Abstract
Intramuscular lipid deposition is important for meat quality improvement. microRNAs and their target mRNAs provide a new approach for studying the mechanism of fat deposition. The present study aimed to investigate the effect of miR-130b duplex (miR-130b-5p, miR-130b-3p) and its target gene KLF3 in regulating goat intramuscular adipocyte differentiation. Goat intramuscular preadipocytes were isolated from 7-d-old male Jianzhou big-ear goats and identified by Oil red O staining after differentiation induction. miR-130b-5p and miR-130b-3p mimics or inhibitors and their corresponding controls were transfected into goat intramuscular preadipocytes, respectively, and differentiation was induced by 50μM oleic acid for 48 h. Oil red O and Bodipy staining indicated that both miR-130b-5p and miR-130b-3p can reduce lipid droplets accumulation and triglyceride (TG) content (P < 0.01). Differentiation markers C/EBPα, C/EBPβ, PPARγ, pref1, fatty acids synthesis markers ACC, FASN, DGAT1, DGAT2, AGPAT6, TIP47, GPAM, ADRP, AP2, SREBP1, and TG markers LPL, ATGL, HSL were assessed by qPCR. All the markers measured were downregulated by miR-130b-5p and miR-130b-3p analog (P < 0.01), suggesting that miR-130b inhibits goat intramuscular adipocyte adipogenic differentiation, fatty acids synthesis, and lipid lipolysis. To examine the mechanism of miR-130b duplex inhibition of lipid deposition, TargetScan, miRDB, and starBase were used to predict the potential targets, KLF3 was found to be the only one intersection. Furthermore, the 3ʹUTR of KLF3 was cloned, qPCR analysis and dual luciferase activity assay showed that both miR-130b-5p and miR-130b-3p could directly regulate KLF3 expression (P < 0.01). In addition, overexpression and interference of KLF3 were conducted, it was found that KLF3 positively regulated lipid droplets accumulation by Oil red O, Bodipy staining, and TG content detection (P < 0.01). Quantitative PCR result indicated that KLF3 overexpression promoted lipid droplets accumulation relative genes C/EBPβ, PPARγ, pref1, ACC, FASN, DGAT1, DGAT2, AGPAT6, TIP47, GPAM, ADRP, SREBP1, LPL, and ATGL expression (P < 0.01). Downregulation of KLF3 inhibited the expression of genes such as C/EBPα, C/EBPβ, PPARγ, pref1, TIP47, GPAM, ADRP, AP2, LPL, and ATGL expression (P < 0.01). Taken together, these results indicate that miR-130b duplex could directly inhibit KLF3 expression, then attenuated adipogenic and TG synthesis genes expression, thus leading to its anti-adipogenic effect.
Keywords: goat, KLF3 gene, lipid accumulation, miR-130b-3p, miR-130b-5p
Results in the present study demonstrate that the miR-130b duplex (miR-130b-3p/miR-130b-5p) expression changed after goat adipocyte differentiation and negatively regulate goat intramuscular preadipocyte lipid droplets accumulation by targeting KLF3 expression.
Introduction
Goats are one of the most important meat livestocks in the world as goat meat is widely consumed (Kannan et al., 2021). Increased intramuscular fat mass is associated with the quality of mutton, including color, juiciness, texture, and tenderness (Ruiz et al., 2001; Pannier et al., 2014; Yang et al., 2021). The process of adipogenesis is divided into different stages, such as growth arrest, mitotic clonal expansion, and differentiation (Wu et al., 2019). Adipogenic differentiation is a complex process, CCAAT enhancer binding proteins and peroxisome proliferator-activated receptors are the vital transcription factors that control this process (Barak et al., 1999; Rosen et al., 1999; MacDougald and Mandrup, 2002; Farmer, 2006; Siersbaek et al., 2010). Although these transcriptional regulators have been shown to play a central role in adipogenesis, identifying the genes and networks involved, as well as exploring the genetic regulatory mechanisms that control fat deposition in goats, will provide a better understanding of goat adipogenesis.
microRNAs (miRNAs) are small (19 to 24 nucleotides), single-stranded, noncoding RNAs that are evolutionarily conserved and participate in almost all biological processes by complimentary binding to the 3ʹ-untranslated region (3ʹUTR) of their target mRNA for cleavage or translation inhibition (Bartel, 2004, 2009; Liu et al., 2017). The posttranscriptional mechanism of downregulation of gene expression depends on the binding target sequence. Increasing evidences suggest that miRNAs are involved in the regulation of adipogenesis. For example, miR-340-5p inhibits sheep adipocyte differentiation by targeting ATF7 (Liu et al., 2020). Both microRNA-23a and microRNA-16-5p promote 3T3-L1 adipocyte differentiation (Shen et al., 2016; Xu et al., 2019). miR-410-3P inhibits adipocyte differentiation by targeting IRS-1 in cancer-associated cachexia patients (Sun et al., 2021). Previous studies revealed that miR-421 and miR-26b-5p expression changed during goat intramuscular adipocyte differentiation (Du et al., 2022; Ma et al., 2021).
Krüppel-like factors (KLF) play a key role in fat formation and lipid metabolism. It was found that Krüppel-like factor 3 (KLF3) belongs to KLF family, and it was reported to play an important role in adipogenesis (Hashmi et al., 2011; Pearson et al., 2011; Zhang et al., 2013). Previous studies showed that KLF3 is a negative regulator in Garcinia cambogia mediated 3T3-L1 cell adipogenesis (Han et al., 2022). Conversely, one study showed a positive correlation between KLF3 expression and IMF content in cattle (Guo et al., 2018). Our recent studies revealed that KLF3 was positively related to goat subcutaneous adipocyte differentiation (He et al., 2021). However, it remains unknown whether KLF3 contributes to goat intramuscular adipogenesis.
While the effect of miR-130b-5p and miR-130b-3p has been previously reported to be involved in the regulation of adipogenesis (Eseberri et al., 2017; Guo et al., 2019; Luo et al., 2022). Based on miRNA expression characteristics before and after differentiation of goat intramuscular adipocytes by RNA sequencing, this study focused on the passenger and guide strands of the miR-130b duplex (passenger strand miR-130b-5p and guide strand miR-130b-3p). We hypothesized that miR-130b-5p and miR-130b-3p could be involved in the differentiation of adipocytes and the regulation of lipid deposition in goats. The involvement of miRNA passenger strands is a novel concept in miRNA biogenesis, and these miRNAs offer the opportunity to discover new regulatory networks in adipocyte. Here, we investigated the differentiation inhibition effects of both miR-130b-3p and miR-130b-5p by targeting KLF3 and their regulation of differentiation-related genes in goat intramuscular adipocyte. This study provides a theoretical basis for the basic research of meat quality improvement.
Materials and Methods
Goat intramuscular preadipocytes isolation, identification, culture, and in vitro differentiation
The goat experiment conducted in this study has been approved in advance by the Ethics Committee of Southwest Minzu University (License No. SMU20160108), all methods are performed according to the guidelines and regulations. Three 7-d-old male goats (Jianzhou big-ear goat; 75% Nubian and 25% Jianyang) weighing 3.2 kg were used as experimental models. Goat intramuscular preadipocytes isolation procedures and culture method were provided in previous studies (Xu et al., 2018; Ma et al., 2021). Briefly, the Longissimus dorsi samples were washed with phosphate buffer saline (PBS) and digested with type I collagenase (Sigma, MO, USA) for 1 h. After digestion, the cells were filtered with a 75-µm strainer and centrifuged at 2,000 rpm for 5 min. The precipitates were treated with red blood cell lysate, centrifuged at 2,000 rpm for 5 min, and suspended with DMEM/F12 (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (FBS, Gemini, Calabasas, USA). The medium was changed 24 h later. The preadipocytes were then identified by Oil red O staining at different time points (3, 24, 48, 72, 96, and 120 h) with differentiation induction (Xu et al., 2021). With the extension of differentiation induction, lipid droplets became larger and accumulation increased. After 120 h of induction, the goat intramuscular preadipocytes differentiated completely (Supplementary Figure S1).
