Abstract
Purpose:
To evaluate effectiveness of omega-3 fatty acid supplements in relieving dry eye symptoms and signs in symptomatic visual display terminal users (VDT).
Methods:
A randomized controlled study was done; eyes of 470 VDT users were randomized to receive four capsules twice daily for 6 months (O3FAgroup), each containing 180 mg of eicosapentaenoic acid and 120 mg docosahexaenoic acid. The O3FA group was compared with another group (n = 480) who received four capsules of a placebo (olive oil) twice daily. Patients were evaluated at baseline, 1, 3, and 6 months, respectively. The primary outcome was improvement in omega-3 index (a measure of EPA and DHA ratio in RBC membrane). Secondary outcomes were improvement dry eye symptoms, Nelson grade on conjunctival impression cytology, Schirmer test values, tear film breakup time (TBUT), and tear film osmolarity. Means of groups (pre-treatment, 1, 3, and 6-months) were compared with repeated measure analysis of variance.
Results:
At baseline, 81% patients had low omega-3 index. In the O3FA group, a significant increase in omega-3 index, improvement in symptoms, reduction in tear film osmolarity, and increase in Schirmer, TBUT, and goblet cell density was observed. These changes were not significant in the placebo group. Improvement in test parameters was significantly (P < 0.001) better in patients with low omega3 index (<4%) subgroup.
Conclusion:
Dietary omega-3 fatty acids are effective for dry eye in VDT users; omega-3 index appears to be the predictor to identify potential dry eye patients who are likely to benefit from oral omega-3 dietary intervention.
Keywords: Goblet cell density, Omega-3 fatty acids, Omega-3 index, tear film osmolarity, visual display terminal users
The prevalence of dry eye in visual display terminal users ranges from 30% to 68.5%.[1,2] A study conducted in the India found that the prevalence of dry eye was 32% in the 20–40 years age group.[3] Prolonged VDT tasks reduce blink rate, blink amplitude, and blink quality and consequently, poor tear film quality. A study by Patel et al.[4] observed that the blink rate decreased from 18.4 blinks/min before computer work to 3.6 blinks/min during computer use.
The lockdown for coronavirus disease 2019 led to an upsurge in computer usage as the office going population worked mostly from home.[5,6] Second, the use of electronic devices for education and entertainment prolonged screen time in young people potentially leading to adverse implications on ocular surface health.[7] A prospective study by Krolo et al.[8] found that face mask wear also exacerbated dry eye symptoms in VDT users. An online survey by Saldanha et al.[9] found that COVID-19-related eye strain has compounded the existing dry eye-related societal burden.
Dry eye disease and ocular surface inflammation are synonymous; in dry eye, ocular surface cells express proinflammatory mediators such as prostaglandins (PGE2), interleukins (IL-1), and leukotrienes (LTB4). Second, chronic ocular surface inflammation alters the epithelial cell morphology and reduces conjunctival goblet cell density.[10]
Some authors are of the opinion that the ratio of O6FA to O3FA determines the overall inflammatory status of the body; a cross-sectional study by Miljanovic’ et al.[11] (Women Health Study) found that a higher ratio of O6FA to O3FA consumption was associated with a significantly increased risk of dry eye disease in women (odds ratio = 2.5).
Omega-6 fatty acids produce proinflammatory mediators like PGE2 and LTB4 and act as substrates for production of resolvins. In contrast, O3FAs block the synthesis of these lipids, IL-1, and tumor necrosis factor-alpha.[12]
There have been conflicting reports in literature regarding the effects of O3FAs in patients with dry eyes. The National Institute of Health (NIH) multicenter, double-blind clinical trial in patients with moderate-to-severe dry eye disease claimed that there was no significant improvement in dry eye parameters in those taking supplements containing 3000 mg O3FAs for 12 months (n = 329) as compared to those receiving olive-oil placebo (n = 170).[13] On the contrary, several randomized double-masked trials conducted in India (n > 2000) observed improvement in dry eye parameters and goblet cell density in patients taking O3FA supplements.[14-17]
In this study, we investigated the effect of consumption of fish-based 2,400 mg/day of oral O3FA for 6 months on red blood cell membrane EPA/DHA content (omega-3 index), tear film osmolarity, dry eye symptoms, Nelson grade on conjunctival impression cytology (CIC), and dry eye parameters. We also evaluated whether omega-3 index could be a predictor to identify potential dry eye patients who are likely to benefit from oral omega-3 dietary intervention.
Methods
A randomized, double-masked, interventional study was done at four referral eye centers in the northern part of the Indian subcontinent. The trial was approved by the institutional review boards and the local ethics committee. Written informed consent was obtained from all the participating patients, and the study followed the tenets of the Declaration of Helsinki. The trial was registered with UMIN clinical trial registry number UMIN000049192.
