Abstract
Objective.
To determine the frequency of subclinical synovitis on musculoskeletal ultrasound (MSUS) in Juvenile Idiopathic Arthritis (JIA) and correlate patient- and provider-reported outcome measures with MSUS synovitis.
Methods:
JIA patients with an active joint count (AJC)>4 underwent a 42-joint MSUS performed at baseline and 3 months. B-mode and Power Doppler images were obtained and scored (range 0–3) for each of the 42-joints. Outcomes evaluated included: physician global assessment (PhGA), patient global assessment (PGA), patient pain, Child Health Assessment Questionnaire (CHAQ), and AJC. Subclinical synovitis was defined as synovitis detected by MSUS only. Generalized Estimation Equations were used to test the relationship between clinical arthritis (positive/negative) and subclinical synovitis (positive/negative). Spearman’s correlation coefficients (rs) were calculated to determine the association between MSUS synovitis and patient and physician-reported outcomes.
Results:
In 30 patients subclinical synovitis was detected in 30% of joints. Clinical arthritis of the fingers, wrists, and knee joints was significantly associated with MSUS synovitis of these joints. PGA and the CHAQ had a moderate (rs: 0.44, p=0.014) to weak (rs: 0.37, p=0.045) correlation with MSUS synovitis. There was a statistically significant strong correlation between MSUS synovitis and PhGA (rs: 0.61, p=0.001), but weak correlation with AJC (rs: 0.37, p=0.048) at the follow-up visit.
Conclusion:
Subclinical synovitis was commonly observed in this cohort of JIA patients. The fair to moderate correlation of MSUS synovitis with patient- and provider-reported outcomes suggests that MSUS assesses a different, possibly more objective, domain not determined by traditional JIA outcome measurements.
Introduction
In children, arthritis or a joint with active inflammation is characterized by presence of either joint swelling or a joint with limited range of motion and pain on motion or tenderness on palpation. Clinically-determined arthritis serves as the current standard assessment to diagnose Juvenile Idiopathic Arthritis (JIA) and is an important outcome measure[1]. JIA, the most common rheumatologic disease in children, can result in irreversible joint damage and detrimental outcomes when not treated adequately. Therefore, timely and accurate detection of arthritis is essential to improve the outcome and quality of life of children with JIA. However, the use of the clinician-determined active joint count to inform the decision-making process is potentially problematic for several reasons. First, JIA commonly presents between the ages of 2–6 years. Given patient reported tenderness is a key component of active arthritis (along with limited range of motion), many toddlers and young children are unable to reliably report JIA symptoms which can contribute to joints being overlooked. Second, the presence of common childhood conditions (e.g. joint hypermobility) complicates the assessment of joint mobility and function. Finally, the clinical assessment of arthritis has poor to moderate interrater agreement[2]. Thus, clinical assessment of arthritis may not be sufficient to diagnose active arthritis and potentially lead to adverse outcomes in JIA.
Ultrasound (US) provides a patient-friendly, convenient, and accessible examination. Musculoskeletal ultrasound (MSUS) is a radiation-free, objective imaging tool that can be used to assess joint inflammation. In adults with rheumatoid arthritis, MSUS has become a valid tool for the assessment of arthritis[3]. Previous publications suggest that MSUS is more sensitive than the clinical exam in the assessment of synovitis[4]. In children, the use of MSUS in JIA is evolving. The definitions of sonographic findings of joints in healthy children as well as in children with JIA have been established[5, 6]. In addition, the need for standardized and validated MSUS scanning protocols and scoring systems in pediatrics has been addressed including the recent publication of a detailed joint-specific scanning protocol and scoring system that was found to be highly reliable[7]. We established the MUSICAL (Musculoskeletal UltraSound In Childhood Arthritis Limited) examination, which represents a set of 10 joints that identified 100% of children with synovitis among a more comprehensive US examination (total of 42 joints). To allow quantification of synovitis, the MUSICAL score was developed. Initial construct validation of this score was done through correlation of MSUS findings with validated clinical composite scores. However, the correlation between physical examination, physician and patient reported outcomes and pediatric-specific MSUS scoring systems needs to be further investigated.