For adipogenic differentiation, the cells were transferred to 12-well plates at a density of 1 × 106 cells per well, after 12 h transfection, standard medium was removed and replaced with adipogenesis induction medium (MEM/F12 [Hyclone] containing 10% FBS [Gemini], 50 μmol•L-1 oleic acid [Sigma]), the culture medium was changed every other day. At the required time points after induction, mature adipocytes, and preadipocytes were distinguished by Oil red O staining and Bodipy staining, or cells were collected for RNA extraction.
Transfection
Small interfering RNA (siRNA, Genepharma, Shanghai, China) against KLF3-1, 5ʹ-CCAUUGUCGGAUAAGUUCUUCCAGA-3ʹ, 5ʹ-UCUGGAAGAACUUAUCCG ACAAUGG -3ʹ; siKLF3-2: 5ʹ-GAUCGAACCACAGAGGACAGAUUAU-3ʹ, 5ʹ-AUAAUCUGUCCUCUGUGGUUCGAUC-3ʹ were synthesized by Invitrogen. The expression plasmid of KLF3 was constructed by inserting expanded KLF3 cDNA (KX247671) fragments into pcDNA3.1 vector (sense primer sequence: 5ʹ-CGGGG TACCATGGATGAACTCATAAACAACT-3ʹ, antisense primer sequence: 5ʹ- ATAAGAATGCGGCCGCTCACACGAGCATGTGGCGC-3ʹ). Cells in 12-well plates at a density of 1 × 106 cells per well had been pre-cultured 2 h in serum-free medium for transfection. Then plasmid or siRNA was introduced into the cells using Lipofectamine 3000 in accordance with the manufacturer’s instruction (Invitrogen, Carlsbad, USA).
The miR-130b-3p mimics, miR-130b-3p inhibitor, miR-130b-5p mimics, miR-130b-5p inhibitor, and respective negative controls (Table 1, Genepharma) as needed were transfected into the goat intramuscular preadipocytes by Lipofectamine 3000 (Invitrogen) and opti-MEM (Gibco BRL Co., Ltd) culture medium according to the manufacturer’s instruction.
Table 1.
The sequences of mimics and inhibitor of goat miR-130b -3p and miR-130b -5p
| miRNA | Classification | Sequence |
|---|---|---|
| NC | Mimic | UUCUCCGAACGUGUCACGUTT ACGUGACACGUUCGGAGAATT |
| Inhibitor | CAGUACUUUUGUGUAGUACAA | |
| miR-130b -3p | Mimic | CAGUGCAAUGAUGAAAGGGCAU GCCCUUUCAUCAUUGCACUGUU |
| Inhibitor | AUGCCCUUUCAUCAUUGCACUG | |
| miR-130b -5p | Mimic | ACUCUUUCCCUGUUGCACUACU UAGUGCAACAGGGAAAGAGUUU |
| Inhibitor | AGUAGUGCAACAGGGAAAGAGU |
Oil red O and Bodipy staining
After transfection of the corresponding fragments into goat intramuscular preadipocytes and inducing differentiation for 48 h, Oil red O and Bodipy were used to detect the formation of lipid droplets, and procedures for staining and quantitative analysis have been provided in our previous study (Xu et al., 2021). Briefly, the cells were washed twice with PBS and fixed with 4% paraformaldehyde at room temperature for 15 min. Next, the cells were washed twice with PBS and stained with Oil red O or Bodipy working solution for 20 min. After taking pictures, Oil red O was dissolved by adding 100% isopropyl alcohol, and absorbance was determined at 490 nm.
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was extracted according to the manufacture of Trizol (TaKaRa, Dalian, China). The RNAs were then reverse transcribed using the RevertAid First Strand cDNA Synthesis Kit (Thermo, Waltham, USA) according to the protocol. Then, qPCR was performed using amplification primers with SYBR Green PCR Master Mix (TaKaRa). We have screened for genes suitable for internal control in goat intramuscular adipocyte, like glyceraldehyde-3-phosphate dehydrogenase, 18S ribosomal RNA, peptidylprolyl isomerase B, ubiquitously expressed prefoldin like chaperone (UXT), actin beta and so on, we found that UXT was stably expressed at different adipogenic differentiation stages. So, UXT (Xu et al, 2018) and U6 (small nuclear RNA) were used as the internal control in this study. The primers for qPCR were listed in Table 2.
Table 2.
The sequences information of specificity primers
| Gene/miRNA | Sequence | T m, °C | GenBank no. |
|---|---|---|---|
| ACC | GGAGACAAACAGGGACCATT ATCAGGGACTGCCGAAAC |
60 | XM_018064169.1 |
| ATGL | GGTGCCAATATCATCGAGGT CACACCCGTGGCAGTCAG |
64 | NM_001285739.1 |
| AP2 | TGAAGTCACTCCAGATGACAGG TGACACATTCCAGCACCAGC |
58 | NM_001285623.1 |
| CEBPα | CCGTGGACAAGAACAGCAAC AGGCGGTCATTGTCACTGGT |
58 | XM_018062278.1 |
| CEBPβ | CAAGAAGACGGTGGACAAGC AACAAGTTCCGCAGGGTG |
66 | XM_018058020.1 |
| DGAT2 | CATGTACACATTCTGCACCGATT TGACCTCCTGCCACCTTTCT |
60 | BT030532.1 |
| FASN | TGTGCAACTGTGCCCTAG GTCCTCTGAGCAGCGTGT |
57 | NM_001285629.1 |
| HSL | AGGGTCATTGCCGACTTCC GTCTCGTTGCGTTTGTAGTGC |
60 | XM_018062484.1 |
| LPL | TCCTGGAGTGACGGAATCTGT GACAGCCAGTCCACCACGAT |
60 | NM_001285607.1 |
| PPARγ | AAGCGTCAGGGTTCCACTATG GAACCTGATGGCGTTATGAGAC | 60 | NM_001285658.1 |
| pref1 | CCGGCTTCATGGATAAGACCT GCCTCGCACTTGTTGAGGAA |
65 | KP686197.1 |
| SREBP1 | AAGTGGTGGGCCTCTCTGA GCAGGGGTTTCTCGGACT | 58 | NM_001285755.1 |
| GPAM | GGAGCAAGCATTGTTGCCAG CAATCAGTCTTCGGCGGGAT |
60 | AY515690 |
| AGPAT6 | AAGCAAGTTGCCCATCCTCA AAACTGTGGCTCCAATTTCGA |
60 | JI861797.1 |
| DGAT1 | CCACTGGGACCTGAGGTGTC GCATCACCACACACCAATTCA |
60 | NM_174693 |
| TIP47 | GGTGGAGGGTCAGGAGAAA TCACGGAACATGGCGAGT |
60 | HQ846826 |
| ADRP | TACGATGATACAGATGAATCCCAC CAGCATTGCGAAGCACAGAGT |
60 | HQ846827 |
| KLF3 | CTCGGTGTCATACCCGTCTAAT CCATCTGCATCCCGTGAGAC |
60 | KU041753.1 |
| UXT | GCAAGTGGATTTGGGCTGTAAC ATGGAGTCCTTGGTGAGGTTGT |
60 | XM_005700842.2 |
| U6 | TGGAACGCTTCACGAATTTGCG GGAACGATACAGAGAAGATTAGC |
60 | |
| miR-130b-3p | CTGGAGCAGTGCAATGATGAAA GTGCAGGGTCCGAGAGGT |
58 | |
| miR-130b-5p | CTGGAGACTCTTTCCCTGTTGC GTGCAGGGTCCGAGAGGT |
58 |
Luciferase reporter assay
The 3ʹUTR of KLF3 containing the wild or mutant binding site of miR-130b-3p and miR-130b-5p was cloned and inserted into the corresponding site of the psiCHECk vector and then co-transfected into 293T cells with miR-130b-3p or miR-10a-5p mimics. Forty-eight hours after transfection, the cells were harvested and the Dual-Luciferase Reporter Assay System kit (Promega, Madison, WI, USA) was used to detect dual luciferase activity after 48 h transfection according to the manufacturer’s instructions.
