Abstract
Background
The tropical plant Ficus microcarpa L. f. cv. Golden Leaves (GL) is a high-light sensitive tropical fig tree in which sun-leaves are yellow and shade-leaves are green. We compared the response of photosynthetic activities to strong light between GL and its wild-type (WT, Ficus microcarpa L. f.).
Results
Field measurements of maximum photosystem II (PSII) efficiency (Fv/Fm) of intact sun-leaves in GL showed that photo synthetic activity was severely photoinhibited during the daytime (Fv/Fm = 0.46) and subsequently recovered in the evening (Fv/Fm = 0.76). In contrast, WT did not show any substantial changes of Fv/Fm values throughout the day (between 0.82 and 0.78). Light dependency of the CO2 assimilation rate in detached shade-leaves of GL showed a response similar to that in WT, suggesting no substantial difference in photosynthetic performance between them. Several indicators of photoinhibition, including declines in PSII reaction center protein (D1) content, Fv/Fm value, and O2 evolution and CO2 assimilation rates, all indicated that GL is much more susceptible to photoinhibition than WT. Kinetics of PAM chlorophyll a fluorescence revealed that nonphotochemical quenching (NPQ) capacity of GL was lower than that of WT.
Conclusion
We conclude that the photosynthetic apparatus of GL is more highly susceptible to photoinhibition than that of WT.
Background
Exposure of leaves to strong light sometimes causes the reduction of photosynthetic efficiency, a phenomenon referred to as photoinhibition [1-3]. The susceptibility of plants to photoinhibition depends on the species and growth light-environments [4]. In general, shade plants or low-light grown plants are more susceptible to photoinhibition than sun plants or high-light grown plants [4]. Since photoinhibition has a potential to lower productivity and plant growth, avoidance of photoinhibition is critical for the fitness and survival of plants in natural habitats [2,5,6].
It is now widely accepted that harmful Reactive Oxygen Species (ROS) produced upon illumination are involved in the mechanism of photoinhibition [7]. Singlet-excited oxygen (1O2) can be generated by the interaction of O2 with triplet-excited chlorophyll (3Chl) formed in the PSII reaction center [8]. Superoxide radical (O2-) is unavoidably produced by the Mehler reaction via electron transfer to O2 at photosystem I. Dismutation of O2- results in the formation of hydrogen peroxide (H2O2) and the reaction between H2O2 and transition metal ions generates hydroxyl radical (•OH) which is the most reactive radical among ROS [7]. These ROS can oxidize molecules in chloroplasts including D1 protein in the PSII reaction center [9] and thiol enzymes in the Calvin-Benson cycle [10,11] to inhibit partial reactions of photosynthesis eventually leading to photoinhibition. Recently, ROS have been shown to also bring about photoinhibition through the inhibition of de novo synthesis of D1 protein of PSII that is essential to recover from photoinhibition [12].
To protect photosynthetic machinery from ROS-mediated photoinhibition, chloroplasts contain a high amount of ascorbate in stroma at 20 to 300 mM [13]. In the stroma, ascorbate contributes to suppress the accumulation of photoproduced-H2O2 by acting as the electron donor for ascorbate peroxidase (APX) which detoxifies H2O2 to H2O [14]. Ascorbate can be also involved in the detoxification of 1O2, O2- and •OH via nonenzymatic reduction [13]. In these reactions, ascorbate is univalently oxidized to monodehydroascorbate (MDA). To maintain ascorbate concentration in the chloroplasts, MDA should be promptly regenerated to ascorbate during illumination. MDA can be reduced to ascorbate by NAD(P)H in a reaction catalyzed by monodehydroascorbate reductase (MDAR) [15] or directly by reduced ferredoxin [16]. MDA, an organic radical, can undergo spontaneous dismutation to produce ascorbate and dehydroascorbate (DHA) in the absence of MDA reduction. DHA can be reduced by glutathione either through nonenzymatic reaction at a slow rate or enzymatic reaction at a much higher rate [17]. Dehydroascorbate reductase (DHAR, EC 1.8.5.1) is considered to be involved in enzymatic DHA reduction in the chloroplasts [17]. Until now, however, direct in vivo evidence showing the physiological significance of DHAR has been yet not available.
We previously reported that leaves of Ficus microcarpa L. f. cv. Golden Leaves (GL), a tropical fig tree, lack heat-stable DHAR activity [18]. In GL, the canopy sun-leaves, which are always exposed to direct sunlight, show characteristic yellow whereas those in wild-type (WT, Ficus microcarpa L. f.) exhibit normal green even when exposed to direct sunlight. The mechanism for yellow leaves production in GL is unknown. In barley, it has been reported that leaves incubated with CO2-enriched air show yellow color due to photoinhibition of photosynthesis [19,20], a phenomenon apparently similar to that observed in GL. We hypothesized that GL possess an incomplete machinery of photosynthesis which is susceptible to photoinhibition. The aim of this study was to directly examine the hypothesis. The side-by-side comparisons shown in this study demonstrate that GL is much more susceptible to photoinhibition of photosynthesis than WT.
