Abstract
Definitive treatment of dry eye disease (DED), one of the commonest ocular surface disorders, has remained elusive despite several recent advances in better diagnostics and the introduction of newer therapeutic molecules. The current treatment paradigms rely heavily on lubricating eye drops and anti-inflammatory agents that may need to be used long-term and are mainly palliative. Research is ongoing not only for a curative treatment option but also to improve the potency and efficacy of existing drug molecules through better formulations and delivery platforms. In the past two decades, significant advancement has been made in terms of preservative-free formulations, biomaterials such as nanosystems and hydrogels, stem cell therapy, and creation of a bioengineered lacrimal gland. This review comprehensively summarizes the newer approaches to DED treatment, which are biomaterials such as nanosystems, hydrogels, and contact lenses for drug delivery, cell and tissue-based regenerative therapy for damaged lacrimal gland and ocular surface, and tissue engineering for developing artificial lacrimal gland. Also, their potential efficacies in animal models or in vitro studies and possible limitations are discussed. The ongoing research looks promising and needs to be supported with clinical efficacy and safety studies for human use.
Keywords: Contact lens, dry eye disease, lacrimal gland regeneration, mesenchymal stem cells, nanomaterials
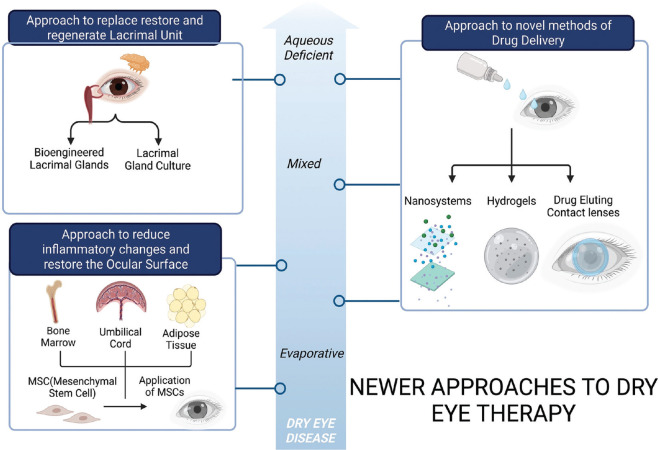
Dry eye disease (DED) is a global health problem affecting millions of individuals approximately 5–50% of the adult population worldwide.[1] According to the Tear Film and Ocular Surface Society (TFOS) and Dry Eye Workshop II (DEWS II) 2017, DED has been classified mainly into three categories: aqueous deficient dry eye (ADDE), evaporative dry eye (EDE), and mixed types based on distinct signs and symptoms.[2] Although the pathogenesis of EDE is related to hyperosmolar tissue damage, there is also the induction of inflammation and loss of epithelial and goblet cells. The existing treatment options for DED are lubricants and drugs that are palliative in nature and have inherent limitations of frequent use and preservative toxicity.[3-5] To address these limitations, different approaches have been explored by various groups in the fields of regenerative medicine, pharmacology, nanotechnology, and biomedical engineering, wherein newer nanosystems, hydrogels, and contact lenses are designed to improve drug delivery to the ocular surface and application of mesenchymal stem cells (MSC) has immunomodulatory effects and epithelial regenerative changes on the ocular surface [Fig. 1]. Either newer drug molecules or new drug delivery devices are researched to circumvent the limitations of current conventional topical ophthalmic drugs that have reduced bioavailability (due to nasolacrimal drainage, reflex tearing, and blinking), drug dilution with tears, non-compliance (frequent instillations), metabolic degradation, and preservatives with potential surface toxicity.[6,7]
Figure 1.
Flow chart to represent various novel strategies to treat Aqueous-Deficient Dry eye Disease (ADDE), Evaporative Dry eye (EDE) and Mixed variant of the disease using nanotechnology, biomedical engineering and regenerative medicine. Approach to newer drug delivery strategies in all variants of the dry eye includes using newer nano drug delivery systems, hydrogels and drug-eluting contact lenses to improve the drug bioavailability at the ocular surface. The approach to improve the ocular surface by immunomodulation, paracrine activity and repair properties of MSC (mesenchymal stem cells) or their secretome is a vast area of research in EDE and the Mixed variant of the disease. Finally approach to regenerate lacrimal glands by bioengineering strategies remains a possible strategy to tackle ADDE
As the DED spectrum ranges from mild to severe disease, pharmacology-based research on newer drug delivery devices addresses mild to moderate DED but the ADDE due to Sjogren’s disease, which can present with more severe disease, needs novel regenerative approaches targeting the lacrimal gland. An alternative curative treatment strategy of regenerating the damaged lacrimal gland, which is responsible for producing the major aqueous component of the tear film, is required [Fig. 1]. The regenerative approach explores either lacrimal gland repair with MSCs or regenerating the lacrimal gland using bioengineering in animal models.[8-12] The paracrine and autocrine effects of the secretome derived from MSCs have shown promising long-term immunomodulatory effects on the ocular surface and corneal epithelial repair.[13-16] This review summarizes the various biomaterials being explored for therapy in DED and the regenerative strategies used for lacrimal gland regeneration as well as ocular surface and their possible therapeutic applications.[5,17]
Nanotechnology and Biomaterials in Dry eye
Nanosystems
Nanotechnology-driven delivery systems have been explored vastly in pharmacotherapeutics and medicine. In ophthalmology, nanomaterials are used for various anterior segment, glaucoma, and posterior segment diseases.[18] A nanoparticle can be lipid-based, polymer-based, or can be made of materials such as silica, carbon (carbon nanotubes), and cobalt/nickel (magnetic). Typically, in the initial days, delivering lipophilic drugs with a hydrophobic formulation was a challenge. Various lipid-based nanocarriers were used to increase the drug availability in the posterior segment ciliary body, choroid, and retina. The lipid-based nanoparticles can be divided based on their core structure having a liquid or vesicle base, which comprises previous colloidal emulsions, such as micelles (central hydrophobic core formed only by the tail ends of a single layer of surfactant molecule), liposomes (liquid core surrounded by double phospholipid layer), and nanoemulsions drop (hydrophobic liquid core oil dispersed in water and stabilized by surfactant with nanoparticles 10 nm–100 nm).[19,20] The positive charge of liposomes helps them adhere to the negatively charged mucin layer in the tear film and they can be coated with an adhesive polymer or dispersed into an adhesive gel to bind with the cornea. Liposome formulations or lipid-containing eye drops for the treatment of dry eyes available in the USA are Vyseo®, Clarimist®, ActiMist, ™ and Tears Again® and can be used for the treatment of patients with mild to moderate evaporative DED. Oil in water nanoemulsions is used as a carrier for lipophilic drugs such as cyclosporin A and Restasis® (nanoemulsion for cyclosporin A). Although these modes of drug delivery improved drug availability, they had certain disadvantages such as physical stability, complicated production method, low percentage entrapment, and cumbersome industrial-scale manufacturing.[19] However, the invention of newer solid lipid nanoparticles (SLN) (consists of lipids in a solid state at room temperature core) and NLC (nanostructured lipid carriers consisting of at least 30% triglycerides, which are in a liquid state at room temperature) in recent years have revolutionized the nano drug delivery systems by being more stable with a longer shelf life up to 3 years, better reproducibility, feasible for large scale manufacturing, better carrier for hydrophobic drugs, lesser immune reactions, and extreme site-specific drug delivery.[21-23] Experimental Cys-NLC formulations of cyclosporin A have shown better drug residence time in rabbits, whereas NLC ophthalmic solutions based on phosphatidylcholine and squalene surfactants have shown to be useful artificial tear products.[24,25] Their nanoscale size have the ability to enhance penetration through the ocular surface layers, protect the active molecules from degradation, and increase the drug residence time on the ocular surface compared to the eye drops formulation.[26,27] Nanosystems in the ocular surface exhibit the adhesive property that prevents degradation by the ocular surface defense mechanisms.[28,29]
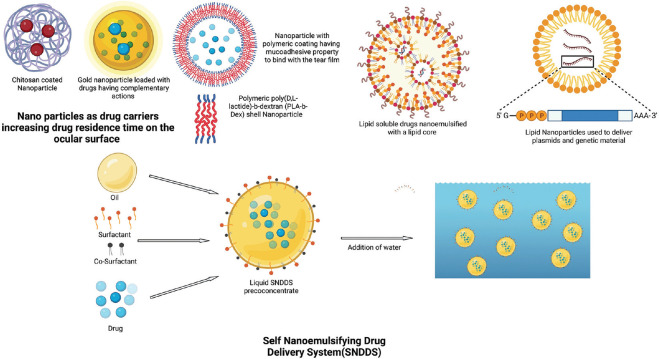
Polymeric nanoparticles are composed of either natural (chitosan, gelatin) or synthetic polymers (polylactic acid and poly L-lysine). They exhibit both biocompatibility and biodegradability. These polymers are non-toxic and eliminated by the liver and provide a customizable surface, which can be used to coat nanoparticles. Li et al.[30] demonstrated gold nanoparticles carrying two drugs with complementary actions such as amfenac (anti-inflammatory) and catechin (antioxidant), which reduced ocular surface inflammation and Reactive oxygen species (ROS) levels in mice models after 4 days of treatment as compared to the commercial cyclosporine (CsA) eye drops. Drug-loaded poly (catechin) shell forms a loose polymeric matrix that helps in loading on gold nanoparticles forming a uniform shell [Fig. 2]. Similarly, cerium oxide has a potential role in controlling ROS activity; therefore, it was encapsulated with chitosan to make it water soluble and be used as an eye drop to alleviate DED. A significant improvement in tear film break-up time (TBUT), ocular surface inflammation, tear volume, and a decrease in intracellular ROS was noted in the rabbit cornea and conjunctiva.[31] Polymeric nanoparticles (NPs) in the form of cationized gelatin have also been used as a plasmid carrier for delivering genes coding MUC5AC in a mice DED model[32] [Fig. 2]. A reduction in inflammatory cytokines and CD4 + T cell infiltration was noted in mice. The nanoparticle poly (D, L-lactide)-b-dextran (PLA-b-Dex) containing cyclosporine reduced the dosing schedule to once a week compared to thrice daily administration of Restasis (Allergan Inc., Irvine, CA) in DED mice model.[33] This study suggests that weekly once administration can maintain the drug’s therapeutic level on the ocular surface.
