Abstract
Dry eye disease encompasses a broad range of etiologies and disease subtypes which have similar clinical manifestations. Medications can cause dry eye disease or symptoms of dryness as a side effect by either interfering with the lacrimal gland or meibomian gland function, or both, and by other mechanisms that affect the ocular surface homeostasis. This is important to know and recognize as eliminating the offending medication can reverse the symptoms and, in many cases, prevent further deterioration of the ocular surface inflammation. This review focuses on drugs like systemic isotretinoin and taxanes, which cause meibomian gland dysfunction; immune checkpoint inhibitors that cause lacrimal gland dysfunction; gliptins and topical antiglaucoma medications that cause cicatrizing conjunctivitis; and epidermal growth factor receptor inhibitors, fibroblast growth factor receptor inhibitors, and belantamab mafodotin, which cause mucosal epitheliopathy. Many of these medications, particularly the newer anticancer agents, have only recently been introduced for clinical use, and knowledge and awareness of their ocular side effects are still evolving. This review aims to update ophthalmologists on the drug-induced causes of dry eye disease or symptoms of dryness, which is avoidable by discontinuation of the incriminating agent or can be mitigated by reducing the dose or frequency of usage.
Keywords: Drug-induced cicatrizing conjunctivitis, drug-induced dry eye disease, meibomian gland dysfunction
As medicine advances, novel classes of medicines emerge unceasingly with the hope to effectively reverse the disease process or prevent further detrimental outcomes. Over the last decades, therapeutic agents have become more targeted toward a specific part or parts of the pathophysiology. Examples included immune checkpoint inhibitors in cancer immunotherapy, epidermal or fibroblastic growth factor receptor inhibitors, and novel chemotherapeutic agents such as S-1, and an ever-expanding array of monoclonal antibodies targeting both neoplastic or inflammatory conditions (belantamab mafodotin for multiple myeloma, and dupilumab for atopic dermatitis). While medication is a known risk factor for ocular surface diseases,[1] the Dry Eye Workshop II report on iatrogenic dry eye focused on conventional medications such as antihypertensive, antidepressants, antihistamines, corticosteroids, or nonsteroidal anti-inflammatory drugs.[2] Like any other treatment-emergent adverse outcomes, incidences of ophthalmic side effects such as dry eyes following the use of a novel medication take time to be reported, usually in an isolated case report or small series. Thus, it is imperative that we review this topic periodically to keep ourselves updated regarding the possible linkage between a drug and ophthalmic manifestations.
Methods
Literature search was performed in PubMed on September 26, 2022 for the following search strategies (1) (Drug OR “Drug induced” OR Toxicit*) AND (“Meibomian Gland” AND (Dysfunction* OR Atrophy)); (2) (Drug OR “Drug induced” OR Toxicit*) AND (“Lacrimal Gland” AND (Dysfunction* OR Atrophy)); (3) (Drug OR “Drug induced”) AND (“cicatri* conjunct*”). Additional search terms included specific drugs “isotretinoin,” “immune checkpoint inhibitor,” “epidermal growth factor receptor inhibitor,” and “fibroblast growth factor receptor inhibitor” in drug-induced ocular surface diseases. Articles published in English were included for this narrative review. Based on the search strategies, 344 titles and abstracts were screened and 65 were considered relevant to this topic. Additional reports which were relevant to this topic were included from the references of selected studies or from authors if they were not identified from the searches.
Results
Drug-induced meibomian gland dysfunction/atrophy
a. Systemic isotretinoin
Isotretinoin, also known as 13-cis-retinoic acid, is a vitamin-A analogue commonly used in the treatment of acne vulgaris as it is known to cause atrophy of the sebaceous glands. As early as the late 1980s, animal studies found that systemic isotretinoin resulted in a reduction in the size and alteration of the structure of meibomian glands in adult rabbits and hamsters.[3-5] Upon histopathological examination, cell necrosis and reduction were identified without any sign of acute or chronic inflammatory reaction.[5] Clinically, the toxicity, however, manifested as blepharoconjunctivitis with crusting of the eyelid margins and erythema of the conjunctiva of the animals. At about the same time, observations of dry eyes, photophobia, contact lens intolerance, and blepharoconjunctivitis were reported in patients taking systemic isotretinoin.[6,7] Mathers et al.[8] prospectively evaluated 11 patients before and after systemic isotretinoin treatment for four months. The group found that meibomian glands became more atrophic and sparse, with an accompanying reduced volume and increased thickness of meibum, and a significant increase in tear osmolarity measured by the Clifton nanoliter osmometer. The same study showed objective evidence that some of these abnormal meibomian glands could return to a more normal appearance after cessation of the isotretinoin treatment, indicating a potentially reversible causal relationship. Later clinical studies which adopted standardized questionnaires such as the Ocular Surface Disease Index and more updated instruments found significantly greater dry eye symptoms and reduced tear film stability, but changes in Schirmer value were inconsistent, with most stating no effect on aqueous production.[9-11] An earlier clinical study reported the disappearance of symptoms and signs of blepharoconjunctivitis one month after the cessation of treatment;[9] however, recent longitudinal studies reported a persistent anatomical and functional effect up to 6 months after discontinuation of isotretinoin.[12,13]
Isotretinoin is a pro-drug that is isomerized to all-trans-retinoic acid, which induces apoptosis in various human cells, including the sebaceous gland and meibomian gland cells. The therapeutic action of isotretinoin lies in the upregulation of the forkhead box class O transcription factors, which were deficient in acne vulgaris and causing pilosebaceous keratinocyte proliferation and sebaceous lipogenesis.[14,15] As treatment-naïve acne vulgaris patients already had a greater predisposition to develop meibomian gland dysfunction (MGD) and ocular surface damage in the first place,[16] ophthalmologists should recognize and provide treatment targeting blepharitis and associated dry eye, especially when these patients are prescribed systemic isotretinoin. [Fig. 1a]
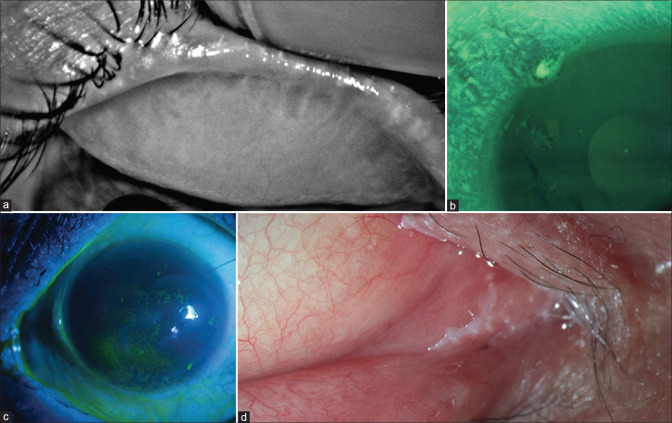
Figure 1.
