Abstract
Evaporative dry eye (EDE) due to meibomian gland dysfunction (MGD) is one of the common clinical problems encountered in ophthalmology. It is a major cause of dry eye disease (DED) and of ocular morbidity. In EDE, inadequate quantity or quality of lipids produced by the meibomian glands leads to faster evaporation of the preocular tear film and symptoms and signs of DED. Although the diagnosis is made using a combination of clinical features and special diagnostic test results, the management of the disease might be challenging as it is often difficult to distinguish EDE from other subtypes of DED. This is critical because the approach to the treatment of DED is guided by identifying the underlying subtype and cause. The traditional treatment of MGD consists of warm compresses, lid massage, and improving lid hygiene, all measures aimed at relieving glandular obstruction and facilitating meibum outflow. In recent years, newer diagnostic imaging modalities and therapies for EDE like vectored thermal pulsation and intense pulsed light therapy have emerged. However, the multitude of management options may confuse the treating ophthalmologist, and a customized rather than a generalized approach is necessary for these patients. This review aims to provide a simplified approach to diagnose EDE due to MGD and to individualize treatment for each patient. The review also emphasizes the role of lifestyle modifications and appropriate counseling so that patients can have realistic expectations and enjoy a better quality of life.
Keywords: Dry eye disease, evaporative dry eye, meibography, meibomian gland, meibomian gland dysfunction
Inflammatory damage to the ocular surface, tear film instability, neurosensory abnormalities, and hyperosmolarity all play a part in the development of dry eye disease (DED), a multifactorial disease of the ocular surface, which is characterized by a loss of tear film homeostasis and is accompanied by ocular symptoms.[1] For classification, determining the predominant DED subtype (aqueous-deficient or evaporative) is crucial. While the second dry eye workshop organized by the tear film and ocular surface society (TFOS DEWS II) stated that these subtypes are believed to be a part of a spectrum of disease rather than being distinct pathophysiological entities, the therapeutic principles are still guided by identifying the main underlying cause.[1] Although these subtypes can coexist in around 40% of cases,[2] aqueous-deficient DED (ADDE) occurs due to lacrimal gland insufficiency,[3] while the main underlying pathology in evaporative dry eye (EDE) is meibomian gland dysfunction (MGD).
According to the international workshop on MGD, it is a chronic, generalized disorder of the meibomian glands that is frequently characterized by terminal duct obstruction and/or qualitative/quantitative alterations in the secretion of the glands.[4] EDE due to MGD is perhaps one of the commonest problems encountered by ophthalmologists. In India, hospital-based studies have reported its prevalence ranging from 42.5% to 57.2%.[2,5,6] There have been significant recent developments in newer diagnostic imaging techniques and therapies for MGD.[7] The multitude of diagnostic platforms and treatment options available can confuse the treating ophthalmologist. It may also be challenging for general ophthalmologists to differentiate EDE from other forms of DED and tailoring the treatment to an individual patient rather than using a generalized approach. This review aims to provide a simplified and logical approach for the management of EDE due to MGD, which is appropriate for use in our daily clinical practice.
Etiopathogenesis and definition of EDE due to MGD
The term “meibomian gland disease” encompasses MGD as well as congenital, neoplastic, and other conditions of the meibomian glands. Other terms used earlier to describe MGD are meibomitis, meibomianitis, and posterior blepharitis. The underlying pathophysiology of MGD is primary obstructive hypertrophy of the meibomian duct epithelium and keratinization of the circular orifice epithelium leading to terminal duct obstruction, abnormal meibomian gland secretion, eyelid inflammation, corneal inflammation and damage, microbiological changes, and EDE. Although MGD can be further subcategorized into hypersecretory, hyposecretory, and obstructive forms, the obstructive type of MGD is the commonest form, and for the purpose of this review, further reference to MGD shall be in context of the obstructive type only.[8] The terms MGD and EDE are also often used synonymously, since there are few other causes of EDE; however, in this review, we will use the term “EDE due to MGD” to allude to the clinical disease and differentiate it from subclinical MGD without obvious EDE.
Risk factors of EDE due to MGD
Several ophthalmic and systemic factors have been implicated as risk factors in the development of EDE due to MGD. Age is an important risk factor for MGD, and there is an increase in the prevalence of EDE with age.[2,9] Other ocular risk factors include anterior blepharitis, contact lens wear, eye make-up or cosmetics, and demodex infestation; the systemic risk factors are androgen deficiency, menopause, rosacea, atopic dermatitis, seborrheic dermatitis, hypertension, smoking, alcohol consumption, and benign prostatic hyperplasia (BPH): systemic medications like antiandrogens, medications for treating BPH, hormone replacement therapy (postmenopausal estrogens and progestins), antihistamines, antidepressants, and retinoids.[9-11] There is also a correlation between higher screen time or exposure to visual display units and the development of MGD.[12-14] When using a computer or other similar device, most people blink up to 60% less frequently than when not using a computer screen. Reduced blinking rate causes the secretions from the meibomian glands to be emptied less frequently, which may eventually result in gland blockage and malfunction of the glands leading to EDE. Incomplete or partial blinks lead to tear film instability by reducing the quantity of secretion of meibum and improper distribution of lipids in the tear film.
Diagnosis of DED
The first step in the management is establishing the diagnosis of DED and confirming EDE due to MGD as the cause. Patients may have MGD without manifest EDE, and in these cases, preventive measures need to be prescribed, while patients with symptomatic EDE due to MGD will need treatment. The diagnosis is based on a combination of symptoms, basic clinical examination, and special diagnostic tests.
Symptoms: Patients presenting with complaints of foreign body sensation, ocular discomfort, burning, tearing, irritation, sensitivity to light, blurring of vision that improves on blinking, and limitation of daily activities like inability to work continuously on a computer screen may be suffering from some form of DED. These DED symptoms are nonspecific for the underlying etiology. However, occupational history of excessive digital device usage and prolonged working hours in low-humidity environments in a young adult may point toward an evaporative etiology. Ocular and systemic drug history has to be noted, especially the use of isotretinoin for acne treatment or the use of hormone replacement therapy. Asthenopic symptoms may coexist if there is a presence of DED or “Computer Vision Syndrome” (CVS) too. Questionnaires like Ocular Surface Disease Index (OSDI), dry eye questionnaire (DEQ), or SPEED questionnaires may be useful to quantify the patient’s symptomatology and are valuable for research but are not mandatory in routine clinical practice.
