Abstract
Bacterial panicle blight (BPB) caused by Burkholderia glumae (BG) has become significantly more prevalent in the rice-growing regions of North India. Based on virulence screening and in vitro quantification of toxoflavin, the BG strains were classified as hyper- (BG1 and BG3), moderate- (BG2, BG4, BG6, BG8, and BG9), and hypo- (BG5, BG7, and BG10) virulent. Plant inoculation assays with cell-free culture filtrate revealed strains with higher toxoflavin-producing ability had higher virulence. Based on 16S rRNA sequence, 6 isolates from Uttar Pradesh were grouped in clad C1; whereas, clad C2 exhibited 4 isolates, two each from Delhi and Uttar Pradesh. Strain BG1 being the most virulent Indian strain from Uttar Pradesh was further profiled for 11 tox genes. We found all the 11 tox genes present in strain BG1. In toxRABCDE cluster, all tox genes showed high similarity to B. glumae BGR1 except toxB, whereas in toxFGHIJ cluster toxF, toxG, toxH and toxI shared maximum similarity to B. glumae 336gr-1. tox genes of BG1 exhibited homology as well as divergence with B. gladioli. The domain prediction and protein association network analysis indicated the possible involvement of tox genes in the toxoflavin biosynthesis. As per our knowledge, this is the first report in India on characterization of tox genes cluster in B. glumae. Altogether, our study unravels a reliable method for identifying and characterizing B. glumae using tox genes and its relationship with disease production.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03660-6.
Keywords: Culture filtrate, Isolates, Symptoms, Quantification, Tox gene
Introduction
Rice is an important staple food crop grown in a wide range of agroclimatic regions all over the world. It is the primary food source for more than a third of the world's population, primarily in Asia (Singh et al. 2012). The bacterial panicle blight (BPB) incited by Burkholderia glumae is one of the emerging diseases of rice. This disease was first identified in Japan as seedling rot and sheath rot based on different stages of development in the rice crop (Goto and Ohata 1956). Since then, several countries throughout the world have reported cases of the disease, viz., Philippines (Cottyn et al. 1996), Taiwan (Chien and Chang 1987), China (Luo et al. 2007), USA (Nandakumar et al. 2009), India (Mondal et al. 2015) and Malaysia (Ramachandran et al. 2021). The disease has affected the rice production in many Asian, African and American countries, and is a classical example of minor disease turning into an emerging plant disease. The infection due to BPB inhibits seed germination, causes floret sterility, grain rot, and abortion (Ham et al. 2011), and severely infected paddy fields have yield losses of up to 75% (Sayler et al. 2006). In India, this disease is causing more damage during panicle emergence stage and in leaf sheath in basmati as well as non-basmati rice varieties. During recent past, many rice-growing regions in northern India, including Delhi, Tarai region of Uttarakhand, Uttar Pradesh and Haryana have been affected with the disease and now the disease has been a serious threat to all the rice-growing areas of the India (Mondal et al. 2015). In spite of being a disease of economic significance the data on yield losses due to BPB is yet to be determined in India.
A number of virulence factors that play significant role in the pathogenesis of B. glumae includes toxoflavin, type III secretion system, flagella, lipase and catalase production (Ham et al. 2011). Burkholderia spp. synthesizes a variety of toxins like toxoflavin, reumycin and fervenulin that exhibit similarity in molecular structure; however, toxoflavin plays major role in pathogenicity (Sato et al. 1989). Initially, the growth of roots and leaves is inhibited by toxoflavin and later during panicle development stage, it induces chlorotic symptom on panicle resulting in grain rot (Iiyama et al. 1995; Sato et al. 1989). In B. glumae, toxoflavin biosynthesis was recorded to start at temperature over 30 °C with maximum production reached at 37 °C, while toxoflavin production was stopped at 25°–28 °C (Matsuda and Sato 1988).
The mechanism of toxoflavin production can be best understood by thorough knowledge on the pathways and genes involved in riboflavin biosynthesis (Bacher 1991). Suzuki et al. (2004) found that the production of toxoflavin is encoded by an operon of five genes toxABCDE (polycistronic operon) located on chromosome 2 of the bacterial genome. Each gene produces components and intermediates that act as the component of toxoflavin biosynthesis pathway. The mechanism starts from GTP and in totality involves five enzymatic reactions. The production of toxoflavin, reumycin or fervenulin gets inhibited if any mutation occurred in the polycistronic operon, proving that the toxoflavin biosynthesis pathway is necessary for the production of all these phytotoxins (Kim et al. 2004; Suzuki et al. 2004). However, toxoflavin transportation is regulated by another set of polycistronic operon i.e., toxFGHI as well as toxR and toxJ gene that acted as the transcriptional activator during toxoflavin biosynthesis (Kim et al. 2004).
The diversity of over 400 strains of B. glumae from different rice-growing areas of the United States was analyzed (Nandakumar et al. 2009). The genetic diversity and geographical distribution of 137 strains of B. glumae from Korea were carried out using transposase-based PCR genomic fingerprinting (Choi et al. 2021). Though, B. glumae is an important rice pathogen, the information on the genome sequence and on phylogenetic relationship are very limited.
The current study aims to determine the toxoflavin, a key virulence factor, production ability of BG strains from severely affected rice-growing regions of North India and to verify the correlation between toxoflavin production and the disease production ability using cell-free culture filtrate. Furthermore, the most virulent strain was selected for profiling and phylogenetic analysis based on 11 toxoflavin biosynthesis linked gene clusters. Since the pathogen was initially discovered in India in 2015, to the best of our knowledge, this is the first detailed report from India on characterization of tox genes in Indian strain, toxoflavin production and its correlation with disease intensity.
Materials and methods
Plant material, bacterial strain and media
During the cropping season, naturally infected rice panicles showing bacterial panicle blight (BPB) symptom were collected from different rice-growing regions of northern India. The rice plants exhibited major symptoms like discoloration of seeds, florets and chaffy grains. The diseased samples were placed in a paper bag, labeled properly and kept in refrigerator at 4 °C for further analysis. From the diseased samples, Burkholderia glumae were straind following standard isolation technique on PSA medium (peptone 10 g, sucrose 10 g, Na-glutamate 1 g and agar 20 gL−1) and King’s B agar medium, pH 6.8 ± 0.2 at 28 ± 1 °C in BOD incubator for 72 h. The single colonies were established from the mother plates and purified through repeated streaking on PSA and LB agar plates. The identification of the pathogen was carried out by studying colony morphology (cell shape, colony color elevation, and margin) after 48 h of incubation using Bergey's Manual of Systematic Bacteriology (Brenner et al. 2015); Gram staining (Schaad 1988) and 16S rRNA sequencing.
Total ten strains of B. glumae were established and used in the present study. The purified strains were deposited in the Indian Type Culture Collection (ITCC, New Delhi) and the accession number obtained against each strain is provided in parenthesis as BG1 (ITCCBQ0004), BG2 (ITCCBQ0005), BG3 (ITCCBQ0006), BG4 (ITCCBQ0007), BG5 (ITCCBQ0001), BG6 (ITCCBQ0008), BG7 (ITCCBQ0002), BG8 (ITCCBQ0010), BG9 (ITCCBQ0009), and BG10 (ITCCBQ0003).
