Abstract
Objective
To determine the SARS-CoV-2 seroprevalence among school workers within the Greater Vancouver area, British Columbia, Canada, after the first Omicron wave.
Design
Cross-sectional study by online questionnaire, with blood serology testing.
Setting
Three main school districts (Vancouver, Richmond and Delta) in the Vancouver metropolitan area.
Participants
Active school staff enrolled from January to April 2022, with serology testing between 27 January and 8 April 2022. Seroprevalence estimates were compared with data obtained from Canadian blood donors weighted over the same sampling period, age, sex and postal code distribution.
Primary and secondary outcomes
SARS-CoV-2 nucleocapsid antibody testing results adjusted for test sensitivity and specificity, and regional variation across school districts using Bayesian models.
Results
Of 1850 school staff enrolled, 65.8% (1214/1845) reported close contact with a COVID-19 case outside the household. Of those close contacts, 51.5% (625/1214) were a student and 54.9% (666/1214) were a coworker. Cumulative incidence of COVID-19 positive testing by self-reported nucleic acid or rapid antigen testing since the beginning of the pandemic was 15.8% (291/1845). In a representative sample of 1620 school staff who completed serology testing (87.6%), the adjusted seroprevalence was 26.5% (95% CrI 23.9% to 29.3%), compared with 32.4% (95% CrI 30.6% to 34.5%) among 7164 blood donors.
Conclusion
Despite frequent COVID-19 exposures reported, SARS-CoV-2 seroprevalence among school staff in this setting remained no greater than the community reference group. Results are consistent with the premise that many infections were acquired outside the school setting, even with Omicron.
Keywords: COVID-19, Public health, EPIDEMIOLOGY, Epidemiology, Schools
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Robust reporting of seroprevalence based on diagnostic serology testing.
Large school staff sample compared with secondary seroprevalence data from a period, demographic and community-matched reference group of blood donors.
Non-random participation may have contributed to selection bias.
The blood donor group could have differed on unmeasured variables related to risk of infection and seroprevalence outcomes.
Introduction
The COVID-19 pandemic had a dramatic impact on schools early in the pandemic, causing major disruptions to the education system, with mental and physical consequences on students and staff.1 2 Earlier studies suggest that while SARS-CoV-2 outbreaks repeatedly occurred in schools, most infections among students and school staff were acquired outside the school setting.3–9 Accordingly, the risk of infection among school staff remained comparable to the community risk during the 2020–2021 school year, while mitigation measures were in place.8 10 11 At the end of 2021, Omicron variants emerged,12 13 rapidly replacing other strains, and causing a massive increase in cases across the world due to its highly transmissible nature and ability to evade vaccine protection against reinfection.14 15 This new wave of infections raised further concerns regarding the safety of the staff in the school setting.
In British Columbia (BC, Canada), relatively low rates of COVID-19 infections had been reported in the earlier phases of the pandemic compared with other areas of the world. By the summer of 2020, ~1% of people living in Vancouver had been infected with SARS-CoV-2.16–18 By March 2021, about 2%–5% of Vancouver residents showed evidence of prior SARS-CoV-2 infection.19 With the emergence of highly transmissible Omicron variants, the province experienced a massive corresponding increase in cases in December 2021.20 21 As new cases exceeded the provincial testing capacity, BC reprioritised viral testing on selected groups around 17 January 2022. Given this change in wide access to testing, it became nearly impossible for health authorities to accurately track the total number of cases in the community during and after the Omicron wave, and thus, the transmission of SARS-CoV-2 within schools. Consequently, we are not aware of any published data on the risk of SARS-CoV-2 infections in the school setting compared with the corresponding risk of infection in the community after Omicron.
The main objective of this study was to determine the seroprevalence of SARS-CoV-2 infections among the staff of three large school districts within the Greater Vancouver area, after the first Omicron wave in BC and Canada, compared with the community seroprevalence, based on secondary data from blood donors collected over the same period, with the same age, sex and residential area.
Materials and methods
Study design and participants
This study is a cross-sectional analysis of data collected from January to April 2022 as part of the second phase of a prospective longitudinal cohort study.19 Participants that were current, full or part-time district staff members from three school districts (Vancouver, Richmond and Delta) within the Greater Vancouver area were originally recruited from 3 February to 31 May 2021. Staff were excluded at the time of initial recruitment in 2021 if they were temporary, on leave, on call with no classroom time or working exclusively in adult education. Staff whose status changed over the course of the study remained eligible to this study phase, unless they dropped out or asked to be withdrawn, or they retired, in which case they were not able to retain their district email address used for study communication.