Signs: Patients presenting with a history and symptoms suggestive of DED, as mentioned above, should then be subjected to a detailed clinical examination. This includes a quick physical examination, slit-lamp examination of the eyes, simple office tests, and some special diagnostics. The diagnosis committee of the International Workshop on MGD recommends a sequence of tests, like tear film breakup time (TBUT), ocular surface staining, Schirmer’s score, and tear volume, to differentiate between MGD-related evaporative DED and ADDE [Table 1].[15,16] Any patient with symptoms of DED with positive ocular surface fluorescein or lissamine green staining, along with low Schirmer’s test scores and/or low TBUT (<10 s), should be considered as a case of DED and treated as such. These basic tests are described first followed by additional investigations to differentiate EDE and ADDE.
Table 1.
Preferred sequence of clinical and diagnostics tests and differentiation between the evaporative dry eye (EDE) and aqueous deficient dry eye (ADDE)
| Sequence of Tests | Asymptomatic MGD | EDE due to MGD | ADDE |
|---|---|---|---|
| Higher scores on symptom questionnaire (DEQ/SPEED/OSDI) | X | ✔ | ✔ |
| Decreased lower tear meniscus height on slit-lamp biomicroscopy | X | X | ✔ |
| Abnormal tear osmolarity (if available) | X | ✔ | ✔ |
| Presence of corneal and conjunctival fluorescein staining | X | ✔ | ✔ |
| Lower Schirmer’s test without anesthesia (or phenol red thread test) | X | X | ✔ |
| Presence of morphologic lid features | ✔ | ✔ | X |
| Changes in meibum expressibility and quality | ✔ | ✔ | X |
| Presence of MG dropout on meibography | X/✔ | ✔ | X |
| Normal direct examination of the palpebral lobe of the lacrimal gland | ✔ | ✔ | X |
Ocular surface staining: Ocular surface damage associated with tear film instability can be quantified by fluorescein staining of the cornea and lissamine green staining of conjunctiva (Oxford scheme and DEWS scheme). MGD shows staining more along the upper and lower lid margins [Fig. 1], whereas ADDE more often causes interpalpebral corneal staining.
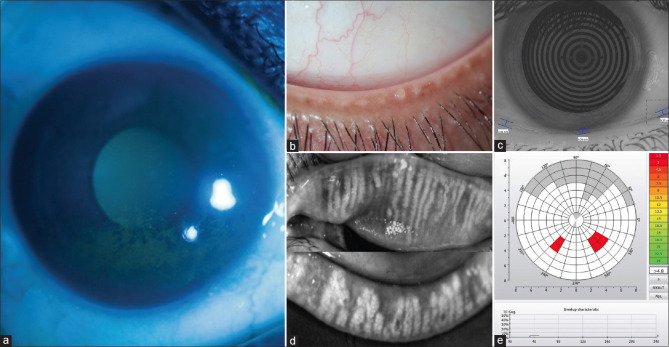
Figure 1.
(a) Typical pattern of corneal fluorescein staining seen under cobalt blue illumination on slit-lamp biomicroscopy in the inferior quadrant adjacent to the lid margin in a case of evaporative dry eye due to meibomian gland dysfunction. (b) Pouting and blocked meibomian gland orifices along the lower lid margin. (c) Reduced tear meniscus height (TMH) as measured by the Keratograph. (d) Infrared imaging of the lids shows areas of meibomian gland dropouts, gland distortion or tortuosity, and gland shortening. (e) Non-invasive-Keratograph-break-up time (NIKBUT) showing abnormal value and pattern
TBUT: TBUT is a surrogate indicator of tear film stability. Using fluorescein, its normal value is more than 15 s; 10–15 s is considered borderline; scores less than 10 s may indicate an inadequate balance between the mucoaqueous and lipid layers of the tear film. However, it does not differentiate between EDE and ADDE.
Clinical clues suggestive of EDE due to MGD
Face: In addition to the ocular and lid features, it is important to look for features of dermatological diseases like acne rosacea and seborrheic dermatitis. Ocular rosacea is a highly underdiagnosed condition and needs an additional degree of suspicion. Almost 90% of ocular rosacea patients (8–50% of all rosacea cases) show eyelid changes including MGD and lid margin inflammation.[16]
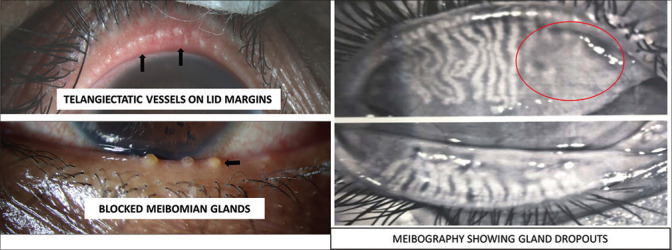
Lids: Examination of the lid margin for any irregularities like thickening, telangiectasia, vascularity, changes in the mucocutaneous junction, collarettes, presence of frothy secretions collected over the lid margin, distichiasis, trichiasis, keratinization, cicatricial changes, and notching is essential to not only diagnose the condition but also to estimate the chronicity of the disease and rule out other lid related diseases. In addition, blink rate, extent of lid excursion, and presence of incomplete or partial blinks are also important determinants of the tear film spread and stability. Abnormality of the eyelid margin can be graded based on lid margin irregularity, vascular engorgement, and glandular orifice obstruction.[15,17]
Meibomian glands: While diagnosing MGD, meibomian gland expression (quantity, quality, and expressibility of meibum) and areas of gland dropout are looked for on slit-lamp biomicroscopy.[17] Meibum quality and expressibility are assessed by applying appropriate digital pressure or using a special handheld instrument to provide standardized force over the eyelid.[18] Usually, all gland openings should be patent and adequate clear fluid is expressed out of the openings. However, in MGD, changes in meibomian lipid composition, such as increased monounsaturated fatty acids and modified fatty acid composition, lead to abnormal lipid behavior like a higher meibum melting temperature resulting in thicker meibum, ductal plugging, capping, stagnation, and pouting of the meibomian gland orifices.[19,20] [Fig. 1b].
