Abstract
Purpose
Low-dose naltrexone (LDN) has increased in popularity as a non-opioid medication that may decrease chronic pain symptoms. LDN is most commonly used to treat fibromyalgia, complex regional pain syndrome (CRPS), and painful diabetic neuropathy. Other studies suggest that LDN provides general symptom reduction in inflammatory conditions such as Crohn’s disease and multiple sclerosis. We reviewed our experience with patients to whom we have prescribed LDN to see what types of painful conditions were most responsive to LDN in our patient population.
Patients and Methods
Charts from patients who came to the Pain Center between 2014 and 2021 were reviewed.
Results
Of the n = 137 patients who were prescribed LDN, 44% had no evidence of ever filling the prescription, and 4.4% of the responses were not charted. Of the remaining who took LDN (n = 70), 64% had some relief and were designated as ‘Responders’. The most common pain diagnosis was neuropathic pain which, when added to the diagnosis of complex regional pain syndrome, accounted for 51% of responders to LDN. Patients who experienced greater than 50% pain relief from LDN were more likely to have the diagnosis of neuropathic pain or complex regional pain syndrome (p = 0.038, Fisher’s Exact Test). There was a significant difference in the diagnosis of patients who responded to LDN. Patients with spondylosis were much less likely to respond to LDN when compared with other diagnoses (p = 0.00435, Chi-Square Test).
Conclusion
Patients with all types of neuropathic pain, including CRPS, were significantly more likely to have pain relief from LDN than patients with spondylosis (p=0.018). The diagnosis of spondylosis was more often associated with a lack of response to LDN than any other diagnosis. Patients may need to have a trial of several weeks before analgesic effects are seen with LDN.
Keywords: low-dose naltrexone, chronic pain, non-opioid analgesia
Introduction
Naltrexone is an oral opioid antagonist, that is used for the treatment of substance use disorder. There is no analgesic indication for naltrexone at the standard 50mg dose. Low-dose naltrexone (LDN), an off-label dose of naltrexone (1–5mg), has been used to treat chronic conditions including chronic pain.1,2 The mechanism of analgesic action of low-dose naltrexone is unclear. Theories about why naltrexone has analgesic properties at low, but not the standard, doses include a rebound increase in opioid receptors after limited receptor blockade by LDN, and anti-inflammatory properties related to LDN’s Toll-Like Receptor 4 (TLR4) antagonism.1 A randomized controlled trial compared LDN’s efficacy to that of tricyclic antidepressants for painful diabetic neuropathy, while another suggested that LDN may reduce fibromyalgia symptoms and complex regional pain syndrome.2,3 A retrospective review suggested LDN is a broad-spectrum analgesic, but it is not clear which type of chronic pain is best suited for a trial of LDN.4 LDN appears to relieve symptoms in patients with inflammatory conditions, such as multiple sclerosis and inflammatory bowel disease.5
We present our experience with the use of LDN for the relief of chronic pain symptoms. The primary hypothesis of this retrospective study was that there are differences in the percentage of patients experiencing pain relief based on type of pain diagnosis. The secondary hypothesis was that side effects would be minimal.
Materials and Methods
After institutional review board approval, we performed an exploratory retrospective chart review of patients in the Emory Pain Center who received LDN prescriptions between 2014 and 2021. We reviewed the records of all patients with prescriptions for LDN and recorded the pain diagnosis, self-reported pain relief, LDN dose, and side effects. Patients who reported at least “some benefit” in terms of pain relief were termed ‘Responders’. Those who reported unsatisfactory to no benefit were termed “Non-responders”. We noted the time between the initial prescription and the first report of pain relief. LDN prescriptions were filled in a compounding pharmacy, and a three-month prescription was used to reduce patient costs. Compounding pharmacies made LDN capsules of differing strengths; doses were titrated from 1 or 1.5mg up to 4 or 4.5mg daily. LDN tablets were later compounded, allowing the flexibility of LDN dose titration with half tablets. Patients on LDN were not prescribed opioids during the time they were taking LDN and were informed that LDN is an opioid antagonist. All data were analyzed with SAS 9.4 (Cary, NC). Comparisons were made with the Fisher's Exact Test or the Chi-Square Test.