Western blot analysis
Proteins from goat intramuscular preadipocytes with or without KLF3 overexpression were extracted using RIPA lysis buffer with 1% protease inhibitor. Twenty micrograms of proteins from each sample were separated by 12% SDS–PAGE, and then transferred to a 0.45-μm PVDF membrane. The antibody of KLF3 was purchased from Thermo Fisher Scientific (1:500, #PA5-30621, Thermo). The antibody of β-actin was purchased from Abways (1:5000, #AB0035, Abways, Shanghai, China). Horseradish peroxidase-conjugated secondary antibody to rabbit IgG (1:5000, #AB0102, Abways). Finally, ECL (Bio-Rad, Hercules, CA, USA) was used to detect target proteins.
Statistical analysis
All data were presented as “means ± SD”. The variance of data was analyzed by SPSS 17.0 (SPSS Inc., Chicago, USA), To assess the significance of differences between 2 groups, we used students t-tests. Differences between multiple groups were assessed by one-way ANOVA analysis. Differences with * indicates the P values were <0.05, ≥0.01, ** indicates that the P values were <0.01. All experiments in our study were carried out in triplicates.
Results
miR-130b-3p and miR-130b-5p expression significantly changed during goat intramuscular adipocyte differentiation
According to our previous RNA-seq results, 112 microRNAs were downregulated after the differentiation of goat intramuscular adipocytes, and miR-130b-3p and miR-10a-5p, as miR130b duplex, both appeared in the downregulated microRNAs (data not shown). Then, goat intramuscular adipocytes were induced to differentiation, and 0, 12, 24, 36, 48, 60, and 96 h post-differentiation were selected as the detection time points during adipogenesis. The expressions of miR-130b-3p and miR-130b-5p were significantly changed during adipogenic differentiation compared with those cells before differentiation (Supplementary Figure S2).
Effects of miR-130b-3p on the accumulation of goat intramuscular adipocyte lipid droplets
To verify the exact role of miR-130b-3p on the differentiation of goat intramuscular adipocytes, primary preadipocytes were isolated from 7-d-old goats and transfected with miR-130b-3p (Figure 1A). Our results showed that strikingly fewer lipid droplets formed in miR-130b-3p overexpression cells than that in mimic NC transfected cells by Oil red O and Bodipy staining, and the adipogenesis defect of miR-130b-3p transfected cells was further confirmed by triglyceride content detection (Figure 1B-D). Moreover, except for CEBP/β, the mRNA levels of the adipogenic markers, CEBP/α, PPARγ, pref1 were significantly downregulated in miR-130b-3p transfected differentiated adipocytes (Figure 1E). Notably, the expression of triglyceride (TG) synthesis genes, including ACC, FASN, DGAT1, DGAT2, AGPAT6, TIP47, GPAM, ADRP, SREBP1 but not AP2 in miR-130b-3p transfected differentiated adipocytes were all markedly decreased, compared with mimic NC transfected cells (Figure 1F). Curiously, compared with the control cells, genes involved in triglyceride lipolysis, like LPL, ATGL, and HSL expression were also significantly downregulated in miR-130b-3p transfected differentiated adipocytes (Figure 1G).
Figure 1.
Effects of miR-130b-3p overexpression on the lipid deposition of goat intramuscular adipocytes. (A) Validation of miR-130b-3p expression in mimics NC and miR-130b-3p transfected preadipocytes by qPCR (48 h after transfection, without differentiation induction). U6 was used as an internal control. (B) The efficiency of adipocyte differentiation was dramatically decreased in miR-130b-3p overexpression cells based on Oil red O staining (magnification: left, 40×, right, 400×). The right panel was the semi-quantitative assessment result. After photographing, the cells were destined in 1 mL 100% isopropanol for 15 min and the Oil red signal was quantified by measuring the absorbance at 490 nm. (C) Representative images of Bodipy staining of lipid droplets in goat differentiated adipocytes (48 h of differentiation) with or without miR-130b-3p overexpression (magnification: 400×). (D) Triglyceride content detection in mimics NC and miR-130b-3p transfected differentiated adipocytes. (E), (F), (G) The mRNA expression level of relative genes in mimics NC and miR-130b-3p transfected differentiated adipocytes were detected by qPCR. UXT was used as an internal control. Data are presented as mean percentage (mean ± SD) of at least three independent experiments. **P < 0.01.
To further investigate whether the lack of endogenous miR-130b-3p affects differentiation, its inhibitor was designed and synthesized to transfect goat intramuscular preadipocyte. Efficiency detection showed that the inhibitor significantly decreased the expression level of miR-130b-3p at 48 h after transfection (Figure 2A). Our data revealed that both the lipid droplets and the triglyceride content were promoted by miR-130b-3p inhibition (Figure 2B-D). Along with the morphology observation, both the adipogenic differentiation genes CEBP/α, CEBP/β, PPARγ, pref1 and the TG synthesis genes, ACC, FASN, DGAT1, DGAT2, AGPAT6, TIP47, GPAM, ADRP, AP2, SREBP1 in miR-130b-3p inhibitor transfected differentiated adipocytes were all significantly increased (Figure 2E and F). Similar to the above results, triglyceride lipolysis genes LPL, ATGL, and HSL expression were also markedly upregulated in miR-130b-3p inhibitor transfected differentiated adipocytes (Figure 2G). Collectively, all these data demonstrate that miR-130b-3p could promote the adipocyte differentiation, which enhances the lipid droplets accumulation in goat intramuscular adipocytes.
Figure 2.
Inhibition of miR-130b-3p promotes lipid deposition in goat intramuscular adipocytes. (A) Validation of miR-130b-3p expression in inhibitor NC and miR-130b-3p inhibitor transfected preadipocytes by qPCR (48 h after transfection, without differentiation induction). U6 was used as an internal control. (B) The efficiency of adipocyte differentiation was dramatically increased in miR-130b-3p downregulation cells based on Oil red O staining (magnification: left, 40×, right, 400×). The right panel was the semi-quantitative assessment result. After photographing, the cells were destined in 1 mL 100% isopropanol for 15 min and the Oil red signal was quantified by measuring the absorbance at 490 nm. (C) Representative images of Bodipy staining of lipid droplets in goat differentiated adipocytes (48 h of differentiation) with or without miR-130b-3p inhibition (magnification: 400×). (D) Triglyceride content detection in mimics NC and miR-130b-3p transfected differentiated adipocytes. (E), (F), (G) The mRNA expression level of relative genes in inhibitor NC and miR-130b-3p inhibitor transfected differentiated adipocytes were detected by qPCR. UXT was used as an internal control. Data are presented as mean percentage (mean ± SD) of at least three independent experiments. **P < 0.01.
miR-130b-5p inhibits goat intramuscular adipocyte differentiation in vitro
In the first study, we found that miR-130b-3p, as the guide strand of the miR-130b duplex is the repressor of adipocyte differentiation, we wondered if its passenger strand, miR-130b-5p will also function in this process. We first performed gain-of-function experiment by using miR-130b-5p mimics transfected into goat intramuscular adipocytes (Figure 3A). As shown in Figure 3B-D, miR-130b-5p overexpression in goat intramuscular adipocytes significantly blocked the adipogenesis. Adipocyte markers and TG synthesis markers, such as CEBP/α, PPARγ, pref1, ACC, FASN, DGAT1, DGAT2, AGPAT6, TIP47, GPAM, ADRP, SREBP1 were also significantly downregulated in miR-130b-5p overexpression cells (Figure 3E, F). Interestingly, TG lipolysis-related genes LPL, ATGL, and HSL expression were observably decreased in miR-130b-5p overexpression goat intramuscular adipocytes too (Figure 3G). These results indicate that miR-130b-5p is involved in the goat adipocyte differentiation process.