Patient selection
A letter was sent to the supervisors in the health management section of regional call-centers, universities, and information technology (IT) companies to explain the study purpose and to request participation in the study. Three universities, three call-centers, and four IT companies responded and agreed to participate in the study; after reviewing the protocol and potential risks and benefits, permission was granted to conduct the study among employees who were willing. Employees were invited by e-mail to answer a questionnaire; this included information such as demographic characteristics, dietary habits (vegetarian/fish consumer), symptoms experienced, total working hours, and average hours spent in VDT work each day during the past year. A maximum of three e-mail reminders were sent. Employees who completed the questionnaire were requested to attend a dry eye clinic for ophthalmic work-up and blood tests. The “Indian Dry Eye Questionnaire” was administered to symptomatic VDT users; the grading of dry eye disease done based on their response to a questionnaire of common dry eye symptoms [Table 1].[14-17] At random, some subjects made to meet the inclusion criteria twice during one visit or during the run-in period.
Table 1.
Dry eye questionnaire and scoring system (DESS©)
| Symptom | Score (maximum 18) | |||
|---|---|---|---|---|
|
| ||||
| Absent (0) | Sometimes (1) | Frequent (2) | Always present (3) | |
| Itching or burning | ||||
| Sandy or gritty sensation | ||||
| Redness | ||||
| Blurring of vision | ||||
| Ocular fatigue | ||||
| Excessive blinking | ||||
aScores of 0-6 were mild, 6.1-12 were moderate, and 12.1-18 indicated severely symptomatic dry eye [13]. Indian Dry Eye Questionnaire
Exclusion Criteria: Patients having current ocular infection, recent refractive surgery, allergic conjunctivitis, contact lens wear, herpetic eye disease, diabetes, and liver diseases were excluded. Patients with inability to swallow soft gel capsules, on aspirin or anti-coagulant therapy, and those allergic to fluorescein were also excluded. Systemic (tetracycline’s and corticosteroids) or topical medications (other than artificial tear supplements) that could affect tear film or meibomian gland functions were discontinued prior to intervention. Moreover, patients were instructed not to use artificial tear preparations, 2 h prior to testing. To minimize seasonal factors that could skew results, most patients were enrolled during the dry-eye season (winter months).
Randomization, masking, and sample size calculation
Sample size calculation was based on inference for means: comparing two independent samples. To calculate the sample size and to compare the mean difference in omega-3 index between both groups, a pilot study was first done on 50 subjects. The mean increase in the omega-3 index in the omega-3 group was 1.2 and, in the placebo, group was 0.9. The common SD was 1.6. Assuming 1:1 randomization, 90% power (alpha = 0.05), and a precision error of 5% to detect a difference of 20% or more in omega-3 index between both groups, the estimated sample size in each group was calculated to be 450 (www.stat.ubc.ca/;rollin/stats/ssize/n2.html).
The allocation codes were generated by disk operating system-based software in the department of community medicine at our institute. The process was stratified according to clinical center with a permuted-block method with randomly chosen block sizes. Patients were randomly allocated to one of the two groups by parallel assignment. The codes were sealed in green envelopes and were opened by a healthcare personnel not involved in patient care.
