Abstract
Dry eye disease (DED) is prevalent in all age groups and is known to cause chronic ocular discomfort and pain, and greatly affects the quality of life. Patients with ocular surface disease (OSD) may also have reduced tear secretion due to lacrimal gland damage, thus leading to aqueous deficient DED. Even with conventional management modalities such as lubricating eyedrops, topical corticosteroids, autologous serum eyedrops, or punctal plugs, many patients continue to suffer from debilitating symptoms. Contact lenses are increasingly being used in OSD providing surface hydration, protection from environmental insults, mechanical damage from abnormal lids, and as a modality for constant drug delivery to the ocular surface. This review describes the role of soft lenses and rigid gas-permeable scleral lenses in the management of DED associated with OSD. The efficacy of contact lenses, lens selection, and optimal lens fit are reviewed for specific indications.
Keywords: Aqueous deficiency dry eye, bandage contact lens, contact lens, dry eye disease, graft-versus-host disease, ocular chemical burns, ocular surface disease, scleral lens, Stevens–Johnson syndrome
Dry eye disease (DED) is a multifactorial disease with symptoms of discomfort, visual disturbance, and tear film instability.[1] It is associated with increased tear film osmolarity, inflammation of the ocular surface, and neurosensory abnormalities in the form of neuropathic pain. The worldwide prevalence of DED ranges from 5–50%.[2,3] Donthineni et al.[4] reported the incidence rate of DED to be 1.58% over 8 years in the elderly age group in the urban Indian population with an equal predisposition for both genders. However, the incidence was significantly more in populations of higher socioeconomic strata. The incidence among children and adolescents was reported to be 0.4%.[4]
Various management options include lubricating eyedrops, topical corticosteroids or cyclosporine, autologous serum eyedrops, punctal plugs, and minor surgical options such as punctal occlusion and tarsorrhaphy, which remain the same despite decades of innovation. Patients with ocular surface disease (OSD) may have associated corneal scarring and neovascularization with poor or unstable tear film contributing to reduced vision. Patients with DED at any stage of the disease suffer from constant discomfort, pain, and role limitation that greatly affect their quality of life.[5] Corneal transplantation is not an option for vision restoration in these patients due to the high risk of failure and poor rates of graft survival.
Contact lenses play an important role in the management of DED. They provide symptomatic relief, provide visual rehabilitation, protect the ocular surface, and keep the ocular surface moist. TFOS DEWS II report recommends the use of contact lenses earlier in the staged management of DED compared to amniotic membrane graft (AMG).[6] In this review, we review the use of contact lenses in the management of DED related to OSD.
Soft Contact Lenses
Soft contact lenses (SCL) are commonly used for refractive error correction.[7] They can be used as an adjunct for treating any ocular disease in which case they might be termed medical contact lenses. Medical contact lenses might incidentally correct refractive errors. SCL when used to protect the ocular surface from mechanical damage by the lids or to treat the underlying condition or aid in the healing of the ocular surface is known as therapeutic contact lenses (TCL)[8] or bandage contact lenses (BCL). The purpose of a BCL is to improve ocular comfort and reduce the effects of an adverse environment. They not only provide mechanical protection to the cornea but also reduce corneal desiccation,[9] improve corneal wound healing,[10,11] and relieve pain.[12-15]
Pain relief
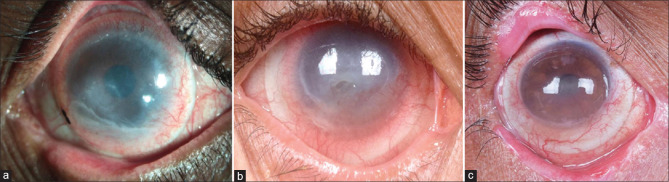
BCLs provide pain relief in various OSDs such as acute or chronic chemical and thermal burns[Fig. 1a, 1b], acute Stevens–Johnson syndrome (SJS)/toxic epidermal necrolysis,[16] and various post-surgical conditions.[12-15] The exact mechanism by which a BCL relieves pain has not been elucidated, but likely involves direct shielding of nociceptors at the ocular surface from environmental stimulation or support of the cellular structure and extracellular matrix elements.[17,18] Timely treatment of any corneal epitheliopathy is considered important for minimizing the risk of developing chronic pain; once there has been centralization of neuropathic pain, a BCL, which might reduce peripheral signaling, may be insufficient for reducing symptoms[17]
Figure 1.
Uses of a bandage contact lens: (a) Right eye of a patient with an acute alkali corneal burn at presentation with corneal and conjunctival epithelial defects. (b) After placement of a bandage contact lens (BCL) and medical management with topical medications, the complete resolution was noted after 5 days. (c) A poorly fitted BCL over keratoprosthesis in the immediate post-operative period shows folds on the BCL along with inferior displacement. (d) BCL placed over keratoprosthesis with a steep fit, showing an air bubble trapped between the BCL and the anterior surface of the cornea
Promoting epithelial healing
In a controlled trial of pressure patch versus BCL, Donnenfeld et al.[10] showed that the use of a BCL significantly shortens the time required for a patient to return to normal activities by aiding in epithelial healing in cases of traumatic epithelial defects. Arora et al.[19] used balafilcon A for overnight wear for persistent epithelial defects (PEDs) and demonstrated that 78.9% of eyes with PEDs healed completely. A similar study was conducted using lotrafilcon A for overnight wear and stated complete healing of PEDs in OSD without sight-threatening complications.[20] A combination of 20% autologous serum eyedrops along with BCLs was also used by Lee et. al, and they reported healing of PEDs in all the eyes with good tolerance to BCLs and no recurrence over a 3-month follow-up period.[21] BCLs, in particular silicone hydrogel (SiHy) lenses, are also effective in promoting healing in ocular chemical injuries.[22] They were also investigated in the treatment of moderate to severe ocular graft-versus-host disease (GVHD) and found to be a safe, tolerable, and effective treatment option for patients who remained symptomatic despite conventional treatments.[23]
In a study of 40 subjects with Sjogren’s syndrome (SS), Li et al.[24] compared the efficacy of BCL to autologous serum and concluded that SiHy lenses were effective in the management of SS-associated DED. At 6 weeks of follow-up, subjects fitted with BCLs had a significant improvement in best-corrected visual acuity (which remained stable for up to 6 weeks after discontinuation of contact lens wear) and significantly improved ocular surface disease index (OSDI) scores, compared with subjects that were treated with autologous serum. Both intervention groups also showed relative improvements in quality-of-life scores, tear break-up time, and corneal staining, compared with baseline. No adverse events were reported in their study group over the entire follow-up period.
The presence of keratoconjunctivitis sicca is the most common ocular manifestation in patients with GVHD. Other ocular findings include punctate epithelial erosions, filamentary keratitis, chronic lid abnormalities with meibomian gland atrophy, lid fibrosis, keratinization, and recurrent corneal epithelial defects leading to corneal ulceration and melt.[25,26] Inamoto et al.[27] used 14–18 mm large diameter hydrogel or SiHy SCL and concluded that these lenses improved vision and objective measures in 50% of patients. SiHy lenses used on a 7-night continuous wear basis for 1 month were reported to have reduced the dry eye symptoms and improved visual acuity.[23]
BCLs have been used in promoting wound healing and aiding in the comfort of patients after cataract surgeries. In two studies that included patients who underwent phacoemulsification and were prescribed BCLs for 1 week, patients reported a decrease in dry eye symptoms with improved OSDI scores. The authors proposed that BCL improved post-operative tear-film stability thus reducing discomfort.[28,29] There are various other studies that describe the use of BCLs in the postoperative period in eyes with penetrating keratoplasty,[30] pterygium surgery,[31,32] post-ptosis surgery,[33] or after keratorefractive surgeries.[34,35]
Corneal protection
In patients with lid conditions such as trichiasis, post–ptosis surgery, and tarsal scarring, these lenses act as a shield and protect the corneal surface from mechanical trauma although the earliest literature describes 20% failure due to superadded infection or advanced corneal pathology.[36,37] Patients with certain OSD, such as SJS may have additional ocular involvement beyond trichiasis and distichiasis in the form of severe dry eyes, punctate epithelial erosions, limbal stem cell deficiency (LSCD), lid margin keratinization, cicatricial entropion, and its associated lid-wiper keratopathy due to mechanical abrasions.[38] Though most literature discusses the role of scleral and mini-scleral lenses, BCLs can be also used successfully in such eyes with epithelial defects to aid in corneal epithelization although this may be used for a short period until epithelization is complete.[39-41]
BCLs also play a vital role in maintaining the hydration of corneas post keratoprosthesis [Fig. 1c, 1d]. They stabilize the tear film over the anterior surface of the graft and prevent it from dehydration, desiccation, dellen formation, and eventual graft melt.[42] Kammerdiener et al.[43] have described that the use of SCL in such eyes is associated with fewer complications.