Dry eye and ocular surface disease caused by various systemic and topical drugs. (a) Isotretinoin-induced complete meibomian gland loss and severe evaporative dry eye; (b) EGFR-induced limbal and peripheral corneal epitheliopathy and dry eye; (c) Gliptin-induced dry eye; (d) Topical decongestant-induced cicatrizing conjunctivitis with medial canthal keratinization
b. Taxanes/S-1/Aromatase Inhibitors
Taxanes are a group of chemotherapeutic agents that are commonly used in the treatment of various cancers. Paclitaxel and docetaxel are two examples. The principal mechanism of the taxane class of drug is by disrupting the microtubules, which are essential to cell division. In 2007, a case report of a 71-year-old male with metastatic prostate cancer who was treated with weekly docetaxel for 3 months developed eye irritation, dry eye, and a chalazion which required an excisional biopsy for determination of its nature.[17] In a recent single center, ten-year retrospective review of patients taking taxanes, Fortes et al.[18] reported 22 patients who experienced an ophthalmic side effect requiring the attention of an eye care provider among 1918 patients who received taxanes from 2010 to 2020. Of which, MGD was the most common side effect that was observed in 5 of the 22 patients. Other adverse effects included canalicular obstruction and cystoid macular edema, which in two cases had led to the cessation of taxane.
Another chemotherapeutic agent S-1 (Taiho Pharmaceutical Co. Ltd, Tokyo, Japan) which contains tegafur (a prodrug of 5-fluorouracil), gimeracil, and oteracil potassium was approved in Japan, Korea, China, and Singapore for the treatment of gastrointestinal, head and neck, breast, and lung cancers.[19] In a single-center Japanese case-control study, severe obstructive MGD was observed in patients within four months after the commencement of S-1 treatment. Irreversible meibomian gland damage was shown on both infrared meibography and in vivo confocal microscopy even after cessation of S-1. The exact mechanism by which S-1 causes damage to the meibomian glands remains unknown. Direct toxicity from the drug in tears is a possibility.[20]
Aromatase inhibitors (AIs) are adjuvant hormonal therapy that is prescribed to women with hormone receptor-positive breast cancer, or ovarian cancer. In the 2010s, observational studies showed that patients who were on systemic AI had greater dry eye symptoms; up to 29% of women treated with AI reported symptoms of ocular irritation or foreign body sensation compared to 9.5% of untreated women.[21] In 2016, 41 hormone receptor-positive breast cancer women on AI (letrozole, anastrozole, and exemestane) were recruited and 42.5% were found to have signs of MGD on slit-lamp examination, which was significantly greater than 12.5% observed in age-matched controls.[22] However, a later case-control study from Australia which recruited a similar number of women on AI did not find any significant difference in the frequency or severity of dry eye or MGD symptoms using multiple questionnaires. The only significant difference on examination was that patients on AI had a lower expressibility of the meibom compared to controls. Otherwise, the two groups were not significantly different in terms of the corneal or conjunctival staining, tear film break-up time, capping of meibomian glands, presence of Marx line, and telangiectasia at lid margins. The results needed to be interpreted with caution as the controls in this study were significantly older than the patients on AI, and that the median duration of AI use was in subjects with a history of chemotherapy was only 0.6 years.[23] A newer study found that the longer duration of AI use and higher tear osmolarity increased the likelihood of dry eye symptoms experienced by the patient.[24] With modern imaging technology, meibomian gland dropout could be assessed quantitatively, and in addition to damage of the meibomian glands, in-vivo confocal microscopy revealed a reduced basal epithelial cell, anterior and posterior keratocytes and endothelial cell densities, as well as long and total sub-basal nerve densities, and increased sub-basal nerve tortuosity during AI treatment in a recent prospective study.[25]
Drug-induced lacrimal gland dysfunction/atrophy
Immune checkpoint inhibitors induced sicca syndrome
Immunotherapy has become one of the novel therapeutics in combating various cancers and immune checkpoint inhibitors are used in treating melanoma, lung, pancreatic cancers, and lymphoma. These agents inhibit the immune checkpoint molecule cytotoxic T-lymphocyte-associated protein 4, programmed death-ligand 1 (PD-L1), and programmed cell death protein 1. Blockades of these molecules aim to increase the immune response from T lymphocytes against tumor cells. As a result of the T-cell activation, immune checkpoint inhibitors are associated with multiple immune-related adverse events, one of which is immune checkpoint inhibitor-induced sicca syndrome. Exocrine gland damages leading to dry mouth and dry eyes were reported as adverse events following the use of nivolumab,[26] durvalumab,[27] and pembroliuzmab.[28] Rheumatic complications were well reported in the literature, especially with regard to the sicca symptoms.[29] However, from the ophthalmologist’s perspective, the dry eye appears to be less common than uveitis. In a 17-year retrospective review between 2000 and 2017, 11 patients were found to have ophthalmic adverse events following the use of immune checkpoint inhibitors. Of these, only one subject reported dry eye, whereas the majority suffered from various degrees of uveitis. Six subjects had to discontinue the immunotherapy and three were related to ocular toxicity. The subject who reported dry eye and blepharitis continued with the immune checkpoint inhibitors.[28]