Additional diagnostic tests of EDE evaluation
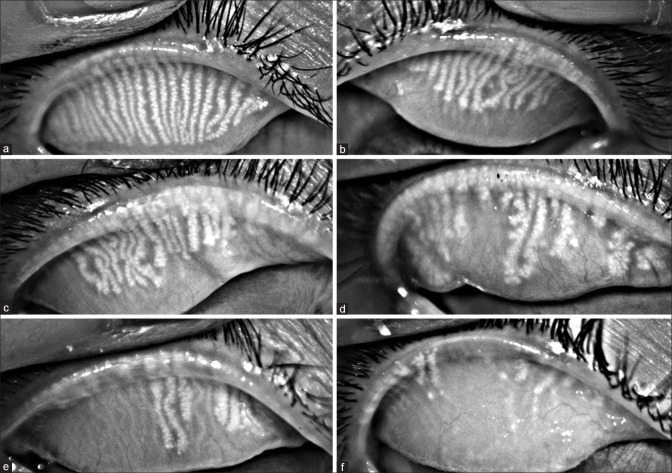
Meibography: Noncontact infrared photography (either by commercially available dedicated meibography devices or by infrared cameras of the autorefractometer) [Fig. 2] facilitates assessment of morphological features of the meibomian gland in vivo. Meibomian gland loss or meibomian gland dropout refers to the loss of acinar tissue detected by meibography.[21,22] The characteristics noted on meibography include gland distortion, gland shortening, and gland dropout, which can be scored [Fig. 2 and 3].[23] According to the DREAM study, meibomian gland loss was significantly less in the upper lid than in the lower lid in patients with DED. Therefore, it is important to evaluate both the upper and lower eyelids as assuming that findings from the upper lid are the same as those from the lower lid is not warranted.[24] A study of the morphological variants of meibomian glands showed the presence of hooked, tortuous, and overlapping glands that had completely normal glandular histology, whereas severely short glands showed atrophic changes with loss of meibocyte differentiation and cellular proliferation [Fig. 2]. Total loss of glandular elements was seen in dropout areas, suggesting that there can be a lot of variation in the morphology and length of the meibomian glands even in the normal population.[25]
Figure 2.
Representative photographs of progressive degrees of meibomian gland loss (a- f), as seen in decreasing number of glands and increase in areas of gland dropouts
Figure 3.
Presence of telangiectatic vessels over the lid margin surrounding the meibomian gland orifices of the upper eyelid and blocked and capped orifices of the lower lid. Meibography showing areas of blocked meibomian glands and their loss or dropouts (marked in the oval)
Noninvasive tear film break up time (NIBUT): Some believe that instilling fluorescein dye is intrusive because it can itself cause instability in the tear film. Thus, NIBUT, a placido disc-based noncontact evaluation of the tear film, was developed [Fig. 1]. Since TBUT and NIBUT levels have been proven to be significantly different in studies, these values cannot be utilized interchangeably. Overall, if the technology is available, it is better to use the average result of several NIBUT measurements.[26-28] Typically in EDE, NIBUT is less than 7-10 seconds. However, similar to TBUT, a low NIBUT alone cannot distinguish between DED subtypes.[29]
Lipid layer thickness: The thickness of the normal tear film lipid layer (TFLL), the most anterior part of the tear film, is approximately 20–160 nm.[30] Lipid layer thickness (LLT) measurement is used to assess thickness, spread time, spread rate, and pattern by broad-spectrum white light interferometry. On recording the movement of particles in the film by interferometry, the lipid layer is seen to spread upward (primarily from the lower reservoir) in the upstroke of the blink and stabilizes after approximately 1–2 s.[31] This changes to 3.54 ± 1.86 s in eyes with lipid tear deficiency.[32] Thinning of the TFLL has been noted in lipid tear deficiency.[33] In normal subjects, the relatively constant appearance of the interferometric pattern of the TFLL over several blink cycles implies the conservation of its architecture from blink to blink. This cycle of stability is shortened in MGD and has been proposed as a measure of MGD-related disease in the dynamic lipid layer interference pattern test.[34,35]
Biomarkers of inflammation: Various inflammatory biomarkers like cytokines and other mediators (TNF-α, IL-6, IL-17a, and IL-8, secretory phospholipase A2, prostaglandin E2, etc.), interleukins; matrix metalloproteinases (MMP-9), Human Leukocytic Antigen- DR isotype (HLA-DR) expression, can be tested by tear sampling or impression cytology and have been found to be deranged in DED.[36] Tests of tear osmolarity and MMP-9 are available as point-of-care objective metrics.[36] These tear film biomarkers have utility in research studies in DED to detect the level of ocular surface inflammation and to evaluate response to therapy in DED due to MGD. However, they may not have relevance in routine clinical practice.
In vitro confocal microscopy (IVCM): The confocal laser-scanning microscope (HRT II RCM Heidelberg Engineering Inc., Heidelberg, Germany, Rostock Cornea Module) is a relatively newer diagnostic modality for MGD. On assessment, increased acinar unit diameter with decreased mean acinar unit density (number of glands/mm2) was found in the MGD group as compared to the control group. The images also indicated that acinar unit enlargement was due to the inspissation of meibum secretions and glandular atrophy with periglandular fibrosis.[37] In another study, severe MGD patients have shown higher corneal nerve reflectivity. Total symptom score negatively correlated with nerve density while positively correlated with nerve reflectivity and dendritic cell density.[38] Reduced nerve density with alteration of subbasal nerve plexus features may be associated with neuropathic pain or dysesthesia due to sensitization of peripheral nerve endings or exposure of the nerves to inflammatory cytokines. IVCM may be of great value in imaging resident Demodex mites in the meibomian gland orifices.[39]
Tests to rule out ADDE
Schirmer’s test: One end of the Whatman 41 filter paper strip (5 mm wide and 35 mm long) is placed over the lateral third of the lower eyelid in each eye. The patient may continue blinking normally or keep the eyes closed. After 5 min, the examiner removes the strips and measures the length of strip wet by tears. To measure the total tear secretion (basal and reflex tear secretion), it is performed without anesthesia and values of more than 15 mm are normal; 5–10 mm are borderline, and less than 5 mm are severely abnormal. This is an indicator of the ADDE component of DED. Schirmer’s test is normal in EDE.