Corneal sealing
SCLs act as a splint in cases of corneal perforation and are effective in sealing them.[44] A study conducted by Lim et al.[45] reported the effective use of SiHy lenses in corneal perforations <2 mm. For larger perforations, fibrin glue and therapeutic BCLs can act as a temporizing measure before penetrating keratoplasty because outcomes for penetrating keratoplasty are often poor in the presence of active inflammation.[46,47] The use of cyanoacrylate glue as a tissue adhesive over the corneal surface can cause discomfort and pain with each lid blink. BCLs act as a mechanical barrier and provide symptomatic relief. Amniotic membrane[44] or Tenon’s patch[48] when used need to be secured with either fibrin glue or sutures. Placement of BCL in these eyes ensures mechanical protection of the graft and prevents its dislodgment with lid movement.[49] BCLs have also been used successfully in managing corneal perforations in eyes with SS.[50]
Drug delivery
Ocular drug delivery involves achieving therapeutic concentrations of medication at the ocular surface. This is traditionally achieved through frequent administration of eye drops. Unfortunately, frequent dosing is inconvenient, not cost-effective, and often leads to patient non-compliance. To overcome these limitations, it is suggested that TCLs may be suitable for controlled and sustained drug delivery due to their extended-wear function and higher bioavailability than eye drop formulations.[51] These lenses, being hydrophilic, can be used for the prolonged release of medication onto the ocular surface.[52,53] Various drug delivery techniques include a soaking method, molecular imprinting, entrapment of drug-laden colloidal nanoparticles, drug plates, ion ligand polymeric systems, and superficial fluid technology.[54-56] Simulation and in vitro experiments have shown promising results—drug delivery from an SCL is more efficient than drug delivery by eye drops, with larger fractional uptake and higher bioavailability.[57-59] Ciolino et al. also described the role of SCLs in the prolonged delivery of different drugs for various indications.[60-62] Commercially available lenses are also available for drug delivery such as HYPER-CL (EyeYon Medical, Ness Tziona, Israel) and Acuvue Theravision with Ketotifen (ATK) (Johnson & Johnson Vision Care, Inc., Jacksonville, Florida, USA). HYPER-CL or hyperosmotic CL has a unique design that forms a cavity between the lens and the cornea where the instilled eyedrop gets trapped and increases the contact time with the cornea. Its use has been reported for corneal edema,[63] Fuch’s endothelial dystrophy,[64] and in patients with SS.[65] ATK contains ketotifen fumarate and is the first drug-eluting contact lens containing an anti-allergic drug. Each lens contains 0.019 mg of ketotifen fumarate, which is released on lens application.[66] Although research on TCLs in drug delivery appears promising, there are currently limited clinical and commercial applications.
Contact lens materials
Depending upon the severity and nature of the OSD, TCLs can be prescribed for short-term (days) or long-term (years) use and may be worn on either a daily wear or extended wear basis. SiHy has very high oxygen permeability, which minimizes the induced hypoxic and hypercapnic stress;[67] hence, can be used for prolonged therapeutic wear especially in eyes with OSDs. Their relatively low water content is useful and performs better as compared to conventional hydrogels.[67,68] Several studies[68-73] have investigated the comfort difference between hydrogels and SiHy; however, the results were contradictory though these studies included the general population and not patients with OSDs.
TCLs are often used for extended wear and hence need to have high oxygen transmissibility (Dk/L) to be suitable. The critical lens oxygen transmissibility needed to limit overnight corneal edema to 4% (the level experienced without a contact lens in place) was found to be 125 × 10–9 (cm · mL O2) (sec · mL · mm Hg).[74] Currently, most silicone–hydrogel lenses, but not hydrogel lenses, fulfill these criteria. Although fitting BCLs, the diameter of the lenses should be 13.5 mm for full corneal coverage, and larger lenses (e.g., Kontur Contact Lens Co., Richmond, California, USA) may be required in certain conditions such as proptosis-related exposure keratopathy, seventh nerve palsy, and after acoustic neuroma surgery. Commercially available therapeutic SiHy lenses frequently have limited base curvature options (usually 2, e.g., 8.4 and 8.8 mm in Johnson and Johnson Acuvue Oasys Lenses, Jacksonville, FL), and hence these lenses may not fit the whole range of corneas with OSDs. Thus, customized lenses might be needed to provide optimal protection and coverage. A few Indian brands provide customization in terms of base curvatures (ranging from 7 mm to 9.80 mm) and diameters (from 10.50 to 20 mm) to meet fitting requirements for the steepest or flattest corneas (Purecon Asfeer, India).
The presence of microbial keratitis (MK) is an absolute contraindication to TCL use with the notable exception of cases where cyanoacrylate glue has been used in an eye with MK with impending perforation. Also, in eyes with neurotrophic cornea/exposure keratopathy with secondary infections, TCLs can be used if a tarsorrhaphy cannot be performed. A relative contraindication of therapeutic lens wear is corneal anesthesia. These patients have reduced pain sensation, lacrimal, and blink reflex, and may be unable to detect symptoms of complications. In these patients, it is permissible to use contact lenses with close follow-up to detect intolerance or early complications. For patients with significant lagophthalmos and exposure keratopathy, localized drying of the contact lens surface might cause discomfort and mechanical abrasions to the ocular surface limiting its use. In-office assessment of retention after 30–60 min and close follow-up are warranted.
Clinical approach to the use of soft contact lenses in DED
For patients with DED, the decision of lens material and the wearing regimen should be carefully made. Before prescribing lenses, blepharitis, meibomian gland dysfunction, and allergy should be addressed. Overnight use of CL will retain inflammatory cells on the ocular surface, thus can exacerbate discomfort and dryness, and is associated with a higher rate of MK, regardless of material. Daily disposable lens wear is recommended, when possible, as this mode of wear is associated with the lowest rates of infectious and inflammatory complications. Rubbing is recommended as part of the daily cleaning regimen for any reusable lens as no rub may not clean the surface and has been associated with an outbreak of MK. No rub solutions have been taken off the market in the United States (US) when it was discovered that two were associated with outbreaks of MK in 2006 and 2007.[75,76] Hydrogel polymers can be classified into ionic and non-ionic materials.[77] Low water content, non-ionic material undergoes less dehydration and deposition and thus has better comfort and a longer wearing time.[77,78] Silicone hydrogel lenses were introduced in the early part of this century to increase oxygen permeability and reduce the likelihood of infection with overnight wear but unfortunately, the rate of infections with these lenses is not lower. SiHy lenses have low water content and high oxygen transmissibility, thus having a lesser degree of lens dehydration.[79] Also, they were reported to have reduced symptoms of dryness and discomfort.[80-82] Various hydrogel and silicone hydrogel polymers available in the market are listed in Table 1.
Table 1.
Details of hydrogel and silicone hydrogel polymers with their ionicity, water content, and Dk value (for soft contact lenses)
| Material | Water content (%) | Dk | Lens variety | Brand name | |
|---|---|---|---|---|---|
|
| |||||
| Hydrogel polymers | |||||
| A | Low water content (<50%) | ||||
| a. Non – ionic | |||||
| Helfilcon A and B | 45 | 12 | Continental Toric* Flexlens* | ||
| Hioxifilcon B | 49 | 15 | Alden* Flexlens* | ||
| Polymacon | 38 | 9 | Soflens 38 Soflens multifocal | Bausch + Lomb | |
| b. Ionic | |||||
| Balafilcon A | 36 | 112 | Purevision | Bausch + Lomb | |
| Deltafilcon A | 43 | 10 | Metrosoft | Bausch + Lomb | |
| B | High water content (>50%) | ||||
| a. Non – ionic | |||||
| Alphafilcon A | 66 | 32 | Soflenstoric | Bausch + Lomb | |
| Hilafilcon B | 59 | 22 | Softlens Daily Disposable Softlens Daily Disposable for astigmatism | Bausch + Lomb Bausch + Lomb | |
| Hioxifilcon A | 59 | 28 | Alden* Extreme* Eyeris* | ||
| Hioxifilcon D | 54 | 21 | Alden* Astera* Extreme* | ||
| Nelfilcon A | 69 | 26 | Dailies AquaComfort Plus Dailies colors Focus Dailies | Alcon | |
| Nesofilcon A | 78 | 42 | BiotrueOneday | Bausch + Lomb | |
| Omafilcon A | 60 | 33 | Proclear 1-Day Misight 1-day | CooperVisionCooperVision | |
| Omafilcon B | 62 | 34 | Proclear Multifocal and Multifocal toric Proclear sphere ProclearToric | CooperVision | |
| b. Ionic | |||||
| Etafilcon A | 58 | 28 | 1-Day Acuvue moist 1-Day Acuvue moist for astigmatism 1-Day Acuvue moist multifocal Acuvue 2 | Johnson & Johnson | |
| Methafilcon A | 55 | 18 | Kontur* | Kontur | |
| Ocufilcon B | 52 | 16 | Continental* | ||
| Ocufilcon D | 55 | 19.7 | Biomedics* | CooperVision | |
| Phemfilcon A | 55 | 16 | Freshlook Colorblends Freshlook colors, Dimensions | Aqualens | |
|
| |||||
| Silicone hydrogel polymers | |||||
|
| |||||
| Comfilcon A | 48 | 128 | Biofinity and all its variants | Coopervision | |
| Delefilcon A | 140 | Dailies Total1 Dailies Total1 Multifocal Dailies Total1 for Astigmatism | Alcon | ||
| Efrofilcon A | 74 | 60 | Kerasoft Thin Rose K2 Soft | Ultravision Menicon | |
| Fanfilcon A | 55 | 90 | Avaira Vitality Avaira Vitality Toric | Coopervision | |
| Lotrafilcon A | 24 | 140 | Air optix Night & Day Aqua | Alcon | |
| Lotrafilcon B | 33 | 110 | Air optix Aqua Air optix Aqua Multifocal Air optix for Astigmatism Air optix Plus HydraGlyde | Alcon | |
| Kalifilcon A | 55 | 107 | Infuse | Bausch + Lomb | |
| Narafilcon A | 46 | 100 | 1-Day AcuvueTruEye | Johnson & Johnson | |
| Olifilcon A | 47 | 175 | Biocurve Spherical Silicone | ||
| Samfilcon A | 46 | 114 | Ultra | Bausch + Lomb | |
| Senofilcon A | 38 | 103 | AcuvueOasys | Johnson & Johnson | |
| Senofilcon C | 41 | 103 | Acuvue Vita | Johnson & Johnson | |
| Somofilcon A | 56 | 60 | Clariti | Coopervision | |
| Stenfilcon A | 54 | 80 | MyDay | Coopervision | |
| Verofilcon | 51 | 90 | Precision | Alcon | |
*These lenses are not commonly available in India
The wettability of the lens material also determines the interaction of the lids with the lens, and thus better wettability adds to the comfortable wearing time.[83] Wettability can be improved by treating the lens with surfactant wetting agents. Comfilcon A, a third-generation SiHy, is inherently wettable and does not require surface treatment to improve its wettability. The contact angle also determines the wettability of the lens surface. The lesser the contact angle of the material, the easier it is for the liquid to spread over and thus better the wettability. Generally, silicone hydrogel lenses have a more rigid modulus and offer easier handling and less adherence than hydrogel lenses. There are no data to suggest the superiority of SiHy lenses over hydrogel lenses in DED although in the US it is only SiHy lenses that are labeled with the indication for use as therapeutic lenses.