Figure 2.
Various Nanoparticle drug delivery systems likely Polymeric nanoparticles with chitosan coating, and gold nanoparticles with mucoadhesive properties are shown to load and deliver the drugs to the ocular surface. Lipid-based nanoparticle drug delivery system for lipid-soluble/hydrophobic drugs in liposome formulation. Plasmids with genetic material are packaged within the lipid core of a nanocapsule by the process of nano-emulsification. Below a mechanism of constituting and working of a self-nano-emulsifying drug delivery system is schematically demonstrated
For the delivery of lipid-soluble drugs, nanocapsules are used for better drug loading. Nanocapsules are composed of a polymeric wall and an oily core.[25,34] CsA was loaded in lipid nanocapsules and better therapeutic effects (increased TBUT, reduced corneal fluorescein score) were observed in the rat DED model compared to Restasis. Dexamethasone-containing nanostructure lipid carriers (NLCs) have also shown prolonged retention time on the rabbit ocular surface with better ocular surface changes in the benzalkonium chloride (BAC)-induced rabbit DED model.[35] Nano wafers is another novel modality of drug delivery, wherein drug such as dexamethasone was loaded onto wells in a nano wafer composed of carboxymethyl-cellulose used as hydrogel showed improved corneal epithelial healing, smoother corneal surface, and increased tear film in a mouse model of dry eye.[36] This decreased the frequency of administration of the drug. Restasis is thermodynamically unstable and requires a large amount of energy in its preparation.[37] Another benefit is reduced burning and stinging sensation associated with Restasis on the ocular surface. Kang et al.[38] showed that cyporin N, a 0.05% CsA nanoemulsion product (Taejoon Pharmaceutical, Seoul, South Korea), had better improvement in TBUT compared to Restasis in primary Sjögren’s syndrome (SS) patients. Another two self assembling nano-emulsifiying drug delivery systems (SNEDDS)-Cyporin-N and T-sporin have also shown comparable efficacy with Restasis in the BAC-induced DED model [Fig. 2].[39]
Nano micelles are colloidal carrier systems ranging between 5 nm and 200 nm in size, which are synthesized from block copolymers and consist of different hydrophobic and hydrophilic monomer units and are called polymeric nano micelles, whereas nanosized micelles are composed of amphiphilic molecules with water-loving head groups (anionic and cationic) and hydrophobic tails and are referred to as surfactant nano micelles.[40] In 2018, the US FDA approved Cequa® (Sun Pharma, Mumbai, India), a preservative-free 0.09% surfactant nano micellar formulation of CsA for DED, which has better stability and cost-effectiveness.[41] The other nanostructured ophthalmic products currently marketed for the treatment of DED are Cyclokat® (preservative-free 0.1% CsA cationic emulsion), Lacrisek® (Vitamin A, Vitamin E liposomal spray), and Artelac Rebalance® (hyaluronic acid, vitamin B12, and viscoelastic eye drops).[42,43] Besides, SYSTANE®, an ophthalmic nanoemulsion for dry eye disease has completed a phase IV clinical trial. Lee et al.[43] developed a polyethylene glycol PEG-catechin nano complex, which showed significant improvement in the tear production, anti-inflammatory properties, and downregulation of IL-1b, IL-6, and TNF alpha expression in the lacrimal gland in the DED mice model. For restoring the lipid layer of the tear film, a nanoscale-dispersed ointment containing a mixture of semi-solid lipids (petrolatum and lanolin) and liquid lipids (medium-chain triglycerides) was explored.[44] A marked decrease in fluorescein staining scores and improved TBUT were found compared to artificial tears.
Hydrogels
Hydrogels are made up of hydrophilic polymer chains that make them absorb a huge quantity of water without getting disintegrated into it and maintaining a three-dimensional (3D) structure. A variety of polymers including natural, semisynthetic, and synthetic can be used to form hydrogels. Hyaluronic acid, chitosan, methylcellulose, poly (vinyl alcohol), and poly (acrylic acid) are some of the ocular lubricating polymers. Hydrogels have tunable physical properties and degradation rates, which provide spatial and temporal control over the environment and the release of encapsulated drugs at the target site of delivery. Their excellent biocompatibility, prolonged drug release action on the ocular surface, good mechanical properties, and the ability to carry and deliver a wide range of drugs from hydrophilic to hydrophobic in its crosslinked matrix make them an emerging drug delivery platform for the treatment of ocular surface diseases.[45-47] Various hydrogel products for DED treatment are available in the market, such as Vidisic® gel (Bausch and Lomb, Rochester, NY, USA), Hylo®gel (URSAPHARM, S aarbrück-en, Germany), GelTears® (Bausch and Lomb, Rochester, NY, USA), Viscotears®(Novartis, Basel, Switzerland), and Clinitas gel®(Altacor, Reading, UK) and a few under clinical trials including VisuXL® gel (VISUfarmaSpA, Rome, Italy), and bovine basic fibroblast growth factor (bFGF) gel (Zhuhai Yisheng Biological Pharmaceutical Co., Ltd., Zhuhai, China). Besides, recent patents on hydrogel products for DED treatment include multi-arm PEG insert with CsA/Dex, PNIPAAm and butyl acrylate plug, Guar gum, PVA, and boric acid drop containing diquafosol sodium.[48-50]
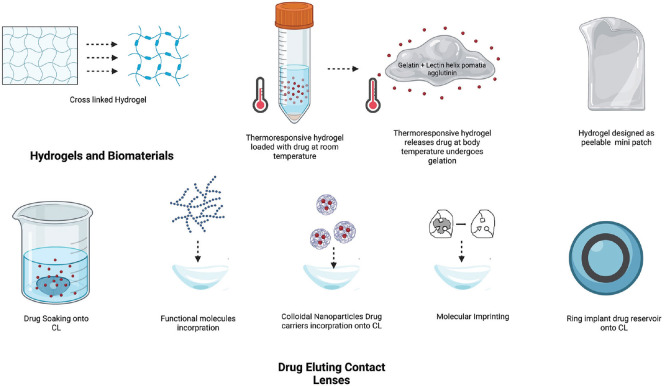
The research on hydrogel-based products for DED treatment continues and groups worldwide have studied various materials. Soft hydrogels containing hyaluronic acid (HA) alone have been explored in a few rabbit DED models.[45,51] Compared to lubricants, cross-linked HA hydrogels significantly improved ocular surface signs and symptoms in canine DED models [Fig. 3].[51] Further, thermo-responsive hydrogels containing mucoadhesive gelatin and lectin helix pomatia agglutinin are loaded with epigallocatechin gallate (EGCG) or FK506 (immunosuppressive agent) and are used in DED management [Fig. 3].[5,52,53] Single use on the DED-induced rabbit ocular surface maintained EGCG bioavailability above the therapeutic level for 14 days.
Figure 3.