Lacrimal gland evaluation: Differentiating between ADDE and other DED causes requires a direct examination of the palpebral lobe of the lacrimal gland. When performing a slit-lamp examination, the palpebral lobe of the lacrimal gland can be evaluated by having the patient look inferonasally as the examiner holds the upper eyelid superotemporally. The palpebral lobe has a convex form, a smooth surface, and a pinkish appearance in healthy people and people with EDE, but in people with ADDE, the lobe shrinks, flattens out, and occasionally develops regions of fibrosis.[40] As a result of symblepharon in cicatrizing conjunctivitis, the gland area is usually not visible. A fluorescein strip placed over the exposed lobe area can also assess tear flow, known as “Direct assessment of tear secretion” or DATS, from the palpebral lobe of the lacrimal gland.[41] There is a considerable decrease in tear flow in ADDE, while in EDE, it is similar to healthy eyes.
Tear volume: Tear meniscus height (TMH) under a slit-lamp or anterior segment optical coherence tomography (OCT) are two methods for measuring tear volume. OCT-based TMH measurement is more accurate and repeatable than slit-lamp-based evaluation [Fig. 1]. Even with OCT, the value can vary greatly depending on the operator, the time since the last blink, the amount of fluorescein previously instilled, the time of day, lighting, temperature, and ambient humidity.[42,43] According to TFOS-DEWS II criteria, the TMH cut off of 0.2 mm provided 98.3% sensitivity and 96.7% specificity in participants who were diagnosed with ADDE.[44]
Staging of MGD
The abnormality of the eyelid margin (Lid Margin Score) is assessed by the following three factors: lid margin irregularity, vascular engorgement, and glandular orifice obstruction assigned one score each. Meibomian gland expression (MG expressibility) is scored according to the secretion seen in all five meibomian glands: 0, all glands; 1 (3–4 glands); 2 (1–2 glands); and 3 (no glands).[15] Meibum quality (Meibum score) is graded as: 0 (clear); 1 (cloudy); 2 (cloudy with debris); and 3 (inspissated, toothpaste-like). Subjectively, the Meiboscore is defined by using the four-point grading scale (0–3): 0 (no dropout); 1 (<1/3 total area dropout); 2 (1/3–2/3 total area dropout); and 3 (>2/3 total area dropout).[45] Semiobjectively, MG dropout is measured and expressed as the percentage when the areas of MG loss are divided by the total area of the everted eyelid.[46] Finally, the total score of the MG grading examinations is calculated (MG score), as an integrated evaluation of the meibomian gland and ranges from 0 to 12. The severity of MGD can be staged using the degree of symptoms, grade of MG involvement, surface staining, and coexisting and accompanying disorders of the ocular surface (Plus disease).[8]
Treatment
Early treatment during the asymptomatic stage of MGD may delay progression to the symptomatic stage and reverse the pathological events of MGD.[15] In the presence of “plus” disease, where MGD may develop in association with ocular surface disease, or secondary to other ocular disorders, concurrent management of comorbid conditions according to standard-of-care protocols may be required.[8,47] Based on the coexistence of ADDE and MGD, the approach to treating patients with various degrees of both these diseases is crafted.
Lifestyle modifications to optimize the work/home environment: Intake of a healthy diet, improvement in body hydration status, adoption of methods to decrease excessive when the evaporation of tear film, to avoid hot, dry environments and to add moisture to the ambient air using a humidifier is advocated; direct blast from air-conditioning or heating vents at home, at work or while driving or traveling in air-conditioned vehicles, is to be avoided. Wraparound-style glasses on windy days and goggles while swimming should be worn. Smoking and passive smoking are to be avoided.
Lid hygiene, warm compresses, lid massage, and gland expression: The effective conventional treatment of MGD consists of the application of warm compresses, improving lid hygiene and removing the obstructed meibum by gentle massage and expression. The melting point of meibum increases in MGD (35°C) as compared to non-MGD (32°C) because of its altered lipid composition. The obstruction of ducts with thick meibomian secretions leads to a stagnant and less dynamic tear film. Increased eyelid temperature helps by decreasing the lipid viscosity, and hence increasing lipid levels of the tear film.[48] This is ideally followed by moderate to firm digital massage of the lids and expression of thicker meibum, thus clearing the meibomian gland ducts blockage, allowing the meibomian glands to produce normal secretions. However, this is a time-consuming and labor-intensive therapy and leads to compliance issues and, finally, lower treatment efficacy. For more inspissated meibum causing severe obstruction, treatment with a manual expression of meibomian glands is done carefully by the clinician using atraumatic specialized expressor forceps.
Lubricant therapy: Even though the use of artificial lubricants or artificial tears (AT) is not the mainstay of treating MGD, the supplementation of the tear film can address the “final common pathway” that mediates a wide range of ocular surface diseases, including EDE (with or without MGD) and aqueous deficient dry eye. The use of emollients in the lubricants may be helpful to a certain extent, e.g., petrolatum, mineral oil, castor oil, hydroxypropyl guar, and sodium hyaluronate. Evidence for treating mixed forms of DED supports the usage of higher-viscosity artificial lubricants in the form of eye drops or gels. However, viscosity should be balanced with a risk of visual blurring. Lubricant eye ointment should be used at bedtime for symptomatic relief as ointments last for a longer duration as compared to eye drops. Also, preservative-induced epithelial toxicity has been reported using detergent-type preservatives, e.g., benzalkonium chloride and may be reduced by using so-called vanishing preservatives like sodium chlorite or sodium perborate, 1.5%. There is also an increase in the use of lipid-containing eye drops or liposomal sprays in some countries. The use of conventional antibiotic eye ointments, with mineral oil or soft paraffin base, may also act as topical lipid supplements in EDE or MGD, as they contain both polar and nonpolar lipids. Ongoing studies consider the utility of emulsion or nano-emulsion-based eye drops, and ointments with tear interference imaging show rapid restructuring of the preexisting lipid tear film.
MiBo thermoflow: MiBo thermoflow (MIBO Medical Group, Dallas, TX, USA) is a model of an electronic heating device, which can warm, massage, and empty clogged meibomian glands on both upper and lower lids simultaneously.[49] The device has an external paddle heated to 42.2°C and applied with a gel buffer to the eyelids.
Intense pulsed light (IPL): IPL therapy uses a noncoherent polychromatic light source with a broad wavelength spectrum of 500–1200 nm and is used for aesthetic or therapeutic purposes in Dermatology.[50] The photothermal effect helps decrease inflammation of the glands and improvement in telangiectasia.[51] However, the exact mechanism of action is still unclear. This is applied through the skin, and the waves get absorbed into the blood vessels near the skin’s surface.[52] These waves are then taken up by the oxy-hemoglobin in the blood cells, leading to local heat generation and coagulation, leading to blood vessel thrombosis. Patients also experience decreased redness due to the resolution of abnormal telangiectatic vessels. Further, IPL also has some antibacterial effects on the treated area.
