Abstract
Bilateral corneal blindness with severe dry eye disease (DED), total limbal stem cell deficiency with underlying corneal stromal scarring and vascularization, combined with adnexal complications secondary to chronic cicatrizing conjunctivitis is a highly complex situation to treat. In such eyes, procedures such as penetrating keratoplasty alone or combined with limbal stem cell transplantation are doomed to fail. In these eyes, keratoprosthesis (Kpro) or an artificial cornea is the most viable option, eliminating corneal blindness even in eyes with autoimmune disorders such as Stevens–Johnson syndrome, ocular mucous membrane pemphigoid, Sjogren’s syndrome, and nonautoimmune disorders such as chemical/thermal ocular burns, all of which are complex pathologies. Performing a Kpro in these eyes also eliminates the need for systemic immunosuppression and may provide relatively early visual recovery. In such eyes, the donor cornea around the central cylinder of the Kpro needs to be covered with a second layer of protection to avoid desiccation and progressive stromal melt of the underlying cornea, which is a common complication in eyes with severe DED. In this review, we will focus on Kpro designs that have been developed to survive in eyes with the hostile environment of severe DED. Their outcomes in such eyes will be discussed.
Keywords: Chronic cicatrizing conjunctivitis, dry eye disease, keratoprosthesis, limbal stem cell deficiency, ocular chemical burns, mucous membrane pemphigoid, Stevens–Johnson syndrome
Severe dry eye disease (DED) can be blinding, especially with coexisting chronic cicatrizing conjunctivitis, concomitant adnexal disorders, and limbal stem cell deficiency (LSCD). Despite the recent advances in lamellar or penetrating keratoplasties (LK/PK) and limbal stem cell transplantation (LSCT) and with the widespread use of systemic immunosuppression, graft survival is still a challenge in eyes with underlying etiologies such as Stevens–Johnson syndrome (SJS), ocular mucous membrane pemphigoid, (MMP), Sjogren’s syndrome, chemical and thermal ocular burns.[1] In such eyes, a keratoprosthesis (Kpro) may be the only option. A Kpro is a synthetic construct that replaces the central portion of the diseased cornea in an eye with end-stage corneal blindness and is used typically in settings where a routine LK or PK has a higher risk of failure.[2] The advantage of the Kpro is relatively early visual recovery without requiring systemic immunosuppression. The concept of an artificial cornea/Kpro dates back to 1789, when a French ophthalmologist Guillaume Pellier de Quengsy first introduced this concept.[1] Since then, over several decades, multiple prosthetic corneas have been developed and modified to improve their survival rates in recipient eyes.
Type of Kpro/Design
The choice of Kpro needs to be individualized for every patient and depends on various factors such as the underlying etiology for corneal blindness, the tear film status, the anatomy of the ocular surface, and the adnexa.[2] Depending on these factors, the Kpros have been classified into two broad categories, that is, type I and type II. In more specific terms, a KPro type I (prototype Boston Kpro type 1 [BKpro-I]) is indicated for visual rehabilitation in eyes with corneal blindness with a wet surface and preferably in eyes with blindness secondary to nonautoimmune diseases.[3] Eyes with end-stage DED, keratinized/dermalized surfaces, disturbed anatomy of the ocular surface, and the eyelids are ideal candidates for type II Kpro [Figs. 1 and 2].[4] In this review, we will focus on different Kpro designs and their outcomes in eyes with corneal blindness in the setting of severe DED. The devices that will be focused on are the osteo-odonto Kpro (OOKP), the Boston Kpro type II (BKpro-II), the Moscow eye microsurgery complex in Russia Kpro (MICOF), the tibial osteo-Kpro (OKP), the LVP Kpro (LV Prasad) (LVP) Kpro, the Lux Kpro, and the Pintucci Kpro [Fig. 3].
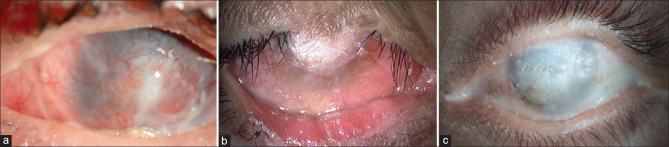
Figure 1.
Preoperative images before Kpro surgery in dry eye disease. (a) Post–toxic epidermal necrolysis, corneal neovascularization with total leukomatous corneal scar is found in eye. A Boston type II Kpro was performed in this eye; the postoperative image is shown in Fig. 3b. (b) Post–acid injury, an eye showing superior symblepharon with total leukomatous corneal scar with dermalization of the corneal surface. A tibial Kpro was performed in this eye; the postoperative image is shown in Fig. 3c. (c) Post-Stevens–Johnson syndrome sequelae. Superior symblepharon with dermalization of the entire ocular surface with underlying total leukomatous corneal scar and severe dry eye disease; an LVP Kpro was performed in this eye. Kpro = keratoprosthesis
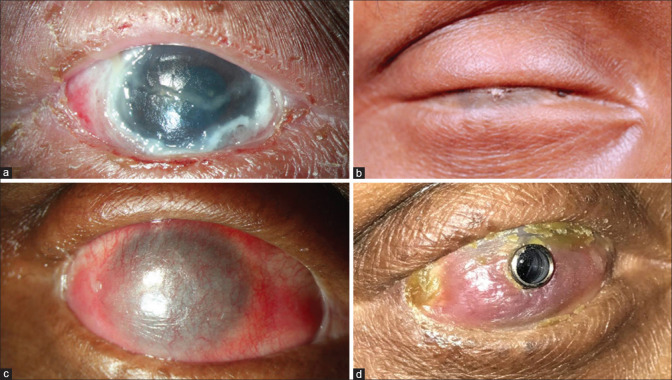
Figure 2.
Different stages of the same eye, from acute stage of cicatrizing conjunctivitis to keratoprosthesis. (a) Right eye of a patient immediately after TEN. (b) Complete ankyloblepharon with dermalization of the ocular surface 2 years after TEN. (c) The postoperative image of the same eye, 3months after the ocular surface mucous membrane grafting was performed. (d) Six months after Lux keratoprosthesis. TEN = toxic epidermal necrolysis
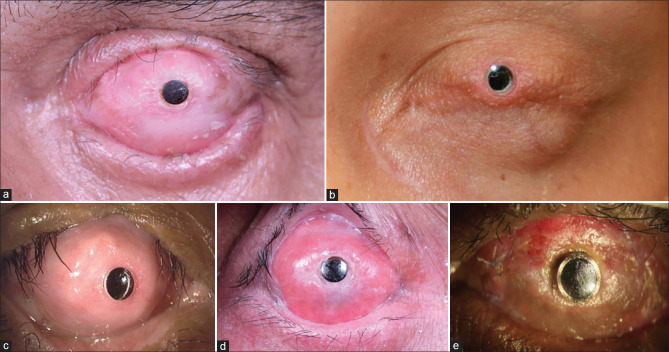
Figure 3.
Different types of keratoprosthesis in dry eye disease secondary to chronic cicatrizing conjunctivitis. (a) Left eye of a patient 5 years after MOOKP was performed for ocular sequelae secondary to SJS. (b) Left eye of a patient 14 years after Boston type II keratoprosthesis was performed for SJS sequelae. (c) Right eye of a patient 5 years after tibial keratoprosthesis was performed for ocular chemical burn secondary to acid injury. (d) Right eye of a patient 4.5 years after LVP keratoprosthesis was performed for SJS sequelae. (e) Right eye of a patient 3 months after Lux keratoprosthesis was performed for SJS sequelae. MOOKP = modified osteo-odonto keratoprosthesis, SJS = Stevens–Johnson syndrome
All Kpro devices currently in use consist of an optic, a central viewing system made of polymethyl methacrylate (PMMA), and a haptic which supports the device in situ.[2] These designs have been described in Table 1. Once implanted, the type I devices are typically covered with a soft contact lens to prevent desiccation and to preserve the corneal epithelial surface (BKpro-I). In type II devices, either the eyelid skin is used to cover the noncentral part of the cornea (BKpro-II) or the buccal/labial mucosal graft may be used (OOKP, tibial OKP, LVP, Lux, and the Pintucci).
Table 1.