Drug-induced keratopathy/epitheliopathy
a. Epidermal growth factor receptor inhibitors
Epidermal growth factor receptor (EGFR), or tyrosine kinase inhibitors, was commonly used in the treatment of lung cancers. Erlotinib, gefitinib, and osimertinib are examples of the first-, second-, and third-generation TKI in the management of lung carcinoma with a known mutation in EGFR. In our eyes, the epidermal growth factor plays an important role in the homeostasis of normal corneal epithelium. The use of EGFR inhibitors has been linked to the development of trichomegaly, punctate epithelial erosions, and corneal ulcerations.[30] [Fig. 1b] Despite aggressive lubricants, topical and systemic corticosteroid, the corneal melting progressed until discontinuation of the inciting medication. Rechallenge with a second-generation TKI resulted in the recurrence of the corneal ulcers.
b. Fibroblast growth factor receptor inhibitors
Fibroblast growth factor receptors are another group of tyrosine kinases consisting of four members (FGFR1 – 4) that are present on the cellular membrane and regulate cell growth, differentiation, migration, and survival. Dysregulation or aberrant expression of FGFR has been associated with cancer development, and thus, the molecule is being researched for its potential as an anticancer therapy.[31] Vinson et al. reported two patients who received two different novel FGFR inhibitors and developed dry eye and trichiasis. The first patient was a 72-year-old woman who developed bilateral diffuse corneal punctate erosions at 6 weeks after two cycles of AZD4547 (an oral, selective inhibitor of FGFR1, 2, and 3). In addition to treatment for dry eye with artificial tears, topical anti-inflammatory, punctal plug, and mechanical lash epilation, this patient had to discontinue the AZD4547 therapy due to worsening of trichiasis and dry eye symptoms. Three months after discontinuation, no aberrant lashes were observed; however, the corneal epitheliopathy remained unchanged. Another patient was a 69-year-old man who developed bilateral, but milder, corneal punctate erosions as well as trichiasis at 6 months following INCB054828 (pemigatinib; inhibitor of FGFR 1-3) treatment. The symptoms and signs improved with topical artificial tears, cyclosporine, and repeated mechanical lash epilation.[32] In earlier phase I/II studies of both AZD4547 and INCB054828, dry skin and mucous membranes (dry mouth at 33.3%,[33] dry eye at 20%[33] in AZD4547 trial; dry eyes at 20.3% of all patients treated with pemigatinib) were reported as one of the more common side effects.[33,34] In pemigatinib, the incidence of dry eye correlated with a greater dose of the medication.[34]
c. Belantamab mafodotin
Belantamab mafodotin is a humanized monoclonal antibody against B-cell maturation antigen conjugated with a cytotoxic agent, maleimidocaproyl monomethl auistatin F. The medication received its FDA approval in 2020 as a treatment for refractory or relapsed multiple myeloma following the Phase 2 randomized open-label study (DREAMM-2) in 2019.[35] In the same study, ophthalmologists were included to study the corneal events during the use of the medication. More than 70% of subjects recruited in this trial developed keratopathy in the form of microcyst-like epithelial changes, with or without symptoms. These changes occurred first in the periphery and progressed toward the center of the cornea, which may affect the vision of the subject.[36] Dry eye symptoms or blurry vision were reported in about half of these subjects. A publication suggested a close collaboration between hematologist/oncologist and eye care professionals in monitoring these treatment-emergent ocular side effects. By grading these corneal events, treatment strategies included dose delays or reductions which took place in the majority of patients recruited in the DREAMM2 study; other included regular use of preservative-free lubricants and avoiding the use of contact lenses.[37] Corticosteroid eye drops are not recommended as they were shown to be ineffective in preventing keratopathy.[35]
Drug-induced cicatrizing conjunctivitis
a. Gliptins
Gliptins, also known as inhibitors of dipeptidyl peptidase-4 (DPP-4 inhibitors), are a class of oral hypoglycemic agents that block the enzyme dipeptidyl peptidase-4 that are gaining popularity in the management of diabetes mellitus. Gliptins are shown to be associated with the development of bullous pemphigoid, among other autoimmune diseases. A recent case series of four patients with gliptin-induced pemphigoid reported clinical features similar to classic bullous pemphigoid with cutaneous and oral ulcers.[38] The onset of the disease was about 2 years after gliptin use. Concurrent ophthalmic involvement was bilateral and included classic signs of drug-induced cicatrizing conjunctivitis such as punctal stenosis and loss of the plica in all four cases. [Fig. 1c] Two cases developed forniceal shortening inferiorly with symblepharon formation. However, unlike ocular involvement in classic bullous pemphigoid, ophthalmic features were of milder severity and all four patients recovered after discontinuation of the gliptins.