Vectored thermal pulsation: The goal of the treatment of MGD is to improve the flow of meibomian gland secretions, thus improving tear film stability. Relieving meibomian gland obstruction is also vital for a successful treatment. In advanced cases, heat and eyelid massage may not be able to cure the disease completely. Lid massage provides partial and temporary relief of obstruction of the meibomian glands and could be painful too. These treatments may be frustrating for patients and ophthalmologists alike. With conventional warm compresses, heat reaches the outer surface of the eyelid only and may be of limited effectiveness for the blocked meibum in the glands in the tarsal plate. With LipiFlow® treatment (TearScience®, Morrisville, NC, USA), heat can be applied to both the upper and lower palpebral conjunctival surfaces in addition to pulsating pressure to the external eyelid at the same time to express the thick meibum from the meibomian glands, thus clearing meibomian gland obstruction in a two-pronged way.[53]
Each eye receives vectored thermal pulsation for 12 min. As a temperature- and pressure-controlled device, this novel treatment for obstructive MGD has combined the benefits of both heat therapy and physical expression. No pressure is transmitted directly onto the eyeball.[54] Adverse effects in the form of eyelid pain, moderate conjunctival congestion, and ocular burning symptoms were reported but resolved in 4 weeks without treatment. A statistically significant mean decrease in corneal staining from baseline to 2 weeks and 4 weeks was observed.[53] It has shown marked improvement in symptoms and signs (based on TBUT, corneal fluorescein staining, and meibomian gland secretion scores).[55] However, not all MGD cases are suitable for this treatment. Patients with widespread MGL can, thus, be identified as unsuitable for such therapies like vectored thermal pulsation and mechanical expression of the liquefied meibum. Table 2 lists the advantages and disadvantages of IPL and LipiFlow. It is also important to understand that multiple sessions of these modalities may be required in some cases and that these are not magic bullets or “one-shot” miracle cures for EDE due to MGD.
Table 2.
Advantages and disadvantages of IPL and LipiFlow
| Characteristics | IPL | LipiFlow |
|---|---|---|
| Automated system | ✔ | ✔ |
| Treatment tool | Pulsed light | Thermal pulses and vectored massage |
| Number of sittings | Need 2-4 sessions to obtain optimal results | One session (12–15 min) only |
| Topical anesthesia | X | ✔ |
| Pain | X | X |
| Cost | Lesser cost | High cost of each treatment |
| Use of postprocedure medications for a lasting relief | ✔ | ✔ |
| Type of eye dryness | Moderate to severe | Mild to moderate |
| Improvement of symptoms | Yes | Yes (less consistent) |
| Antimicrobial effect | ✔ | X |
| Long-term effect | ✔ | ✔ |
| Reduction in the appearance of wrinkles and rosacea | ✔ | X |
| Type of skin | All skin types if paired with LLLT treatment | All skin types |
| Post-treatment recovery time | None | None |
| Need for post-treatment artificial tears | Significantly reduced | Necessary |
| Contraindicated in a patient using photosensitizing drugs | Yes | No |
| Use in pregnant patient | X | ✔ |
Intraductal meibomian gland probing is a relatively nontraumatic, slit-lamp-based procedure where mechanical opening and dilatation of the natural orifices and ducts of the meibomian glands and removal of abnormal meibum secretions are performed, leading to rapid and lasting relief of MGD symptoms.[56] Under topical anesthesia, patients are treated with the 2 mm probe at the slit lamp initially. Then, the 4 mm probe is subsequently used for deeper probing. Post probing, reestablishment of orifice and central ducts leads to relief of symptoms. Some disadvantages such as variable discomfort and orifice hemorrhage maybe seen during the procedure but resolve eventually.[57]
Following any meibomian gland-targeted intervention for MGD, like probing, thermal vectored treatment, or IPL, the patient needs topical medications, frequent lid hygiene, and continuation of warm compresses to prevent blocking of the meibomian ducts and easy expression of meibum.
Oral tetracycline/doxycycline: If chronic symptoms and signs of MGD are not adequately controlled by eyelid cleansing or meibomian gland expression (especially in patients with ocular rosacea), daily oral doxycycline, minocycline, or tetracycline may be helpful and tapered when clinical improvement is documented.[58] These drugs limit bacterial colonization and reduce inflammation of the lid margin. In women of childbearing age and children less than 8 years of age, these may be substituted by oral erythromycin or azithromycin.[59] The side-effect profile and contraindications for the use of tetracyclines should be kept in mind while prescribing them.
Topical antibiotic (azithromycin) ointment: Commensal and other lid bacteria (like Staphylococcus epidermidis, Staphylococcus aureus, Propionibacterium acnes, etc.) produce lipolytic enzymes (lipases and esterases), hydrolyze the meibum lipids, increase their melting temperature, and release free fatty acids and inflammatory mediators to destabilize the tear film.[60] Topical azithromycin, a broad-spectrum macrolide antibiotic, is a potentially effective and well-tolerated treatment for MGD, leading to clinical control or relief of symptoms and signs of MGD, improvement in meibomian gland orifice plugging, favorable tissue penetration to the eyelid, good pharmacokinetics for a daily dose, and a sustained delivery mechanism system with potent ocular anti-inflammatory properties (decreased inflammatory cytokine levels such as interleukin-6 and interleukin-8), as well as improvement in lipid behavior of meibomian gland secretion.[61-63]
Oral omega-3 fatty acids: Modifying dietary lipid intake can influence meibum lipid composition as a treatment of MGD. Therefore, it is recommended that oral supplementation with omega-3 essential fatty acids could be a possible MGD therapy in improving some clinical symptoms and signs, as well as changes in meibum content.[64] The breakdown of omega-3 essential fatty acids in the body may lead to suppression of inflammation, whereas the breakdown of omega-6 essential fatty acids produces molecules promoting inflammation.[65] Omega-3 and omega-6 fatty acids compete for the same enzymes in the inflammatory pathway, mediated by the anti-inflammatory agents. So, the metabolism of omega-3 essential fatty acids could inhibit the metabolism of omega-6 essential fatty acids, thus leading to decreased proinflammatory cascade in the eyelid. However, patients who were given supplements containing 3000 mg of n-3 fatty acids for 12 months did not fare any better than those who received a placebo.[66]
Topical anti-inflammatory therapy with corticosteroids: Classic anti-inflammatory treatments are used in combination with lid hygiene, warm compresses, and topical antibiotics for a short time in MGD with posterior eyelid margin inflammation.[37] Topical cyclosporine and/or punctal plugs may also be helpful in managing coexisting aqueous tear deficiency. Topical anti-inflammatory therapy with corticosteroids has shown to be effective in the treatment of severe MGD by suppressing the migration of inflammatory cells and inhibiting the release of several cytokines.[67] However, corticosteroids might induce some complications such as cataracts, steroid-induced ocular hypertension, and opportunistic superinfections and must be used with caution and supervision.
