Abstract
Purpose:
This study aims to investigate the effects of maqui-berry extract (MBE) in improving signs and symptoms of dry eye disease (DED) along with ocular surface inflammation in patients with DED.
Methods:
Twenty patients were randomly assigned to a MBE or a placebo group (PLC). DED parameters including Schirmer’s test 1 (ST1), tear film break-up time (TBUT), ocular surface disease index (OSDI), and corneal staining were assessed before treatment and 2 months post-treatment. Tear fluid samples before and after treatment from a subset of these patients were collected from the study subjects using sterile Schirmer’s strips, and the levels of interleukin (IL)-1β, IL-10, IL-6, IL-17A, tumor necrosis factor-α (TNFα), matrix metalloproteinase-9 (MMP9), soluble intercellular adhesion molecule-1 (sICAM1), and vascular endothelial growth factor-A (VEGF-A) were measured using a microfluidic cartridge-based multiplex ELISA.
Results:
The MBE group demonstrated a significant (p < 0.05) decrease in OSDI scores along with a significant increase in Schirmer’s test 1 compared to the PLC group. No significant change in TBUT and corneal staining was observed between the study groups. Levels of proinflammatory factors such as IL-1β, IL-6, IL-17A, TNFα, and MMP9 were observed to be significantly reduced, along with a significant increase in IL-10 levels following treatment in the MBE group compared with the PLC group.
Conclusion:
Consumption of MBE resulted in the resolution of DED signs and symptoms, along with a reduction in ocular surface inflammation.
Keywords: Cytokines, delphinidin, dry eye disease, inflammation, Maqui berry, tear fluid
Dry eye disease (DED) is a multifactorial condition with a complicated etiopathogenesis and is often broadly divided into insufficient tear production and excessive tear evaporation and with both.[1] Irrespective of the underlying causative conditions, DED is associated with ocular surface inflammation.[1] The role of conventional pharmacological treatment which includes lubricating topical formulations and immunomodulators is well known; however, there may be other mechanisms to reduce ocular surface inflammation.[2] Some patients do not respond well to currently available therapies, and hence newer strategies do become necessary. Maqui berry (Aristotelia chilensis) which is grown in the Patagonia region of Chile is rich in anthocyanins and has been used traditionally to treat inflammation in various parts of the body.[3,4] Delphinidin-3,5-O-diglucoside is a specific compound in maqui berries.[3,4] Anthocyanins in maqui berry are delphinidin derivatives with antioxidant activities and, thus, anti-inflammatory in nature.[3,4] The possible anti-inflammatory effect could also be explained due to the inhibitory effect of delphinol on NFkB activation, which is responsible for the production of proinflammatory factors.[5]
An animal study demonstrated that the consumption of maqui berry inhibited the decline in lacrimal fluid production and prevented damages to corneal and lacrimal gland tissues in a DED animal model.[6] Additionally, it has also been demonstrated to increase tear production, thereby improving overall DED-related symptoms in a previous human pilot study.[7] Owing to the lack of a placebo group in the pilot trial, the evidence related to the beneficial effects of maqui berry on tear production and alleviation of DED signs and symptoms remains insufficient.
Hence, this study was conducted to investigate the effects of maqui-berry extract (MBE) on DED clinical parameters and ocular surface inflammation in patients with DED.
Methods
Study cohort and design
The study was a prospective interventional study which was undertaken in a tertiary care eye hospital in India, between July 2022 and September 2022. The study was approved by the institutional ethics committee. The study followed the tenets of the Declaration of Helsinki. The selection criteria of this study were as follows: (a) patients aged 30–50 years; (b) presence of eye dryness symptoms; with ocular surface disease index (OSDI) >18, tear film break-up time (TBUT) ≤10 but ≥5 s, with Schirmer’s test 1 (ST1) value ≤10 mm/5 min but ≥5 mm/5 min. The exclusion criteria included patients diagnosed with severe dry eye syndrome with Schirmer 1 <5/5 min, taking any medication for DED (provided that a patient has not taken the medication for 4 weeks before the study and does not take the medication during the study period), current eye disease or a history of eye disease, currently using eye drops for the treatment of any eye diseases, previous corneal surgeries, presence of Sjögren’s syndrome, allergy, or chronic asthma, allergies to medications and/or products related to the study substance; pregnancy, lactation. Prohibited medications during the study were topical cyclosporine or any other ophthalmic medication, including artificial tears, antihistamines, corticosteroids, or mast cell stabilizers.