The aims of this study are to 1. Determine the frequency of subclinical synovitis in peripheral joints as per MSUS, 2. Evaluate the association between clinical findings of arthritis and MSUS findings of synovitis, and 3. Examine the relationship between MSUS synovitis and standardized physician and patient-reported outcomes among children with active JIA.
PATIENTS AND METHODS
Patients
Children with a diagnosis of JIA and clinical arthritis of 4 or more joints were recruited from the rheumatology clinic of Children’s Health Care of Atlanta, Cincinnati Children’s Hospital Medical Center, and Nationwide Children’s Hospital between November 2017 and August 2019[7]. Clinical arthritis was defined by the presence of either joint swelling or the presence of joint tenderness and limited range of motion. Patients were excluded if they had an intraarticular corticosteroid injection within 4 weeks prior to the baseline visit or had started oral steroids or a conventional or biologic disease-modifying antirheumatic drug (DMARDs) in the week prior to the first MSUS examination. The study was approved by the Institutional Review Board of each center and informed consent was obtained from all patients.
Clinical assessment
A joint examination was done at baseline and 3 months later to assess the active joint count (AJC) or presence of clinical arthritis of the shoulders, elbows, wrists, fingers, hips, knees, ankles, and toe joints and to document presence of tendon involvement or tenosynovitis by a “joint exam certified” pediatric rheumatologist according to the Pediatric Rheumatology Collaborative Study Group (PRCSG)[8]. At baseline, disease subtype according to ILAR categories[7] was recorded. Information collected during each visit included general demographic and disease characteristics, current medications, a pain Visual Analogue Scale (VAS; 0= no pain to 10= maximum pain), patient/parent global assessment (PGA) VAS (0 = very good to 10= very poor), physician global assessment of disease activity (PhGA) VAS (0= no activity to 10= maximum activity), and the Childhood Health Assessment Questionnaire (CHAQ). The CHAQ is a valid, arthritis specific measure that evaluates functional disability[8].
Ultrasound assessment
A complete MSUS examination of 42 joints was done (at both baseline and 3 months follow-up): bilateral shoulder, elbow, wrist, metacarpophalangeal (MCP) 1–5, proximal interphalangeal (PIP) 1–5, hip, knee, ankle and metatarsophalangeal (MTP) 1–5 joints during each study visit. The MSUS examination was performed by an expert American College of Rheumatology Musculoskeletal Ultrasound (ACR RhMSUS) certified pediatric rheumatologist (PVF, TT, EO) who was blinded to physical examination findings and the participant’s symptoms. Real time MSUS examinations were performed at all sites using a General Electric (GE) Ultrasound System Logiq S8 XDclear machine equipped with a 6–15 MHz multifrequency linear transducer and a footprint linear transducer of 4–18 MHz frequency. The setting for B-mode and color-coded power doppler (PD) were standardized among all GE US machines[7]. However, the images of the 3 first subjects enrolled in the study were collected on a MyLab alpha machine (Esaote S.p.A., Genoa, Italy) with a multifrequency linear transducer of 6–18 MHz. Still images for analysis were taken at the point of maximum findings as determined by the sonographer.
Interrater reliability among 4 readers (PVF, TT, EO, JR) was assessed prior to the scoring of the images[7]. Once excellent reliability was met for all views collected (Intraclass correlation coefficient >0.75) the total number of images was equally distributed among the 4 readers for scoring purposes. Readers were blinded to the clinical data and used a pediatric-specific semiquantitative scoring system ranging from 0-normal to 3-severe published recently[7]. Subclinical synovitis was defined as B-mode grade 2 (moderate) and 3 (severe) MSUS findings, or PD-mode of any grade within an area of B-mode findings grade 1 or above in a joint deemed normal on clinical exam.