Various designs of type II keratoprosthesis categorized based on the haptic material
| Optic | Haptic | |
|---|---|---|
| A. Biocompatible | ||
| BKpro-II | PMMA | Titanium |
| LVP Kpro | PMMA | PMMA |
| Lux Kpro | PMMA | Titanium |
| MICOF | PMMA | Titanium |
| B. Biointegrated | ||
| Pintucci Kpro | PMMA | Dacron mesh |
| C. Biological | ||
| Tibial OKP | PMMA | Tibial bone |
| OOKP | PMMA | Canine tooth and surrounding alveolar bone |
BKpro-II=Boston type II keratoprosthesis, MICOF=Moscow eye microsurgery complex in Russia, OKP=osteo-keratoprosthesis, OOKP=osteo-odonto keratoprosthesis, PMMA=polymethyl methacrylate
Currently, Boston Kpro is the most widely used Kpro worldwide.[5] The outcomes of BKpro-I are widely published, and outcomes have been generally favorable in eyes with a wet surface, while the outcomes in certain etiologies such as SJS and MMP have been less favorable.[6–10] This expert group does not recommend the use of BKpro-I in patients with severe DED, especially secondary to autoimmune conditions such as SJS and MMP. Hence, there is a need to focus on the other designs of Kpro which have better anatomical and functional outcomes in such eyes with LSCD, concomitant DED, and adnexal pathologies.
The Modified OOKP
In 1963, Strampelli introduced the first Kpro suitable to be performed in human eyes, the OOKP.[11] Modifications made by Falcinelli over several years allowed for longer retention and reduced the rates of postoperative complications. This device then came to be known as the modified OOKP (MOOKP).[12] A detailed description of these modifications and the rationale behind these surgical adaptations have been discussed elsewhere.[13]
The MOOKP is a heterotrophic autograft. This epicorneal Kpro is constructed by placement of a PMMA optical cylinder within an excised biological support (haptic) derived from live human tissue (the autologous osteodental lamina) and is implanted in two stages, in conjunction with a full-thickness oral mucosal graft.[12,14–17] The biological behavior of the implant is similar to a dental implant, where the MOOKP is fixed by the surrounding periosteum to the anterior corneal and scleral surfaces and covered by the mucosal graft, which acts as a source of vascular supply and micronutrients required for its survival.
Though the MOOKP is an invasive and a technically challenging surgery, its principal strength lies in its retention, with rates varying from of 66% to 85% at 10–18 years following implantation, despite an adverse ocular surface environment in most instances.[15,18] Such promising long-term anatomical outcomes have been attributed to several biological properties of the MOOKP design.[19-22]
Indications and contraindications
The recommendations for selecting an ideal case for the MOOKP were agreed upon by the participants of the Modified OOKP Teaching Group in “The Rome–Vienna Protocol.”[12] The MOOKP surgery is ideally performed in patients with bilateral corneal blindness with preserved retinal and optic nerve function. The surgery should ideally be performed first in the eye with poorer visual acuity (VA) among the two, but with intact light perception. Eyes without previous PK or multiple anterior segment interventions before MOOKP have better outcomes. The surgery is performed only in one eye, without intervening in the other “spare eye.” The latter can be operated upon in the future if the Kpro fails in the first operated eye.[12]
The surgery is avoided in the pediatric age group (age <17 years) due to high turnover of the bone with faster resorption of the osteodental lamina within months post-surgery.[12] The surgery is also avoided in patients who may be unwilling to accept the cosmetic outcome or who may be unable to comply with multiple follow-up visits. The surgery is also deferred in edentulous patients or patients with an unsuitable tooth for harvest.
Preoperative evaluation[12]
Ophthalmological evaluation: A clear history to ascertain the lens status and previous anterior segment interventions should be elicited. Clinical evaluation is performed to ensure light perception and accurate projection of rays, eye closure, status of the conjunctival fornices, and the intraocular pressure (IOP). A B-scan ultrasound is essential to rule out any posterior segment pathology such as retinal detachment or optic nerve cupping. An A-scan is required for axial length measurements to determine a power for the optical cylinder.
Oral evaluation:[12,17] The condition of the buccal and labial mucosa should be assessed preoperatively. Presence of a vital single tooth (preferably an upper canine) can be assessed by an ophthalmologist, but a detailed evaluation should be performed by an experienced oromaxillofacial surgeon. An orthopantomography is required to assist in the selection of the individual tooth.
Systemic evaluation: A thorough systemic evaluation is performed to ensure fitness for general anesthesia.
Surgical technique[12,17]
The MOOKP is a multi-staged procedure that requires a multidisciplinary approach by the ophthalmologist and a dental surgeon. To maintain uniformity, the surgical steps were dictated by the Rome–Vienna protocol. Stage 1 involves the preparation of the globe, where Stage 1A constitutes removal of all the ocular surface epithelia and then securing a buccal mucosal graft over the entire ocular surface. Stage 1B involves harvesting a tooth and the surrounding intact alveolar bone, which is then drilled and secured to support the PMMA optical cylinder, to form a biological skirt around the device. The implant is then inserted in the subcutaneous or submucosal space in the contralateral orbito-zygomatic area. Stage 2 is performed after 2–4 months, after a fibrovascular capsule has formed around the implant and the buccal mucosal graft is well vascularized. The implant is harvested from the subcutaneous or submuscular pouch. The buccal mucosa covering the ocular surface is reflected inferiorly by careful dissection. The central cornea is trephined to create an opening for the optical cylinder of the implant. A 360° iridodialysis is performed, the crystalline lens is removed, and a thorough anterior vitrectomy is performed. The implant is then inserted and sutured to the corneal surface, with the posterior optic protruding into the eye through the central cornea opening. The reflected buccal mucosal flap is reposited, sutured, and a central opening of 3 mm is trephined. This enables the anterior optic to protrude through the mucosa. Multiple variations in the surgical technique have been described by Liu et al.,[16] Iyer et al.,[19] Fukuda et al.,[20] Tan et al.[21]
Postoperative care[12,16]
After each stage, topical and systemic antibiotics are advised. After the second stage, oral corticosteroids are advised to reduce intraocular inflammation. IOP should be assessed at every visit by digital palpation. If the IOP is high, this is treated by administering oral acetazolamide. Topical antibiotic and lubricating eye drops should be continued lifelong. Follow-up examination should be performed every 3 months postoperatively, including assessment of the VA, visual field, IOP by digital palpation, and fundus. A change in refraction, appearance of the buccal mucous membrane, condition of the lamina, and stability of the optical cylinder should be assessed for at every visit.
Outcomes
The demographic data from studies highlighting the anatomical and functional outcomes of OOKP in DED have been presented in Table 2. Common etiological conditions for which MOOKP was performed have been listed in Table 3.
Table 2.
Demographic details, anatomical and functional outcomes in eyes which have received a modified osteo-odonto keratoprosthesis (studies with >25 eyes)
| Authors, year | Number or eyes | Mean age (years, range) | Follow-up duration (range), months | Anatomical success | Functional outcome |
|---|---|---|---|---|---|
| Marchi et al.[14] 1994 | 85 | 45 (26-74) | <120 | @5 years: 100% @20 years: 98% |
BCVA ≥20/40: 46% |
| Falcinelli et al.[15] 2005 | 181 | 54.3 (20-83) | 144 (12-300) | @8 years: >90% @18 years: 85% |
NA |
| Hille et al.[12] 2005 | 232 | NA | 112.8 (3-348) | @5 years: 96.5% @10 years: 94.1% @20 years : 88.8% |
NA |
| Liu et al.[16] 2008 | 36 | 51 (19-87) | 46.8 (6-108) | @5 years: 67% @9 years: 56% |
BCVA ≥20/40: 53% BCVA ≥20/200: 78% |
| Michael et al.[18] 2008 | 145 | 43 (NA) | 100.8 (0-360) | @10 years: 66% @18 years: 59% |
NA |
| Iyer et al.[19] 2010 | 50 | 26.5 (20-52) | 15.38 (1-54) | 96%^ | BCVA ≥20/60: 33/50 (66%) BCVA ≥20/20: 15/50 (45.45%) |
| De La Paz et al.[24] 2011 | 145 | 43 (10-81) | 100.8 (1-360) | @5 years: 73% @10 years: 60% |
BCVA of 20/20 (0.05 logMAR) @5 years: 50% @10 years: 39% |
| Tan et al.[21] 2012 | 35 | 41 (23-72) | 37.4 (3-84) | @5 years: 100% | BCVA ≥20/60: 60% |
| Iannetti et al.[23] 2022 | 82 | 34 (13-59) | 328 (28.8-624) | 94%^ | BCVA ≥20/200: @5 years: 85% @10 years: 81% @30 years: 55% |
BCVA=Best-corrected visual acuity, logMAR=Log of minimum angle or resolution. Anatomical success: no loss or explantation of the keratoprosthesis for the entire duration of follow-up, ^=Anatomical success is described as the percentage of eyes retaining the device till the last follow-up
Table 3.