Figure 3.
miR-130b-5p overexpression inhibits the lipid accumulation of goat intramuscular adipocytes. (A) Validation of miR-130b-5p expression in mimics NC and miR-130b-3p transfected preadipocytes by qPCR (48 h after transfection, without differentiation induction). U6 was used as an internal control. (B) The efficiency of adipocyte differentiation was dramatically decreased in miR-130b-5p overexpression cells based on Oil red O staining (magnification: left, 40×, right, 400×). The right panel was the semi-quantitative assessment result. After photographing, the cells were destined in 1 mL 100% isopropanol for 15 min and the Oil red signal was quantified by measuring the absorbance at 490 nm. (C) Representative images of Bodipy staining of lipid droplets in goat differentiated adipocytes (48 h of differentiation) with or without miR-130b-5p overexpression (magnification: 400×). (D) Triglyceride content detection in mimics NC and miR-130b-5p transfected differentiated adipocytes. (E), (F), (G) The mRNA expression level of relative genes in mimics NC and miR-130b-5p transfected differentiated adipocytes were detected by qPCR. UXT was used as an internal control. Data are presented as mean percentage (mean ± SD) of at least three independent experiments. **P < 0.01.
In the inhibitor study, the expression of miR-130b-5p was downregulated by its inhibitor (Figure 4A). As expected, our data showed a significant increase in lipid droplets accumulation and TG content in the miR-130b-5p inhibitor group (Figure 4B-D). Simultaneously, adipogenic and TG synthesis markers, like CEBP/α, CEBP/β, PPARγ, pref1, ACC, FASN, DGAT1, DGAT2, AGPAT6, TIP47, GPAM, ADRP, AP2, SREBP1 were also upregulated in the miR-130b-5p inhibitor group, which confirmed the above findings (Figure 4E and F). Next, we examined the TG lipolysis markers, such as LPL, ATGL, and HSL, and observed upregulation in the miR-130b-5p inhibitor group (Figure 4G). Therefore, these experiments confirmed the inhibition role of miR-130b-5p in adipogenic differentiation.
Figure 4.
Inhibition of miR-130b-5p promotes lipid deposition in goat intramuscular adipocytes. (A) Validation of miR-130b-5p expression in inhibitor NC and miR-130b-5p inhibitor transfected preadipocytes by qPCR (48 h after transfection, without differentiation induction). U6 was used as an internal control. (B) The efficiency of adipocyte differentiation was dramatically increased in miR-130b-5p downregulation cells based on Oil red O staining (magnification: left, 40×, right, 400×). The right panel was the semi-quantitative assessment result. After photographing, the cells were destined in 1 mL 100% isopropanol for 15 min and the Oil red signal was quantified by measuring the absorbance at 490 nm. (C) Representative images of Bodipy staining of lipid droplets in goat differentiated adipocytes (48 h of differentiation) with or without miR-130b-5p inhibition (magnification: 400×). (D) Triglyceride content detection in mimics NC and miR-130b-5p transfected differentiated adipocytes. (E), (F), (G), The mRNA expression level of relative genes in inhibitor NC and miR-130b-5p inhibitor transfected differentiated adipocytes were detected by qPCR. UXT was used as an internal control. Data are presented as mean percentage (mean ± SD) of at least three independent experiments. **P < 0.01.
KLF3 is a direct target gene of miR-130b-3p and miR-130b-5p
To elucidate the molecular mechanism of miR-130b-3p and miR-130b-5p inhibiting goat adipocyte differentiation. TargetScan, miRDB, and starBase were used to predict the potential targets of bovine miR-130b-3p and miR-130b-5p. KLF3 was found to be the only one intersection, and the binding sequence of miR-130b was found in the 3ʹUTR region of bovine KLF3 mRNA (Figure 5A). Then we hypothesized that KLF3 may be the target gene of miR-130b-3p and miR-130b-5p in goat. To validate this hypothesis, we also cloned the 3ʹUTR of goat KLF3 gene, and 172, 78, and 81 miRNAs were predicted to target KLF3 3ʹUTR by TargetScan, miRDB, and starBase bioinformatics algorithms, respectively. The three microRNAs in the intersection include miR-130b-3p and miR-130b-5p (Figure 5B), the binding site of miR-130b-3p and miR-130b-5p on KLF3 3ʹUTR were shown in the up panel of Figure 5D and E.
Figure 5.
Both miR-130b-3p and miR-130b-5p directly regulate KLF3 expression. (A) TargetScan, miRDB, and starBase were used to predict the potential targets of bovine miR-130b-3p and miR-130b-5p, the lower panel was the binding sequence. (B) TargetScan, miRDB, and starBase were used to predict miRNAs that could target goat KLF3. (C) Quantitative PCR validation of KLF3 expression in miR-130b-3p or miR-130b-5p mimics or inhibitor transfected preadipocytes. UXT was used as an internal control. Wild-type and mutant binding site of (D) miR-130b-3p and miR-130b-5p (E) in the 3ʹUTR of KLF3 mRNA. (F) Dual luciferase reporter assays using vectors encoding putative miR-130b-3p and miR-130b-5p target sites in the KLF3 3ʹUTRs for both wild-type and mutant regions. Renilla luciferase was used to normalize the firefly luciferase values. Data are presented as mean percentage (mean ± SD) of at least three independent experiments. **P < 0.01.
microRNAs could bind to the 3ʹ-untranslated region (3ʹUTR) of their target mRNAs for mRNA destabilization or translation inhibition (Bushati and Cohen, 2007). To explore the relationship between miR-130b-3p, miR-130b-5p and KLF3, the expression of KLF3 in miR-130b-3p or miR-130b-5p inhibition or overexpression preadipocytes were detected by qPCR. Congruously, KLF3 expression was significantly upregulated in miR-130b-3p and miR-130b-5p inhibition cells, while the expression of KLF3 was significantly downregulated in miR-130b-3p and miR-130b-5p overexpression cells (Figure 5C). To elucidate whether KLF3 was directly regulated by miR-130b-3p and miR-130b-5p, the luciferase reporter plasmid containing the wildtype or mutant 3ʹUTR of goat KLF3 was constructed (Figure 5D and E). As shown in Figure 5F, when co-transfected with miR-130b-3p or miR-130b-5p mimics, the relative luciferase activity of the reporter containing the wild-type 3ʹUTR of KLF3 was significantly decreased. On the contrary, the luciferase activity of the mutant-type 3ʹUTR was similar between the miR-130b-3p or miR-130b-5p mimics and control mimics (Figure 5F). Taken together, these data indicate that KLF3 was directly regulated by both miR-130b-3p and miR-130b-5p.