Virulence screening using cell-free culture filtrate
Ten B. glumae strains (BG1–BG10) were grown in nutrient broth culture of 50 ml. The one loopful of previously grown bacterial strains on King’s B media was aseptically transferred to King’s B broth culture and incubated for 24 h at 150 rpm at 37 °C in a shaker. After incubation, OD600 value was recorded as 0.1 (= 108 CFU ml−1). The broth culture was subjected to centrifugation at 6000 rpm for 10 min and the culture filtrates were harvested. The supernatant was filtered using 0.45 m and 0.20 m pore size membrane filters (Axiva co) and stored in the refrigerator as a stock solution. With the help of a sterile syringe, the rice plants were injected with freshly prepared culture filtrate solution @ 1 ml plant−1 at boot leaf stage. After 7–10 days, the artificially inoculated rice plants produced lesion identical to those observed during natural rice infection in the field. At 10–15 days of post inoculation, the panicle emerged from the inoculated boot leaf. The emerged panicles showed light brown discoloration similar to BPB symptoms. The plants were observed for symptom expression until maturity, and disease scoring was carried out using standard procedure (Nandakumar et al. 2009).
Extraction of toxoflavin
Each BG strain was inoculated into 100 ml King’S B broth and incubated at 37 °C for 4 days with shaking (150 rpm). All the strains were inoculated simultaneously and kept under similar environment so that they all grow at the same rate. Each culture filtrate (100 mL) was extracted thrice with chloroform (20 mL × 3) following liquid–liquid partitioning method as described previously (Kumar et al. 2021). The lower heavy chlorinated solvent layer was passed through the anhydrous sodium sulfate (5 g, each) to remove traces of moisture, if any. Chloroform was allowed to evaporate under vacuo using a rotary evaporator (Heidolph, Germany) below 35 °C to achieve the residues, which was further dissolved in LC–MS/MS grade ACN and filtered through syringe filters (0.22 µ pore size) before injection.
16S rRNA gene-based phylogeny of B. glumae strains
The genomic DNA of ten B. glumae strains (BG1 to BG10) were extracted by CTAB method following an earlier described procedure (Murray and Thompson 1980). The gDNA was used as template for 16S rRNA based PCR identification. The genomic region of 1498 bp was amplified using universal primers (27F: 5′-AGAGTTTGATCCTGGCTCAG-3′) and (1492R: 5′-GGTTACCTTGTTACGACTT-3′). A 25 µl reaction volume was prepared with 5.0 µl of 5X buffer, 2.5 µl of 25 mM MgCl2, 1 µl of 10 mM dNTPs, 1 µl each of 10 µm forward and reverse primers of 16S rRNA, 1.0 µl of DNA template, 0.25 µl of Taq polymerase (Takara Bio) and 13.25 µl of nuclease-free water. The amplification was performed in a thermal cycler with an initial denaturation step at 95 °C for 5 min followed by 35 cycles each of denaturation at 94 °C for 1 min, annealing at 58 °C for 1 min and extension at 72 °C for 90 s reaction and the reaction was terminated by final elongation at 72 °C for 10 min.
The PCR-amplified 16S rRNA products of ten B. glumae strains (BG1–BG10) were outsourced for sequencing to AgriGenome Labs Private Ltd. The gene sequences were end-trimmed, edited and contig-assembled using DNA baser (http://www.dnabaser.com/download/DNA-Baser-sequence-assembler) and a sequence of ≈ 1300–1500 bp was submitted after trimming. The NCBI database (https://www.ncbi.nlm.nih.gov/search/) was used to determine nucleotide sequence identity and the closest match confirmed the bacterial identity.
The MEGA X software was used to perform phylogenetic analysis based on 16S rRNA gene sequences (Kumar et al. 2018) following the maximum likelihood method and the Tamura-Nei model (Tamura and Nei 1993). Missing data and gaps in all positions were removed, and the bootstrap confidence intervals were calculated using 1,000 replicates. The reference sequences of B. gladioli and B. glumae obtained from the NCBI Genbank database were included in the phylogenetic analysis for comparison. Erwinia amylovora was chosen as an outgroup (Table 1).
Table 1.
Details of B. glumae isolates, tox genes, reference genes and tox gene-specific primers used in the study
| Sr. no | Isolates in this study | Place of collection | GenBank accession number | Name of tox genes | Primers (5′- to -3′) | Gene locus in reference genome | Product Size submitted (bp) | Accession number of submitted tox genes | Reference of tox genes |
|---|---|---|---|---|---|---|---|---|---|
| 1. | BG1 | Uttar Pradesh | OP113931 | toxR | ATGAATAATCTGAAGCGGATCGACC | 314–943 | 630 | ON807150 | AB040403 |
| TTACCTCGCACGGGGCCGGCTATTC | |||||||||
| 2. | BG2 | Uttar Pradesh | OP113932 | toxA | ATGAGTACGACAGCTCGATACGATT | 1693–2320 | 628 | ON807151 | AB040403 |
| AAGCCGGGCGGATGGGTGGCGATG | |||||||||
| 3. | BG3 | Uttar Pradesh | OP113933 | toxB | GATCATTCCGGTGTGAGCATCCGC | 2614–3111 | 498 | ON807152 | AB040403 |
| TCATTGCATTTCTCCGTTGAGCTT | |||||||||
| 4. | BG4 | Uttar Pradesh | OP113934 | toxC | GATTGCCCATCGCAGCCCGATCAGC | 3316–4776 | 1461 | ON807153 | AB040403 |
| GGATCGCCGGCACGCGGTAAACCTG | |||||||||
| 5. | BG5 | Delhi | OP113935 | toxD | ATGACCATCAAGTTGGCTGACAATC | 5037–5728 | 692 | ON807154 | AB040403 |
| CCGGAAGCCCATCGCGTAGATGTT | |||||||||
| 6. | BG6 | Delhi | OP113936 | toxE | ATGGCGAAGGCGCTGGAGTTGGCGG | 6075–7002 | 932 | ON807155 | AB040403 |
| CGAACCGCGCCAGGATCCGCAGGTC | |||||||||
| 7. | BG7 | Uttar Pradesh | OP113937 | toxF | ATG CCA ACC TCT CTT TCG | 2,421,774–2,422,349 | 576 | OP209184 | CP023204.1 |
| TCA GGT GTG GGG ATG CGC | |||||||||
| 8. | BG8 | Uttar Pradesh | OP113938 | toxG | AAC TGA TTC TTG TCG GCA CCG | 2,422,389–2,423,528 | 1013 | OP209180 | CP023204.1 |
| TCA TTG CGC CGT CTC CGC TG | |||||||||
| 9. | BG9 | Uttar Pradesh | OP113939 | toxH | ATG AAC ACC CAC CGC TCG CAG CCG G | 2,423,525–2,426,617 | 3011 | OP209183 | CP023204.1 |
| TCA ATG CTG GGT GGC GAG CAG | |||||||||
| 10. | BG10 | Uttar Pradesh | OP113940 | toxI | CGC GGC GGC CCT GCT GCT CGC | 2,426,689–2,428,209 | 1481 | OP209181 | CP023204.1 |
| GCG TGG CGG AAT CAT TTT GCC | |||||||||
| toxJ | GTG GTC GAG ATA TTT GGC AA | 2,429,015–2,429,845 | 810 | OP209182 | CP023204.1 | ||||
| TCA CGA TTC GTC GAG CA |
| Reference strain used in the study | |||
|---|---|---|---|
| Sr. no | Reference strain | Place of collection | GenBank accession number |
| 1 | Burkholderia glumae strain 336gr-1 chromosome 2 | South Korea | CP023204 |
| 2 | Burkholderia glumae strain P 1–22-1 16S ribosomal RNA | – | NR_029211 |
| 3 | B. glumae LMG 2196 = ATCC 33617 | Japan | CP009435 |
| 4 | B. plantarii CIP 105769 | – | NR_116151 |
| 5 | B. gladioli Co14 chromosome 1 | China | CP033430 |
| 6 | Erwinia amylovora strain DSM 30165 | – | NR_041970 |
bp base pairs
Detection and molecular characterization of tox genes
Eleven primer sets targeting tox gene cluster that covers cluster associated with toxoflavin biosynthesis (viz., toxA, toxB, toxC, toxD, toxE), cluster associated with toxoflavin transport (toxF, toxG, toxH, toxI) and cluster associated with transcriptional (Kim et al. 2004) regulation (toxJ and toxR) located across the genome of B. glumae (AB040403) were designed using NCBI blast and OligoCalc. The target regions were amplified by conventional PCR (Kibbe 2007) (Table 1).