Although most studies have reported no episode of MK with the use of TCL in OSD irrespective of the use of topical antibiotics, there is a need to exercise caution when prescribing them in an eye with a compromised ocular surface.[36] There are studies that report no episode of MK with the use of TCL in OSD, irrespective of the use of prophylactic antibiotics.[23,24,27] Many, but not all, clinicians opt to use prophylactic antibiotics whenever extended wear of a soft contact lens is prescribed on a therapeutic basis. Issues that might factor into the decision whether or not to use prophylactic antibiotics include the potential for emerging microbial resistance, toxicity, cost, concurrent use of topical steroids, underlying immunocompromise, and the presence of a geographical epithelial defect. A few authors recommend the use of prophylactic antibiotics when using TCL with keratoprosthesis to reduce the risk of MK.[84,85] Some also recommend the use of 5% povidone–iodine in addition to prophylactic fluoroquinolone to reduce the risk of fungal colonization as well.[86] In long-term BCL wear, periodic replacement to avoid protein and microbial build-up is prudent. The patient should be informed about the potential risks, signs, and symptoms of infection and the need for follow-up visits at regular intervals.
Scleral Lenses
Scleral contact lenses (ScCL) vault the cornea and limbus and rest on the sclera. The space between the lens and cornea is occupied by a fluid reservoir, which acts as a liquid bandage. These lenses thus protect the ocular surface not only from desiccation but also from the mechanical effect of eyelids. There are various nomenclatures used by different manufacturers to describe these lenses. They are called semi-scleral (13.6–14.9 mm), mini-scleral (15–18), or large scleral (18.1–24 mm) lenses based on their diameter.[87] The Scleral Lens Education Society (SLS) 2013 described an internationally recognized nomenclature, which was based on the resting or landing zone and classified lenses as corneal, corneoscleral, and scleral lenses.[87] SLS recommends avoiding the classification based on diameter as it may create confusion in cases of extremely large or small corneas. Scleral lenses can be further classified as mini-scleral and large scleral lenses based on central corneal clearance and fitting characteristics with respect to horizontal visible iris diameter (HVID). A lens that is up to 6 mm larger than HVID is called a mini-scleral lens (i.e., a diameter extending no more than 3 mm on either side of the cornea), whereas large scleral lenses are the ones with diameters more than 6 mm than HVID.[87] In addition, smaller-diameter mini-scleral lenses typically have less central corneal clearance compared to a large scleral lens.
ScCLs play an important role in eyes with DED. They are indicated for the correction of refractive error secondary to the irregular corneal surface,[88] for symptomatic relief, protection of the ocular surface, healing of epithelial defects,[89] and as a medium for constant drug delivery[90] to the ocular surface. Various authors have described the efficacy of ScCLs in general for DED[91] as well as for various conditions causing dry eyes including primary and secondary SS,[92] SJS,[93-95] GVHD,[96-98] exposure keratopathy,[99,100] neurotrophic keratopathy,[100] ocular cicatricial pemphigoid, atopic keratoconjunctivitis, and chemical and thermal injury. Alipour et al.[101] reported the reduction of discomfort with mini-scleral lenses, the need for the use of lubricants, and the improvement of visual acuity in eyes with moderate to severe DED.
Sjogren’s Syndrome
Reports from the 1970s describe the use of ScCL in SS but do not mention the benefits of dry eye symptomatology.[102,103] Study showed improvement in OSDI scores between pre- and post-SCL use in eyes with SS.[92,104] Weber et al.[92] reported that the use of ScCLs significantly reduces tear hyperosmolarity and corneal staining; however, they did not describe its use for SS separately. Tear hyperosmolarity depicts either rapid evaporation of tears or low aqueous tear secretion or both. ScCLs cover the cornea and conjunctiva, and ensure constant contact between the fluid reservoir and the cornea, thus protecting the cornea from dehydration. It also protects the ocular surface from mechanical trauma caused by irregular, or keratinized eyelids and misdirected eyelashes. They also showed that these changes were not significant with 6 months of ScCL wear but were significant when they were worn for a longer period of 12 months. This shows that the duration of ScCL wear may be an important factor. ScCLs do not affect the status of meibomian glands or tear meniscus height, suggesting that though these lenses may reduce the evaporation of tears from the ocular surface, they do not play a role in tear film stabilization. Fluid ventilation may be important for success with ScCLs in DED as described in some reports.[88,91,105,106] Weber et al.[107] studied impression cytology of patients of SS who wore ScCLs and found an increase in an inflammatory response in these eyes. Thus, further studies are needed to support the success of ScCLs in these patients.
Stevens–Johnson syndrome
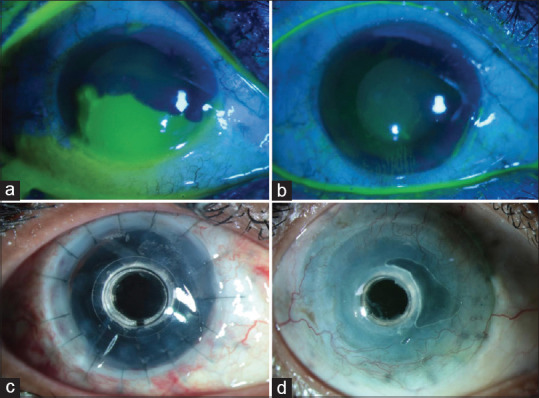
Patients with SJS develop several chronic sequelae in the form of lid margin keratinization causing lid-wiper keratopathy, cicatricial entropion, trichiasis, distichiasis, diffuse superficial punctate keratopathy, partial or total LSCD, and severe Aqueous deficient dry eye (ADDE).[108] ScCLs have proved to be effective in reducing the discomfort and photophobia in these eyes [Fig. 2a, 2b]. They have been reported to reduce the OSDI score and improve the NEI VFQ-25 composite score by 300%.[93] Though the primary indication for advising ScCL in SJS is to relieve the discomfort and improve the symptomatology caused secondary to dry eyes and the lid-wiper effect of the abnormal lid margins, these lenses also improve the functional visual acuity in such patients.[91,94] Wang et al.[109] described the feasibility of fitting PROSE lenses (prosthetic replacement of ocular surface ecosystem; Boston Sight, Needham, Massachusetts, USA) in pediatric patients of SJS, and reported significant improvement in vision. Although studies have shown that the surgical procedure of mucous membrane grafting for lid margin keratinization in eyes with SJS halts associated keratopathy, the outcomes are better in conjunction with the use of ScCLs [Fig. 2c].[110,111]
Figure 2.
Fitting of scleral lenses in patients with Stevens–Johnson syndrome (SJS) sequelae: (a) Left eye of a patient with SJS sequelae showing corneal vascularisation, stromal scarring in the inferonasal cornea, conjunctivalization inferiorly from 5 to 8 o’clock with a well-centered scleral lens, the two black dots on the lens suggests the left-sided laterality of the scleral lens for easy identification of the lens by the patient. (b) The right eye of a patient with SJS sequelae showing a scleral lens, and total limbal stem cell deficiency with corneal scarring was seen although significant visual improvement was not seen with scleral lenses, the patient’s photophobia was reduced and she was comfortable with the scleral lens. (c) Right eye of a patient with SJS sequelae fitted with a scleral lens post mucous membrane grafting in the upper and lower eyelids
Persistent epithelial defects
Rosenthal et al.[112] described the use of ScCL in the treatment of PEDs that were otherwise resistant to other treatment modalities such as the autologous serum, AMG, or tarsorrhaphies. They added prophylactic antibiotics and corticosteroids to the fluid reservoir to reduce surface inflammation and the risk of MK. Four out of 14 patients developed MK despite the use of antibiotics.[112] Lim et al.[89] conducted a study where a fourth-generation fluoroquinolone was used as a prophylactic antibiotic in 20 eyes of 19 patients and none developed MK. The fluid reservoir between the cornea and contact lens not only constantly bathes the ocular surface but also acts as the source of constant drug delivery to the compromised epithelium and thus promotes its healing. The use of 20% autologous serum eyedrops is known to resolve 43% of PEDs in 2 weeks and 62% of PEDs in 1 month.[113] With the use of ScCLs, 46% of PEDs healed within 2 weeks.[112] Ciralsky et al.[114] and Khan et al.[115] reported 100% resolution of PEDs within 2 weeks of continuous ScCL wear. However, PEDs recurred in four out of eight eyes when they were shifted from 24 h lens wear to continuous daytime-only wear, which resolved again when they were shifted to 24 h lens wear.[114] Xu et al.[116] reported that the addition of autologous hematopoietic eyedrops in the reservoir helped in faster healing of PED within 2–4 weeks. Kumar et al.[117] reported the use of mini-scleral lenses for the treatment of PED in a case of mucous membrane pemphigoid. When prescribing ScCL for eyes with PEDs, close monitoring is essential to evaluate epithelial healing and rule out secondary infection.
Exposure and neurotrophic keratopathy
ScCL has been used in patients with lid loss or lid deformity leading to corneal desiccation causing exposure keratopathy. It can occur secondary to trauma, chemical or thermal burns, lid malposition, Bell’s palsy, or proptosis. These patients are at risk of recurrent epithelial defects, corneal vascularization, corneal thinning, corneal melt, and eventual perforation. TCLs have a tendency for dehydration, and desiccation and are often lost in such eyes.[118] ScCL has proved to be useful in preventing further desiccation, providing hydration to the cornea, as well as aid in the healing of epithelial defects. These lenses can be used as an alternative to tarsorrhaphies in long-standing exposure and neurotrophic keratopathy.[100] Chaudhary et al.[119] have reported successful use of ScCL in eyes with keratoprosthesis with total lid loss secondary to chemical injury and stated that ScCL can be used safely in such eyes in the interim to buy time for definitive surgical interventions.