Hydrogels with tunable physical properties can be customised in different shapes and consistency thickness for drug delivery by cross-linking or smart designing with CAD software to make a thin patch for application over the ocular surface. Thermoresponsive hydrogels shrink at higher temperatures at the target site in the organ and allow the release of the drug particles as the degrade. Below various techniques to load the drug over the CL(contact lens) are schematically represented
Hydrogels have also been explored as intracanalicular plugs (hydroxybutyl chitosan [HBC]) for blocking the lacrimal outflow system.[54] Clinical trials in eight humans showed improvement in TBUT and ocular surface disease index (OSDI) scores but no comparison with available plugs was done. Hydrogels are also under research for use as a mini eye patch with the ability to convert visible light into heat to stimulate lacrimal tear secretion to treat dry eyes.[55]
Drug-eluting contact lenses
Drug-eluting contact lenses are advantageous for their prolonged drug release, improved bioavailability, and increased patient comfort and compliance in comparison to conventional eye drops. Research on drug delivery through contact lens (CL) picked up its pace in the 2000s with the advancement in material science understanding. Conventional hydrogels and silicone hydrogels are the two major materials used for developing soft contact lenses. The ocular drugs are loaded in the contact lenses by various strategies such as soaking (wherein the CLs are soaked in drug solution), direct incorporation into the CL matrix, nanocarriers, molecular imprinting (wherein a high drug affinity sites are created in the CL matrix), ring implant (which includes the incorporation of the drug-infused ring into the CL polymer matrix), and vitamin E barrier (creating transport diffusion barriers for drugs). Soaking is the simplest method to obtain therapeutic CLs. This method retains its therapeutic effect for a few hours to a few days.[56,57]
Lubricants and anti-inflammatory drugs are explored for delivery via contact lenses (CL) where a constant release of the drug has been achieved for a longer time, ranging from 48 h to 15 days in in vitro studies.[58-60] Hyaluronic acid-loaded CLs have improved epithelial defect healing in BAC-induced rabbit DED compared to untreated rabbit eyes; however, a comparison with HA drops without CL would give more information about its efficacy. The use of poly (vinylpyrrolidone) (PVP-K90)-coated CLs has shown a high tear volume in the animal study compared to the uncoated ones and commercial eye drop solution. The techniques used for loading CLs are soaking, molecular imprinting, ring implant, and incorporation of nanocarriers and functional molecules [Fig. 3]. Anti-inflammatory drugs such as cyclosporine A, dexamethasone, and betamethasone have also been incorporated into CLs, with the ability to release the drugs for a long period of up to 14 days.[61-63] Vitamin E-soaked lenses have been loaded with steroids such as betamethasone and ciprofloxacin and have shown better extended-release. Non-steroidal anti-inflammatory drugs (NSAIDs) such as flurbiprofen sodium, diclofenac sodium, and ketorolac tromethamine have also been studied for extended delivery through vitamin E cationic surfactant-loaded commercial silicone hydrogel (SiH) CLs.[64]
Approach to reduce inflammatory changes and restore the ocular surface
The field of regenerative medicine has significantly advanced in recent years, and in the scenario of DED, there have been developments in understanding the mechanisms and actions of stem cells, their immunomodulatory effects over the ocular surface, cell-to-cell interaction, regenerative capacity to re-epithelialize corneal defects and re-establish secretory functions of lacrimal glands.
Applications of stem cells in DED and ocular surface inflammation
Dry eye and other ocular surface inflammatory diseases are characterized by increased surface inflammation, tear film, epithelial abnormalities, hyperosmolarity, and oxidative stress. The applications of stem cells derived from various sources (limbal epithelial stem cells, iPSCs, and MSCs) and their paracrine functions have been explored extensively. Induced pluripotent stem cells (iPSCs) are derived from skin or blood cells and reprogrammed back to an embryonic stage of pluripotency, from where they can be grown into any desired cell lineage.[65]The iPSC-derived RPE cells have recently been used for the treatment of ARMD; however, in DED, these cells can be used to regenerate lacrimal glands though there is no ongoing direct trial to prove the same.[66] iPSC-conditioned media has been used by a group to regenerate lacrimal glands in a mouse model of DED.[67] However, currently, high tumorigenic potential, high cost, and regulation involved in culturing and applying iPSCs are limitations of this technique on a larger scale.[65] Similarly, human amniotic membrane epithelial cells are being investigated for the regeneration of salivary glands and can be used in the future to regenerate lacrimal glands. This has been demonstrated in certain double-chamber systems, wherein the human amniotic membrane epithelial cells trans-differentiate into acinar cells of the salivary gland.[68]
Typically, allogenic or autologous MSCs can be derived from the bone marrow, adipose cells, umbilical cord, Wharton’s jelly, and dentine pulp. The ability of stem cells to modulate innate and adaptive immune systems, and secrete growth factors and anti-inflammatory factors in response to inflammation make them an attractive therapeutic modality. MSCs are capable of cell-to-cell interaction, secreting immunomodulatory factors such as indoleamine 2,3-dioxygenase, interleukins-1β, -6, -8, transforming growth factor-β, nitric oxide, ciliary neurotrophic factor, glial cell line-derived neurotrophic factor, vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) in response to pro-inflammatory stimuli such as IFN-gamma and various interleukins.[14,69,70] Their paracrine activity makes them an ideal candidate to be administered systemically. MSCs can be applied topically or injected subconjunctivally. Their paracrine activity makes them an ideal modality to inject systemically. Beyond ophthalmology, MSC application in the field of orthopedics has been seen with the JOINTSTEM phase 2b clinical trial, wherein autologous adipose-derived MSC were injected intra-articularly in patients to promote cartilage growth, and results have shown no side effects, significant improvement in function and pain.[71] In the field of cardiology, PERISCOPE clinical trials have tested the use of MSCs derived from the allogenic umbilical cord, Wharton’s jelly or autologous bone marrow was seen for safety and efficacy in patients with infarcted myocardial tissue and chronic ischemic cardiomyopathy.[72] Autologous MSCs have also shown short-term benefits with improvement in symptoms in patients with multiple sclerosis and are being tried for other neurological disorders such as stroke, Alzheimer’s disease, and amyotrophic lateral sclerosis.[73-75] MSCs have also shown faster wound healing properties when they were tried on patients with diabetic foot ulcers and amputation stumps.[76] Recently, MSCs have been tried in various clinical trials in COVID-19 patients to control the cytokine storm and improve outcomes in acute respiratory distress syndrome and multiple organ failure.[77,78]
Studies by various groups have shown faster healing and corneal epithelial regeneration after the application of MSCs in various animal models of corneal wound healing and chemical burns.[79-84] Of the two types of dry eye disease, evaporative and aqueous deficiency, stem cells and their role have been studied in the later in the past few years. Xu et al.[85] showed inhibitory effects of intravenously administered MSCs on experimental animal models and clinical SS in patients, wherein it was seen that T cells directed toward Treg and Th2 suppressed Th17 and Tfh responses, and alleviated disease symptoms. Beyazyıldız et al.[86] demonstrated that topical application of MSCs was safe and effective in a BAC-induced dry eye disease model in rats. They showed significant improvement in aqueous tear volume, ocular surface evaluation tests, and engraftment of MSCs within the conjunctival goblet cells and meibomian glands in the rats. This was speculated to be due to homing characteristics of MSCs, paracrine effects, differentiation of topically transferred MSCs to goblet cells or glandular cells, or stimulation of repair mechanisms of damaged goblet cells of conjunctiva. The microvillus structure of the epithelium was maintained and there was no neutrophilic infiltration observed.
Dietrich et al.[8] have demonstrated a surgically induced model of aqueous deficiency dry eye by duct ligation for 3 days in mice, which showed clinical signs of acute ADDE with impaired functional LG tissue and tear physiology with minor impact on the ocular surface. The MSC cell population used was unique in the way that it belonged to the native tissue of the same species in this case murine LG from eGFP mice. In comparison with direct saline injection within the LG, extrinsic tissue-specific MSCs showed accelerated regeneration with an enhanced amount of vital acinar structures, an increase in the total area of viable lacrimal gland tissue, earlier decline in apoptotic cells, a modulated macrophage invasion, and a lower number of proliferating cells during acute inflammation, a lower expression of TNFα, and an increased expression of IL-6. This approach showed the possible benefits of extrinsic MSCs in severe aqueous deficiency dry eye individuals with poor intrinsic LG regenerative capacity.