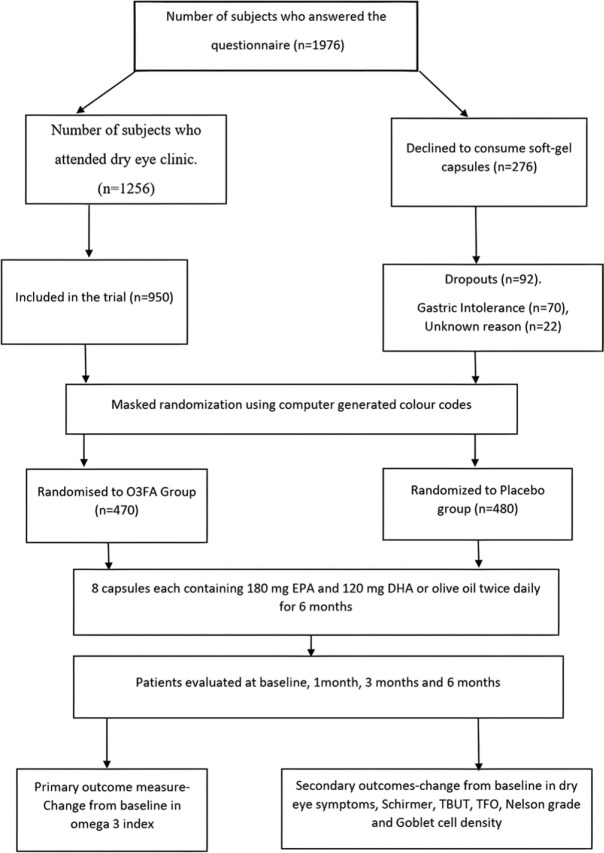
The FDA has established an acceptable daily intake (ADI) of 3 g (3000 mg) per person of combined EPA and DHA from natural fish oil sources in either oil or capsule form. In both trial groups, the regimen was four soft-gel capsules twice daily. In the O3FA group, capsule contained received 180 mg of EPA and 120 mg docosahexaenoic acid (DHA) (total 1,440 mg of EPA +960 mg DHA/day). In the placebo group, olive oil capsules were oleic acid 70%, linoleic acid 18%, and palmitic acid 12%, respectively. The capsules were administered twice daily for 180 days. The subjects were masked to the contents. The two types of capsules and packs were like each other. The subjects were instructed to return the bottles of study capsules at the 1-month visit, and any unused capsules were counted to determine patient compliance with the study protocol, wherein another pack with 240 capsules were provided to them. The subjects were instructed to eat a normal diet (devoid of additional dietary supplements and extra allowances for fatty fish) and not to consume over the counter antioxidants. Fig.1 shows the flowchart for enrolment, randomization, intervention, follow-up, and analysis.
Figure 1.
Flowchart showing patients’ enrolment, randomization, intervention, follow-up, and analysis
Outcome measures
Patients were seen at baseline, 1 month, 3 months, and 6 months after the start of dietary supplementation. The primary outcome measure was the change from baseline in omega-3 index (a measure of the amount of EPA and DHA in the blood, specifically the red blood cell membranes).
Secondary outcomes were subjective dry eye symptoms (a reduction from baseline representing an improvement). A score of 0–3 was assigned to dry eye-related symptoms such as ocular fatigue, blurring of vision, itching, or burning, sandy or gritty sensation, and redness, respectively (DESS); when symptom-free, 0; sometimes present, 1; frequently present, 2; and always present, 3. A score of 0–6 was mild, 6.1–12 moderate, and 12.1–18, severely symptomatic dry eye [Table 1].
Other outcome measures were a change in tear film osmolarity, the Schirmer test value (increase in the amount of wetting representing an improvement), TBUT (increased time [in sec] representing an improvement), and Nelson grade at day 180 (reduction in the grade representing an improvement).
Omega-3 Index
For Omega-3 testing, collaboration was done with a laboratory with highest quality certifications. The facility was College of American Pathologist (CAP) certified, and the testing was National Accreditation Board for Testing and Calibration Laboratories (NABL) accredited. All samples reached the designated laboratory within 12 hours’ time frame as per WHO-IATA guidelines, barcoded, and transported in thermocol box with frozen cool packs. All patients were advised 10–12 hours of fasting for the test. They were instructed to avoid alcohol 24–48 hours before testing. The phlebotomy workforce collected specimens from home. A whole blood sample was taken, and a turnaround time of 8–10 days was given to all patients for reports.
Tear function tests
The participants were instructed to visit the dry eye clinic in the morning, and all the tests were performed at the same time of the day (between 10 AM and 12 PM) in a dimly lit room. The independent investigator (A.A.) was masked to the information obtained from the questionnaire. One eye of each patient was selected at random for examination.
TBUT was performed as described previously.[18] Three readings were taken in succession and averaged. A TBUT of less than 10 s was considered consistent with dry eye.
The subject then waited for another 30 min, and a Schirmer test with anesthesia (0.4% oxybuprocaine hydrochloride) was performed with eyes closed. The length of wetting less than 6 mm was considered consistent with dry eye. To ensure uniformity and eliminate bias, CIC was performed by a single examiner who was masked to information obtained from the questionnaire.
CIC was performed as per the technique described previously.[14-17] At least 10 high-power fields were examined for goblet cells and epithelial cells. Grading and scoring were done according to the criteria suggested by Nelson et al.[19]
Statistical analysis
Statistical analysis was performed on an intent-to-treat basis using IBM, SPSS Statistics version 29 (IBM Inc.). Independent t-tests were performed to ensure group similarities at baseline. Chi-square tests were used for proportions. Linear regression with a robust variance estimator was used to compare mean change in continuous variable between the two groups. A one-way repeated-measures analysis of variance (ANOVA) was conducted to determine whether there were significant differences in mean test values over the course of 6 months of intervention (O3FA or placebo). A P value less than 0.005 was considered statistically significant. A multiple regression was done to ascertain the effect of the daily hr spent at VDT and the independent variables over 6 months of dietary intervention (omega-3 index, tear film osmolarity, Schirmer test score, TBUT, and Nelson grade).