Recalcitrant cases: In a certain subset of patients, symptoms may be severe and unaccompanied by equivalent signs. This is sometimes referred to as “corneal pain without stain” with normal tear film parameters and is often misdiagnosed as DED. It is postulated to be due to injury to or disease of the peripheral corneal nerves. A ”proparacaine challenge test” can be used to distinguish between central and peripheral nervous system origins for ocular neuropathic pain to determine treatments. If a patient experiences complete or partial relief with topical 0.5% proparacaine hydrochloride, a peripheral or combined form, respectively, may be diagnosed. If there is no relief or a worsening of symptoms, then the patient has central sensitization of pain and is very challenging to treat.[68]
In patients with recalcitrant blepharitis not responding to therapy, the possibility of carcinoma or immune-mediated diseases should be considered, particularly if the blepharitis is associated with a loss of eyelashes, nodular mass, and/or conjunctival cicatricial changes along with localized crusting and scaling of the dermis. Early diagnosis and appropriate treatment can prevent disfigurement and may be lifesaving. Ulceration, extensive scarring, lash loss, or yellow conjunctival nodules surrounded by intense inflammation may suggest the presence of an eyelid tumor. The most frequently encountered malignant tumors involving the eyelids are basal cell carcinoma, squamous cell carcinoma, melanoma, and sebaceous carcinoma. Moderate to severe cases with pain, or cases unresponsive to treatment or with visual loss, or orbital involvement or systemic disease are suspected; timely referral to a specialist ophthalmologist who is knowledgeable and experienced in the management of these entities is recommended. Pediatric patients should be referred sooner.
Counseling and Preventive Aspects
One of the most important aspects of caring for patients with EDE due to MGD is the need to give them more “clinic chair time”. It is important to explain the chronic nature of EDE, its pathogenesis, and the deleterious effects of multiple physician consultations or “doctor shopping” without complying with treatment. It is imperative for patients to have realistic expectations (e.g., symptoms may relatively improve but can rarely be eliminated), and they should be conducive to lifestyle modifications if diagnosed with MGD. This includes changes in diet and work/home environments like improving ambient humidity to minimize tear evaporation. The patients should also be educated about the possible contributory effect of their systemic medications and about the warning signs of surface damage which they need to watch out for. Contact lens wearers should be cautioned about the chances of developing intolerance and the risk of corneal infection if the associated MGD does not receive appropriate treatment.[69] Emphasis should be laid on frequent follow-ups and immediate return to the clinic in the presence of any warning signs in the eyes. The patient’s compliance and understanding of the disease must be periodically reassessed to prevent a vicious cycle of dissatisfaction. A small subset of patients may even benefit from professional counseling as an aid in coping with the chronic disease state. Pre- and postoperative gentle and patient counseling and treatment may be necessary for patients undergoing a cataract or refractive surgery. In patients with MGD and coexistent CVS, it is advisable to follow the 20-20-20 rule and perform “strategic blinking,” which means to take frequent short blinking breaks when engaging in activities that typically precipitate symptoms, like excessive screen usage. It may also be beneficial to adjust the display screen’s brightness, use appropriate viewing angulation, enable night mode in digital devices, use ambient light or background illumination, and to switch over to e-book readers and try to limit or decrease screen time and practice “squeeze blinking.”
Conclusion
Although a common condition that affects millions worldwide, the management of EDE due to MGD remains challenging. It is not unusual for a treating ophthalmologist to become perplexed by the variety of diagnostic tools and therapeutic options available. Additionally, it may be difficult to distinguish EDE from other types of DED and to customize treatment for each patient rather than taking a broad approach. This review is specifically intended for general ophthalmologists to orient them toward helping them diagnose EDE, differentiate it from ADDE, and provide a simplified treatment approach including the adequate emphasis on prevention and patient counseling.
Financial support and sponsorship
Hyderabad Eye Research Foundation (HERF), Hyderabad, Telangana, India.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Donthineni PR, Kammari P, Shanbhag SS, Singh V, Das AV, Basu S. Incidence, demographics, types and risk factors of dry eye disease in India:Electronic medical records driven big data analytics report I. Ocul Surf. 2019;17:250–6. doi: 10.1016/j.jtos.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Doctor MB, Basu S. Lacrimal gland insufficiency in aqueous deficiency dry eye disease:Recent Advances in pathogenesis, diagnosis, and treatment. Semin Ophthalmol. 2022;37:801–12. doi: 10.1080/08820538.2022.2075706. [DOI] [PubMed] [Google Scholar]
- 4.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, et al. The international workshop on meibomian gland dysfunction:Report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–7. doi: 10.1167/iovs.10-6997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee S, Agrawal D, Sharma A. Meibomian gland dysfunction in a hospital-based population in central India. Cornea. 2020;39:634–9. doi: 10.1097/ICO.0000000000002217. [DOI] [PubMed] [Google Scholar]
