Abstract
This study reviewed the efficacy and safety of intense pulsed light (IPL) for the treatment of dry eye disease (DED). The PubMed database was used to conduct the literature search, which used the keywords “intense pulsed light” and “dry eye disease”. After the authors evaluated the articles for relevancy, 49 articles were reviewed. In general, all treatment modalities were proven to be clinically effective in reducing dry eye (DE) signs and symptoms; however, the level of improvement and persistence of outcomes differed amongst them. Meta-analysis indicated significant improvement in the Ocular Surface Disease Index (OSDI) scores post-treatment with a standardized mean difference (SMD) = −1.63; confidence interval (CI): −2.42 to −0.84. Moreover, a meta-analysis indicated a significant improvement in tear break-up time (TBUT) test values with SMD = 1.77; CI: 0.49 to 3.05. Research suggests that additive therapies, such as meibomian gland expression (MGX), sodium hyaluronate eye drops, heated eye mask, warm compress, lid hygiene, lid margin scrub, eyelid massage, antibiotic drops, cyclosporine drops, omega-3 supplements, steroid drops, and warm compresses along with IPL, have been found to work in tandem for greater effectiveness; however, in clinical practice, its feasibility and cost-effectiveness have to be taken into consideration. Current findings suggest that IPL therapy is suitable when lifestyle modifications such as reducing or eliminating the use of contact lenses, lubricating eye drops/gels, and warm compresses/eye masks fail to improve signs and symptoms of DE. Moreover, patients with compliance issues have been shown to benefit well as the effects of IPL therapy is sustained for over several months. DED is a multifactorial disorder, and IPL therapy has been found to be safe and efficient in reducing its signs and symptoms of meibomian gland dysfunction (MGD)-related DE. Although the treatment protocol varies among authors, current findings suggest that IPL has a positive effect on the signs and symptoms of MGD-related DE. However, patients in the early stages can benefit more from IPL therapy. Moreover, IPL has a better maintenance impact when used in conjunction with other traditional therapies. Further research is needed to assess cost-utility analysis for IPL.
Keywords: Dry eye, intense pulsed light, meibomian gland dysfunction
Dry eye disease (DED) is a multifactorial disorder that manifests on the ocular surface as loss of homeostasis of the tear film, ocular symptoms, tear film instability, hyperosmolarity, ocular surface inflammation, damage, and neurosensory abnormalities.[1] The primary symptoms of patients with dry eye (DE) are dryness, discomfort, foreign body sensation, burning sensation, and decreased visual quality, which has the tendency to affect their quality of life.[2,3] Depending on the demographics surveyed, it is estimated that the prevalence of DE ranges from 5% to 50%.[4] Because of deleterious lifestyle changes, an aging population, chronic illness, and the use of certain medications, the prevalence of DED has continued to increase, burdening the healthcare system and expenses year over year.[5]
According to the Tear Film and Ocular Surface Society’s Dry Eye Workshop II (TFOS DEWS II), DED is divided into the aqueous deficient dry eye (ADDE), evaporative dry eye (EDE) due to meibomian gland dysfunction (MGD), and a combination of ADDE and EDE. Varying severity of hypo-secretion of meibum by the meibomian glands (MG) is considered the most likely cause of EDE [Fig. 1].[6] Conventional treatments such as preservative-free drops, omega-3, and fatty acid supplementation can be used for mild disease. For moderate DED, high viscosity eye drops and gel or ointment, warm compresses, eyelid massage,[7] eyelid expression, and lacrimal plugs have been proven to be useful. In severe DED, serum tears,[8] topical azithromycin,[9] topical cyclosporine,[10] oral doxycycline,[11] cholinergic agonist,[12,13] and amniotic membrane biologic corneal bandage lens,[14] have shown to improve the signs and symptoms of DED.[15] However, in the long term, their efficacy is not satisfactory due to the patient’s lack of treatment adherence.[16,17]
Figure 1.
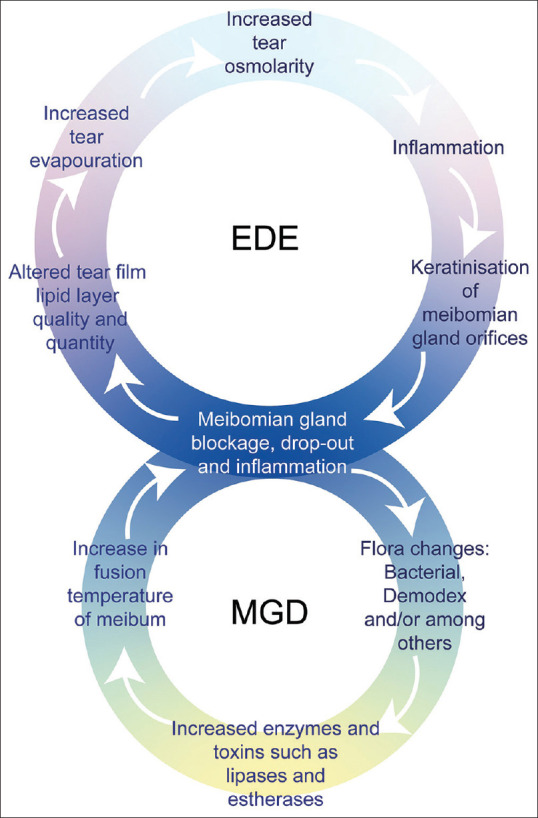
Vicious cycle of evaporative dry eye (EDE) and meibomian gland dysfunction (MGD)
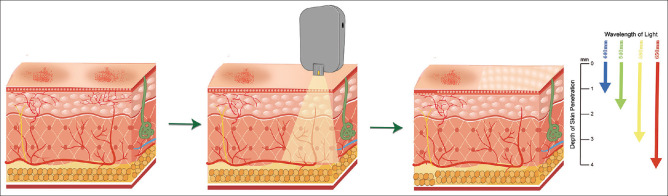
Intense pulsed light (IPL) has mostly been utilized as a dermatological treatment for conditions such as facial rosacea, facial erythema, acne, and seborrheic keratosis throughout the last few decades.[18] In 2015, Toyos et al.[19] reported the use of IPL for treating MGD to improve the signs and symptoms of DED. Two years later, the TFOS DEWS II report listed IPL as an option for treating DED.[20] Based on this, numerous studies have evaluated the potential use of IPL for treating the signs and symptoms of primary and secondary forms of DED [Fig. 2]. The purpose of this review is to summarize relevant studies and evaluate the efficacy of IPL for treating DE.
Figure 2.