Indications for performing the osteo-odonto keratoprosthesis in eyes with dry eye disease (studies with >25 eyes)
| Marchi et al.[14] 1994 n=85 | Falcinelli et al.[15] 2005 n=181 | Hille et al.[12] 2005 n=234 | Liu et al.[16]2008 n=36 | Michael et al.[18] 2008 n=145 | Iyer et al.[19] 2010 n=50 | De La Paz et al.[24] 2011 n=145 | Tan et al.[21] 2012 n=35 | Iannetti et al.[23] 2022 n=82 | |
|---|---|---|---|---|---|---|---|---|---|
| Chemical/thermal burns | 44 (52%) | 68 (38%) | 84 (36%) | 6 (7%) | 77 (53%) | 22 (44%) | 77 (53%) | 13 (37%) | 58 (70.7%) |
| SJS | 10 (11%) | 4 (2.5%) | 16 (44%) | 22 (15%) | 24 (48%) | 22 (15%) | 16 (46%) | 7 (8.5%) | |
| MMP | 39 (21.5%) | 5 (14%) | 8 (5.5%) | 3 (6%) | 8 (6%) | 2 (6%) | |||
| GVHD | 1 (0.5%) | 1 (3%) | |||||||
| Sjogren’s syndrome | 11 (6%) | 1 (3%) | |||||||
| Xerosis | 12 (14%) | 96 (41%) | 1 (3%) | ||||||
| Lyell syndrome | 6 (3.5%) |
GVHD=Graft versus host disease, SJS=Stevens-Johnson syndrome, MMP=Mucous membrane pemphigoid
a. Anatomical outcomes:
The long-term anatomical outcomes of OOKP have been promising with the device being retained in 89%–98% of eyes over a 20-year follow-up period.[12,14] Compared to previous studies, Iannetti et al.[23] reported a higher retention rate of 99% at 20 years and 94% at 30 years. De la Paz et al.[24] reported worse outcomes describing a 10-year anatomical survival of 60% following MOOKP. When the indications for surgery were compared, eyes with MMP had relatively unfavorable results compared to eyes with thermal ocular burns.[24]
b. Functional outcomes:
In most studies, the best functional outcomes were seen in SJS followed by chemical burns and worse functional outcomes were seen in eyes with thermal injury and MMP.[19-23] Eyes with thermal injury had poorer VA due to associated optic neuropathy and retinal dysfunction from the initial injury.[12,14,18,24] Cicatricial disorders such as SJS cause a more localized damage predominantly affecting the ocular surface, in comparison to chemical burns, where the agent may penetrate the surface and damage the intraocular structures, also causing glaucoma, thus limiting functional success.[23]
Complications
The various intraoperative and postoperative complications encountered during MOOKP implantation have been listed in Table 4. Complications due to mucous membrane graft disorders (thinning, ulceration, necrosis) were seen in the post-implantation stage (after stage 2) in 4%–48% of cases across all studies.[13,16,18,19,22-24] Glaucoma is a vision-threatening postoperative complication common to all Kpro devices. Around 50% of patients with an indication for OOKP had preexisting glaucoma.[25,26] The highest incidence of de novo glaucoma following implantation was reported by Iannetti et al.,[23] where 66% eyes developed glaucoma postoperatively. Assessment of glaucoma is difficult in these eyes, as evidence of the disease on visual field examination is seen only during the advanced stages.[27] Laminar resorption is a unique and daunting complication of MOOKP that threatens device retention. The reported incidence ranges between 19% and 40%,[13,16,19] with higher rates in children and young adults and in patients in whom a tibial or allograft lamina has been used. Compared to other Kpro designs, there was a relatively low incidence of endophthalmitis (0–8%) among all the studies reviewed.[21,23]
Table 4.
Intra- and postoperative complications seen during the various stages of osteo-odonto keratoprosthesis implantation
| A. Intra- and postoperative complications after stage 1 | |
|---|---|
|
| |
| Intraoperative | Postoperative |
| A. Intraoperative in stage 1A (preparation of the globe) | A. Mucosal |
| Corneal/scleral perforation Damage to the parotid duct while harvesting the buccal mucosa | Persistent paresthesia in the lower lip (site of donor graft) |
| Mucous membrane defect while harvesting the graft | Submucosal scar band |
| B. Intraoperative in stage 1B (preparation of the tooth) | B. After stage 1A |
| Maxillary sinus perforation/oroantral fistula | Trophic alterations in the mucosa |
| Damage to adjacent teeth and the oral structures | Secondary glaucoma |
| Fracture of the mandible | Choroidal detachment |
| Dental unit loss | Retinal detachment |
| C. After stage 1B | |
| Absorption of lamina | |
| Infection of lamina in the submucosal space | |
|
| |
| B. Intra- and postoperative complications after stage 2 | |
|
| |
| Intraoperative | Postoperative |
|
| |
| Suprachoroidal hemorrhage | Mucosal ulceration/defect/thinning |
| Choroidal detachment | Mucosal overgrowth |
| Vitreous hemorrhage | Laminar resorption/OOAL necrosis |
| Infected lamina | |
| Hypotony | |
| Glaucoma | |
| Retinal detachment | |
| Vitreous hemorrhage | |
| Choroidal detachment | |
| Sterile vitritis | |
| Endophthalmitis | |
| Retroprosthetic membrane | |
| Retroprosthetic fistula | |
| Optic cylinder instability | |
| Expulsion of the optic cylinder | |
| Expulsion of the prosthesis | |
| Phthisis bulbi | |
| Epiretinal membrane | |
OOAL=Osteo-odonto alveolar ligament
The Boston Type II Kpro
The BKpro-II (Massachusetts Eye and Ear, Boston, MA, USA) is a therapeutic alternative for visual rehabilitation in eyes with total LSCD with an absent tear film, surface keratinization, symblepharon, and obliterated conjunctival fornices that interfere with adequate closure of eyelids.[28] The most recent BKpro-II design consists of a double-plated/collar button–shaped device with a PMMA front plate and a slitted, titanium, “click-on” backplate with 16 holes.[4,29,30] The PMMA front plate has a protruding cylinder of 2 mm length supporting through-the-lid placement. The overall length of the device is 4.7 mm and it gives a 40° field of vision. Both aphakic and pseudophakic versions are available.[4,31]
Indications and preoperative evaluation
The indications for BKpro-II are similar to those for MOOKP, but there must be intact eyelids, meaning that patients with vertical shortening of their eyelids due to previous chemical trauma may not be candidates. Relatively normal eyelids are, therefore, a relative prerequisite for patients undergoing BKpro-II implantation.[4,31,32] The surgery is generally not performed in patients unless the better eye has a VA <20/400, but with accurate light projection. B-scan ultrasound and A-scan biometry should be performed in all eyes, as described for MOOKP. The BKpro-II is an alternative to MOOKP, particularly in elderly patients lacking a healthy canine tooth or in patients with poor oral and dental hygiene.
Surgical technique[9,33]
The surgery is preferably performed under general anesthesia. First, all ocular surface mucosa is removed to prevent fistulas and inclusion cysts later. The recipient cornea is trephined, typically with an 8 mm corneal trephine. For Kpro assembly, a donor corneal carrier tissue is trephined with a trephine size 0.5 mm larger than the host trephination. A central 3 mm hole is punched. The graft is then slid over the stem of the Kpro, and the backplate is pushed into place with the assembly tool. Extracapsular lens extraction is performed in phakic eyes, and these are left aphakic, whereas in pseudophakic eyes, the IOL can be left in situ, if it is well centered, or removed. If the eye is to be left aphakic, a temporary Kpro such as the Eckardt Kpro (Dutch Ophthalmic Research Center [DORC], Zuidland, The Netherlands) is sutured and a pars plana vitrectomy (PPV) is performed. In most cases, a glaucoma drainage device (GDD), such as the Ahmed glaucoma valve (AGV), is implanted through the pars plana. The assembled Kpro is sutured into the corneal wound as one would for a PK, and the upper and the lower tarsal plates on either side are sutured, thus locking the lids on both sides of the Kpro horizontally, exposing the protruding optic. The upper and lower eyelid skin is then sutured, and an opening is fashioned in the upper eyelid for the Kpro to protrude. Postoperatively, topical antibiotics and corticosteroids are prescribed and continued.