b. Dupilumab
Dupilumab is a human monoclonal antibody that inhibits interleukin 4 and interleukin 13 by binding antagonistically to the alpha subunit of the interleukin-4 receptor (IL-4Rα), which are found predominantly on T lymphocytes. This results in a downregulation of type 2 T helper cells which mediate the inflammation in atopic diseases. The drug has been licensed for the treatment of atopic dermatitis, as well as moderate-to-severe asthma, chronic rhinosinusitis, eosinophilic esophagitis, and recently prurigo nodularis. Ocular diseases associated with dupilumab were poorly understood but thought to be multifactorial in their pathogenesis. There were four small case series/reports regarding dupilumab-associated cicatrizing conjunctivitis totaling seven patients (mean: 52.6 ± 17.5 years old, range 19–72).[39-42] All cases were bilateral and the onset of conjunctivitis ranged from 2 weeks to 1 year after starting dupilumab. While most patients’ ocular condition stabilized after discontinuation or reduction in dose or frequency of administration of dupilumab, some required topical immunosuppressant and one patient suffered bilateral limbal stem cell deficiency despite treatment because of prolonged conjunctival inflammation. To avoid such side effects associated with dupilumab, alternative treatments have been proposed such as Janus Kinase proteins inhibitor in atopic dermatitis, which are intracellular proteins and downstream of the interleukin receptor.[43]
c. Levamisole
Igwe et al.[44] reported a single case of middle age woman with levamisole-induced bilateral cicatrizing conjunctivitis after misuse of cocaine adulterated with lemivasole. Levamisole is an antihelminthic originally used to treat worm infestations in both humans and livestock.[45] Other indications included cancers such as colorectal carcinoma and hematological malignancy. Levamisole has been used to adulterate cocaine and its use was associated with complications such as agranulocytosis and vasculitis.[46,47]
d. Lamotrigine
Lamotrigine is an anticonvulsant that belongs to the class of sodium channel blockers that is commonly used in the treatment of seizures or bipolar affective disorder. While the drug is known for hypersensitivity reaction and its rare association with Stevens–Johnson Syndrome/toxic epidermal necrolysis,[48] one case of isolated conjunctivitis without systemic dermatological involvement was reported. Praharaj et al.[49] described a 32-year-old man with a diagnosis of bipolar affective disorder who developed bilateral conjunctival congestion with mucous discharge and itch after 2 weeks of lamotrigine at 150 mg/day. Conjunctivitis subsided few days after lamotrigine was discontinued. As the drug was withdrawn soon after its onset, no cicatricial feature was reported. Another report of visual loss secondary to cicatrizing conjunctivitis following the use of lamotrigine was a 32-year-old lady who had a petit mal seizure breakthrough while on sodium valproate.[50] One week after starting lamotrigine, she developed an itchy rash on the back and face, which gradually spread to all limbs and mucous membranes, and the diagnosis of toxic epidermal necrosis was made. Despite the immediate discontinuation of lamotrigine, intensive topical and systemic anti-inflammatory treatment for her conjunctivitis, she developed progressive conjunctival scarring resulting in tear film insufficiency and recurrent painful corneal erosions.
e. Topical ophthalmic medications and preservatives
Conjunctival cauterization has been reported to occur with the chronic use of topical mydriatics, cycloplegics, vasoconstrictors [Fig. 1d], and most commonly due to antiglaucoma medications (AGM). Almost all kinds of AGM (beta blockers, epinephrine, and alpha agonists) with or without preservatives are known to be associated with DICC.[51] [Fig. 2] In a case series of 42 eyes of 23 patients with AGM-induced DICC, from India and the United Kingdom, all subjects reacted to preserved AGM with one exception, who also reacted to nonpreserved AGM.[52] At diagnosis, >70% of eyes showed punctate scarring, inflammation, and fornical shortening. Nearly 83% had MGD and at least two eyes had severe aqueous tear deficiency. Long-term glaucoma management is a complex balance between the efficacy and safety of topical medication and the attempt to achieve drug tolerability and then patient’s compliance. Despite such aim, 49–59% of these patients still have ocular surface discomfort, ranging from occasional burning sensation at instillation up to a severe ocular surface disease.[53,54]
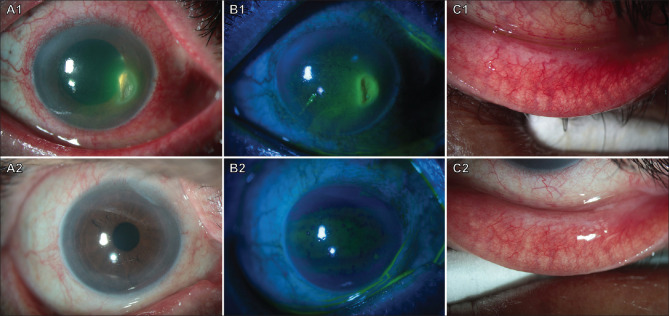
Figure 2.
