Abstract
Vitamin D is a steroid hormone that has widespread role in human physiology, not only in the maintenance of calcium homeostasis but also in immunomodulation, cellular differentiation, and proliferation. The immunomodulatory effects of vitamin D are well known and are applicable to the ocular surface immune cells and structural cells. The role of vitamin D in ocular surface conditions such as dry eye disease (DED), keratoconus (KC), and post-surgical outcomes has received widespread and well-deserved attention. Vitamin D supplementation is shown to improve DED clinically as well as in experimental models. The anti-inflammatory properties may be crucial in the treatment of ocular surface conditions such as DED and KC. Vitamin D plays a multifaceted role in corneal wound healing with its anti-inflammatory and extracellular matrix remodeling properties. In this review, we discuss how to approach patients with DED and those undergoing refractive surgery with the available basic and clinical knowledge on the role of vitamin D in these conditions. We aim to highlight the importance of clinically harnessing vitamin D-mediated natural immuno-inflammatory modulation in combination with currently available standard of care strategies to reduce the morbidity and disease duration associated with ocular surface diseases.
Keywords: Dry eye disease, keratoconus, vitamin D, vitamin D deficiency
The initial interest in vitamin D was purely for its role in calcium and mineral homeostasis in skeletal tissues. At the time of its discovery, vitamin D was considered as a vitamin sourced from food to treat rickets.[1] However, evidence started pouring in from multiple specialties of medicine on its role in immune and inflammation modulation, sustenance of epithelial barrier integrity, and cellular homeostasis. Hence, vitamin D is now categorized as an endocrine mediator. In this article, we outline the existing basic and clinical knowledge on the role of vitamin D in the pathophysiology of ocular surface conditions such as dry eye disease (DED), keratoconus (KC), etc., and the treatment suggestions for vitamin D deficiency (VDD) in these patients.
Source and Metabolism of Vitamin D
There are two sources of vitamin D available to humans: dietary sources and local synthesis in skin. The dietary sources include foods that naturally contain vitamin D such as fish, egg yolk, and liver.[2] Many nations have arranged for the fortification of commonly used foods like milk, breakfast cereals, and orange juice.[3] The metabolites used for vitamin D fortification are either ergocalciferol (D2) or cholecalciferol (D3).[2] Endogenously, vitamin D3 is synthesized from 7-dehydrocholesterol in the skin through photo-conversion under the influence of ultraviolet B radiation. It is then transported to the liver where it is converted to 25 hydroxyvitamin D3 (25-OH-D3) by the enzyme 25-hydroxylase.[4] It then undergoes further modification in the proximal convoluted tubules of kidneys to 1,25 – (OH) 2D3 (calcitriol, active form of vitamin D) by the enzyme 25-OH-1 alpha hydroxylase.[5] This enzyme is encoded by the gene CYP27B1, whose expression is stimulated by the parathyroid hormone, depending on the serum concentrations of calcium and phosphorous.[6]
Metabolism of vitamin D in ocular surface and other peripheral tissues
Extrarenal synthesis of 1,25-(OH)2D3 has been demonstrated in many tissues including the skin, respiratory system, breast, colon, endometrium, and prostate.[7-10] Similarly, the eye has many cell types including corneal epithelial cells, endothelial cells, scleral fibroblasts, and retinal pigment epithelial cells which express both 1α–hydroxylase and 25α–hydroxylase, the enzymes responsible for the activation of vitamin D3.[10,11] These observations establish that many ocular structures have machinery for metabolizing vitamin D3 into its active metabolites.
Mechanisms of Action of Vitamin D
The cellular effects of vitamin D are predominantly mediated by its interaction with the vitamin D receptor (VDR) and subsequent transcriptional regulation in the nucleus.[12] Nevertheless, there are certain cellular events that are too rapid in response to vitamin D, such as induction of ion channel activity, intracellular cell signaling, and related transcriptional activity while the VDR is still localized in the cell periphery, hence, considered to be the non-genomic effects of vitamin D.[13]
Genomic actions of vitamin D - Vitamin D receptor
The vitamin D receptor (VDR) is a nuclear receptor that heterodimerizes with retinoid- X receptor, after binding with vitamin D.[14] The ligand-bound heterodimer then interacts with the vitamin D responsive element (VDRE) genes. After the interaction with the transcription factors, together with co-activators or co-repressors, the ligand-bound VDR-RXR complex either increases or decreases the transcription of a multitude of target genes.[12,15]
Non-genomic actions of vitamin D
Based on the cell type, vitamin D interacts with and activates ion channels (calcium, chloride), various intracellular signaling molecules such as phospholipase C (PLC), phospholipase A2 (PLA2), phosphatidylinositol-3 kinase (PI3K), and protein kinases such as MAP kinases among others.[13] These activated molecules further interact with transcription factors to regulate the expression of a variety of genes. Another non-genomic action of calcitriol (active form of vitamin D) is to influence the gene expression mediated by factors such as IFNα and TNFα by regulating VDR binding with signaling factors such as STAT1 and IKKβ that is critical in mediating the effects of IFNα and TNFα.[16]
Status of Vitamin D in Ocular Surface
Tear fluid vitamin D
Tear fluid contains higher levels of 25-OH-D3 than the serum in healthy human subjects.[17] The ocular surface including corneal and conjunctival epithelium is capable of metabolizing vitamin D. Since the serum concentrations of vitamin D are different from its tear levels, it is prudent to measure the tear fluid vitamin D levels, while assessing the association between vitamin D and ocular diseases.