Graft-vs-host disease (GVHD)
Because patients with GVHD frequently suffer from severe dry eyes, they complain of foreign body sensation, photophobia, dryness, and blurring of vision and they may present with corneal epithelial defects, vascularization, corneal scarring, and LSCD.[26] ScCLs play a therapeutic role and aid in the healing and stabilization of the ocular surface and improve symptomatology in these conditions.[97] Jacobs et al.[98] described that patients with GVHD with severe DED reported the highest improvement in pain and photophobia, and 73% of them felt improvement in the quality of life with ScCL use. A questionnaire-based study was conducted by Bligdon et al.,[120] in which the patients with GVHD were asked about the symptoms, transplant history, and their experience related to the use of ScCLs. They stated that patients reported improvement in terms of pain relief and improved quality of life. Sixty-three percent of these patients had never heard of ScCLs before. This study highlights that even though literature describes the beneficial effects of these lenses, they are underutilized.
Neuropathic pain
These lenses have also proved their worth in relieving severe neuropathic pain even when the surface looks relatively healthy. The rationale behind the use of these lenses is to form a shield around the corneal nociceptors with the fluid bandage forming a barrier from the surrounding stimulus, thus reducing the peripheral nociceptor signaling.[121] These lenses when used early in the disease course can help reverse chronic pain.[122] Later, once the chronic pain is established, these lenses may not be well tolerated due to secondary hyperalgesia.[17]
Lens selection
When selecting a trial lens, the first parameter to choose is the diameter of the lens. Because the major indication of ScCL in patients with DED is symptomatic relief and protection of the ocular surface from desiccation, a general notion can be that the larger the diameter the better the protection against desiccation. However, it is not clear that a larger diameter lens gives better symptomatic relief. It is also important to consider the extent of palpebral aperture widening and the presence of associated symblepharon or forniceal shortening as they will greatly influence the selection of the lens diameter. The diameter should be selected such that the haptic ends before the start of symblepharon, which otherwise can cause edge lift and air bubble trap in the reservoir. This will worsen the corneal damage from desiccation and dehydration. The presence of a tarsorrhaphy should also be taken into consideration as it will reduce the palpebral fissure height. It should be kept in mind, that as we move farther away from the limbus, the greater the scleral asymmetry or toricity, which correlates with the muscle insertion.[87] The sagittal height or vault should be selected large enough to provide optimum central corneal and limbal clearance 360 degrees. The ideal way of assessing the depth of the central fluid reservoir is by comparing it with ScCL thickness using an optical section on a slit lamp or anterior segment–optical coherence tomography. Central corneal clearance reduces by 80–100 microns in the first 4 h of wear.[123,124] Changes in the vault height noted thereafter are insignificant.[123] The viscosity of fluid used to fill the reservoir does not affect the amount of lens settling on the eye.[125] Even the change in the subjective over-refraction becomes non-significant after 6–8 h of lens wear.[126] Kumar et al.[127] graded criteria for optimal fitting of ScCL. These include a central corneal clearance of 200–400 microns and a limbal clearance of 100–200 microns. The lens haptic supports the lens weight and distributes it over the landing area. The larger the haptic zone, the better the weight distribution, and the minimal would be its compression effect on the underlying blood vessels. An optimal fit would be with no whitish band of blanching on the sclera, and without blockage of major or minor vessels. Optimal edge alignment should neither have lens impingement or “sink in” nor “edge off” or edge lift effect on the conjunctival surface.
In the case of a sealed ScCL, the central vault or central corneal clearance determines the oxygen that reaches the cornea. A larger ScCL diameter can accommodate more fluid in the reservoir and can form a thicker tear film behind the lens. Theoretically, according to Michaud et al.,[128] for a sealed lens, a lens with a thickness of >350 microns, made of a material with Dk 150 and a tear film thickness of >250 microns can induce corneal edema under an open eye condition. However, Pullum and Stapleton reported that the mean corneal edema was 3% with an ScCL thickness of 0.6 mm in a material with a Dk of 115.[129] Oxygen availability at the ocular surface can be improved by fluid ventilation. Fluid ventilation (tear exchange) with tears at the ocular surface that are exposed to atmospheric oxygen is more likely to occur with larger lenses with non-spherical bearing haptic such as can be designed for PROSE lenses, with EyePrint Pro (Lakewood, Colorado, USA), and with commercially available ScCLs that have the option for toric or quadrant specific haptics. Some of these lenses can also incorporate ventilating channels in their posterior surface. Small diameter limbal CLs (13–14 mm) can also be designed and fitted to allow this fluid ventilation as described by Sotozono et al.[130] Fluid ventilation increases oxygen availability and reduces the likelihood of seal-off or suction, which is a mechanical challenge at the bearing point and to the epithelial tight junctions under the vaulting lenses. Eyes with fragile epithelium, such as occurs in OSD benefit from this lack of mechanical challenge as well as extra oxygen transmission under a fluid-ventilated lens as opposed to a sealed lens. In such lenses, the height of the reservoir is irrelevant.
The term RGP/gas permeable is considered obsolete as now all commercially available lenses are invariably gas permeable and allow high levels of oxygen to pass through and reach the underlying cornea. Details of various lens materials currently available are given in Table 2.
Table 2.
Different lens materials, their generic names, Dk value, contact angle, and refractive index (for scleral and corneal gas-permeable lenses)
| Material name | Generic name | Dk value | Contact angle (in degree) | Refractive index |
|---|---|---|---|---|
| PMMA | 0 | |||
| Boston II | Itafocon A | 12 | 20 | 1.47 |
| Boston ES | Enflufocon A | 18 | 52 | 1.44 |
| Boston IV | Itafocon B | 19 | 17 | 1.47 |
| Boston Equalens | Itaflourofocon A | 47 | 30 | 1.44 |
| Boston EO | Enflufocon B | 58 | 49 | 1.43 |
| Boston Equalens II | Oprifocon A | 85 | 30 | 1.42 |
| Boston XO | Hexafocon A | 100 | 49 | 1.42 |
| Boston XO2 | Hexafocon B | 141 | 38 | 1.42 |
| Contamac | ||||
| Optimum Classic | Roflufocon A | 26 | 12 | 1.45 |
| Optimum Comfort | Roflufocon C | 65 | 6 | 1.44 |
| Optimum Extra | Roflufocon D | 100 | 3 | 1.43 |
| Optimum Extreme | Roflufocon E | 125 | 6 | 1.43 |
| Optimum Infinite | Tisilfocon A | 180 | 6 | 1.44 |
Limbal vault is an equally important parameter to be assessed. An ScCL fitting with limbal bearing can induce discomfort due to pressure on the highly sensitive sensory nerve fibers.[131] In addition, a limbal bearing can lead to recurrent epithelial breakdown and subepithelial scarring.[132] ScCLs often get displaced inferiorly or inferotemporally, thus causing limbal bearing along the superior and superonasal quadrants. Often, a limbal bearing is unavoidable if the fitter only has access to smaller and spherical lenses. Walker et al.[131] recommend a limbal touch of less than 20% along its entire circumference.
While prescribing ScCLs to patients with DED, the decision for material selection is also important. Though there are materials that provide a Dk of 140 or higher, there are other factors that must be considered. Patients with DED often complain of discomfort and dryness of the lens. Contact angle or the wettability of the lens material should be taken into consideration while prescribing lenses to these patients. The lesser the contact angle for a given material, the better its wettability. A material with higher Dk tends to have a larger wetting angle, thus having poor surface wetting, leading to dryness and discomfort for the wearer.[133] They are also less resistant to scratches[134] and therefore require frequent replacements. In addition, the higher the Dk value, the higher likelihood of the silicone content increasing the risk of surface deposits.[134]
The wettability of a lens can be improved by plasma treatment by up to 40%. The process consists of bombarding the lens with ionized oxygen to create a hydrophilic surface and reducing the wetting angle.[135] Another approach to increase wettability and CL comfort is through polyethylene glycol polymer (PEG) coating following a polymer coating.[136] Hydra-PEG is a 90% water-based polymer mix that covalently bonds with the surface of the lens, creates a wetting surface over the lens, and separates it from the ocular surface and the tear film.[137] Studies have reported that it not only reduces the contact angle by 50% but also reduces lipid and protein deposition.[136] Both plasma treatment and PEG coating diminish with daily lens handling and cleaning. Therefore, patients need to be instructed to use specific lens cleaning and storage solution to increase their longevity. Patients who experience ocular dryness, discomfort with ScCL use, heavy surface depositors, and those who experience midday fogging are likely to get benefitted the most.[137] It is recommended to avoid alcohol-based or abrasive solutions and tap water for cleaning and storing these PEG-treated lenses as they can damage the surface and reduce the benefits of coatings.[137] To increase the longevity of the plasma treatment, it is recommended to avoid storing these lenses dry, and instead, keep them soaked in the recommended solutions.