For this second study phase, staff who participated in the first study phase and original recruitment in 2021 (n=2538)19 were emailed again on 26 January 2022, with subsequent reminder emails in the following weeks. Recruitment ended on 31 March 2022. Blood samples were collected for serology testing between 27 January and 8 April 2022, shortly after the first Omicron wave in BC (online supplemental figure 1). Comparative community data were obtained from Canadian blood donors who did not have COVID-19 at the time of serology collection, which occurred between 1 January and 31 March 2022.
bmjopen-2022-071228supp001.pdf (545.4KB, pdf)
Study setting
The Vancouver, Richmond and Delta school districts include 186 schools (150 elementary and 36 secondary) distributed in the greater metropolitan area of Vancouver, BC, Canada. Together, they serve a population of ~935 000 (2.6 million people live in the greater urban area). During the 2021–2022 school year, schools remained open all year long, as usual, except for planned holidays. COVID-19 mitigation measures implemented in district schools and indications for viral testing are detailed in online supplemental appendix 1.
Data collection
Questionnaire data
Data on sociodemographic factors, occupation, health status and history of COVID-19 exposures, testing, behaviour (eg, masking) and vaccination were collected from the school staff via an online questionnaire.19 To assess exposure to a COVID-19 case, participants were asked if a household member tested positive for COVID-19 and if anyone outside their household with whom they had close contact (defined as within 2 m and for 2 min or longer) had ever tested positive for COVID-19. Participants were asked if the close contact was a family member from outside the household, a friend, a coworker, a student or someone else (check all that apply). COVID-19 exposures were asked since the beginning of the pandemic (ie, a positive test, vaccination and exposure to a case since January 2020). An additional questionnaire that asked about mental health is not reported in this paper.
Serology testing
Venous blood samples were collected at clinics set up in participating Vancouver schools, at the BC Children’s Hospital or outpatient clinical laboratories in the Greater Vancouver area. Samples were sent to the Canadian Blood Services national laboratory in Ottawa, Canada, for testing. Testing for anti-nucleocapsid (N) protein SARS-CoV-2 antibodies was performed using the Health Canada and Food and Drug Administration-licensed qualitative total antibody Roche Elecsys Anti-SARS-CoV-2 anti-nucleocapsid assay (Roche, USA) on a Cobas e601 analyser. Of note, the same serology assay was used and tested at the same facility for both school staff and blood donors. Specimens were considered reactive at a cut-off index ≥1.00 (online supplemental figure 2). N antibodies persist in blood with assay sensitivity maintained until at least a year after infection.22
Secondary blood donor data
SARS-CoV-2 seroprevalence among blood donor data was obtained through a data request to Canadian Blood Services, on venous blood samples collected at outpatient clinics during routine donations. Seroprevalence data in blood donors living in the same geographical area as the school staff (by first two postal code digits), collected between 1 January and 31 March 2022, were weighted according to a proportionally identical age, sex, sampling month and first two postal code digits distribution as the school sample, with the SE and 95% CIs calculated using the weighted frequencies. Both the school staff and the blood donors were asked to follow the public health recommendations for quarantine duration, if they had active COVID-19, before they could provide a blood sample. Almost all blood donors (~99%) were fully vaccinated, as confirmed also by spike serology testing.
Bias minimisation strategies
To ensure recruitment bias was minimised, we deployed extensive resources in an active recruitment and facilitation strategy to capture a maximum number of school staff over a defined recruitment period. Before the study began, district leaders, teachers and student support workers, and parent associations were engaged actively. Weekly meetings occurred with school district representatives from study launching until publication, allowing to adapt our advertisement strategy with school district in real time during the recruitment phase (eg, reminder emails, etc). For serology sampling, blood collection sites were set up in a variety of geographically dispersed schools over lunch and after work or participants could attend one of over 100 private community clinics open on weekends or the BC Children’s Hospital (for collection both within and outside normal working hours). A full-time study coordinator was hired to maintain contact with participants 7 days/week and facilitate blood collection with flexible hours, including driving around the city to meet the few participants who were unable to attend the blood clinics. Participants received a $20 incentive and their serology results. Based on data from local health authorities on COVID-19 cases by school, participants were evenly distributed across schools with high and low reported COVID-19 cases within the Vancouver district, as we reported earlier.19
Statistical analyses
To estimate the seroprevalence among education workers, a Bayesian analysis was conducted to account for test specificity and sensitivity, and incorporating a hierarchical design to account also for variation in prevalence between school districts. For this analysis, 182 positive out of a total 205 (viral test-positive) infected samples (based on our own unpublished data in a fully vaccinated adult population), and 10 432 negative out of a total 10 453 uninfected samples (obtained before December 2019, based on data from the manufacturer, at: https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html) were incorporated using a binomial likelihood to account for the true evidence of test sensitivity and specificity. Weakly informative priors were selected for the baseline prevalence, logit sensitivity, logit specificity and scale of the regional variance. Two thousand samples were generated from the posterior with 1000 warm-up iterations and no thinning across four chains using the No-U-Turn Sampler (NUTS) sampling algorithm. Models accounting for variation across each school were also produced. Effective sample size, Gelman-Rubin statistic and visual inspection of posterior sample chains were used to determine convergence, mixing and adequate sample size. Results were reported using the expectation under the posterior with 95% credible intervals (95% CrI). Details of the Bayesian model are further explained in online supplemental appendix 2. Bayesian analyses were conducted in R V.4.1.0 and Stan V.2.21.1.