Vitamin D receptor expression on ocular surface
As mentioned earlier, the ocular surface epithelial cells including corneal epithelium express VDR,[11] and vitamin D influences many cellular functions of these epithelial cells including the barrier function,[11,18] response to inflammation and infections.[19]
Vitamin D and Ocular Surface Physiology
Regulation of inflammation
Vitamin D is an established endogenous modulator of immune response and is known for its anti-inflammatory effects.[20] Calcitriol (1,25-(OH) 2D3) is shown to inhibit the hyperosmotic stress-induced cellular inflammation in human corneal epithelial cells (HCECs).[21] Vitamin D is also demonstrated to modify the toll-like receptor (TLR)-mediated inflammation and reduce the release of pro-inflammatory cytokines in HCECs.[19,22] Our group previously demonstrated that 1,25-(OH)2D3 in addition to the reduction of hyperosmotic stress-induced inflammatory gene expression, calcitriol (with genistein) was able to prevent hyperosmotic stress-induced VDR degradation in HCECs.[23] Topical application of 1,25-(OH)2D3 in animal models with dry eye reduced the corneal inflammation and improved clinical signs.[24] 1,25-(OH)2D3 complex inhibits maturation of dendritic cells (DC) and improves the tolerance of these cells by modulating the cytokine and chemokine production.[25] Serum vitamin D levels were shown to be inversely correlated to corneal dendritic cell density (DCD), potentiating the immunoregulatory role of this vitamin.[26]
Epithelial barrier function
1,25(OH)2D3 plays a favorable role in maintaining epithelial gap junction integrity. Lu et al.[18] demonstrated that 1,25(OH) 2D3 and 24R,25(OH) 2D3 enhanced gap junction connectivity in HCECs and cultured mouse primary epithelial cells. Activated VDR suppresses the activity of beta-catenin and epithelial cell proliferation.[27] 25(OH) D3- and 1,25(OH) 2D3-treated HCECs and rabbit corneal epithelial cells showed enhanced corneal epithelial barrier function.
Tear Fluid Homeostasis and Hyperosmolarity
Effect of Vitamin D on corneal epithelial cells under hyperosmotic stress
Tear hyperosmolarity plays a critical role in the pathogenesis of DED. The ocular surface of patients with DED showed increased expression of TonEBP (tonicity-responsive enhancer-binding protein, an osmoresponsive factor), inflammatory factors and reduced expression of VDR, suggestive of hyperosmotic stress-induced degradation of VDR, and increased inflammation. Our team has demonstrated that 1,25(OH)2D3 along with genistein reduced the TonEBP, inflammatory gene expression, and mitigated the VDR degradation.[23] Genistein is an isoflavone (present in soybeans) that has diverse biological activities including reduction of inflammation, ion channel modulation, and it also prevents stress-induced vitamin D receptor degradation. 1,25(OH)2D3 was shown to induce autophagy, suppress inflammation, reduce the oxidative stress, and protect HCECs from hyperosmotic stress-induced effects. Vitamin D supplementation through intramuscular injection reduced the tear film osmolarity in patients with VDD.[28]
Vitamin D and Pain Modulation
We have earlier showed that serum 25-OH-D3 levels were inversely correlated with the ocular surface disease index (OSDI) scores in patients with evaporative DED.[26] We have also reported that patients with DED showed lower tear fluid anti-nociceptive factors and low serum vitamin D.[29]
Vitamin D Deficiency – Hypovitaminosis D
The serum 25-OH-D3 levels are considered to assess vitamin D status since it is a longer lasting metabolite with higher levels than 1,25(OH)2D3. The threshold serum levels of 25-OH-D3 below which VDD to be considered have been changed over the years.[30,31] During earlier days of our knowledge on role vitamin D in the maintenance of bone health, presence or absence of rickets was considered as the sign of VDD.[30] The threshold levels of 25-OH-D3 to be considered as normal are derived based on the relationship between 25-OH-D3 and parathyroid hormone (PTH). PTH closely regulates the serum calcium and phosphorous levels. The serum levels of PTH are negatively correlated to that of 25-OH-D3 until the latter reaches the levels of 30-40 ng/milliliter (ml) (75–100 nano mol/liter). The PTH levels reach their lowest beyond these concentrations of the 25-OH-D3. Hence, the serum 25-OH-D3 concentrations of 30 ng/ml are considered as normal.[31,32] The serum levels of 21–29 ng/ml are considered as relative insufficiency, and those less than 20 ng/ml are considered as deficiency.[33]
Prevalence of vitamin D deficiency
The prevalence of VDD is reported to be different in countries with different levels of exposure to sunlight. The reported prevalence of VDD in India at community level was between 50 and 90%.[34] The high prevalence of VDD in healthy individuals is of concern.[35] Shukla et al.[35] reported the prevalence of VDD as 93% among healthy individuals who enrolled under preventive health checkup in an urban hospital. Healthy subjects of all age groups and both genders ranging from newborns, adolescents to post-menopausal women are reported to have high prevalence of VDD.[36-39] Similarly, multiple studies showed high prevalence of VDD among patients with various illnesses, including glucose intolerance, thyroid dysfunction, and chronic kidney disease.[40-42]
Etiology and risk factors of vitamin D deficiency
The etiology and risk factors of VDD are mentioned in Table 1. These etiologies can be categorized into those resulting in reduced bio-synthesis of vitamin D, reduced oral intake, bio-availability, impaired metabolism, and increased loss of vitamin D from the body. The vitamin D supplementation should be given more emphasis in the groups of individuals who are at higher risk of VDD including those with malabsorption syndromes, high rates of catabolism, parathyroid hormone anomalies, etc., [Table 2].
Table 1.
Vitamin D deficiency: Risk factors and etiopathogenetic mechanisms
| Pathophysiology | Etiology | Conditions |
|---|---|---|
| Reduced synthesis of vitamin D3 in skin | Reduced exposure to ultraviolet B (UV B) rays Increased absorption of UV B rays | Areas with low sun exposure[43] Sunscreen use[44,45] |
| Increased melanin pigment in the skin | ||
| Reduced oral intake and bio-availability of vitamin D from food sources | Low vitamin D3 in the foods Reduced bio-availability due to high amount of phytates and phosphates Malabsorption syndromes | Vegetarian food sources, unfortified milk High-fiber foods Cystic fibrosis, celiac disease |
| Impaired metabolism of vitamin D | Decreased synthesis of 25-OH-D3 | Liver disorders |
| Decreased synthesis of 1,25(OH) 2D3 | Chronic renal disorders | |
| Increased de-activation of 25-OH-D3 and 1,25(OH) 2D3 and conversion to calcitroic acid | Drug-induced HAART, glucocorticoids | |
| Increased loss of vitamin D | Increased urinary excretion of 25-OH-D3 | Nephrotic syndrome |
D3 - calcitriol; HAART - Highly active antiretroviral therapy
Table 2.