Lens care
Poor lens wetting and midday fogging (MDF) are common problems that were faced by these patients with severe dry eyes [Fig. 3a, 3b].[131,138] Eyewash with preservative-free saline before lens application in the morning might be useful for patients who use lubricating ointment at bedtime. Cases, where patients complain of blurring of vision or MDF within a few hours of lens wear, should be assessed for non-wetting of the lens, drying of the anterior surface of the lens in the form of front surface debris deposition, and debris collection in the fluid reservoir. Poor lens wetting can be improved with plasma treatment or hydra-peg coating as described above. Drying of the anterior lens surface may need frequent instillation of either preservative-free normal saline or lubricating eyedrops that do not cause blurring of the vision after instillation. In our experience, patients feel less drying of the lens and better visual quality with carboxymethylcellulose 0.5% or 1% over hydroxymethyl cellulose preparation. MDF occurs secondary to the accumulation of tear film debris in the fluid reservoir. An ScCL fitting with an optimal haptic or landing zone fit tends to have a better approximation of the lens edge with the scleral contour, reduced debris accumulation, and MDF. Lenses with toric peripheral haptics help provide a good alignment of the haptics compared to the spherical periphery and thus improve the wear time and comfort of the patients.[139] MDF can also be reduced by using a more viscous preservative-free fluid in the reservoir. However, no studies have been published yet, related to oxygen permeability, hypoxia-related complications, or lens removal regimens in these cases. Lens cleaning can be done with any alcohol-based cleaning solutions; however, alcohol-free or non-abrasive solutions are preferred for the lenses with hydra-PEG coating.
Figure 3.
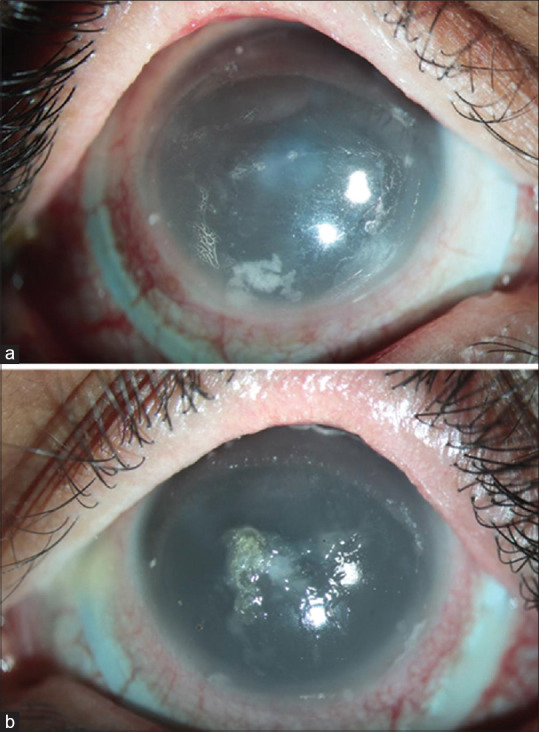
Diffuse slit-lamp image of the right and left eye of a patient with Stevens–Johnson syndrome sequelae after 4 h of scleral lens wear showing (a) entrapped debris in the vault causing midday fogging (right eye) and (b) drying of the anterior lens surface with mucin deposits on the anterior lens surface (left eye)
Conclusion
Contact lenses play an important role in the management of DED. BCLs and rigid gas permeable ScCLs not only aid in visual rehabilitation but are also useful for therapeutic indications and provide symptomatic relief. BCLs are useful in epithelial healing and maintenance. Lens care and hygiene require careful attention and the potential risk of complications in the form of MK should be kept in mind, particularly if extended wear is prescribed. ScCLs can be useful in instances of severe DED in which BCL fails, but wear may be limited by MDF and dryness of the anterior surface of the lens. This possibility should be reviewed with patients. Adequate training in ScCL insertion and removal, as well as patient motivation, are critical for success with ScCLs in DED.
Financial support and sponsorship
For the authors SC, DG, SB, and SSS, this work was funded by the Hyderabad Eye Research Foundation in Hyderabad, India. The sponsoring organization had no role in the design or conduct of this research.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lemp MA, Foulks GN. The definition and classification of dry eye disease. Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang G-H, Klein BE, Klein R, et al. Dry eye in the beaver dam offspring study:Prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. Tfos dews ii epidemiology report. OculSurf. 2017;15:334–65. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Donthineni PR, Kammari P, Shanbhag SS, Singh V, Das AV, Basu S. Incidence, demographics, types and risk factors of dry eye disease in India:Electronic medical records driven big data analytics report I. Ocul Surf. 2019;17:250–6. doi: 10.1016/j.jtos.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Na K-S, Han K, Park Y-G, Na C, Joo C-K. Depression, stress, quality of life, and dry eye disease in Korean women:A population-based study. Cornea. 2015;34:733–8. doi: 10.1097/ICO.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 6.Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15:802–12. doi: 10.1016/j.jtos.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Morgan P WC, Tranoudis I, Helland M, Efron N. International contact lens prescribing in 2021: Contact Lens Spectrum. [[Last Accessed on 2022 Sep 26]];2022 37:32–38. [Available from: https://www.clspectrum.com/issues/2022/january-2022/international-contact-lens-prescribing-in-2021.] [Google Scholar]
- 8.Lim L, Lim EWL. Therapeutic contact lenses in the treatment of corneal and ocular surface diseases-A review. Asia Pac J Ophthalmol (Phila) 2020;9:524–32. doi: 10.1097/APO.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 9.Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628. doi: 10.1016/j.jtos.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Donnenfeld ED, Selkin BA, Perry HD, Moadel K, Selkin GT, Cohen AJ, et al. Controlled evaluation of a bandage contact lens and a topical nonsteroidal anti-inflammatory drug in treating traumatic corneal abrasions. Ophthalmology. 1995;102:979–84. doi: 10.1016/s0161-6420(95)30926-8. [DOI] [PubMed] [Google Scholar]
- 11.Salz JJ, Reader AL, Schwartz LJ, Van Le K. Treatment of corneal abrasions with soft contact lenses and topical diclofenac. J Refract Corneal Surg. 1994;10:640–6. [PubMed] [Google Scholar]
- 12.Montero J, Sparholt J, Mely R. Retrospective case series of therapeutic applications of a lotrafilcon A silicone hydrogel soft contact lens. Eye Contact Lens. 2003;29((1 Suppl)):S54–6. discussion S7-9, S192-4. [PubMed] [Google Scholar]
- 13.Rashad R, Weed MC, Quinn N, Chen VM. Extended wear bandage contact lenses decrease pain and preserve vision in patients with epidermolysis bullosa:Case series and review of literature. Ocul Immunol Inflamm. 2020;28:379–83. doi: 10.1080/09273948.2019.1587472. [DOI] [PubMed] [Google Scholar]
- 14.Miller DD, Hasan SA, Simmons NL, Stewart MW. Recurrent corneal erosion:A comprehensive review. Clin Ophthalmol. 2019;13:325–35. doi: 10.2147/OPTH.S157430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahad MA, Anandan M, Tah V, Dhingra S, Leyland M. Randomized controlled study of ocular lubrication versus bandage contact lens in the primary treatment of recurrent corneal erosion syndrome. Cornea. 2013;32:1311–4. doi: 10.1097/ICO.0b013e31829dec39. [DOI] [PubMed] [Google Scholar]
- 16.Visser E-S, Van der Linden BJ, Otten HM, Van der Lelij A, Visser R. Medical applications and outcomes of bitangential scleral lenses. Optom Vis Sci. 2013;90:1078–85. doi: 10.1097/OPX.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 17.Goyal S, Hamrah P. Understanding neuropathic corneal pain--gaps and current therapeutic approaches. Semin Ophthalmol. 2016;31:59–70. doi: 10.3109/08820538.2015.1114853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain:An important yet underevaluated feature of dry eye. Eye (Lond) 2015;29:301–12. doi: 10.1038/eye.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora R, Jain S, Monga S, Narayanan R, Raina UK, Mehta DK. Efficacy of continuous wear PureVision contact lenses for therapeutic use. Cont Lens Anterior Eye. 2004;27:39–43. doi: 10.1016/j.clae.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Kanpolat A, Uçakhan ÖÖ. Therapeutic use of Focus®Night &Day8482contact lenses. Cornea. 2003;22:726–34. doi: 10.1097/00003226-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y-K, Lin Y-C, Tsai S-H, Chen W-L, Chen Y-M. Therapeutic outcomes of combined topical autologous serum eye drops with silicone–hydrogel soft contact lenses in the treatment of corneal persistent epithelial defects:A preliminary study. Cont Lens Anterior Eye. 2016;39:425–30. doi: 10.1016/j.clae.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Baradaran-Rafii A, Eslani M, Haq Z, Shirzadeh E, Huvard MJ, Djalilian AR. Current and upcoming therapies for ocular surface chemical injuries. Ocul Surf. 2017;15:48–64. doi: 10.1016/j.jtos.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo PA, Bouchard CS, Galasso JM. Extended-wear silicone hydrogel soft contact lenses in the management of moderate to severe dry eye signs and symptoms secondary to graft-versus-host disease. Eye Contact Lens. 2007;33:144–7. doi: 10.1097/01.icl.0000244154.76214.2d. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Zhang X, Zheng Q, Zhu Y, Wang H, Ma H, et al. Comparative evaluation of silicone hydrogel contact lenses and autologous serum for management of Sjogren syndrome-associated dry eye. Cornea. 2015;34:1072–8. doi: 10.1097/ICO.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 25.Riemens A, te Boome L, Imhof S, Kuball J, Rothova A. Current insights into ocular graft-versus-host disease. Curr Opin Ophthalmol. 2010;21:485–94. doi: 10.1097/ICU.0b013e32833eab64. [DOI] [PubMed] [Google Scholar]