To compare seroprevalence within school types, occupations and quartiles of contact hours with students, separate mixed effects logistic regression models, with school district as a random effect and age and sex as covariates, were used to obtain ORs, 95% CIs and p values. A p value <0.05 was considered statistically significant. Statistical analyses were done on cases with complete data; all variables had <1.0% missing data. Descriptive statistics were run using STATA V.17.0.
Patient and public involvement
During the study we corresponded daily with some of the study participants. District leaders (CO’R) and a liaison (Kathy O’Sullivan) were engaged before and during the study through weekly meetings, until after publication, to facilitate, provide support and seek feedback on study procedures. At the end, results were shared with study participants in a newsletter, with Canadian public health agencies and publicly as a preprint (https://www.medrxiv.org/content/10.1101/2022.07.04.22277230v1).
Results
Characteristics of school staff sample
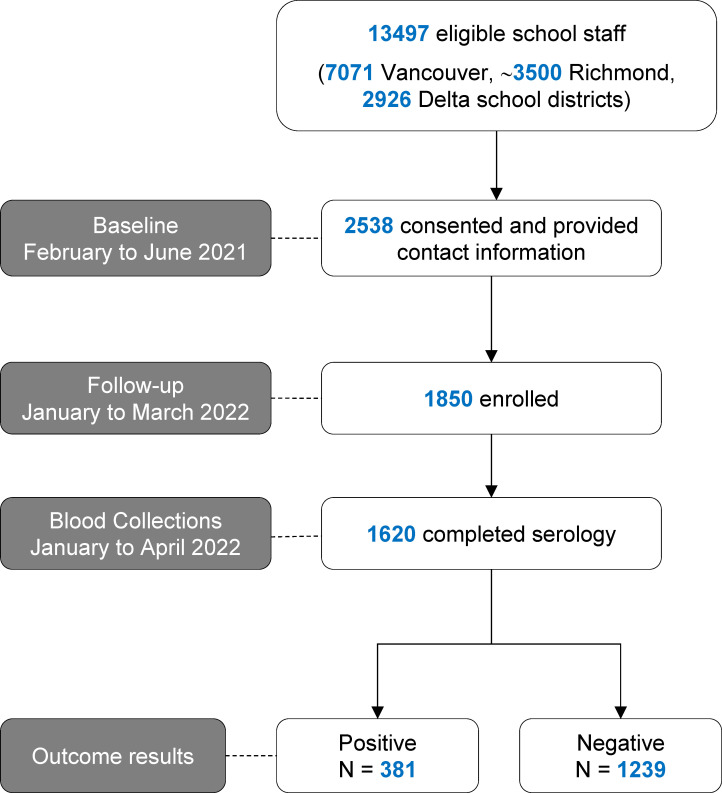
In total, 1850 school staff were enrolled (figure 1). Among enrolled staff, 80.7% (1485/1841 participants with complete data for this variable) were classroom workers with a median contact time with students of 20.0 (IQR: 6.0–30.0) hours/week. Most staff (1809/1830) had received at least two doses of a COVID-19 vaccine at the time of enrolment. The sample included staff from 185 schools out of 186 schools within the three school districts and lacked representation from one elementary school in the Richmond school district. Overall, the school staff enrolled were representative of the school district populations in terms of age, proportion of classroom staff and residence area distribution, except for a higher proportion of females (80.8%) in the school staff sample versus the proportion of females (68.4%) in the entire Vancouver school district population (online supplemental table 1).
Figure 1.
Flow diagram for enrolment of school staff study sample.
COVID-19 exposures among school staff
The potential sources of close contact with COVID-19 cases since the beginning of the pandemic among school staff are shown in table 1.
Table 1.