Before (top row) and after (bottom row) photographs of a patient with severe inflammation, dryness, and corneal ulceration due to chronic use of topical timolol maleate eyedrops. The top row shows the presence of conjunctival inflammation in both bulbar and palpebral regions (A1, C1), and a paracentral corneal ulceration with epithelial defect (B1). The bottom row shows the resolution of conjunctival inflammation (A2, C2) and corneal ulceration (B2) following the withdrawal of the medication and use of preservative-free lubricants
Therefore, such patients, which suffer from a severe, invalidating but silent glaucoma disease, jump into an underestimated highly symptomatic, although less invalidating, ocular surface disease, which limits them in daily life activity and worsens their quality of vision and life.[55]
However, an ocular surface disease associated with hypotensive eye drops significantly affects patients’ quality of daily life, patients’ adherence to medication, and it affects surgical glaucoma treatment outcome.[56]
Glaucoma-associated tear film dysfunction and dry eye symptoms can lead to a reduced quality of vision, along the day among the days, and is typically worse during attention-requiring activities, such as driving or working on a digital screen.[57] The diagnosis can be made clinically when there is a rapid resolution of symptoms and signs of inflammation, usually within 1–16 weeks, after withdrawal of suspected inciting medications. Meanwhile, the intraocular pressure should be controlled by temporary substitution of oral carbonic anhydrase inhibitors.
Ocular surface glaucoma-related diseases are mostly related to inflammatory reactions to the preservatives and occasionally allergic reactions to some medication or preservative, but also to long-lasting chronic inflammatory insults, which may lead to epithelial damage and metaplasia, goblet cell loss with subsequent tear instability, superficial punctate keratitis, and cicatrizing sequelae such as pseudopemphigoid.[58]
Thus, the combination of two or more drugs results in a marked decrease in goblet cells and an increase in intraepithelial pale cells, macrophages, lymphocytes, and fibroblasts with severe profibrotic changes related to the tissue remodelling.[59] If the response to medication withdrawal is uncertain, or the progression of inflammation and scarring continues, then patients must be evaluated to exclude concurrent (or drug induced) mucous membrane pemphigoid, and other potential causes of cicatrizing conjunctivitis, for which the treatment and prognosis are different.
Finally, these cicatrizing conjunctival changes significantly reduce the success rate of surgical procedures such as trabeculectomy which are sometimes required in such patients who are prescribed topical corticosteroid or glaucomatous medications.[60,61] Therefore, prompt management of the ocular surface disease, by using preservative-free lubricating eyedrops, which artificially regenerate tear film mucin matrix, would help improve tolerability and compliance to glaucoma medications and therapeutic outcome.
Possible but unproven associations
a. Terpinen-4-ol (Tea tree oil extract)
Terpinen-4-ol (T4O), or commonly referred to as a tea tree oil extract, is a component of the tea tree oil that has been shown effective in the treatment of demodex infestations. Demodex is a parasite found in eyelash follicles or meibomian glands and is frequently implicated as a cause of both anterior and posterior blepharitis. The application of T4O is often in the form of a sterile lid wipe impregnated with the chemical (e.g. Blephademodex T4O at 2.5%, Thea Pharmaceuticals). A laboratory cell study utilizing immortalized human meibomian gland epithelial cells (IHMGEC) and various concentrations of T4O revealed a dose- and time-dependent decrease in cell survival of the IHMGEC. The number of IHMGEC was significantly reduced after 5 days exposure at the lowest-tested concentration of 0.01%, indicating toxicity to the meibomian gland cells even at 100 folds lower concentration.[62]
b. 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) Reductase Inhibitors
HMG-CoA reductase (HMGCR) inhibitors are commonly known as statins (examples, atorvastatin, rosuvastatin, and simvastatin). They are a group of medicines that lower the serum level of low-density lipoprotein (LDL) cholesterol and reduce the risks associated with atherosclerosis and cardiovascular diseases. Dyslipidemia is known for its association with moderate to severe MGD.[63] MGD patients were found to have higher HDL cholesterol that contributed to an elevated total cholesterol level compared to healthy controls. Nonetheless, according to the findings from the Blue Mountains Eye Study III in Sydney, participants who had hypercholesterolemia (total cholesterol >5.5 mmol/L) did not report more dry eye symptoms than those without hypercholesterolemia. Neither LDL-cholesterol nor HDL-cholesterol level was significantly correlated with dry eye symptoms. However, patients who were taking statins had a twofold odds of reporting one or more moderate to severe symptoms of dry eye disease, indicating for the first time a potential association between oral statin use and dry eye disease.
The enzyme HMGCR was found to be present in the meibomian gland epithelial cells, sebocytes of Zeis, and pilosebaceous glands in full-thickness human eyelid specimens. HMGCR was also expressed in the vascular endothelium. This has led to the proposition of topical statin in the management of blepharitis. In a prospective study comparing subjects on statin treatment and those without, both groups showed deterioration of meibomian gland structure and meibom quality with time. But in this study, no significant difference was detected in any of the dry eye symptoms and signs between the two groups.