Outcomes
The demographic data from studies highlighting anatomical and functional outcomes of the BKpro-II are presented in Table 5. The most common surgical indications in published studies were SJS and MMP, followed by ocular chemical/thermal burns.[4,31–33]
Table 5.
Demographic details, anatomical and functional outcomes of eyes which have undergone Boston Type II keratoprosthesis
| Authors, year | No. of eyes | Mean age (years, range) | Follow-up duration (range), months | Type of BKpro II no. of eyes (%) | Etiology No. of eyes (%) | Anatomical success | Functional outcome No. of eyes (%) |
|---|---|---|---|---|---|---|---|
| Pujari et al.[4]2011 | 29 | 61 (43-79) | 44.4 (11-78) | NA | SJS: 12 (41.4%) Chemical/thermal burns: 1 (3.4%) MMP: 15 (51.7%) Others: 1 (3.4%) | 51.7% (15/29) | BCVA ≥20/200 Achieved: 79.3% (23/29) Maintained: 46.2% (6/13) (13/29 eyes followed up for >5 years) BCVA ≥20/30: 34.5% (10/29) |
| Lee et al.[31] 2017 | 48 | 58.9 (43-75) | 70.2 (6-237.6) | PMMA backplate: 33 (68.6%) Titanium backplate: 15 (31.3) | SJS: 20 (41.7%) Chemical/thermal burns: 4 (8.3%) MMP: 2 (4.2%) Sjogren’s syndrome: 1 (2.1%) Kearns-Sayre syndrome: 1 (2.1%) | 50% (24/48) | BCVA ≥20/200 Achieved: 91.7% (44/48) Maintained: 37.5% (18/48) BCVA ≥20/50 Achieved: 75% (36/48) |
| Iyer et al.[32] 2019 | 10 | 46.7 (22-75) | 33 (12-54) | NA | SJS: 8 (80%) Chemical/thermal burns: 1 (10%) MMP: 1 (10%) | 90% (9/10) | BCVA ≥20/200 Achieved: 90% (9/10) Maintained: 70% (7/10) BCVA ≥20/30 Achieved: 80% (8/10) Maintained: 60% (6/10) |
| Saini et al.[33] 2022 | 56 | 59.7 (42-76) | 45.8 (0.2-134.7) | Click-on BKpro-II | SJS: 28 (49.1%) Chemical/thermal burns: 3 (5.7%) MMP: 22 (39.6%) Sjogren’s syndrome: 1 (1.9%) | 73.2% (41/56) | BCVA ≥20/200 Achieved: 89.3% (50/56) Maintained : 50% (9/18)* |
BCVA=best-corrected visual acuity, BKpro-II=Boston keratoprosthesis type II, Kpro=keratoprosthesis, MMP=Mucous membrane pemphigoid, NA=not available, PMMA=polymethyl methacrylate, SJS=Stevens-Johnson syndrome. *18/56 eyes retained the Kpro for > 5 years.
a. Anatomical outcomes
Anatomical success reported by Lee et al.[31] was 50% (24/48 eyes) over an average follow-up time of 5.9 ± 5.2 years, which was comparable to that reported by Pujari et al.,[4] where the anatomical retention rate was 51.7% (17/29 eyes) over an average follow-up of 107.9 person-years. Though Iyer et al.[32] reported a higher anatomical success rate of 90% (9/10 eyes), their mean duration of follow-up was lesser (2.75 years) than those in the studies of Pujari et al.[4] and Lee et al.[31] These studies have analyzed outcomes of the previously used older design BKpro-II. When compared to these results, the anatomical retention of the new BKpro-II device was superior, as reported by Saini et al.[33] Of the 56 eyes that underwent BKpro-II (new click-on design) implantation, only 15 eyes (26.8%) required replacement of the device, while the remaining 41 eyes (73.2%) retained the device for the entire duration of follow-up (48.5 months, range: 0.2–134.7). Compared to type I Kpro implants, which have a retention rate of 65%–100% over 5 years, the retention rates of type II implants are less (50%–73%).[31,34]
b. Visual outcomes
Across all the studies, significant improvement of VA was seen, with 79%–92% eyes achieving best-corrected visual acuity (BCVA) ≥20/200 postoperatively, over an average follow-up of 2.75–5.85 years. Saini et al.[33] evaluated visual outcomes in eyes which underwent replacement of the older design BKpro-II with the newer click-on design BKpro-II and compared them to eyes which did not have a previous BKpro-II.[33] They reported superior outcomes in eyes which did not have a previous BKpro-II. Saini et al. [33] also reported that among the eyes with no previous BKpro-II, the visual outcomes were comparable in the eyes which underwent replacement with new BKpro-II device versus the eyes which did not, concluding that a repeat intervention with the current, newer design BKpro-II was not a risk factor for irreversible loss of vision in these eyes.[33] They concluded that BKpro-II could be repeated safely when indicated, without causing irreversible loss of vision. The functional outcomes were comparable to the post-MOOKP 5-year outcomes reported by Iannetti et al.,[23] where 85% eyes achieved BCVA of 20/200 or better. Lee et al.[31] concluded that a more robust postoperative follow-up and concomitant GDD procedures resulted in better functional outcomes.
Postoperative complications
The postoperative complications reported after BKpro-II implantation across various studies have been listed in Table 6. Recent changes to the antibiotic regimen post-Kpro implantation have reduced the incidence of postoperative endophthalmitis, making glaucoma the most common sight-threatening postoperative complication.[25,35] Preexisting glaucoma may worsen, or new glaucoma may develop after the surgery, especially in eyes with ocular chemical/thermal burns.[4,29] Topical antiglaucoma medications are not absorbed after a type-II Kpro, but oral acetazolamide is a safe option in such patients.[36] In studies where PPV with AGV implantation has been performed in all eyes, the prevalence of glaucoma is low.[32] The published literature, therefore, supports concomitant GDD implantation in all patients undergoing BKpro-II implantation.[32]
Table 6.
Postoperative complications seen after Boston type II keratoprosthesis
| Pujari et al.[4] 2011 n=29 | Lee et al.[31] 2017 n=48 | Iyer et al.[32] 2019 n=10 | Saini et al.[33] 2022 n=56 | |
|---|---|---|---|---|
| Revision of tarsorrhaphy/skin overgrowth on the nub | 10 (34.5%) | 25 (52%) | 2 (20%) | |
| Wound leak | 10 (34.5%) | 6 (10.7%) | ||
| Retroprosthetic membrane | 14 (48.3%) | 29 (60.4%) | 1 (10%) | 6 (10.7%) |
| Sterile vitritis | 12 (25%) | 13 (23.2%) | ||
| Cystoid macular edema | 5 (8.9%) | |||
| Vitreous hemorrhage | 2 (4.2%) | 9 (16%) | ||
| Retinal detachment | 8 (27.6%) | 3 (6.3%) | 1 (10%) | 17 (25%) |
| Choroidal detachment | 2 (8.7%) | 4 (8.3%) | 17 (30.3%) | |
| End-stage glaucoma (progression or de novo) | 2 (8.7%) | 17 (35.4%) | 23 (41.1%) | |
| Endophthalmitis | 1 (4.3%) | 3 (6.3%) | ||
| Keratoprosthesis extrusion | 24 (50%) | 15 (26.7%) |
The Moscow Eye Microsurgery Complex in Russia Kpro
The MICOF Kpro is an alternative used for visual rehabilitation in eyes with a compromised ocular surface with concomitant DED.[37] Long-term outcomes have been published from Russia and China.[37–41] The MICOF Kpro (dioptric power: +55–+62 D) consists of a central rigid PMMA optical cylinder (2.5 mm) and a titanium haptic that is placed in lamellar fashion. The cylinder length is available from 2.2 to 2.4 mm and can be selected depending on the corneal thickness.[37]
Surgical procedure[37]
The surgery may be performed under local anesthesia. Implantation of the MICOF device consists of two stages, performed 3 months apart. In stage 1, the supporting titanium haptic is inserted into a lamellar intracorneal pocket. Superior bulbar conjunctiva and half the limbus is dissected to a depth of about 8 mm (two-thirds of the corneal depth) to create a pocket of approximately 6 × 8 mm, where the supporting titanium plate is inserted. In patients with inadequate corneal thickness (<1.5 mm), the cornea is reinforced with autologous auricular cartilage, after inserting the titanium plate. Three months later, the second stage of the surgery is performed. Once the pars planar irrigation is established to achieve ideal globe tension, a 2.5-mm-diameter section of the corneal tissue is trephined from the center of the frame. The PMMA core is unscrewed, and the underlying corneal tissue of 2.2 mm diameter is removed.