Results
Patient selection: A total of 1976 subjects responded to our email questionnaire. Out of these, 1256 subjects attended the dry eye clinics of the four referral eye centers. Two hundred and seventy-six subjects declined to participated in the study as they were strict vegetarians and had reservation on consumption the fish-oil (n = 176) and/or soft-gel placebo capsules (n = 100), respectively. A total of 950 (49.6%) patients were recruited for participation in the trial. Four hundred seventy patients were assigned to the O3FA group and 480 patients to the placebo group.
Adherence to the study protocol: In the O3FA group, 84 (17.8%) patients were found irregular with their supplements due to fish-burps and gastric intolerance at first follow-up visit. This was severe enough to warrant discontinuation of treatment in 70 (14.9%) patients. In the placebo group, 24 (5%) patients complained of skin rashes after consumption of soft-gel capsules. However, these were not severe enough to warrant discontinuation of treatment. Another 22 (4.6%) patients declined to participate due to unknown reasons. All dropouts (n = 70 + 22 = 92) were included for analysis based on the last-observation-carried-forward method.
Baseline characteristics: Table 2 shows the mean age, gender, VDT time, mean omega-3 index, mean baseline symptom score, Schirmer score, TBUT, tear film osmolarity, Nelson grade, and goblet cell density before random allocation in both groups; the intergroup differences regarding these variables did not statistically differ at baseline.
Table 2.
Baseline characteristics of patients
| Parameter | O3FA group | Placebo group | Mean difference | P |
|---|---|---|---|---|
| Age | 26.5±2.4 | 25.8±2.6 | 0.7 | 0.092 |
| Male | 225 | 242 | 17 | 0.302* |
| Female | 255 | 238 | 17 | |
| VDT time | 8.2±1.3 | 8.4±1.6 | 0.2 | 0.067 |
| Omega-3 index | 3.5±0.7 | 3.4±0.6 | 0.1 | 0.056 |
| Symptom score | 8.1±2.7 | 7.9±2.6 | 0.63 | 0.716 |
| Schirmer | 12.9±4.6 | 12.5±4.3 | 0.4 | 0.171 |
| TBUT | 7.8±2.2 | 8±1.8 | 0.2 | 0.369 |
| TFO | 322±3 | 320±3.2 | 2.0 | 0.964 |
| Nelson grade | 1.23±0.8 | 1.2±0.7 | 0.03 | 0.056 |
| GCD | 567±223 | 570±220 | 3.0 | 0.856 |
| Follow-up (months) | 11.6±2.4 | 11.2±2.7 | 0.4 | 0.356 |
*Chi-square tests, visual display terminal users (VDT), tear film breakup time (TBUT), tear film osmolarity (TFO), goblet cell density (GCD)
At baseline, in the placebo group, 80% patients had low omega-3 index (less than 4%), 20% patients had abnormal Schirmer scores, 48% had abnormal TBUT, 44% had increased tear film osmolarity, and 30% had abnormal cytology. In the O3FA group, 82% patients had low omega-3 index, 22% patients had abnormal Schirmer scores, 46% had abnormal TBUT, 42% had increased tear film osmolarity, and 34% had abnormal cytology; examination of CIC specimens under a light microscope revealed an increase in the nuclear–cytoplasmic ratio of non-secretory epithelial cells along with reduction in goblet cell density [Fig. 2].
Figure 2.