- 26.Nassar A, Tabbara KF, Aljurf M. Ocular manifestations of graft-versus-host disease. Saudi J Ophthalmol. 2013;27:215–22. doi: 10.1016/j.sjopt.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inamoto Y, Sun Y-C, Flowers ME, Carpenter PA, Martin PJ, Li P, et al. Bandage soft contact lenses for ocular graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:2002–7. doi: 10.1016/j.bbmt.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi D-N, Song H, Ding T, Qiu W-Q, Wang W. Evaluation of the safety and efficacy of therapeutic bandage contact lenses on post-cataract surgery patients. Int J Ophthalmol. 2018;11:230–4. doi: 10.18240/ijo.2018.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Yuan R, Sun M, Chen X, Lin S, Ye J, et al. Efficacy of an ocular bandage contact lens for the treatment of dry eye after phacoemulsification. BMC Ophthalmol. 2019;19:1–6. doi: 10.1186/s12886-018-1023-8. doi:10.1186/s12886-018-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu Y, Liu J, Tseng SC. Ocular surface deficits contributing to persistent epithelial defect after penetrating keratoplasty. Cornea. 2012;31:723–9. doi: 10.1097/ICO.0b013e31821142ee. [DOI] [PubMed] [Google Scholar]
- 31.Yeung SN, Lichtinger A, Kim P, Elbaz U, Ku J, Teichman J, et al. Efficacy and safety of patching vs bandage lens on postoperative pain following pterygium surgery. Eye (Lond) 2015;29:295–6. doi: 10.1038/eye.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prat D, Zloto O, Ben Artsi E, Ben Simon GJ. Therapeutic contact lenses vs. tight bandage patching and pain following pterygium excision:A prospective randomized controlled study. Graefes Arch Clin Exp Ophthalmol. 2018;256:2143–8. doi: 10.1007/s00417-018-4118-2. [DOI] [PubMed] [Google Scholar]
- 33.Patel BC, Patipa M, Anderson RL, McLeish W. Management of postblepharoplasty lower eyelid retraction with hard palate grafts and lateral tarsal strip. Plast Reconstruct Surg. 1997;99:1251–60. doi: 10.1097/00006534-199704001-00007. [DOI] [PubMed] [Google Scholar]
- 34.Lin P-Y, Wu C-C, Lee S-M. Combined phototherapeutic keratectomy and therapeutic contact lens for recurrent erosions in bullous keratopathy. Br J Ophthalmol. 2001;85:908–11. doi: 10.1136/bjo.85.8.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szaflik JP, Ambroziak AM, Szaflik J. Therapeutic use of a lotrafilcon A silicone hydrogel soft contact lens as a bandage after LASEK surgery. Eye Contact Lens. 2004;30:59–62. doi: 10.1097/01.ICL.0000107181.42704.D8. [DOI] [PubMed] [Google Scholar]
- 36.Dada V, Kalra V, Angra S. Role of soft contact lens in ocular surface problems. Indian J Ophthalmol. 1984;32:519. [PubMed] [Google Scholar]
- 37.Adam RS, Harvey JT, Gould JN, Suntheralingam S, Farrokhyar F. The role of postoperative bandage contact lens in patients undergoing fasanella-servat ptosis repair. Ophthalmic Plast Reconstr Surg. 2021;37:61–4. doi: 10.1097/IOP.0000000000001690. [DOI] [PubMed] [Google Scholar]
- 38.Sotozono C, Ang LP, Koizumi N, Higashihara H, Ueta M, Inatomi T, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens–Johnson syndrome. Ophthalmology. 2007;114:1294–302. doi: 10.1016/j.ophtha.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Schulz E. The application of highly hydrophilic contact lenses in the Fuchs-Steven-Johnson syndrome (author's transl) Klin Monbl Augenheilkd. 1979;174:113–8. [PubMed] [Google Scholar]
- 40.Haefliger I, Vysniauskiene I, Pimentel A, Soares E, Piffaretti J-M. Free autologous buccal mucosal graft transplantation to treat ocular complications after toxic epidermal necrolysis:Case report. Klin Monbl Augenheilkd. 2004;221:395–7. doi: 10.1055/s-2004-812852. [DOI] [PubMed] [Google Scholar]
- 41.Isawi H, Dhaliwal DK. Corneal melting and perforation in Stevens Johnson syndrome following topical bromfenac use. J Cataract Refract Surg. 2007;33:1644–6. doi: 10.1016/j.jcrs.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 42.Thomas M, Shorter E, Joslin CE, McMahon TJ, Cortina MS. Contact lens use in patients with Boston keratoprosthesis type 1:Fitting, management, and complications. Eye Contact Lens. 2015;41:334–40. doi: 10.1097/ICL.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 43.Kammerdiener LL, Speiser JL, Aquavella JV, Harissi-Dagher M, Dohlman CH, Chodosh J, et al. Protective effect of soft contact lenses after Boston keratoprosthesis. Br J Ophthalmol. 2016;100:549–52. doi: 10.1136/bjophthalmol-2014-306396. [DOI] [PubMed] [Google Scholar]
- 44.Jhanji V, Young AL, Mehta JS, Sharma N, Agarwal T, Vajpayee RB. Management of corneal perforation. Surv Ophthalmol. 2011;56:522–38. doi: 10.1016/j.survophthal.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Lim L, Tan DT, Chan WK. Therapeutic use of Bausch &Lomb PureVision contact lenses. CLAO J. 2001;27:179–85. [PubMed] [Google Scholar]
- 46.Sharma A, Kaur R, Kumar S, Gupta P, Pandav S, Patnaik B, et al. Fibrin glue versus N-butyl-2-cyanoacrylate in corneal perforations. Ophthalmology. 2003;110:291–8. doi: 10.1016/S0161-6420(02)01558-0. [DOI] [PubMed] [Google Scholar]
- 47.Rana M, Savant V. A brief review of techniques used to seal corneal perforation using cyanoacrylate tissue adhesive. Cont Lens Anterior Eye. 2013;36:156–8. doi: 10.1016/j.clae.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Kate A, Vyas S, Bafna RK, Sharma N, Basu S. Tenon's Patch Graft:A Review of Indications, Surgical Technique, Outcomes and Complications. Semin Ophthalmol. 2022;37:462–70. doi: 10.1080/08820538.2021.2017470. [DOI] [PubMed] [Google Scholar]
- 49.Gris O, del Campo Z, Wolley-Dod C, Güell JL, Bruix A, Calatayud M, et al. Amniotic membrane implantation as a therapeutic contact lens for the treatment of epithelial disorders. Cornea. 2002;21:22–7. doi: 10.1097/00003226-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Pfister RR, Murphy GE. Corneal ulceration and perforation associated with Sjögren's syndrome. Arch Ophthalmol. 1980;98:89–94. doi: 10.1001/archopht.1980.01020030091006. [DOI] [PubMed] [Google Scholar]
- 51.Peng CC, Burke MT, Carbia BE, Plummer C, Chauhan A. Extended drug delivery by contact lenses for glaucoma therapy. J Control Release. 2012;162:152–8. doi: 10.1016/j.jconrel.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 52.Peterson RC, Wolffsohn JS, Nick J, Winterton L, Lally J. Clinical performance of daily disposable soft contact lenses using sustained release technology. Cont Lens Anterior Eye. 2006;29:127–34. doi: 10.1016/j.clae.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Schrader S, Wedel T, Moll R, Geerling G. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefes Arch Clin Exp Ophthalmol. 2006;244:1345–9. doi: 10.1007/s00417-006-0257-y. [DOI] [PubMed] [Google Scholar]
- 54.Xinming L, Yingde C, Lloyd AW, Mikhalovsky SV, Sandeman SR, Howel CA, et al. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems:A review. Cont Lens Anterior Eye. 2008;31:57–64. doi: 10.1016/j.clae.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Guzman-Aranguez A, Colligris B, Pintor J. Contact lenses:Promising devices for ocular drug delivery. J Ocul Pharmacol Ther. 2013;29:189–99. doi: 10.1089/jop.2012.0212. [DOI] [PubMed] [Google Scholar]
- 56.Carvalho IM, Marques CS, Oliveira RS, Coelho PB, Costa PC, Ferreira DC. Sustained drug release by contact lenses for glaucoma treatment-a review. J Control Release. 2015;202:76–82. doi: 10.1016/j.jconrel.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 57.Li C-C, Chauhan A. Modeling ophthalmic drug delivery by soaked contact lenses. Industrial &engineering chemistry research. 2006;45:3718–34. [Google Scholar]
- 58.Li C, Chauhan A. Ocular transport model for ophthalmic delivery of timolol through p-HEMA contact lenses. J Drug Deliv Sci Technol. 2007;17:69–79. [Google Scholar]
- 59.Kim J, Chauhan A. Dexamethasone transport and ocular delivery from poly (hydroxyethyl methacrylate) gels. Int J Pharm. 2008;353:205–22. doi: 10.1016/j.ijpharm.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 60.Bengani LC, Kobashi H, Ross AE, Zhai H, Salvador-Culla B, Tulsan R, et al. Steroid-eluting contact lenses for corneal and intraocular inflammation. Acta Biomater. 2020;116:149–61. doi: 10.1016/j.actbio.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciolino JB, Dohlman CH, Kohane DS. Contact lenses for drug delivery. Semin Ophthalmol. 2009;24:156–60. doi: 10.1080/08820530902802161. [DOI] [PubMed] [Google Scholar]