Illustration depicting intense pulsed light (IPL) skin penetration
IPL Manufacturers
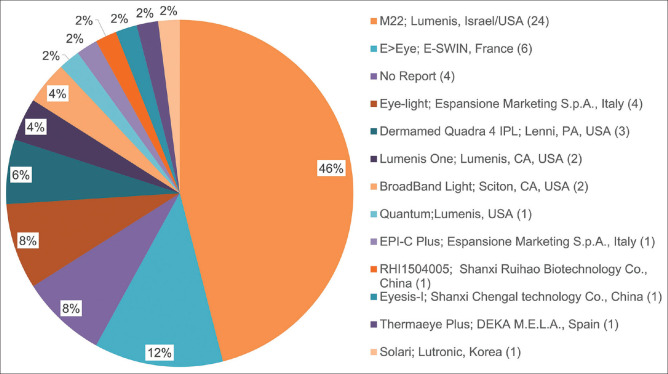
The current IPL manufacturers for ocular surface therapy are displayed in Fig. 3. The multiple homogenously sculpted light pulses (E > Eye; E-SWIN, Paris, France) is stated to be the first IPL devices certified for the treatment of MGD.[21,22] The DreaMed Quadra4 IPL (DermaMed Solutions, LLC, Lenni, PA) is another device that has been reported to treat DE by selecting the “dry eye mode.”[23,24] In addition, various clinical studies using Lumenis One (Lumenis, Inc., Santa Clara, CA), Thermaeye Plus (DEKA M.E.L.A. Spa, MDS Medical Technologies SL, Spain), and BroadBand Light (Sciton Palo Alto, CA, USA).[25-28] Eye-light® (Espansione Marketing SPA., Bologna, Italy) CE-marked device is a relatively new instrument for MGD, which has low-level light therapy (LLLT) mode.[29-32] The Lumenis M22 system (M22; Yokneam, Israel/America) combines optimal pulse technology (OPT) with a modular laser multi-application platform and is the most researched and published device for DE-related disorders.[33,34]
Figure 3.
IPL dry eye research publications
IPL Treatment Process
IPL system uses a xenon lamp to emit light with a usual wavelength of between 500 and 1200 nm, which is aimed onto the cutaneous face sebaceous glands.[35] The intensity of IPL treatment needs to be selected according to the Fitzpatrick skin scale (from I to VI). Ultrasound gel is used to cover the targeted periorbital skin around the eyes before treatment. Routine treatment areas for IPL include the skin below the lower eyelid and the temples on both sides [Fig. 4]. In certain circumstances, IPL with a small spot size is applied directly to the upper eyelid to alleviate upper eyelid symptoms [Fig. 4].[27,36,37] Opaque goggles are required to cover both eyes during treatment. Currently, because there is no consensus on the frequency and duration of IPL therapy, clinician and publication usually have a 2 to 6-week interval between IPL therapy for DE and to achieve better treatment results, patients usually require numerous IPL sessions. After treatment, meibomian gland expression (MGX) or eye drops are used by some physicians for follow-up treatment.
Figure 4.

Routine treatment areas of IPL for EDE and MGD
Mechanism of Action
Enhances MG function
Liquification of meibum
The photothermal effect has been postulated to enhance the lipid layer of the tear film as the heat generated by IPL can pass through the thin skin surrounding the eyes and into the MG,[38] thereby making the meibum less viscous as the temperature is raised. The meibum in the duct softens, flows, and secretes more, making it easier to evenly distribute it across the ocular surface.[19]
Promoting changes to MG architecture
According to Yin et al.,[34] IPL therapy not only improved the macrostructure but also the microstructure of MG, as the activity of acinar cells was stimulated by IPL, which thereby improved the microstructure of MG.
Effects on inflammatory markers and immune cell
Inflammation plays a key role in both the early and late stages of EDE.[39] Multiple aspects affecting the stability and osmotic pressure of the tear film can cause ocular surface damage and provoke an inflammatory reaction, which can further damage the ocular surface. Irritation, tear film instability, tear production, and ocular surface integrity are linked to cytokine and chemokine levels.[40] IPL disrupts the inflammatory cycle by enhancing anti-inflammatory factors or suppressing pro-inflammatory factors.[41,42] Although the mechanism is not fully understood, D’Souza et al.’s[43] report suggests that IPL can reduce the levels of molecular factors, including IL-1b, IL-17F, and MMP-9/TIMP1 ratio and immune cell including B cells in the tears of DE patients. These observations suggest a decrease in ocular surface inflammation validating the improvement in ocular surface health metrics.
Demodex and IPL
Reports suggest that Demodex mites can cause MGD-related DE.[44,45] Demodex mite proliferation can lead to a rise in Bacillus, which produces a number of inflammatory reactions on the ocular surface.[46] Simultaneously, it clogs the meibomian gland pores and limits the release of meibum.[47] IPL applied on the ocular region has been reported to induce coagulation and necrosis in Demodex mites, resulting in a therapeutic effect.[48,49] These changes have been proposed to be beneficial for improving lipid secretion.
Destruction of abnormal blood vessels
In patients with DED, eyelid telangiectasia is a prevalent symptom.[50] According to reports, dilated capillaries generate inflammatory mediators, which travel through local blood circulation to the eyelid and induce MGD.[19] The pulsed light energy emitted by IPL can be preferentially absorbed by the chromophore of hemoglobin and act on abnormal blood vessels near the eyelid margin and conjunctiva, causing local destruction of superficial abnormal blood vessels, reducing the source of inflammatory mediators, thereby improving the symptoms of MGD and DED [Fig. 2].[51]
Inhibit Metalloproteinases
Metalloproteinases (MMPs) are proteins involved in the pathogenesis of DE and these enzymes contribute to extracellular matrix remodeling. The therapeutic use of IPL has been found to lower the concentration of MMPs in tear samples of participants.[52]
Photomodulation
Photomodulation is the process by which light induces changes in cells at the gene or protein level. As the eyelid skin lexes with age, it can lead to rough lid margins or incomplete lid closure, resulting in decreased lipid secretion. IPL stimulates mitochondria to increase adenosine triphosphate production,[53] which promotes the proliferation of fibroblasts and upregulates the synthesis of collagen fibers to achieve the effect of skin rejuvenation treatment.[54,55] Furthermore, significant improvements in molecular and cellular factors have also been reported.[43]
Neurotrophic effect
Nerve injury has been studied to be improved by low-power laser irradiation.[56,57] Fiber sprouting and neuronal cell migration from aggregates were aided by irradiation with a 780 nm laser.[58] A faster and better regeneration of the wounded nerve is achieved by increasing the rate of axon development and myelinization.
Methods
Since 2015, IPL has gotten a lot of interest as a therapy option for DED. The PubMed database was used to conduct the literature search, with the keywords “intense pulsed light” and “dry eye disease.” We conducted this search in March 2022 and returned 83 results. This review focused on any original research relevant to IPL for the treatment of DED. The articles for the meta-analysis were filtered by the following exclusion criteria: (a) articles written in languages than English, (b) review articles, (c) letters to the editor, and (d) meta-analysis articles. For a study to be included in the meta-analysis, it was required to be an RCT comparing the effect of IPL therapy for MGD with control, and they must report their advent events. The study was required to evaluate the following outcome measures: Ocular Surface Disease Index (OSDI) score and non-invasive tear break-up time (NITBUT) score. In the end, five studies were included in this review article [Fig. 5].
Figure 5.