Of the 20 patients recruited, 12 patients (24 eyes) were randomized into the group which received MBE in the form of tablets (Maqvue™-Lavue pharmaceuticals), and 8 patients (16 eyes) were randomized into the placebo group (PLC). The mean age in the MBE group was 35 ± 3.4 years with 67% males and 33% females. The mean age in the PLC group was 34 ± 4.2 years with 63% males and 37% females.
The supplements given were indistinguishable brown capsules containing MBE or placebo. The capsules were approved as identical to each other in color, odor, and flavor, although active capsules contained 120 mg of dextrin and 30 mg of MBE in powder containing up to 10.5 mg of total anthocyanins, up to 7.5 mg of total delphinidins, and delphinidin-3,5-O-diglucoside, while placebo capsules were inert gelatin capsules which contained 180 mg of dextrin and did not induce inflammation. All patients were administrated one capsule twice per day either with MBE or placebo before food in the morning and night for 4 weeks followed by one capsule once daily in the morning before food for 4 weeks. The study follow-up was divided into pretreatment initiation and 2 months postinitiation of treatment.
Clinical parameters
Ocular surface and dry eye evaluation include the assessment of symptoms using the OSDI questionnaire (Allergan, Dublin, Ireland)[8] Schirmer’s test without anesthesia (ST1), TBUT, and corneal fluorescein staining. Schirmer’s test 1 was performed using sterile Schirmer’s strips (Contacare Ophthalmics and Diagnostics, Vadodara, Gujarat, India). TBUT and ocular surface staining were performed using fluorescein strips (Contacare Ophthalmics and Diagnostics). Objective and subjective measures were done at baseline visit, and 2 months post initiation of treatment. Objective assessments also included Schirmer’s test without anesthesia (ST1), TBUT, corneal fluorescein score. Subjective assessments included the OSDI questionnaire to measure ocular discomfort.[8,9]
In addition, tear fluid samples were collected from a subset of patients which included 12 eyes of the MBE group and 8 eyes of the PLC group at baseline and 2 months to measure the concentration of tear-soluble factors.
Tear fluid sample collection
Tear fluid samples before and after treatment were collected from the study subjects using sterile Schirmer’s strips (Contacare Ophthalmics and Diagnostics, Vadodara, Gujarat, India) by following Schirmer’s test 1 protocol. Following the wetting of the Schirmer’s strip by the tear fluid, the wetting length was noted and the Schirmer’s strip was then stored in a sterile microcentrifuge tube at − 80°C until further processing.
Tear soluble factor measurements in the clinics
The levels of interleukin (IL)-1b, IL-10, IL-6, IL-17A, tumor necrosis factor-α (TNFα), matrix metalloproteinase 9 (MMP9), soluble intercellular adhesion molecule 1 (sICAM1), and vascular endothelial growth factor-A (VEGF-A) were measured in the tear fluid samples collected using a microfluidic cartridge-based multiplex ELISA kit (Bio-M Pathfinder, NovoMol-Dx, India, a customized version of the Ella™ Automated ELISA system, Bio-Techne® Corporation, Minnesota, USA). Briefly, 300 ml of extraction buffer was added to the microcentrifuge tube containing the Schirmer’s strip and was agitated for 5 min at room temperature. Subsequently, 50 ml of the eluted tear sample was added to the sample well, and 1 ml of wash buffer was put into the buffer well in the kit’s cartridge. The cartridge was then placed into the device, and a run was initiated. At the end of the run, the absolute concentration of the specific factors was determined based on the standard curve which was built into the cartridge. The Schirmer’s strip wetting length for the respective sample and extraction buffer volume was used to adjust the absolute concentration to determine to final concentration for the specific factors for every sample.
Statistical analysis
The normal distribution of the data was determined by the Shapiro-Wilk normality test. The statistical significance between matched data of pre-treatment and post-treatment parameters was analyzed by the Wilcoxon matched-pairs signed rank test. The statistical significance between the treatment groups for the various parameters was analysed by Mann Whitney test. P value <0.05 was considered to be statistically significant.