The PCR amplification condition was first optimized using the designed primer sets to verify the desired amplicons. The amplification was performed in a thermal cycler (Gradient thermocycler, C-1000TM, BIORAD); The optimized PCR reaction mixture consisted of 1.0 µl of 10 mM dNTPs, 5.0 µl of 5 × Taq buffer, 2.50 µl of 25 mM MgCl2, 0.50 µl each of forward and reverse primers (10 µm stock), 1.0 µl of 100 ng DNA, 14.25 µl of nuclease-free water and 0.25 µl of Taq polymerase (Takara bio) for detection of toxA, toxB, toxD, toxE, toxF, toxG, toxH and toxJ genes; whereas, the PCR reaction mixture consisted of 0.2 µl of 10X PCR buffer (Mg2+), 2.0 µl of 10 mM dNTPs, 1.0 µl each of 10 µm stock of forward and reverse primers, 1.0 µl of 100 ng DNA, 17.75 µl of nuclease-free water and 0.25 µl of Taq polymerase (Takara bio) for detection of toxC, toxI and toxR genes.
For toxA amplification, initial denaturation at 94 °C for 1 min followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s, and extension at 72 °C for 1 min and the reaction was terminated by final elongation at 72 °C for 5 min. For toxB, toxD, toxE, toxF, toxG, toxJ amplification, the reaction condition was similar to that of toxA except that annealing temperature was adjusted to 54 °C, 57 °C, 58 °C, 56 °C, 56 °C, 54 °C, respectively. The toxH gene was amplified using initial denaturation at 94 °C for 30 s followed by 35 cycles each consisting of denaturation at 98 °C for 10 s, annealing at 64 °C for 30 s and extension at 72 °C for 3.10 min followed by final elongation at 72 °C for 10 min. For, toxC and toxI amplification, the initial denaturation was at 98 °C for 10 s followed by 35 cycles each consisting of denaturation at 98 °C for 10 secs, elongation at 57 °C for 1.30 min and extension at 72 °C for 1 min and the reaction was terminated by final elongation at 72 °C for 5 min. For toxR amplification, the PCR condition was similar to that of toxC except that annealing temperature adjusted to 64 °C for 30 s. The PCR products were visualized on 1% agarose gel stained with ethidium bromide in TAE buffer at 75 V through gel electrophoresis.
Phylogenetic analysis using tox genes
To determine the phylogeny, the sequences of tox genes were aligned using Muscle algorithm; Mega X software was used to calculate phylogenetic distance and to build a phylogenetic tree. The evolutionary history was inferred through the maximum likelihood method with 1000 bootstrap replicates in Mega X software.
Domain prediction and string analysis of tox genes
The gene sequences obtained from Sanger sequencing were used as template for converting it to a protein sequence through AUGUSTUS webserver (https://bioinf.uni-greifswald.de/augustus/). Using NCBI Batch CDD search (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi), all these protein sequences were examined for the presence of toxoflavin protein and other domains (Marchler-Bauer et al. 2017). The protein association network for all the tox genes was developed to find the important proteins to which a given Tox protein may interact through STRING database v10 (Szklarczyk et al. 2015).
Results
Morphological analysis
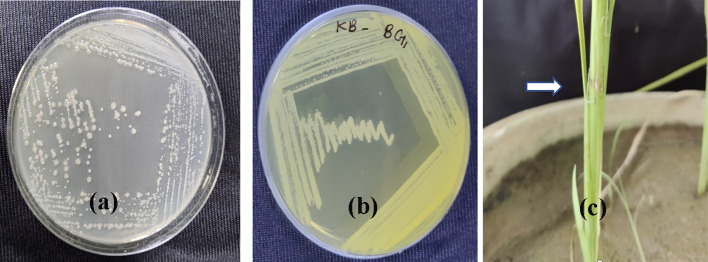
The bacteria produced glistening colonies grayish-white in color after 48 h of incubation period. The colonies were circular in shape and flat in elevation (Fig. 1a). They produced light-yellow-colored pigment (Fig. 1b) on King’s B agar, which is typically a distinguishing character associated with B. glumae. The bacteria showed Gram-negative reaction.
Fig. 1.
Growth of Burkholderia glumae BG1 strain on a PSA media b King’s B agar media c lesion formation at the point of inoculation in boot leaf
Virulence screening using culture filtrate
The Pusa basmati 1 plants started developing symptom 15 days after inoculation at the flag leaf initiation stage. The healthy control plants didn’t show any disease symptom. The boot leaf first showed the lesions at the point of inoculation (Fig. 1c), which later extended to the entire flag leaf and subsequently to panicles. The chaffy grains developed during panicle emergence in boot leaf; however, partially or fully discolored grains were also observed in some inoculated plants. All the strains were pathogenic with varied level of virulence as observed upon challenge inoculation on rice plants, viz., two strains BG3 and BG1 were hypervirulent, five strains BG2, BG4, BG6, BG8, BG9 were moderately virulent and three strains BG5, BG7 and BG10 were hypovirulent (Fig. 2).
Fig.2.