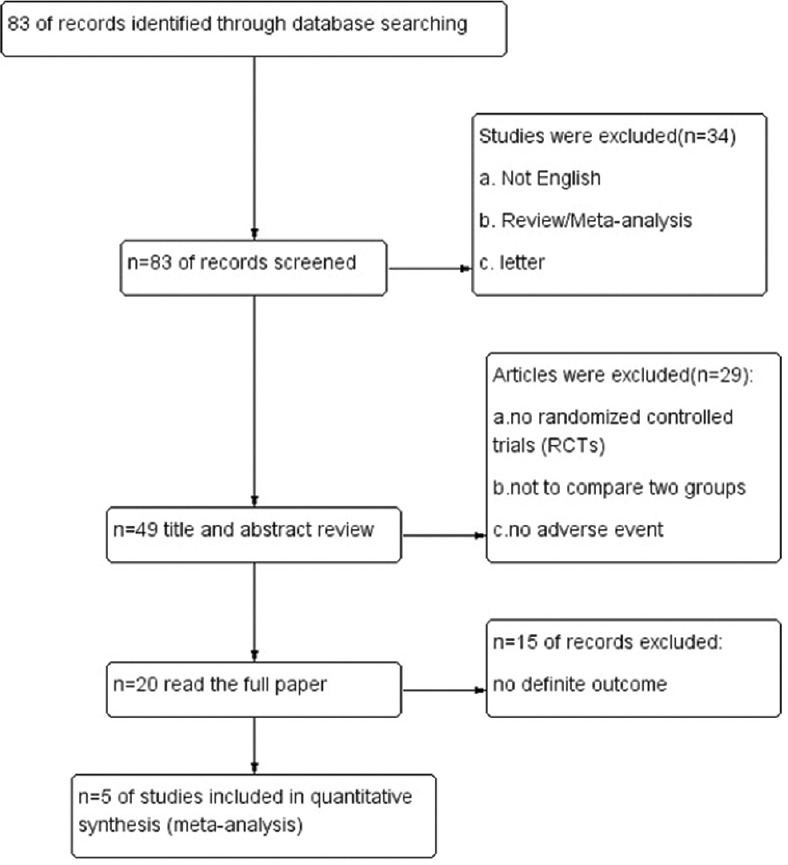
Flowchart of selection of studies for inclusion in the meta-analysis
Statistical Analysis
Statistical analysis was performed using Review Manager [RevMan, version 5.3; Nordic Cochrane Centre (Cochrane Collaboration)]. Pooled estimates for the continuous outcome measures were reported as the standard mean difference (SMD) with a 95% confidence interval (CI). The SMD was generated using the mean and standard deviation (SD) reported in the individual RCTs included in the meta-analysis. P <0.05 was considered to indicate statistical significance. Heterogeneity was examined using Tau and Higgins’ I2 and it governed whether a fixed-effects model (I2 value ≤50%) or a random-effects model (I2 value >50%) was used.
Results
Changes in overall OSDI scores
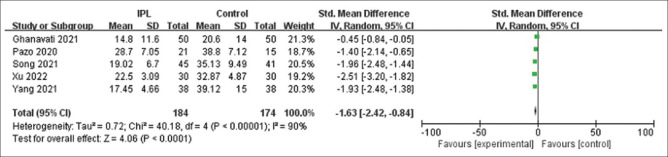
Fig. 6 is a forest plot displaying the changes in the overall OSDI scores after IPL treatment reported by five studies at various follow-up periods (ranging from <1 month to >6 months). After examining the forest plot for OSDI scores, the meta-analysis results indicated a significant improvement in OSDI values after IPL with (SMD) = −1.63; confidence interval (CI): −2.42 to −0.84.
Figure 6.
OSDI forest map after follow-up
Impact on TBUT
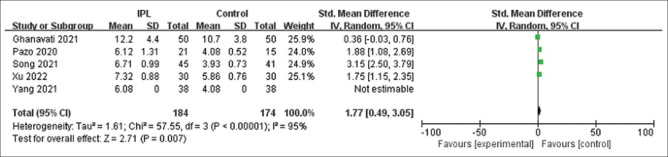
Fig. 7 is a forest plot displaying the changes in TBUT values after IPL treatment reported by five studies at various follow-up periods (ranging from <1 month to >6 months). After examining the forest plot for TBUT, the meta-analysis results indicated a significant increase in TBUT test values after IPL with SMD = 1.77; CI: 0.49 to 3.05.
Figure 7.
TBUT forest map after follow-up
Indications for IPL in the treatment of DED
Initially, IPL was used to treat MGD-related DE (MGD-DE), either alone or in combination with other treatments such as meibomian gland expression, artificial tears, low-level light (LLL), warm compresses, and antibiotics. Its therapeutic range was further broadened, and some studies revealed that IPL might be beneficial for symptoms of patients with Sjögren’s syndrome-related DE (SS-DE), contact lens-related DE (CL-DE), and laser refractive surgery-related DE, among other conditions.
MGD-DE
MGD is one of the most common causes of EDE.[59] It can produce a series of ocular surface inflammation and irritation due to the inhibited secretion of sebaceous glands in the upper and lower eyelids.
Single therapy
Craig et al.[22] used a double-blind, randomized controlled study to analyze the efficacy of IPL in the treatment of MGD. Twenty-eight patients received a total of three treatments on days 1, 15, and 45. Results reported significant improvement in subjective symptoms of DE, NIBUT, and lipid layer grade. Although the findings imply that IPL improves tear film quality and lowers DE symptoms, the trial did not have a long follow-up time.
In another study, Yin et al.[34] aimed to clarify the specific effects of IPL on MG. Comparisons were made with a control group treated with eyelid hygiene and the results showed that OSDI scores, conjunctiva staining (CS), TBUT, and MG function were all improved after IPL treatment. It is worth noting that the study looked at MG microstructure indices and discovered that changes in OSDI were linked to the MG acinar unit. There is a positive association between changes in OSDI and changes in MG acinar unit density. The researchers believe that IPL manages MGD by improving the microstructure of MG, which may be one of the therapeutic mechanisms of IPL.
A randomized controlled trial by Yan et al.[60] compared the efficacy of IPL therapy with MGX plus warm compresses for MGD. The results showed that the short-term and long-term efficacy indicators of IPL in the treatment of MGD DE were better than MGX combined with warm compresses. Furthermore, patients treated with IPL were more satisfied than controls at 7 days and 30 days follow-ups. No adverse events were reported in this study.
IPL can significantly improve vision quality in addition to relieving DE symptoms according to Fan et al.[61] The quality of vision (QOV) questionnaire was utilized to assess 64 participants who had two IPL sessions spaced 3 weeks apart. The results revealed that the patients’ subjective OSDI and QOV scores were greatly improved following therapy, mainly manifested as glare, halos, blurred vision, and blurred vision were significantly improved.
To further explore factors influencing clinical outcomes of IPL, Chen et al.[62] followed 48 MGD patients for 120 days. The characteristics of effective and ineffective IPL therapy outcomes were evaluated in this study. The analysis found that the effective rate of IPL treatment was higher in patients with younger age, higher baseline OSDI, higher baseline Schirmer’s I test, and moderate MGD. The incidence of effective IPL decreased with increasing age.[63,64] Surprisingly, there was no discernible correlation between mild MGD and effective IPL, and the authors explained that individuals with mild MGD had more MG expression capacity to improve than those with severe MGD, resulting in less OSDI improvement following IPL in mild MGD patients. These results need to be further verified by large-scale multicenter experiments.
Zhao et al.[65] used lipidomic analysis of meibomian gland secretions to discover changes in lipid profiles caused by IPL. In total, 323 lipid compounds were discovered. After IPL irradiation, lipids such as (O-acyl)-w-hydroxy fatty acids (OAHFA), triglycerides (TG), lysophosphatidylcholine (LPC), also called lysolecithins (LPC), ceramides (Cer), and others were shown to be altered in MGD patients. In these patients, IPL treatment provides therapeutic results by improving deleterious tear film lipid alterations. This study found that the expression of lipids by the meibomian glands can be used to assess the effectiveness of IPL therapy or other therapies for MGD.