Self-reported exposure to COVID-19 cases and other markers of potential exposure to COVID-19 at home and at school among school staff
| Variable | n* | School staff (n=1850) |
n* | Serology sample (n=1620) |
| COVID-19 case among household members†, % Yes, n (%) | 1842 | 424 (23.0) | 1612 | 354 (22.0) |
| Live with children <18 years, n (%) | 1845 | 758 (41.1) | 1620 | 666 (41.1) |
| Live with essential worker, n (%) | 1845 | 662 (35.9) | 1610 | 572 (35.5) |
| Occupation of other household members working in an essential service: Agriculture and food production |
11 (1.7%) | 9 (1.6%) | ||
| Community services (waste disposal, emergency response, postal) | 45 (6.8%) | 41 (7.2%) | ||
| Construction, maintenance, skilled trades | 89 (13.4%) | 72 (12.6%) | ||
| Consumer products (hardware, safety, vehicle, sales, garden centres) | 11 (1.7%) | 9 (1.5%) | ||
| Financial services (banking, real estate, insurance) | 20 (3.0%) | 18 (3.2%) | ||
| Food (grocery, convenience, liquor, restaurant) | 68 (16.8%) | 60 (10.5%) | ||
| Healthcare | 111 (16.6%) | 91 (15.9%) | ||
| Social services, education, research | 287 (43.4%) | 253 (44.2%) | ||
| Manufacturing, resources, energy, utilities | 19 (2.9%) | 18 (3.2%) | ||
| Services (pharmacy, gas station, delivery, funeral, vet, etc) | 22 (3.3%) | 19 (3.3%) | ||
| Sports (professional) | 5 (0.8%) | 5 (0.9%) | ||
| Supply chain and transportation | 37 (5.6%) | 32 (5.6%) | ||
| Telecommunications and IT (including the media) | 11 (1.7%) | 10 (1.8%) | ||
| Other | 87 (13.1%) | 77 (13.5%) | ||
| Close contact‡ with a COVID-19 case outside household, n (%) | 1845 | 1214 (65.8) | 1615 | 1045 (64.7) |
| Another school staff member/work colleague | 666 (54.9) | 569 (54.5) | ||
| Student in classroom setting | 625 (51.5) | 533 (51.0) | ||
| Friend | 553 (45.6) | 461 (44.1) | ||
| Family (non-household member) | 417 (34.4) | 349 (33.4) | ||
| Other | 93 (7.7) | 81 (7.8) | ||
| Wear a mask in public places§, % Always or Often, n (%) | 1839 | 1824 (99.2) | 1611 | 1604 (99.6) |
| Coworkers wear masks§, % Always or Usually, n (%) | 1826 | 1776 (97.3) | 1603 | 1560 (97.3) |
| Students wear masks§, % Always or Usually, n (%) | 1702 | 1502 (88.3) | 1491 | 1323 (88.7) |
| Elementary | 1043 | 892 (85.5) | 917 | 788 (85.9) |
| Secondary | 568 | 535 (94.2) | 494 | 472 (95.5) |
*Number of participants with data available for this variable.
†‘Since the last survey, has anyone in your household (not counting yourself) ever tested positive for COVID-19? ([Yes], [Not applicable, I live alone], [No one has been tested], [No, they tested negative], [Not sure, waiting for the result])’.
‡Reported contact with a known COVID-19 case within 2 m and for >2 min.
§Questions about masking were as follows: ‘How often have you worn a mask in public places in the past three months? (Never, Rarely, Occasionally, Often, Always)’; ‘To the best of your knowledge, how often do your co-workers wear a mask in your presence? (Never, Occasionally, Usually, Always)’; ‘To the best of your knowledge, how often do students in your school wear a mask in your presence? (Never, Occasionally, Usually, Always)’.
IT, information technology.
About one-third (662/1845) of staff reported living with an essential worker, 41.1% (758/1845) had children and 23.0% (424/1842) reported a COVID-19 case in the household (table 1). Two-thirds (1214/1845) reported close contact with a COVID-19 case outside the household, of which about half of these close contacts were with a case at school either in a student (625/1214) or coworker (666/1214). Half (960/1845) of school staff reported no close contact with a school case. Most staff reported that coworkers and students wore masks often or always at school in the past 3 months (table 1).
The self-reported cumulative incidence of COVID-19 diagnosed by nucleic acid or rapid antigen testing among the school staff since the beginning of the pandemic was 15.8% (291/1845; table 2).
Table 2.
Cumulative incidence of self-reported COVID-19 infections among school staff
| Variable | n* | School staff (n=1850) |
n* | Completed serology testing (n=1620) |
| Reported having COVID-19 symptoms†, n (%) | 1845 | 301 (16.3) | 1615 | 252 (15.6) |
| Number tested for COVID-19 (PCR), n (%) | 766 (41.5) | 668 (41.4) | ||
| At least one positive COVID-19 PCR test | 120 (6.5) | 99 (6.1) | ||
| More than one positive COVID-19 PCR test | 4 (0.2) | 2 (0.1) | ||
| All negative COVID-19 PCR tests | 639 (35.0) | 565 (35.3) | ||
| Did not know/could not remember test result | 33 (1.8) | 33 (4.3) | ||
| Number who tested positive by rapid test, n (%) | 1842 | 206 (11.8) | 1614 | 165 (10.2) |
| Number who tested positive by any test, n (%) | 1845 | 291 (15.8) | 1615 | 212 (13.1) |
| Hospitalised for COVID-19, n (%) | 1845 | 1 (0.01) | 1615 | 1 (0.06) |
*Participants with non-missing data available.
†Participants who reported that they had COVID-19 symptoms in response to: ‘Do you think you’ve had covid? (Yes/No). If yes, why?’.
One school staff required hospitalisation for COVID-19.
SARS-CoV-2 seroprevalence
Of the 1850 school staff enrolled prospectively, 1620 (87.6%) underwent serology testing with a median testing date of 14 February 2022 (online supplemental figure 1). The characteristics of the 1620 school staff who completed serology testing were representative of the 1850 school staff enrolled (table 3).
Table 3.
