Abstract
Purpose
Pronounced underuse of radiotherapy (RT) in muscle-invasive bladder cancer (MIBC) is reported. This study aims to assess the awareness about the role of RT in different MIBC settings and see whether this has increased since 2017.
Materials and Methods
We reviewed the bladder cancer guidelines of the EAU, ESMO, NCCN, NICE, and AUA/ASCO/ASTRO/SUO, focusing on the role of RT in MIBC. In 2017, we evaluated the use of RT in MIBC in Belgium. This raised awareness about the indications of RT in different MIBC settings. Here, we present a retrospective pattern of care analysis of the RT use for MIBC patients at our center from January 2012 until December 2021. Frequency of RT use, patient, disease and treatment characteristics were compared between two 5-year periods (2012–2016 and 2017–2021).
Results
Review of the guidelines suggested that RT can be used as a treatment option in most MIBC settings. However, differences between guideline recommendations existed and high-level evidence was often lacking. Overall, 221 unique MIBC patients received RT at our center. RT use for MIBC was 39% higher in the second 5-year period (Between the same periods, the number of new MIBC registrations increased with 26%). The most pronounced increase, ie, 529%, was observed in the primary setting and was in parallel with patient preference becoming the main indication for RT. Participation in clinical trials seems to have had an important impact on the frequency of RT use in the adjuvant and metastatic setting.
Conclusion
We provide a critical overview of the RT indications in MIBC as recommended by the international guidelines. Increased awareness about RT as a treatment option in MIBC seems to have an impact on the treatment choice in clinical practice, as was observed in our tertiary center.
Keywords: urothelial carcinoma, patterns of care, patient preference, radiation, underutilization
Introduction
Neo-adjuvant chemotherapy, followed by surgery, and in case of high-risk residual disease, adjuvant immunotherapy, is recognised as standard of care treatment for muscle-invasive bladder cancer (MIBC).1 The role of radiotherapy (RT) in the management of MIBC is more frequently debated. Despite being one of the cancers with the most frequent evidence-based indications for RT, its actual utilization in the treatment of bladder cancer remains limited.2 In Belgium, for instance, an important gap was observed between the optimal and actual use of RT in bladder cancer patients.3 In 2017, a survey conducted among Belgian urologists, medical oncologists and radiation oncologists confirmed the underutilization of RT in MIBC in all treatment settings. This survey suggests that providing an overview of the available guidelines can increase awareness of RT’s role in MIBC.4 Since then, our tertiary institution has made several efforts to raise awareness about RT as a treatment option for MIBC patients. These efforts include increasing research on RT for MIBC (including conducting the aforementioned survey) and giving more attention to RT as a MIBC treatment option at our multi-disciplinary tumor board meetings. The current study aims to provide a critical overview of the RT indications in MIBC as currently recommended by several guidelines. The study also aims to evaluate whether the raised awareness about the role of RT in MIBC impacted the use of RT in MIBC in daily practice at a tertiary center.
Materials and Methods
Guideline Review
We identified the most recent guideline versions of the European Association of Urology (EAU), the European Society for Medical Oncology (ESMO), the National Comprehensive Cancer Network (NCCN), and the National Institute for Health and Care Excellence (NICE).5–8 Also, the collaborative guideline of the American Urological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Urologic Oncology (AUA/ASCO/ASTRO/SUO) was included in this review.9 The full-text documents were compared and guideline modifications (if updated) since our previous assessment in the survey of 2017 were evaluated.