Combination with other therapy
In 2015, Toyos et al.[19] employed the IPL technique for the first time to assess the efficacy of the treatment of MGD. Eighty-seven percent of patients improved tear breakup time after four IPL plus MGX treatments and achieved better satisfaction. Although 13 patients experienced minor adverse events, such as blisters, skin redness, and forehead hair loss, this study offers a novel therapeutic option for DED. Subsequently, Vegunta et al.[24] reported a similar retrospective study showing significant improvement in 89% of patients following combined treatment with IPL and MGX. The investigators speculate that although treatment is unlikely to benefit end-stage MGD, early IPL plus MGX therapy may be beneficial to these patients.
The following year, Dell et al.[66] evaluated the effect of IPL combined with MGX on 40 patients with moderate to severe MGD. Except for lipid layer thickness (LLT), all examined outcome measures such as corneal fluorescein staining (CFS), MG score (MGS), TBUT, and standard patient evaluation of eye dryness (SPEED) scores were significantly improved after 15 weeks. Results suggest that IPL combined with MGX reduces the severity of symptoms in patients with moderate to severe MGD. The limitation of this study is that the treatment effect of MGX and IPL cannot be distinguished due to the lack of a control group. In contrast, the study by Rong et al.[67] is more rigorous, which employed IPL plus MGX to treat 44 patients with MGD in a double-blind, randomized, controlled study. After three treatments, the DE symptoms, TBUT, MG secretion function, and ocular surface conditions improved. This study concluded that IPL combined with MGX is safe and effective in the treatment of MGD.
To determine whether the combination of MGX has a better effect, Chen et al.[68] conducted a clinical trial of IPL, MGX, and IPL + MGX groups. After the first treatment, the OSDI, TBUT, and MG indexes of the three groups were significantly improved; however, the duration of improvements differed. Several parameters in the IPL + MGX group continued to improve during the 3-month follow-up. In the IPL group, improvements in meibomian gland secretion function and TBUT were maintained for 3 months, whereas most parameters in the MGX group were not maintained at 3 months of follow-up. According to the authors, MGX has a synergistic effect, making the combination treatment more effective.
In contrast to traditional IPL treatment regions, Toyos et al.[36] evaluated the safety and efficacy of IPL in relieving symptoms in patients with upper MGD. After four treatments using a new small-spot IPL device on the upper eyelid skin, 19 patients experienced a considerable reduction in subjective symptoms and had no adverse effects. However, MGX and other medications were combined in the IPL treatment procedure, and there was no control group included in their study. Therefore, a larger sample size study is needed to confirm their findings. Similarly, Li et al.[27] applied IPL on the upper eyelid of some patients with severe upper eyelid symptoms. Thirty patients were divided into the traditional IPL group and an additional upper eyelid group and after therapy, the symptoms of DE in both groups were improved; however, BUT, OSDI score and patient satisfaction of the latter were better than those of traditional IPL therapy. These findings showed that adding upper eyelid IPL therapy may be a feasible option for patients with refractory upper eyelid DE symptoms.
Low-level light therapy (LLLT), which relies on photobiomodulation has been used in combination with IPL therapy. Stonecipher et al. reviewed 230 patients treated with LLLT combined with IPL therapy.[32] According to their findings, both MG function and OSDI scores improved significantly following therapy. However, there are some limitations to the study. The lack of a control group cannot determine the treatment effect of a particular therapy. In addition, Pérez-Silguero et al.[31] and Marta et al.[29] verified that IPL combined with LLLT in the treatment of MGD can improve the thickness of the lipid layer and reduce the symptoms of DE.
Gao et al.[42] measured OSDI, TBUT, meibomian mass, and lacrimal gland cytokine levels to compare the anti-inflammatory effects of IPL and tobramycin/dexamethasone plus warm compress. The OSDI score, TBUT, CFS, and meibomian gland expression (MGE) improved in both groups after treatment; however, IPL was able to lower IL-17A and IL-1 levels. Gao et al.[42] suggests that the change in IL-17A and IL-1 levels from negative to positive can be used to monitor the duration of IPL’s clinical effect.
Recently, Wu et al.[69] investigated the efficacy of IPL combined with deproteinized calf blood extract (DCBE) eye drops in the treatment of DED with allodynia. This study included 23 patients with four treatments every 4 weeks. The results showed that visual analog scale (VAS), OSDI, Ocular Pain Assessment Scale (OPAS), patient health questionnaire-9 items, Athens Insomnia scale, generalized anxiety disorder (GAD-7), CFS, MG quality (MGQ), and expression scores were significantly reduced. Schirmer’s I test and TBUT significantly increased after the treatment. The study concluded that IPL combined with DCBE eye drops for four treatments is an effective treatment for relieving eye pain in DED patients, in addition to repairing the brand density of the corneal nerve and improving substance P levels. Although not proven, these findings suggest that there could be a relationship between IPL treatment and corneal nerve repair.
Post-LASIK refractory dry eye
LASIK surgery is a common corneal surgery for myopia correction. Postoperative DE occurs due to damage to the corneal nerves during surgery.[70] Patients can experience ocular discomfort, along with disturbances in their quality of vision following LASIK surgery. Some patients cannot improve after traditional drug treatment and suffer from long-term problems.[71] Pazo et al. conducted a prospective study of IPL in the treatment of post-Lasik refractory DE and discovered that IPL treatment improved the symptoms of post-Lasik refractory DE and reduced dependence on artificial tears.[72] One year later, their team employed IPL combined with 0.1% sodium hyaluronate and heated eye mask for post-Lasik DE.[73] Combination therapy was considerably superior to IPL only in enhancing MG functions and lowering symptoms and indicators of DE in this prospective randomized controlled trial, which demonstrated an increase in tear-film lipid layer (TFLL) in 100 patients after two sessions.
Sjögren’s syndrome
Sjögren’s syndrome (SS) is a chronic autoimmune disease in which the lacrimal gland is one of the glands affected, leading to severe DE symptoms.[74] IPL plus LLLT was first evaluated for the treatment of SS-DE in a prospective study by Di Marino et al.[30] After one treatment, 20 SS patients showed significant improvement in OSDI and BUT, but no improvement in tear secretion. They speculated that the treatment failed to stimulate the lacrimal glands and reduced tear evaporation. Recently, Huo et al.[75] studied 27 SS-DE patients who received three IPL plus MGX treatments, and the treatment group showed more significant improvement in the OSDI score, NBUT, CFS, MGX, and MGQ compared with the control group. Contrary to the results of Marino’s study, Schirmer’s I test and TMH were also significantly improved at the 12-week follow-up. This study suggests that the reduction of inflammation and improvement of the ocular surface after IPL treatment can initiate a cascade involving improved activation of corneal sensory nerve endings and enhanced tear secretion.
Contact lens-related dry eye
Contact lens (CL) wearers often suffer from dryness, discomfort, and pain.[76] DED is the most common cause of CL termination. The commencement of an inflammatory cascade on the ocular surface, resulting in tear film hyperosmolarity in CL users, is regarded to be one of the major mechanisms of CL-DE. The use of IPL in the treatment of CL-DE was initially reported by Yang et al.[77] The results of this randomized controlled trial revealed that two cycles of IPL treatment increased both MG function as indicated by an enhanced NITBUT and TFLL along with the clinical symptoms of DED. In this trial, no adverse effects were reported. The latest study from Xu et al.[33] compared IPL and Heated Eye Mask (HEM) in 60 patients with CL-DE. Both groups saw significant improvements in NITBUT, OSDI score, TFLL score, and MGQ. In comparison with the control group treated with HEM, IPL treatment was more effective in enhancing the overall stability of the tear film and minimizing the requirement for artificial tears. No adverse events were disclosed throughout the study.