Results
Photoinhibition in the field
GL is a cultivar of the tropical fig tree Ficus microcarpa L. f. (WT) that is natively distributed in subtropical/tropical regions of Asia and Oceania. A significant characteristic of GL is the presence of yellow leaves that contain high amounts of flavonoids and negligible amounts of chlorophyll and carotenoids [21]. The reduced content of photosynthetic pigments in the yellow leaves can be increased by shading them from high-light. In shade conditions, therefore, GL exhibits normal green leaves which cannot be morphologically distinguished from WT leaves (Fig. 1). We have suggested that high susceptibility of GL to photoinhibition could explain the phenomenon [21]. However, there was no direct evidence available to show the susceptibility of GL to photoinhibition.
Figure 1.
Photograph of Ficus microcarpa L. f. (WT) and Ficus microcarpa L. f. cv. Golden Leaves (GL). Each branch was collected from canopy of mature trees grown in the field. Characteristic yellow leaves are observed only in sun-leaves of GL.
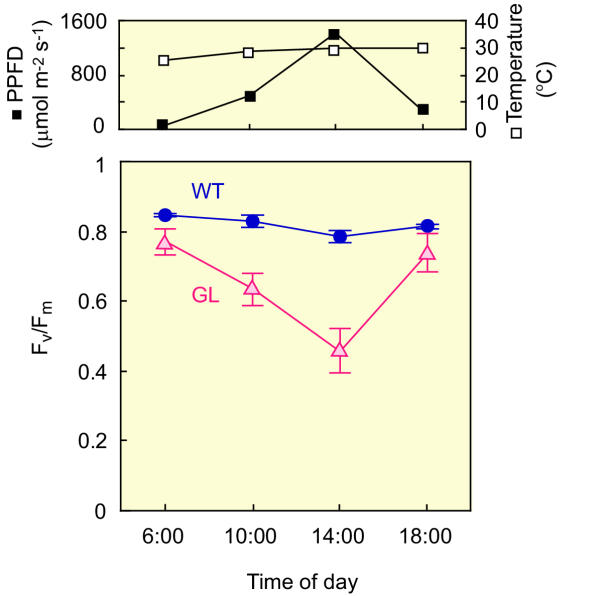
Figure 2 shows diurnal change of maximum PSII efficiency (Fv/Fm) in field grown attached sun-leaves collected from the canopy. Chlorophyll content of sun-leaves in GL (yellow) was 0.05 g m-2 and that in WT (green) was 0.44 g m-2. There was no significant difference between GL and WT in Fv/Fm values measured early in the morning (6 a.m.). The value of Fv/Fm in GL decreased from 0.77 (6 a.m.) to 0.46 (2 p.m.) and subsequently recovered to 0.74 in the evening (6 p.m.). In contrast to GL, Fv/Fm values in WT were almost constant throughout a day. Shade-leaves of GL(green), collected from the inside of tree, did not show any decrease in the Fv/Fm value (data not shown). These data obtained in the field conditions suggest that GL is highly susceptible to high-light.
Figure 2.
Photoinhibition of GL observed in the field-grown conditions. Top panel, a typical diurnal change in PPFD (▪) on the leaves and atmospheric temperature (□) in the field. Bottom panel, a diurnal change in maximum PSII efficiency of intact attached leaves in the field. (•, blue), WT; (Δ red), GL. Fv/Fm value was measured as PSII efficiency with a portable chlorophyll a fluorometer (PAM-2000). Each data point is the mean of 10 separate measurements ± SD.
Photosynthetic performance
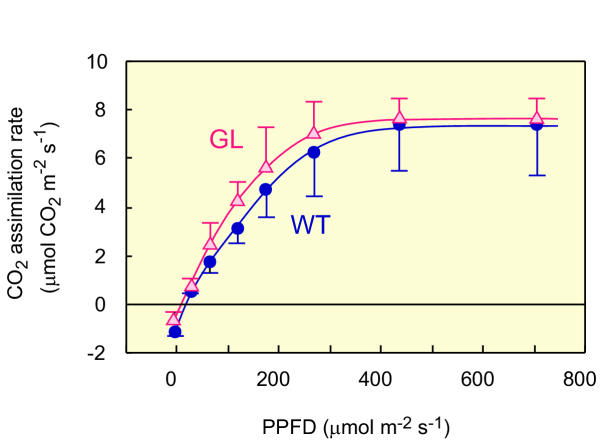
We compared photosynthetic performance between GL and WT to confirm that the fundamental photosynthetic machinery of GL is normal. Shade-leaves of GL and WT, both of which are green, were used for this purpose. Figure 3 shows the light response curves of CO2 assimilation rate (based on surface area) in detached shade-leaves. The chlorophyll content of shade-leaves in GL (green) was 0.58 g Chl m-2 and that in WT (green) was 0.70 g Chl m-2. GL showed a very similar light-response curve to WT and there was no substantial difference in the maximum activity of CO2 assimilation between them. These results clearly demonstrate that the photosynthetic capacity of GL is almost identical to that of WT, suggesting that photosynthetic machinery of GL is functionally not defective.