Statistical analysis
We performed descriptive statistics to characterize the assessments of clinical arthritis and subclinical synovitis using frequencies (percentages) for categorical and dichotomous variables, and means (standard deviations, SD) or medians (interquartile ranges, IR) for continuous variables depending on the distribution of the data. The MSUS models or joint sets (i.e., 42-joint set, reduced joint-set, and MUSICAL examination joint-set) of the MUSICAL study were used to evaluate the correlation between MSUS and patient- and physician-reported outcomes[7]. Briefly, the 42-joint set included all 42-joints scanned. The reduced joint-set identified a combination of 10-joints able to detect 100% of subjects with B-mode synovitis within the 42-joint set. The identification of the key views contributing to the reduced joint-set resulted in the MUSICAL examination joint-set. Data from baseline as well as follow-up visits were used for analysis. Generalized Estimation Equations were used to run a repeated measure logistic regression (controlling for within subject correlation) to test the relationship between clinical arthritis (positive/negative) and subclinical synovitis (positive/negative). Odds ratios and confidence intervals reported from this analysis. Wilcoxon Signed-Rank Test was used to test whether there were significant changes in the number of joints with subclinical synovitis from baseline to follow up visit per each individual patient. Strength of the association between MSUS synovitis, patient and physician-reported outcomes were calculated using Spearman correlation. P-value was considered significant if <0.05[9]. All data were analyzed using SAS v9.4©, Cary, NC.
RESULTS
Of the 30 JIA patients enrolled in the study 70% were female with a median age of 14 years (IQR: 12–16) (Supplementary Table 1). MSUS detected subclinical synovitis in 15% (hip joint) to 40% (MCP joints) of the joints (Figure 1). Not only moderate but also severe findings of MSUS synovitis were present in some joints with subclinical synovitis (Supplementary Figure 1). The presence of joint swelling rather than joint pain and limited range of motion was the main abnormality documented on the joints with clinical arthritis that were not detected by MSUS. Given that this was a cohort of newly diagnosed patients (median duration of arthritis 