Contraindications
Some patients are not suitable for IPL therapy due to safety concerns. According to the review, they usually include the following categories: 1). existing ocular trauma, infectious diseases, recent surgical history, 2). skin defects, pigmentation, moles, scars in the treatment area, and skin cancer, 3). autoimmune diseases and skin allergies, 4). pregnancy or lactation, 5). Fitzpatrick skin type V or VI (these two types of skin can also receive IPL therapy after adjusting the spot size and reducing the energy of the device).[26]
Adverse events
We reviewed 49 studies, adverse events were not recorded in 14 studies; no adverse events were reported in 30 studies, and transient adverse events were recorded in 5 studies [Fig. 8]. A total of 2,114 patients in the report received IPL, and 53 patients (2.5%) experienced mild adverse events [Fig. 9]. Mild pain, localized eyelash loss, erythema, edema, hyperpigmentation, corneal/conjunctival abrasion, and hair loss at the brow and forehead were the most common symptoms.[19,28,37,67,78] These symptoms went away in a short time, and no long-term or serious adverse events were noted. It is worth noting that some adverse events are caused by improper operations by doctors.[67] According to the guidelines for care by the European Society for Laser Dermatology, human error is responsible for 30% of common adverse effects.[79] Adverse occurrences can be reduced by well-trained clinicians. Overall, IPL therapy is safe for MGD-related DE treatment.
Figure 8.
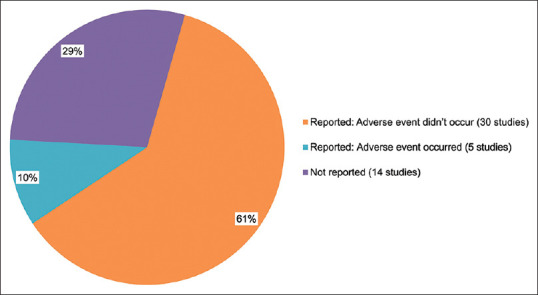
Adverse event report in all studies
Figure 9.
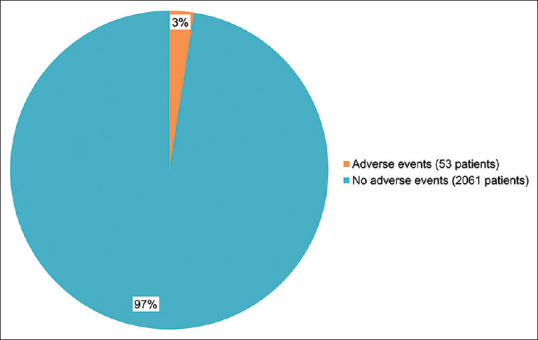
Adverse events in reported studies
Cost benefit
The cost of IPL is considered a major limitation as its not covered by most insurances, and maintenance treatments are often needed annually or bi-annually. There are a growing number of device-based therapies that help relieve signs and symptoms of DED, their costs range from LipiFlow (Johnson and Johnson Vision)[80] costing about $900 per session, iLux (Alcon),[80]a handheld device that provides warm massage to the eyelids costs between $600 and $700 per session, and IPL therapy can cost about $400 per session.[81] Although these costs can vary depending upon the region and therapy provider, IPL therapy, which must be performed several times, is not accessible to the masses.[82] In the future, it may be feasible due to economies of scale to produce and provide IPL therapy at a reasonable cost.[83-85]
Discussion
DE is one of the common diseases in ophthalmology clinics, and it is more common in women, Asians, and the elderly.[86-88] Mixed dysfunction mechanisms were present in 80% of patients, rather than pure aqueous tear production or excessive evaporation.[2,89] EDE due to various underlying conditions is one of the common causes of DE. Through this study, we were able to examine the effectiveness of IPL as an effective treatment, and the results indicated a significant increase in TBUT and overall OSDI at all follow-up periods.
DED is a multifactorial disorder, including MGD, ocular surgery, contact lens wear, and SS.[30,33,34,43,72,73,75,77] The management of DED is a major challenge for ophthalmology practitioners because long-term improvement is difficult to maintain with traditional treatment modalities. Fortunately, IPL has been shown to be effective in the treatment of MGD-related DED and provides an alternative therapy choice. In the literature review, all findings suggest that IPL can improve ocular surface parameters and MGD-related DE symptoms and it is a safe treatment modality.[62,78,90] At the same time, the treatment method is also dynamic, and higher benefits can be obtained when combined with other traditional treatment methods.[29,33,43,61,68,69]
IPL instrument update has acquired good therapeutic efficacy with the advancement of technology as several recent studies have shown that the improved IPL devices can be directly applied on the skin of the upper and lower eyelids, which was not possible several years ago due to safety concerns.[26,27,36,37,91] However, clinicians should avoid the treatment on darkly pigmented skin.
The majority of existing studies suggest that the effect of IPL treatment can be maintained for at least 3 months.[61,68] However, to consolidate the treatment effect for a long time, it is necessary to receive repeated treatment at regular intervals. Furthermore, a unified standard or protocol has not yet been developed. Concurrently, the principal mechanism of IPL treatment is still unclear, and further verification is required based on the current assumption. In addition, the high cost of treatment is a barrier for some patients, especially for those in less developed economies. These flaws need to be addressed for the progress of IPL as a suitable therapy.
Conclusion
IPL therapy has been proven to be a safe and effective option for reducing the signs and symptoms of MGD-related DE. It is beneficial for mild to severe forms of DE; however, early treatment is considered better. In addition, IPL has a better maintenance impact when used in conjunction with other traditional therapies. Further research is needed to understand the additional mechanism of action at a cellular and physiological level.
Financial support and sponsorship
This study was entirely funded by He Eye Specialist Hospital, Shenyang, China. No support was received for the publication of this article.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was supported by He Eye Specialist Hospital, Shenyang, China. The authors have no proprietary interest in any of the products mentioned in this article.