Thorough anterior vitrectomy, complete iridectomy, and lens extraction are performed through the pars plana route, following which the PMMA optical cylinder is screwed onto the central frame. Postoperatively, topical corticosteroids and antibiotic eye drops are prescribed. Autologous auricular cartilage may be used as a reinforcement, either prophylactically in thin corneas or postoperatively in eyes with corneal melt.[41,42] The cartilage improves the lifespan with a resorption rate of less than 1% over 1 year postoperatively.[42]
Outcomes
a. Anatomical outcomes
The anatomical outcomes have been listed in Table 7. Device retention rate ranged from 81.2% to 100% over a mean follow-up of 60.4 months (range: 34.7–100.5).[37–41] These were comparable to the anatomical retention rates of MOOKP (88.8% over a 20-year follow-up).[12] Early reinforcement using autologous auricular cartilage reduces the risk of device extrusion, improving the long-term outcomes.[37,38]
Table 7.
Demographic details, anatomical and functional outcomes of eyes which have undergone MICOF keratoprosthesis (including >15 eyes)
| Authors, year | No. of eyes | Mean age (years, range) | Follow-up duration (range), months | Etiology No. of eyes (%) | Anatomical success No. of eyes (%) | Functional outcome | Complications No. of eyes (%) |
|---|---|---|---|---|---|---|---|
| Huang et al.[37] 2011 | 85 | 44.9 (19-80) | 34.7 (3-107) | Alkali burns: 39 (45.9%) Thermal burn: 20 (23.5%) Acid burn: 1 (12.9%) SJS: 10 (11.8%) MMP: 5 (5.9%) | 69/85 (81.2%) | NA | RPM: 39 (45.9%) Corneal leak/melt: 16 (19%) |
| Huang et al.[38] 2012 | 14 | 67 (29-80) | 46.8 (10-93.6) | SJS: 7 (50%) MMP: 4 (28.5%) Sjogren’s: 3 (21.4%) | 14/14 (100%) | BCVA ≥20/200 Achieved: 13/14 (93%) Maintained: 9/13 (69%) BCVA ≥20/40: Achieved: 43% Maintained: none | Corneal leak/melt: 7 (50%) Conjunctival membrane overgrowth: 2 (14%) |
| Wang et al.[39] 2015 | 90 | 40 (8-64) | 58.22 (1-145) | Alkali burns: 51 (56.6%) Acid burns: 18 (20%) Thermal burns: 21 (23.33%) | 87/90 (96.67%) | BCVA ≥20/200 Achieved: 73/90 (81.11%) Maintained: NA BCVA ≥20/40: Achieved: 39/90 (43.33%) Maintained: NA | Corneal leak/melt: 36 (40%) Conjunctival membrane overgrowth: 28 (31.11%) |
| Ma et al.[40] 2017 | 14 | 61.5 (27-87) | 62 (13-144) | SJS: 14 (100%) | 13 (92.8%) | BCVA ≥20/200 Achieved: 13/14 (92.9%) Maintained: 10/14 (71.4%) BCVA ≥20/40: Achieved: 8/14 (57.1%) Maintained: 5 (35.7%) | Corneal leak or melt: 10 (71.4%) RPM: 4 (28.6%) |
| Wang et al.[41] 2021 | 91 | 46.7 (17-90) | 100.56 (60-207) | Chemical or thermal burns: 62 (68.1%) Explosive injuries: 11 (12.1%) SJS: 9 (10.0%) Sjogren’s syndrome: 4 (4.4%) MMP: 3 (3.3%) Multiple failed PK: 2 (2.2%) | 77/91 (84.6%) | BCVA ≥20/200 Achieved: 41/90 (45%) Maintained: 32/91 (35.2%) | Conjunctival membrane overgrowth: 29 (31.9%) RPM: 14 (15.4%) |
BCVA=best-corrected visual acuity, MICOF=the Moscow eye microsurgery complex in Russia, MMP=mucous membrane pemphigoid, NA=not applicable, PK=penetrating keratoplasty, RD=retinal detachment, RPM=retroprosthetic membrane, SJS=Stevens-Johnson syndrome
b. Visual outcomes
As per the reported literature, majority of the patients undergoing MICOF implantation had significant improvement in the vision , with 81-93%eyes acheiving BCVA ≥ 20/200.[37–41] VA outcomes in autoimmune DED were highlighted in the study by Huang et al.[38] in 2012. In their study, 69% (9/13) eyes maintained BCVA ≥20/200 over 3.9 years. Also, 43% patients achieved BCVA ≥20/40, but none of the eyes maintained this vision through the last follow-up. Visual outcomes were inferior in eyes with autoimmune disease compared to ocular burns.[38,40]
Complications
Compared to other Kpro devices, the prevalence of stromal melt appears to be higher after MICOF Kpro implantation. Stromal tissue melt was seen in 10%–34.5% of eyes following BKpro-II implantation, and mucosal necrosis was seen in 28% of eyes following MOOKP implantation.[4,16] Anterior lamina thinning and haptic exposure were seen in 50% (7/14) of eyes during an average follow-up period of 12.5 months.[38] Across all the studies, the highest incidence of corneal melt following MICOF implantation was reported by Ma et al.[40] They reported peri-cylindrical melt in 10/14 eyes (71.4%) with SJS sequelae, all of which were treated with auto-auricular cartilage inforcement and had a stable anatomical retention at the last visit (92.8%). The reported incidence of corneal melt in this study (71.4%) was significantly higher than the 34.5% reported by Pujari et al.[4] with BKpro-II implantation and 50% reported by Basu et al.[22] following MOOKP implantation. Huang et al.[38] reported the highest number of prophylactic reinforcements being performed in 42.8% (6/14) of eyes. The majority of their patients (50%) had SJS. They reported a 100% anatomical retention rate, thus proving the beneficial effects of prophylactic reinforcements. Progressive glaucoma is a sight-threatening complication commonly seen after MICOF implantation. The MICOF device has a small optic (about 2.2 mm diameter), making it difficult to assess the optic nerve postoperatively.[38] Huang et al. reported the highest incidence of glaucoma in eyes with alkali burns (58.3%), followed by SJS and thermal burns (16.7% each).[37]
Tibial Osteo-Kpro
As described above, in OOKP, the surgeon harvests the patient’s own canine tooth and the surrounding alveolar bone to form the haptic supporting a PMMA optical cylinder made of PMMA.[12] As the procedure involves using the patient’s own tooth, the risk of immunologic rejection is negligible. Some patients may not have a healthy canine tooth due to advanced age or may have poor oral and dental hygiene not permitting MOOKP surgery. In 1985, Temprano modified Strampelli’s technique by using autologous tibial bone lamina to form the haptic to support the PMMA cylinder.[24] The anatomical and functional outcomes of tibial OKP are, in general, inferior to MOOKP.[18,24] This is attributed to the presence of alveolar bone and dentine in MOOKP surgery, leading to a lower resorption rate compared to tibial laminae. However, tibial OKP is a less-invasive procedure than MOOKP and eliminates the risk of oroantral fistula and damage to adjacent oral structures.[43]
Surgical technique[43]
The surgical technique is the same as that for OOKP, except for the step in which the Kpro is assembled. A vertical skin incision is made over the tibial bone at the level of the superior one-third of the leg. The subcutaneous tissue is dissected to expose the anterior surface of the tibial bone. A tibial bone graft is harvested, measuring 10 mm in diameter and 3 mm in thickness. After assembly of the tibial OKP, it is left in the subcutaneous space of the inferior orbital region for 3 months to allow growth of a fibrovascular capsule. The rest of the surgical technique is similar to that of OOKP.[12,17]
Surgical outcomes
Surgical outcomes in the two studies comparing the two approaches, that is, tibial OKP versus MOOKP, were published by De la Paz et al.[24] and Charoenrook et al.[44] These have been described in Table 8.