Going ahead from the learnings of this animal study, Hansen et al.[10] conducted a first clinical trial wherein the safety and therapeutic efficacy of allogenic adipose-derived MSCs in seven Sjogrens disease patients was studied. The MSC suspension was directly injected into the LG capsule up to 50% of the LG volume as detected on the MRI imaging of the gland, and the outcomes were measured by looking for adverse reactions to the injection, changes in the OSDI score, tear osmolarity, TBUT, Oxford grade, Schirmer’s I test, and development of donor-specific anti-HLA antibodies. No adverse events were noted to the injection, and overall there were improvements in all the parameters in the study eye. The changes seen were hypothesized to be due to the anti-inflammatory activity of MSCs and this treatment was more likely to have an effect in patients with early phases of the DED than in later phases with the presence of cicatricial conjunctivitis.
Recently, topical application of exosomes containing bioactive molecules and micro RNA (miR-204) secreted by MSCs was explored in a group of Graft versus Host disease (GVHD) patients, wherein the exosomes act like the secretome of the MSCs and were shown to improve the signs of symptoms of dry eye.[87] The miR-204 targeted interleukin-6 receptor (IL-6R) to suppress the activation of the IL-6/IL-6R/Stat3 pathway, inducing the inflammatory M1 macrophages to shift toward the immunosuppressive M2 phenotype, which was desirable in GVHD patients. Similarly, topical application of autologous platelet-rich plasma in a prospective non-randomized clinical study showed improvement in dry eye signs in 322/368 patients. In comparison with autologous serum, the platelets secrete various growth factors, which are responsible for ocular surface reconstruction.[88]
Approach to replace restore and regenerate Ocular Surface and Lacrimal unit
Lacrimal gland regeneration
For patients with severe DED, lacrimal gland function can be restored by either transplanting the artificially grown lacrimal gland having a secretory activity or by transplanting autologous salivary glands or allogenic lacrimal gland transplantation. The first two options have been tried in animal and human eyes, respectively, whereas the third option is still a creative imagination. Salivary gland transplantation for severe dry eye disease (<2 mm) achieved some improvement in tear volume but the dependency on lubricants remains and ocular surface changes did not reverse remarkably. Autologous minor and submandibular salivary glands have been transplanted but hyposmolar tears of the submandibular gland and very small changes in tear volume with minor salivary gland transplant limit its widespread use. Porcine lacrimal gland shares similar morphology, ductal system, and vascular supply as the human lacrimal gland. The idea of using it as a xenograft was put forth. However, tear composition and immune rejection other than regulatory issues would limit its further exploration. The transplantation of a donor human lacrimal gland could be a possibility in the future as subconjunctival lacrimal gland transplantation has shown good results in the surgical dacryoadenectomy rabbit DED model. However, the transplanted gland was 70 mg in volume and Schirmer values were assessed at 1 month only.
Bioengineered lacrimal glands
The lacrimal gland is derived from the surface ectoderm during the ontogenesis in the embryo.[89-91]The invagination of surface ectoderm forms an epithelial component and surrounding mesenchyme forms myoepithelial cells.[92] We can replicate this in vitro with the use of epithelial and mesenchymal progenitor cells.[93] Lacrimal gland revival with the use of stem cells can repair the lacrimal glands, which have some leftover function. In eyes with absolute tear deficiency, the whole lacrimal gland is non-functional and demonstrates no remaining tissue in vivo. Hence, we need to develop an in vitro technique that can fabricate a 3D fully functional bioengineered organ, which has transplantation potential.[94] The existing bioengineered tissues and organs are composites of polymers and cells.[95] Computer-aided design (CAD) and computer-aided manufacturing (CAM) technologies have developed fully functional organs and their substitutes for replacing organs, such as the eyes, heart, liver, and kidneys.[96,97]
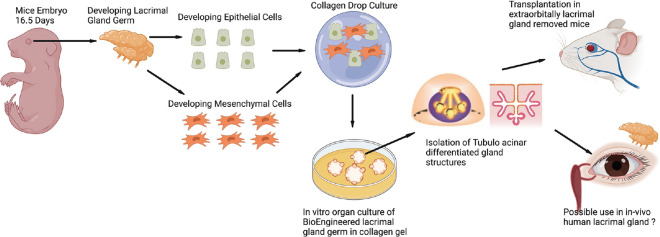
Bioengineering using the organ germ method was used initially for developing tooth and hair follicles.[98] In vitro bioengineered organ germ method involves compartmentalization between epithelial and mesenchymal cells.[99] The compartmentalized epithelial and mesenchymal cells are cultured at a high cell density and then grown in a type I collagen gel as a bioengineered organ germ.[92] [Fig. 4]. The collagen drop method and co-culture of mice LG cells and Harderian gland’s dissociated epithelial and mesenchymal cells were employed for lacrimal gland regeneration.[100] Branching morphogenesis was successfully observed in this culture after 3 days, along with stalk elongation and cleft-like structure formation. The mouse embryonic epithelial and mesenchymal cells were obtained from 16.5 embryonic days and were cultured using the collagen drop method. The cultured cells along with endothelial cells were seeded on matrigel and spheroids with acinar cell-like morphology were seen. The spheroids with duct were transplanted into a mouse model (no extra orbital gland) where the duct was coapted with intraorbital lacrimal gland duct segment [Fig. 4]. There occurred changes in corneal epithelial staining. The histology confirmed the morphological similarity with the lacrimal gland but it needs proof of in vivo secretory function and nerve stimulatory response.[100] Likewise, these 3D bioengineered functional organ regeneration techniques were also employed in several other studies and screened for their potential in lacrimal gland regeneration.[101,102] The development of a bioengineered LG organ germ offers a successful model for transplantation as it has shown a sufficient level of secretory potential.[100]
Figure 4.
Bioengineered organ germ method to culture Lacrimal gland from the lacrimal gland germ cells of mice embryo 16.5 days old. The epithelial and mesenchymal cells are isolated and co-cultured using a collagen drop method. Over weeks tubule acinar structures are identified and these can be transplanted into the diseased glands
Lacrimal gland culture
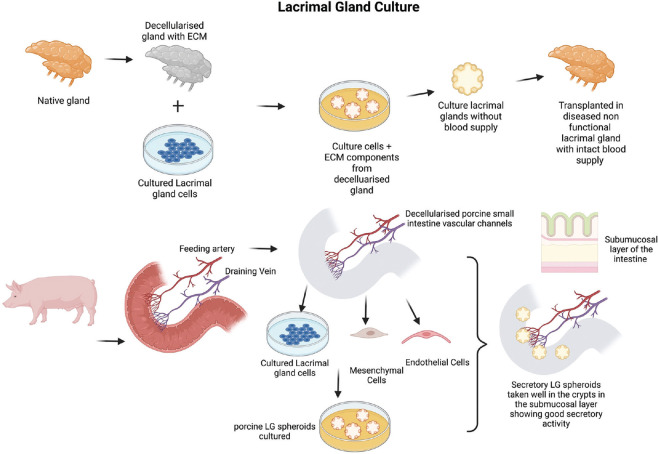
Lacrimal gland obtained from mice, rabbits, and humans has been cultured and grown in vitro in two- and three-dimension in the form of monolayers, spheroids (3D aggregation of cells), and organoids (3D with central secretory lumen) under appropriate culture media conditions.[11,12,103] Very few studies have transplanted the cultured spheroids or organoids into the animal models of DED with encouraging short-term results on viability. Their effect on overall ocular surface health and dry eye improvement is yet to be studied. To be able to regenerate the lacrimal gland with transplantation potential, a 3D scaffold could be a better option. Scaffold can be either artificially printed material or decellularized human or animal tissue. Decellularized tissue contains an extracellular matrix and tissue-specific microenvironment without any immunogenic material. Hence, decellularized lacrimal gland or another tissue scaffold can be used. Extracellular matrix offers a better substrate for cell adhesion, growth, and proliferation as seen in many studies. Culturing lacrimal glands in decellularized tissue requires bioreactors. Porcine and rabbit lacrimal glands have been decellularized and repopulated with cultured epithelial cells [Fig. 5]. Acinar-like and ductal structures could be seen in these gland tissues growing over decellularized scaffold [Fig. 5].[11] Thus, there is a possibility of culturing cells derived from the human lacrimal gland on the decellularized lacrimal gland. However, these artificially engineered lacrimal glands can only be placed into the damaged lacrimal gland as they lack vascular supply for de-novo transplantation.
Figure 5.