Pattern of Care Analysis
After approval by our Ethics Committee (Ghent University Hospital: ONZ-2022-0209), we retrospectively reviewed the medical charts of MIBC patients treated with RT at Ghent University Hospital between 1 January 2012 and 31 December 2021. Due to the retrospective nature of the study, the Ethics Committee waived the requirement for written informed consent. Patient data was maintained with confidentiality. Before analysis, all confidential patient information was removed. The ethical principles established by the Declaration of Helsinki were respected. Patient characteristics (age and sex), disease characteristics (histology, stage according to UICC TNM Classification 8 edition) and treatment characteristics (start/stop date, total dose, total number of fractions, concomitant treatment, and trial inclusion) were collected. Treatment practice was evaluated in the curative and palliative setting. The curative setting consists of patients treated with primary RT, adjuvant RT, perioperative RT (patients treated with neoadjuvant RT followed by bladder-sparing surgery and brachytherapy) and with RT for local recurrent disease. For patients treated with primary RT, the reason for referral to the RT department was extracted from the patients’ electronic health record. Predefined reasons were patient preference (patient could choose between RT and surgery, and opted for RT), medical preference (patient was suitable for surgery, but the multidisciplinary tumor board preferred RT), and inoperability (patient was not suited for surgery). The palliative setting consists of patients treated with RT to palliate symptomatic pelvic or extra-pelvic disease and patients treated with metastasis-directed radiotherapy (MDRT), ie, metastatic disease treated with high-dose radiotherapy with the intent to eradicate individual metastatic lesions. Frequency of RT use for each indication was evaluated per year between the first (2012–2016: Group A) and second (2017–2021: Group B) 5-year period of the analyzed decade. 2017 was chosen as cut-off to compare the pre- and post-survey period. As reference, the number of newly registered MIBC patients at our hospital during these 2 periods was obtained from the Cancer Registry. Further, patient, disease, and treatment characteristics were compared between the 5-year periods. Continuous variables were compared using the independent sample t-test and categorical variables with the Chi-square test. Statistical significance was reached at p ≤ 0.05 in all tests performed. Data were analyzed by SPSS statistics 28 (IBM SPSS, Chicago, IL).
Results
Guideline Review
In Table 1–3, an overview of the role of RT in MIBC as recommended by the current guidelines is presented. Characteristics of the consulted guidelines, including the system used for evidence and recommendation grading, are summarized in Supplementary Table 1.
Table 1.
Overview of the Role of RT as Primary Treatment for MIBC, as Recommend by the Consulted Guidelines
| Guideline* | Evidence/ Recommendation | Level/ Strength |
|---|---|---|
| Primary radiotherapy | ||
| EAU | Radiochemotherapy/ Multi-modality treatment | |
| In selected patient population, long-term survival rates of TMT are comparable to those of early cystectomy.5 | LE: 2B | |
| Offer surgical intervention or TMT to appropriate candidates as primary curative therapeutic approaches since they are more effective than RT alone.5 | SR: Strong | |
| Offer TMT as an alternative to selected, well informed and compliant patients, especially for whom RC is not an option or not acceptable†.5 | SR: Strong | |
| In patients with clinical T4 or clinical N+ disease (regional), radical CRT can be offered accepting that this may be palliative rather than curative in outcome (EAU-ESMO consensus statement).5 | NA | |
| CRT should be given to improve local control in cases of inoperable locally advanced tumours (EAU-ESMO consensus statements).5 | NA | |
| Radiotherapy-alone | ||
| External beam RT alone should only be considered as therapeutic option when the patient is unfit for cystectomy.5 | LE: 3 | |
| Do not offer RT alone as primary treatment for localised bladder cancer | SR: Strong | |
| EBRT can be an alternative treatment in patients unfit for radical surgery or concurrent chemotherapy.5 | NA | |
| AUA/ ASCO/ ASTRO/ SUO | Radiochemotherapy/ Multi-modality treatment | |
| A multi-modal bladder preserving approach with its merits and disadvantages should be discussed in each individual case. The studies that evaluate curative bladder preserving strategies, as a general rule, have highly select patient populations. The Panel found no strong evidence to determine whether or not immediate cystectomy improved survival when compared to initial bladder sparing protocols that employ salvage cystectomy as therapy for persistent bladder cancer.9 | NA | |
| The Panel believes that multi-modal bladder preserving therapy is the preferred treatment in those patients who desire bladder preservation and understand the unique risks associated with this approach and/or those who are medically unfit for surgery.9 | NA | |