Results
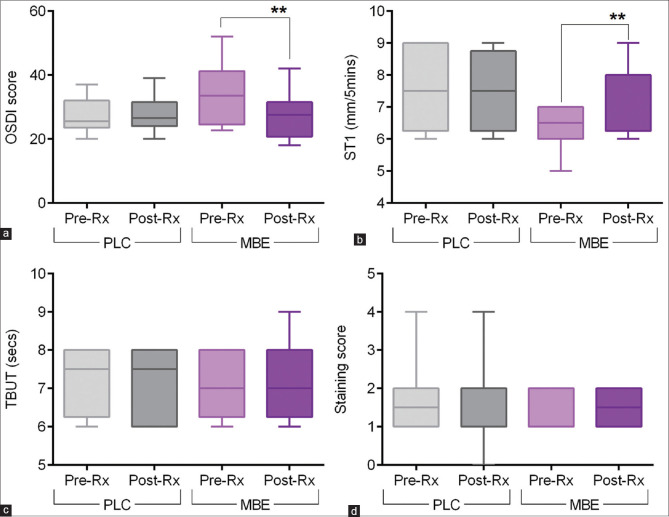
The eyes of the study subjects on MBE tablets – MBE showed improvement in some of the dry eye parameters compared to the eyes of subjects on placebo tablets – PLC. [Figs. 1–3]. The OSDI score was observed to be significantly decreased post-treatment compared to pre-treatment scores in the eyes of subjects on MBE [Fig. 1a]. No significant difference in the OSDI score was observed between pre- and post-treatment in the PLC group [Fig. 1a]. A higher proportion (75%) of eyes of subjects on MBE treatment showed a reduction in the OSDI scores following treatment, whereas 25% of the eyes of subjects showed a reduction in the OSDI scores following PLC treatment [Fig. 2a]. The fold difference in the OSDI score between pre- and post-treatment was significantly lower in the MBE-treated group compared to the PLC group [Fig. 3a]. Schirmer’s test 1 (ST1) value was observed to be significantly increased post-treatment compared to pre-treatment scores in the eyes of subjects on MBE [Fig. 1b]. No significant difference in the ST1 was observed between pre- and post-treatment ST1 values in the PLC group [Fig. 1b]. A higher proportion (67%) of eyes of subjects on MBE treatment showed an increase in ST1 values following treatment, whereas none of the eyes of subjects showed an increase in ST1 values following PLC treatment [Fig. 2b]. The fold difference in the increase in ST1 values pre- and post-treatment was significantly higher in the MBE-treated group compared to the PLC group [Fig. 3b]. No significant improvement or changes in the TBUT [Figs. 1c and 3c] and staining score [Figs. 1d and 3d] were observed following treatment compared to pre-treatment values in both MBE and PLC groups.
Figure 1.
Effect of maqui-berry extract (MBE) treatment on OSDI score, TBUT, Schirmer’s test 1, and staining score in study subjects. The box and whiskers plots indicate minimum, first quartile, median, third quartile, and maximum values of the OSDI score (a), Schirmer’s test 1 – ST1 values (b), tear break up time – TBUT (c), and staining score (d) before (pre-Rx) and after (post-Rx) treatment from subjects under MBE or under placebo (PLC) treatment. MBE – 24 eyes; PLC – 16 eyes. **p < 0.01; Wilcoxon matched-pairs signed rank test
Figure 3.