High-risk groups of individuals for vitamin D deficiency
| Celiac disease, malabsorption syndromes |
| Hypo and hyperparathyroidism |
| Chronic liver failure, chronic renal failure |
| Obese individuals, post-bariatric surgery |
| Pregnancy and lactating women |
Vitamin D deficiency and Ocular Surface Pathologies
Dry eye disease (DED)
Association between hypovitaminosis D and DED
Few questionnaire-based public health and nutrition surveys previously reported no significant association between serum 25-OH-D3 levels and the risk of DED.[46] The diagnosis of DED was made based on OSDI score, or a self-reported diagnosis of DED in the past. These surveys have limitations in diagnosing the subjects with DED based solely on the patient reported questionnaires without an evaluation of tear film status. A case–control study which tested the subjects’ Schirmer-1 test scores, tear breakup time, and OSDI scores, however, reported a significant association between serum 25-OH-D3 and DED incidence.[48] A recent meta-analysis showed that patients with VDD had higher mean OSDI scores and lower Schirmer’s test scores suggesting higher symptomatic disturbances and lower tear secretion.[49] Similarly, patients with DED had lower mean serum vitamin D levels than controls.[49]
Hypovitaminosis D and aqueous deficient DED
Vitamin D, being an endogenous immunoregulator, may have a protective role in the pathogenesis of Sjogren’s syndrome.[50] Serum 25-OH-D3 levels may be associated with severity of DED in patients with Sjogren’s syndrome.[47]
Hypovitaminosis D and evaporative dry eye
An inverse correlation was reported between serum 25-OH-D3 levels and OSDI scores in patients with evaporative DED, suggestive of a possible role of vitamin D in pain modulation and regulation of inflammation.[29] Patients with low serum vitamin D levels showed lower TBUT and Schirmer’s test scores than controls.[51]
Vitamin D and Keratoconus
Ectasia is a manifestation of dysregulated extracellular matrix remodeling (ECM). Even though initially considered as a non-inflammatory disease, tear fluid of patients with keratoconus has demonstrated elevated inflammatory mediators.[52-54] Multiple inflammatory cytokines, including IL-1β,[55] TNFα,[56] IL-6,[57,58] IL-17A,[59] IFNγ,[60] and MMPs,[58] that are capable of influencing ECM remodeling are elevated in the ocular surface tissues or tear fluid of keratoconus patients. Vitamin D, an established endogenous modulator of immune and inflammatory processes, positively regulates the ECM metabolism. Patients with keratoconus, particularly those with progressive disease, are shown to have lower serum levels of vitamin D than the normal population.[61-63] Our group has shown decreased expression of VDR in epithelium over the ectatic zones of cornea in keratoconus.[64] We further demonstrated that vitamin D addition helps induce VDR in epithelial cells under oxidative stress.[64] Clinically, Knapp in 1939 showed that vitamin D supplementation caused flattening of the cone in six patients with keratoconus.[65] Future studies should explore the benefits of vitamin D supplementation in treatment of keratoconus.
Vitamin D and Post-photorefractive Keratectomy (PRK) Haze
Corneal wound healing is an orchestrated response to any kind of injury that involves cytokine and growth factor-mediated interaction between epithelium, stroma, immune cells, and nerves.[66] Vitamin D has a complex integrated role on the corneal wound healing response. It is shown to delay the re-epithelization after acute injuries,[67,68] but such effect is shown to be concentration and the micro-environment dependent.[67] We have previously shown a significant association and higher risk of developing haze after PRK with VDD.[69] Further research should be able to throw light on the clinical applications of vitamin D in the management of post-refractive surgery deviant outcomes.
Clinical Relevance and Management
Vitamin D replacement therapy and dry eye syndrome
Patients with DED should be pro-actively screened for vitamin D status. Those with VDD should be treated as per the standard guidelines. The daily recommended dose of vitamin D supplementation depends on age of the individual, presence of risk factors for VDD, and the status of the vitamin D sufficiency. Table 3 shows the recommended daily intakes of vitamin D for healthy individuals of different ages and those at risk of deficiency. The Institute of Medicine (IOM), USA, and the US Endocrine Society (ES) gave slightly differing definitions of VDD, 20 ng is considered as deficiency as per IOM, whereas ES considers serum vitamin D less than 30 ng as deficiency. This dissimilarity in the considerations of deficiency explains the differences in the recommended dietary intakes between the two committees. The Indian Council of Medical Research (ICMR) suggests recommended daily intake of 400 IU/day.[70] But, this amount of intake may be inadequate due to changes in lifestyle and increased time spent indoors by the population. The upper tolerable limit of supplement dose (oral 4000 IU per day in healthy individuals) should also be kept under consideration to avoid the risk of toxicity.[33] The daily supplement dose in healthy individuals is lower than the doses recommended for the deficient individuals. This difference in the doses should be considered before suggesting the supplementation since high doses of supplementation in healthy individuals with normal serum levels of vitamin D can cause vitamin toxicity. Similarly, one should strictly adhere to the duration of high dose supplementation in vitamin D-deficient individuals, since prolonged high dose supplementation could lead to vitamin D toxicity and hypercalcemia.[71,72]
Table 3.