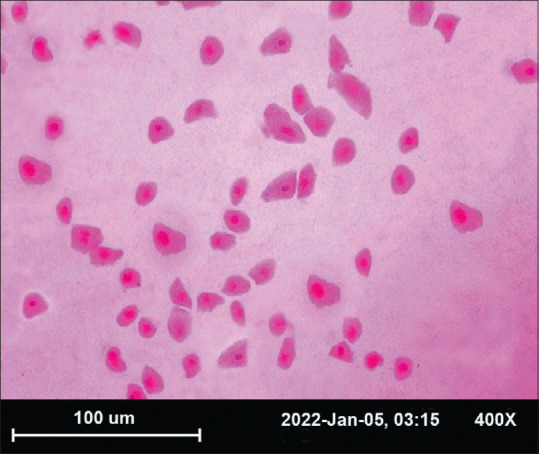
Photomicrograph of impression cytology specimen, stained with periodic acid-Schiff, and hematoxylin-eosin at X 400 with squamous metaplasia. Showing both normal cells (NC) and increased nuclear–cytoplasmic ratio (SM)
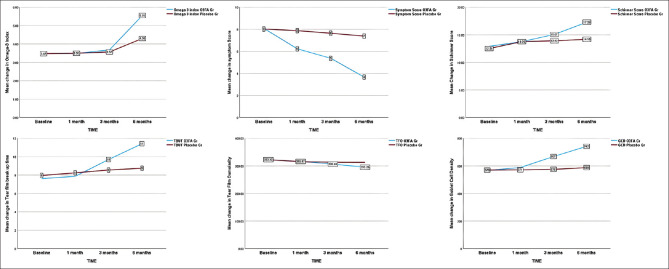
Omega-3 index: In the O3FA group, repeated-measures ANOVA revealed that there was a significant change in mean omega-3 index over 6 months of intervention (F [1, 459] = 2341, P < 0.001). The post hoc test revealed that O3FA dietary intervention did not elicited a significant increase (P < 0.001) in mean omega-3 index from baseline to 1 month (ANOVA, P = 0.976) and baseline to 3 months (ANOVA, P = 0.345) of intervention, respectively (mean scores of 3.45 ± 0.7, 3.5 ± 0.7, and 3.7 ± 0.4, respectively). After intervention (at 6 months), the omega-3 index increased to 5.5, which was significantly different (P < 0.001) from the score before treatment and after 3 months. In the placebo group, mean change in omega-3 index was not significant over 6 months of intervention (F [1.498, 717] =541, P = 0.245).
Dry eye symptoms: In the O3FA group, repeated-measures ANOVA revealed that there was a significant change in the mean symptom score over 6 months of intervention (F [1.971, 944] =854, P < 0.001). The post hoc test revealed that O3FA dietary intervention elicited a significant reduction (P < 0.001) in symptoms at all time points. In the placebo group, mean symptom scores did not differ significantly over time (F [1.971, 1437] =854, P = 0.121).
Schirmer Test: Repeated-measures ANOVA in the O3FA group revealed there was a significant increase in the mean Schirmer scores over 6 months of intervention (F [3, 1437] =1268, P < 0.001). On post hoc analysis, the change from baseline in Schirmer test score was not statistically significant at 1 month. However, there was a significant (P = 0.005) increase at 3 months and 6 months, respectively (mean scores of 12.9 ± 4.6 at baseline, to 15 ± 4.5 at 3 months, and 17.3 ± 5 at 6 months). In the placebo group, the mean Schirmer scores were not significantly different at 6 months (F [3, 1437] =319, P = 0.256).
Tear film breakup time: In the O3FA group, there was a significant increase in the mean TBUT scores at 6 months (F [3, 1437 = 1107, P < 0.001). On post hoc analysis, the change in TBUT scores at 1 month was not statistically significant from baseline (mean score of 7.6 ± 2.2 at baseline vs 7.8 ± 1.9 sec at 1 month). However, after intervention, TBUT increased to 11.2 ± 1.9 sec at 6 months, which was significantly different (P < 0.001) from that at baseline and after 3 months (9.3 ± 2.4). In the placebo group, mean TBUT scores did not differ significantly at 6 months (F [1, 479] =1108, P = 0.121).
Tear film osmolarity: In the O3FA group, the mean tear film osmolarity scores were significantly different at 6 months (F [3, 1437 = 1706, P < 0.001). The post hoc test revealed that O3FA intervention did not elicit a significant reduction in tear film osmolarity from baseline to 1 month (mean score of 322 ± 33.2 vs 316 ± 35.8). At 3 months, the mean tear film osmolarity was 306 ± 30.8 which significantly (P < 0.001) decreased to 295 ± 30.6 at 6 months. In the placebo group, mean tear film osmolarity did not differ significantly at 6 months (F [3, 479] =798, P = 0.345).
Conjunctival impression cytology: Repeated-measures ANOVA in the O3FA group revealed that there was a significant increase in goblet cell density over time (F [3, 1437] = 445, P < 0.001). The post hoc test revealed that the O3FA intervention did not elicit a significant change (P = 0.345) in the goblet cell density (GCD) from pre-treatment to 1 month of intervention (mean score of 567 ± 223 vs. 588 ± 430). The GCD increased to 741 ± 221 at 6 months, which was significantly different (P < 0.001) from the density before treatment and after 3 months. In the placebo group, mean scores for the GCD were not significantly different at 6 months (F [3, 1437] = 255, P = 0.115).
Fig. 3 depicts a line diagram comparing the mean change in omega-3 index, symptoms, Schirmer, TBUT, tear film osmolarity, and goblet cell density, respectively, between the two groups over 6 months of dietary intervention.
Figure 3.