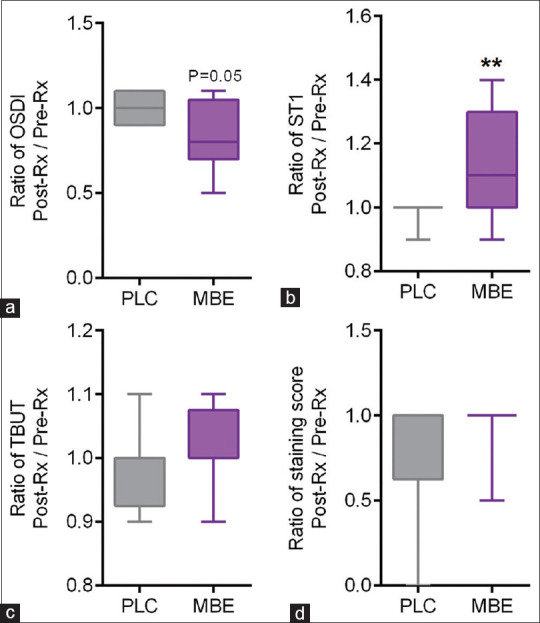
Fold changes in OSDI score, TBUT, Schirmer’s test 1, and staining score in study subjects following placebo or maqui-berry extract treatment (MBE). The box and whiskers plots indicate minimum, first quartile, median, third quartile, and maximum fold difference or ratio of the OSDI score (a), Schirmer’s test 1 – ST1 values (b), tear break up time – TBUT (c), and staining score (d) following treatment in subjects under MBE compared to those under placebo (PLC) treatment. The ratio of change or fold difference for each of the parameters was determined by dividing the post-treatment values by the pre-treatment value for every study subject. MBE – 24 eyes; PLC – 16 eyes. **p < 0.01; Mann–Whitney test
Figure 2.
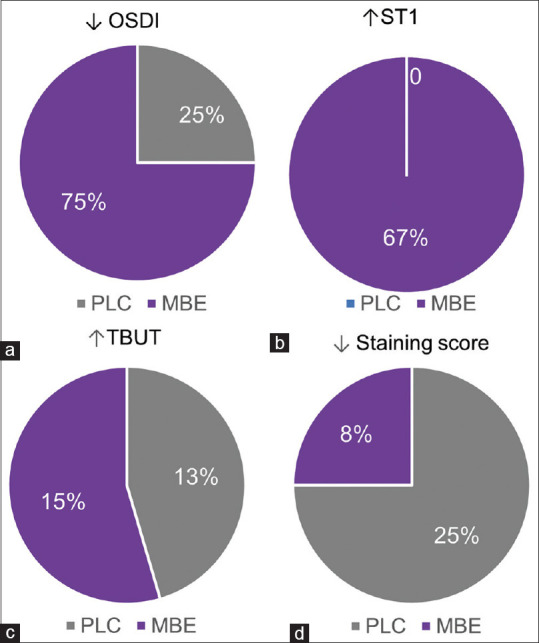
Proportion of eyes that exhibited beneficial changes in OSDI score, TBUT, Schirmer’s test 1, and staining score in study subjects following placebo or maqui-berry extract treatment (MBE). The pie charts indicate the percentage of eyes that showed a decrease in OSDI score (a), increase in Schirmer’s test 1 – ST1 values (b), decrease in tear break up time – TBUT (c), and decrease in staining score (d) after treatment compared to pretreatment values in subjects under MBE or under placebo (PLC) treatment
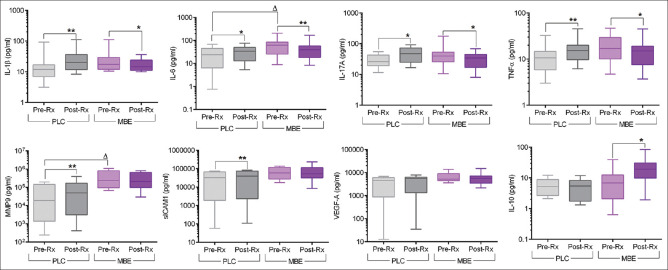
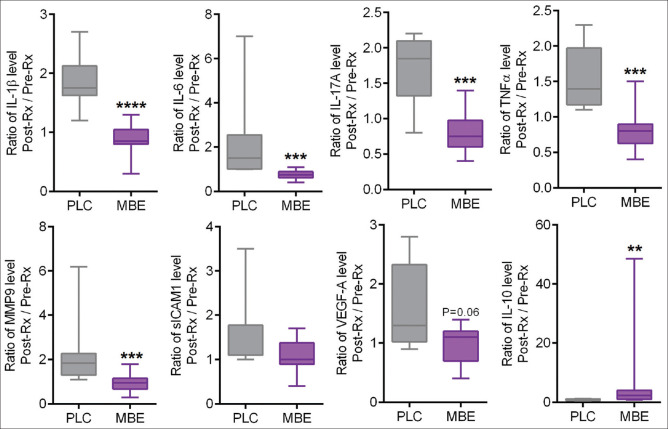
Tear fluid samples were collected to assess tear inflammatory factor levels from the study subjects before and after the treatment with MBE or PLC. The levels of pro-inflammatory factors such as IL-1β, IL-6, IL-17A, and TNFα were observed to be significantly reduced following treatment with MBE compared to pre-treatment levels [Fig. 4]. It is noteworthy that an anti-inflammatory factor, IL-10, was observed to be significantly increased following treatment with MBE compared to pre-treatment levels [Fig. 4]. On the other hand, an increase in the levels of IL-1β, IL-6, IL-17A, TNFα, MMP9, and sICAM1 was observed in the post-treatment tear samples compared to pre-treatment samples in the PLC group [Fig. 4]. The fold difference in the levels of IL-1β, IL-6, IL-17A, TNFα, MMP9, and VEGF between pre- and post-treatment was significantly lower in the MBE-treated group compared to the PLC group [Fig. 5]. The fold difference in the levels of IL-10 between pre- and post-treatment was significantly higher in the MBE-treated group compared to the PLC group [Fig. 5].