Figure 3.
Light-response curves for the rate of photosynthetic CO2 assimilation in WT and GL. Shade-leaves, which exhibited identical green coloration in both WT and GL, were used for measurements. Light intensities of irradiation were changed from high to low. (•, blue), WT; (Δ, red), GL. Points are the means of three (•) or four (Δ) separate measurements ± SD.
Photoinhibition in shade-leaves of Golden Leaves
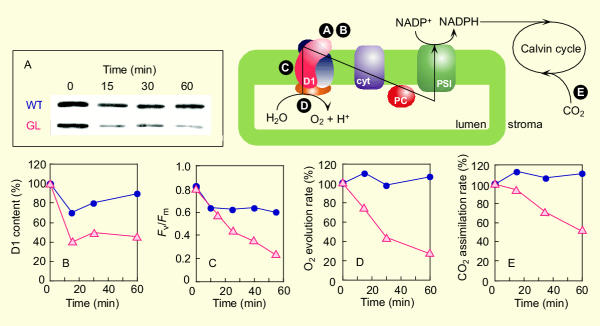
Figure 4 shows effects of high-light on several indicators of photoinhibition: D1 protein content, Fv/Fm value, O2 evolution rate and CO2 assimilation rate. Before high-light exposure, there was no substantial difference between WT and GL shade-leaves in D1 protein content on protein basis (Fig. 4A) and Fv/Fm value (Fig. 4C). GL showed a significant decrease in the D1 protein content and Fv/Fm value upon high-light illumination (Fig. 4A,4B,4C). Similar to the indicators specific for PSII activity, those for net photosynthetic activity (i.e. O2 evolution and CO2 assimilation rates) also decreased upon high-light illumination (Fig. 4D,4E). In contrast to these responses observed in GL, WT showed only a small decrease in D1 protein content and Fv/Fm value (Fig. 4A,4B,4C). The O2 evolution and CO2 assimilation rates in WT did not change even under high-light condition (Fig. 4D,4E).
Figure 4.
High-light induced photoinhibition of GL assessed by several parameters. Shade-leaves were exposed to high-light (2300 μmol m-2 s-1) at zero time after preexposure to medium-light (1000 μmol m-2 s-1) for 30 min. A schematic illustration shows the outline of photosynthesis including the electron transport process in thylakoid membranes. Letters (A-D) in filled circles, which correspond to those of the panels A-D, represent the sites where the photo synthetic parameters can be measured. A, Immuno blotting of the D1 protein contained in leaf-extract. B, Degradation of the in vivo D1 protein induced by high-light. Each point was plotted using a relative density of band on the gel as shown in A. C, Decline of Fv/Fm induced by high-light. Note that the time indicated represents total illumination time. D, High-light induced inhibition of the activity of O2 evolution from intact detached leaves. E, High-light induced inhibition of the activity of CO2 assimilation in intact detached leaves. Points are taken by a separate measurement in different leaves (A, B, D) or a leaf (C, E). (•, blue), WT; (Δ, red), GL.