References
- 1.Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15:539–74. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Uchino M, Uchino Y, Dogru M, Kawashima M, Yokoi N, Komuro A, et al. Dry eye disease and work productivity loss in visual display users:The Osaka study. Am J Ophthalmol. 2014;157:294–300. doi: 10.1016/j.ajo.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–12. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States:A decision tree analysis. Cornea. 2011;30:379–87. doi: 10.1097/ICO.0b013e3181f7f363. [DOI] [PubMed] [Google Scholar]
- 6.Chhadva P, Goldhardt R, Galor A. Meibomian gland disease:The role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124:S20–6. doi: 10.1016/j.ophtha.2017.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MTM, Feng J, Wong J, Turnbull PR, Craig JP. Randomised trial of the clinical utility of an eyelid massage device for the management of meibomian gland dysfunction. Cont Lens Anterior Eye. 2019;42:620–4. doi: 10.1016/j.clae.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Kojima T, Ishida R, Dogru M, Goto E, Matsumoto Y, Kaido M, et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease:A prospective randomized case-control study. Am J Ophthalmol. 2005;139:242–6. doi: 10.1016/j.ajo.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 9.Vernhardsdottir RR, Magno MS, Hynnekleiv L, Lagali N, Dartt DA, Vehof J, et al. Antibiotic treatment for dry eye disease related to meibomian gland dysfunction and blepharitis –A review. Ocul Surf. 2022;26:211–21. doi: 10.1016/j.jtos.2022.08.010. [DOI] [PubMed] [Google Scholar]
- 10.de Paiva CS, Pflugfelder SC, Ng SM, Akpek EK. Topical cyclosporine a therapy for dry eye syndrome. Cochrane Database Syst Rev. 2019;9:CD010051. doi: 10.1002/14651858.CD010051.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foulks GN, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction:A comparative clinical and spectroscopic pilot study. Cornea. 2013;32:44–53. doi: 10.1097/ICO.0b013e318254205f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papas AS, Sherrer YS, Charney M, Golden HE, Medsger TA, Walsh BT, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren's syndrome patients with oral pilocarpine:A randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169–77. doi: 10.1097/01.rhu.0000135553.08057.21. [DOI] [PubMed] [Google Scholar]
- 13.Pflugfelder SC, Cao A, Galor A, Nichols KK, Cohen NA, Dalton M. Nicotinic acetylcholine receptor stimulation:A new approach for stimulating tear secretion in dry eye disease. Ocul Surf. 2022;25:58–64. doi: 10.1016/j.jtos.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Mead O, Tighe S, Tseng S. Amniotic membrane transplantation for managing dry eye and neurotrophic keratitis. Taiwan J Ophthalmol. 2020;10:13–21. doi: 10.4103/tjo.tjo_5_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolffsohn JS, TravéHuarte S, Jones L, Craig JP, Wang MTM. Clinical practice patterns in the management of dry eye disease:A TFOS international survey. Ocul Surf. 2021;21:78–86. doi: 10.1016/j.jtos.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Simmons PA, Carlisle-Wilcox C, Chen R, Liu H, Vehige JG. Efficacy, safety, and acceptability of a lipid-based artificial tear formulation:A randomized, controlled, multicenter clinical trial. Clin Ther. 2015;37:858–68. doi: 10.1016/j.clinthera.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Maïssa C, Guillon M, Simmons P, Vehige J. Effect of castor oil emulsion eyedrops on tear film composition and stability. Cont Lens Anterior Eye. 2010;33:76–82. doi: 10.1016/j.clae.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Piccolo D, Di Marcantonio D, Crisman G, Cannarozzo G, Sannino M, Chiricozzi A, et al. Unconventional use of intense pulsed light. Biomed Res Int. 2014;2014:618206. doi: 10.1155/2014/618206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction;A 3-year retrospective study. Photomed Laser Surg. 2015;33:41–6. doi: 10.1089/pho.2014.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones L, Downie LE, Korb D, Benitez-del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Zarei-Ghanavati S, Hassanzadeh S, Azimi Khorasani A, Ehsaei A, Bakhtiari E. Efficacy of five-flash intense pulsed light therapy technique in patients with meibomian gland dysfunction. Clin Exp Optom. 2021;105:687–93. doi: 10.1080/08164622.2021.1976595. [DOI] [PubMed] [Google Scholar]
- 22.Craig JP, Chen YH, Turnbull PRK. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2015;56:1965–70. doi: 10.1167/iovs.14-15764. [DOI] [PubMed] [Google Scholar]
- 23.Gupta PK, Vora GK, Matossian C, Kim M, Stinnett S. Outcomes of intense pulsed light therapy for treatment of evaporative dry eye disease. Can J Ophthalmol. 2016;51:249–53. doi: 10.1016/j.jcjo.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Vegunta S, Patel D, Shen JF. Combination therapy of intense pulsed light therapy and meibomian gland expression (IPL/MGX) can improve dry eye symptoms and meibomian gland function in patients with refractory dry eye:A retrospective analysis. Cornea. 2016;35:318–22. doi: 10.1097/ICO.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 25.Murtaza F, Toameh D, Al-Habib S, Maini R, Chiu HH, Tam ES, et al. Safety and efficacy of broadband intense pulsed light therapy for dry eye disease with meibomian gland dysfunction. Clin Ophthalmol. 2021;15:3983–91. doi: 10.2147/OPTH.S331289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergés C, Salgado-Borges J, de Ribot FM. Prospective evaluation of a new intense pulsed light, thermaeye plus, in the treatment of dry eye disease due to meibomian gland dysfunction. J Optom. 2021;14:103–13. doi: 10.1016/j.optom.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, Lin S-B, Zhang MZ, Cheng B. Preliminary assessment of intense pulsed light treatment on the upper eyelids for meibomian gland dysfunction. Photobiomodul Photomed Laser Surg. 2020;38:249–54. doi: 10.1089/photob.2019.4689. [DOI] [PubMed] [Google Scholar]
- 28.Li D, Lin S-B, Cheng B. Intense pulsed light treatment for meibomian gland dysfunction in Skin Types III/IV. Photobiomodul Photomed Laser Surg. 2019;37:70–6. doi: 10.1089/photob.2018.4509. [DOI] [PubMed] [Google Scholar]
- 29.Marta A, Baptista PM, Marques JH, Almeida D, José D, Sousa P, et al. Intense pulsed plus low-level light therapy in meibomian gland dysfunction. Clin Ophthalmol. 2021;15:2803–11. doi: 10.2147/OPTH.S318885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Marino M, Conigliaro P, Aiello F, Valeri C, Giannini C, Mancino R, et al. Combined low-level light therapy and intense pulsed light therapy for the treatment of dry eye in patients with Sjögren's syndrome. J Ophthalmol. 2021;2021:2023246. doi: 10.1155/2021/2023246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez-Silguero MA, Pérez-Silguero D, Rivero-Santana A, Bernal-Blasco MI, Encinas-Pisa P. Combined intense pulsed light and low-level light therapy for the treatment of dry eye:A retrospective before–after study with one-year follow-up. Clin Ophthalmol. 2021;15:2133–40. doi: 10.2147/OPTH.S307020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stonecipher K, Abell TG, Chotiner B, Chotiner E, Potvin R. Combined low level light therapy and intense pulsed light therapy for the treatment of meibomian gland dysfunction. Clin Ophthalmol. 2019;13:993–9. doi: 10.2147/OPTH.S213664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Wu Y, Song Y, Zhang Q, Qin G, Yang L, et al. Comparison between heated eye mask and intense pulsed light treatment for contact lens-related dry eye. Photobiomodul Photomed Laser Surg. 2022;40:189–97. doi: 10.1089/photob.2021.0094. [DOI] [PubMed] [Google Scholar]