Figure 4.
Effect of maqui-berry extract (MBE) treatment on the tear soluble factor levels in study subjects. The box and whiskers plots indicate minimum, first quartile, median, third quartile, and maximum values of the IL-1β, IL-6, IL-17A, TNFα, MMP9, sICAM1, VEGF, and IL-10 levels in the tear samples collected before (pre-Rx) and after (post-Rx) treatment from subjects under MBE or under placebo (PLC) treatment. MBE – 12 eyes; PLC – 8 eyes. **p < 0.01, Wilcoxon matched-pairs signed rank test; Dp < 0.05, Mann–Whitney test. IL – interleukin, tumor necrosis factor-a (TNF-a), matrix metalloproteinase-9 (MMP-9), soluble intercellular adhesion molecule-1 (sICAM-1), and vascular endothelial growth factor-A (VEGF-A)
Figure 5.
Fold changes in tear soluble factors in study subjects following placebo or maqui-berry extract treatment (MBE). The box and whiskers plots indicate minimum, first quartile, median, third quartile, and maximum fold difference or ratio of IL-1β, IL-6, IL-17A, TNFα, MMP9, sICAM1, VEGF, and IL-10 levels following treatment in subjects under MBE treatment compared to those under placebo (PLC) treatment. The ratio of change or fold difference for each of the soluble factor’s concentrations was determined by dividing the post-treatment values by the pretreatment value for every study subject. MBE – 12 eyes; PLC – 8 eyes. **p < 0.01, ***p < 0.001, ****p < 0.0001, Mann–Whitney test. IL – interleukin, tumor necrosis factor-a (TNF-a), matrix metalloproteinase-9 (MMP-9), soluble intercellular adhesion molecule-1 (sICAM-1), and vascular endothelial growth factor-A (VEGF-A)
Discussion
This study looked at the effect of the consumption of a standardized MBE on DED parameters. The results showed a significant increase in tear production and resolution in ocular surface discomfort. There have been several studies which have demonstrated the consumption of maqui berry in animal models and humans. Nakamura et al.[6] showed the oral administration of a MBE stabilized the lacrimation secretion resulting from dry air and partial damage to the cornea and lacrimal gland tissue in vitro. Hitoe et al.[7] suggested that the intake of both 30 and 60 mg/day of MBE considerably enhanced the amount of tear fluid after 4 and 8 weeks in humans with DED. They concluded that an increase in the lacrimal fluid could be stimulated by delphinidin-3,5-O-diglucoside in MBE, a compound known to inhibit the production of reactive oxygen species (ROS) in the lacrimal gland tissue and known to suppress lacrimal gland tissue dysfunction, thereby preventing DED-related symptoms.[7,10]
Based on the OSDI questionnaire in this study, MBE intake alleviated subjective symptoms associated with DED like what was seen in our study where patients in the MBE group had a significant reduction in OSDI.[7] OSDI results are consistent with those of the previous MaquiBright® pilot trial and demonstrated improvement in ocular symptoms.[7] In addition, there was also a reduction in subjective symptoms associated with eye fatigue/discomfort, demonstrating that MBE alleviated ocular surface discomfort and dry eye-related symptoms. The rise in tear secretion observed by the consumption of MBE probably reduced general subjective symptoms associated with DED.