Above is a schematic representation of tissue-based lacrimal gland reconstruction- Extracellular matrix obtained from the decellularised lacrimal gland is repopulated with primary lacrimal gland cells to generate lacrimal gland constructs which are transplanted into the diseased gland with intact blood supply. Below is a schematic representation of developing a vascularised Lacrimal gland construct/spheroid/organoid grown within a segment of the porcine small intestine (jejunum) with intact vascular channels stemming from the iliac artery and veins. The cultured primary lacrimal gland cells along with MSCs and endothelial cells are grown in the submucosal region of the crypts of the intestine. These vascular channels can be connected to a bioreactor with perfusion of culture media mediated through pressure sensors and peristaltic pumps to maintain the nutrition of the constructs and develop viable blood vessels
Researchers have been able to create a scaffold with a blood supply. A biological vascularized scaffold (BioVaSc) is a decellularized segment of the porcine intestine along with its arteriovenous blood supply (Mertsching). The decellularized tissue can be used for culturing any tissue-specific cells where vascular channels and intestinal lumen are infused constantly via a bioreactor. The BioVaSc could be reendothelialized with autologous porcine endothelial cells after 3 weeks in a dynamic culture,[104,105] were successfully implanted with vascular anastomosis to the pig’s external iliac artery and vein. BioVaSc re-endothelized with human autologous peripheral blood mononuclear cells could be successfully implanted into a patient’s upper arm and maintained its integrity without any thrombosis at 1 week.[106] In vitro, BioVaSc, and bioreactor systems are used for creating a liver-like tissue, a fully functional vascularized human skin model, or a human cardiac patch.[107-109] Porcine intestine is bigger in size for ocular use; hence, murine BioVaSc from rat intestine was generated. The Chinese hamster ovary (CHO) cells embedded in a collagen hydrogel inside the lumen of the mBioVaSc maintained secretory activity until 1 week.[110] For the lacrimal gland, the luminal part of BioVaSc without vessels, that is, SIS-muc (small intestinal sub-mucosa with preserved mucosa) was used as a scaffold for growing LG epithelial cells under dynamic culture conditions [Fig. 5].[12] For creating a 3D structure mimicking LG, spheroids containing acinar epithelial cells, mesenchymal stem cells, and endothelial cells were cultured in the lumen [Fig. 5]. The crypts of SIS-muc got seeded well with the spheroids and showed secretory properties.[111] The bioreactor system provides a pulsatile flow and sufficient nutrient supply for supporting growth, expansion, and functional maturation in vitro.[109,110] With reendothelized vascular channels and lumen containing lacrimal gland organoids, it is possible to transplant them into the eye after establishing vascular anastomosis.
Future Perspectives and Unmet Needs
Research and development breakthrough in biomaterials technology opens up a promising area of research in DED. There is a distinct advantage over conventional administration routes, but there is a scope for further improvement in their design and efficacy of the biomaterials and studying the effect of nanosystems particle size, composition, and surface charge on pharmacokinetic studies.[112] Further standardization to improve large-scale manufacturing, long-term studies, and clinical trials to determine their ocular toxicity and biocompatibility will help in bringing these therapies to the clinic. Similarly, further studies are needed to understand the in vivo behavior and relationship of drugs with hydrogels and drug-eluting contact lenses in human DED patients.
In contrast, the regeneration of the complete lacrimal gland in the future looks promising. Future directions would be to look into the understanding of the genes regulating lacrimal gland development, identifying cell sources, and also important transcription factors, which can induce LG regeneration from induced pluripotent stem cells. MSCs derived from lacrimal gland tissue are multipotent and can support the growth of lacrimal gland acinar and epithelial cells; however, it is difficult to isolate a potent and pure colony of these cells.[113] Although bioengineered glands have been transplanted successfully in animals, their therapeutic efficacy in humans needs to be tested. Identification of the appropriate progenitor cells in human LG and establishing protocols to isolate, culture, and maintain these cell lines for injection in damaged human lacrimal glands could be the next step in translational research. Detailed invivo studies are essential to study clinical applicability of cultured lacrimal glands.
Most stem cell work has shown short-term benefits in improvement in the secretion of residual acinar cells of salivary glands in animals, the results of which can be extrapolated to the lacrimal glands; however, this needs to be studied separately. Also, it is important to explore various techniques that can evaluate lacrimal gland anatomy and cellular structure and function in patients for the clinical application of therapies.
MSCs through various routes have shown significant advantages in their use to decrease inflammation and immunomodulation of the ocular surface and in the future, establishing standard protocols for isolating appropriate cell sources, types, and manufacturing for commercial use could be a way ahead.
Conclusion
The new emerging therapies discussed in this review have the potential to decrease the burden of disease due to DED and provide a definite advantage over the conventional palliative therapy of applying lubricant eye drops. They also provide a solution to the advanced stages of the disease by addressing the root cause.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Aragona P, Giannaccare G, Mencucci R, Rubino P, Cantera E, Rolando M. Modern approach to the treatment of dry eye, a complex multifactorial disease:A P. I. C. A. S. S. O. board review. Br J Ophthalmol. 2021;105:446–53. doi: 10.1136/bjophthalmol-2019-315747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. TFOS Int Dry Eye Workshop DEWS II. 2017;15:438–510. doi: 10.1016/j.jtos.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Asbell PA. Increasing importance of dry eye syndrome and the ideal artificial tear:Consensus views from a roundtable discussion. Curr Med Res Opin. 2006;22:2149–57. doi: 10.1185/030079906X132640. [DOI] [PubMed] [Google Scholar]
- 4.Thacker M, Tseng CL, Chang CY, Jakfar S, Chen HY, Lin FH. Mucoadhesivebletillastriata polysaccharide-based artificial tears to relieve symptoms and inflammation in rabbit with dry eyes syndrome. Polymers. 2020;12:1465. doi: 10.3390/polym12071465. doi:10.3390/polym12071465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Q, Al-Sheikh O, Elisseeff JH, Grant MP. Biomaterials and tissue engineering strategies for conjunctival reconstruction and dry eye treatment. Middle East Afr J Ophthalmol. 2015;22:428–34. doi: 10.4103/0974-9233.167818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachu RD, Chowdhury P, Al-Saedi ZHF, Karla PK, Boddu SHS. Ocular drug delivery barriers-role of nanocarriersin the treatment of anterior segment ocular diseases. Pharmaceutics. 2018;10:E28. doi: 10.3390/pharmaceutics10010028. doi:10.3390/pharmaceutics10010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzman-Aranguez A, Colligris B, Pintor J. Contact lenses:Promising devices for ocular drug delivery. J OculPharmacolTher Off J Assoc Ocul Pharmacol Ther. 2013;29:189–99. doi: 10.1089/jop.2012.0212. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich J, Ott L, Roth M, Witt J, Geerling G, Mertsch S, et al. MSC transplantation improves lacrimal gland regeneration after surgically induced dry eye disease in mice. Sci Rep. 2019;9:18299. doi: 10.1038/s41598-019-54840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich J, Roth M, König S, Geerling G, Mertsch S, Schrader S. Analysis of lacrimal gland derived mesenchymal stem cell secretome and its impact on epithelial cell survival. Stem Cell Res. 2019;38:101477. doi: 10.1016/j.scr.2019.101477. doi:10.1016/j.scr.2019.101477. [DOI] [PubMed] [Google Scholar]
- 10.Møller-Hansen M, Larsen AC, Toft PB, Lynggaard CD, Schwartz C, Bruunsgaard H, et al. Safety and feasibility of mesenchymal stem cell therapy in patients with aqueous deficient dry eye disease. Ocul Surf. 2021;19:43–52. doi: 10.1016/j.jtos.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Spaniol K, Metzger M, Roth M, Greve B, Mertsch S, Geerling G, et al. Engineering of a secretory active three-dimensional lacrimal gland construct on the basis of decellularized lacrimal gland tissue. Tissue Eng Part A. 2015;21:2605–17. doi: 10.1089/ten.TEA.2014.0694. [DOI] [PubMed] [Google Scholar]