Conclusion
Numerous novel therapeutic agents were now reported to be associated with ocular surface changes, including dry eye symptoms either because of MGD, lacrimal gland atrophy, scarring of the conjunctiva, or aberrant lash growth see Table 1. While these complaints may seem minute and remotely related to drug use to patients who are already suffering major illnesses such as malignancy or severe inflammatory conditions, it is the responsibility of the attending ophthalmologist to obtain an accurate drug history and identify the potential causation to reverse the iatrogenic damages. One of the key tasks is to delineate the onset of ocular symptoms in relation to the commencement of any new medication; ophthalmologists need to keep themselves updated regarding the systemic medical condition and constantly keep in mind the possibility of drug-induced dry eyes in the era of biologics and cancer immunotherapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. J Ophthalmol. 2012;2012:285851. doi: 10.1155/2012/285851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15:511–38. doi: 10.1016/j.jtos.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Lambert RW, Smith RE. Pathogenesis of blepharoconjunctivitis complicating 13-cis-retinoic acid (isotretinoin) therapy in a laboratory model. Invest Ophthalmol Vis Sci. 1988;29:1559–64. [PubMed] [Google Scholar]
- 4.Lambert RW, Smith RE. Effects of 13-cis-retinoic acid on the hamster meibomian gland. J Invest Dermatol. 1989;92:321–5. doi: 10.1111/1523-1747.ep12277122. [DOI] [PubMed] [Google Scholar]
- 5.Kremer I, Gaton DD, David M, Gaton E, Shapiro A. Toxic effects of systemic retinoids on meibomian glands. Ophthalmic Res. 1994;26:124–8. doi: 10.1159/000267402. [DOI] [PubMed] [Google Scholar]
- 6.Blackman HJ, Peck GL, Olsen TG, Bergsma DR. Blepharoconjunctivitis:a side effect of 13-cis-retinoic acid therapy for dermatologic diseases. Ophthalmology. 1979;86:753–9. doi: 10.1016/s0161-6420(79)35468-9. [DOI] [PubMed] [Google Scholar]
- 7.Fraunfelder FT, LaBraico JM, Meyer SM. Adverse ocular reactions possibly associated with isotretinoin. Am J Ophthalmol. 1985;100:534–7. doi: 10.1016/0002-9394(85)90676-2. [DOI] [PubMed] [Google Scholar]
- 8.Mathers WD, Shields WJ, Sachdev MS, Petroll WM, Jester JV. Meibomian gland morphology and tear osmolarity:changes with Accutane therapy. Cornea. 1991;10:286–90. doi: 10.1097/00003226-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Bozkurt B, Irkec MT, Atakan N, Orhan M, Geyik PO. Lacrimal function and ocular complications in patients treated with systemic isotretinoin. Eur J Ophthalmol. 2002;12:173–6. doi: 10.1177/112067210201200316. [DOI] [PubMed] [Google Scholar]
- 10.Caglar C, Senel E, Sabancilar E, Durmus M. Reduced ocular surface disease index (OSDI) scores in patients with isotretinoin treatment. Int Ophthalmol. 2017;37:197–202. doi: 10.1007/s10792-016-0263-y. [DOI] [PubMed] [Google Scholar]
- 11.Duzgun E, Ozkur E. The effect of oral isotretinoin therapy on meibomian gland morphology and dry eye tests. J Dermatolog Treat. 2022;33:762–8. doi: 10.1080/09546634.2020.1774041. [DOI] [PubMed] [Google Scholar]
- 12.Tanriverdi C, Nurozler Tabakci B, Donmez S. Longitudinal assessment of meibomian glands and tear film layer in systemic isotretinoin treatment. Eur J Ophthalmol. 2021:11206721211018361. doi: 10.1177/11206721211018361. doi:10.1177/11206721211018361. [DOI] [PubMed] [Google Scholar]
- 13.Gurlevik U, Kemeriz F, Yasar E. The effect of isotretinoin on meibomian glands in eyes:a pilot study. Int Ophthalmol. 2022;42:2071–8. doi: 10.1007/s10792-021-02205-1. [DOI] [PubMed] [Google Scholar]
- 14.Melnik BC. The role of transcription factor FoxO1 in the pathogenesis of acne vulgaris and the mode of isotretinoin action. G Ital Dermatol Venereol. 2010;145:559–71. [PubMed] [Google Scholar]
- 15.Melnik BC. Isotretinoin and FoxO1:A scientific hypothesis. Dermatoendocrinol. 2011;3:141–65. doi: 10.4161/derm.3.3.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koca S, Oral AY. Assessments of the ocular surface and meibomian gland morphology in patients with treatment-I acne vulgaris. Arq Bras Oftalmol. 2022 doi: 10.5935/0004-2749.20230025. S0004-27492022005004208. doi:10.5935/0004-2749.20230025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Silliman CG, Trump DL. Docetaxel-induced meibomian duct inflammation and blockage leading to chalazion formation. Prostate Cancer Prostatic Dis. 2007;10:396–7. doi: 10.1038/sj.pcan.4500939. [DOI] [PubMed] [Google Scholar]
- 18.Fortes BH, Liou H, Dalvin LA. Ophthalmic adverse effects of taxanes:The Mayo Clinic experience. Eur J Ophthalmol. 2022;32:602–11. doi: 10.1177/1120672120969045. [DOI] [PubMed] [Google Scholar]
- 19.Chhetri P, Giri A, Shakya S, Shakya S, Sapkota B, Pramod KC. Current development of anti-cancer drug S-1. J Clin Diagn Res. 2016;10:XE01–5. doi: 10.7860/JCDR/2016/19345.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto Y, Dogru M, Sato EA, Ibrahim OMA, Tatematsu Y, Ogawa Y, et al. S-1 induces meibomian gland dysfunction. Ophthalmology. 2010;117:1275.e4–7. doi: 10.1016/j.ophtha.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 21.Turaka K, Nottage JM, Hammersmith KM, Nagra PK, Rapuano CJ. Dry eye syndrome in aromatase inhibitor users. Clin Exp Ophthalmol. 2013;41:239–43. doi: 10.1111/j.1442-9071.2012.02865.x. [DOI] [PubMed] [Google Scholar]