Results
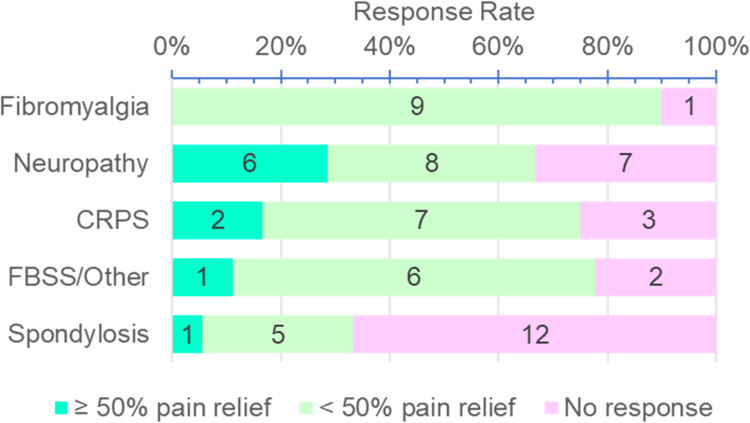
One hundred thirty-seven patients at the Pain Center were prescribed LDN over the seven-year period. The vast majority (83%) were women. Of the patients who were prescribed LDN, 44% had no evidence of ever filling the prescription, and 4.4% of the responses were not charted. Of the remaining who took LDN (n = 70), 64% had at least some relief and were designated as “Responders”. We documented the pain diagnoses of all the patients who received LDN (Table 1). Although the diagnosis of complex regional pain syndrome (CRPS) is a form of neuropathic pain, CRPS was separated out, given previous reports of pain relief by LDN in this specific patient population. The top three diagnoses of Responders included neuropathic pain (31%), complex regional pain syndrome (20%), and fibromyalgia (20%) (Figure 1). Half (48%) of the Non-responders were diagnosed with spondylosis, making it the most common diagnosis of those who did not respond to LDN. The most common pain diagnosis of Responders was neuropathic pain which, when added to the diagnosis of complex regional pain syndrome, accounted for 51% of responders to LDN. Patients who experienced ≥50% pain relief from LDN were more likely to have the diagnosis of neuropathic pain or CRPS (p = 0.038, Fisher's Exact Test). There was a significant difference in the diagnosis of patients who responded to LDN. Patients with spondylosis were much less likely to respond to LDN when compared with other diagnoses (p = 0.00435, Chi-Square Test). Patients with all types of neuropathic pain, including CRPS, were significantly more likely to have pain relief than patients with spondylosis (p = 0.018, Fisher's Exact Test). It took more than a month for the majority of patients to get initial pain relief. Most (72%) of the patients responded in a time frame between one and three months and 12% did not get pain relief until after three months of taking LDN. Of the patients who had pain relief, six reported side effects, which resolved spontaneously or abated by changing the time of day that the medication was taken. The half-life of LDN is six hours and there is a theoretical rebound increase in endorphins, opioid receptor number and efficacy after six hours that results in pain relief. Taking LDN at night takes advantage of this proposed mechanism, enhancing the effect of LDN during the day. Side effects, such as difficulty sleeping or vivid dreams, were usually resolved by switching to daytime dosing. There is no evidence that LDN is more effective when dosed during the day or at night. The most common side effect was vivid dreams; conversely, three patients reported that LDN improved their sleep.
Table 1.
Characteristics of Responders and Non-Responders
| Variable, N (%) | Responder | Non-Responder | Total | p-value | |||
|---|---|---|---|---|---|---|---|
| >50% Relief | <50% Relief | Total | |||||
| TOTALa | 10 (14%) | 35 (50%) | 45 (64%) | 25 (36%) | 70 | ||
| Pain Diagnosis | 0.0045b | ||||||
| Neuropathic pain/ neuropathy/radicular pain | 6 (29%) | 8 (38%) | 14 (67%) | 7 (33%) | 21 | ||
| CRPS | 2 (17%) | 7 (58%) | 9 (75%) | 3 (25%) | 12 | ||
| Fibromyalgia | 0 | 9 (90%) | 9 (90%) | 1 (10%) | 10 | ||
| FBSS | 1 (14%) | 4 (57%) | 5 (71%) | 2 (29%) | 7 | ||
| Spondylosis/axial LBP | 1 (6%) | 5 (28%) | 6 (33%) | 12 (67%) | 18 | ||
| Cancer Pain | 0 | 1 (100%) | 1 (100%) | 0 | 1 | ||
| Other | 0 | 1 (100%) | 1 (100%) | 0 | 1 | ||
| Sex | 1.0c | ||||||
| Male | 3 (27%) | 4 (36%) | 7 (64%) | 4 (36%) | 11 | ||
| Female | 7 (12%) | 31 (53%) | 38 (64%) | 21 (36%) | 59 | ||
| Length of Time | To First Response | To Stop | NA | ||||
| < 1 month | 7 (16%) | 7 (28%) | 14 | ||||
| 1–3 months | 31 (72%) | 13 (52%) | 44 | ||||
| 3–6 months | 5 (12%) | 3 (12%) | 8 | ||||
| 6–9 months | 0 | 2 (8%) | 2 | ||||
| Not Documented | 2 (NA) | 2 | |||||
| Side Effects | 6 (+3) | 6 | NA | ||||
| Vivid Dreams | 3 | ||||||
| Nightmares | 1 | ||||||
| Night Sweats | |||||||
| Allergy | |||||||
| Difficulty Sleeping | 1 (d/c’d) | ||||||
| Rash | 1 (d/c’d) | ||||||
| Nausea | 2 (d/c’d) | ||||||
| Headaches | 1 | ||||||
| Chest Heaviness | 1(d/c’d) | ||||||
| Face Twitching | 1 (d/c’d) | ||||||
| Other | 1 | ||||||
| Improved Sleep | 3 | ||||||