Virulence screening of B. glumae strains using culture filtrate
In vitro toxoflavin production
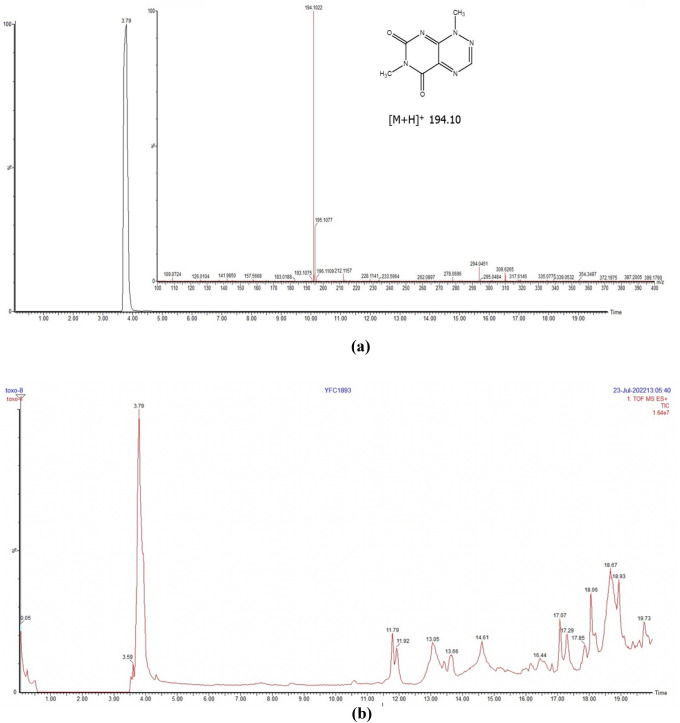
All the ten strains of B. glumae grown on King’s B broth produced yellow color which is an indication that the bacteria produced toxoflavin. The toxoflavin produced by each strain was quantified by UPLC–QTOF-ESI–MS/MS. The content of toxoflavin produced by the bacteria was estimated using calibration curve. An UPLC method was developed under gradient condition using ACN and H2O to separate toxoflavin in the bacterial samples. In UPLC, toxoflavin was detected at Rt 3.79 min. Total ion chromatogram (TIC) in mass spectral analysis revealed exact molecular adduct ion peak at m/z 194.10 (Fig. 3a). UPLC–QTOF-MS spectra of a representative bacterial strain (Fig. 3b) showed various peaks corresponding to its metabolomic profile. Among these, toxoflavin was identified and estimated. The in vitro toxoflavin production varied substantially across the strains. Highest toxoflavin was produced by BG3 strain (867 µg/mL) followed by BG1 strain (796 µg/mL); whereas, lowest toxoflavin was produced by BG5 strain i.e., 160 µg/mL (Table 2). However, in the pathogenicity assay, all the strains that produced high amounts of toxoflavin also produced disease in the rice plants; and higher intensity of disease was associated with BG3 and BG1 strains. Thus, based on the estimated toxoflavin content and the intensity of disease production, the bacterial strains BG3 and BG1 can be classified as hypervirulent, whereas BG5 strain as hypovirulent.
Fig. 3.
a Total ion chromatogram of toxoflavin at M/Z 194.10 b UPLC–QTOF-MS spectra of bacterial strain BG8
Table 2.
Relationship between the bacterial strain and toxoflavin production as analyzed in UPLC–QTOF-ESI–MS/MS
| Sr. no | Bacterial strain | Toxoflavin produced (µg/mL) |
|---|---|---|
| 1 | BG1 | 795.68 ± 31.28 |
| 2 | BG2 | 555.15 ± 19.74 |
| 3 | BG3 | 866.50 ± 46.36 |
| 4 | BG4 | 406.96 ± 11.98 |
| 5 | BG5 | 159.64 ± 8.05 |
| 6 | BG6 | 484.22 ± 15.42 |
| 7 | BG7 | 220.95 ± 29.56 |
| 8 | BG8 | 507.98 ± 26.30 |
| 9 | BG9 | 638.55 ± 10.83 |
| 10 | BG10 | 215.58 ± 25.65 |
16S rRNA sequencing and phylogenetic analysis
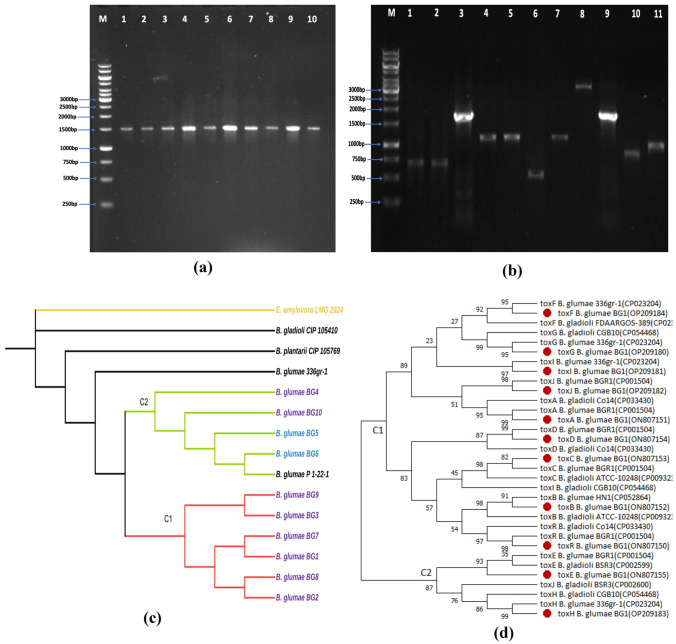
16S rRNA region (1500 bp) was amplified from all the 10 B. glumae strains (Fig. 4a). The BLASTn was employed to compare the 16S rRNA gene sequences with the available sequences in the NCBI GenBank database. A total of six strains (BG1, BG2, BG3, BG7, BG8 and BG9) showed highest nucleotide similarity of 99.78%, 99.79%, 99.79%, 99.78%, 99.79% and 99.79%, respectively, to the B. glumae strain 336gr (CP023204.1) from USA and four strains BG4, BG5, BG6 and BG10 showed 99.93%, 99.87%, 99.87% and 99.93% similarity to B. glumae strain P 1-22-1 16S (NR029211) from USA. The 16S rRNA sequences of BG1–BG10 strains generated in the present study were deposited in the GenBank having the accession number OP113931, OP113932, OP113933, OP113934, OP113935, OP113936, OP113937, OP113938, OP113939, OP113940, respectively.
Fig. 4.
a PCR Amplification of 16 s rRNA fragments from 10 B. glumae strains (1500 bp). Lane M: 1 kb Gene Ruler ladder, Lane 2: BG1, Lane 3: BG2, Lane 4: BG3, Lane 5: BG4, Lane 6: BG5, Lane 7: BG6, Lane 8: BG7, Lane 9: BG8, Lane 10: BG9, Lane 11: BG10. b PCR Amplification of 11 tox genes of B. glumae (BG1) using gene-specific primers. Lane M: 1 kb ladder (Gene Ruler). Lane 2: toxA (683 bp), 3: toxB (639 bp), 4: toxC (1682 bp), 5: toxD (960 bp), 6: toxE (1069 bp), 7: toxF (576 bp), 8: toxG (1127 bp), 9: toxH (3093 bp), 10: toxI (1496 bp), 11: toxJ (833 bp), 12: toxR (1002 bp). c Phylogenetic tree of Burkholderia spp based on the maximum likelihood analysis of the 16S rRNA gene sequences constructed using MEGA X. The strains accession numbers are listed in Table 1. Numbers at nodes represent percentage bootstrap values of 1,000 replicates. E. amylovora served as an outgroup. d Phylogenetic tree of of Burkholderia spp based on the maximum likelihood analysis of the twelve tox genes of BG1 strain (toxA, toxB, toxC, toxD, toxE, toxF, toxG, toxH, toxI, toxJ and tox R) constructed using MEGA X.The bootstrap values are shown at nodes
The phylogenetic analysis was carried out by comparing 16S rRNA sequences of 10 B. glumae strains with 4 sequences from Burkholderia spp. E. amylovora LMG 2024 sequence was kept as an outgroup (Fig. 4c). The similarity identity matrix of 10 B. glumae strains and other Burkholderia spp. strains (retrieved from NCBI database) was prepared using 16S rRNA sequence alignment analysis. Among the Indian B. glumae strains, BG1, BG2, BG3, BG7, BG8, and BG9 shared 100% sequence identity; while, strains BG4, BG5, BG6, and BG10 shared 100% similarity. The phylogenetic analysis revealed that all B. glumae strains from India descended from B. glumae 336gr-1 and were classified into two clades, C1 and C2; C1 included strains BG1, BG2, BG3, BG7, BG8 and BG9 collected from Uttar Pradesh sharing 99.78–99.79% resemblance to the reference strain B. glumae strain 336gr-1 from USA; whereas, clad C2 grouped BG4, BG5, BG6, BG10 (Delhi and UP strains) exhibited 99.87–99.93% similarity to the B. glumae P 1–22-1. Strains B. gladioli CIP 105410, B. plantarii CIP 105769 and E. amylovora LMG 2024 formed a separate outgroup. The B. gladioli and B. plantarii exhibited 99.98–99.75% similarity to all the B. glumae strains.