Table 8.
Demographic details, anatomical and functional outcomes of eyes post-tibial keratoprosthesis
| Authors, year | No. of eyes | Mean age (years, range) | Follow-up duration (range), months | Etiology No. of eyes (%) | Anatomical success | Functional success |
|---|---|---|---|---|---|---|
| De la Paz et al.[24] 2011 | 82 | 53 (14-86) | 42 (1-150) | Chemical/thermal burns: 31 (38%) | 65.1% @ 5 years | 29% @ 5 years |
| SJS: 9 (11%) | 48% @ 10 years | 17% @ 10 years | ||||
| MMP: 17 (21%) | ||||||
| Trachoma: 1 (2%) | ||||||
| Others: 23 (28%) | ||||||
| Charoenrook et al.[44] 2018 | 113 | 52.8 (14-86) | 50.4 (NA) | Chemical and thermal burns: | 69.5% @ 5 years | 33% @ 5 years |
| 36 (31.8%) | 53.5% @ 10 years | 19.2% @ 10 years | ||||
| MMP: 28 (24.5%) | 42.8% @ 15 years | 12% @ 15 years | ||||
| SJS: 7 (6.2%) | ||||||
| Trachoma: 5 (4.4%) |
MMP=Mucous membrane pemphigoid, SJS=Stevens-Johnson syndrome
a. Anatomical and functional outcomes
De la Paz et al.[24] demonstrated a 10-year anatomical retention for MOOKP of 60% compared to tibial OKP with 48% retention. The 10-year functional outcomes, defined as VA ≥20/200, for MOOKP (39%) were statistically superior to those of tibial OKP (17%). This was in accordance with the long-term outcomes published by Charoenrook et al.[44] in 2018, in comparing the two techniques. The 10-year anatomical success of OOKP (67%) was superior to tibial OKP (54%) but did not reach statistical significance. The 10-year functional outcomes of OOKP (49%) were significantly better than those of tibial OKP (25%). In that study, the underlying indication for surgery was not associated with the likelihood of success or failure of either of the surgeries. However, the probability of complications at 10-year follow-up was significantly higher in tibial OKP recipient eyes (65%) compared to MOOKP recipient eyes (40%), with mucous membrane necrosis and retroprosthetic membrane (RPM) formation being the most common complications.
b. Outcomes of tibial OKP based on primary diagnosis:
Charoenrook et al.[43] published the outcomes of tibial OKP in eyes with different underlying indications for surgery. The best 5-year anatomical survival was seen in eyes with chemical (81.3%) and thermal injuries (80%) and the worst outcomes were seen in eyes with SJS (49%). Extrusion of the Kpro was seen in 31 eyes (27.4%), and the most common underlying etiologies were MMP (9/31, 29%) and SJS (8/31, 25.8%). The majority of the device extrusions (75% in SJS eyes, 55% in MMP eyes) were seen within the first 2 years postoperatively. Buccal mucosal necrosis was judged to be the most common predisposing factor leading to device extrusion and anatomical failure. This was comparable to the outcomes for MOOKP published by Iyer et al.,[19] where eyes in patients post-SJS had a higher incidence of mucosal necrosis and laminar resorption, causing Kpro extrusion.
LVP Keratoprosthesis
Another complication in patients with MOOKP is mucosal overgrowth, a problem particularly common in patients whose underlying diagnosis is SJS (13%–33%).[16,22] To overcome this issue, Basu et al.[45] modified the design of the BKpro-I to elongate the anterior optical cylinder, creating a device known as the LVP Kpro. In 57 eyes with a mean follow-up of 28 months, anatomical success was achieved in 81% eyes, superior to the outcomes of BKpro-II (51.7%) reported by Pujari et al.[4] but less than with MOOKP reported by Hille et al.[12] (96.5% at 5 years postoperatively). The functional outcomes of LVP Kpro (36% maintained BCVA ≥20/200 at the final follow-up) were inferior to those reported by Saini et al.[33] (50% maintained BCVA ≥20/200 at 5 years) following implantation of the new design BKpro-II and by Liu et al.[16] (53% maintained BCVA ≥20/200 at 5 years) following MOOKP implantation.
With LVP Kpro, the incidence of mucosal overgrowth was only 10%, which is less than the reported incidence following MOOKP implantation.[22] LVP Kpro has a few advantages over MOOKP.[45] It is surgically a significantly easier procedure, can be easily repeated, and permits absorption of topical medications to control the IOP and intraocular inflammation. Also, it can be performed in children.
Lux Kpro
Lux Kpro (Lux, derived from Latin for light), is a three-piece device consisting of a cone-shaped PMMA optic encased in a titanium sleeve and titanium backplate having a diameter of 7.8 mm.[46] The design was intended to incorporate positive aspects of both MOOKP and BKpro-II, with the titanium sleeve intended to afford better biocompatibility than BKpro-II and with the putative advantage of covering the device with a mucous membrane graft rather than needing to suture the eyelids closed. Like BKpro-II, the Lux Kpro requires a carrier donor corneal graft. However, it can be performed in eyes without normal eyelids. Like the MOOKP, the Lux is covered by a buccal mucosal graft, while implantation does not require a healthy tooth. The surgical technique is similar to BKpro-II surgery, with the exception of requiring a previous mucous membrane graft.
Surgical outcomes: A single study published by Bakshi et al.[46] [Table 9] reports the short-term outcomes of Lux Kpro in nine eyes over a mean follow-up of 18.7 months. The anatomical outcomes were promising, with 100% eyes retaining the device and achieving VA of ≥20/200 within 18.7 months postoperatively. Progressing of preexisting glaucoma was seen in 50% eyes (2/4). None of the patients developed de novo glaucoma. None of the eyes developed peri-cylindrical retraction of the mucous graft, thus explaining the higher rate of anatomical success. This has been attributed to the presence of a polished titanium sleeve, such that the host tissue is never in direct contact with PMMA. The smaller backplate diameter of 7.8 mm (vs. 8.5 mm in BKpro-II) enables better fit in eyes with smaller corneal diameters, commonly seen in eyes with chemical burns, and even in eyes which are pre-phthisical.
Table 9.
Demographic details, anatomical and functional outcomes, and the most common complication of the largest studies published on the different types of keratoprosthesis devices preferred in patients with dry eye disease
| Type of Kpro | Study, year | Number of eyes | Most common etiology No. of eyes (%) | Duration of follow-up in months | Anatomical outcomes | Functional outcomes | Most common complication No. of eyes (%) |
|---|---|---|---|---|---|---|---|
| MOOKP | Iannetti et al.[23] 2022 | 82 | SJS: 58 (70.7%) | 328 (28.8-624) | 94%a | BCVA ≥20/200: @5 years: 85% @10 years: 81% @30 years: 55% | Glaucoma De novo: 41/62 (66%) |
| Boston type II | Lee et al.[31] 2016 | 48 | SJS: 20 (41.7%) MMP: 20 (41.7%) | 70.2 (6-237.6) | 50%a | BCVA ≥20/200: 37.5% | RPM: 29/48 (60.4%) |
| MICOF | Wang et al.[39] 2015 | 90 | Alkali burns: 51 (56.6%) | 58.22 (1-145) | 96.67%a | BCVA ≥20/200: 81.11% | Corneal leak or melt: 36/90 (40%) |
| Tibial | Charoenrook et al.[43] 2016 | 113 | Chemical and thermal burns: 36 (31.8%) | 504 (1-38.8) | 69.5% @ 5 years 53.5% @ 10 years 42.8% @ 15 years | BCVA ≥20/20 33%@ 5 years 19.2%@ 10 years | Keratoprosthesis extrusion: 31/113 (27.4%) |
| LVP | Basu et al.[45] 2018 | 58 | SJS: 49 (84.4%) | 28 (12-67) | 81%a | BCVA ≥20/400: 37.6%@ 3 years | RPM: 25/58 (43%) |
| Lux | Bakshi et al.[46] 2020 | 9 | SJS: 5 (55.5%) | 18.7 (7-28) | 100%a | BCVA ≥20/200: 100% | RPM: 3/9 (33.3%) |
| Pintucci | Maskati and Maskati[48] 2006 | 31 | Chemical burns: 11 (35.4%) | 38.4 (6-84) | 100%a | BCVA ≥20/200: 13% | Glaucoma: 3/31 (9.7%) |
BCVA=best-corrected visual acuity, MICOF=the Moscow eye microsurgery complex in Russia, MMP=mucous membrane pemphigoid, MOOKP=modified osteo-odonto keratoprosthesis, RPM=retroprosthetic membrane, SJS=Stevens-Johnson syndrome. aAnatomical outcomes were defined as the number of eyes that retained the keratoprosthesis at last follow-up
Pintucci Kpro
Kpro extrusion is thought to occur with any of the above devices because of an absence of or failure of biointegration.[12] In 1979, Pintucci developed a biointegratable haptic using Dacron felt.[47] When implanted subcutaneously under the lower lid for a period of 40 days, the Dacron skirt showed three-dimensional colonization by host connective tissue, effectively filling all the spaces in the Dacron mesh and along the attached optical cylinder, suggesting a reduced likelihood of aqueous leak and secondary infection once implanted in the cornea.