- 22.Chatziralli I, Sergentanis T, Zagouri F, Chrysikos D, Ladas I, Zografos GC, et al. Ocular surface disease in breast cancer patients using aromatase inhibitors. Breast J. 2016;22:561–3. doi: 10.1111/tbj.12633. [DOI] [PubMed] [Google Scholar]
- 23.Gibson E, Stapleton F, Dear R, Wolffsohn JS, Golebiowski B. Dry eye signs and symptoms in aromatase inhibitor treatment and the relationship with pain. Ocul Surf. 2020;18:108–13. doi: 10.1016/j.jtos.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Khoo P, Groeneveld T, Boyle F, O'Neill S, Forster B, Watson SL. Dry eye signs and symptoms in patients on aromatase inhibitor therapy. Eye (Lond) 2022;36:766–72. doi: 10.1038/s41433-021-01538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agin A, Kocabeyoglu S, Yucel Gencoglu A, Aksoy S, Karakaya J, Irkec M. The effects of systemic aromatase inhibitors on meibomian glands and corneal structure. Eye (Lond) 2022;36:1185–93. doi: 10.1038/s41433-021-01612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yildirim OA, Poyraz K, Erdur E, Can C, Gundogan C, Guzel Y, et al. Nivolumab-related dry mouth and dry eye:Cross-sectional study. Cancer Invest. 2021;39:797–807. doi: 10.1080/07357907.2021.1971241. [DOI] [PubMed] [Google Scholar]
- 27.Pringle S, van der Vegt B, Wang X, van Bakelen N, Hiltermann TJN, Spijkervet FKL, et al. Lack of conventional acinar cells in parotid salivary gland of patient taking an anti-PD-L1 immune checkpoint inhibitor. Front Oncol. 2020;10:420. doi: 10.3389/fonc.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee J, et al. Ocular adverse events following use of immune checkpoint inhibitors for metastatic malignancies. Ocul Immunol Inflamm. 2020;28:854–9. doi: 10.1080/09273948.2019.1583347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris JA, Huang K, Miloslavsky E, Hanna GJ. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oral Dis. 2022;28:2083–92. doi: 10.1111/odi.14000. [DOI] [PubMed] [Google Scholar]
- 30.Kam KW, Wong PPY, Young AL. Tyrosine kinase inhibitor-induced corneal ulcers. Lancet Oncol. 2019;20:e65. doi: 10.1016/S1470-2045(18)30520-5. doi:10.1016/S1470-2045 (18) 30520-5. [DOI] [PubMed] [Google Scholar]
- 31.Dai S, Zhou Z, Chen Z, Xu G, Chen Y. Fibroblast growth factor receptors (FGFRs):Structures and small molecule inhibitors. Cells. 2019;8:614. doi: 10.3390/cells8060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinson KB, Gillette WM, Baston CF, Leahey AB. Trichiasis and dry eye syndrome in two patients on novel fibroblast growth factor receptor inhibitor therapies. Am J Ophthalmol Case Rep. 2020;19:100818. doi: 10.1016/j.ajoc.2020.100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paik PK, Shen R, Berger MF, Ferry D, Soria J, Mathewson A, et al. A Phase Ib open-label multicenter study of AZD4547 in patients with advanced squamous cell lung cancers. Clin Cancer Res. 2017;23:5366–73. doi: 10.1158/1078-0432.CCR-17-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subbiah V, Iannotti NO, Gutierrez M, Smith DC, Feliz L, Lihou CF, et al. FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann Oncol. 2022;33:522–33. doi: 10.1016/j.annonc.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2):A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21:207–21. doi: 10.1016/S1470-2045(19)30788-0. [DOI] [PubMed] [Google Scholar]
- 36.Farooq AV, Degli Esposti S, Popat R, Thulasi P, Lonial S, Nooka AK, et al. Corneal epithelial findings in patients with multiple myeloma treated with antibody-drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol Ther. 2020;9:889–911. doi: 10.1007/s40123-020-00280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lonial S, Nooka AK, Thulasi P, Badros AZ, Jeng BH, Callander NS, et al. Management of belantamab mafodotin-associated corneal events in patients with relapsed or refractory multiple myeloma (RRMM) Blood Cancer J. 2021;11:103. doi: 10.1038/s41408-021-00494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kate A, Doctor MB, Basu S. Drug-induced pemphigoid:Clinical presentation, diagnosis, and management of gliptin-associated cicatrizing conjunctivitis. Ocul Surf. 2022;26:50–2. doi: 10.1016/j.jtos.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Reddy AK, Hauswirth SG, Gregory DG, Liao SD, Palestine AG. Dupilumab-associated cicatrizing ocular disease. Am J Ophthalmol Case Rep. 2022;26:101528. doi: 10.1016/j.ajoc.2022.101528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta U, Farid M. Dupilumab induced limbal stem cell deficiency. Int Med Case Rep J. 2021;14:275–8. doi: 10.2147/IMCRJ.S308583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liberman P, Shifera AS, Berkenstock M. Dupilumab-associated conjunctivitis in patients with atopic dermatitis. Cornea. 2020;39:784–6. doi: 10.1097/ICO.0000000000002262. [DOI] [PubMed] [Google Scholar]