Line diagram showing mean change in omega-3 index, dry eye symptom score, Schirmer score, tear film breakup time, tear film osmolarity, goblet cell density, and Nelson grade between O3FA and placebo groups at baseline, 1 month, 3 months, and 6 months, respectively
Correlations: At baseline [Table 3], there was a significant (P < 0.001) and inverse correlation between omega-3 index, dry eye symptoms, and tear film osmolarity in O3FA and placebo groups. A significant (P < 0.001) and positive correlation was observed between omega-3 index, tear film breakup time, Schirmer score, and goblet cell density in both the groups.
Table 3.
Correlations between Omega-3 index and study parameters at baseline
| Parameter | Omega-3 Group | Placebo group | ||
|---|---|---|---|---|
|
|
|
|||
| Pearson’s correlation coefficient (r) with 95% CI | P | Pearson’s correlation coefficient (r) with 95% CI | P | |
| Symptom score | -0.440 (-0.509 to -0.365) | <0.001 | -0.406 (-0.478 to -0.329) | <0.001 |
| Schirmer | 0.412 (0.335 to 0.484) | <0.001 | 0.204 (0.116 to 0.288) | <0.001 |
| TBUT | 0.307 (0.223 to 0.386) | <0.001 | 0.404 (0.327 to 0.477) | <0.001 |
| Tear film osmolarity | -0.158 (-0.244 to -0.069) | <0.001 | -0.315 (-0.392 to -0.231) | <0.001 |
| Goblet cell density | 0.547 (0.481 to 0.607) | <0.001 | 0.390 (0.312 to 0.464) | <0.001 |
CI (95% confidence interval)
Subgroup Analysis: After 6 months of dietary intervention [Table 4], subjects with omega-3 index <4 had significantly better reduction in symptom score, tear film osmolarity, and Nelson grade as compared to patients with omega-3 index >4. Moreover, there was significantly better increase in Schirmer score, TBUT, and goblet cell density in this subgroup of patients (omega-3 index less than 4). In the placebo group, the difference was not statistically significant [Table 5].
Table 4.
Omega-3 index and study parameters at 6 months in O3FA group
| Parameter | Omega-3 index (O3FA group) | P | |
|---|---|---|---|
|
| |||
| Less than 4 | More than 4 | ||
| Symptom score (6M) | 3.5±1.8 | 3.8±1.7 | 0.043 |
| Schirmer score (6M) | 18±4.6 | 16.4±5.4 | <0.001 |
| TBUT (6M) | 12.4±1.7 | 10.4±1.9 | <0.001 |
| TFO (6M) | 290±6.6 | 298±6.6 | <0.001 |
| Nelson Grade (6M) | 0.55±0.5 | 0.71±0.5 | 0.002 |
| GCD (6M) | 770±209 | 711±230 | <0.005 |
Table 5.
Omega-3 index and study parameters at 6 months in placebo group
| Parameter | Omega-3 index (O3FA Placebo Group) | P | |
|---|---|---|---|
|
| |||
| Less than 4 | More than 4 | ||
| Symptom score (6M) | 7.4±2.3 | 7.3±2.1 | 0.610 |
| Schirmer score (6M) | 14.2±4.3 | 14±4.5 | 0.963 |
| TBUT (6M) | 8.7±2 | 8.6±1.8 | 0.514 |
| TFO (6M) | 312±4.5 | 310±4 | 0.096 |
| Nelson Grade (6M) | 0.95±0.6 | 1±0.6 | 0.310 |
| GCD (6M) | 587±220 | 590±216 | 0.902 |
Chi-square tests, visual display terminal users (VDT), tear film breakup time (TBUT), tear film osmolarity (TFO), goblet cell density (GCD)
Follow-up: The mean follow-up in O3FA group was 11.6 ± 2.4, and in placebo group was 11.2 ± 2.7 months (independent t-test, P = 0.345), respectively. The compliance to follow-up (completed follow-up visits) was 90% patients in O3FA group and 87% patients in placebo group.
Discussion
In our randomized, multicenter clinical trial of 6 months of daily oral supplementation with 2400 mg omega-3 fatty acids for dry eye in symptomatic VDT users, there was a significant change in omega-3 index, dry eye symptoms and tear film tests in patients which received active omega supplement (O3FA group). The change was not significant in patients who received olive oil (placebo group).
It was observed that 80% patients in our study sample had low omega -3 index at baseline. In O3FA group, there was a significant increase in the omega -3 index at 6 months. In terms of percentage, omega-3 index increased by 63% in O3FA group indicating a high level of adherence to the treatment protocol. The baseline omega-3 index correlated significantly (P < 0.001) and inversely with dry eye symptoms and tear film osmolarity and positively with Schirmer, TBUT, and goblet cell density. Our results suggest that improvement in dry eye symptoms and tear film indices were more marked in the subcategory of patients with omega-3 index <4%.