| For patients with newly diagnosed non-metastatic MIBC who desire to retain their bladder, and for those with significant comorbidities for whom RC is not a treatment option, clinicians should offer bladder preserving therapy when clinically appropriate.9 | Clinical Principle | |
| Radiotherapy-alone | ||
| For patients with MIBC, clinicians should not offer RT alone as a curative treatment.9 | LE: C SR: Strong |
|
| ESMO | Organ-preservation therapy with RT, as part of multimodal schema for MIBC, is a reasonable option for patients seeking an alternative to RC† and an option for those who are medically unfit for surgery.6 | II, B |
| NCCN | Radiochemotherapy/ Multi-modality treatment | |
| Bladder-preserving approaches are reasonable alternatives to RC for patients who are medically unfit for surgery and those seeking an alternative to RC.7 | NA | |
| Stage II (T2, N0) and Stage IIIA (T3-T4a, N0; T1-T4a, N1).7 Stage IIB (T1-T4a, N2-3) and IVA (T4b, any N, M0).7 |
LE: 1 LE: 2A |
|
| Radiotherapy-alone | ||
| RT alone is inferior to RT combined with chemotherapy for patients with an invasive bladder tumor, and is not considered standard for patients who can tolerate combined therapy.7 | NA | |
| RT alone is only indicated for those who cannot tolerate a cystectomy or chemotherapy because of medical comorbidities.7 | NA | |
| Stage II (T2, N0) and Stage IIIA (T3-T4a, N0; T1-T4a, N1) (note: CRT preferred).7 | LE: 2A | |
| NICE | Radiochemotherapy/ Multi-modality treatment | |
| Offer a choice of RC or RT with a radiosensitiser to people with urothelial MIBC for whom radical therapy is suitable Ensure that the choice is based on a full discussion between the person and a urologist who performs RC, a clinical oncologist and a clinical nurse specialist.8 | LE: Very low-low | |
Notes: *Characteristics of the consulted guidelines, including the system used for evidence and recommendation grading, are summarized in Supplementary Table 1. †Underlined text indicates modifications to the guidelines (since our previous review in 2017) which encourage a treatment choice based on patient preference.
Abbreviations: AUA/ASCO/ASTRO/SUO, American Urological Association/American Society of Clinical Oncology/American Society of Radiation Oncology/Society of Urologic Oncology; CRT, chemoradiotherapy; EAU, European Association of Urology; EBRT, external beam radiotherapy; ESMO, European Society of Medical Oncology; LE, level of evidence; M, metastasis; MIBC, muscle-invasive bladder cancer; N, nodes; NA, not available; NCCN, National Comprehensive Cancer Network; NICE, National Institute for Health and Care Excellence; RC, radical cystectomy; RT, radiotherapy; SR, strength rating; T, tumor; TMT, trimodality therapy.
Table 2.
Overview of the Role of RT in MIBC in the Neoadjuvant, Adjuvant and Recurrent Treatment Setting, as Recommend by the Consulted Guidelines
| Guideline* | Evidence/ Recommendation | Level/ Strength |
|---|---|---|
| Neoadjuvant radiotherapy | ||
| EAU | No contemporary data exists to support that pre-operative RT for operable MIBC increases survival.5 | LE: 2A |
| Pre-operative RT for operable MIBC, using a dose of 45–50Gy in fractions of 1.8–2Gy, results in down-staging after 4 to 6 weeks.5 | LE: 2 | |
| Limited high-quality evidence supports the use of pre-operative RT to decrease local recurrence of MIBC after RC.5 | LE: 3 | |
| Do not offer pre-operative RT for operable MIBC since it will only result in down-staging, but will not improve survival.5 | SR: Strong | |
| Do not offer pre-operative RT when subsequent RC with urinary diversion is planned.5 | SR: Strong | |
| NCCN | For invasive tumors, consider low-dose preoperative RT prior to segmental cystectomy.7 | 2B |
| Adjuvant radiotherapy | ||
| EAU | Addition of adjuvant RT to chemotherapy is associated with an improvement in local relapse-free survival following cystectomy for locally-advanced bladder cancer (pT3b–4, or node-positive).5 | LE: 2A |
| Consider offering adjuvant RT in addition to chemotherapy following RC, based on pathologic risk (pT3b–4 or positive nodes or positive margins).5 | SR: Weak | |
| ESMO | Adjuvant RT (with or without radiosensitising chemotherapy) is not standard treatment of patients with MIBC.6 | III, C |
| NCCN | Based on pathologic risk, consider adjuvant RT in selected patients (pT3- 4, positive nodes/margins).7 | LE: 2B |
| Local recurrence/ persistent disease | ||
| EAU | Offer RT, chemotherapy and possibly surgery as options for treatment, either alone or in combination.5 | SR: Strong |
| NCCN | Subsequent-line therapy for metastatic disease or local recurrence includes CRT (if no previous RT), or RT.7 | LE: 2A |
| RT alone can also be considered as a subsequent-line therapy for patients with metastatic disease or local recurrence following cystectomy, especially in selected cases with regional only recurrence or with clinical symptoms.7 | LE: 2A | |
Notes: *Characteristics of the consulted guidelines, including the system used for evidence and recommendation grading, are summarized in Supplementary Table 1. No RT recommendations provided for the neoadjuvant, adjuvant and recurrent MIBC setting by the AUA/ASCO/ASTRO/SUO and NICE guidelines.