1 month [CI=0–34]) and treatment was initiated in most of them, the frequency of joints detected with clinical arthritis and subclinical synovitis at the follow-up visit was lower than at baseline (Table 1, Supplementary Figure 2). Most strikingly, only 30% of the ankle joints reported as normal by clinical assessment were found to be normal by MSUS examination. A higher but still fairly low percentage of 40–50% of PIP, MCP, wrist, elbow and knee joints which were normal on clinical exam were also found to be normal on MSUS.
Figure 1.
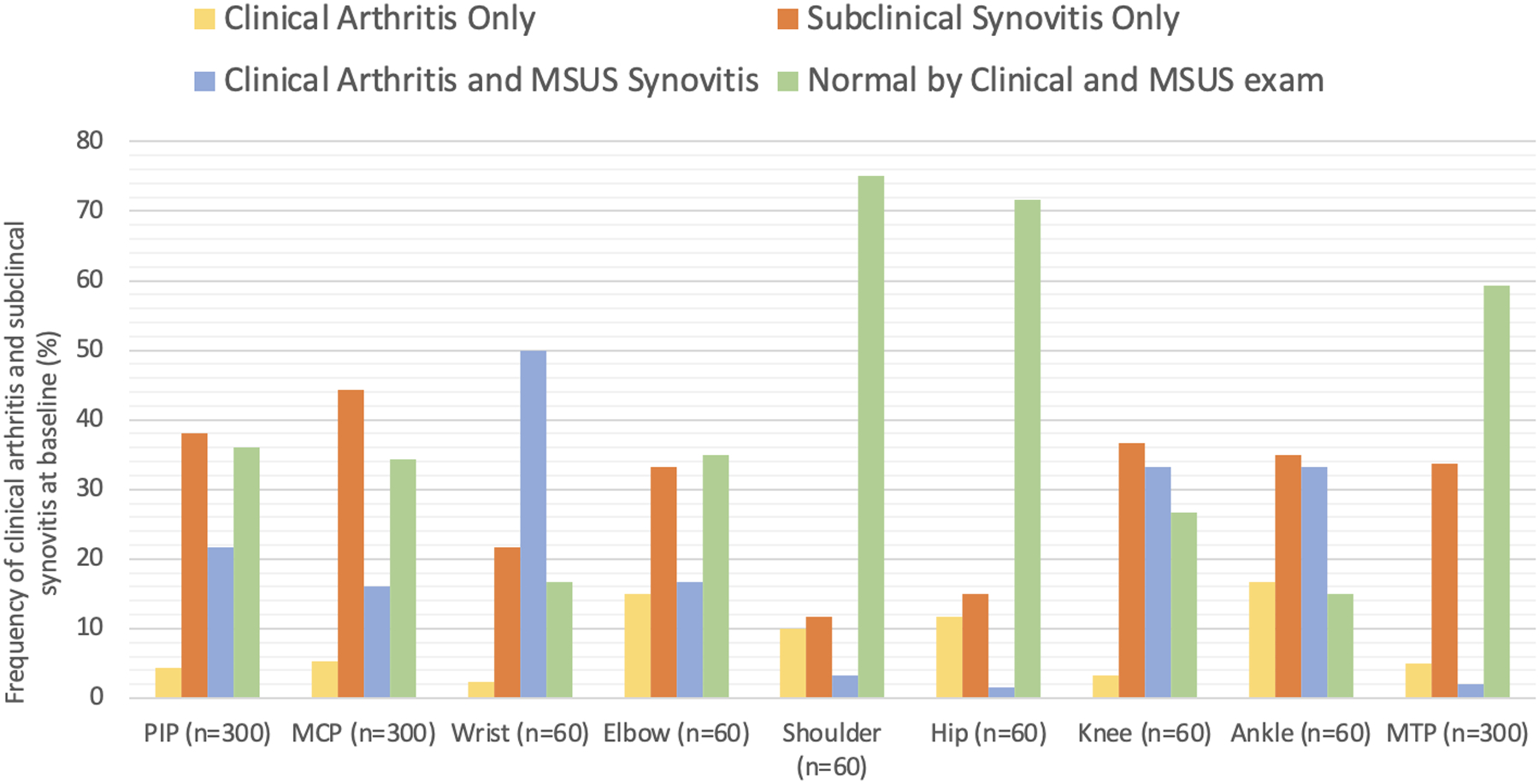
Frequency of clinical arthritis and subclinical synovitis at baseline visit. n = 30 participants. PIP: proximal interphalangeal joint, MCP: metacarpophalangeal joint, MTP: metatarsophalangeal joints. MSUS: musculoskeletal ultrasound
Table 1.
Frequency of Subclinical Synovitis in Peripheral Joints
| Baseline visita | |||
|---|---|---|---|
| Joints with clinical arthritis only (%) | Joints with subclinical synovitis only (%) | Joints with clinical arthritis and MSUS synovitis (%) | |
| PIP (n=300) | 13 (4.3) | 114 (38) | 65 (21.7) |
| MCP (n=300) | 16 (5.3) | 133 (44.3) | 48 (16) |
| Wrist (n=60) | 7 (2.3) | 13 (21.7) | 30 (50) |
| Elbow (n=60) | 9 (15) | 20 (33.3) | 10 (16.7) |
| Shoulder (n=60) | 6 (10) | 7 (11.7) | 2 (3.3) |
| Hip (n=60) | 7 (11.7) | 9 (15) | 1 (1.6) |
| Knee (n=60) | 2 (3.3) | 22 (36.7) | 20 (33.3) |
| Ankle (n=60) | 10 (16.7) | 21 (35) | 20 (33.3) |
| MTP (n=300) | 15 (5) | 101 (33.7) | 6 (2) |
| Follow-up visitb | |||
| Joints with clinical arthritis only (%) | Joints with subclinical synovitis only (%) | Joints with clinical arthritis and MSUS synovitis (%) | |
| PIP (n=290) | 15 (5.2) | 90 (31) | 46 (15.9) |
| MCP (n=290) | 21 (7.2) | 94 (32.4) | 33 (11.4) |
| Wrist (n=58) | 7 (12) | 13 (22.4) | 22 (37.9) |
| Elbow (n=58) | 5 (8.6) | 20 (34.9) | 10 (17.2) |
| Shoulder (n=58) | 3 (5.1) | 7 (12) | 2 (3.4) |
| Hip (n=58) | 4 (6.9) | 4 (6.9) | N/A |
| Knee (n=58) | 1 (1.7) | 17 (29.3) | 12 (20.7) |
| Ankle (n=58) | 8 (13.8) | 15 (25.9) | 15 (25.9) |
| MTP (n=290) | 16 (5.5) | 82 (28.3) | 6 (2) |
Baseline visit involved a total of 30 participants;
Follow-up visit includes 29 participants. PIP: proximal interphalangeal joint, MCP: metacarpal phalangeal joint, MTP: metatarsophalangeal joint, MSUS: musculoskeletal ultrasound, N/A: not applicable.