A meta-analysis of 17 randomized controlled trials evaluating efficacy of omega-3 fatty acids in dry eye (n = 3363) was conducted by Giannaccare et al.[20] by using a random-effects model. The authors observed a higher improvement of dry eye symptoms and TBUT in studies conducted in India. In these studies, there was no evidence of publication bias. Moreover, a sensitivity analyses done by the authors indicated robustness of results in these studies. However, the they did not comment on the source of heterogeneity between trials conducted in the subcontinent and the American population.
Direct comparison of the present study with other placebo-controlled trials is limited by several factors.[12,21-23] The DREAM trial was conducted in American population with different dietary practices as compared to Indians. In India, fish especially cold-water fish is not an essential component of north Indian diet. Moreover, Indian fishes like Rohu, Catla, Pangas, and Magur have a significantly lower (P < 0.05) omega-3 content in comparison to Salmon, Tuna, Sardines, and Mackerel.[24] Lack of fatty fish in diet and a predominantly vegetarian diet comprising small quantities of omega-3 alpha linoleic acid (ALA) obtained from dark green leafy vegetables and soybean oil in vegetarians are unlikely to provide acceptable O3FA levels in these subjects. This could partially explain the low baseline omega-3 index seen in our study sample (both groups).
The present study enrolled substantially larger controls than other studies (n = 480). Most other studies enrolled fewer than 200 controls making comparisons less valid in the treatment group. Most trials including the DREAM trial considered improvement in symptoms as primary outcome based on ocular surface disease index (OSDI) scale. On the contrary, we evaluated symptoms as secondary outcome using the dry eye scoring system (DESS) scale. The DESS scale consists of six items covering itching or burning, sandy or gritty sensation, redness, blurring of vision, ocular fatigue, or excessive blinking. On the other hand, OSDI is a 12-item scale evaluating symptoms and two additional subscales for visual function and environmental trigger. Both scales are different in concept and units of measurement. Wu et al.[25] observed that most Indian studies have evaluated effect of omega-3 on dry eye symptoms using DESS because country difference is a potential factor leading to heterogeneity due to different dietary practices between India and other countries. We believe that country difference and measurement difference cannot be separated until both scales are compared by future studies.[14-17]
The significantly better improvement in dry eye symptoms and tear film indices in patients with low omega-3 index following dietary supplementation suggest role and effectiveness of omega-3 fatty acids in dry eye patients. Having said this, further validation of omega-3 index is needed in populations with different dietary practices and dry eye subsets.
Conclusion
In conclusion, dietary omega-3 fatty acid supplements are effective in relieving dry eye symptoms and improving dry eye indices in symptomatic VDT users in comparison to olive oil placebo. The benefit seems to be more marked in patients with low omega-3 index. Country difference may be the source of heterogeneity in the pooled effect of Omega-3 on dry eye symptom score.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank <www.indianmedicalstats.com> for statistical analysis.
References
- 1.Courtin R, Pereira B, Naughton G, Chamoux A, Chiambaretta F, Lanhers C, et al. Prevalence of dry eye disease in visual display terminal workers: A systematic review and meta-analysis. BMJ Open. 2016;6:e009675. doi: 10.1136/bmjopen-2015-009675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian L, Wei W. Identified risk factors for dry eye syndrome: A systematic review and meta-analysis. PLoS One. 2022;17:e0271267. doi: 10.1371/journal.pone.0271267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Titiyal JS, Falera RC, Kaur M, Sharma V, Sharma N. Prevalence, and risk factors of dry eye disease in North India: Ocular surface disease index-based cross-sectional hospital study. Indian J Ophthalmol. 2018;66:207–11. doi: 10.4103/ijo.IJO_698_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel S, Henderson R, Bradley L, Galloway B, Hunter L. Effect of visual display unit use on blink rate and tear stability. Optom Vis Sci. 1991;68:888–92. doi: 10.1097/00006324-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Napoli PE, Nioi M, Fossarello M. The “Quarantine Dry Eye”: The Lockdown for coronavirus disease 2019 and its implications for ocular surface health. Risk Manag Healthc Policy. 