KLF3 positively regulate adipogenesis in goat intramuscular adipocyte
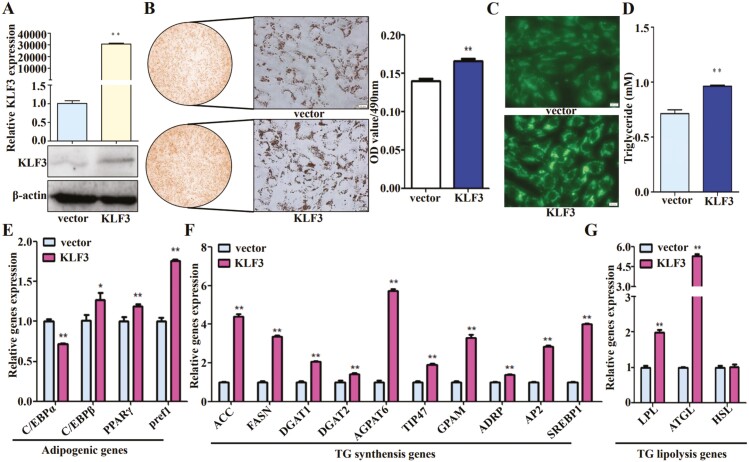
To explore the potential role of KLF3 in goat intramuscular adipocyte differentiation and lipid droplets accumulation, we first examined KLF3 expression in adipocytes at different stages of differentiation, and the results showed that KLF3 expression was significantly altered after adipocyte differentiation compared with that before differentiation (Figure S3). Then, KLF3 expression plasmids were constructed as described in the methods and materials, and were transfected into goat intramuscular preadipocyte. The efficiency was validated by qPCR and Western blot analysis, and the result indicated that the plasmid could significantly upregulate KLF3 expression (Figure 6A). Lipid droplets accumulation in adipocytes were measured by Oil red O and Bodipy staining, as well as triglyceride content detection, all these results showed that KLF3 could promote adipogenesis (Figure 6B-D). Since the expression of relevant molecules is also an indication of the cell state, adipogenic markers (CEBP/α, CEBP/β, PPARγ, pref1), TG synthesis genes (ACC, FASN, DGAT1, DGAT2, AGPAT6, TIP47, GPAM, ADRP, AP2, SREBP1), TG lipolysis genes (LPL, ATGL, and HSL) expression were examined. Quantitative PCR assay revealed that except for CEBP/α and HSL, the expression of other genes was upregulated by overexpressed KLF3 (Figure 6E-G).
Figure 6.
Gain-of function of KLF3 promotes the lipid deposition of goat intramuscular adipocytes. (A) qPCR and WB validation of KLF3 expression in vector and KLF3 plasmid transfected preadipocytes. UXT (qPCR) or β-actin (WB) was used as an internal control (48 h after transfection, without differentiation induction). (B) The efficiency of adipocyte differentiation was dramatically increased in KLF3 overexpression cells based on Oil red O staining (magnification: left, 40×, right, 400×). The right panel was the semi-quantitative assessment result. After photographing, the cells were destined in 1 mL 100% isopropanol for 15 min and the Oil red signal was quantified by measuring the absorbance at 490 nm. (C) Representative images of Bodipy staining of lipid droplets in goat differentiated adipocytes (48 h of differentiation) with or without KLF3 overexpression (magnification: 400×). (D) Triglyceride content detection in vector and KLF3 expression plasmid transfected differentiated adipocytes. (E), (F), (G) The mRNA expression level of relative genes in vector and KLF3 expression plasmid transfected differentiated adipocytes were detected by qPCR. UXT was used as an internal control. Data are presented as mean percentage (mean ± SD) of at least three independent experiments. *P < 0.05, **P < 0.01.
To further verify the role of KLF3 in adipocyte differentiation, two siRNA especially targeting KLF3 were synthesized by Invitrogen and transfected into goat intramuscular preadipocyte, respectively. The silencing effect validation results revealed that both siRNAs could especially downregulate the expression of KLF3 (Figure 7A). Because of its stronger silencing effect, siKLF3-2 was selected for the subsequent experiments in this study. Our results showed that the adipogenic differentiation of goat intramuscular adipocyte was weakened while silencing KLF3 (Figure 7B-D). Meanwhile, the mRNA level of adipogenic markers (CEBP/α, CEBP/β, PPARγ, pref1), TG synthesis genes (TIP47, GPAM, ADRP, AP2), triglyceride lipolysis genes (LPL, ATGL) expression were significantly downregulated after KLF3 interference (Figure 7E-G). These results indicate that overexpression of KLF3 promoted the accumulation of intramuscular fat in goats.
Figure 7.
Loss-of function of KLF3 inhibits goat intramuscular adipocyte lipid deposition. (A) qPCR validation of KLF3 expression in siNC and KLF3 siRNA transfected preadipocytes. UXT was used as an internal control (48 h after transfection, without differentiation induction). (B) The efficiency of adipocyte differentiation was dramatically decreased in KLF3 downregulation cells based on Oil red O staining (magnification: left, 40×, right, 400×). The right panel was the semi-quantitative assessment result. After photographing, the cells were destined in 1 mL 100% isopropanol for 15 min and the Oil red signal was quantified by measuring the absorbance at 490 nm. (C) Representative images of Bodipy staining of lipid droplets in goat differentiated adipocytes (48 h of differentiation) with or without KLF3 interference (magnification: 400×). (D) Triglyceride content detection in siNC and KLF3 siRNA transfected differentiated adipocytes. (E), (F), (G) The mRNA expression level of relative genes in siNC and KLF3 siRNA transfected differentiated adipocytes were detected by qPCR. UXT was used as an internal control. Data are presented as mean percentage (mean ± SD) of at least three independent experiments. **P < 0.01.
KLF3 overexpression could rescue the intramuscular fat deposition inhibited by miR-130b-3p or miR-130b-5p overexpression
Having clarified the function of miR-130b-3p, miR-130b-5p, and KLF3 in goat intramuscular adipocyte differentiation, this allowed us to examine whether KLF3 plays a key role in the adipogenesis inhibition regulated by miR-130b-3p and miR-130b-5p overexpression. First, we overexpressed KLF3 in miR-130b-3p or miR-130b-5p overexpression adipocytes respectively (Figures 8A, B and 9A, B). As expected, miR-130b-3p and miR-130b-5p strongly decreased the adipogenesis and this inhibition was rescued by KLF3 overexpression (Figures 8C and 9C). miR-130b-3p overexpression decreased the mRNA levels of adipogenic markers (CEBP/α, CEBP/β, PPARγ, pref1), TG synthesis genes (DGAT1, DGAT2, TIP47, GPAM, ADRP), TG lipolysis genes (ATGL and HSL), which was rescued by KLF3 overexpression (Figure 8D-F). Also, miR-130b-5p inhibited adipogenic markers (CEBP/α, CEBP/β), TG synthesis genes (FASN, DGAT2, AGPAT6, GPAM, AP2, SREBP1), TG lipolysis genes (ATGL) expression and KLF3 rescued their expression as well (Figure 9D-F). These results, together with previous studies, indicate that miR-130b-3p and miR-130b-5p inhibit the differentiation of goat intramuscular adipocytes by targeting KLF3.
Figure 8.
The role of KLF3 in miR-130b-3p inhibited adipocyte differentiation. Quantitative PCR validation of (A) miR-130b-3p and (B) KLF3 expression in miR-130b-3p overexpressed preadipocytes with or without KLF3 overexpression (48 h after transfection, without differentiation induction). (C) Representative images of Oil red O (the left panel) (magnification: left, 40×, right, 400×) and Bodipy (the right panel) staining of lipid droplets in miR-130b-3p overexpressed goat differentiated adipocytes (48 h of differentiation) with or without KLF3 overexpression (magnification: 400×). (E), (F), (G) The mRNA expression level of relative genes in mimics NC+vector, miR-130b-3p+vector, and miR-130b-3p+KLF3 goat differentiated adipocytes. Data are presented as mean percentage (mean ± SD) of at least three independent experiments. *P < 0.05, **P < 0.01, ns indicates no significance.
Figure 9.