The MSUS detection of synovitis in clinically active joints varied. For instance, 90% of the active knee joints on clinical exam were found to have MSUS findings of synovitis; however, only 12.5% of hip and 25% of shoulder joints with clinical arthritis were abnormal on MSUS (Table 1). The odds of having MSUS examination findings consistent with synovitis when physical examination is positive for arthritis is presented in Supplementary Table 2. As expected, at follow-up a significant decrease in subclinical synovitis was found especially at the PIP, MCP, knee, and ankle joints, as well as the total joint count per participant (Supplementary Table 3).
Clinically, tenosynovitis was found in the ankle, wrist, and finger tendon group(s). In addition to these tendon groups, MSUS revealed the presence of tenosynovitis in the biceps tendon in the absence of shoulder arthritis. Overall, tenosynovitis was detected more often by MSUS than by physical examination (Supplementary Table 4).
The correlations between MSUS synovitis, patient- and physician-reported outcomes are presented in Table 2. At baseline, there was a statistically significant moderate correlation between PGA and MSUS synovitis particularly with the MUSICAL joint-set, and a weak agreement between the CHAQ with the MUSICAL joint-set. At the follow-up visit, AJC was found to have a weak correlation with the 42-joint MSUS joint-set. PhGA rather than PGA was found to have a moderate correlation with MSUS synovitis as defined by both the 42-joint set and the MUSICAL joint-set.
Table 2.
Correlation Between MSUS Synovitis and Patient- and Physician-Reported Measures of Disease Activity and Damage
| Baseline MSUS synovitis | Follow-up MSUS synovitis | |||
|---|---|---|---|---|
| 42-joint set (p-value) | MUSICAL joint-set (p-value) | 42-joint set (p-value) | MUSICAL joint-set (p-value) | |
| Pain VAS | 0.27 (0.158) | 0.28 (0.145) | 0.02 (0.928) | 0.11 (0.563) |
| PGA | 0.37 (0.047)* | 0.44 (0.014)* | 0.22 (0.248) | 0.30 (0.084) |
| CHAQ | 0.27 (0.153) | 0.37 (0.045)* | 0.04 (0.844) | 0.10 (0.590) |
| AJC | 0.12 (0.528) | 0.24 (0.209) | 0.37 (0.048)* | 0.25 (0.184) |
| PhGA | 0.30 (0.112) | 0.22 (0.254) | 0.61 (<0.001)* | 0.49 (0.017)* |
MSUS synovitis: musculoskeletal ultrasound synovitis defined as B-mode grade 2 and 3 MSUS findings with any PD-mode grade 0, 1, 2, and 3 MSUS findings, Pain VAS: patient pain visual analogue scale, PGA: patient/parent global assessment, CHAQ: Child Health Assessment Questionnaire, AJC: active joint count, PhGA: physician global assessment, 42-joint set: included a total of 42-joint MSUS, MUSICAL joint-set: includes a total of 10-joint MSUS. Strength of the correlation calculated using Spearman correlation as follows: very weak: 0.0–0.19, weak: 0.2–0.39, moderate 0.4–0.59, strong 0.6–0.79, very strong 0.8–1.0.
p-value significant (<0.05).
DISCUSSION
In a cohort of recently diagnosed polyarticular course JIA patients, pathologic sonographic findings consistent with inflammatory arthritis were found in approximately half of the imaged joints. MSUS detected synovitis in approximately 2/3 of joints determined to have arthritis by clinical examination. Subclinical synovitis, defined as the presence of moderate to severe B-mode MSUS synovitis findings, was not detected clinically by an experienced rheumatology provider in about a third of the joints examined. Moreover, about 50% of the joints with subclinical synovitis had severe rather than moderate findings of sonographic synovitis. Pediatric rheumatology providers could therefore be missing marked joint abnormalities in 15% of joints or more. This is especially true for the ankle region which is relatively complex. The frequency of subclinical synovitis observed in our study is in line with previously reported abnormal MSUS findings in 30% of joints assessed as normal by physical examination[4]. However, unlike previous studies and to minimize bias in the interpretation of MSUS findings, we used stringent and standardized interpretation criteria[7].