Characteristics of school staff sample
| Variable | n* | Completed questionnaire (n=1850) |
n* | Completed serology testing (n=1620) |
| Age (mean±SD) | 1850 | 46.9±10.2 | 1620 | 47.5±10.1 |
| Sex, % female, n (%) | 1842 | 1510 (82.0) | 1616 | 1331 (82.4) |
| Canadians of indigenous origin, n (%) | 1845 | 33 (1.8) | 1617 | 27 (1.7) |
| Ethnicity, n (%) | 1850 | 1620 | ||
| White, Caucasian | 1303 (70.4) | 1118 (69.2) | ||
| South Asian | 68 (3.7) | 59 (3.7) | ||
| Chinese | 309 (16.7) | 288 (17.8) | ||
| Black | 10 (0.5) | 8 (0.5) | ||
| Filipino | 31 (1.7) | 28 (1.7) | ||
| Latin American | 27 (1.5) | 22 (1.4) | ||
| Arab | 5 (0.3) | 3 (0.2) | ||
| Southeast Asian | 31 (1.7) | 28 (1.7) | ||
| West Asian | 1 (0.2) | 3 (0.2) | ||
| Korean | 14 (0.8) | 13 (0.8) | ||
| Japanese | 47 (2.5) | 42 (2.6) | ||
| Other/no answer | 56 (3.0) | 45 (2.8) | ||
| Classroom workers†, n (%) | 1841 | 1485 (80.7) | 1611 | 1292 (80.2) |
| Contact time with students (hours/week), median (IQR)‡ | 1810 | 20.0 (6.0–30.0) | 1585 | 20.0 (6.0–30.0) |
| Vaccination status, n (%) | 1830 | 1613 | ||
| Not vaccinated | 15 (0.8) | 13 (0.8) | ||
| One dose | 6 (0.3) | 6 (0.4) | ||
| Two doses | 249 (13.6) | 213 (13.2) | ||
| Three or more doses | 1560 (85.3) | 1382 (85.6) | ||
| School level, n (%) | 1837 | 1609 | ||
| Elementary | 1081 (58.9) | 947 (58.9) | ||
| Secondary | 587 (32.0) | 509 (31.6) | ||
| Work at multiple levels | 59 (3.2) | 51 (3.2) | ||
| School district office/other | 110 (6.0) | 102 (6.3) | ||
| At least one comorbidity§, n (%) | 1845 | 491 (26.6) | 1620 | 434 (26.8) |
| Smoker, n (%) | 1836 | 26 (1.4) | 1608 | 22 (1.4) |
*Participants with non-missing data available.
†Including participants who reported being a teacher, teacher librarian, resource teacher, student support worker or family and youth worker in response to the question: ‘What is your job title? (Teacher, Teacher Librarian, Resource Teacher, Student Support Worker, Family and Youth Worker, Administrator [Principal, Vice Principal], Administrative Assistant, Maintenance Staff, School Board Office Staff, Other)’, and those who responded ‘Other’ and listed student-facing positions such as student aide, counsellor, speech language pathologist, etc.
‡29 staff indicated that they were on leave at the time they completed the survey and their responses were omitted from the contact time with students variable.
§Included the following conditions: hypertension, diabetes, asthma, chronic lung disease, chronic heart disease, chronic kidney disease, liver disease, cancer, chronic blood disorder, immunosuppression, chronic neurological disorder.
Of the 1620 school staff who underwent serology testing, 381 (23.5%) were positive by nucleocapsid antibodies, with a marginal difference in seroprevalence between females (23.9%; 95% CI 21.6% to 26.3%) and males (21.9%; 95% CI 17.2% to 27.2%). Of those who tested positive, 272 (71.4%) believed they had COVID-19 and 194 (50.9%) reported a previous positive viral test. The sampled posterior for the Bayesian seroprevalence model showed good convergence with Gelman-Rubin statistic 0.999–1 for all parameters. The unadjusted seroprevalence was 23.7% (95% CrI 21.7% to 26.0%) among all school staff, which was comparable after adjustment by school (online supplemental figure 3).
In mixed effects logistic regression models examining group differences in seropositivity by school level, occupation and contact with students, the unadjusted seroprevalence among all school staff was similar to staff working in a classroom setting, between staff working in elementary and secondary schools and among staff categorised by quartile of time spent in contact with students (table 4).
Table 4.
COVID-19 seroprevalence by school education level and occupation
| Seropositivity | ||
| n (%)† | OR* | |
| School level | ||
| Elementary | 220 (23.2) | Reference |
| Secondary | 123 (24.1) | 1.10 (0.85–1.43) |
| Multiple/mixed levels | 11 (21.6) | 0.87 (0.44–1.74) |
| School board office | 21 (24.1) | 1.14 (0.68–1.91) |
| Occupation | ||
| Classroom worker | 306 (23.7) | Reference |
| Other | 73 (22.9) | 1.07 (0.79–1.43) |
| Contact with students (hours/week), quartiles | ||
| <6 | 85 (22.9) | Reference |
| 6 to <20 | 93 (22.0) | 0.91 (0.65–1.27) |
| 20 to <30 | 87 (27.0) | 1.18 (0.83–1.67) |
| >30 | 114 (23.1) | 0.89 (0.64–1.24) |
| Total | 381 (23.5) | n/a |
*Individual mixed effects logistic regression models examining group differences in seropositivity by school level, occupation and contact with students were non-significant (p>0.05).