Treatment regimen for vitamin D deficiency and supplementation protocol
| Age | Balasubramanian et al.[73,74] | Holick et al.[33] | |||
|---|---|---|---|---|---|
|
|
|
||||
| Daily regimen (12 weeks) | Weekly regimen (6 weeks) | Daily regimen (6 weeks) | Weekly regimen (6 weeks) | Daily intake in healthy individuals | |
| 1-18 years | 3000-6,000IU | 60,000 IU | 2000-6000 IU | 50,000 IU | 400 IU |
| >18 years | 6000 IU | 60,000 IU | 6000 IU | 50,000 IU | 400-600 IU |
| Patients with obesity, malabsorption syndromes, or on medications affecting vitamin D metabolism (>18 years) | 600-10,000 IU | 6000-10,000 IU | 600-2000 IU | ||
IU - International Units
Route of administration
Oral formulations are shown to rapidly increase the serum 25(OH)D and 1,25 – (OH)2D3 levels than similar doses of intramuscular injection.[75,76] The delayed bio-availability of intramuscular route could be due to the oily nature of the depot injection and the deposition at the injection site.[77] Guidelines addressing prevention and treatment of rickets and VDD preferred oral route to intramuscular injection.[78] Nevertheless, intramuscular injections are the treatment of choice in patients with malabsorption syndromes affecting alimentary tract and hepato-biliary system, including patients post-bariatric surgery.[79] In addition, intramuscular injections result in more sustained long-term increase in serum levels of vitamin D.[76] Hence, we believe that the vitamin D supplementation through intramuscular route in individuals with severe vitamin D deficiency (serum levels <10 ng/ml) leads to more sustained improvement in the serum vitamin D levels, with beneficial effects on ocular surface VDR expression and dampening of ocular surface inflammation in patients with DED and keratoconus.[23,64]
Dosage and duration of administration
Daily, weekly, or large single-dosage (Stoss therapy) schedules can be followed for the treatment of VDD. Weekly semi-large doses for a duration of 8–12 weeks are the most commonly suggested dosing schedule.[33,73,78] The efficacy, tolerability, and safety of single megadose (of variable proportions) have been established through multiple studies, and such dosing practice should be an informed, individualized decision by the physician and the patient.[80,81] The two available forms of vitamin D supplements, ergocalciferol (D2) and cholecalciferol (D3), are equally effective with daily dosing, but cholecalciferol is preferable in weekly or single megadosing schedules.[78]
Vitamin D Toxicity
Although rare, vitamin D toxicity presents with severe, dramatic cases of life-threatening symptoms. There is a “U”-shaped biological response to the serum levels of vitamin D with deleterious effects at very low and high serum concentrations, including mortality, cardiovascular effects, and certain cancers.[71] Institute of Medicine (IOM) advises caution against maintaining serum vitamin D levels more than 50 ng/ml.[82] Stoss therapy, which involves single large bolus doses oral or intramuscular vitamin D, has higher risk of resulting in dangerously high serum levels of vitamin D and symptoms of toxicity, than the weekly or daily supplementation regimen.[83,84] The clinicians treating VDD should be well aware of the dosing regimen and risks of toxicity of vitamin D supplementation. The maximum doses for daily, weekly, and Stoss therapy are 6000IU, 60,000 IU, and 6,00,000 IU, respectively.[33,73]
Approach to a patient with DED [Fig. 1]
Figure 1.
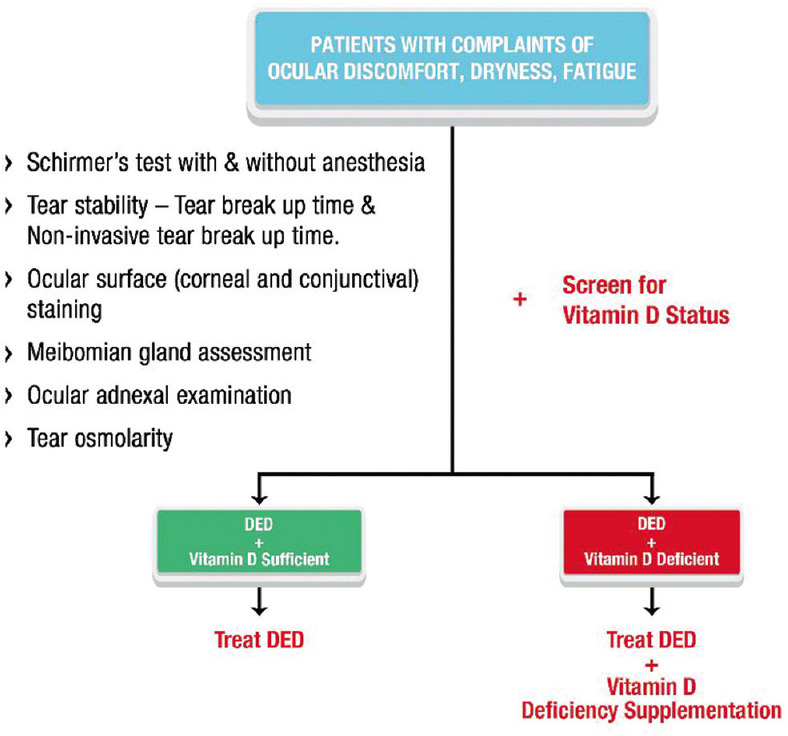
Algorithmic approach to patients with DED. The figure depicts the approach to manage patients with DED and vitamin D deficiency
Any patient with complaints of ocular discomfort, itching, dryness, and fatigue should be evaluated for possible DED, with the following clinical tests.
Schirmer’s test with and without anesthesia
Tear stability – Tear breakup time and non-invasive tear breakup time.
Ocular surface (corneal and conjunctival) staining
Meibomian gland assessment
Ocular adnexal examination
Tear osmolarity (if available).
Along with the ocular examination, patients should be screened for VDD. Based on the tear film metrics, if aqueous-deficient DED is suspected, they should be screened for autoimmune etiology. The mode and dose of vitamin D supplementation depend on the status of the serum vitamin D. Table 2 shows the suggested vitamin D supplementation strategy for patients with DED and VDD. The algorithmic approach is explained in Figs. 1 and 3.
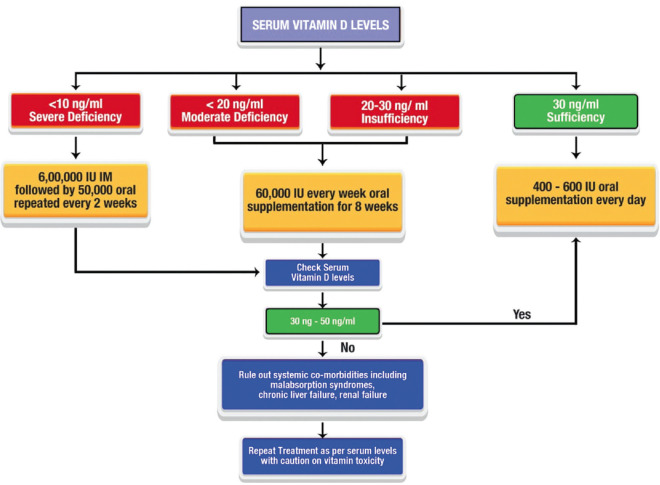
Figure 3.
Approach to management of vitamin D deficiency. Guidelines to treat vitamin D deficiency in adults with DED
Considerations in prerefractive surgery patients [Fig 2]
Figure 2.