Molecular characterization of tox genes
The PCR amplification using primer sets targeting 11 different tox genes viz., toxA, toxB, toxC, toxD, toxE, toxF, toxG, toxH, toxI toxJ, and toxR produced amplicons of size 683 bp, 639 bp, 1682 bp, 960 bp, 1069 bp, 576 bp, 1127 bp, 3093 bp, 1496 bp and 833 bp, 1002 bp, respectively (Fig. 4b). All 11 tox genes of BG1 strain (being the most virulent one) were sequenced and the FASTA sequences were end-trimmed, edited, and contigs were assembled using DNA baser (http://www.dnabaser.com/download/DNA-Baser-sequence-assembler) and the partial sequence was submitted to NCBI databank (Table 1). The BLASTn was used to compare these partial sequences to the available sequences in the NCBI database. The toxA, toxD, toxH and toxR gene sequences were found to be 100% identical with the B. glumae strain 336gr-1 (CP023204) of rice from USA; whereas, toxB, toxC, toxF, toxG, toxI and toxJ gene sequences showed 99.81%, 99.73%, 99.48%, 99.31%, 98.99% and 98.77% homology, respectively, to the same strain. The toxE gene sequence exhibited 100% similarity to the closest B. glumae rice strain (AB040403) from Japan as well as other global strains of Burkholderia spp. (Table 3).
Table 3.
Results of tox gene sequence of BG1 strain and gene bank accession numbers using BLASTn algorithm
| Name of gene | Closest related strain in NCBI database | Accession number | Similarity (%) | Geographical origin | Host plant |
|---|---|---|---|---|---|
| toxR | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 100 | South Korea | Rice |
| toxA | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 100 | South Korea | Rice |
| toxB | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 99.80 | South Korea | Rice |
| toxC | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 99.73 | South Korea | Rice |
| toxD | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 100 | South Korea | Rice |
| toxE | Burkholderia glumae toxoflavin biosynthesis related gene cluster | AB040403 | 100 | Japan | Rice |
| toxF | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 99.48 | South Korea | Rice |
| toxG | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 99.31 | South Korea | Rice |
| toxH | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 100 | South Korea | Rice |
| toxI | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 98.99 | South Korea | Rice |
| toxJ | Burkholderia glumae strain 336gr-1 chromosome 2, complete sequence | CP023204 | 98.77 | South Korea | Rice |
Phylogenetic analysis based on tox genes
The tox genes of Indian strain BG1 shared a varied level of similarity with other global strains of Burkholderia spp. For the construction of phylogenetic tree, the 11 tox gene sequences of BG1 strain were aligned individually with the respective tox gene sequences of other strains of Burkholderia spp. (collected from the NCBI database). Based on NCBI data, all the tox family genes were found to be located on chromosome 2. The tox genes showed various groupings in the resultant phylogenetic tree forming two major clads: clad C1 having toxA, toxB, toxC, toxD, toxF, toxG, toxI, toxJ and toxR genes and C2 clustering toxE and toxH genes (Fig. 4d). toxA sequence (ON807151) of Indian BG1 strain grouped with toxA sequence of B. glumae BGR1; similarly, the sequences of toxC, toxD, toxE, toxJ and toxR showed highest similarity to NCBI strain B. glumae BGR1 while sequences of toxF, toxG, toxH and toxI shared highest similarity with B. glumae 336gr-1. All the clusters were supported by high bootstrap probabilities (> 90%).
The sequences of toxA, toxD and toxR showed highest similarity to B. gladioli Co14 and the sequences of toxB and toxC showed highest similarity to B. gladioli ATCC-10248 indicating a close phylogenetic relationship between the two species. The toxG, toxH and toxI genes matched most closely with the respective tox genes of B. gladioli CGB10; whereas, toxE and toxJ genes showed highest matching with respective tox genes of B. gladioli BSR3. However, gene sequence of toxI and toxJ did not form grouping with B. gladioli as the respective gene sequence of B. gladioli showed divergence and grouped with toxC and toxJ, respectively. The gene sequence identity of tox genes with the different NCBI accessions ranged from 98.65 to 100%. The missing homologs and gaps were assessed with other accessions of B. glumae from the NCBI database and are presented in supplementary table 1.
Domain prediction of the tox genes
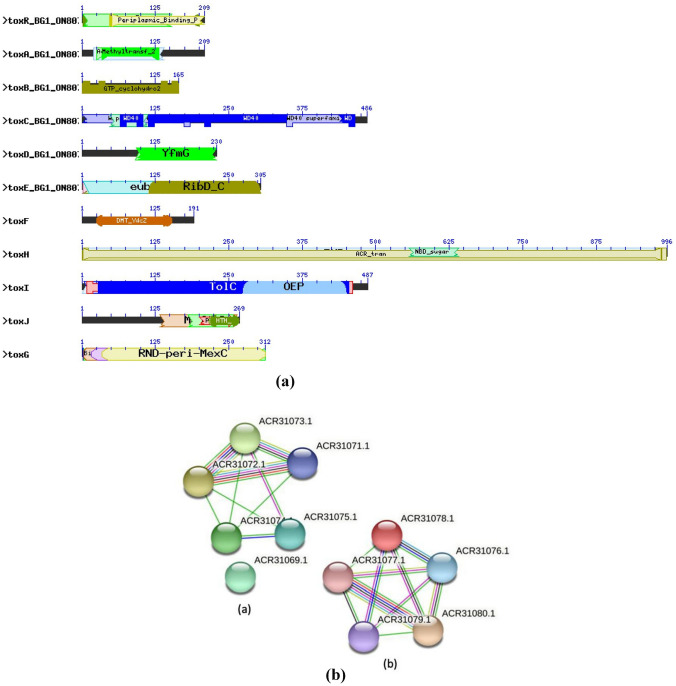
The toxoflavin production pathway shares a common relationship with the riboflavin biosynthetic pathway as both starts with the precursor GTP. The toxoflavin biosynthesis is regulated by a polycistronic operon containing five genes i.e., toxABCDE (Suzuki et al. 2004). The toxA showed domain similarity to methyltransferases which suggested that toxA may have role to catalyze methylation during toxoflavin biosynthesis (Fig. 5a). Likewise, toxB and toxE gene displayed identity with ribA and ribD domain, respectively. Both the domains are known to be associated with riboflavin biosynthesis. Since, the initial steps of toxoflavin and riboflavin biosynthesis are almost similar, it is predicted that toxB and toxE homologs catalyze the first step of toxoflavin biosynthesis (Taura et al. 1992). The toxC gene shared domain similarity to WD40. The WD40 domain functions as a protein–DNA or protein–protein interaction platform. The WD40 domain-containing proteins can form β-propeller structures, thus can serve as a platform for the stable or reversible association of intermediates (Fong et al. 1986). The toxD exhibited identity with FGE-sulfatase domain. The sulfatases are essential for degradation and remodeling of sulfate esters and formylglycine (FGly); hence, it may be involved with toxoflavin production.