Abbreviations: AUA/ASCO/ASTRO/SUO, American Urological Association/American Society of Clinical Oncology/American Society of Radiation Oncology/Society of Urologic Oncology; CRT, chemoradiotherapy; EAU, European Association of Urology; ESMO, European Society of Medical Oncology; LE, level of evidence; MIBC, muscle-invasive bladder cancer; NCCN, National Comprehensive Cancer Network; NICE, National Institute for Health and Care Excellence; PT, pathologic tumor stage; RC, radical cystectomy; RT, radiotherapy; SR, strength rating; T, tumor.
Table 3.
Overview of the Role of RT as Palliative Treatment in MIBC, as Recommend by the Consulted Guidelines
| Guideline* | Evidence/ Recommendation | Level/ Strength |
|---|---|---|
| Palliative radiotherapy | ||
| EAU | RT can also be used to stop bleeding from the tumour when local control cannot be achieved by transurethral manipulation because of extensive local tumour growth.5 | LE: 3 |
| Local recurrence: offer RT, chemotherapy and possibly surgery as options for treatment, either alone or in combination.5 | SR: Strong | |
| Distant recurrence: Offer chemotherapy as the first option, and consider metastasectomy or RT in case of unique metastasis site.5 | SR: Strong | |
| ESMO | Palliative RT can be offered for palliation (bleeding, pain).6 | III, C |
| NCCN | RT alone can also be considered as a subsequent-line therapy for patients with metastatic disease or local recurrence following cystectomy, especially in selected cases with regional only recurrence or with clinical symptoms.7 | LE: 2A |
| Subsequent-line therapy for metastatic disease or local recurrence includes CRT (if no previous RT), or RT.7 | LE: 2A | |
| Concurrent CRT or RT alone should be considered for local palliation in patients with metastatic disease.7 | LE: 2A | |
| NICE | Offer palliative hypofractionated RT to people with symptoms of haematuria, dysuria, urinary frequency or nocturia caused by advanced bladder cancer that is unsuitable for potentially curative treatment.8 | LE: very low-high |
| Consider hypofractionated RT or embolisation for people with intractable bleeding caused by incurable bladder cancer.8 | LE: very low | |
| Pelvic pain: Consider, in addition to best supportive care, 1 or more of the following to treat pelvic pain caused by incurable bladder cancer: -hypofractionated RT if the person has not had pelvic RT.8 | LE: very low | |
Notes: *Characteristics of the consulted guidelines, including the system used for evidence and recommendation grading, are summarized in Supplementary Table 1. No RT recommendations provided for the palliative MIBC setting by the AUA/ASCO/ASTRO/SUO guideline.