- 34.Yin Y, Liu N, Gong L, Song N. Changes in the meibomian gland after exposure to intense pulsed light in meibomian gland dysfunction (MGD) patients. Curr Eye Res. 2018;43:308–13. doi: 10.1080/02713683.2017.1406525. [DOI] [PubMed] [Google Scholar]
- 35.Heymann WR. Intense pulsed light. J Am Acad Dermatol. 2007;56:466–7. doi: 10.1016/j.jaad.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 36.Toyos R, Toyos M, Willcox J, Mulliniks H, Hoover J. Evaluation of the safety and efficacy of intense pulsed light treatment with meibomian gland expression of the upper eyelids for dry eye disease. Photobiomodul Photomed Laser Surg. 2019;37:527–31. doi: 10.1089/photob.2018.4599. [DOI] [PubMed] [Google Scholar]
- 37.Zhang-Nunes S, Guo S, Lee D, Chang J, Nguyen A. Safety and efficacy of an augmented intense pulse light protocol for dry eye syndrome and blepharitis. Photobiomodul Photomed Laser Surg. 2021;39:178–84. doi: 10.1089/photob.2020.4913. [DOI] [PubMed] [Google Scholar]
- 38.Nagymihályi A, Dikstein S, Tiffany JM. The influence of eyelid temperature on the delivery of meibomian oil. Exp Eye Res. 2004;78:367–70. doi: 10.1016/s0014-4835(03)00197-0. [DOI] [PubMed] [Google Scholar]
- 39.Enríquez-de-Salamanca A, Castellanos E, Stern ME, Fernández I, Carreño E, García-Vázquez C, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–73. [PMC free article] [PubMed] [Google Scholar]
- 40.Stevenson W, Chauhan SK, Dana R. Dry eye disease:An immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Liu J, Liu C, Piao J, Yang W, An N, et al. Effects of intense pulsed light treatment on tear cytokines and clinical outcomes in meibomian gland dysfunction. PLoS One. 2021;16:e0256533. doi: 10.1371/journal.pone.0256533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao YF, Liu RJ, Li YX, Huang C, Liu YY, Hu CX, et al. Comparison of anti-inflammatory effects of intense pulsed light with tobramycin/dexamethasone plus warm compress on dry eye associated meibomian gland dysfunction. Int J Ophthalmol. 2019;12:1708–13. doi: 10.18240/ijo.2019.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Souza S, Padmanabhan Nair A, Iyappan G, Dickman MM, Thakur P, Mullick R, et al. Clinical and molecular outcomes after combined intense pulsed light therapy with low-level light therapy in recalcitrant evaporative dry eye disease with meibomian gland dysfunction. Cornea. 2022;41:1080–7. doi: 10.1097/ICO.0000000000002954. [DOI] [PubMed] [Google Scholar]
- 44.Szkaradkiewicz A, Chudzicka-Strugala I, Karpiński TM, Goślińska-Pawlowska O, Tulecka T, Chudzicki W, et al. Bacillus oleronius and Demodex mite infestation in patients with chronic blepharitis. Clin Microbiol Infect. 2012;18:1020–5. doi: 10.1111/j.1469-0691.2011.03704.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Sheha H, Tseng SCG. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10:505–10. doi: 10.1097/ACI.0b013e32833df9f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacey N, Delaney S, Kavanagh K, Powell FC. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474–81. doi: 10.1111/j.1365-2133.2007.08028.x. [DOI] [PubMed] [Google Scholar]
- 47.Baudouin C, Messmer EM, Aragona P, Geerling G, Akova YA, Benítez-Del-Castillo J, et al. Revisiting the vicious circle of dry eye disease:A focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100:300–6. doi: 10.1136/bjophthalmol-2015-307415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prieto VG, Sadick NS, Lloreta J, Nicholson J, Shea CR. Effects of intense pulsed light on sun-damaged human skin, routine, and ultrastructural analysis. Lasers Surg Med. 2002;30:82–5. doi: 10.1002/lsm.10042. [DOI] [PubMed] [Google Scholar]
- 49.Giannaccare G, Taroni L, Senni C, Scorcia V. Intense pulsed light therapy in the treatment of meibomian gland dysfunction:Current perspectives. Clin Optom (Auckl) 2019;11:113–26. doi: 10.2147/OPTO.S217639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–65. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Bäumler W, Vural E, Landthaler M, Muzzi F, Shafirstein G. The effects of intense pulsed light (IPL) on blood vessels investigated by mathematical modeling. Lasers Surg Med. 2007;39:132–9. doi: 10.1002/lsm.20408. [DOI] [PubMed] [Google Scholar]
- 52.Wong WR, Shyu WL, Tsai JW, Hsu KH, Lee HY, Pang JHS. Intense pulsed light modulates the expressions of MMP-2, MMP-14 and TIMP-2 in skin dermal fibroblasts cultured within contracted collagen lattices. J Dermatol Sci. 2008;51:70–3. doi: 10.1016/j.jdermsci.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 53.Cote S, Zhang AC, Ahmadzai V, Maleken A, Li C, Oppedisano J, et al. Intense pulsed light (IPL) therapy for the treatment of meibomian gland dysfunction. Cochrane Database Syst Rev. 2020;3:CD013559. doi: 10.1002/14651858.CD013559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barolet D, Roberge CJ, Auger FA, Boucher A, Germain L. Regulation of skin collagen metabolism in vitro using a pulsed 660 nm led light source:Clinical correlation with a single-blinded study. J Invest Dermatol. 2009;129:2751–9. doi: 10.1038/jid.2009.186. [DOI] [PubMed] [Google Scholar]
- 55.Goldberg DJ. Current trends in intense pulsed light. J Clin Aesthet Dermatol. 2012;5:45–53. [PMC free article] [PubMed] [Google Scholar]
- 56.Shin DH, Lee E, Hyun JK, Lee SJ, Chang YP, Kim JW, et al. Growth-associated protein-43 is elevated in the injured rat sciatic nerve after low power laser irradiation. Neurosci Lett. 2003;344:71–4. doi: 10.1016/s0304-3940(03)00354-9. [DOI] [PubMed] [Google Scholar]
- 57.Manstein CH. Effect of low level laser treatment on neurosensory deficits subsequent to sagittal split ramus osteotomy. Plast Reconstr Surg. 1998;101:1424. doi: 10.1016/s1079-2104(96)80215-0. [DOI] [PubMed] [Google Scholar]
- 58.Rochklnd S, El-Ani D, Nevo Z, Shahar A. Increase of neuronal sprouting and migration using 780 nm laser phototherapy as procedure for cell therapy. Lasers Surg Med. 2009;41:277–81. doi: 10.1002/lsm.20757. [DOI] [PubMed] [Google Scholar]
- 59.Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction:Executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–9. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan S, Wu Y. Efficacy and safety of intense pulsed light therapy for dry eye caused by meibomian gland dysfunction:A randomised trial. Ann Palliat Med. 2021;10:7857–65. doi: 10.21037/apm-21-1303. [DOI] [PubMed] [Google Scholar]
- 61.Fan Q, Pazo EE, You Y, Zhang C, Zhang C, Xu L, et al. Subjective quality of vision in evaporative dry eye patients after intense pulsed light. Photobiomodul Photomed Laser Surg. 2020;38:444–51. doi: 10.1089/photob.2019.4788. [DOI] [PubMed] [Google Scholar]