Many studies investigating the onset mechanism of dry eyes have attributed the condition not only to lacrimal dysfunction but also to external factors such as a decrease in blinking, computer and visual display terminal use, and several other factors.[11-14] An increase in ROS causes inflammation in corneal epithelial cells, which, in turn, decreases the stability of the tear film layer.[15] Some animal studies have confirmed a reduction of blinking frequency in animals with dry eyes, further augmenting ROS production and the presence of ROS in the tear film.[16,17] Hence, the consumption of MBE, a botanical extract with antioxidant properties, causes an increase in the amount of lacrimal fluid, with a reduction of ROS formation in the lacrimal gland tissue, and an improvement in the subjective symptoms of eye discomfort and fatigue.[17]
Although the consumption of MBE increased the amount of tear fluid based on the results of the Schirmer’s test, no significant changes were observed in the TBUT. This lack of effect might have resulted from different underlying etiology as aqueous deficient and evaporative, and both differ in the underlying mechanism of the onset.[18] Although participants in this study might not have had significant evaporative dry eyes, the Schirmer’s test results revealed that their tear fluid quantity was between 5 and 10 mm/5 min and TBUT was between 5 and 10 s, which is still lower than normal (moderate evaporative eye dryness). Thus, the effect of MBE intake on the TBUT time was not statistically significant. Therefore, further studies on the role of MBE are needed to investigate TBUT in patients experiencing excessive evaporative DED.
This is the first study to look at tear inflammatory factor levels following the use of MBE, and the levels of pro-inflammatory factors such as IL-1β, IL-6, IL-17A, TNFα, and MMP9 were observed to be significantly reduced following treatment with MBE compared to pretreatment levels. The reduction observed in the inflammatory cytokines on the ocular surface could possibly be due to the inhibitory effect of delphinol on NFkB activation, a key transcription factor responsible for the production of proinflammatory factors.[5,19] We, therefore, speculate that the patients with DED might be experiencing eye pain or discomfort-related symptoms or abnormal nociceptive response due to disruption in the pro- and antinociceptive factor balance on the ocular surface and MBE seems to be reducing these proinflammatory/nociceptive cytokines. IL-1β, a major proinflammatory cytokine, activates nociceptors to generate action potentials and induces pain on the ocular surface.[20] IL-17A, a key factor in inflammatory disorders, is also involved in nociception as its receptors are expressed by nociceptor neurons.[21] Interestingly, IL-10, which has been documented for its potent antinociceptive function and has being harnessed in the management of pain,[22] is an anti-inflammatory cytokine which was observed to be significantly increased following treatment with MBE, highlighting an important anti-inflammatory property in addition to increase in tear levels.
The limitation was a smaller sample size and that we did not include patients who had severe DED as these patients could not be stopped on topical medications to assess the efficacy. Also, the cohort of patients selected were restricted not to only aqueous deficiency DED on which MBE is known to act on but also included both evaporative and aqueous deficiency (mixed dry eye). MBE influences increased the tear fluid generation and diminished subjective symptoms of eye fatigue/discomfort, along with a potent anti-inflammatory effect on the ocular surface. Thus, future studies should be done to investigate the impact of MBE on eye dryness, in a larger cohort of patients with possible additive effects to existing topical medications across grades of DEDs, and this can provide clinicians with an important new oral medication with a unique mechanism of action in DED.