Reduced NPQ capacity in Golden Leaves
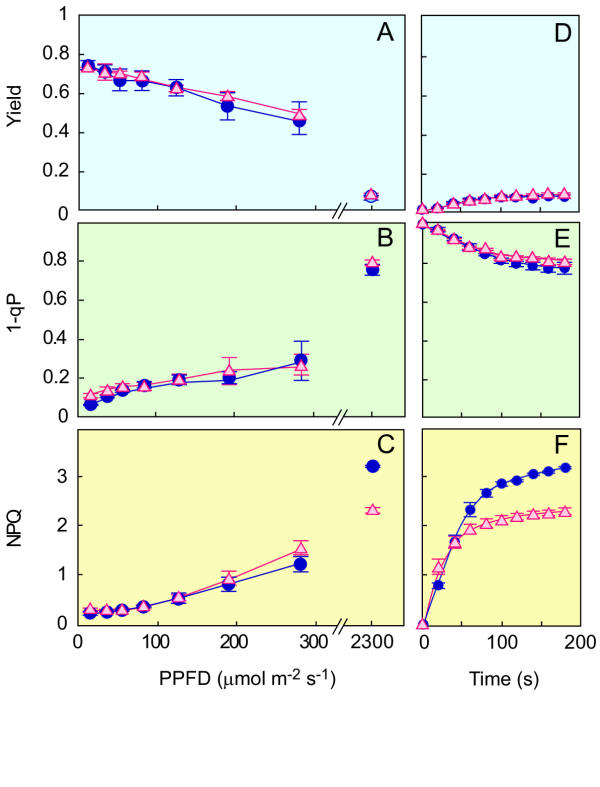
When plants are exposed to high-light that exceeds the capacity of photosynthesis, excess absorbed light energy can be safely dissipated as heat [22]. This energy dissipation can be measured as the NPQ of PAM chlorophyll a fluorescence analysis. NPQ includes the quantum yield of PSII as an index of heat energy dissipation, photoinactivation of PSII and distribution of photon acceptance between PSII and PSI due to the state transition. It is known that the sate transition can be controlled by the redox state of QA[23], which can be measured as 1-qP. Figure 5 shows that there is no significant difference between GL and WT in yield and 1-qP values. Furthermore, measurements of the electron transport rate (ETR) showed essentially no difference in the sensitivity of PSII photoinactivation between GL and WT (data not shown). However, GL showed a much lower level of NPQ compared to WT (Fig. 5C,5F). These results suggest that low NPQ in GL can be attributed to dysfunction of the heat dissipation mechanism.
Figure 5.
Yield of PSII (A, D), 1-qp reflecting the reduction state of the qa pool (B, E), NPQ (C, F) in shade-leaves of WT (•, blue) and GL (Δ, red). Each measurement was made after the exposure of leaves to actinic light for 3 min at different intensities (A-C). In D, E and F, high light (2300 μmol m-2 s-1) was used as actinic light to record time courses of Yield (D), 1-qP (E), and NPQ (F). Points are presented as the means of 5 (A-C) or 3 (D-F) separate measurements ± SEM.
Discussion
This study has demonstrated that photosynthetic activities of GL are highly susceptible to photoinhibition under high-light conditions (Figs. 2, 4). There was essentially no difference in photosynthetic efficiency between GL and WT at least up to 700 μmol m-2 s-1 for a short time (Fig. 3), but NPQ in GL was lower than that in WT at 2300 μmol m-2 s-1 (Fig. 5C,5F). It has been reported that a npq1 mutant of Arabidopsis thaliana shows increased susceptibility to photoinhibition, but is unaffected in short-term photosynthetic O2 evolution and CO2 assimilation [24,25]. For the operation of NPQ, special xanthophyll pigments in the light-harvesting complexes of PSII are known to have critical roles [26]. The extent of NPQ in plants is strongly correlated with the levels of zeaxanthin and antheraxanthin that are formed from violaxanthin by the enzyme violaxanthin deepoxidase (VDE) located on the luminal side of thylakoid membranes [22]. In excessive light conditions, VDE is activated and converts violaxanthin to zeaxanthin via antheraxanthin when acidification in thylakoid lumen reaches a critical threshold [27]. The activity of VDE is also regulated by the concentration of ascorbate in the lumen because VDE can utilize only ascorbate as a reductant [28]. It has been shown that decreases in ascorbate availability severely affect VDE activity in vivo[28]. Because thylakoid membranes do not have any active transport mechanisms for ascorbate, the concentration of ascorbate in the stroma determines that in the lumen by a passive diffusion mechanism [29]. In Arabidopsis, it has been clearly demonstrated that NPQ performance in vivo is reduced by a mutation that causes ascorbate deficiency [13]. We previously reported that the ascorbate content of GL is severally decreased in daytime under field conditions [30]. Thus, it is reasonable to assume that decrease of ascorbate content would be a cause of dysfunction of NPQ in GL.
Among antioxidant enzymes, we have shown that GL lacks heat-stable DHAR activity [18]. Plant cells possess several kinds of enzymes that exhibit DHAR activity: thioredoxin [31], glutaredoxin [32] and disulfide isomerase [33]. Recently, two distinct chloroplast proteins that exhibit DHAR activity have been isolated from spinach; one is Kunitz-type trypsin inhibitor [31] and the other is specific DHAR [34]. Because both chloroplastic DHARs show higher heat-stable activity than non-chloroplastic DHAR [31,34], GL may lack chloroplastic DHAR(s) though molecular information is not available. In fact, heat-stable activity cannot be detected in nonphotosynthetic organs such as fruits, that do not include chloroplasts [18]. Although chloroplastic DHAR(s) have been presumed to function in the ascorbate regeneration system for maintaining the ascorbate concentration in the chloroplasts [7], the physiological significance of DHAR in chloroplasts is still controversial [35-38]. Since the activity of stromal MDAR is high, the spontaneous disproportionation from MDA to DHA and ascorbate may not occur under in vivo situations. Therefore, there is an argument that DHAR might be dispensable for the ascorbate-glutathione cycle to protect chloroplasts from high-light stress [36]. However, there is another location in chloroplasts where the stromal enzymes cannot directly regenerate ascorbate, namely, the lumen space.