- 42.Levine RM, Tattersall IW, Gaudio PA, King BA. cicatrizing blepharoconjunctivitis occurring during dupilumab treatment and a proposed algorithm for its management. JAMA Dermatol. 2018;154:1485–6. doi: 10.1001/jamadermatol.2018.3427. [DOI] [PubMed] [Google Scholar]
- 43.Arkwright PD, Koplin JJ. Impact of a decade of research into atopic dermatitis. J Allergy Clin Immunol Pract. 2023;11:63–71. doi: 10.1016/j.jaip.2022.09.021. [DOI] [PubMed] [Google Scholar]
- 44.Igwe CN, Robinson F, Jones SM. A novel case of ocular cicatricial pemphigoid induced by levamisole-adulterated cocaine. Eur J Ophthalmol. 2021;31((1_suppl)):11–5. doi: 10.1177/1120672120964756. [DOI] [PubMed] [Google Scholar]
- 45.Moser W, Schindler C, Keiser J. Efficacy of recommended drugs against soil transmitted helminths:systematic review and network meta-analysis. BMJ. 2017;358:j4307. doi: 10.1136/bmj.j4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-Puigdollers A, Just-Sarobe M, Pastor Jane L. Cutaneous and mucosal conditions associated with cocaine use. Actas Dermosifiliogr. 2023;114:125–31. doi: 10.1016/j.ad.2022.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Macias Alonso B, Bashyam AM, Eginli AN, Ahn CS, McMichael AJ. Recurrent antineutrophil cytoplasmic antibody-associated vasculitis induced by levamisole-adulterated cocaine. Am J Dermatopathol. 2021;43:443–5. doi: 10.1097/DAD.0000000000001837. [DOI] [PubMed] [Google Scholar]
- 48.Bloom R, Amber KT. Identifying the incidence of rash, Stevens-Johnson syndrome and toxic epidermal necrolysis in patients taking lamotrigine:A systematic review of 122 randomized controlled trials. An Bras Dermatol. 2017;92:139–41. doi: 10.1590/abd1806-4841.20175070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Praharaj SK, Sarkhel S, Akhtar S. Lamotrigine-induced conjunctivitis. Gen Hosp Psychiatry. 2011;33:e3–4. doi: 10.1016/j.genhosppsych.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 50.McDonald MA, Favilla I. Visual loss in a patient with lamotrigine-induced cicatrizing conjunctivitis. Clin Exp Ophthalmol. 2003;31:541–3. doi: 10.1046/j.1442-9071.2003.00717.x. [DOI] [PubMed] [Google Scholar]
- 51.Kahana A, Marcet MM, Albert DM, Thliveris AT. Drug-induced cicatrising granulomatous conjunctivitis. Br J Ophthalmol. 2007;91:691–2. doi: 10.1136/bjo.2006.099085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh S, Donthineni PR, Shanbhag SS, Senthil S, Ong HS, Dart JK, et al. Drug induced cicatrizing conjunctivitis:A case series with review of etiopathogenesis, diagnosis and management. Ocul Surf. 2022;24:83–92. doi: 10.1016/j.jtos.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Prum BE, Jr, Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, et al. Primary open-angle glaucoma preferred practice PatIn((R)) guidelines. Ophthalmology. 2016;123:P41–111. doi: 10.1016/j.ophtha.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 54.Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618–21. doi: 10.1097/ICO.0b013e3181c325b2. [DOI] [PubMed] [Google Scholar]
- 55.Wong ABC, Wang MTM, Liu K, Prime ZJ, Danesh-Meyer HV, Craig JP. Exploring topical anti-glaucoma medication effects on the ocular surface in the context of the current understanding of dry eye. Ocul Surf. 2018;16:289–93. doi: 10.1016/j.jtos.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Anwar Z, Wellik SR, Galor A. Glaucoma therapy and ocular surface disease:Current literature and recommendations. Curr Opin Ophthalmol. 2013;24:136–43. doi: 10.1097/ICU.0b013e32835c8aba. [DOI] [PubMed] [Google Scholar]
- 57.Di Zazzo A, Roberti G, Mashaghi A, Abud TB, Pavese D, Bonini S. Use of topical cannabinomimetic palmitoylethanolamide in ocular surface disease associated with antiglaucoma medications. J Ocul Pharmacol Ther. 2017;33:670–7. doi: 10.1089/jop.2016.0117. [DOI] [PubMed] [Google Scholar]
- 58.Stewart WC, Stewart JA, Nelson LA. Ocular surface disease in patients with ocular hypertension and glaucoma. Curr Eye Res. 2011;36:391–8. doi: 10.3109/02713683.2011.562340. [DOI] [PubMed] [Google Scholar]
- 59.Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops:The good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–34. doi: 10.1016/j.preteyeres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112:1446–54. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]
- 61.Senthil S, Rao HL, Ali MH, Krishnamurthy R, Dikshit S, Choudhari N, et al. Long-term outcomes and risk factors for failure of glaucoma filtering surgery in eyes with vernal keratoconjunctivitis and steroid-induced glaucoma. Indian J Ophthalmol. 2022;70:820–5. doi: 10.4103/ijo.IJO_1897_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen D, Wang J, Sullivan DA, Kam WR, Liu Y. Effects of Terpinen-4-ol on meibomian gland epithelial cells in vitro. Cornea. 2020;39:1541–6. doi: 10.1097/ICO.0000000000002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dao AH, Spindle JD, Harp BA, Jacob A, Chuang AZ, Yee RW. Association of dyslipidemia in moderate to severe meibomian gland dysfunction. Am J Ophthalmol. 2010;150:371–5 e1. doi: 10.1016/j.ajo.2010.04.016. [DOI] [PubMed] [Google Scholar]