- 6.Yadav S, Gupta N, Makwana T, Vanathi M, Tandon R. Noninvasive ocular surface analyzer as an adjunct in diagnosis and estimating prevalence of meibomian gland dysfunction:Hospital-based comparative study. Ind J Ophthalmol. 2022;70:1539–45. doi: 10.4103/ijo.IJO_2245_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narang P. Commentary:Meibomian gland dysfunction:Taking a deep dive into the ocular surface parameters. Ind J Ophthalmol. 2022;70:1548. doi: 10.4103/ijo.IJO_742_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The International Workshop on meibomian gland dysfunction:Executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–9. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–65. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan DA, Rocha EM, Aragona P, Clayton JA, Ding J, Golebiowski B, et al. TFOS DEWS II sex, gender, and hormones report. Ocul Surf. 2017;15:284–333. doi: 10.1016/j.jtos.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Gomes JAP, Azar DT, Baudouin C, Efron N, Hirayama M, Horwath-Winter J, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15:511–38. doi: 10.1016/j.jtos.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Wang Y, Dong N, Yang F, Lin Z, Shang X, et al. Meibomian gland dysfunction determines the severity of the dry eye conditions in visual display terminal workers. PLoS One. 2014;9:e105575. doi: 10.1371/journal.pone.0105575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenga C, Aragona P, Cacciola A, Spinella R, Di Nola C, Ferreri F, et al. Meibomian gland dysfunction and ocular discomfort in video display terminal workers. Eye (Lond) 2008;22:91–5. doi: 10.1038/sj.eye.6703025. [DOI] [PubMed] [Google Scholar]
- 14.Mehra D, Galor A. Digital screen use and dry eye:A review. Asia Pac J Ophthalmol (Phila) 2020;9:491–7. doi: 10.1097/APO.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, et al. The international workshop on meibomian gland dysfunction:Report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–49. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction:Report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005. doi: 10.1167/iovs.10-6997e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geerling G, Baudouin C, Aragona P, Rolando M, Boboridis KG, Benítez-Del-Castillo JM, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction:Proceedings of the OCEAN group meeting. Ocul Surf. 2017;15:179–92. doi: 10.1016/j.jtos.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Powell DR, Nichols JJ, Nichols KK. Inter-examiner reliability in meibomian gland dysfunction assessment. Invest Ophthalmol Vis Sci. 2012;53:3120–5. doi: 10.1167/iovs.12-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dougherty JM, Osgood JK, McCulley JP. The role of wax and sterol ester fatty acids in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:1932–7. [PubMed] [Google Scholar]
- 20.Ong BL, Larke JR. Meibomian gland dysfunction:Some clinical, biochemical and physical observations. Ophthalmic Physiol Opt. 1990;10:144–8. doi: 10.1111/j.1475-1313.1990.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee JM, Jeon YJ, Kim KY, Hwang KY, Kwon YA, Koh K. Ocular surface analysis:A comparison between the LipiView® II and IDRA® . Eur J Ophthalmol. 2021;31:2300–6. doi: 10.1177/1120672120969035. [DOI] [PubMed] [Google Scholar]
- 22.Arita R, Fukuoka S, Kawashima M. Proposed algorithm for management of meibomian gland dysfunction based on noninvasive meibography. J Clin Med. 2021;10:65. doi: 10.3390/jcm10010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao J, Adil MY, Chen X, Utheim ØA, Ræder S, Tønseth KA, et al. Functional and morphological evaluation of meibomian glands in the assessment of meibomian gland dysfunction subtype and severity. Am J Ophthalmol. 2020;209:160–7. doi: 10.1016/j.ajo.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Daniel E, Maguire MG, Pistilli M, Bunya VY, Massaro-Giordano GM, Smith E, et al. Grading and baseline characteristics of meibomian glands in meibography images and their clinical associations in the Dry Eye Assessment and Management (DREAM) study. Ocul Surf. 2019;17:491–501. doi: 10.1016/j.jtos.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Naidu GC, Vemuganti G, Basu S. Morphological variants of meibomian glands:Correlation of meibography features with histopathology findings. Br J Ophthalmol. 2023;107:195–200. doi: 10.1136/bjophthalmol-2021-318876. [DOI] [PubMed] [Google Scholar]
- 26.Hong J, Sun X, Wei A, Cui X, Li Y, Qian T, et al. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea. 2013;32:716–21. doi: 10.1097/ICO.0b013e3182714425. [DOI] [PubMed] [Google Scholar]
- 27.Fuller DG, Potts K, Kim J. Noninvasive tear breakup times and ocular surface disease. Optom Vis Sci. 2013;90:1086–91. doi: 10.1097/OPX.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 28.Cho P, Douthwaite W. The relation between invasive and noninvasive tear break-up time. Optom Vis Sci. 1995;72:17–22. doi: 10.1097/00006324-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Zhao S, Le Q. Analysis of the first tear film break-up point in Sjögren's syndrome and non-Sjögren's syndrome dry eye patients. BMC Ophthalmol. 2022;22:1. doi: 10.1186/s12886-021-02233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–60. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 31.King-Smith PE, Fink BA, Nichols JJ, Nichols KK, Braun RJ, McFadden GB. The contribution of lipid layer movement to tear film thinning and breakup. Invest Ophthalmol Vis Sci. 2009;50:2747–56. doi: 10.1167/iovs.08-2459. [DOI] [PubMed] [Google Scholar]
- 32.Goto E, Tseng SC. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Arch Ophthalmol. 2003;121:173–80. doi: 10.1001/archopht.121.2.173. [DOI] [PubMed] [Google Scholar]
- 33.Goto E, Tseng SC. Kinetic analysis of tear interference images in aqueous tear deficiency dry eye before and after punctal occlusion. Invest Ophthalmol Vis Sci. 2003;44:1897–905. doi: 10.1167/iovs.02-0818. [DOI] [PubMed] [Google Scholar]
- 34.Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res. 1998;17:565–96. doi: 10.1016/s1350-9462(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 35.Ousler GW, 3rd, Hagberg KW, Schindelar M, Welch D, Abelson MB. The ocular protection index. Cornea. 2008;27:509–13. doi: 10.1097/ICO.0b013e31816583f6. [DOI] [PubMed] [Google Scholar]
- 36.Roy NS, Wei Y, Kuklinski E, Asbell PA. The growing need for validated biomarkers and endpoints for dry eye clinical research. Invest Ophthalmol Vis Sci. 2017;58:BIO1–9. doi: 10.1167/iovs.17-21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto Y, Sato EA, Ibrahim OM, Dogru M, Tsubota K. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–71. [PMC free article] [PubMed] [Google Scholar]