- 62.Ciolino JB, Hoare TR, Iwata NG, Behlau I, Dohlman CH, Langer R, et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009;50:3346–52. doi: 10.1167/iovs.08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daphna O, Mimouni M, Keshet Y, Ben Ishai M, Barequet IS, Knyazer B, et al. Therapeutic HL-contact lens versus standard bandage contact lens for corneal edema:A prospective, multicenter, randomized, crossover study. J Ophthalmol. 2020;2020:8410920. doi: 10.1155/2020/8410920. doi:10.1155/2020/8410920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erdinest N, London N, Levinger N. Therapeutic contact lens for Fuchs endothelial corneal dystrophy:Monitoring with Scheimpflug tomopraphy. Am J Ophthalmol Case Rep. 2022;25:101242. doi: 10.1016/j.ajoc.2021.101242. doi:10.1016/j.ajoc.2021.101242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romano V, Romano D, Semeraro P, Forbice E, Iaria A, Pizzolante T, et al. Therapeutic Hyper-CL soft contact lens in Sjögren's syndrome. Am J Ophthalmol Case Rep. 2022;28:101685. doi: 10.1016/j.ajoc.2022.101685. doi: 10.1016/j.ajoc. 2022.101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ono J, Toshida H. Use of Ketotifen fumarate-eluting daily disposable soft contact lens in management of ocular allergy:Literature review and report of two cases. Cureus. 2022;14:e27093. doi: 10.7759/cureus.27093. doi:10.7759/cureus. 27093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim N, Vogt U. Comparison of conventional and silicone hydrogel contact lenses for bullous keratoplasty. Eye Contact Lens. 2006;32:250–3. doi: 10.1097/01.icl.0000219499.24304.d3. [DOI] [PubMed] [Google Scholar]
- 68.Ozkan J, Papas E. Lubricant effects on low Dk and silicone hydrogel lens comfort. Optom Vis Sci. 2008;85:773–7. doi: 10.1097/OPX.0b013e3181819f37. [DOI] [PubMed] [Google Scholar]
- 69.Fonn D, Dumbleton K. Dryness and discomfort with silicone hydrogel contact lenses. Eye Contact Lens. 2003;29((1 Suppl)):S101–4. doi: 10.1097/00140068-200301001-00028. discussion S15-8, S92-4. [DOI] [PubMed] [Google Scholar]
- 70.Cheung SW, Cho P, Chan B, Choy C, Ng V. A comparative study of biweekly disposable contact lenses:Silicone hydrogel versus hydrogel. Clin Exp Optom. 2007;90:124–31. doi: 10.1111/j.1444-0938.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 71.Martin R, de Juan V, Rodriguez G, Martin S, Fonseca S. Initial comfort of lotrafilcon A silicone hydrogel contact lenses versus etafilcon A contact lenses for extended wear. Cont Lens Anterior Eye. 2007;30:23–8. doi: 10.1016/j.clae.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Ousler GW, 3rd, Anderson RT, Osborn KE. The effect of senofilcon A contact lenses compared to habitual contact lenses on ocular discomfort during exposure to a controlled adverse environment. Curr Med Res Opin. 2008;24:335–41. doi: 10.1185/030079908x260826. [DOI] [PubMed] [Google Scholar]
- 73.Maissa C, Guillon M, Garofalo RJ. Contact lens-induced circumlimbal staining in silicone hydrogel contact lenses worn on a daily wear basis. Eye Contact Lens. 2012;38:16–26. doi: 10.1097/ICL.0b013e31823bad46. [DOI] [PubMed] [Google Scholar]
- 74.Fonn D, Bruce AS. A review of the Holden-Mertz criteria for critical oxygen transmission. Eye Contact Lens. 2005;31:247–51. doi: 10.1097/01.icl.0000182488.70745.1d. [DOI] [PubMed] [Google Scholar]
- 75.Mok KH, Cheung RWL, Wong BK-H, Yip KK, Lee VW-H. Effectiveness of no-rub contact lens cleaning on protein removal:A pilot study. Optom Vis Sci. 2004;81:468–70. doi: 10.1097/01.opx.0000135098.07502.9f. [DOI] [PubMed] [Google Scholar]
- 76.Patel A, Hammersmith K. Contact lens-related microbial keratitis:Recent outbreaks. Curr Opin Ophthalmol. 2008;19:302–6. doi: 10.1097/ICU.0b013e3283045e74. [DOI] [PubMed] [Google Scholar]
- 77.Maissa C, Franklin V, Guillon M, Tighe B. Influence of contact lens material surface characteristics and replacement frequency on protein and lipid deposition. Optom Vis Sci. 1998;75:697–705. doi: 10.1097/00006324-199809000-00026. [DOI] [PubMed] [Google Scholar]
- 78.Minarik L, Rapp J. Protein deposits on individual hydrophilic contact lenses:Effects of water and ionicity. CLAO J. 1989;15:185–8. [PubMed] [Google Scholar]
- 79.Morgan PB, Efron N. In vivo dehydration of silicone hydrogel contact lenses. Eye Contact Lens. 2003;29:173–6. doi: 10.1097/01.ICL.0000072825.23491.59. [DOI] [PubMed] [Google Scholar]
- 80.Riley C, Young G, Chalmers R. Prevalence of ocular surface symptoms, signs, and uncomfortable hours of wear in contact lens wearers:The effect of refitting with daily-wear silicone hydrogel lenses (senofilcon a) Eye Contact Lens. 2006;32:281–6. doi: 10.1097/01.icl.0000224522.04723.7a. [DOI] [PubMed] [Google Scholar]
- 81.Long B, McNally J. The clinical performance of a silicone hydrogel lens for daily wear in an Asian population. Eye Contact Lens. 2006;32:65–71. doi: 10.1097/01.icl.0000175915.01688.ba. [DOI] [PubMed] [Google Scholar]
- 82.Chalmers RL, Dillehay S, Long B, Barr JT, Bergenske P, Donshik P, et al. Impact of previous extended and daily wear schedules on signs and symptoms with high Dk lotrafilcon A lenses. Optom Vis Sci. 2005;82:549–54. doi: 10.1097/00006324-200506000-00019. [DOI] [PubMed] [Google Scholar]
- 83.Tonge S, Jones L, Goodall S, Tighe B. The ex vivo wettability of soft contact lenses. Curr Eye Res. 2001;23:51–9. doi: 10.1076/ceyr.23.1.51.5418. [DOI] [PubMed] [Google Scholar]
- 84.Farooq AV, Hou JH, Jassim S, Haq Z, Tu EY, de la Cruz J, et al. Biofilm formation on bandage contact lenses worn by patients with the Boston type 1 keratoprosthesis:A pilot comparison study of prophylactic topical vancomycin 15 mg/mL and linezolid 0.2% Eye Contact Lens. 2018;44:S106–9. doi: 10.1097/ICL.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 85.Lee SH, Mannis MJ, Shapiro B, Li JY, Polage C, Smith W. Evaluation of microbial flora in eyes with a Boston type 1 Keratoprosthesis. Cornea. 2013;32:1537–9. doi: 10.1097/ICO.0b013e3182a81992. [DOI] [PubMed] [Google Scholar]
- 86.Magalhães FP, do Nascimento HM, Ecker DJ, Sannes-Lowery KA, Sampath R, Rosenblatt MI, et al. Microbiota evaluation of patients with a Boston type I keratoprosthesis treated with topical 0.5% moxifloxacin and 5% povidone–iodine. Cornea. 2013;32:407–11. doi: 10.1097/ICO.0b013e31824a8b9b. [DOI] [PubMed] [Google Scholar]
- 87.Van der Worp E. Pacific University; 2010. A Guide to Scleral Lens Fitting: College of Optometry. [Google Scholar]
- 88.Bavinger JC, DeLoss K, Mian SI. Scleral lens use in dry eye syndrome. Curr Opin Ophthalmol. 2015;26:319–24. doi: 10.1097/ICU.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 89.Lim P, Ridges R, Jacobs DS, Rosenthal P. Treatment of persistent corneal epithelial defect with overnight wear of a prosthetic device for the ocular surface. Am J Ophthalmol. 2013;156:1095–101. doi: 10.1016/j.ajo.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 90.Jacobs DS. Update on scleral lenses. Curr Opin Ophthalmol. 2008;19:298–301. doi: 10.1097/ICU.0b013e328302cc4f. [DOI] [PubMed] [Google Scholar]
- 91.Stason WB, Razavi M, Jacobs DS, Shepard DS, Suaya JA, Johns L, et al. Clinical benefits of the Boston ocular surface prosthesis. Am J Ophthalmol. 2010;149:54–61.e2. doi: 10.1016/j.ajo.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 92.La Porta Weber S, Becco de Souza R, Gomes J, Hofling-Lima A. The use of the esclera scleral contact lens in the treatment of moderate to severe dry eye disease. Am J Ophthalmol. 2015;163:167–73.e1. doi: 10.1016/j.ajo.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 93.Tougeron-Brousseau B, Delcampe A, Gueudry J, Vera L, Doan S, Hoang-Xuan T, et al. Vision-related function after scleral lens fitting in ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. 2009;148:852–9.e2. doi: 10.1016/j.ajo.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 94.Rathi VM, Mandathara PS, Dumpati S, Vaddavalli PK, Sangwan VS. Boston ocular surface prosthesis:An Indian experience. Indian J Ophthalmol. 2011;59:279–81. doi: 10.4103/0301-4738.81994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Papakostas TD, Le H-G, Chodosh J, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of Stevens–Johnson syndrome/toxic epidermal necrolysis. Ophthalmology. 2015;122:248–53. doi: 10.1016/j.ophtha.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 96.Takahide K, Parker PM, Wu M, Hwang WY, Carpenter PA, Moravec C, et al. Use of fluid-ventilated, gas-permeable scleral lens for management of severe keratoconjunctivitis sicca secondary to chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1016–21. doi: 10.1016/j.bbmt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schornack MM, Baratz KH, Patel SV, Maguire LJ. Jupiter scleral lenses in the management of chronic graft versus host disease. Eye Contact Lens. 2008;34:302–5. doi: 10.1097/ICL.0b013e318188e205. [DOI] [PubMed] [Google Scholar]
- 98.Jacobs DS, Rosenthal P. Boston scleral lens prosthetic device for treatment of severe dry eye in chronic graft-versus-host disease. Cornea. 2007;26:1195–9. doi: 10.1097/ICO.0b013e318155743d. [DOI] [PubMed] [Google Scholar]
- 99.Grey F, Carley F, Biswas S, Tromans C. Scleral contact lens management of bilateral exposure and neurotrophic keratopathy. Cont Lens Anterior Eye. 2012;35:288–91. doi: 10.1016/j.clae.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 100.Weyns M, Koppen C, Tassignon M-J. Scleral contact lenses as an alternative to tarsorrhaphy for the long-term management of combined exposure and neurotrophic keratopathy. Cornea. 2013;32:359–61. doi: 10.1097/ICO.0b013e31825fed01. [DOI] [PubMed] [Google Scholar]