In the lumen, oxidation of ascorbate by xanthophylls, α-tochopherol and PSII result in the production of DHA and all of these reactions could be promoted under high-light conditions [13,35]. Therefore, stromal DHAR would be essential to reduce DHA produced in the lumen side. In this context, chloroplastic DHAR must be physiologically indispensable for photoprotection mechanisms particularly when leaves are exposed to high-light stress conditions. This is consistent with previous reports that DHAR activity in leaves of A. thaliana is increased by high-light conditions [39]. Thus, we consider that ascorbate availability of GL is decreased by high-light due to a lack of DHAR activity which eventually results in lowered performance of NPQ (Fig. 5C,5F). Ascorbate is involved in photoprotection not only through NPQ but also primarily through ROS scavenging [7]. It should be noted that high susceptibility of GL to photoinhibition could be also explained by a low ROS scavenging capacity which may result in photodamage of target molecules in chloroplast [7] and the inhibition of D1 de novo synthesis [12].
The mechanism for yellow leaf production is still unknown. Sun-leaves of GL contain low amounts of chlorophyll and carotenoids but shade-leaves show comparable amount of those to WT, implying that exhibition of yellow leaves in GL is associated with a long-termed irradiation of high-light [21]. When leaves of GL were exposed to high-light, they showed significant symptoms of photoinhibition (Figs. 2, 4). It has been suggested that long-termed photoinhibition (or chronic photoinhibition) can lead photobleaching of photo synthetic pigments such as chlorophyll and carotenoids [3]. The npq1 Arabidopsis mutant which is partially defective in NPQ has been reported to show the photobleaching of chlorophyll in high-light conditions [40], a phenomenon apparently very similar to that observed in GL. It is plausible that photoinhibition is involved in an early stage of the yellow leaf-producing mechanism. Unlike herbaceous plants, the leaf-yellowing phenomenon does not cause senescence or cell death [21]. It has been shown that flavonoids accumulated in leaves could protect tissues from oxidative damage by complementing the ascorbate-glutathione cycle [15,41,42]. In addition to the ROS scavenging function, flavonoids have long been known to be effective sunscreen pigments particularly against UV radiation [43]. Increased flavonoid pigments in GL may play roles including ROS scavenging function, UV-protection and light-attenuation for shadeleaves. The mechanisms of photoinhibition susceptibility and, in particular of high-light tolerance in GL leaves remain to be fully clarified. Although it is difficult to apply molecular biology techniques to GL, we consider that the tropical tree could provide a unique opportunity for examining the important determinants of survival in high-light environments.
Materials and Methods
Plant materials
Plant materials were Ficus microcarpa L. f. cv. Golden Leaves (GL) and Ficus microcarpa L. f. (WT) grown in the campus of the University of the Ryukyus on Okinawa island (26'15"N, 127'45"E) in Japan. The study period was from March to June in 1999–2000. The photo synthetic photon flux density (PPFD) on the plant canopy was c.a. 2500 μmol m-2 s-1 in full sunlight conditions during the period. There was no significant difference in growth conditions of PPFD between WT and GL. Green leaves, grown under light conditions of about 100 μmol m-2 s-1 as the maximum, were harvested and used for laboratory experiments as shade-leaves. To avoid the drought of leaves, leaf petioles were continuously kept in distilled water throughout the measurements. To activate the photosynthetic activity, detached leaves were exposed to an illumination of 1000 μmol m-2 s-1 for 30 min before experiments.
Illumination of leaves
Leaves were illuminated with white light from three halogen lumps (400 W). Leaf temperature was maintained at 30°C with an electric fan or a thermostatically controlled water bath during illumination. Various light intensities were obtained by placing wire screens in front of the light source.
Measurements of chlorophyll fluorescence
Chlorophyll fluorescence was measured with a PAM-2000 chlorophyll fluorometer system under atmospheric conditions (Heinz Walz, Effeltrich, Germany). After a dark adaptation period of 15 min, minimum fluorescence (Fo) was determined by a weak red light. Maximum fluorescence of dark-adapted leaf (Fm) was measured during a subsequent saturating light pulse of white light (8000 μmol m-2 s-1 for 0.4 s). Maximum fluorescence (Fm') and steady-state fluorescence (Fs) of illuminated leaf were measured upon a subsequent saturating light pulse of white light (8000 μmol m-2 s-1, for 0.4 s) to determine NPQ (Fm-Fm')/Fm'), yield of PSII [(Fm'-Fs)/Fm] and qP (Fm'-Fs)/Fm'-Fo) [44,45]. A 650 nm light-emitting-diode (LED) equipped with PAM-2000 was used for the illumination of leaf as actinic light.