Previous studies looking to determine the significance of subclinical synovitis in JIA[10, 11] were limited due to the lack of validated and standardized pediatric specific sonographic assessment and scoring systems. They often included heterogeneous study cohorts with a wide range of disease and remission duration. Imaging findings in a patient who entered remission 3 months prior may be different than after 2 years in remission. In the current study the use of pediatric-specific sonographic definitions of synovitis[5], standardized scanning protocols, reliable scoring systems[12] and a longitudinal assessment in a homogenous cohort of newly diagnosed JIA patients with polyarticular onset provides a strong framework to support further studies examining therapeutic and prognostic implications of subclinical synovitis. Growing evidence suggest that timely management of JIA is related to better disease outcomes[13], as imprecise detection of joint involvement and the degree of inflammation in children with JIA may increase the risk of irreversible joint damage, poor disease prognosis and impaired quality of life. The high frequency of subclinical synovitis found in our cohort raises concerns for missed opportunities to implement treatment plans that appropriately addressed the needs of patients and that ultimately may result in improved outcomes. The statistically significant decrease in the frequency of MSUS synovitis from baseline to follow-up suggest that it is a sensitive measurement responsive to therapeutic interventions.
Our study underlines the lack of sensitivity of physical examination detecting tendon involvement. Subclinical tenosynovitis was found in 20% of potential sites. In addition, none of the findings of MSUS tenosynovitis were detected on physical exam - neither as clinical arthritis nor clinical tenosynovitis. Precise establishment of the location of joint and tendon inflammation may have significant treatment implications[14].
Our prior work demonstrated that the MUSICAL joint-set has a statistically significant moderate correlation with the clinical Juvenile Arthritis Disease Activity Score-10 joints (cJADAS-10), a validated disease activity measurement in JIA[7]. The discordance found in this study between patient- and physician-reported measures of disease activity and MSUS, also differing between baseline and follow up for correlations with the same measure, may be related to poor agreement in the PhGA among providers[15]. It also may be due to inherent limitations of these measures to assess disease activity reliably in individual patients as opposed to entire cohorts and highlights the role of MSUS complementing the clinical assessment of any peripheral joint in JIA. Future longitudinal cohort studies are indicated to elucidate the correlation between radiologic findings of synovitis, patient/parent and provider determined measurements of disease activity and chronicity at different disease stages. Magni-Mazoni et al[4] reported low and even negative correlation between MSUS features of synovitis and PGA, pain assessment and PhGA. Similarly, in our study, pain assessment was found not to correlate with MSUS synovitis at either study visit. Cognitive, emotional and situational factors might exaggerate or minimize self-report ratings of pain and wellbeing[16]. MSUS could serve as a more objective outcome measure.
The relatively small sample size without full representation of JIA subtypes used for the present analysis is a limitation of this study. However, our analysis included 1260 and 1218 joints examined by MSUS at enrollment and follow-up visits respectively. If taken by anatomical site, this offers information of 300 MCP, PIP and MTP joints as well as 60 wrist, elbow, shoulder, knee and ankle joints at 2 time points, which to our knowledge is one of the largest pediatric cohorts reported. In an effort to avoid overestimation of the joint disease burden by MSUS, we chose stringent cutoff values to delineate presence of sonographic synovitis following previously established definitions of sonographic features of synovitis in children[5, 6].
The present study suggests that the information provided by standardized MSUS assessment could complement the available instruments for measuring JIA disease activity and therefore strengthen the medical decision-making process to improve the outcomes of children with JIA.
Supplementary Material
Significance and Innovations.
Subclinical synovitis detected by ultrasound is frequently encountered in children with juvenile arthritis.
Clinical arthritis of the finger, wrist, and knee joints is associated with evidence of MSUS synovitis of these joints.
Subclinical synovitis and tenosynovitis is currently underrepresented in the active joint count total for monitoring JIA disease activity. The addition of MSUS may better reflect disease activity.
Ultrasound determined synovitis has strong to weak correlation with patient- and provider-reported outcomes suggesting that ultrasound may provide an important adjunct to the clinical evaluation of the patient.
Acknowledgments
Project funded by a Childhood Arthritis and Rheumatology Research Alliance – Arthritis Foundation Small Grant; Dr. Vega-Fernandez was supported by the Center for Clinical & Translational Science & Training (CCTST) at the University of Cincinnati funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant 2UL1TR001425-05A1; the National Center for Advancing Translational Sciences of the NIH, under Award Number 2KL2TR001426-05A1, and the National Institutes of Arthritis and Musculoskeletal Skin Diseases under Award - Number P30AR076316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors wish to acknowledge CARRA and the ongoing Arthritis Foundation financial support of CARRA.