- 101.Alipour F, Kheirkhah A, Behrouz MJ. Use of mini scleral contact lenses in moderate to severe dry eye. Cont Lens Anterior Eye. 2012;35:272–6. doi: 10.1016/j.clae.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 102.Gould HL. Therapeutic effect of flush fitting scleral lenses and hydrogel bandage lenses. Int Surg. 1973;58:469–72. [PubMed] [Google Scholar]
- 103.Gould HL. The dry eye and scleral contact lenses. Am J Ophthalmol. 1970;70:37–41. doi: 10.1016/0002-9394(70)90666-5. [DOI] [PubMed] [Google Scholar]
- 104.Gilbard JP, Farris RL. Tear osmolarity and ocular surface disease in keratoconjunctivitis sicca. Arch Ophthalmol. 1979;97:1642–6. doi: 10.1001/archopht.1979.01020020210003. [DOI] [PubMed] [Google Scholar]
- 105.Chahal HS, Estrada M, Sindt CW, Boehme JA, Greiner MA, Nerad JA, et al. Scleral contact lenses in an academic oculoplastics clinic:Epidemiology and emerging considerations. Ophthalmic Plast Reconstr Surg. 2018;34:231–6. doi: 10.1097/IOP.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 106.Rosenthal P, Cotter J. The Boston Scleral Lens in the management of severe ocular surface disease. Ophthalmol Clin North Am. 2003;16:89–93. doi: 10.1016/s0896-1549(02)00067-6. [DOI] [PubMed] [Google Scholar]
- 107.Weber SLP, Hazarbassanov RM, Nasaré A, Gomes JÁP, Hofling-Lima AL. Conjunctival impression cytology evaluation of patients with dry eye disease using scleral contact lenses. Cont Lens Anterior Eye. 2017;40:151–6. doi: 10.1016/j.clae.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 108.Kohanim S, Palioura S, Saeed HN, Akpek EK, Amescua G, Basu S, et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis–a comprehensive review and guide to therapy. II. Ophthalmic disease. Ocul Surf. 2016;14:168–88. doi: 10.1016/j.jtos.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 109.Wang Y, Rao R, Jacobs DS, Saeed HN. Prosthetic replacement of the ocular surface ecosystem treatment for ocular surface disease in pediatric patients with Stevens-Johnson syndrome. Am J Ophthalmol. 2019;201:1–8. doi: 10.1016/j.ajo.2019.01.006. doi:10.1016/j.ajo.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Basu S, Shanbhag SS, Gokani A, Kedar R, Bahuguna C, Sangwan VS. Chronic ocular sequelae of Stevens-Johnson syndrome in children:Long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. 2018;189:17–28. doi: 10.1016/j.ajo.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 111.Shanbhag SS, Shah S, Singh M, Bahuguna C, Donthineni PR, Basu S. Lid-related keratopathy in Stevens-Johnson syndrome:Natural course and impact of therapeutic interventions in children and adults. Am J Ophthalmol. 2020;219:357–65. doi: 10.1016/j.ajo.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 112.Rosenthal P, Cotter JM, Baum J. Treatment of persistent corneal epithelial defect with extended wear of a fluid-ventilated gas-permeable scleral contact lens. Am J Ophthalmol. 2000;130:33–41. doi: 10.1016/s0002-9394(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 113.Tsubota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999;106:1984–9. doi: 10.1016/S0161-6420(99)90412-8. [DOI] [PubMed] [Google Scholar]
- 114.Ciralsky JB, Chapman KO, Rosenblatt MI, Sood P, Fernandez AGA, Lee MN, et al. Treatment of refractory persistent corneal epithelial defects:A standardized approach using continuous wear PROSE therapy. Ocul Immunol Inflamm. 2015;23:219–24. doi: 10.3109/09273948.2014.894084. [DOI] [PubMed] [Google Scholar]
- 115.Khan M, Manuel K, Vegas B, Yadav S, Hemmati R, Al-Mohtaseb Z. Case series:Extended wear of rigid gas permeable scleral contact lenses for the treatment of persistent corneal epithelial defects. Cont Lens Anterior Eye. 2019;42:117–22. doi: 10.1016/j.clae.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 116.He X, Donaldson KE, Perez VL, Sotomayor P. Case series:Overnight wear of scleral lens for persistent epithelial defects. Optom Vis Sci. 2018;95:70–5. doi: 10.1097/OPX.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 117.Kumar M, Shetty R, Jayadev C. Role of mini-scleral lens in mucous membrane pemphigoid. Indian J Ophthalmol. 2017;65:320. doi: 10.4103/ijo.IJO_730_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Williams ZR, Aquavella JV. Management of exposure keratopathy associated with severe craniofacial trauma. J Cataract Refract Surg. 2007;33:1647–50. doi: 10.1016/j.jcrs.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 119.Chaudhary S, Chatterjee S, Jain N, Basu S. Scleral contact lenses for optimal visual recovery in a case of severe acid burn with total lagophthalmos. BMJ Case Rep. 2022;15:e248384. doi: 10.1136/bcr-2021-248384. doi:10.1136/bcr-2021-248384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bligdon SM, Colarusso BA, Ganjei AY, Kwok A, Luo ZK, Brocks D. Scleral lens and prosthetic replacement of the ocular surface ecosystem utilization in ocular Graft-versus-Host Disease:A survey study. Clin Ophthalmol (Auckland, NZ) 2021;15:4829–38. doi: 10.2147/OPTH.S337824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain:Is it real? Ocul Surf. 2009;7:28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- 122.Theophanous C, Jacobs DS, Hamrah P. Corneal neuralgia after LASIK. OptomVis Sci. 2015;92:e233–e40. doi: 10.1097/OPX.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 123.Vincent SJ, Alonso-Caneiro D, Collins MJ. The temporal dynamics of miniscleral contact lenses:Central corneal clearance and centration. Cont Lens Anterior Eye. 2018;41:162–8. doi: 10.1016/j.clae.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 124.Otchere H, Jones LW, Sorbara L. Effect of time on scleral lens settling and change in corneal clearance. Optom Vis Sci. 2017;94:908–13. doi: 10.1097/OPX.0000000000001111. [DOI] [PubMed] [Google Scholar]
- 125.Courey C, Michaud L. Variation of clearance considering viscosity of the solution used in the reservoir and following scleral lens wear over time. Cont Lens Anterior Eye. 2017;40:260–6. doi: 10.1016/j.clae.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 126.Bray C, Britton S, Yeung D, Haines L, Sorbara L. Change in over-refraction after scleral lens settling on average corneas. Ophthalmic Physiol Opt. 2017;37:467–72. doi: 10.1111/opo.12380. [DOI] [PubMed] [Google Scholar]
- 127.Kumar P, Carrasquillo KG, Chaudhary S, Basu S. A multi-parameter grading system for optimal fitting of scleral contact lenses. F1000Res. 2022;11:6. doi: 10.12688/f1000research.74638.1. doi:10.12688/f1000research. 74638.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Michaud L, Van Der Worp E, Brazeau D, Warde R, Giasson CJ. Predicting estimates of oxygen transmissibility for scleral lenses. Cont Lens Anterior Eye. 2012;35:266–71. doi: 10.1016/j.clae.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 129.Pullum K, Stapleton F. Scleral lens induced corneal swelling:What is the effect of varying Dk and lens thickness? CLAO J. 1997;23:259–63. [PubMed] [Google Scholar]
- 130.Sotozono C, Yamauchi N, Maeda S, Kinoshita S. Tear exchangeable limbal rigid contact lens for ocular sequelae resulting from Stevens-Johnson syndrome or toxic epidermal necrolysis. Am J Ophthalmol. 2014;158:983–93.e1. doi: 10.1016/j.ajo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 131.Walker MK, Bergmanson JP, Miller WL, Marsack JD, Johnson LA. Complications and fitting challenges associated with scleral contact lenses:A review. Cont Lens Anterior Eye. 2016;39:88–96. doi: 10.1016/j.clae.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 132.Severinsky B, Behrman S, Frucht-Pery J, Solomon A. Scleral contact lenses for visual rehabilitation after penetrating keratoplasty:Long term outcomes. Cont Lens Anterior Eye. 2014;37:196–202. doi: 10.1016/j.clae.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 133.Bennett ES, Henry VA. Lippincott Williams & Wilkins; 2019. Clinical Manual of Contact Lenses. [Google Scholar]
- 134.Tranoudis I, Efron N. Scratch resistance of rigid contact lens materials. Ophthalmic Physiol Opt. 1996;16:303–9. [PubMed] [Google Scholar]
- 135.Shin HS, Jang JK, Kwon YS, Mah KC. Surface modification of rigid gas permeable contact lens treated by using a low-temperature plasma in air. J Korean Phys Soc. 2009;55:2436–40. [Google Scholar]
- 136.Sato T, Kobayashi K, Tanigawa H, Uno K. The effect of the poly (ethylene glycol) chain on surface exchange of rigid gas-permeable contact lenses. Eye Contact Lens. 2002;28:181–5. doi: 10.1097/01.ICL.0000024160.18315.F7. [DOI] [PubMed] [Google Scholar]
- 137.Sindt CW. Tangible Hydra-PEG: A novel custom contact lens coating technology designed to improve patient comfort and satisfaction. Whitepaper[Google Scholar] 2016. Available from: https://blanchardlab.com/wp-content/uploads/2018/01/White-paper-from-Tangible-Science-website-2017-05-09.pdf .
- 138.Schornack MM, Fogt J, Harthan J, Nau CB, Nau A, Cao D, et al. Factors associated with patient-reported midday fogging in established scleral lens wearers. Cont Lens Anterior Eye. 2020;43:602–8. doi: 10.1016/j.clae.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 139.Visser E-S, Visser R, Van Lier HJ. Advantages of toric scleral lenses. Optom Vis Sci. 2006;83:233–6. doi: 10.1097/01.opx.0000214297.38421.15. [DOI] [PubMed] [Google Scholar]