- 12.Massie I, Spaniol K, Barbian A, Poschmann G, Stühler K, Geerling G, et al. Evaluation of decellularized porcine jejunum as a matrix for lacrimal gland reconstruction in vitro for treatment of dry eye syndrome. Invest Ophthalmol Vis Sci. 2017;58:5564–74. doi: 10.1167/iovs.16-20759. [DOI] [PubMed] [Google Scholar]
- 13.Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260. doi: 10.1186/s12967-014-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs:Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013:732742. doi: 10.1155/2013/732742. doi:10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omoto M, Katikireddy KR, Rezazadeh A, Dohlman TH, Chauhan SK. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Invest Ophthalmol Vis Sci. 2014;55:6631–8. doi: 10.1167/iovs.14-15413. [DOI] [PubMed] [Google Scholar]
- 16.Orozco Morales ML, Marsit NM, McIntosh OD, Hopkinson A, Sidney LE. Anti-inflammatory potential of human corneal stroma-derived stem cells determined by a novel in vitro corneal epithelial injury model. World J Stem Cells. 2019;11:84–99. doi: 10.4252/wjsc.v11.i2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams R, Lace R, Kennedy S, Doherty K, Levis H. Biomaterials for regenerative medicine approaches for the anterior segment of the eye. AdvHealthc Mater. 2018;7:1701328. doi: 10.1002/adhm.201701328. [DOI] [PubMed] [Google Scholar]
- 18.Khiev D, Mohamed ZA, Vichare R, Paulson R, Bhatia S, Mohapatra S, et al. Emerging nano-formulations and nanomedicines applications for ocular drug delivery. Nanomaterials. 2021;11:173. doi: 10.3390/nano11010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, et al. A brief review on solid lipid nanoparticles:Part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10:26777–91. doi: 10.1039/d0ra03491f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balamurugan K, Chintamani P. Lipid nano particulate drug delivery:An overview of the emerging trend. Pharma Innov J. 2018;7:779–89. [Google Scholar]
- 21.Mehnert W, Mäder K. Solid lipid nanoparticles:Production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–96. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 22.Lim SB, Banerjee A, Önyüksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J Controlled Release. 2012;163:34–45. doi: 10.1016/j.jconrel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Dhakad RS, Tekade RK, Jain NK. Cancer targeting potential of folate targeted nanocarrier under comparative influence of tretinoin and dexamethasone. Curr Drug Deliv. 2013;10:477–91. doi: 10.2174/1567201811310040012. [DOI] [PubMed] [Google Scholar]
- 24.Shen J, Deng Y, Jin X, Ping Q, Su Z, Li L. Thiolated nanostructured lipid carriers as a potential ocular drug delivery system for cyclosporine A:Improving in vivo ocular distribution. Int J Pharm. 2010;402:248–53. doi: 10.1016/j.ijpharm.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Niamprem P, Teapavarapruk P, Srinivas SP, Tiyaboonchai W. Impact of nanostructured lipid carriers as an artificial tear film in a rabbit evaporative dry eye model. Cornea. 2019;38:485–91. doi: 10.1097/ICO.0000000000001867. [DOI] [PubMed] [Google Scholar]
- 26.Janagam DR, Wu L, Lowe TL. Nanoparticles for drug delivery to the anterior segment of the eye. Adv Drug Deliv Rev. 2017;122:31–64. doi: 10.1016/j.addr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirza AZ, Siddiqui FA. Nanomedicine and drug delivery:A mini review. Int Nano Lett. 2014;4:94. [Google Scholar]
- 28.Lynch C, Kondiah PPD, Choonara YE, du Toit LC, Ally N, Pillay V. Advances in biodegradable nano-sized polymer-based ocular drug delivery. Polymers. 2019;11:E1371. doi: 10.3390/polym11081371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang HY, Wang MC, Chen ZY, Chiu WY, Chen KH, Lin IC, et al. Gelatin-epigallocatechingallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int J Nanomedicine. 2018;13:7251–73. doi: 10.2147/IJN.S173198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li YJ, Luo LJ, Harroun SG, Wei SC, Unnikrishnan B, Chang HT, et al. Synergistically dual-functional nano eye-drops for simultaneous anti-inflammatory and anti-oxidative treatment of dry eye disease. Nanoscale. 2019;11:5580–94. doi: 10.1039/c9nr00376b. [DOI] [PubMed] [Google Scholar]
- 31.Yu F, Zheng M, Zhang AY, Han Z. A cerium oxide loaded glycol chitosan nano-system for the treatment of dry eye disease. J Control Release Off J Control Release Soc. 2019;315:40–54. doi: 10.1016/j.jconrel.2019.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contreras-Ruiz L, Zorzi GK, Hileeto D, López-García A, Calonge M, Seijo B, et al. A nanomedicine to treat ocular surface inflammation:Performance on an experimental dry eye murine model. Gene Ther. 2013;20:467–77. doi: 10.1038/gt.2012.56. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Dozois MD, Chang CN, Ahmad A, Ng DLT, Hileeto D, et al. Prolonged ocular retention of mucoadhesive nanoparticle eye drop formulation enables treatment of eye diseases using significantly reduced dosage. Mol Pharm. 2016;13:2897–905. doi: 10.1021/acs.molpharmaceut.6b00445. [DOI] [PubMed] [Google Scholar]
- 34.Zhang A, Sun R, Ran M, Deng Y, Ge Y, Zhu Y, et al. A novel eyes topical drug delivery system:CsA-LNC for the treatment of DED. Pharm Res. 2020;37 doi: 10.1007/s11095-020-02872-2. [DOI] [PubMed] [Google Scholar]
- 35.Tan G, Li J, Song Y, Yu Y, Liu D, Pan W. Phenylboronic acid-tethered chondroitin sulfate-based mucoadhesive nanostructured lipid carriers for the treatment of dry eye syndrome. Acta Biomater. 2019;99:350–62. doi: 10.1016/j.actbio.2019.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Coursey TG, Henriksson JT, Marcano DC, Shin CS, Isenhart LC, Ahmed F, et al. Dexamethasone nanowafer as an effective therapy for dry eye disease. J Control Release Off J Control Release Soc. 2015;213:168–74. doi: 10.1016/j.jconrel.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine a delivery to the eye:A comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Pharm Verfahrenstechnik EV. 2017;117:14–28. doi: 10.1016/j.ejpb.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Kang MJ, Kim YH, Chou M, Hwang J, Cheon EJ, Lee HJ, et al. Evaluation of the efficacy and safety of a novel 0.05% cyclosporin a topical nanoemulsion in primary Sjögren'ssyndrome dry eye. Ocul Immunol Inflamm. 2020;28:370–8. doi: 10.1080/09273948.2019.1587470. [DOI] [PubMed] [Google Scholar]
- 39.Bang SP, Yeon CY, Adhikari N, Neupane S, Kim H, Lee DC, et al. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS One. 2019;14:e0224805. doi: 10.1371/journal.pone.0224805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cholkar K, Patel A, DuttVadlapudi A. K, Mitra A. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Pat Nanomed. 2012;2:82–95. doi: 10.2174/1877912311202020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldberg DF, Malhotra RP, Schechter BA, Justice A, Weiss SL, Sheppard JD. A phase 3, randomized, double-masked study of OTX-101 ophthalmic solution 0.09% in the treatment of dry eye disease. Ophthalmology. 2019;126:1230–7. doi: 10.1016/j.ophtha.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 42.Gorantla S, Rapalli VK, Waghule T, Singh PP, Dubey SK, Saha RN, et al. Nanocarriers for ocular drug delivery:Current status and translational opportunity. RSC Adv. 2020;10:27835–55. doi: 10.1039/d0ra04971a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H, Shim W, Kim CE, Choi SY, Lee H, Yang J. Therapeutic efficacy of nanocomplex of poly (ethylene glycol) and catechin for dry eye disease in a mouse model. Invest Ophthalmol Vis Sci. 2017;58:1682–91. doi: 10.1167/iovs.16-20843. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W, Wang Y, Lee BTK, Liu C, Wei G, Lu W. A novel nanoscale-dispersed eye ointment for the treatment of dry eye disease. Nanotechnology. 2014;25:125101. doi: 10.1088/0957-4484/25/12/125101. doi:10.1088/0957-4484/25/12/125101. [DOI] [PubMed] [Google Scholar]