The role of KLF3 in miR-130b-5p inhibited adipocyte differentiation. Quantitative PCR validation of (A) miR-130b-5p and (B) KLF3 expression in miR-130b-5p overexpressed preadipocytes with or without KLF3 overexpression (48 h after transfection, without differentiation induction). (C) Representative images of Oil red O (the left panel) (magnification: left, 40×, right, 400×) and Bodipy (the right panel) staining of lipid droplets in miR-130b-5p overexpressed goat differentiated adipocytes (48 h of differentiation) with or without KLF3 overexpression (magnification: 400×). (E), (F), (G) The mRNA expression level of relative genes in mimics NC+vector, miR-130b-5p+vector, and miR-130b-5p+KLF3 goat differentiated adipocytes. Data are presented as mean percentage (mean ± SD) of at least three independent experiments. *P < 0.05, **P < 0.01, ns indicates no significance.
Discussion
Intramuscular fat content contributes to the color, tenderness, juiciness, and flavor of the meat, which is an important factor affecting meat quality (Ruiz et al., 2001; Hamill et al., 2012; Pannier et al., 2014; Wang et al., 2021; Yang et al., 2021; Yao et al., 2022). Intramuscular fat is mainly distributed in the muscle layers and muscle fibers, and also exists in the muscle cytoplasm in the form of lipid droplets, including intracellular phospholipids, triglycerides, and cholesterol. Intramuscular fat content is an important quantitative trait, which is affected by both nutritional and genetic factors, such as mRNA and miRNAs (Zhang et al., 2019). In recent decades, more attention has been paid to the roles of miRNAs in adipogenic differentiation. Therefore, finding differentially expressed miRNAs and their downstream target genes is the molecular basis of meat quality improvement. In this study, we focused on both strands of miR-130b (miR-130b-5p and miR-130b-3p) for that these miRNAs were significantly downregulated in mature goat intramuscular adipocytes.
Previous studies showed that the passenger strands, miR-130b-5p may be crucial for inducing the adipogenic differentiation of human adipose tissue-derived stromal stem cells (Guo and Cao, 2019). However, the role of miR130b-5p and miR130b-3p in adipogenesis of economic meat animals has not been reported. The present data indicate that both strands of miR-130b (miR-130b-3p and miR-130b-5p) could inhibit intramuscular adipocyte differentiation thus decrease lipid droplets accumulation in goat. Interestingly, based on the qPCR results, we found that the expression trends of genes related to adipocyte differentiation, TG synthesis and lipolysis detected in this study were basically the same in miR-130b (miR-130b-3p and miR-130b-5p)/KLF3 regulated adipogenesis (Figures 1E-G, 2E-G, 3E-G, 4E-G, 6E-G, 7E-G). However, cells showed increased or decreased lipid accumulation, contrary to the trend of TG lipolysis genes expression. It was speculated that the rate of TG lipolysis was less than the rate of lipid synthesis, so the cells showed increased accumulation of lipid droplets. Whether or not miR-130b duplex has the same role in fat deposition in other species, it is indispensable to elucidate the molecular mechanism by which it regulates intramuscular fat deposition in goats.
It was reported that KLF3 was relevant to adipogenesis in mice (Sue et al., 2008; Ahmed and Gaffen, 2013; Guo et al., 2013; Han et al., 2022), and according to the prediction by bioinformatics, KLF3 is a potential target gene for miR-130b-3p and miR-130b-5p in goats. Our study demonstrated that KLF3 is a positive regulator of adipocyte differentiation and a key target gene of goat adipogenesis regulated by miR-130b-3p and miR-130b-5p for the first time. Together with the previous findings, this study confirmed that KLF3 may be involved in the regulation of multispecies adipogenesis. However, the specific mechanism of KLF3 in the regulation of lipid differentiation remains to be further studied.
Interestingly, it was found that after gene overexpression or interference, the expression trend of downstream-related genes was consistent in the above two types of cells. For example, CEBP/β gene expression was upregulated in response to miR-130b-3p and miR-130b-5p overexpression or endogenous expression inhibition (Figures 1E, 2E, 3E, and 4E). At the same time, we also found that the gene expression may be unchanged in the cells of the overexpression group, but significantly up or downregulated in the cells of the interference group. For example, AP2 did not present a significant change in the cells with miR-130b-3p and miR-130b-5p overexpression, but was downregulated in the cells of interference with miR-130b-3p and miR-130b-5p (Figures 1G, 2G, 3G, and 4G). The same result was observed in the rescue experiment of this study. Also, the same problem has been found in previous studies (Guo et al., 2020; Xu et al., 2021; Chen et al., 2022). In addition, pref-1, a well-known negative regulator of adipogenesis, was positively correlated with miR130b duplex (miR-130b-3p and miR-130b-5p) and KLF3 regulated goat intramuscular adipocyte differentiation. This may be because the upstream genes of pref-1 expression changed and then regulated its expression. Because one of the cell behaviors is the result of synergistic action of multiple genes, so, pref-1 expression is positively correlated with goat intramuscular adipocyte differentiation in this study. All the results above indicate the complex effects of miRNA and molecules on adipocytes and signaling pathways, the aim of this study was to identify key miRNAs, its target gene, and downstream lipids droplets accumulation relative mRNAs in goat intramuscular adipocytes.
This study focused on how miR-130b-5p and miR-130b-3p regulate lipid deposition through adipocyte differentiation. Our findings indicate that these two microRNAs could target KLF3 gene expression then inhibit intramuscular adipocyte differentiation in goats. However, it remains to be further explored whether miR-130b-5p and miR-130b-3p still regulate fat deposition in adipocytes of other tissues of goats or adipocytes of other species through this mechanism. At the same time, whether miR-130b-5p and miR-130b-3p have an effect on lipid deposition through other ways, such as cell proliferation, also needs to be further confirmed. In addition, the specific molecular mechanisms involved in regulating adipocyte differentiation by miR-130b-5p and miR-130b-3p are worthy of further investigation.
Conclusion
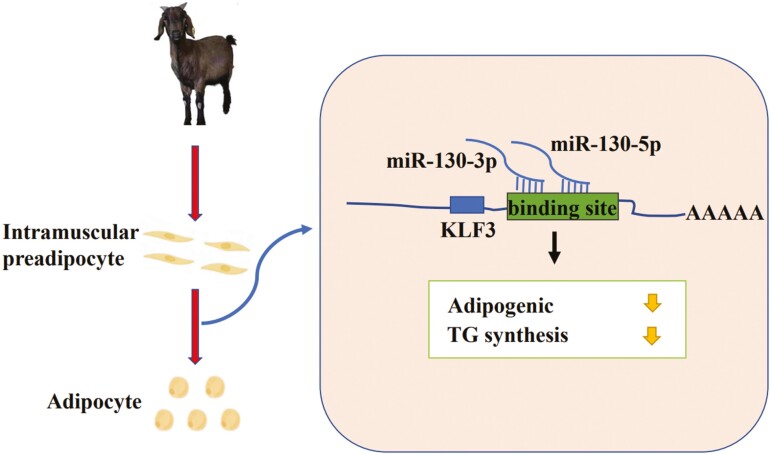
In conclusion, the present study provides the first evidence that both strands of miR-130b (miR-130b-5p and miR-130b-3p) play an important role in adipogenesis in goat intramuscular adipocytes, acting to directly inhibit the transcriptional activity of a key transcription factor in the phase of adipogenic differentiation, KLF3. The aberrant expression of KLF3 enhanced lipid droplets accumulation, suggesting that KLF3 could be a promising target for the selection of fat deposition traits in goats (Figure 10). Our approach, discovery of anti-adipogenesis miRNAs and their target mRNAs, will contribute to exploring the selection of this molecular breeding.
Figure 10.
miR-130b-5p and miR-130b-3p inhibit goat intramuscular adipocyte differentiation by targeting KLF3.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (32072723), Sichuan Science and Technology Program (2022JDTD0030), and the Fundamental Research Funds for the Central Universities, Southwest Minzu University (2021057).