Footnotes
Financial Interest: The authors have no financial interests to report.
Contributor Information
Patricia Vega-Fernandez, Department of Pediatrics, University of Cincinnati, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Edward J. Oberle, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH, USA.
Michael Henrickson, Department of Pediatrics, University of Cincinnati, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Jennifer Huggins, Department of Pediatrics, University of Cincinnati, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Sampath Prahalad, Department of Pediatrics, Emory University, Division of Rheumatology, Children’s Hospital of Atlanta, Atlanta, GA.
Amy Cassedy, Department of Pediatrics, University of Cincinnati, Division of Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
Johannes Roth, Department of Pediatrics, Children’s Hospital Eastern Ontario, Ottawa, CAN.
Tracy V. Ting, Department of Pediatrics, University of Cincinnati, Division of Rheumatology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio.
References
- 1.Palman J, et al. , Update on the epidemiology, risk factors and disease outcomes of Juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol, 2018. 32(2): p. 206–222. [DOI] [PubMed] [Google Scholar]
- 2.Scott IC and Scott DL, Joint counts in inflammatory arthritis. Clin Exp Rheumatol, 2014. 32(5 Suppl 85): p. S-7–12. [PubMed] [Google Scholar]
- 3.Naredo E, et al. , Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum, 2007. 57(1): p. 116–24. [DOI] [PubMed] [Google Scholar]
- 4.Magni-Manzoni S, et al. , Comparison of clinical versus ultrasound-determined synovitis in juvenile idiopathic arthritis. Arthritis Rheum, 2009. 61(11): p. 1497–504. [DOI] [PubMed] [Google Scholar]
- 5.Roth J, et al. , Preliminary Definitions for the Sonographic Features of Synovitis in Children. Arthritis Care Res (Hoboken), 2017. 69(8): p. 1217–1223. [DOI] [PubMed] [Google Scholar]
- 6.Collado P, et al. , Amendment of the OMERACT ultrasound definitions of joints’ features in healthy children when using the DOPPLER technique. Pediatr Rheumatol Online J, 2018. 16(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vega-Fernandez P, et al. , The MUSICAL pediatric ultrasound examination - a comprehensive, reliable, time efficient assessment of synovitis. Arthritis Care Res (Hoboken), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consolaro A, et al. , Clinical outcome measures in juvenile idiopathic arthritis. Pediatr Rheumatol Online J, 2016. 14(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukaka MM, Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J, 2012. 24(3): p. 69–71. [PMC free article] [PubMed] [Google Scholar]
- 10.De Lucia O, et al. , Baseline ultrasound examination as possible predictor of relapse in patients affected by juvenile idiopathic arthritis (JIA). Ann Rheum Dis, 2018. 77(10): p. 1426–1431. [DOI] [PubMed] [Google Scholar]
- 11.Lanni S, et al. , Ultrasound changes in synovial abnormalities induced by treatment in juvenile idiopathic arthritis. Clin Exp Rheumatol, 2018. 36(2): p. 329–334. [PubMed] [Google Scholar]
- 12.Vega-Fernandez P, T.T., Oberle E, Figueroa J, McCracken C, Roth J, Musculoskeletal Ultrasound Study in Childhood Arthritis: A Limited Examination [abstract]. Arthritis Rheumatol, 2020. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong MS, et al. , Improved disease course associated with early initiation of biologics in untreated polyarticular Juvenile Idiopathic Arthritis: A trajectory analysis of the STOP-JIA study. Arthritis Rheumatol, 2021. [DOI] [PubMed] [Google Scholar]
- 14.Lanni S, et al. , Comparison between clinical and ultrasound assessment of the ankle region in juvenile idiopathic arthritis. Arthritis Care Res (Hoboken), 2020. [DOI] [PubMed] [Google Scholar]
- 15.Taylor J, et al. , Lack of Concordance in Interrater Scoring of the Provider’s Global Assessment of Children With Juvenile Idiopathic Arthritis With Low Disease Activity. Arthritis Care Res (Hoboken), 2018. 70(1): p. 162–166. [DOI] [PubMed] [Google Scholar]
- 16.Stinson JN, et al. , Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain, 2006. 125(1–2): p. 143–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