- 62.Chen C, Chen D, Chou YY, Long Q. Factors influencing the clinical outcomes of intense pulsed light for meibomian gland dysfunction. Medicine (United States) 2021;100:e28166. doi: 10.1097/MD.0000000000028166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction:Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–78. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villani E, Canton V, Magnani F, Viola F, Nucci P, Ratiglia R. The aging meibomian gland:An in vivo confocal study. Invest Ophthalmol Vis Sci. 2013;54:4735–40. doi: 10.1167/iovs.13-11914. [DOI] [PubMed] [Google Scholar]
- 65.Zhao H, Wu SN, Shao Y, Xiao D, Tang LY, Cheng Z, et al. Lipidomics profiles revealed alterations in patients with meibomian gland dysfunction after exposure to intense pulsed light. Front Neurol. 2022;13:1–13. doi: 10.3389/fneur.2022.827544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dell SJ, Gaster RN, Barbarino SC, Cunningham DN. Prospective evaluation of intense pulsed light and meibomian gland expression efficacy on relieving signs and symptoms of dry eye disease due to meibomian gland dysfunction. Clin Ophthalmol. 2017;11:817–27. doi: 10.2147/OPTH.S130706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rong B, Tang Y, Tu P, Liu R, Qiao J, Song W, et al. Intense pulsed light applied directly on eyelids combined with meibomian gland expression to treat meibomian gland dysfunction. Photomed Laser Surg. 2018;36:326–32. doi: 10.1089/pho.2017.4402. [DOI] [PubMed] [Google Scholar]
- 68.Chen Y, Li J, Wu Y, Lin X, Deng X, Yun-E Z. Comparative evaluation in intense pulsed light therapy combined with or without meibomian gland expression for the treatment of meibomian gland dysfunction. Curr Eye Res. 2021;46:1125–31. doi: 10.1080/02713683.2020.1867750. [DOI] [PubMed] [Google Scholar]
- 69.Wu Y, Mou Y, Zhang Y, Han Y, Lin L, Huo Y, et al. Efficacy of intense pulsed light combined blood extract eye drops for treatment of nociceptive pain in dry eye patients. J Clin Med. 2022;11:1312. doi: 10.3390/jcm11051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toda I. Dry eye after LASIK. Invest Ophthalmol Vis Sci. 2018;59:DES109–15. doi: 10.1167/iovs.17-23538. [DOI] [PubMed] [Google Scholar]
- 71.Pietilä J, Huhtala A, Mäkinen P, Nättinen J, Rajala T, Salmenhaara K, et al. Uncorrected visual acuity, postoperative astigmatism, and dry eye symptoms are major determinants of patient satisfaction:A comparative, real-life study of femtosecond laser in situ keratomileusis and small incision lenticule extraction for myopia. Clin Ophthalmol. 2018;12:1741–55. doi: 10.2147/OPTH.S172894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pazo EE, Huang H, Fan Q, Zhang C, Yue Y, Yang L, et al. Intense pulse light for treating post-LASIK refractory dry eye. Photobiomodul Photomed Laser Surg. 2021;39:155–63. doi: 10.1089/photob.2020.4931. [DOI] [PubMed] [Google Scholar]
- 73.Wu Y, Xu L, Song Y, Zhang Q, Qin G, Yang L, et al. Management of post-LASIK dry eye with intense pulsed light in combination with 0.1% sodium hyaluronate and heated eye mask. Ophthalmol Ther. 2022;11:161–76. doi: 10.1007/s40123-021-00418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitsias DI, Kapsogeorgou EK, Moutsopoulos HM. Sjögren's syndrome:Why autoimmune epithelitis? Oral Dis. 2006;12:523–32. doi: 10.1111/j.1601-0825.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 75.Huo Y, Wan Q, Hou X, Zhang Z, Zhao J, Wu Z, et al. Therapeutic effect of intense pulsed light in patients with Sjögren's syndrome related dry eye. J Clin Med. 2022;11:1377. doi: 10.3390/jcm11051377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kojima T. Contact lens-associated dry eye disease:Recent advances worldwide and in Japan. Invest Ophthalmol Vis Sci. 2018;59:DES102–8. doi: 10.1167/iovs.17-23685. [DOI] [PubMed] [Google Scholar]
- 77.Yang L, Pazo EE, Zhang Q, Wu Y, Song Y, Qin G, et al. Treatment of contact lens related dry eye with intense pulsed light. Cont Lens Anterior Eye. 2022;45:101449. doi: 10.1016/j.clae.2021.101449. [DOI] [PubMed] [Google Scholar]
- 78.Yurttaser Ocak S, Karakus S, Ocak OB, Cakir A, Bolukbasi S, Erden B, et al. Intense pulse light therapy treatment for refractory dry eye disease due to meibomian gland dysfunction. Int Ophthalmol. 2020;40:1135–41. doi: 10.1007/s10792-019-01278-3. [DOI] [PubMed] [Google Scholar]
- 79.Adamič M, Troilius A, Adatto M, Drosner M, Dahmane R. Vascular lasers and IPLS:Guidelines for care from the European Society for Laser Dermatology (ESLD) J Cosmet Laser Ther. 2007;9:113–24. doi: 10.1080/14764170701280693. [DOI] [PubMed] [Google Scholar]
- 80.Tauber J, Owen J, Bloomenstein M, Hovanesian J, Bullimore MA. Comparison of the iLUX and the LipiFlow for the treatment of meibomian gland dysfunction and symptoms:A randomized clinical trial. Clin Ophthalmol. 2020;14:405–18. doi: 10.2147/OPTH.S234008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.12 Devices for Treating Dry Eyes-American Academy of Ophthalmology [Internet] [Last accessed on 2022 Nov 05]. Available from:https://www.aao.org/eye-health/tips-prevention/how-to-treat-dry-eye-devices .
- 82.Intense Pulsed Light:For Treating Dry Eye [Internet] [Last accessed on 2022 Nov 05]. Available from:https://www.reviewofophthalmology.com/article/intense-pulsed-light-for-treating-dry-eye .
- 83.Nayak Karopadi A, Mason G, Rettore E, Ronco C. The role of economies of scale in the cost of dialysis across the world:A macroeconomic perspective. Nephrol Dial Transplantat. 2014;29:885–92. doi: 10.1093/ndt/gft528. [DOI] [PubMed] [Google Scholar]
- 84.Indust MMRIET, 2010 undefined. Economies of scale and hospital productivity:An empirical analysis of medical area level panel data. rieti.go.jp [Internet] 2010. [Last accessed on 2022 Nov 05]. Available from:https://www.rieti.go.jp/jp/publications/dp/10e050.pdf .
- 85.Freeman M, Savva N, Scholtes S. Economies of scale and scope in hospitals:An empirical study of volume spillovers. Manage Sci. 2021;67:673–97. [Google Scholar]
- 86.Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan:The Shihpai eye study. Ophthalmology. 2003;110:1096–101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 87.Jie Y, Xu L, Wu YY, Jonas JB. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye. 2009;23:688–93. doi: 10.1038/sj.eye.6703101. [DOI] [PubMed] [Google Scholar]
- 88.Uchino M, Dogru M, Yagi Y, Goto E, Tomita M, Kon T, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci. 2006;83:797–802. doi: 10.1097/01.opx.0000232814.39651.fa. [DOI] [PubMed] [Google Scholar]
- 89.Arita R, Fukuoka S, Mizoguchi T, Morishige N. Multicenter study of intense pulsed light for patients with refractory aqueous-deficient dry eye accompanied by mild meibomian gland dysfunction. J Clin Med. 2020;9:1–13. doi: 10.3390/jcm9113467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang Y, Liu R, Tu P, Song W, Qiao J, Yan X, et al. A retrospective study of treatment outcomes and prognostic factors of intense pulsed light therapy combined with meibomian gland expression in patients with meibomian gland dysfunction. Eye Contact Lens. 2021;47:38–44. doi: 10.1097/ICL.0000000000000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Min JS, Yoon SH, Kim KY, Jun I, Kim EK, Kim TI, et al. Treatment effect and pain during treatment with intense pulsed-light therapy according to the light guide in patients with meibomian gland dysfunction. Cornea. 2022;41:177–82. doi: 10.1097/ICO.0000000000002859. [DOI] [PubMed] [Google Scholar]