Notes: aN=70 patients total, of whom n=25 (36%) had no response and n=45 (64%) had a response. Of the n=45 who had a response, n=10 (14% of total) had ≥50% response while n=35 (50% of total) had <50% response. bChi-Square Test: Response 2 groups; Disease in 3: spondylosis, fibromyalgia, other. Excludes cancer/other. cFisher’s Exact Test, Responder vs Non-responder.
Abbreviations: FBSS, Failed Back Surgery Syndrome; LBP, Lower Back Pain; CRPS, Complex Regional Pain Syndrome. d/c’d, Discontinued LDN due to side effect.
Figure 1.
LDN response rate. Spondylosis patients were significantly less likely to show a positive response to Low-Dose Naltrexone than patients with other pain types (p=0.00435, Chi-Square Test).
Abbreviations: CRPS, Chronic Regional Pain Syndrome; FBSS, Failed Back Surgery Pain Syndrome.
Discussion
LDN has been associated with symptom relief in some autoimmune, inflammatory and pain conditions.6 TLR4 inhibition by LDN is one of the proposed mechanisms for LDN’s analgesic properties.5 TLRs are transmembrane pattern recognition receptors that can be found on microglia and are involved in central immune signaling. In addition to pattern recognition, TLR receptors can be activated by endogenous ligands and are involved in the body’s response to inflammation and pain.7 TLR4 is thought to have an important role in neuropathic pain and has been described as an emerging therapeutic target for chronic pain.7,8 LDN has long been thought to relieve pain with rebound increase in endogenous opioids after temporary opioid receptor blockade.1 A recent study has cast doubt on this proposed mechanism of action, finding no evidence of LDN’s effect on opioid receptor sensitivity, production of β-endorphin precursor or β-endorphin release in the plasma.9 This leaves TLR4 inhibition as a significant mechanism of action for analgesia with LDN.
In our study, neuropathic pain was the most common pain diagnosis associated with pain relief. Neuropathic pain and CRPS together accounted for over half of the patients who responded to LDN. In the randomized, double-blind, placebo-controlled trial of LDN for the treatment of pain, fibromyalgia patients had overall symptom improvement with significant improvement in mood and life satisfaction and modest reduction in pain.10 We also observed this in many of our patients who remarked they felt overall improved symptoms that contributed to their quality of life, yet their reduction in pain score was modest. This makes analgesia associated with LDN particularly challenging to quantify as it is often difficult to separate from an overall sense of wellbeing. We used the term “Responder” to include patients who reported some pain improvement. We then quantified patients who reported at least 50% improvement in their pain to see who had the most benefit from LDN. Arthritic conditions, such as spondylosis, were less likely to respond to LDN in comparison with other diagnoses and patients with all types of neuropathic pain, including CRPS, were significantly more likely to have ≥50% pain relief over other types of pain.
When we looked more closely at Responders, we found that most did not see the analgesic effects of the LDN for 1 to 3 months, with some patients (12%) taking over three months to start seeing effects. This has changed the way that we discuss LDN with our patients. Naltrexone has no therapeutic indication in the United States, aside from treatment of substance use disorder. The off-label dose of 1–5 mg of naltrexone (LDN) is compounded and not currently covered by most insurance companies or available in retail pharmacies. Having to pay out of pocket was one of the reasons some patients stated to one of the authors (AMMB) that they decided not to fill the LDN prescription. When prescribing LDN, we prescribe as a three-month prescription, as that is the least expensive method. We encourage patients to complete the prescription before deciding on its efficacy as we have noted that it may take several weeks in some patients to get effect. We feel that informing patients about this aspect of LDN has increased the number of patients in our clinic who have received benefit from LDN. We have also narrowed the patient conditions that we treat with LDN to patients with fibromyalgia, pain associated with inflammatory conditions, such as multiple sclerosis, and neuropathic pain conditions.