Fig. 5.
a Conserved domain structure of tox genes present in B. glumae (BG1). b STRING analysis showing interaction network of a toxABCDE genes b toxFGHIJR genes
The toxF gene shared homology with domain DMT_YdcZ which acts as putative inner membrane exporter. The toxG gene is found to have RND-peri-MexC domain which generally pump out drugs rapidly into the periplasm. The toxH gene is shown to have ACR_tran domain and members of this family are integral membrane proteins; whereas, toxI is predicted to be OEP domain and associated with outer membrane efflux proteins. The toxR gene is found to possess periplasmic binding domain and LysR domain (Fig. 5a). LysR is the transcriptional activator of lysA and the C-terminal substrate-binding domain of LysR exhibits structural similarity to the type 2 periplasmic binding proteins (Maddocks and Oyston 2008). The periplasmic binding proteins (PBPs) are involved in a large number of fundamental processes viz., quorum sensing, transport and chemotaxis.
Based on the domain function, the four genes present in the tox operon toxFGHI are found to be engaged in toxoflavin transport (Kim et al. 2004). Therefore, we can infer that these genes are either involved in the transport of the intermediate product or the final product, i.e., toxoflavin.
STRING analysis for tox genes
Currently, the molecular basis of toxoflavin production by B. glumae is not fully understood; however, preliminary studies on tox operons and comparing the homologous proteins involved in toxoflavin biosynthesis, one can predict the possible molecular interaction that leads to toxoflavin production. As depicted in STRING analysis, the tox operon contains set of five polycistronic genes viz., toxABCDE that are involved in toxoflavin biosynthesis (Fig. 5b). The toxB (ACR31077.1) interacts with toxC (ACR31078.1), toxD (ACR31079.1) and toxE (ACR31080.1). We found possible interaction between toxB and toxE. The toxB and toxE gene exhibited similarity with ribA and ribD domains, respectively; both the domains have high sequence similarity to guanosine triphosphate (GTP) cyclohydrolase II proteins (e.g., RibA and deaminase/reductase proteins, RibD) involved in riboflavin biosynthesis. As toxB and toxE homologs initiate the first steps of riboflavin biosynthesis, so the first step of toxoflavin biosynthesis are predicted to be analogous, with GTP serving as the initial substrate (Bacher et al. 2000). Similarly, toxC showed to interact with toxD; toxC gene is found to be producing WD40 protein. The inherent common function of all WD40-repeat proteins are to coordinate multi-protein complex assemblies with the repeating units acting as a rigid scaffold for protein complexes (Stirnimann et al. 2010); whereas toxD exhibited identity with FGE-sulfatase domain, which is associated with degradation and remodeling of sulfate esters by converting cysteine to a 2-formylglycyl residue (FGly). Thus, toxC and toxD gene singly or together may be associated with coordinating multi-protein complex assemblies or scaffolding of intermediates during toxoflavin synthesis. The toxA gene produces protein belonging to methyltransferase family. There are two methyl groups present in toxoflavin derived from S-adenosylmethionine (Levenberg and Linton 1966), the toxA gene can be predicted to play role in methylations during toxoflavin biosynthesis as out of all tox operon, toxA is the only protein product involved in methyltransferase activity.
Based on the STRING analysis, toxR (ACR31075.1) interacts with toxF (ACR31074.1), toxG (ACR31073.1) and toxH (ACR31072.1). toxR being the first gene of the operon, can play the start point role for the toxin production. Besides being the first gene of the operon, toxR interacts with toxF (inner membrane transporter protein as it contains DMT ydc2 domain), toxG (RND-mfp, i.e., multi-drug efflux transporter) and toxH (putative rnd efflux transporter) which is an indicative that toxR is either an activator of entire operon or involved in toxF, toxG and toxH mediated transport of the toxoflavin produced. Although toxI (ACR31071.1) is not found to be interacting with toxR, but its interaction with other tox genes like toxF, toxG and toxH again implies its role in toxin transportation. Moreover, the other regulator, toxJ (ACR 31069.1) possesses HTH_LuxR domain and not interacting with any other gene. Most luxR-type regulators are associated with quorum sensing and act as transcription activators; hence, it can be predicted that toxJ may be helpful in activation of tox operons. However, both operons require the transcriptional activator toxJ whose expression is controlled by quorum sensing (Goo et al. 2015).
Discussion
The bacterial panicle blight of rice is a newly emerging rice disease occurring in mild to epiphytotic levels in different rice-growing regions of North India (Mondal et al. 2015). Despite preliminary investigation on identification and characterization of pathogen (Mondal et al. 2015; Gowda et al. 2022), no further studies on the disease with reference to virulence profiling of strains and factors governing virulence to B. glumae like toxoflavin. In the present study, phenotypic as well as molecular characterization of the BG strains from hot spot rice-growing areas of North India was carried out. The purified B. glumae colonies exhibited typical grayish-white color on PSA media and yellow on King’s B medium (Fig. 1a, b) confirming their identity as B. glumae. The production of the bright yellow diffusible pigment was indicative of toxoflavin, the key virulence factor associated with B. glumae (Mondal et al. 2015; Ham et al. 2011). In vitro quantification of toxoflavin production by the strains revealed substantial variations across the tested strains of B. glumae. The plant assay using cell-free culture filtrate of B. glumae deciphered the virulence profile of the strains: BG3 and BG1 as hypervirulent; BG2, BG6, BG8 and BG9 as moderately virulent while strains BG5, BG7 and BG10 as hypovirulent (Fig. 2). Interestingly, the virulence grouping (based on plant assay) has direct correlation with quantity of toxoflavin production by the strains, as evident from quantification assay. Such as, based on virulence and in vitro toxoflavin quantification, strains BG1 and BG3 were concurrently categorized as hypervirulent. Hence, we could establish a correlation between higher the toxoflavin production with the higher disease intensity. Our finding further corroborates the fact that toxoflavin is the major virulence factor for B. glumae (Kim et al. 2004). In vitro quantification of toxoflavin, thus, is validated as an important marker to verify the virulence status of a given strain. Likewise, the virulence categorization based on infiltration of cell-free culture filtrate of B. glumae on susceptible rice plants reflects the relative toxoflavin production ability of the strains. Additionally, quantifying the amount of toxoflavin produced together under in vivo is critical in correlating with pathogenicity as plant pathogenic bacteria have evolved to produce varying amount of virulence factors under natural condition (Leonard et al. 2017).
The 16S rRNA from all eleven B. glumae strains were PCR amplified using specific primers that yielded an amplicon of size ≈ 1.50 kb, as previously reported (Mondal et al. 2015). Subsequent analysis with 16S rRNA gene sequence confirmed high degree of similarity among the B. glumae strains, which indicates the high degree of sequence conserveness within species, but variable between species (Takeuchi et al. 1997).
In phylogeny study, strains from similar geographical region grouped into similar clads, i.e., all the strains collected from Uttar Pradesh, namely BG1, BG2, BG3, BG7, BG8 and BG9 grouped together into the clad C1; Likewise, Delhi strains BG4, BG5, BG6 and BG10 and grouped into the clad C2. This resemblance suggests the geographical origin plays important role in determining the genetic make-up of a particular bacterial strain vis-a-vis their 16S rRNA sequences. Similar findings of 16S rRNA and gyrB gene-based grouping in the phylogenetic trees was observed, where all B. glumae strains were found to be similar and belonged to the same clade (Ramachandran et al. 2021).