The surgical procedure involves two stages performed 2 months apart. In the first stage, the ocular surface is reconstructed with an oral mucous membrane graft and a KPro is placed under the skin and the orbicularis oculi muscle. Two to three months later, the colonized KPro is removed and implanted. In 1995, Pintucci et al.[47] reported the outcomes in 20 eyes, with 13 eyes (62%) retaining the device for more than 2 years with significant improvement in VA. Later, Maskati and Maskati[48] described the outcomes in 31 eyes with a follow-up period of 3.2 years [Table 9]. All the eyes retained the device for the entire duration of follow-up. The functional outcomes (4/31, 13% achieving BCVA ≥ 20/200) were inferior to other devices.[12,31] None of the eyes developed RPM formation, endophthalmitis, or extrusion. This was attributed to the fact that the connective tissue within the Dacron mesh led to effective biointegration.
Conclusion
We have summarized the outcomes of different Kpro devices in recent use for visual rehabilitation in patients with bilateral end-stage corneal blindness with DED, where corneal transplantation, limbal transplantation, or type 1 devices have shown poor results. The best long-term anatomical and functional outcomes have been reported with MOOKP. However, due to the complexity of the procedure, multiple stages, need for involvement of a dental or a maxillofacial surgeon, and difficulty in managing complications, this procedure is performed in only a few centers in the world. Kpro designs have, therefore, been in continual evolution to overcome these issues in order to make the surgery accessible for more patients. The outcomes of currently used devices and approaches are far from perfect, but given that the alternative without surgery is permanent blindness, these procedures offer hope of restored vision to those who otherwise would have none.
Financial support and sponsorship
For authors PRD, SB, SSS: This work was funded by the Hyderabad Eye Research Foundation, Hyderabad, India. The sponsoring organization had no role in the design or conduct of this research.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank the Centro de Oftalmología Barraquer, Barcelona, Spain for providing Figures 1b and 3c for this review.
References
- 1.Chirila TV, Hicks CR. The origins of the artificial cornea:Pellier de Quengsy and his contribution to the modern concept of keratoprosthesis. Gesnerus. 1999;56:96–106. [PubMed] [Google Scholar]
- 2.Iyer G, Srinivasan B, Agarwal S, Talele D, Rishi E, Rishi P, et al. Keratoprosthesis:Current global scenario and a broad Indian perspective. Indian J Ophthalmol. 2018;66:620–9. doi: 10.4103/ijo.IJO_22_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeed HN, Shanbhag S, Chodosh J. The Boston keratoprosthesis. Curr Opin Ophthalmol. 2017;28:390–6. doi: 10.1097/ICU.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 4.Pujari S, Siddique SS, Dohlman CH, Chodosh J. The Boston keratoprosthesis Type II:The Massachusetts eye and ear infirmary experience. Cornea. 2011;30:1298–303. doi: 10.1097/ICO.0b013e318215207c. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JK, Basak SK, Padilla MD, Yu F, Aldave AJ. International outcomes of the Boston Type I Keratoprosthesis in Stevens–Johnson syndrome. Cornea. 2015;34:1387–94. doi: 10.1097/ICO.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 6.Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2011;30:1187–94. doi: 10.1097/ICO.0b013e3182114467. [DOI] [PubMed] [Google Scholar]
- 7.Aravena C, Bozkurt TK, Yu F, Aldave AJ. Long-Term Outcomes of the Boston Type I Keratoprosthesis in the Management of Corneal Limbal Stem Cell Deficiency. Cornea. 2016;35:1156–64. doi: 10.1097/ICO.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 8.Shanbhag SS, Senthil S, Mohamed A, Basu S. Outcomes of the Boston type 1 and the Aurolab keratoprosthesis in eyes with limbal stem cell deficiency. Br J Ophthalmol. 2021;105:473–8. doi: 10.1136/bjophthalmol-2020-316369. [DOI] [PubMed] [Google Scholar]
- 9.Sayegh RR, Ang LP, Foster CS, Dohlman CH. The Boston Keratoprosthesis in Stevens-Johnson Syndrome. Am J Ophthalmol. 2008;145:438–44. doi: 10.1016/j.ajo.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Palioura S, Kim B, Dohlman CH, Chodosh J. The Boston keratoprosthesis type I in mucous membrane pemphigoid. Cornea. 2013;32:956–61. doi: 10.1097/ICO.0b013e318286fd73. [DOI] [PubMed] [Google Scholar]
- 11.Strampelli B. [Osteo-odontokeratoprosthesis. Ann Ottalmol Clin Ocul. 1963;89:1039–44. [PubMed] [Google Scholar]
- 12.Hille K, Grabner G, Liu C, Colliardo P, Falcinelli G, Taloni M, et al. Standards for modified osteoodontokeratoprosthesis (OOKP) surgery according to strampelli and falcinelli:The Rome-Vienna protocol. Cornea. 2005;24:895–908. doi: 10.1097/01.ico.0000157401.81408.62. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz-Morales G, Loya-Garcia D, Colorado-Zavala MF, Gomez-Elizondo DE, Soifer M, Srinivasan B, et al. The evolution of the modified osteo-odonto-keratoprosthesis, its reliability, and long-term visual rehabilitation prognosis:An analytical review. Ocul Surf. 2022;24:129–44. doi: 10.1016/j.jtos.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Marchi V, Ricci R, Pecorella I, Ciardi A, Di Tondo U. Osteo-odonto-keratoprosthesis. Description of surgical technique with results in 85 patients. Cornea. 1994;13:125–30. [PubMed] [Google Scholar]
- 15.Falcinelli G, Falsini B, Taloni M, Colliardo P, Falcinelli G. Modified osteo-odonto-keratoprosthesis for treatment of corneal blindness:Long-term anatomical and functional outcomes in 181 cases. Arch Ophthalmol. 2005;123:1319–29. doi: 10.1001/archopht.123.10.1319. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Okera S, Tandon R, Herold J, Hull C, Thorp S. Visual rehabilitation in end-stage inflammatory ocular surface disease with the osteo-odonto-keratoprosthesis:Results from the UK. Br J Ophthalmol. 2008;92:1211–7. doi: 10.1136/bjo.2007.130567. [DOI] [PubMed] [Google Scholar]
- 17.Tay AB, Tan DT, Lye KW, Theng J, Parthasarathy A, Por YM. Osteo-odonto-keratoprosthesis surgery:A combined ocular–oral procedure for ocular blindness. Int J Oral Maxillofac Surg. 2007;36:807–13. doi: 10.1016/j.ijom.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Michael R, Charoenrook V, de la Paz MF, Hitzl W, Temprano J, Barraquer RI. Long-term functional and anatomical results of osteo- and osteoodonto-keratoprosthesis. Graefes Arch Clin Exp Ophthalmol. 2008;246:1133–7. doi: 10.1007/s00417-008-0850-3. [DOI] [PubMed] [Google Scholar]
- 19.Iyer G, Pillai VS, Srinivasan B, Falcinelli G, Padmanabhan P, Guruswami S, et al. Modified osteo-odonto keratoprosthesis-The Indian experience-results of the first 50 cases. Cornea. 2010;29:771–6. doi: 10.1097/ICO.0b013e3181ca31fc. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda M, Hamada S, Liu C, Shimomura Y. Osteo-odonto-keratoprosthesis in Japan. Cornea. 2008;27((Suppl 1)):S56–61. doi: 10.1097/ICO.0b013e31817f1fe4. [DOI] [PubMed] [Google Scholar]