Conclusion
Thus, consumption of maqui-berry extract resulted in significant improvement of DED signs and symptoms, along with a reduction in ocular surface inflammation based on tear biomarker profiling. It can prove to be a useful addition to existing treatment options in the form of oral supplementation in patients with dry eye disease to enhance and improve the ocular surface health.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15:802–12. doi: 10.1016/j.jtos.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Misle E, Garrido E, Contardo H, González W. Maqui Aristotelia chilensis (Mol.) Stuntz]-the amazing chilean tree: A review. J Agric Sci Technol. 2011;1:473–82. [Google Scholar]
- 4.Muñoz O, Christen P, Cretton S, Backhouse N, Torres V, Correa O, et al. Chemical study and anti-inflammatory, analgesic and antioxidant activities of the leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. J Pharm Pharmacol. 2011;63:849–59. doi: 10.1111/j.2042-7158.2011.01280.x. [DOI] [PubMed] [Google Scholar]
- 5.Krum SA, Chang J, Miranda-Carboni G, Wang CY. Novel functions for NFkB: Inhibition of bone formation. Nat Rev Rheumatol. 2010;6:607–11. doi: 10.1038/nrrheum.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura S, Tanaka J, Imada T, Shimoda H, Tsubota K. Delphinidin 3,5-O-diglucoside, a constituent of the maqui berry (Aristotelia chilensis) anthocyanin, restores tear secretion in a rat dry eye model. J Funct Foods. 2014;10:346–54. [Google Scholar]
- 7.Hitoe S, Tanaka J, Shimoda H. MaquiBright™standardized maqui berry extract significantly increases tear fluid production and ameliorates dry eye-related symptoms in a clinical pilot trial. Panminerva Med. 2014;56(3 Suppl 1):1–6. [PubMed] [Google Scholar]
- 8.Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–74. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Miller KL, Walt JG, Mink DR, Satram-Hoang S, Wilson SE, Perry HD, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128:94–101. doi: 10.1001/archophthalmol.2009.356. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka J, Kadekaru T, Ogawa K, Hitoe S, Shimoda H, Hara H. Maqui berry (Aristotelia chilensis) and the constituent delphinidin glycoside inhibit photoreceptor cell death induced by visible light. Food Chem. 2013;139:129–37. doi: 10.1016/j.foodchem.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Tsubota K, Nakamori K. Dry eyes and video display terminals. N Engl J Med. 1993;328:584. doi: 10.1056/NEJM199302253280817. [DOI] [PubMed] [Google Scholar]
- 12.Tsubota K, Toda I, Nakamori K. Poor illumination, VDTs, and desiccated eyes. Lancet. 1996;347:768–9. doi: 10.1016/s0140-6736(96)90122-1. [DOI] [PubMed] [Google Scholar]
- 13.Schlote T, Kadner G, Freudenthaler N. Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefes Arch Clin Exp Ophthalmol. 2004;242:306–12. doi: 10.1007/s00417-003-0845-z. [DOI] [PubMed] [Google Scholar]
- 14.Wolkoff P, Nøjgaard JK, Troiano P, Piccoli B. Eye complaints in the office environment: Precorneal tear film integrity influenced by eye blinking efficiency. Occup Environ Med. 2005;62:4–12. doi: 10.1136/oem.2004.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Augustin AJ, Spitznas M, Kaviani N, Meller D, Koch FH, Grus F, et al. Oxidative reactions in the tear fluid of patients suffering from dry eyes. Graefes Arch Clin Exp Ophthalmol. 1995;233:694–8. doi: 10.1007/BF00164671. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura S, Shibuya M, Nakashima H, Imagawa T, Uehara M, Tsubota K. D-beta-hydroxybutyrate protects against corneal epithelial disorders in a rat dry eye model with jogging board. Invest Ophthalmol Vis Sci. 2005;46:2379–87. doi: 10.1167/iovs.04-1344. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura S, Shibuya M, Nakashima H, Hisamura R, Masuda N, Imagawa T, et al. Involvement of oxidative stress on corneal epithelial alterations in a blink-suppressed dry eye. Invest Ophthalmol Vis Sci. 2007;48:1552–8. doi: 10.1167/iovs.06-1027. [DOI] [PubMed] [Google Scholar]
- 18.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea. 2012;31:472–8. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- 19.Nagaoka M, Maeda T, Chatani M, Handa K, Yamakawa T, Kiyohara S, et al. A Delphinidin-enriched Maqui berry extract improves bone metabolism and protects against bone loss in osteopenic mouse models. Antioxidants (Basel) 2019;8:386. doi: 10.3390/antiox8090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CF, Moalem-Taylor G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J Pain. 2011;12:370–83. doi: 10.1016/j.jpain.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Milligan ED, Penzkover KR, Soderquist RG, Mahoney MJ. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation. 2012;15:520–6. doi: 10.1111/j.1525-1403.2012.00462.x. discussion 526. [DOI] [PMC free article] [PubMed] [Google Scholar]