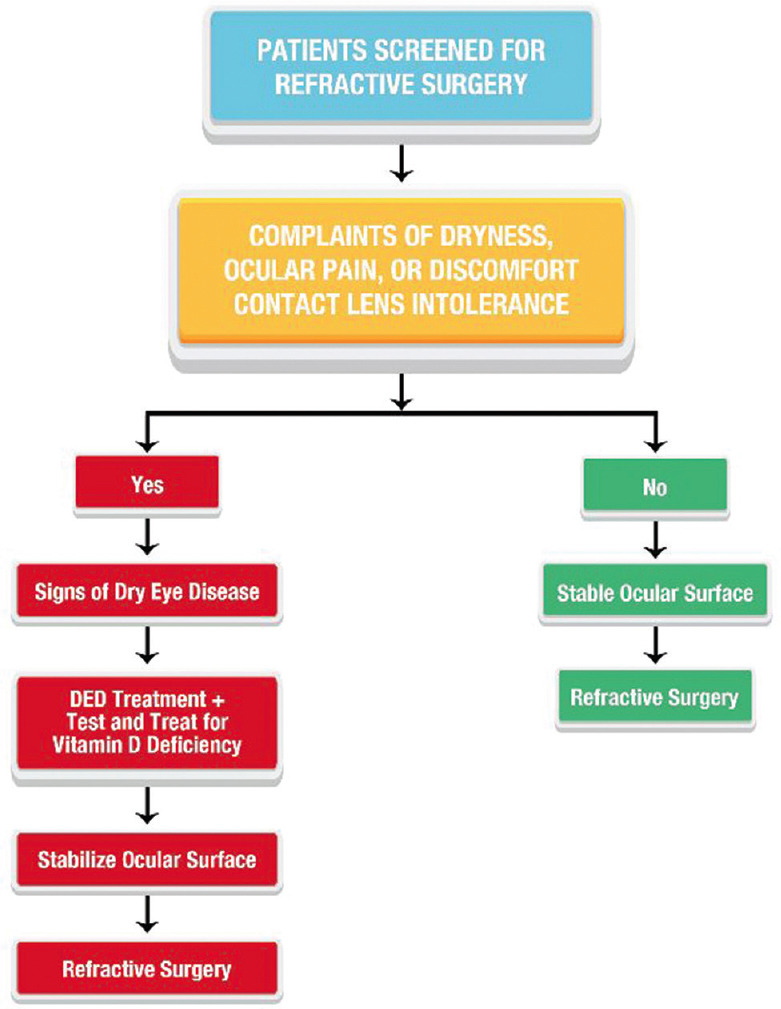
Preoperative management of patients undergoing refractive surgery. Guideline to preoperative management of patients undergoing refractive surgery to ensure ideal post-surgical outcomes
An optimal ocular surface health should be ensured before proceeding with refractive surgery to achieve optimal post-surgical outcomes. If these patients show signs of DED, higher ocular surface inflammation, or meibomian gland dysfunction (MGD), they should be treated for the underlying pathology and screened for VDD. Ocular surface inflammation and tear fluid pro-nociceptive factors are shown to increase in individuals with low serum vitamin D levels. The sub-clinical ocular surface inflammation even without overt DED in individuals with VDD could potentially increase the risk of post-refractive surgery complications like regression, post-photorefractive keratectomy haze, pain, etc., Hence, these patients should undergo refractive surgery after ensuring vitamin D sufficiency and ocular surface stabilization.
Our Clinical Experience with the Treatment of DED with Vitamin D Supplementation
At our dry eye clinic (at a tertiary referral center for DED), 423 patients with “pain without stain,” those with symptoms of DED but no corroborative clinical signs, were evaluated. Among these, 345 patients had high OSDI scores at presentation, and 296 of them had serum vitamin D levels lower than 20ng/ml. They were treated with vitamin D supplementation as per the algorithm mentioned in Fig. 3 along with topical lubricants (sodium hyaluronate 0.1%). The OSDI scores significantly improved in 275 patients, three months following treatment with vitamin D supplements. In addition to these cohort-based analyses, anecdotally we observed improvement in Schirmer and TBUT scores in patients with both aqueous deficiency and evaporative DED. This improvement could be due the augmentation of endogenous immuno-inflammatory dampening process by vitamin D, as these immune-inflammatory factors are critical contributors to the pathogenic mechanisms that result in reduction in tear secretion, tear film stability, and threshold for pain.
Conclusion
To conclude, VDD is one of the significant contributors to the pathogenesis of DED. Following these guidelines, and effectively managing VDD in individuals with DED, will prevent worsening of DED and post-operative complications. Ensuring vitamin D sufficiency in patients with ocular ailments is a clinically wise prophylactic strategy as we are augmenting human body’s own defense and disease resolution mechanism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This work was supported by Narayana Nethralaya Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this abstract.