Measurements of gas exchange
CO2 assimilation was determined by the difference in CO2 concentration between inlet and outlet of the assimilation chamber with a CO2 gas analyzer (LI-6251; LiCor, Inc., Lincoln, Nebraska) in 0.036% CO2 and 21% O2[46]. Measurements of CO2 assimilation rates were carried out under an illumination of 2300 μmol m-2 s-1, except for light response curve measurements. Approximately 25 cm2 of leaf were employed for an experiment. The air in the chamber was stirred rapidly to maintain a high boundary layer conductance for CO2 diffusion. O2 evolution was determined using a gas-phase Walker-type oxygen electrode system (Model LD-1; Hansatech, Norfolk, U.K.) in 5% CO2 and 21% O2[47]. Measurements of O2 evolution were carried out on 4 cm2 of leaf disc under an illumination of 600 μmol m-2 s-1.
Measurements of D1 protein content
Leaf (0.5 g) was homogenized for 30 s at 0°C in a grinding medium containing 50 mM potassium phosphate (pH 7.0), 5 mM phenylmethylsulfonyl fluoride and 5% (w/v) polyvinylpolypyrrolidone. The homogenate (4 ml) was mixed with 4 ml of 125 mM Tris (pH 6.8) containing 5% (w/v) SDS, and then incubated at 80°C for 3 min to solubilize proteins. After cooling down the sample to room temperature, aggregates were removed by the centrifugation at 1,500 × g for 30 s. The supernatant (800 μl) was mixed with the same amount of a solution that contained 0.4 M sucrose, 8 M urea, 5 mM EDTA and 5% (w/v) 2-mercaptoethanol.
SDS-PAGE was carried out according to a previously reported method [48]. Polyacrylamide gels containing 6.0 M urea were used for the stacking gel (4.5 %) and separation gel (13 %). Samples that contained 8 μg protein were loaded in each lane. Immunoblot analysis was performed using specific polyclonal antibodies raised against the D1 protein [48]. The intensities of the protein bands were determined with NIH image system version 1.61 (NIH, USA).
List of abbreviations used
APX, ascorbate peroxidase; 1Chl, singlet excited chlorophyll; 3Chl, triplet excited chlorophyll; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; D1, the reaction center-forming protein of photosystem II; Fv/Fm, ratio of variable to maximum chlorophyll a fluorescence; GL, Golden Leaves; MDA, monodehydroascorbate; MDAR, monodehydroascorbate reductase; NPQ, nonphotochemical quenching; •OH, hydroxyl radical; 1O2, singlet excited O2; O2-, superoxide radical; PPFD, photosynthetic photon flux density; ROS, reactive oxygen species; WT, wild-type.
Acknowledgments
Acknowledgements
We are grateful to Dr. M. F. Cohen of University of the Ryukyus for critical reading of the manuscript. This work was supported by a Grant-in-Aid for Scientific Research (B) and (C) from Japan Society for the Promotion of Science to HY.
Contributor Information
Shunichi Takahashi, Email: takahashishunichi@hotmail.com.
Ayumu Tamashiro, Email: ayumu@ii-okinawa.ne.jp.
Yasuko Sakihama, Email: yasuko@ii-okinawa.ne.jp.
Yasusi Yamamoto, Email: yasusiya@cc.okyama-u.ac.jp.
Yoshinobu Kawamitsu, Email: kawamitu@agr.u-ryukyu.ac.jp.
Hideo Yamasaki, Email: yamasaki@comb.u-ryukyu.ac.jp.