- 45.Yu Y, Chow DWY, Lau CML, Zhou G, Back W, Xu J, et al. A bioinspired synthetic soft hydrogel for the treatment of dry eye. Bioeng Transl Med. 2021;6:e10227. doi: 10.1002/btm2.10227. doi:10.1002/btm2.10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, Cao Y, Wang P. Recent advances in hydrogels for the diagnosis and treatment of dry eye disease. Gels. 2022;8:816. doi: 10.3390/gels8120816. doi:10.3390/gels8120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams DL, Mann BK. A crosslinked HA-based hydrogel ameliorates dry eye symptoms in dogs. Int J Biomater. 2013;2013:e460437. doi: 10.1155/2013/460437. doi:10.1155/2013/460437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan H, DeFail A, Rubin JP, Chu CR, Marra KG. Novel multi-arm PEG-based hydrogels for tissue engineering. J Biomed Mater Res A. 2010;92:979–87. doi: 10.1002/jbm.a.32438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekerdt BL, Fuentes CM, Lei Y, Adil MM, Ramasubramanian A, Segalman RA, et al. Thermoreversiblehyaluronic acid-PNIPAAmhydrogel systems for 3D stem cell culture. AdvHealthc Mater. 2018;7:e1800225. doi: 10.1002/adhm.201800225. doi:10.1002/adhm. 201800225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ailincai D, Dorobanţu AM, Dima B, Irimiciuc Ştefan A, Lupaşcu C, Agop M, et al. Poly (vinyl alcohol boric acid)-Diclofenacsodium salt drug delivery systems:Experimental and theoretical studies. J Immunol Res. 2020;2020:3124304. doi: 10.1155/2020/3124304. doi:10.1155/2020/3124304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams DL, Mann BK. Efficacy of a crosslinked hyaluronic acid-based hydrogel as a tear film supplement:A masked controlled study. PloS One. 2014;9:e99766. doi: 10.1371/journal.pone.0099766. doi:10.1371/journal.pone.0099766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buwalda SJ, Boere KWM, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE. Hydrogels in a historical perspective:From simple networks to smart materials. J Control Release Off J Control Release Soc. 2014;190:254–73. doi: 10.1016/j.jconrel.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 53.Han Y, Jiang L, Shi H, Xu C, Liu M, Li Q, et al. Effectiveness of an ocular adhesive polyhedral oligomericsilsesquioxane hybrid thermo-responsive FK506 hydrogel in a murine model of dry eye. Bioact Mater. 2022;9:77–91. doi: 10.1016/j.bioactmat.2021.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin T, Lu Y, Zhang X, Gong L, Wei C. Treatment of dry eye by intracanalicular injection of a thermosensitive chitosan-based hydrogel:Evaluation of biosafety and availability. Biomater Sci. 2018;6:3160–9. doi: 10.1039/c8bm01047a. [DOI] [PubMed] [Google Scholar]
- 55.Pang Y, Wei C, Li R, Wu Y, Liu W, Wang F, et al. Photothermal conversion hydrogel based mini-eye patch for relieving dry eye with long-term use of the light-emitting screen. Int J Nanomedicine. 2019;14:5125–33. doi: 10.2147/IJN.S192407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ciolino JB, Hoare TR, Iwata NG, Behlau I, Dohlman CH, Langer R, et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009;50:3346–52. doi: 10.1167/iovs.08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rykowska I, Nowak I, Nowak R. Soft contact lenses as drug delivery systems:A review. Molecules. 2021;26:5577. doi: 10.3390/molecules26185577. doi:10.3390/molecules 26185577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maulvi FA, Patel PJ, Soni PD, Desai AR, Desai DT, Shukla MR, et al. Novel poly (vinylpyrrolidone)-coated silicone contact lenses to improve tear volume during lens wear:in vitro and in vivo studies. ACS Omega. 2020;5:18148–54. doi: 10.1021/acsomega.0c01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maulvi FA, Soni TG, Shah DO. Extended release of hyaluronic acid from hydrogel contact lenses for dry eye syndrome. J BiomaterSciPolym Ed. 2015;26:1035–50. doi: 10.1080/09205063.2015.1072902. [DOI] [PubMed] [Google Scholar]
- 60.Akbari E, Imani R, Shokrollahi P, HeidariKeshel S. Preparation of nanoparticle-containing ring-implanted poly (Vinyl Alcohol) contact lens for sustained release of hyaluronic acid. Macromol Biosci. 2021;21:e2100043. doi: 10.1002/mabi.202100043. doi:10.1002/mabi. 202100043. [DOI] [PubMed] [Google Scholar]
- 61.Guzman-Aranguez A, Fonseca B, Carracedo G, Martin-Gil A, Martinez-Aguila A, Pintor J. Dry eye treatment based on contact lens drug delivery:A review. Eye Contact Lens. 2016;42:280–8. doi: 10.1097/ICL.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 62.Peng CC, Chauhan A. Extended cyclosporine delivery by silicone-hydrogel contact lenses. J Control Release Off J Control Release Soc. 2011;154:267–74. doi: 10.1016/j.jconrel.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 63.Choi JH, Li Y, Jin R, Shrestha T, Choi JS, Lee WJ, et al. The efficiency of cyclosporine A-eluting contact lenses for the treatment of dry eye. Curr Eye Res. 2019;44:486–96. doi: 10.1080/02713683.2018.1563702. [DOI] [PubMed] [Google Scholar]
- 64.Torres-Luna C, Hu N, Tammareddy T, Domszy R, Yang J, Wang NS, et al. Extended delivery of non-steroidal anti-inflammatory drugs through contact lenses loaded with Vitamin E and cationic surfactants. Contact Lens Anterior Eye J Br Contact Lens Assoc. 2019;42:546–52. doi: 10.1016/j.clae.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 65.Cao S, Loh K, Pei Y, Zhang W, Han J. Overcoming barriers to the clinical utilization of iPSCs:Reprogramming efficiency, safety and quality. Protein Cell. 2012;3:834–45. doi: 10.1007/s13238-012-2078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–46. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Deng C, Qian J, Zhang M, Li X. Improvement of radiotherapy-induced lacrimal gland injury by induced pluripotent stem cell-derived conditioned medium via MDK and inhibition of the p38/JNK pathway. Int J Mol Sci. 2014;15:18407–21. doi: 10.3390/ijms151018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang GL, Zhang NN, Wang JS, Yao L, Zhao YJ, Wang YY. Transdifferentiation of human amniotic epithelial cells into acinar cells using a double-chamber system. Cellular Reprogram. 2012;14:377–83. doi: 10.1089/cell.2011.0096. [DOI] [PubMed] [Google Scholar]
- 69.Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW, et al. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-g. E Bio Medicine. 2018;28:261–73. doi: 10.1016/j.ebiom.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotentmesenchymal stromal cells—The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054–61. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis:A phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8:504–11. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.FundacióInstitut Germans Trias i Pujol. Pericardial Matrix WithMesenchymal Stem Cells for the Treatment of Patients With Infarcted Myocardial Tissue (The PERISCOPE Trial). clinicaltrials.gov. 2022. [Last accessed on 2023 Feb 09]. Report No.: NCT03798353. Available from:https://clinicaltrials.gov/ct2/show/NCT03798353 .
- 73.Karussis D. Phase 2 Trial to Investigate the Clinical Efficacy and the Optimal Administration (Based on the Immunological, Clinical and Neuroradiological Effects) of Autologous Mesenchymal Bone Marrow Stem Cells in Active and Progressive Multiple Sclerosis. clinicaltrials.gov. 2019. [Last accessed on 2023 Feb 09]. Report No.: NCT02166021. Available from:https://clinicaltrials.gov/ct2/show/NCT02166021 .
- 74.CHABiotech CO.. Ltd. A Randomized, Double-blind, Placebo-controlled, Phase I/IIa Clinical Trial for Evaluation of the Safety and Potential Therapeutic Effects After Intravenous Transplantation of Human Umbilical Cord-derived Mesenchymal Stem Cells in Patients With Cerebral Infarction. clinicaltrials.gov. 2019. [Last accessed on 2023 Feb 09]. Report No.: NCT02378974. Available from:https://clinicaltrials.gov/ct2/show/NCT02378974 .
- 75.Nature Cell Co. Ltd. A Phase 1/2, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of AstroStem, Autologous Adipose Tissue Derived Mesenchymal Stem Cells, in Patients With Alzheimer's Disease. clinicaltrials.gov. 2021. [Last accessed on 2023 Feb 09]. Report No.: NCT03117738. Available from:https://clinicaltrials.gov/ct2/show/NCT03117738 .
- 76.Borsari GT. Local Inoculation of Autologous Micro-fragmented Adipose Tissue in the Treatment of Minor Amputations of Diabetic Foot:A Randomized Controlled Trial. clinicaltrials.gov. 2018. [Last accessed on 2023 Feb 09]. Report No.: NCT03276312. Available from:https://clinicaltrials.gov/ct2/show/NCT03276312 .
- 77.Thomas Advanced Medical LLC. A Pilot Phase Study Evaluating the Effects of a Single Mesenchymal Stem Cell Injection in Patients With Suspected or Confirmed COVID-19 Infection and Healthcare Providers Exposed to Coronavirus Patients. clinicaltrials.gov. 2020. [Last accessed on 2023 Feb 09]. Report No.: NCT04573270. Available from:https://clinicaltrials.gov/ct2/show/NCT04573270 .
- 78.State-Financed Health Facility “Samara Regional Medical CenterDinasty.”The Protocol of Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Two-Sided Pneumonia. clinicaltrials.gov. 2020. [Last accessed on 2023 Feb 09]. Report No.: NCT04491240. Available from:https://clinicaltrials.gov/ct2/show/NCT04491240 .
