJ. 2015;7647:1–25. doi: 10.1136/bmj.g7647 [DOI] [Google Scholar]
- 25.Modisakeng C, Matlala M, Godman B, Meyer JC. Medicine shortages and challenges with the procurement process among public sector hospitals in South Africa; findings and implications. MBC Heal Serv Res. 2020;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagonza J, Rutebemberwa E, Eckmanns T, Ekirapa-kiracho E, McCartney G. What influences availability of medicines for the community management of childhood illnesses in central Uganda ? Implications for scaling up the integrated community case management programme. BMC Public Health. 2015;15:1–8. doi: 10.1186/s12889-015-2525-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandani Y, Noel M, Pomeroy A, Andersson S, Pahl MK, Williams T. Factors affecting availability of essential medicines among community health workers in Ethiopia, Malawi, and Rwanda: solving the last mile puzzle. Am J Trop Med Hyg. 2012;87(Suppl 5):120–126. doi: 10.4269/ajtmh.2012.11-0781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanda MK, Iravo MA. Access factors affecting supply chain efficiency of medical supplies in public health centres in Kenya: a case study of public health centres in Elgeyo marakwet count. Int J Acad Res Account Financ Mang Sci. 2015;5(2):32–41. doi: 10.6007/IJARAFMS/v5-i2/1560 [DOI] [Google Scholar]
- 29.Brhlikova P, Maigetter K, Murison J, Agaba AG, Tusiimire J, Pollock AM. Registration and local production of essential medicines in Uganda. J Pharm Policy Pract. 2020;13:1–8. doi: 10.1186/s40545-019-0198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Droti B, Neill KPO, Mathai M, Yao D, Dovlo T, Robertson J. Poor availability of essential medicines for women and children threatens progress towards Sustainable Development Goal 3 in Africa. BMJ Glob Heal. 2019;4:1–10. doi: 10.1136/bmjgh-2018-001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bas van der H. Drug pricing and access to essential medicines; 2000.
- 32.Cameron A, Ewen M, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2008;373:1–22. doi: 10.1016/S0140-6736(08)61762-6 [DOI] [PubMed] [Google Scholar]
- 33.Osei-Assibey A, Akweongo P. Assessment of availability of essential medicines in Sekondi-Takoradi metropolis. J Biomed Pharm Res. 2017;6:100–111. [Google Scholar]
- 34.Selvaraj S. Ensuring Access to Medicines in East Africa: Lessons from India. New Delhi, India: Observational Research Foundation; 2019. [Google Scholar]
- 35.Ongarora D, Karumbi J, Minnaard W, Abuga K, Okungu V. Medicine prices, availability, and affordability in private health facilities in low-income settlements in Nairobi County, Kenya. Pharmacy. 2019;1–14. doi: 10.3390/pharmacy7020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kefale B. Explore the challenge in medicines registration process in Ethiopia: qualitative phenomenological study; 2020.
- 37.Baker BK. Patents, pricing, and access to essential medicines in developing countries. Am Med Assoc J Ethics. 2009;2009:527–532. [DOI] [PubMed] [Google Scholar]
- 38.Moran M, Strub-wourgaft N, Guzman J, Boulet P, Wu L, Pecoul B. Registering new drugs for low-income countries: the African challenge registering new drugs for low-income countries: the African challenge. PLoS Med. 2011;8:20–23. doi: 10.1371/journal.pmed.1000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schöpperle A. Analysis of challenges of medical supply chains in sub-Saharan Africa regarding inventory management and transport and distribution; 2013.
- 40.Mohammadshahi M, Sakha MA, Zarei L, Karimi M, Peiravian F. Factors affecting medicine-induced demand and preventive strategies: a scoping review. Shiraz E Medical J. 2019;20(10):1–12. doi: 10.5812/semj.87079.Review [DOI] [Google Scholar]
- 41.Kirigia JM, Barry SP. Health challenges in Africa and the way forward. BioMed Cent. 2008;6–8. doi: 10.1186/1755-7682-1-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zondi S. Overcoming Africa’s health burden: challenges and prospects; 2010.
- 43.Muhammad F, Abdulkareem JH, Chowdhury AB. Major public health problems in Nigeria: a review. South East Asia J Public Heal. 2017;7:6–11. doi: 10.3329/seajph.v7i1.34672 [DOI] [Google Scholar]