References
- Demming-Adams B, Adams WW. Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. doi: 10.1146/annurev.arplant.43.1.599. [DOI] [Google Scholar]
- Long SP, Humphries S, Falkowski PG. Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:633–662. doi: 10.1146/annurev.arplant.45.1.633. [DOI] [Google Scholar]
- Powles SB. Photoinhibition of photosynthesis induced by visible light. Ann Rev Plant Physiol. 1984;35:15–44. doi: 10.1146/annurev.arplant.35.1.15. [DOI] [Google Scholar]
- Osmond CB. What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker NR, Bowyer JR, editor. Photoinhibition of photosynthesis: from molecular mechanisms to the field. Oxford, BIOS Scientific Publishers; 1994. pp. 1–24. [Google Scholar]
- Ball MC. The role of photoinhibition during tree seedling establishment at low temperatures. In: Baker NR, Bowyer JR, editor. Photoinhibition of photosynthesis: from molecular mechanisms to the field. Oxford, BIOS Scientific Publishers; 1994. pp. 365–376. [Google Scholar]
- Kitao M, Lei TT, Koike T, Tobita H, Maruyama Y, Matsumoto Y, Ang LH. Temperature response and photoinhibition investigated by chlorophyll fluorescence measurements for four distinct species of dipterocarp trees. Physiol Plant. 2000;109:284–290. doi: 10.1034/j.1399-3054.2000.100309.x10.1034/j.1399-3054.2000.100309.x. [DOI] [Google Scholar]
- Asada K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Macpherson AN, Telfer A, Barber J, Truscott TG. Direct detection of singlet oxygen from isolated photosystem II reaction centers. Biochim Biophys Acta. 1993;1143:301–309. doi: 10.1016/0005-2728(93)90201-P. [DOI] [Google Scholar]
- Yamamoto Y. Quality control of photosystem II. Plant Cell Physiol. 2001;42:121–128. doi: 10.1093/pcp/pce022. [DOI] [PubMed] [Google Scholar]
- Kaiser WM. Reversible inhibition of the Calvin cycle and activation of oxidative pentose phosphate cycle in isolated intact chloroplasts by hydrogen peroxide. Planta. 1979;145:377–382. doi: 10.1007/BF00388364. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Otsubo T, Kondo N. Participation of hydrogen peroxide in the inactivation of Calvin-cycle SH enzymes in SO2-fumgated spinach leaves. Plant Cell Physiol. 1982;23:1009–1018. [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001;20:5587–5594. doi: 10.1093/emboj/20.20.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. Ascorbate biosynthesis and function in photoprotection. Phil Trans R Soc Lond B. 2000;355:1455–1464. doi: 10.1098/rstb.2000.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. Ascorbate peroxidase – a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 1992;85:235–241. doi: 10.1034/j.1399-3054.1992.850216.x10.1034/j.1399-3054.1992.850216.x. [DOI] [Google Scholar]
- Sakihama Y, Mano J, Sano S, Asada K, Yamasaki H. Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem Biophys Res Commun. 2000;279:949–954. doi: 10.1006/bbrc.2000.4053. [DOI] [PubMed] [Google Scholar]
- Miyake C, Asada K. Ferredoxin-dependent photoreduction of the monodehydroascorbate radical in spinach thylakoids. Plant Cell Physiol. 1994;35:539–549. [Google Scholar]
- Hossain MA, Asada K. Purification of dehydroascorbate reductase from spinach and its characterization as a thiol enzyme. Plant Cell Physiol. 1984;25:85–92. [Google Scholar]
- Yamasaki H, Takahashi S, Heshiki R. The tropical fig Ficus microcarpa L. f. cv. Golden Leaves lacks heat-stable dehydroascorbate reductase activity. Plant Cell Physiol. 1999;40:640–646. [Google Scholar]
- Sicher RC. Yellowing and photosynthetic decline of barley primary leaves in response to atmospheric CO2 enrichment. Physiol Plant. 1998;103:193–200. doi: 10.1034/j.1399-3054.1998.1030206.x10.1034/j.1399-3054.1998.1030206.x. [DOI] [Google Scholar]
- Sicher RC. Photosystem-II activity is decreased by yellowing of barley primary leaves during growth in elevated carbon dioxide. Int J Plant Sci. 1999;160:849–854. doi: 10.1086/314182. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Heshiki R, Ikehara N. Leaf-goldenning induced by high light in Ficus microcarpa L. f., a tropical fig. J Plant Res. 1995;108:171–180. [Google Scholar]
- Gilmore AM. Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant. 1997;99:197–209. doi: 10.1034/j.1399-3054.1997.990127.x10.1034/j.1399-3054.1997.990127.x. [DOI] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol. 1991;42:313–349. doi: 10.1146/annurev.arplant.42.1.313. [DOI] [Google Scholar]
- Niyogi KK, Grossman AR, Bjorkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy converiion. Plant Cell. 1998;10:1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AM. Xanthophyll cycle-dependent nonphotochemical quenching in Photosystem II: Mechanistic insights gained from Arabidopsis thaliana L. mutants that lack violaxanthin deepoxidase activity and/or lutein. Photosynth Res. 2001;67:89–101. doi: 10.1023/A:1010657000548. [DOI] [PubMed] [Google Scholar]