Abbreviations:
- 3ʹUTR
3ʹ-untranslated region
- ACC
acetyl-CoA carboxylase
- ADRP
adipose differentiation-related protein
- AGPAT6
Sn-1-acylglycerol-3-phosphate O-acyltransferase 6
- AP2
fatty acid binding protein 4
- ATGL
adipose triglyceride lipase
- C/EBPα
CCAAT enhancer binding protein alpha
- C/EBPβ
CCAAT enhancer binding protein beta
- DGAT1
diacylgycerol acyltransferase 1
- DGAT2
diacylgycerol acyltransferase 2
- FASN
fatty acid synthase;
- FBS
fetal bovine serum
- GPAM
glycerol-3-phosphate acyltransferase
- HSL
hormone-sensitive lipase
- IMF
intramuscular fat
- KLF
Krüppel-like factors
- KLF3
Kruppel-like factor 3
- LPL
lipoprotein lipase
- PBS
phosphate-buffer saline
- PPARγ
peroxisome proliferative activated receptor gamma
- pref1
preadipocyte factor 1
- qPCR
quantitative real-time polymerase chain reaction
- siRNA
small interfering RNA
- SREBP1
sterol regulatory element binding protein 1
- TG
triglyceride
- TIP47
tail interacting protein of 47 kDa
- UXT
ubiquitously expressed prefoldin like chaperone
Contributor Information
Yanyan Li, Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization, Ministry of Education, Southwest Minzu University, Chengdu, China; Key Laboratory of Sichuan Province for Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Exploitation, Southwest Minzu University, Chengdu, China.
Changsheng He, Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization, Ministry of Education, Southwest Minzu University, Chengdu, China; Key Laboratory of Sichuan Province for Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Exploitation, Southwest Minzu University, Chengdu, China; College of Animal Science and Veterinary Medicine, Southwest Minzu University, Chengdu, China.
Li Ran, Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization, Ministry of Education, Southwest Minzu University, Chengdu, China; Key Laboratory of Sichuan Province for Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Exploitation, Southwest Minzu University, Chengdu, China; College of Animal Science and Veterinary Medicine, Southwest Minzu University, Chengdu, China.
Yong Wang, Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization, Ministry of Education, Southwest Minzu University, Chengdu, China; Key Laboratory of Sichuan Province for Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Exploitation, Southwest Minzu University, Chengdu, China; College of Animal Science and Veterinary Medicine, Southwest Minzu University, Chengdu, China.
Yan Xiong, Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization, Ministry of Education, Southwest Minzu University, Chengdu, China; Key Laboratory of Sichuan Province for Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Exploitation, Southwest Minzu University, Chengdu, China; College of Animal Science and Veterinary Medicine, Southwest Minzu University, Chengdu, China.
Youli Wang, Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization, Ministry of Education, Southwest Minzu University, Chengdu, China; Key Laboratory of Sichuan Province for Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Exploitation, Southwest Minzu University, Chengdu, China.
Jiangjiang Zhu, Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization, Ministry of Education, Southwest Minzu University, Chengdu, China; College of Animal Science and Veterinary Medicine, Southwest Minzu University, Chengdu, China.
Yaqiu Lin, Key Laboratory of Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Utilization, Ministry of Education, Southwest Minzu University, Chengdu, China; Key Laboratory of Sichuan Province for Qinghai-Tibetan Plateau Animal Genetic Resource Reservation and Exploitation, Southwest Minzu University, Chengdu, China; College of Animal Science and Veterinary Medicine, Southwest Minzu University, Chengdu, China.
Conflicts of Interest Statement
The authors declare that they have no competing interests.
Authors’ Contributions
Yanyan Li, Changsheng He, Yaqiu Lin: conceptualization, methodology, and writing original draft. Yong Wang, Yan Xiong, Youli Wang, Jiangjiang Zhu: methodology. Changsheng He and Li Ran: investigation and analysis. Yaqiu Lin: resources, supervision and project administration. Yanyan Li, Changsheng He, Li Ran, Yaqiu Lin: review and editing.
Literature Cited
- Ahmed, M., and Gaffen S. L.. . 2013. IL-17 inhibits adipogenesis in part via C/EBPα, PPARγ and Krüppel-like factors. Cytokine 61(3):898–905. doi: 10.1016/j.cyto.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak, Y., Nelson M. C., Ong E. S., Jones Y. Z., Ruiz-Lozano P., Chien K. R., Koder A., and Evans R. M.. . 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Bartel, D. P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel, D. P. 2009. MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati, N., and Cohen S. M.. . 2007. microRNA functions. Annu. Rev. Cell Dev. Biol. 23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- Chen, D., Lin Y., Zhao N., Wang Y., and Li Y.. . 2022. Hoxa5 inhibits the proliferation and induces adipogenic differentiation of subcutaneous preadipocytes in goats. Animals (Basel) 12:1859. doi: 10.3390/ani12141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y., Ma J., Wang Y., Zhu J., Li Y., Meng Q., and Lin Y.. . 2022. MiR-421 regulates goat intramuscular preadipocytes differentiation via targeting FGF13. Anim. Biotechnol. 33(6):1333–1343. doi: 10.1080/10495398.2021.1898414. [DOI] [PubMed] [Google Scholar]
- Eseberri, I., Lasa A., Miranda J., Gracia A., and Portillo M. P.. . 2017. Potential miRNA involvement in the anti-adipogenic effect of resveratrol and its metabolites. PLoS One 12:e0184875. doi: 10.1371/journal.pone.0184875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, S. R. 2006. Transcriptional control of adipocyte formation. Cell Metab. 4(4):263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Z., and Cao Y.. . 2019. An lncRNA-miRNA-mRNA ceRNA network for adipocyte differentiation from human adipose-derived stem cells. Mol. Med. Rep. 19(5):4271–4287. doi: 10.3892/mmr.2019.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, L., Huang J. X., Liu Y., Li X., Zhou S. R., Qian S. W., Liu Y., Zhu H., Huang H. Y., Dang Y. J., . et al. 2013. Transactivation of Atg4b by C/EBPβ promotes autophagy to facilitate adipogenesis. Mol. Cell. Biol. 33:3180–3190. doi: 10.1128/MCB.00193-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, H., Raza S. H. A., Schreurs N. M., Khan R., Wei D., Wang L., Zhang S., Zhang L., Wu S., and UIIah I., . et al. 2018. Genetic variants in the promoter region of the KLF3 gene associated with fat deposition in Qinchuan cattle. Gene. 672:50–55. doi: 10.1016/j.gene.2018.06.022. [DOI] [PubMed] [Google Scholar]