References
- 1.McCollum EV, Simmonds N, Becker JE, Shipley PG. Studies on experimental rickets. J Biol Chem. 1922;53:293–312. [PubMed] [Google Scholar]
- 2.Lamberg-Allardt C. Vitamin D in foods and as supplements. Prog Biophys Mol Biol. 2006;92:33–8. doi: 10.1016/j.pbiomolbio.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Raulio S, Erlund I, Männistö S, Sarlio-Lähteenkorva S, Sundvall J, Tapanainen H, et al. Successful nutrition policy:Improvement of vitamin D intake and status in Finnish adults over the last decade. Eur J Public Health. 2017;27:268–73. doi: 10.1093/eurpub/ckw154. [DOI] [PubMed] [Google Scholar]
- 4.Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of cytochrome P450 2R1:A microsomal vitamin D 25-hydroxylase *. J Biol Chem. 2003;278:38084–93. doi: 10.1074/jbc.M307028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunette MG, Chan M, Ferriere C, Roberts KD. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978;276:287–9. doi: 10.1038/276287a0. [DOI] [PubMed] [Google Scholar]
- 6.DeLuca HF. Vitamin D:The vitamin and the hormone. Fed Proc. 1974;33:2211–9. [PubMed] [Google Scholar]
- 7.Flanagan JN, Young MV, Persons KS, Wang L, Mathieu JS, Whitlatch LW, et al. Vitamin D metabolism in human prostate cells:Implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006;26:2567–72. [PubMed] [Google Scholar]
- 8.Larriba MJ, Ordóñez-Morán P, Chicote I, Martín-Fernández G, Puig I, Muñoz A, et al. Vitamin D receptor deficiency enhances Wnt/β-Catenin signaling and tumor burden in colon cancer. PLoS One. 2011;6:e23524. doi: 10.1371/journal.pone.0023524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form:Potential effects on host defense. J Immunol. 2008;181:7090–9. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsalem JA, Patel D, Susarla R, Coca-Prados M, Bland R, Walker EA, et al. Characterization of Vitamin D production by human ocular barrier cells. Investig Opthalmol Vis Sci. 2014;55:2140. doi: 10.1167/iovs.13-13019. [DOI] [PubMed] [Google Scholar]
- 11.Yin Z, Pintea V, Lin Y, Hammock BD, Watsky MA. Vitamin D enhances corneal epithelial barrier function. Investig Opthalmol Vis Sci. 2011;52:7359. doi: 10.1167/iovs.11-7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, Shevde NK, Pike JW. 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res. 2004;20:305–17. doi: 10.1359/JBMR.041112. [DOI] [PubMed] [Google Scholar]
- 13.Hii C, Ferrante A. The non-genomic actions of vitamin D. Nutrients. 2016;8:135. doi: 10.3390/nu8030135. doi:10.3390/nu8030135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demay MB. Mechanism of vitamin D receptor action. Ann N Y Acad Sci. 2006;1068:204–13. doi: 10.1196/annals.1346.026. [DOI] [PubMed] [Google Scholar]
- 15.Hirota Y, Suhara Y, Osakabe N, Sakaki T, Okano T. 25-hydroxyvitamin D3 may function via genomic and non-genomic actions. Anat Physiol. 2017;7 doi: 10.4172/2161-0940.1000278. [Google Scholar]
- 16.Lange CM, Gouttenoire J, Duong FHT, Morikawa K, Heim MH, Moradpour D. Vitamin D receptor and Jak-STAT signaling crosstalk results in calcitriol-mediated increase of hepatocellular response to IFN-α. J Immunol. 2014;192:6037–44. doi: 10.4049/jimmunol.1302296. [DOI] [PubMed] [Google Scholar]
- 17.Sethu S, Shetty R, Deshpande K, Pahuja N, Chinnappaiah N, Agarwal A, et al. Correlation between tear fluid and serum vitamin D levels. Eye Vis Lond Engl. 2016;3:22. doi: 10.1186/s40662-016-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X, Chen Z, Vick S, Watsky MA. Vitamin D receptor and metabolite effects on corneal epithelial cell gap junction proteins. Exp Eye Res. 2019;187:107776. doi: 10.1016/j.exer.2019.107776. doi:10.1016/j.exer.2019.107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reins RY, Baidouri H, McDermott AM. Vitamin D activation and function in human corneal epithelial cells during TLR-induced inflammation. Investig Opthalmol Vis Sci. 2015;56:7715–27. doi: 10.1167/iovs.15-17768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55:96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y, Zhang J, Xiang J, Li Y, Wu D, Xu J. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019;21:101093. doi: 10.1016/j.redox.2018.101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reins RY, Mesmar F, Williams C, McDermott AM. Vitamin D induces global gene transcription in human corneal epithelial cells:Implications for corneal inflammation. Investig Opthalmol Vis Sci. 2016;57:2689–98. doi: 10.1167/iovs.16-19237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panigrahi T, D'Souza S, Shetty R, Padmanabhan Nair A, Ghosh A, Jacob Remington Nelson E, et al. Genistein-calcitriol mitigates hyperosmotic stress-induced TonEBP, CFTR dysfunction, VDR degradation and inflammation in dry eye disease. Clin Transl Sci. 2021;14:288–98. doi: 10.1111/cts.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Dai Y, Wu D, Xu J. Calcitriol, the active metabolite of vitamin D3, inhibits dry eye related corneal inflammation in vivo and in vitro. Ocul Immunol Inflamm. 2019;27:257–65. doi: 10.1080/09273948.2017.1372486. [DOI] [PubMed] [Google Scholar]
- 25.Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7:8127–51. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shetty R, Sethu S, Deshmukh R, Deshpande K, Ghosh A, Agrawal A, et al. Corneal dendritic cell density is associated with subbasal nerve plexus features, ocular surface disease index, and serum vitamin D in evaporative dry eye disease. BioMed Res Int. 2016;2016:4369750. doi: 10.1155/2016/4369750. doi:10.1155/2016/4369750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang YG, Wu S, Sun J. Vitamin D, vitamin D receptor and tissue barriers. Tissue Barriers. 2013;1:e23118. doi: 10.4161/tisb.23118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kizilgul M, Kan S, Ozcelik O, Beysel S, Apaydin M, Ucan B, et al. Vitamin D replacement improves tear osmolarity in patients with vitamin D deficiency. Semin Ophthalmol. 2018;33:589–94. doi: 10.1080/08820538.2017.1358752. [DOI] [PubMed] [Google Scholar]
- 29.Khamar P, Nair AP, Shetty R, Vaidya T, Subramani M, Ponnalagu M, et al. Dysregulated tear fluid nociception-associated factors, corneal dendritic cell density, and vitamin D levels in evaporative dry eye. Investig Opthalmol Vis Sci. 2019;60:2532–42. doi: 10.1167/iovs.19-26914. [DOI] [PubMed] [Google Scholar]