- Gilmore AM, Yamasaki H. 9-aminoacridine and dibucaine exhibit competitive interactions and complicated inhibitory effects that interfere with measurements of Δ pH and xanthophyll cycle-dependent photosystem II energy dissipation. Photosynth Res. 1998;57:159–174. doi: 10.1023/A:1006065931183. [DOI] [Google Scholar]
- Bratt CE, Arvidsson P-O, Carlsson M, Åkerlund H-E. Regulation of violaxanthin de-epoxidase activity by pH and ascorbate concentration. Photosynth Res. 1995;45:169–175. doi: 10.1007/BF00032588. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Leiandais M. A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cell. J Plant Physiol. 1996;148:391–398. [Google Scholar]
- Yamasaki H, Heshiki R, Yamasu T, Sakihama Y, Ikehara N. Physiological significance of the ascorbate regenerating system for the high-light tolerance of chloroplasts. In: Mathis P, editor. Photosynthesis: from light to biosphere. Dordrecht, Kluwer Academic; 1995. pp. 291–294. [Google Scholar]
- Trümper S, Follmann H, Häberlein I. A novel dehydroascorbate reductase from spinach chloroplasts homologous to plant trypsin inhibitor. FEBS Lett. 1994;352:159–162. doi: 10.1016/0014-5793(94)00947-3. [DOI] [PubMed] [Google Scholar]
- Sha S, Minakuchi K, Higaki N, Sato K, Ohtsuki K, Kurata A, Yoshikawa H, Kotaru M, Masumura T, Ichihara K, Tanaka K. Purification and characterization of glutaredoxin (thioltransferase) from rice (Oryza sativa L.). J Biochem. 1997;121:842–848. doi: 10.1093/oxfordjournals.jbchem.a021663. [DOI] [PubMed] [Google Scholar]
- Wells WW, Xu DP. Dehydroascorbate reduction. J Bioenerg Biomembr. 1994;26:369–77. doi: 10.1007/BF00762777. [DOI] [PubMed] [Google Scholar]
- Shimaoka T, Yokota A, Miyake C. Purification and characterization of chloroplast dehydroascorbate reductase from spinach leaves. Plant Cell Physiol. 2000;41:1110–1118. doi: 10.1093/pcp/pcd035. [DOI] [PubMed] [Google Scholar]
- Mano J, Ushimaru T, Asada K. Ascorbate in thylakoid lumen as an endogenous electron donor to Photosystem II: Protection of thylakoids from photoinhibition and regeneration of ascorbate in stroma by dehydroascorbate reductase. Photosynth Res. 1997;53:197–204. doi: 10.1023/A:1005832022204. [DOI] [Google Scholar]
- Morell S, Follmann H, DeTullio M, Häberlein I. Dehydroascorbate and dehydroascorbate reductase are phantom indicators of oxidative stress in plants. FEBS Lett. 1997;414:567–570. doi: 10.1016/S0014-5793(97)01074-0. [DOI] [PubMed] [Google Scholar]
- Morell S, Follmann H, De Tullio M, Häberlein I. Dehydroascorbate reduction: the phantom remaining. FEBS Lett. 1998;425:530–531. doi: 10.1016/S0014-5793(98)00282-8. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Mullineaux PM. The presence of dehydroascorbate and dehydroascorbate reductase in plant tissue. FEBS Lett. 1998;425:528–529. doi: 10.1016/S0014-5793(98)00281-6. [DOI] [PubMed] [Google Scholar]
- Kubo A, Aono M, Nakajima N, Saji H, Tanaka K, Kondo N. Differential responses in activity of antioxidant enzymes to different environmental stresses in Arabidopsis thaliana. J Plant Res. 1999;112:279–290. [Google Scholar]
- Havaux M, Bonfils J-P, Lütz C, Niyogi KK. Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol. 2000;124:273–284. doi: 10.1104/pp.124.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Ikehara N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997;115:1405–1412. doi: 10.1104/pp.115.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H. A function of colour. Trends Plant Sci. 1997;2:7–8. doi: 10.1016/S1360-1385(97)82729-X. [DOI] [Google Scholar]
- Cohen MF, Sakihama Y, Yamasaki H. Roles of plant flavonoids in interactions with microbes: from protection against pathogens to the mediation of mutualism. In: Pandalai SG, editor. In:Recent research developments in plant physiology. Trivandrum, Research signpost; 2001. pp. 157–173. [Google Scholar]
- Schreiber U. Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer. Photosynth Res. 1986;9:261–272. doi: 10.1007/BF00029749. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Agata W, Kawamitsu Y, Hakoyama S, Shima S. A system for measuring leaf gas exchange based on regulating vapour pressure difference. Photosynth Res. 1986;9:345–357. doi: 10.1007/BF00029799. [DOI] [PubMed] [Google Scholar]
- Kawamitsu Y, Boyer JS. Photosynthesis and carbon storage between tides in a brown alga, Fucus vesiculosus L. Marine Biol. 1999;133:361–369. doi: 10.1007/s002270050475. [DOI] [Google Scholar]
- Yamamoto Y, Akasaka T. Degradation of antenna chlorophyll-binding protein CP43 during photoinhibition of photosystem II. Biochemistry. 1995;34:9038–9045. doi: 10.1021/bi00028a012. [DOI] [PubMed] [Google Scholar]