†Cases by group based on those with non-missing data for each variable (<1% missing data); therefore, cases by variable do not add up to total cases.
n/a, not available.
After taking into account the sensitivity and specificity of the serology test and regional variation, the adjusted seroprevalence was 26.5% (95% CrI 23.9% to 29.3%) among the staff of all school districts and 25.8% (95% CrI 22.9%–28.8%) after weighting by school. In comparison, the period-weighted, sex-weighted, age-weighted and residency location-weighted seroprevalence was 32.4% (95% CrI 30.6% to 34.5%) among 7164 blood donors, after taking into account the sensitivity and specificity of the serology test (online supplemental table 2). When examined for each district separately, seroprevalence rates were similar (online supplemental table 3).
Discussion
This study found that one-quarter of school staff working within three of the largest school districts in the Greater Vancouver area in BC, Canada (serving approximately 83 000 students, and altogether, representing about one-third of all public school staff in BC), showed evidence of a past SARS-CoV-2 infection. The seroprevalence among the school staff was not higher compared with the community, represented by a demographically and regionally similar group of blood donors. To the best of our knowledge, this study is the first to report seroprevalence estimates among school staff after the emergence of the highly transmissible Omicron variants. Findings are in keeping with other Canadian data19 23 and with a systematic review and meta-analysis performed in May 2021, of screening, contact tracing and seroprevalence studies from other areas of the world,24 before the emergence of more transmissible SARS-CoV-2 variants. A major strength of this study is that it used sensitive antibody testing to account for asymptomatic SARS-CoV-2 infections that may not have come to clinical attention, using N-based serology testing,25 in a large, representative Canadian sample of school staff.
About one-quarter of all COVID-19 cases reported in BC during the study period occurred in the regional health authority where the participating school districts are located. Despite a 10-fold increase in seroprevalence among school staff after the first Omicron wave in BC, compared with seroprevalence data obtained during the 2020–2021 school year,19 these findings suggest that the risk of SARS-CoV-2 infection in a highly vaccinated cohort of school workers remained no greater than the risk of infection in a group with similar demographics in the community. These findings contrast with the abundance of COVID-19 cases reported within schools throughout Canada, Europe and the USA and with concerns expressed in the media in BC and around the world throughout the pandemic. Understandably, hearing about COVID-19 cases in schools fuelled a high level of stress among the staff and students attending those schools and their families.2 26 Thus, it is important to provide tangible evidence to support or refute public claims. Despite frequent COVID-19 exposures in schools the data here support that many school COVID-19 cases were acquired outside, rather than within the school setting.8 27 28
The statistically lower seroprevalence among school staff compared with the blood donor reference group in this study should be interpreted with caution. While blood donors are a particularly healthy group and may not be representative of the general population, they are likely representative of school staff compared with other socioeconomic groups at higher risk of COVID-19.29 30 However, it is important to state that the current study was not designed to determine whether the risk of COVID-19 infection in schools could be lower than the community. Potentially, some of the differences may be explained by differences in vaccination rates between school workers and the general population. Most importantly, the CrIs used to present the estimates in each group depend on the sensitivity of the serology tests for which there are little data in the context of a vaccinated population or Omicron. In another study, the crude seroprevalence in residents 30–59 years old of the Vancouver metropolitan area was 44.2% (265/600; 95% CI 40.2% to 48.2%),31 whereas in comparison, it was 36.8% (25/68; 95% CI 25.4% to 49.3%) when we consider only school staff in our study of the same age range sampled over the exact same period between 13 and 24 March 2022. This further supports that the risk of infection among the school staff in this study was no higher than the risk in the community.
Limitations
This study has limitations. First, although we invested substantial efforts to facilitate blood sampling among school staff we cannot exclude a recruitment bias due to the non-random participation. However, the data comparing the staff sample with the whole district workforce suggest that these differences are negligible and likely due to chance. Our sample did include a higher proportion of females than the whole district workforce; in another study where samples were collected between 13 and 24 March 2022 among Vancouver metropolitan area residents, seroprevalence estimates were similar between females (43.1%) and males (41.9%).31 In the current study, crude seroprevalence estimates were also remarkably similar between females and males, so the difference in representation between the school staff sample and the district workforce is unlikely to have significantly impacted the results. Third, data were collected through self-reported questionnaires; therefore, recall biases may have impacted how participants responded, such as when reporting their masking behaviours. Although blood donors tend to be more health conscious than the general population, they are likely representative of school workers. Fourth, we did not obtain data on blood donors, so we cannot exclude that they may have differed by risk of infection. Finally, this study was conducted before mask mandates were lifted in schools in BC, so ongoing monitoring is warranted to determine if these conclusions will continue to hold true in later phases of the pandemic and restriction measures.