- 30.Hewison M. An update on vitamin D and human immunity:An update on vitamin D. Clin Endocrinol (Oxf) 2012;76:315–25. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 32.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency:An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 34.Aparna P, Muthathal S, Nongkynrih B, Gupta SK. Vitamin D deficiency in India. J Fam Med Prim Care. 2018;7:324–30. doi: 10.4103/jfmpc.jfmpc_78_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla K, Sharma S, Gupta A, Raizada A, Vinayak K. Current scenario of prevalence of vitamin D deficiency in ostensibly healthy Indian population:A hospital based retrospective study. Indian J Clin Biochem. 2016;31:452–7. doi: 10.1007/s12291-016-0552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar RK, Das H, Girish SV, Nevilebasappa A. Prevalence of vitamin D deficiency among newborns. Indian Pediatr. 2020;57:258–9. [PubMed] [Google Scholar]
- 37.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of Vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 38.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–7. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 39.Sahu M, Bhatia V, Aggarwal A, Rawat V, Saxena P, Pandey A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in northern India. Clin Endocrinol (Oxf) 2009;70:680–4. doi: 10.1111/j.1365-2265.2008.03360.x. [DOI] [PubMed] [Google Scholar]
- 40.Jabbar Z, Aggarwal PK, Chandel N, Kohli HS, Gupta KL, Sakhuja V, et al. High prevalence of vitamin D deficiency in north Indian adults is exacerbated in those with chronic kidney disease. Nephrology. 2009;14:345–9. doi: 10.1111/j.1440-1797.2008.01082.x. [DOI] [PubMed] [Google Scholar]
- 41.Modi KD, Ahmed MI, Chandwani R, Kumar KVSH. Prevalence of vitamin D deficiency across the spectrum of glucose intolerance. J Diabetes Metab Disord. 2015;14:54. doi: 10.1186/s40200-015-0179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goswami R, Marwaha RK, Gupta N, Tandon N, Sreenivas V, Tomar N, et al. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians:A community-based survey. Br J Nutr. 2009;102:382–6. doi: 10.1017/S0007114509220824. [DOI] [PubMed] [Google Scholar]
- 43.Ladizesky M, Lu Z, Oliveri B, Roman NS, Diaz S, Holick MF, et al. Solar ultraviolet B radiation and photoproduction of vitamin D3 in central and southern areas of argentina. J Bone Miner Res. 2009;10:545–9. doi: 10.1002/jbmr.5650100406. [DOI] [PubMed] [Google Scholar]
- 44.Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol. 2009;161:732–6. doi: 10.1111/j.1365-2133.2009.09332.x. [DOI] [PubMed] [Google Scholar]
- 45.Neale RE, Khan SR, Lucas RM, Waterhouse M, Whiteman DC, Olsen CM. The effect of sunscreen on vitamin D:A review. Br J Dermatol. 2019;181:907–15. doi: 10.1111/bjd.17980. [DOI] [PubMed] [Google Scholar]
- 46.Jeon DH, Yeom H, Yang J, Song JS, Lee HK, Kim HC. Are serum vitamin D levels associated with dry eye disease?Results from the study group for environmental eye disease. J Prev Med Pub Health. 2017;50:369–76. doi: 10.3961/jpmph.17.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JH, Kim SJ, Byun YS, Lee J, Park SH, Chung SH. The association of serum vitamin D level with the severity of dry eye parameters in primary Sjögren syndrome. Cornea. 2020;39:702–5. doi: 10.1097/ICO.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 48.Meng YF, Lu J, Xing Q, Tao JJ, Xiao P. Lower serum vitamin D level was associated with risk of dry eye syndrome. Med Sci Monit. 2017;23:2211–6. doi: 10.12659/MSM.901857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Dong Y, Wang Y. Vitamin D deficiency is associated with dry eye syndrome:A systematic review and meta-analysis. Acta Ophthalmol (Copenh) 2020;98:749–54. doi: 10.1111/aos.14470. [DOI] [PubMed] [Google Scholar]
- 50.Baldini C, Delle Sedie A, Luciano N, Pepe P, Ferro F, Talarico R, et al. Vitamin D in “early”primary Sjögren's syndrome:Does it play a role in influencing disease phenotypes?Rheumatol Int. 2014;34:1159–64. doi: 10.1007/s00296-013-2872-3. [DOI] [PubMed] [Google Scholar]
- 51.Kurtul BE, Özer PA, Aydinli MS. The association of vitamin D deficiency with tear break-up time and Schirmer testing in non-Sjögren dry eye. Eye. 2015;29:1081–4. doi: 10.1038/eye.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shetty R, D'Souza S, Khamar P, Ghosh A, Nuijts RMMA, Sethu S. Biochemical markers and alterations in keratoconus. Asia-Pac J Ophthalmol (Phila) 2020;9:533–40. doi: 10.1097/APO.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 53.Loh IP, Sherwin T. Is keratoconus an inflammatory disease?The implication of inflammatory pathways. Ocul Immunol Inflamm. 2022;30:246–55. doi: 10.1080/09273948.2020.1780271. [DOI] [PubMed] [Google Scholar]
- 54.D'Souza S, Nair AP, Sahu GR, Vaidya T, Shetty R, Khamar P, et al. Keratoconus patients exhibit a distinct ocular surface immune cell and inflammatory profile. Sci Rep. 2021;11:20891. doi: 10.1038/s41598-021-99805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shetty R, Deshmukh R, Ghosh A, Sethu S, Jayadev C. Altered tear inflammatory profile in Indian keratoconus patients-The 2015 Col Rangachari Award paper. Indian J Ophthalmol. 2017;65:1105–8. doi: 10.4103/ijo.IJO_233_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ionescu IC, Corbu CG, Tanase C, Ionita G, Nicula C, Coviltir V, et al. Overexpression of tear inflammatory cytokines as additional finding in keratoconus patients and their first degree family members. Mediators Inflamm. 2018;2018:1–9. doi: 10.1155/2018/4285268. doi:10.1155/2018/4285268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shetty R, Ghosh A, Lim RR, Subramani M, Mihir K, Reshma AR, et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci. 2015;56:738–50. doi: 10.1167/iovs.14-14831. [DOI] [PubMed] [Google Scholar]
- 58.Balasubramanian SA, Mohan S, Pye DC, Willcox MDP. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol (Copenh) 2012;90:e303–9. doi: 10.1111/j.1755-3768.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 59.Jun AS, Cope L, Speck C, Feng X, Lee S, Meng H, et al. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One. 2011;6:e16437. doi: 10.1371/journal.pone.0016437. doi:10.1371/journal.pone. 0016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fodor M, Vitályos G, Losonczy G, Hassan Z, Pásztor D, Gogolák P, et al. Tear mediators NGF along with IL-13 predict keratoconus progression. Ocul Immunol Inflamm. 2021;29:1090–101. doi: 10.1080/09273948.2020.1716024. [DOI] [PubMed] [Google Scholar]