- 38.Fu J, Chou Y, Hao R, Jiang X, Liu Y, Li X. Evaluation of ocular surface impairment in meibomian gland dysfunction of varying severity using a comprehensive grading scale. Medicine (Baltimore) 2019;98:e16547. doi: 10.1097/MD.0000000000016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randon M, Liang H, El Hamdaoui M, Tahiri R, Batellier L, Denoyer A, et al. In vivo confocal microscopy as a novel and reliable tool for the diagnosis of Demodex eyelid infestation. Br J Ophthalmol. 2015;99:336–41. doi: 10.1136/bjophthalmol-2014-305671. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Shanbhag SS, Basu S. Palpebral lobe of the human lacrimal gland:Morphometric analysis in normal versus dry eyes. Br J Ophthalmol. 2021;105:1352–7. doi: 10.1136/bjophthalmol-2020-316929. [DOI] [PubMed] [Google Scholar]
- 41.Singh S, Shanbhag SS, Basu S. Tear secretion from the lacrimal gland:Variations in normal versus dry eyes. Br J Ophthalmol. 2022;106:772–6. doi: 10.1136/bjophthalmol-2020-318159. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Srivastav S, Modiwala Z, Ali MH, Basu S. Repeatability, reproducibility and agreement between three different diagnostic imaging platforms for tear film evaluation of normal and dry eye disease. Eye (Lond) 2022 doi: 10.1038/s41433-022-02281-2. doi:10.1038/s41433-022-02281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu X, Gong L, Lu Y, Jin H, Robitaille M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjögren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmol. 2012;90:e359–66. doi: 10.1111/j.1755-3768.2012.02413.x. [DOI] [PubMed] [Google Scholar]
- 44.Singh A, Vanathi M, Kishore A, Gupta N, Tandon R. Evaluation of strip meniscometry, tear meniscus height and depth in the diagnosis of dry eye disease in asian Indian eyes. Ocul Surf. 2019;17:747–52. doi: 10.1016/j.jtos.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–5. doi: 10.1016/j.ophtha.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 46.Srinivasan S, Menzies K, Sorbara L, Jones L. Infrared imaging of meibomian gland structure using a novel keratograph. Opt Vision Sci. 2012;89:788–94. doi: 10.1097/OPX.0b013e318253de93. [DOI] [PubMed] [Google Scholar]
- 47.Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol (Auckland, NZ) 2013;7:1797–803. doi: 10.2147/OPTH.S33182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olson MC, Korb DR, Greiner JV. Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact Lens. 2003;29:96–9. doi: 10.1097/01.ICL.0000060998.20142.8D. [DOI] [PubMed] [Google Scholar]
- 49.Li S, Yang K, Wang J, Li S, Zhu L, Feng J, et al. Effect of a novel thermostatic device on meibomian gland dysfunction:A randomized controlled trial in chinese patients. Ophthalmol Ther. 2022;11:261–70. doi: 10.1007/s40123-021-00431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg DJ. Current trends in intense pulsed light. J Clin Aesthet Dermatol. 2012;5:45–53. [PMC free article] [PubMed] [Google Scholar]
- 51.D'Souza S, Padmanabhan Nair A, Iyappan G, Dickman MM, Thakur P, Mullick R, et al. Clinical and molecular outcomes after combined intense pulsed light therapy with low-level light therapy in recalcitrant evaporative dry eye disease with meibomian gland dysfunction. Cornea. 2022;41:1080–7. doi: 10.1097/ICO.0000000000002954. [DOI] [PubMed] [Google Scholar]
- 52.Bäumler W, Vural E, Landthaler M, Muzzi F, Shafirstein G. The effects of intense pulsed light (IPL) on blood vessels investigated by mathematical modeling. Lasers Surg Med. 2007;39:132–9. doi: 10.1002/lsm.20408. [DOI] [PubMed] [Google Scholar]
- 53.Lane SS, DuBiner HB, Epstein RJ, Ernest PH, Greiner JV, Hardten DR, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31:396–404. doi: 10.1097/ICO.0b013e318239aaea. [DOI] [PubMed] [Google Scholar]
- 54.Greiner JV. A single LipiFlow® Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res. 2012;37:272–8. doi: 10.3109/02713683.2011.631721. [DOI] [PubMed] [Google Scholar]
- 55.Friedland BR, Fleming CP, Blackie CA, Korb DR. A novel thermodynamic treatment for meibomian gland dysfunction. Curr Eye Res. 2011;36:79–87. doi: 10.3109/02713683.2010.509529. [DOI] [PubMed] [Google Scholar]
- 56.Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010;29:1145–52. doi: 10.1097/ICO.0b013e3181d836f3. [DOI] [PubMed] [Google Scholar]
- 57.Wladis EJ. Intraductal meibomian gland probing in the management of ocular rosacea. Ophthal Plast Reconstr Surg. 2012;28:416–8. doi: 10.1097/IOP.0b013e3182627ebc. [DOI] [PubMed] [Google Scholar]
- 58.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombolà L, Carnuccio R, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292:156–63. [PubMed] [Google Scholar]
- 59.Dougherty JM, McCulley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991;32:2970–5. [PubMed] [Google Scholar]
- 60.Suzuki T. Inflamed obstructive meibomian gland dysfunction causes ocular surface inflammation. Invest Ophthalmol Vis Sci. 2018;59:DES94–101. doi: 10.1167/iovs.17-23345. [DOI] [PubMed] [Google Scholar]
- 61.Foulks GN, Borchman D, Yappert M, Kim SH, McKay JW. Topical azithromycin therapy for meibomian gland dysfunction:Clinical response and lipid alterations. Cornea. 2010;29:781–8. doi: 10.1097/ICO.0b013e3181cda38f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedlaender MH, Protzko E. Clinical development of 1% azithromycin in DuraSite, a topical azalide anti-infective for ocular surface therapy. Clin Ophthalmol. 2007;1:3–10. [PMC free article] [PubMed] [Google Scholar]
- 63.Aghai ZH, Kode A, Saslow JG, Nakhla T, Farhath S, Stahl GE, et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res. 2007;62:483–8. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 64.Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:336–56. [PMC free article] [PubMed] [Google Scholar]
- 65.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–24. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 66.Dry Eye Assessment and Management Study Research Group. Asbell PA, Maguire MG, Pistilli M, Ying GS, Szczotka-Flynn LB, et al. n-3 fatty acid supplementation for the treatment of dry eye disease. N Engl J Med. 2018;378:1681–90. doi: 10.1056/NEJMoa1709691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jackson WB. Blepharitis:Current strategies for diagnosis and management. Can J Ophthalmol. 2008;43:170–9. doi: 10.1139/i08-016. [DOI] [PubMed] [Google Scholar]
- 68.Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100:128–34. doi: 10.1136/bjophthalmol-2014-306280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Downie LE, Craig JP. Tear film evaluation and management in soft contact lens wear:A systematic approach. Clin Exp Optom. 2017;100:438–58. doi: 10.1111/cxo.12597. [DOI] [PubMed] [Google Scholar]