It is worth noting that no previous reports in India on tox gene-based identification of B. glumae strains have been published. Here, we report the partial sequences of gene clusters, namely toxABCDE, toxFGH and toxJR genes. Toxoflavin biosynthesis is a polycistronic harboring five genes, toxA–toxE (Suzuki et al. 2004). Later another polycistronic operon, toxFGHI coding for toxoflavin transport was reported (Kim et al. 2004). B. glumae strain BG1 exhibited maximum similarity (> 99%) with B. glumae rice strains 336gr-1 from USA (CP023204), from Japan (AB040403) as well as with other global strains. Evidently, the disease had its first report in 2015 in India and was not prevailing until then. The high genetic relatedness to the strains of USA, Japan and USA highlighting the high possibility of across continental movements during seed exchange, as asymptomatic seeds are huge reservoir of B. glumae (Zhu et al. 2008).
The BG1 strain, being one of the virulent strains, was further studied for tox gene analysis. The strains BG1 and BG3 were similar based on 16S rRNA sequences while there was no significant difference in toxoflavin production between them as compared to other strains. BG1 and BG3 strains shared 100% 16S rRNA sequence identity. In virulence screening using culture filtrate, there was slight difference in disease score of BG1 and BG3 strains; however, pathogenic variability analysis using bacterial cell, BG1 was found to be hypervirulent than BG3 (unpublished data). These findings led to the subsequent selection of BG1 for tox gene investigation. The comparative analysis of toxoflavin gene sequence of BG1 strain with the other strains of Burkholderia spp. demonstrated its similarity with other global strains of B. gladioli and B. glumae. This indicates that important virulence factors like tox genes is highly conserved across the strains of B. glumae globally. Similar phylogenetic relationship among the global strains B. gladioli and B. glumae using tox gene was established (Hussain et al. 2020). However, exact biosynthetic pathway of toxoflavin is still unknown. In our study, domain prediction and protein network analysis sheds light on possible role of tox gene intermediates in toxoflavin biosynthesis.
Conclusion
Our study brings an overview on the major Indian B. glumae strains from severely affected rice belts of North India with respect to genetic variability, toxoflavin-producing ability and their correlation with pathogenicity. Further, the most virulent strain BG1 was profiled and phylogenetically analyzed based on 11 tox genes. We were able to group the BG strains into hypovirulent and hypervirulent categories using quantitative analysis for toxoflavin and vis-a-vis disease production ability. Through PCR-based screening, the amplification of all 11 tox genes was indicative of the virulence nature of Indian strain BG1. Moreover, the domain prediction and protein network analysis of tox genes present in the BG1 strain sheds light on molecular networking among tox genes and its intermediates in toxoflavin biosynthesis. Altogether, our study unravels a reliable method for identifying and characterizing B. glumae using tox genes and its relationship with disease production. As the pathogen is of quarantine importance, this knowledge can be used for the monitoring the disease globally. However, comparative genomics approaches with whole genome sequence information would provide better resolution in corroborating the variations among the strains.
Accession numbers
| NCBI Accession number | |
|---|---|
| Submitted 16Sr RNA sequences | |
| BG1 | OP113931 |
| BG2 | OP113932 |
| BG3 | OP113933 |
| BG4 | OP113934 |
| BG5 | OP113935 |
| BG6 | OP113936 |
| BG7 | OP113937 |
| BG8 | OP113938 |
| BG9 | OP113939 |
| BG10 | OP113940 |
| Submitted tox gene sequences | |
| toxR | ON807150 |
| toxA | ON807151 |
| toxB | ON807152 |
| toxC | ON807153 |
| toxD | ON807154 |
| toxE | ON807155 |
| toxF | OP209184 |
| toxG | OP209180 |
| toxH | OP209183 |
| toxI | OP209181 |
| toxJ | OP209182 |
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the Plant Bacteriology lab, Division of Plant Pathology, Indian Agricultural Research Institute, New Delhi for providing all the necessary infrastructure and materials so that the study could be conducted. No external financial assistance was provided for the study.
Author contributions
KKM: Conceptualized the research, supervised, analyzed the data and edited the MS; SK, ALM: Associated with data analysis, writing of draft manuscript; AK Estimation of toxoflavin; SK: performed research activities including BG isolation and DNA extraction; AK, SB, ALM, MS: Virulence analysis, Submission of the tox gene and 16 s sequence data; MS, RER, KNS, SK: amplification of the tox genes, and CM, TG, AK: maintained the BG strain, helped in raising the rice crops and inoculation.
Funding
The authors declare that no funds, grants, or other support were received during the research work and preparation of this manuscript.
Data availability
All the 16srRNA sequence data and tox gene sequence data has been submitted to NCBI under the accession number mentioned in the Table 1.
Declarations
Conflict of interest
The authors declare no conflict of interest/competing interests.
Ethical approval and consent to participate:
Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material complies with relevant institutional (ICAR—Indian Institute of Agricultural Research, New Delhi, India), national, and international guidelines and legislations.
Consent for publication
Not applicable
Contributor Information
Sanjeev Kumar, Email: sanjv007@gmail.com.
Kalyan K. Mondal, Email: mondal_kk@rediffmail.com
Thungri Ghoshal, Email: ghoshal.thungri@gmail.com.
Aditya Kulshreshtha, Email: aditya.ihbt@gmail.com.
B. Sreenayana, Email: sreenayanabhaskar95@gmail.com
M. Amrutha Lakshmi, Email: amruthavvk@gmail.com.
S. Mrutyunjaya, Email: mrutyu.in@gmail.com
E. R. Rashmi, Email: rashmier.6666@gmail.com
N. S. Kalaivanan, Email: kalaivanan.tnau@gmail.com
Aditi Kundu, Email: chem.aditi@gmail.com.
Chandra Mani, Email: cmbarthwal@gmail.com.