- 79.Yao L, Bai H. Review:Mesenchymal stem cells and corneal reconstruction. Mol Vis. 2013;19:2237–43. [PMC free article] [PubMed] [Google Scholar]
- 80.Yao L, Li Z rong, Su W ru, Li Y ping, Lin M li, Zhang WX, et al. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012;7:e30842. doi: 10.1371/journal.pone.0030842. doi:10.1371/journal.pone.0030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma Y, Xu Y, Xiao Z, Yang W, Zhang C, Song E, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24:315–21. doi: 10.1634/stemcells.2005-0046. [DOI] [PubMed] [Google Scholar]
- 82.Guo T, Wang W, Zhang J, Chen X, Li BZ, Li LS. [Experimental study on repairing damage of corneal surface by mesenchymal stem cells transplantation. Zhonghua Yan Ke Za Zhi Chin J Ophthalmol. 2006;42:246–50. [PubMed] [Google Scholar]
- 83.Gallazzi A, Ghinelli E, Carito G, Isacchi G, Scadden DT. Topical application of BM–derived stem cells enhances the repair of corneal injuries. Invest Ophthalmol Vis Sci. 2004;45:1432. [Google Scholar]
- 84.Agorogiannis GI, Alexaki VI, Castana O, Kymionis GD. Topical application of autologous adipose-derived mesenchymal stem cells (MSCs) for persistent sterile corneal epithelial defect. Graefes Arch Clin Exp Ophthalmol. 2012;250:455–7. doi: 10.1007/s00417-011-1841-3. [DOI] [PubMed] [Google Scholar]
- 85.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142–51. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beyazyıldız E, Pınarlı FA, Beyazyıldız Ö, Hekimoğlu ER, Acar U, Demir MN, et al. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. 2014;2014:e250230. doi: 10.1155/2014/250230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou T, He C, Lai P, Yang Z, Liu Y, Xu H, et al. miR-204-containing exosomes ameliorate GVHD-associated dry eye disease. Sci Adv. 2022;8:eabj9617. doi: 10.1126/sciadv.abj9617. doi:10.1126/sciadv.abj9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alio JL, Rodriguez AE, Ferreira-Oliveira R, Wróbel-Dudzińska D, Abdelghany AA. Treatment of dry eye disease with autologous platelet-rich plasma:A prospective, interventional, non-randomized study. Ophthalmol Ther. 2017;6:285–93. doi: 10.1007/s40123-017-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cvekl A, Zhang X. Signaling and gene regulatory networks in mammalian lens development. Trends Genet. 2017;33:677–702. doi: 10.1016/j.tig.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ogawa M, Tsuji T. Functional salivary gland regeneration as the next generation of organ replacement regenerative therapy. Odontology. 2015;103:248–57. doi: 10.1007/s10266-015-0210-9. [DOI] [PubMed] [Google Scholar]
- 91.Oshima M, Tsuji T. Regenerative Engineering and Developmental Biology. CRC Press;Boca Raton; 2017. Functional ectodermal organ regeneration based on epithelial and mesenchymal interactions. [Google Scholar]
- 92.Takeo M, Tsuji T. Organ regeneration based on developmental biology:Past and future. Curr Opin Genet Dev. 2018;52:42–7. doi: 10.1016/j.gde.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 93.Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes:Insights into tube formation, elongation, and elaboration. Dev Biol. 2010;341:34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cui J, Wang H, Zheng Z, Shi Q, Sun T, Huang Q, et al. Fabrication of perfusable 3D hepatic lobule-like constructs through assembly of multiple cell type laden hydrogel microstructures. Biofabrication. 2018;11:015016 doi: 10.1088/1758-5090/aaf3c9. doi:10.1088/1758-5090/aaf3c9. [DOI] [PubMed] [Google Scholar]
- 95.Bhat S, Kumar A. Biomaterials and bioengineering tomorrow's healthcare. Biomatter. 2013;3:e24717. doi: 10.4161/biom.24717. doi:10.4161/biom. 24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang YS, Yue K, Aleman J, Mollazadeh-Moghaddam K, Bakht SM, Yang J, et al. 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng. 2017;45:148–63. doi: 10.1007/s10439-016-1612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X. Intelligent freeform manufacturing of complex organs. Artif Organs. 2012;36:951–61. doi: 10.1111/j.1525-1594.2012.01499.x. [DOI] [PubMed] [Google Scholar]
- 98.Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T, et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun. 2012;3:784. doi: 10.1038/ncomms1784. doi:10.1038/ncomms1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–30. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 100.Hirayama M, Tsubota K, Tsuji T. Generation of a bioengineered lacrimal gland by using the organ germ method. Methods Mol Biol Clifton NJ. 2017;1597:153–65. doi: 10.1007/978-1-4939-6949-4_11. [DOI] [PubMed] [Google Scholar]
- 101.Bannier-Hélaouët M, Post Y, Korving J, TraniBustos M, Gehart H, Begthel H, et al. Exploring the human lacrimal gland using organoids and single-cell sequencing. Cell Stem Cell. 2021;28:1221–1232.e7. doi: 10.1016/j.stem.2021.02.024. [DOI] [PubMed] [Google Scholar]
- 102.Dietrich J, Massie I, Roth M, Geerling G, Mertsch S, Schrader S. Development of causative treatment strategies for lacrimal gland insufficiency by tissue engineering and cell therapy. Part 1:Regeneration of lacrimal gland tissue:Can we stimulate lacrimal gland renewal in vivo? Curr Eye Res. 2016;41:1131–42. doi: 10.3109/02713683.2016.1148741. [DOI] [PubMed] [Google Scholar]
- 103.Jeong SY, Choi WH, Jeon SG, Lee S, Park JM, Park M, et al. Establishment of functional epithelial organoids from human lacrimal glands. Stem Cell Res Ther. 2021;12:247. doi: 10.1186/s13287-021-02133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walles T, Giere B, Hofmann M, Schanz J, Hofmann F, Mertsching H, et al. Experimental generation of a tissue-engineered functional and vascularized trachea. J Thorac Cardio vasc Surg. 2004;128:900–6. doi: 10.1016/j.jtcvs.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 105.Schultheiss D, Gabouev AI, Cebotari S, Tudorache I, Walles T, Schlote N, et al. Biological vascularized matrix for bladder tissue engineering:Matrix preparation, reseeding technique and short-term implantation in a porcine model. J Urol. 2005;173:276–80. doi: 10.1097/01.ju.0000145882.80339.18. [DOI] [PubMed] [Google Scholar]
- 106.Mertsching H, Walles T, Hofmann M, Schanz J, Knapp WH. Engineering of a vascularized scaffold for artificial tissue and organ generation. Biomaterials. 2005;26:6610–7. doi: 10.1016/j.biomaterials.2005.04.048. [DOI] [PubMed] [Google Scholar]
- 107.Linke K, Schanz J, Hansmann J, Walles T, Brunner H, Mertsching H. Engineered liver-like tissue on a capillarized matrix for applied research. Tissue Eng. 2007;13:2699–707. doi: 10.1089/ten.2006.0388. [DOI] [PubMed] [Google Scholar]
- 108.Groeber F, Engelhardt L, Lange J, Kurdyn S, Schmid FF, Rücker C, et al. A first vascularized skin equivalent as an alternative to animal experimentation. ALTEX-Altern Anim Exp. 2016;33:415–22. doi: 10.14573/altex.1604041. [DOI] [PubMed] [Google Scholar]
- 109.Schürlein S, Al Hijailan R, Weigel T, Kadari A, Rücker C, Edenhofer F, et al. Generation of a human cardiac patch based on a reendothelialized biological scaffold (BioVaSc) Adv Biosyst. 2017;1:1600005. doi:10.1002/adbi. 201600005. [Google Scholar]
- 110.Kress S, Baur J, Otto C, Burkard N, Braspenning J, Walles H, et al. Evaluation of a miniaturized biologically vascularized scaffold in vitro and in vivo. Sci Rep. 2018;8:4719. doi: 10.1038/s41598-018-22688-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Massie I, Spaniol K, Barbian A, Geerling G, Metzger M, Schrader S. Development of lacrimal gland spheroids for lacrimal gland tissue regeneration. J Tissue Eng Regen Med. 2018;12:e2001–9. doi: 10.1002/term.2631. [DOI] [PubMed] [Google Scholar]
- 112.Thacker M, Singh V, Basu S, Singh S. Biomaterials for dry eye disease treatment:Current overview and future perspectives. Exp Eye Res. 2023;226:109339. doi: 10.1016/j.exer.2022.109339. [DOI] [PubMed] [Google Scholar]
- 113.Veernala I, Jaffet J, Fried J, Mertsch S, Schrader S, Basu S, et al. Lacrimal gland regeneration:The unmet challenges and promise for dry eye therapy. Ocul Surf. 2022;25:129–141. doi: 10.1016/j.jtos.2022.06.005. [DOI] [PubMed] [Google Scholar]