There are limitations to this study, including missing data points inherent to a retrospective case series. Many did not return to the clinic and were lost to follow-up. Due to the retrospective nature of the study, documentation of pain relief was inconsistent and often described in adjectives. Many of these patients were also on other non-opioid pain medications. If the patient was taking LDN but there was no specific attribution of their symptom improvement to LDN, we did not count their pain relief as related to LDN. This may have resulted in underestimating LDN efficacy. Because LDN is rarely covered by insurance, we often switched pharmacies to find the most cost-effective choices for our patients.
Conclusion
LDN has been used to relieve symptoms in certain inflammatory pain conditions and has been most commonly associated with the treatment of symptoms from fibromyalgia. A barrier to the use of LDN is that it is currently not covered by most insurance companies. There may be benefit to advising patients at the time of initial LDN prescribing that it may take several weeks before any noticeable analgesic effect is seen. LDN was very well tolerated in our patient population. The diagnosis of spondylosis was most likely to be associated with a lack of analgesic response to LDN. Patients with neuropathic pain, including CRPS, were most likely to get pain relief from LDN, and were also most likely to have more than 50% reduction in their reported pain symptoms. LDN appears to be a safe alternative to other non-opioid medications for certain types of pain, including neuropathic pain, CRPS and fibromyalgia.
Funding Statement
No external funding sources.
Ethics and Consent
IRB Approval: IRB #104165. An IRB Waiver of Consent was obtained in accordance HHS’ HIPPA regulation by the Emory Institutional Review Board (IRB). Our manuscript complies with the Declaration of Helsinki.
Disclosure
No conflicts of interest reported by any of the authors in this work.
References
- 1.Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33(4):451–459. doi: 10.1007/s10067-014-2517-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatfield E, Phillips K, Swidan S, Ashman L. Use of low-dose naltrexone in the management of chronic pain conditions: a systematic review. J Am Dent Assoc. 2020;151(12):891–902.e1. doi: 10.1016/j.adaj.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan A, Dutta P, Bansal D, Chakrabarti A, Bhansali AK, Hota D. Efficacy and safety of low-dose naltrexone in painful diabetic neuropathy: a randomized, double-blind, active-control, crossover clinical trial. J Diabetes. 2021;13(10):770–778. doi: 10.1111/1753-0407.13202 [DOI] [PubMed] [Google Scholar]
- 4.Martin SJ, McAnally HB, Okediji P, Rogosnitzky M. Low-dose naltrexone, an opioid-receptor antagonist, is a broad-spectrum analgesic: a retrospective cohort study. Pain Manag. 2022;12:699–709. doi: 10.2217/pmt-2021-0122 [DOI] [PubMed] [Google Scholar]
- 5.Kim PS, Fishman MA. Low-dose naltrexone for chronic pain: update and systemic review. Curr Pain Headache Rep. 2020;24(10):64. doi: 10.1007/s11916-020-00898-0 [DOI] [PubMed] [Google Scholar]
- 6.Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72(3):333–337. doi: 10.1016/j.mehy.2008.06.048 [DOI] [PubMed] [Google Scholar]
- 7.Nicotra L, Loram LC, Watkins LR, Hutchinson MR. Toll-like receptors in chronic pain. Exp Neurol. 2012;234(2):316–329. doi: 10.1016/j.expneurol.2011.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno K, Woller SA, Miller YI, et al. Targeting toll-like receptor-4 (TLR4)-an emerging therapeutic target for persistent pain states. Pain. 2018;159(10):1908–1915. doi: 10.1097/j.pain.0000000000001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metz MJ, Daimon CM, Hentges ST. Reported Benefits of Low-Dose Naltrexone Appear to Be Independent of the Endogenous Opioid System Involving Proopiomelanocortin Neurons and β-Endorphin. eNeuro. 2021;8(3):ENEURO.0087–21.2021. doi: 10.1523/ENEURO.0087-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younger J, Noor N, McCue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65(2):529–538. doi: 10.1002/art.37734 [DOI] [PubMed] [Google Scholar]