2021;14:1629–36. doi: 10.2147/RMHP.S277067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh S, Rhee MK. COVID-19 and dry eye. Eye Contact Lens. 2021;47:317–22. doi: 10.1097/ICL.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 7.Alnahdi W, Hadrawi M, Danish E, Alghamdi A, Taher N, Alfaraidi AT, et al. Relationship between screen time and dry eye symptoms during the COVID-19 pandemic in the pediatric population of the Western Region of Saudi Arabia. Cureus. 2022;14:e31015. doi: 10.7759/cureus.31015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krolo I, Blazeka M, Merdzo I, Vrtar I, Sabol I, Petric-Vickovic I. Mask-associated dry eye during COVID-19 pandemic-how face masks contribute to dry eye disease symptoms. Med Arch. 2021;75:144–8. doi: 10.5455/medarh.2021.75.144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saldanha IJ, Petris R, Makara M, Channa P, Akpek EK. Impact of the COVID-19 pandemic on eye strain and dry eye symptoms. Ocul Surf. 2021;22:38–46. doi: 10.1016/j.jtos.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, Yu C, Dong H, Mu Y, Zhang R, Zhang Q, et al. Recent developments about the pathogenesis of dry eye disease: Based on immune inflammatory mechanisms. Front Pharmacol. 2021;12:732887. doi: 10.3389/fphar.2021.732887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miljanović B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–93. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Name MA, Savoye M, Chick JM, Galuppo BT, Feldstein AE, Pierpont B, et al. A Low w-6 to w-3 PUFA Ratio (n-6: n-3 PUFA) diet to treat fatty liver disease in obese youth. J Nutr. 2020;150:2314–21. doi: 10.1093/jn/nxaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asbell PA, Maguire MG, Pistilli M, Ying GS, Szczotka-Flynn LB, et al. Dry Eye Assessment and Management Study Research Group. n-3 fatty acid supplementation for the treatment of dry eye disease. N Engl J Med. 2018;378:1681–90. doi: 10.1056/NEJMoa1709691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhargava R, Kumar P, Kumar M, Mehra N, Mishra A. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int J Ophthalmol. 2013;6:811–6. doi: 10.3980/j.issn.2222-3959.2013.06.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhargava R, Kumar P, Phogat H, Kaur A, Kumar M. Oral omega-3 fatty acids treatment in computer vision syndrome related dry eye. Cont Lens Anterior Eye. 2015;38:206–10. doi: 10.1016/j.clae.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Bhargava R, Chandra M, Bansal U, Singh D, Ranjan S, Sharma S. A randomized controlled trial of omega 3 fatty acids in rosacea patients with dry eye symptoms. Curr Eye Res. 2016;41:1274–80. doi: 10.3109/02713683.2015.1122810. [DOI] [PubMed] [Google Scholar]
- 17.Bhargava R, Kumar P. Oral omega-3 fatty acid treatment for dry eye in contact lens wearers. Cornea. 2015;34:413–20. doi: 10.1097/ICO.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 18.Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. Clao J. 2015;21:221–32. [PubMed] [Google Scholar]
- 19.Nelson JD, Havener VR, Cameron JD. Cellulose acetate impressions of the ocular surface. Dry eye states. Arch Ophthalmol. 1983;101:1869–72. doi: 10.1001/archopht.1983.01040020871007. [DOI] [PubMed] [Google Scholar]
- 20.Giannaccare G, Pellegrini M, Sebastiani S, Bernabei F, Roda M, Taroni L, et al. Efficacy of Omega-3 fatty acid supplementation for treatment of dry eye disease: A Meta-analysis of randomized clinical trials. Cornea. 2019;38:565–73. doi: 10.1097/ICO.0000000000001884. [DOI] [PubMed] [Google Scholar]
- 21.Kangari H, Eftekhari MH, Sardari S, Hashemi H, Salamzadeh J, Ghassemi-Broumand M, et al. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology. 2013;120:2191–6. doi: 10.1016/j.ophtha.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Epitropoulos AT, Donnenfeld ED, Shah ZA, Holland EJ, Gross M, Faulkner WJ, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35:1185–91. doi: 10.1097/ICO.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deinema LA, Vingrys AJ, Wong CY, Jackson DC, Chinnery HR, Downie LE. A randomized, double-masked, placebo-controlled clinical trial of two forms of omega-3 supplements for treating dry eye disease. Ophthalmology. 2017;124:43–52. doi: 10.1016/j.ophtha.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 24.Jakhar KA, Pal AK, Reddy DA, Sahu NP, Venkateshwarlu G, Vardia HK. Fatty acids composition of some selected Indian fishes. Afr J Basic Appl Sci. 2012;4:155–60. [Google Scholar]
- 25.Wu CL, Tuan HI, Kang YN. What causes heterogeneity in the pooled effect of Omega-3 on dry eye symptom score? Countries or measurements? Cornea. 2019;38:e54. doi: 10.1097/ICO.0000000000002108. [DOI] [PubMed] [Google Scholar]