- 21.Tan A, Tan DT, Tan XW, Mehta JS. Osteo-odonto keratoprosthesis:Systematic review of surgical outcomes and complication rates. Ocul Surf. 2012;10:15–25. doi: 10.1016/j.jtos.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Basu S, Pillai VS, Sangwan VS. Mucosal complications of modified osteo-odonto keratoprosthesis in chronic Stevens-Johnson syndrome. Am J Ophthalmol. 2013;156:867–73.e2. doi: 10.1016/j.ajo.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Iannetti L, Liberali M, Armentano M, Alisi L, Visioli G, Mastromarino D, et al. Osteo-odonto-keratoprosthesis according to strampelli original technique:A retrospective study with up to 30 years of follow-up. Am J Ophthalmol. 2022;242:56–68. doi: 10.1016/j.ajo.2022.05.015. [DOI] [PubMed] [Google Scholar]
- 24.De La Paz MF, De Toledo JÁ, Charoenrook V, Sel S, Temprano J, Barraquer RI, et al. Impact of clinical factors on the long-term functional and anatomic outcomes of osteo-odonto-keratoprosthesis and tibial bone keratoprosthesis. Am J Ophthalmol. 2011;151:829–39.e1. doi: 10.1016/j.ajo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology. 1998;105:751–7. doi: 10.1016/S0161-6420(98)94034-9. [DOI] [PubMed] [Google Scholar]
- 26.Iyer G, Srinivasan B, Agarwal S, Shetty R, Krishnamoorthy S, Balekudaru S, et al. Glaucoma in modified osteo-odonto-keratoprosthesis eyes:Role of additional stage 1A and Ahmed glaucoma drainage device–technique and timing. Am J Ophthalmol. 2015;159:482–9.e2. doi: 10.1016/j.ajo.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Falcinelli GC, Falsini B, Taloni M, Piccardi M, Falcinelli G. Detection of glaucomatous damage in patients with osteo-odontokeratoprosthesis. Br J Ophthalmol. 1995;79:129–34. doi: 10.1136/bjo.79.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dohlman CH, Harissi-Dagher M, Khan BF, Sippel K, Aquavella JV, Graney JM. Introduction to the use of the Boston keratoprosthesis. Expert Rev Ophthalmol. 2006;1:41–8. [Google Scholar]
- 29.Todani A, Ciolino JB, Ament JD, Colby KA, Pineda R, Belin MW, et al. Titanium back plate for a PMMA keratoprosthesis:Clinical outcomes. Graefes Arch Clin Exp Ophthalmol. 2011;249:1515–8. doi: 10.1007/s00417-011-1684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harissi-Dagher M, Khan BF, Schaumberg DA, Dohlman CH. Importance of nutrition to corneal grafts when used as a carrier of the Boston Keratoprosthesis. Cornea. 2007;26:564–8. doi: 10.1097/ICO.0b013e318041f0a6. [DOI] [PubMed] [Google Scholar]
- 31.Lee R, Khoueir Z, Tsikata E, Chodosh J, Dohlman CH, Chen TC. Long-term visual outcomes and complications of Boston keratoprosthesis Type II implantation. Ophthalmology. 2017;124:27–35. doi: 10.1016/j.ophtha.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Iyer G, Srinivasan B, Agarwal S, Ravindran R, Rishi E, Rishi P, et al. Boston Type 2 keratoprosthesis- mid term outcomes from a tertiary eye care centre in India. Ocul Surf. 2019;17:50–4. doi: 10.1016/j.jtos.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Saini C, Chen TC, Young LH, Vavvas DG, Vangel M, Papaliodis GN, et al. Restoration of vision in severe, cicatricial, ocular surface disease with the Boston keratoprosthesis Type II. Am J Ophthalmol. 2022;243:42–54. doi: 10.1016/j.ajo.2022.06.022. [DOI] [PubMed] [Google Scholar]
- 34.Lee WB, Shtein RM, Kaufman SC, Deng SX, Rosenblatt MI. Boston keratoprosthesis:Outcomes and complications:A report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:1504–11. doi: 10.1016/j.ophtha.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Durand ML, Dohlman CH. Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis. Cornea. 2009;28:896–901. doi: 10.1097/ICO.0b013e3181983982. [DOI] [PubMed] [Google Scholar]
- 36.Kumar R, Dohlman CH, Chodosh J. Oral acetazolamide after Boston keratoprosthesis in Stevens-Johnson syndrome. BMC Res Notes. 2012;5:205. doi: 10.1186/1756-0500-5-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Yu J, Liu L, Du G, Song J, Guo H. Moscow eye microsurgery complex in Russia keratoprosthesis in Beijing. Ophthalmology. 2011;118:41–6. doi: 10.1016/j.ophtha.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Dong Y, Wang L, Du G, Yu J, Song J, et al. Long-term outcomes of MICOF keratoprosthesis in the end stage of autoimmune dry eyes:An experience in China. Br J Ophthalmol. 2012;96:28–33. doi: 10.1136/bjo.2010.193029. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Huang Y, Du G, Dong Y, Guo H, Wang D, et al. Long-term outcomes and complications of Moscow Eye Microsurgery Complex in Russia (MICOF) keratoprosthesis following ocular surface burns:Clinical experience in China. Br J Ophthalmol. 2015;99:1669–74. doi: 10.1136/bjophthalmol-2014-306115. [DOI] [PubMed] [Google Scholar]
- 40.Ma X, Xiang R, Meng X, Qin L, Wu Y, Tain L, et al. Russian Keratoprosthesis in Stevens–Johnson syndrome. Cornea. 2017;36:304–9. doi: 10.1097/ICO.0000000000001094. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, He X, Wang Q, Wu T, Liu A, Huang Y. Long-term outcomes of the MICOF keratoprosthesis surgery. Ocul Surf. 2021;21:178–85. doi: 10.1016/j.jtos.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Wee JH, Park MH, Oh S, Jin HR. Complications associated with autologous rib cartilage use in rhinoplasty:A meta-analysis. JAMA Facial Plast Surg. 2015;17:49–55. doi: 10.1001/jamafacial.2014.914. [DOI] [PubMed] [Google Scholar]
- 43.Charoenrook V, Michael R, de la Paz MF, Ding A, Barraquer RI, Temprano J. Osteokeratoprosthesis using tibial bone:Surgical technique and outcomes. Ocul Surf. 2016;14:495–506. doi: 10.1016/j.jtos.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Charoenrook V, Michael R, de la Paz MF, Temprano J, Barraquer RI. Comparison of long-term results between osteo-odonto-keratoprosthesis and tibial bone keratoprosthesis. Ocul Surf. 2018;16:259–64. doi: 10.1016/j.jtos.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Basu S, Nagpal R, Serna-Ojeda JC, Bhalekar S, Bagga B, Sangwan V. LVP keratoprosthesis: Anatomical and functional outcomes in bilateral end-stage corneal blindness. Br J Ophthalmol. 2018 doi: 10.1136/bjophthalmol-2017-311649. bjophthalmol-2017-311649. doi: 10.1136/bjophthalmol-2017-311649. [DOI] [PubMed] [Google Scholar]
- 46.Bakshi SK, Graney J, Paschalis EI, Agarwal S, Basu S, Iyer G, et al. Design and outcomes of a novel keratoprosthesis:Addressing unmet needs in end-stage cicatricial corneal blindness. Cornea. 2020;39:484–90. doi: 10.1097/ICO.0000000000002207. [DOI] [PubMed] [Google Scholar]
- 47.Pintucci S, Pintucci F, Cecconi M, Caiazza S. New Dacron tissue colonisable keratoprosthesis:Clinical experience. Br J Ophthalmol. 1995;79:825–9. doi: 10.1136/bjo.79.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maskati QB, Maskati BT. Asian experience with the Pintucci keratoprosthesis. Indian J Ophthalmol. 2006;54:89–94. doi: 10.4103/0301-4738.25828. [DOI] [PubMed] [Google Scholar]