- Guo, Y., Tan J., Xiong W., Chen S., Fan L., and Li Y.. . 2020. Notch3 promotes 3T3-L1 pre-adipocytes differentiation by up-regulating the expression of LARS to activate the mTOR pathway. J. Cell. Mol. Med. 24:1116–1127. doi: 10.1111/jcmm.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill, R. M., McBryan J., McGee C., Mullen A. M., Sweeney T., Talbot A., Cairns M. T., and Davey G. C.. . 2012. Functional analysis of muscle gene expression profiles associated with tenderness and intramuscular fat content in pork. Meat Sci. 92:440–450. doi: 10.1016/j.meatsci.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Han, J. H., Jang K. W., and Myung C. S.. . 2022. Garcinia cambogia attenuates adipogenesis by affecting CEBPB and SQSTM1/p62-mediated selective autophagic degradation of KLF3 through RPS6KA1 and STAT3 suppression. Autophagy 18:518–539. doi: 10.1080/15548627.2021.1936356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi S., Zhang J., Siddiqui S. S., Parhar R. S., Bakheet R., Al-Mohanna. F.. 2011. Partner in fat metabolism: Role of KLFs in fat burning and reproductive behavior. 3 Biotech. 1:59–72. doi: 10.1007/s13205-011-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, C., Wang Y., Xu Q., Xiong Y., Zhu J., and Lin Y.. . 2021. Overexpression of Krueppel like factor 3 promotes subcutaneous adipocytes differentiation in goat Capra hircus. Anim. Sci. J. 92:e13514. doi: 10.1111/asj.13514. [DOI] [PubMed] [Google Scholar]
- Kannan, G., Mahapatra A. K., and Degala H. L.. . 2021. Preharvest Management and Postharvest Intervention Strategies to Reduce Escherichia coli Contamination in Goat Meat: A Review. Animals 11:2943. doi: 10.3390/ani11102943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. W., Meng J., Cui J., and Luan Y. S.. . 2017. Characterization and function of microRNA*s in plants. Front. Plant Sci. 8:2200. doi: 10.3389/fpls.2017.02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., Li B., Qiao L., Liu J., Ren D., and Liu W.. . 2020. miR-340-5p inhibits sheep adipocyte differentiation by targeting ATF7. Anim. Sci. J. 91(1):e13462. doi: 10.1111/asj.13462. [DOI] [PubMed] [Google Scholar]
- Luo, W., Kim Y., Jensen M. E., Herlea-Pana O., Wang W., M. C. Rudolph, Friedman J. E., Chernausek S. D., and Jiang S.. . 2022. miR-130b/301b is a negative regulator of beige adipogenesis and energy metabolism in vitro and in vivo. Diabetes 71:2360–2371. doi: 10.2337/db22-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J., Lin Y., Zhu J., Huang K., and Wang Y.. . 2021. MiR-26b-5p regulates the preadipocyte differentiation by targeting FGF21 in goats. In Vitro Cell. Dev. Biol. Anim. 57:257–263. doi: 10.1007/s11626-020-00493-y. [DOI] [PubMed] [Google Scholar]
- MacDougald, O. A., and Mandrup S. A.. . 2002. Adipogenesis: forces that tip the scales. Trends Endocrinol. Metab. 13(1):5–11. doi: 10.1016/s1043-2760(01)00517-3. [DOI] [PubMed] [Google Scholar]
- Pannier, L., Gardner G. E., Pearce K. L., McDonagh M., Ball A. J., Jacob R. H., and Pethick D. W.. . 2014. Associations of sire estimated breeding values and objective meat quality measurements with sensory scores in Australian lamb. Meat Sci. 96:1076–1087. doi: 10.1016/j.meatsci.2013.07.037. [DOI] [PubMed] [Google Scholar]
- Pearson, R. C. M., Funnell A. P. W., and Crossley M.. . 2011. The mammalian zinc finger transcription factor Krüppel-like factor 3 (KLF3/BKLF). IUBMB Life 63:86–93. doi: 10.1002/iub.422. [DOI] [PubMed] [Google Scholar]
- Rosen, E. D., Sarraf P., Troy A. E., Bradwin G., Moore K., Milstone D. S., Spiegelman B. M., and Mortensen R. M.. . 1999. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Ruiz, J. A., Guerrero L., Arnau J., Guardia M. D., and Esteve-Garcia E.. . 2001. Descriptive sensory analysis of meat from broilers fed diets containing vitamin E or beta-carotene as antioxidants and different supplemental fats. Poult. Sci. 80:976–982. doi: 10.1093/ps/80.7.976. [DOI] [PubMed] [Google Scholar]
- Shen, L., Zhang Y., Du J., Chen L., Luo J., Li X., Li M., Tang G., Zhang S., and Zhu L.. . 2016. MicroRNA-23a regulates 3T3-L1 adipocyte differentiation. Gene 575:761–764. doi: 10.1016/j.gene.2015.09.060. [DOI] [PubMed] [Google Scholar]
- Siersbaek, R., Nielsen R., and Mandrup S.. . 2010. PPARgamma in adipocyte differentiation and metabolism--novel insights from genome-wide studies. FEBS Lett. 584:3242–3249. doi: 10.1016/j.febslet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Sue, N., Jack B. H., Eaton S. A., Pearson R. C., Funnell A. P., Turner J., Czolij R., Denyer G., Bao S., Molero-Navajas J. C., . et al. 2008. Targeted disruption of the basic Krüppel-like factor gene (Klf3) reveals a role in adipogenesis. Mol. Cell. Biol. 28:3967–3978. doi: 10.1128/MCB.01942-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, D., Ding Z., Shen L., Yang F., Han J., and Wu G.. . 2021. miR-410-3P inhibits adipocyte differentiation by targeting IRS-1 in cancer-associated cachexia patients. Lipids Health Dis. 20:115. doi: 10.1186/s12944-021-01530-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L., Huang Y., Wang Y., and Shan T.. . 2021. Effects of polyunsaturated fatty acids supplementation on the meat quality of pigs: a meta-analysis. Front Nutr. 8:746765. doi: 10.3389/fnut.2021.746765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. Y., Chen C. W., Chen L. K., Chou H. Y., Chang C. L., and Juan C. C.. . 2019. Curcumin attenuates adipogenesis by inducing preadipocyte apoptosis and inhibiting adipocyte differentiation. Nutrients 11:2307. doi: 10.3390/nu11102307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q., Lin S., Wang Y., Zhu J., and Lin Y.. . 2018. Fibroblast growth factor 10 (FGF10) promotes the adipogenesis of intramuscular preadipocytes in goat. Mol. Biol. Rep. 45:1881–1888. doi: 10.1007/s11033-018-4334-1. [DOI] [PubMed] [Google Scholar]
- Xu, J., Zhang L., Shu G., and Wang B.. . 2019. microRNA-16-5p promotes 3T3-L1 adipocyte differentiation through regulating EPT1. Biochem. Biophys. Res. Commun. 514:1251–1256. doi: 10.1016/j.bbrc.2019.04.179. [DOI] [PubMed] [Google Scholar]
- Xu, Q., Wang Y., Li X., Du Y., Li Y., Zhu J., and Lin Y.. . 2021. miR-10a-5p inhibits the differentiation of goat intramuscular preadipocytes by targeting KLF8 in goats. Front. Mol. Biosci. 8:700078. doi: 10.3389/fmolb.2021.700078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, T., Wang X., Wen M., Zhao H., Liu G., Chen X., Tian G., Cai J., Jia G., . et al. 2021. Effect of manganese supplementation on the carcass traits, meat quality, intramuscular fat, and tissue manganese accumulation of Pekin duck. Poult. Sci. 100:101064. doi: 10.1016/j.psj.2021.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, D., Su R., Zhang Y., Wang B., Hou Y., Luo Y., Sun L., Guo Y., and Jin Y.. . 2022. Impact of dietary lactobacillus supplementation on intramuscular fat deposition and meat quality of Sunit sheep. J. Food Biochem. 46:e14207. doi: 10.1111/jfbc.14207. [DOI] [PubMed] [Google Scholar]
- Zhang, J., Hashmi S., Cheema F., Al-Nasser N., Bakheet R., Parhar R. S., Al-Mohanna F., Gaugler R., Hussain M. M., and Hashmi S.. . 2013. Regulation of lipoprotein assembly, secretion and fatty acid β-oxidation by Krüppel-like transcription factor, klf. J. Mol. Biol. 425:2641–2655. doi: 10.1016/j.jmb.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M., Li F., Sun J. W., Li D. H., Li W. T., Jiang R. R., Li Z. J., Liu X. J., Han R. L., Li G. X., . et al. 2019. LncRNA IMFNCR promotes intramuscular adipocyte differentiation by sponging miR-128-3p and miR-27b-3p. Front. Genet. 10:42. doi: 10.3389/fgene.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.