- 61.Akkaya S, Ulusoy DM. Serum vitamin D levels in patients with keratoconus. Ocul Immunol Inflamm. 2020;28:348–53. doi: 10.1080/09273948.2019.1604002. [DOI] [PubMed] [Google Scholar]
- 62.Aslan MG, Fındık H, Okutucu M, Aydın E, Oruç Y, Arpa M, et al. Serum 25-hydroxy vitamin D, vitamin B12, and folic acid levels in progressive and nonprogressive keratoconus. Cornea. 2021;40:334–41. doi: 10.1097/ICO.0000000000002475. [DOI] [PubMed] [Google Scholar]
- 63.Gupta P, Pathak M, Thakur B, Fogla R, Agarwal A, Ram J. Association of keratoconus with serum levels of 25-hydroxyvitamin D and antioxidant trace elements:A systematic review and meta-analysis. Indian J Ophthalmol. 2022;70:2818–24. doi: 10.4103/ijo.IJO_3216_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shivakumar S, S R, Ghosh A, Jeyabalan N. Vitamin D enhances the autophagic lysosomal clearance in oxidatively stressed human corneal epithelial cells:A therapeutic intervention for keratoconus. Invest Ophthalmol Vis Sci. 2019;60:2819. [Google Scholar]
- 65.Knapp AA. Results of vitamin-D-complex treatment of keratoconus:Preliminary?study. Am J Ophthalmol. 1939;22:289–92. [Google Scholar]
- 66.Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Hong J, Lee J. The corneal wound healing response. Prog Retin Eye Res. 2001;20:625–37. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 67.Reins RY, Hanlon SD, Magadi S, McDermott AM. Effects of topically applied vitamin D during corneal wound healing. PLoS One. 2016;11:e0152889. doi: 10.1371/journal.pone.0152889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sel S, Trau S, Paulsen F, Kalinski T, Stangl GI, Nass N. 1,25-dihydroxyvitamin D3 inhibits corneal wound healing in an ex-vivo mouse model. Graefes Arch Clin Exp Ophthalmol. 2016;254:717–24. doi: 10.1007/s00417-016-3267-4. [DOI] [PubMed] [Google Scholar]
- 69.Kundu G, D'Souza S, Lalgudi VG, Arora V, Chhabra A, Deshpande K, et al. Photorefractive keratectomy (PRK) Prediction, Examination, tReatment, Follow-up, Evaluation, Chronic Treatment (PERFECT) protocol-A new algorithmic approach for managing post PRK haze. Indian J Ophthalmol. 2020;68:2950–5. doi: 10.4103/ijo.IJO_2623_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nutrient Requirements and Recommended Dietary Allowances for Indians. Jamai-Osmania PO, Hyderabad: National Institute of Nutrition, Indian Council of Medical Research; 2009. A Report of the Expert Group of the Indian Council of Medical Research. [Google Scholar]
- 71.Vogiatzi MG, Jacobson-Dickman E, DeBoer MD. for the Drugs, and Therapeutics Committee of the Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: A review of current literature. J Clin Endocrinol Metab. 2014;99:1132–41. doi: 10.1210/jc.2013-3655. [DOI] [PubMed] [Google Scholar]
- 72.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–56. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 73.Khadilkar A, Khadilkar V, Chinnappa J, Rathi N, Khadgawat R, Balasubramanian S, et al. Prevention and treatment of vitamin D and calcium deficiency in children and adolescents:Indian Academy of Pediatrics (IAP) guidelines. Indian Pediatr. 2017;54:567–73. doi: 10.1007/s13312-017-1070-x. [DOI] [PubMed] [Google Scholar]
- 74.Balasubramanian S, Dhanalakshmi K, Amperayani S. Vitamin D deficiency in childhood—A review of current guidelines on diagnosis and management. Indian Pediatr. 2013;50:669–75. doi: 10.1007/s13312-013-0200-3. [DOI] [PubMed] [Google Scholar]
- 75.Zabihiyeganeh M, Jahed A, Nojomi M. Treatment of hypovitaminosis D with pharmacologic doses of cholecalciferol, oral vs intramuscular;an open labeled RCT. Clin Endocrinol (Oxf) 2013;78:210–6. doi: 10.1111/j.1365-2265.2012.04518.x. [DOI] [PubMed] [Google Scholar]
- 76.Cipriani C, Romagnoli E, Pepe J, Russo S, Carlucci L, Piemonte S, et al. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol:Implications for treatment and prophylaxis. J Clin Endocrinol Metab. 2013;98:2709–15. doi: 10.1210/jc.2013-1586. [DOI] [PubMed] [Google Scholar]
- 77.Whyte MP, Haddad JG, Walters DD, Stamp TCB. Vitamin D bioavailability:Serum 25-hydroxyvitamin D levels in man after oral, subcutaneous, intramuscular, and intravenous vitamin D administration. J Clin Endocrinol Metab. 1979;48:906–11. doi: 10.1210/jcem-48-6-906. [DOI] [PubMed] [Google Scholar]
- 78.Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. Horm Res Paediatr. 2016;85:83–106. doi: 10.1159/000443136. [DOI] [PubMed] [Google Scholar]
- 79.Einarsdóttir K, Preen DB, Clay TD, Kiely L, Holman CDJ, Cohen LD. Effect of a single 'megadose'intramuscular vitamin D (600,000 IU) injection on vitamin D concentrations and bone mineral density following biliopancreatic diversion surgery. Obes Surg. 2010;20:732–7. doi: 10.1007/s11695-009-0024-3. [DOI] [PubMed] [Google Scholar]
- 80.Leventis P, Kiely PDW. The tolerability and biochemical effects of high-dose bolus vitamin D2 and D3 supplementation in patients with vitamin D insufficiency. Scand J Rheumatol. 2009;38:149–53. doi: 10.1080/03009740802419081. [DOI] [PubMed] [Google Scholar]
- 81.Diamond TH, Ho KW, Rohl PG, Meerkin M. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency:Efficacy and safety data. Med J Aust. 2005;183:10–2. doi: 10.5694/j.1326-5377.2005.tb06879.x. [DOI] [PubMed] [Google Scholar]
- 82.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine:What clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–8. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Markestad T, Hesse V, Siebenhuner M, Jahreis G, Aksnes L, Plenert W, et al. Intermittent high-dose vitamin D prophylaxis during infancy:Effect on vitamin D metabolites, calcium, and phosphorus. Am J Clin Nutr. 1987;46:652–8. doi: 10.1093/ajcn/46.4.652. [DOI] [PubMed] [Google Scholar]
- 84.Cesur Y, Çaksen H, Gündem A, Kirimi E, Odabaş D. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. J Pediatr Endocrinol Metab. 2003;16:1105–9. doi: 10.1515/jpem.2003.16.8.1105. [DOI] [PubMed] [Google Scholar]