Conclusions
In conclusion, this study confirmed that a substantial proportion (26.5%) of sampled school staff working in three Metro Vancouver public school districts were infected with SARS-CoV-2 after a major and first Omicron wave in BC. Taking a conservative approach and considering the limitations of this study, these findings suggest that the risk of SARS-CoV-2 infection among school staff was not significantly higher than the risk of the community after the first wave of infections with Omicron in BC in early 2022.
Supplementary Material
Acknowledgments
We thank the school staff who participated in the study and have been working tirelessly during this pandemic, and the district leadership, particularly Suzanne Hoffman and David Nelson for providing full support during this study; Kathy O’Sullivan for ongoing work reviewing district communications, documents and liaising with school partners throughout the study; Esther Alonso-Prieto for help with the human resource and financial management of this study; Brandon Bates, John Bhullar and the Chemistry Laboratory staff at Children’s and Women’s Hospitals and the BC Children’s Hospital Biobank staff for help with sample collection and processing; the district and BC Children’s Hospital communication teams, VCH’s Office of the Chief Medical Health Officer, the BC Centre for Disease Control; Steven Drews and Qi-Long Yi for consultation with data from Canadian Blood Services. We thank LifeLabs for partnering with us commercially to collect a portion of the blood samples.
Footnotes
Twitter: @PAscal_M_Lavoie
Contributors: LCM and PML obtained funding for this study. AWW, DMG, SMH, MAI, DC, PML and LCM designed the study. LM set up and coordinated the recruitment of participants. EB created the data management platform. BP processed the blood samples under the supervision of PML and VEB. MAI performed the statistical analyses. FR helped review the literature. SFO'B provided the matched data from Canadian blood donors. AWW and MP performed the data analyses. CO'R facilitated the communications within the Vancouver district. AWW and PML wrote the first draft of the manuscript. All other authors revised the manuscript and approved its final version. PML is responsible for the overall content as the guarantor.
Funding: The study was funded by the Government of Canada’s COVID-19 Immunity Task Force (to PML and LCM as coprincipal applicants; award number AWD-016994). PML and LCM received a salary from the British Columbia Children’s Hospital (BCCH) Foundation through the Investigator Grant Award Program. The BC Children’s Hospital Healthy Starts Theme provided some seed funding at the beginning of the study.
Competing interests: CO'R is an employee of the Vancouver School District, but the latter was not involved in the design, analysis, interpretation of data or the drafting of this manuscript. LifeLabs played no role in the study other than providing a service for the collection of blood samples.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Deidentified participant data and data dictionaries will be made available after publication through requests to the Government of Canada’s COVID-19 Immunity Task Force.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by The University of British Columbia Children’s and Women’s Research Ethics Board (H20-03593). Participants gave informed consent to participate in the study before taking part.
References
- 1.Levinson M, Cevik M, Lipsitch M. Reopening primary schools during the pandemic. N Engl J Med 2020;383:981–5. 10.1056/NEJMms2024920 [DOI] [PubMed] [Google Scholar]
- 2.Hutchison SM, Watts A, Gadermann A, et al. School staff and teachers during the second year of COVID-19: higher anxiety symptoms, higher psychological distress, and poorer mental health compared to the general population. J Affect Disord Rep 2022;8:100335. 10.1016/j.jadr.2022.100335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macartney K, Quinn HE, Pillsbury AJ, et al. Transmission of SARS-Cov-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc 2020;4:807–16. 10.1016/S2352-4642(20)30251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yung CF, Kam K-Q, Nadua KD, et al. Novel Coronavirus 2019 transmission risk in educational settings. Clin Infect Dis 2021;72:1055–8. 10.1093/cid/ciaa794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ismail SA, Saliba V, Lopez Bernal J, et al. SARS-Cov-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis 2021;21:344–53. 10.1016/S1473-3099(20)30882-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-Cov-2 infections in schools. Pediatrics 2021;147. 10.1542/peds.2020-048090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma JK, Thamkittikasem J, Whittemore K, et al. COVID-19 infections among students and staff in New York city public schools. Pediatrics 2021;147:e2021050605. 10.1542/peds.2021-050605 [DOI] [PubMed] [Google Scholar]