References
- Bacher A. Biosynthesis of flavins. In: Muller F, editor. Chemistry and biochemistry of flavoenzymes. Boca Raton: Chemical Rubber Co.; 1991. pp. 215–259. [Google Scholar]
- Bacher A, Eberhardt S, Fischer M, Kis K, Richter G. Biosynthesis of vitamin B2 (riboflavin) Annu Rev Nutr. 2000;20:153–167. doi: 10.1146/annurev.nutr.20.1.153. [DOI] [PubMed] [Google Scholar]
- Brenner DJ, Krieg NR, Staley JT. Bergey’s manual of systematic bacteriology. 2. Springer; 2015. [Google Scholar]
- ChienChang CCYC. The susceptibility of rice plants at different growth stages and of 21 commercial rice varieties to Pseudomonas glumae. Agric Sci China. 1987;36(3):302–310. [Google Scholar]
- Choi O, Kim S, Kang B, Lee Y, Bae J, Kim J. Genetic Diversity and Distribution of Korean Strains of Burkholderia glumae. Plant Dis. 2021;5(5):1398–1407. doi: 10.1094/PDIS-08-20-1795-RE. [DOI] [PubMed] [Google Scholar]
- Cottyn B, Cerez MT, Outryve VMF, Barroga J, Swings J, Mew WT. Bacterial diseases of rice. I. Pathogenic bacteria associated with sheath rot complex and grain discoloration of rice in the Philippines. Plant Dis. 1996;80(4):429–437. doi: 10.1094/PD-80-0429. [DOI] [Google Scholar]
- Fong HK, Hurley JB, Hopkins RS, Miake-Lye R, Johnson MS, Doolittle RF, Simon MI. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci. 1986;83:2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo E, An JH, Kang Y, Hwang I. Control of bacterial metabolism by quorum sensing. Trends Microbiol. 2015;23(9):567–576. doi: 10.1016/j.tim.2015.05.007. [DOI] [PubMed] [Google Scholar]
- GotoOhata K KK. A new bacterial disease of rice (abstract in Japanese) Ann Phytopathol Soc Jpn. 1956;21(1956):46–47. [Google Scholar]
- Gowda HR, Tripathi R, Tewari R, Vishunavat K. Morphological and molecular characterization of Burkholderia glumae causing panicle blight of paddy. Physiol Mol Plant Pathol. 2022;117:101755. doi: 10.1016/j.pmpp.2021.101755. [DOI] [Google Scholar]
- Ham JH, Melanson RA, Rush MC. Burkholderia glumae: Next major pathogen of rice? Mol Plant Pathol. 2011;12:329–339. doi: 10.1111/j.1364-3703.2010.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Shahbaz M, Tariq M, Ibrahim M, Hong X, Naeem F, Khalid Z, Raza HMZ, Bo Z, Bin L. Genome re-seqeunce and analysis of Burkholderia glumae strain AU6208 and evidence of toxoflavin: a potential bacterial toxin. Comput Biol Chem. 2020;86:107245. doi: 10.1016/j.compbiolchem.2020.107245. [DOI] [PubMed] [Google Scholar]
- Iiyama K, Furuya N, Takanami Y, Matsuyama N. A role of phytotoxin in virulence of Pseudomonas glumae Kurita et Tabei. Ann Phytopathol Soc Jpn. 1995;61:470–476. doi: 10.3186/jjphytopath.61.470. [DOI] [Google Scholar]
- Kibbe WA. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 2007;35:W43–W46. doi: 10.1093/nar/gkm234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim JG, Kang Y, Jang JY, Jog GJ, Lim JY, Kim S, Suga H, Nagamatsu T, Hwang I. Quorum sensing and the LysR-type transcriptional activator ToxR regulate toxoflavin biosynthesis and transport in Burkholderia glumae. Mol Microbiol. 2004;54:921–934. doi: 10.1111/j.1365-2958.2004.04338.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Kundu A, Dutta A, Saha S, Das A, Bhowmik A. Chemo-profiling of bioactive metabolites from Chaetomium globosum for biocontrol of Sclerotinia rot and plant growth promotion. Fungal Biol. 2021;125(3):167–176. doi: 10.1016/j.funbio.2020.07.009. [DOI] [PubMed] [Google Scholar]
- Leonard S, Hommais F, Nasser W, Reverchon S. Plantphytopathogen interactions: bacterial responses to environmental and plant stimuli. Environ Microbiol. 2017;19:1689–1716. doi: 10.1111/1462-2920.13611. [DOI] [PubMed] [Google Scholar]
- Levenberg B, Linton SN. On the biosynthesis of toxoflavin, an azapteridine antibiotic produced by Pseudomonas cocovenenans. J Biol Chem. 1966;241:846–852. doi: 10.1016/S0021-9258(18)96842-0. [DOI] [PubMed] [Google Scholar]
- Luo J, Xie G, Li B, Lihui X. First report of Burkholderia glumae straind from symptomless rice seeds in China. Plant Dis. 2007;91:10. doi: 10.1094/PDIA-91-10-1363B. [DOI] [PubMed] [Google Scholar]
- Maddocks SE, Oyston PC. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiol. 2008;154:3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lu F, Marchler GH, Song JS, Thanki N, Wang Z, Yamashita RA, Zhang D, Zheng C, Geer LY, Bryant SH. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45(D1):D200–D203. doi: 10.1093/nar/gkw1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda I, Sato Z. Relation between pathogenicity and pigment productivity in the causal agent of bacterial grain rot of rice. Ann Phytopathol Soc Jpn. 1988;54:378. doi: 10.3186/jjphytopath.54.378. [DOI] [Google Scholar]
- Mondal KK, Mani C, Verma G. Emergence of bacterial panicle blight caused by Burkholderia glumae in North India. Plant Dis. 2015;99(9):1268. doi: 10.1094/PDIS-01-15-0094-PDN. [DOI] [Google Scholar]
- Murray M, Thompson W. Rapid isolation of higher weight DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar R, Shahjahan AKM, Yuan XL, Dickstein ER, Groth DE, Clark CA, Cartwright RD, Rush MC. Burkholderia glumae and B. gladioli cause bacterial panicle blight in rice in the Southern United States. Plant Dis. 2009;93(9):896–905. doi: 10.1094/PDIS-93-9-0896. [DOI] [PubMed] [Google Scholar]
- Ramachandran K, Vijaya SI, Ahmad FN. Characterization and identification of Burkholderia glumae as the causal pathogen of bacterial panicle blight of rice (Oryza sativa L.) in Malaysian rice granaries. J Gen Plant Pathol. 2021;87:164–169. doi: 10.1007/s10327-021-00991-1. [DOI] [Google Scholar]
- Sato Z, Koiso Y, Iwasaki S, Matsuda I, Shirata A. Toxins produced by Pseudomonas glumae. Ann Phytopathol Soc Jpn. 1989;55:353–356. doi: 10.3186/jjphytopath.55.353. [DOI] [Google Scholar]
- Sayler RJ, Cartwright RD, Yang Y. Genetic characterization and real time PCR detection of Burkholderia glumae, a newly emerging bacterial pathogen of rice in the United States. Plant Dis. 2006;90:603–610. doi: 10.1094/PD-90-0603. [DOI] [PubMed] [Google Scholar]
- Schaad NW, Laboratory guide for identification of plant pathogenic bacteria. St. Paul: American Phytopathological Society; 1988. [Google Scholar]
- Singh KM, Jha A, Meena M, Singh R. Constraints of rainfed rice production in India: an overview. In: Shetty PK, Hegde MR, Mahadevappa M, editors. Innovations in rice production (1st edn) Bangalore, India: National Institute of Advance Studies, Indian Institute of Science Campus; 2012. [Google Scholar]
- Stirnimann CU, Petsalaki E, Russell RB, Müller CW. WD40 proteins propel cellular networks. Trends Biochem Sci. 2010;35(10):565–574. doi: 10.1016/j.tibs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Sawada HA, Zegami K, Tsuchiya K. Molecular characterization of the tox operon involved in toxoflavin biosynthesis of Burkholderia glumae. J Gen Plant Pathol. 2004;70:97–107. doi: 10.1007/s10327-003-0096-1. [DOI] [Google Scholar]
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von MC. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Sawada H, Suzuki F, Matsuda I. Specific detection for Burkholderia plantarii and B. glumae by PCR using primers selected from the 16S–23S rDNA spacer regions. Ann Phytopathol Soc Jpn. 1997;63:455–462. doi: 10.3186/jjphytopath.63.455. [DOI] [Google Scholar]
- TamuraNei KM. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Taura T, Ueguchi C, Shiba K, Ito K. Insertional disruption of the nusB (ssyB) gene leads to cold-sensitive growth of Escherichia coli and suppression of the secY24 mutation. Mol Gen Genet. 1992;234:429–432. doi: 10.1007/BF00538702. [DOI] [PubMed] [Google Scholar]
- Zhu B, Lou MM, Huai Y, Xie G, Luo J, Xu L. Isolation and identification of Burkholderia glumae from symptomless rice seeds. Rice Sci. 2008;15:145–149. doi: 10.1016/S1672-6308(08)60033-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the 16srRNA sequence data and tox gene sequence data has been submitted to NCBI under the accession number mentioned in the Table 1.