- 8.Bark D, Dhillon N, St-Jean M, et al. SARS-Cov-2 transmission in kindergarten to grade 12 schools in the Vancouver Coastal health region: a descriptive epidemiologic study. CMAJ Open 2021;9:E810–7. 10.9778/cmajo.20210106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullard J, Funk D, Dust K, et al. Infectivity of severe acute respiratory syndrome Coronavirus 2 in children compared with adults. CMAJ 2021;193:E601–6. 10.1503/cmaj.210263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladhani SN, Baawuah F, Beckmann J, et al. SARS-Cov-2 infection and transmission in primary schools in England in June-December, 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health 2021;5:417–27. 10.1016/S2352-4642(21)00061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres JP, Piñera C, De La Maza V, et al. Severe acute respiratory syndrome Coronavirus 2 antibody prevalence in blood in a large school community subject to a Coronavirus disease 2019 outbreak: a cross-sectional study. Clin Infect Dis 2021;73:e458–65. 10.1093/cid/ciaa955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karim SSA, Karim QA. Omicron SARS-Cov-2 variant: a new chapter in the COVID-19 pandemic. Lancet 2021;398:2126–8. 10.1016/S0140-6736(21)02758-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Cheng G. Sequence analysis of the emerging SARS-Cov-2 variant Omicron in South Africa. J Med Virol 2022;94:1728–33. 10.1002/jmv.27516 [DOI] [PubMed] [Google Scholar]
- 14.Lu L, Mok BW, Chen LL, et al. Neutralization of SARS-Cov-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin Infect Dis 2021. 10.1093/cid/ciab1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-Cov-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022;399:924–44. 10.1016/S0140-6736(22)00152-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majdoubi A, Michalski C, O’Connell SE, et al. A majority of uninfected adults show preexisting antibody reactivity against SARS-Cov-2. JCI Insight 2021;6:e146316. 10.1172/jci.insight.146316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skowronski DM, Sekirov I, Sabaiduc S, et al. Low SARS-Cov-2 Sero-prevalence based on Anonymized residual sero-survey before and after first wave measures in British Columbia, Canada, March-May 2020. Infectious Diseases (except HIV/AIDS) [Preprint] 2020. 10.1101/2020.07.13.20153148 [DOI]
- 18.Saeed S, Drews SJ, Pambrun C, et al. SARS-Cov-2 Seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion 2021;61:862–72. 10.1111/trf.16296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldfarb DM, Mâsse LC, Watts AW, et al. SARS-Cov-2 Seroprevalence among Vancouver public school staff in British Columbia, Canada: a cross-sectional study. BMJ Open 2022;12:e057846. 10.1136/bmjopen-2021-057846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reedman CN, Drews SJ, Yi Q-L, et al. Changing patterns of SARS-Cov-2 Seroprevalence among Canadian blood donors during the vaccine era. Microbiol Spectr 2022;10:e00339-22. 10.1128/spectrum.00339-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-19 Immunity Task Force . Seroprevalence in Canada. 2021. Available: https://www.covid19immunitytaskforce.ca/results-blood-donation-organizations/ [Accessed 31 Mar 2023].
- 22.Favresse J, Douxfils J. Evaluations of SARS-Cov-2 serological assay performance need inclusion of long-term samples. J Clin Microbiol 2021;59:e00487-21. 10.1128/JCM.00487-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman BL, Fischer K, Maunder R, et al. Study of the epidemiology of COVID-19 in Ontario elementary and secondary school education workers: an interim analysis following the first school year. Can J Public Health 2022;113:185–95. 10.17269/s41997-022-00613-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caini S, Martinoli C, La Vecchia C, et al. SARS-Cov-2 circulation in the school setting: a systematic review and meta-analysis. Int J Environ Res Public Health 2022;19:5384. 10.3390/ijerph19095384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunau B, O’Brien SF, Kirkham TL, et al. A prospective observational cohort comparison of SARS-Cov-2 seroprevalence between paramedics and matched blood donors in Canada during the COVID-19 pandemic. Ann Emerg Med 2022;80:38–45. 10.1016/j.annemergmed.2022.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts AW, Hutchison SM, Bettinger JA, et al. COVID-19 vaccine intentions and perceptions among public school staff of the greater Vancouver metropolitan area, British Columbia, Canada. Front Public Health 2022;10:832444. 10.3389/fpubh.2022.832444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzpatrick T, Wilton A, Cohen E, et al. School reopening and COVID-19 in the community: evidence from a natural experiment in Ontario, Canada. Health Affairs 2022;41:864–72. 10.1377/hlthaff.2021.01676 [DOI] [PubMed] [Google Scholar]
- 28.Choi A, Mâsse LC, Bardwell S, et al. Symptomatic and asymptomatic transmission of SARS-Cov-2 in K-12 schools, British Columbia, April to June 2021. Microbiol Spectr 2022;10:e00622–22. 10.1128/spectrum.00622-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro Dopico X, Muschiol S, Christian M, et al. Seropositivity in blood donors and pregnant women during the first year of SARS-Cov-2 transmission in Stockholm, Sweden. J Intern Med 2021;290:666–76. 10.1111/joim.13304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant R, Dub T, Andrianou X, et al. SARS-Cov-2 population-based Seroprevalence studies in Europe: a Scoping review. BMJ Open 2021;11:e045425. 10.1136/bmjopen-2020-045425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skowronski DM, Kaweski SE, Irvine MA, et al. Serial cross-sectional estimation of vaccine-and infection-induced SARS-Cov-2 Seroprevalence in British Columbia, Canada. CMAJ 2022;194:E1599–609. 10.1503/cmaj.221335 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-071228supp001.pdf (545.4KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Deidentified participant data and data dictionaries will be made available after publication through requests to the Government of Canada’s COVID-19 Immunity Task Force.