Abbreviations: AUA/ASCO/ASTRO/SUO, American Urological Association/American Society of Clinical Oncology/American Society of Radiation Oncology/Society of Urologic Oncology; CRT, chemoradiotherapy; EAU, European Association of Urology; ESMO, European Society of Medical Oncology; LE, level of evidence; MIBC, muscle-invasive bladder cancer; NCCN, National Comprehensive Cancer Network; NICE, National Institute for Health and Care Excellence; RT, radiotherapy; SR, strength rating.
Pattern of Care Analysis
Between January 2012 and December 2021, a total of 221 unique patients were treated for MIBC at our department of radiation oncology. The number of patients treated with RT increased with 39% between the first (Group A: N=109) and second (Group B: N=151) 5-year period. The number of newly registered MIBC patients at our hospital increased between the same periods with 26% (2012–2016: N=72 and 2017–2021: N=91) (note: registration of bladder cancer patients was based on the TNM-classification at initial diagnosis and was not updated in case of disease progression or recurrence). An overview of the number of MIBC patients treated with RT per 5-year period is shown in Table 4. Comparison of the patient, disease and treatment characteristics per 5-year period is shown in Table 5.
Table 4.
Number of Patients Treated with Radiotherapy per Treatment Setting per 5-Year Period, Ie, (2012–2016) and (2017–2021)
| Treatment Setting | Group A | Group B |
|---|---|---|
| 2012–2016 | 2017–2021 | |
| (n=109) | (n=151) | |
| Curative | 45 | 72 |
| -Primary | 7 | 44 |
| -Adjuvant | 25 | 25 |
| -Peri-operative | 9 | 1 |
| -Local recurrent | 4 | 2 |
| Palliative | 64 | 79 |
| -Pelvic palliative | 15 | 18 |
| -Extra-pelvic palliative | 42 | 32 |
| -Metastasis directed therapy | 7 | 29 |
Table 5.
Comparison of the Patient, Disease and Treatment Characteristics Between (2012–2016) and (2017–2021), Univariate Analysis
| Characteristics | Group A | Group B | P value* |
|---|---|---|---|
| 2012–2016 | 2017–2021 | ||
| (n=109) | (n=151) | ||
| Patient-related | |||
| -Mean age, years (SD) | 68 (12.3) | 71 (10.9) | 0.07 |
| -Gender | 0.5 | ||
| Male | 83 (76%) | 120 (79%) | |
| Female | 26 (24%) | 31 (21%) | |
| Disease-related | |||
| -Histology | 0.3 | ||
| Urothelial | 91 (83%) | 136 (90%) | |
| Other | 13 (12%) | 12 (7.9%) | |
| Unknown | 5 (4.6%) | 3 (2.0%) | |
| -Stage† | 0.3 | ||
| Stage II | 16 (15%) | 33 (22%) | |
| Stage III | 30 (28%) | 39 (26%) | |
| Stage IV | 63 (58%) | 79 (52%) | |
| Treatment-related | |||
| -Intent | 0.3 | ||
| Curative | 45 (41%) | 72 (48%) | |
| Palliative | 64 (59%) | 79 (52%) |
Notes: †Stage according to UICC TNM Classification 8 ed. *Chi-square test for proportions and the independent t-test for continuous variables, statistical significance defined as P ≤ 0.05.
Abbreviation: SD, standard deviation.
Curative Setting
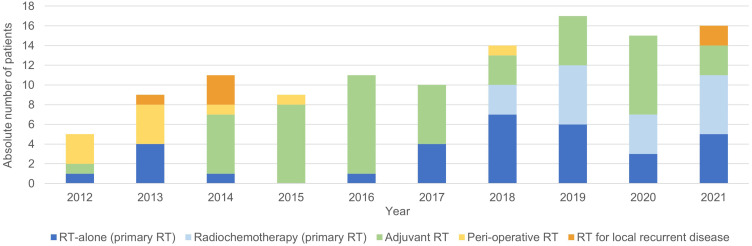
During the analyzed decade, one hundred and seventeen MIBC patients received RT with curative intent. An overview of the number of patients per type of treatment, per year, is shown in Figure 1.
Figure 1.
Overview of the absolute number of muscle-invasive bladder cancer patients treated with radiotherapy (RT) in the curative setting, per type of treatment, per year.
Primary RT
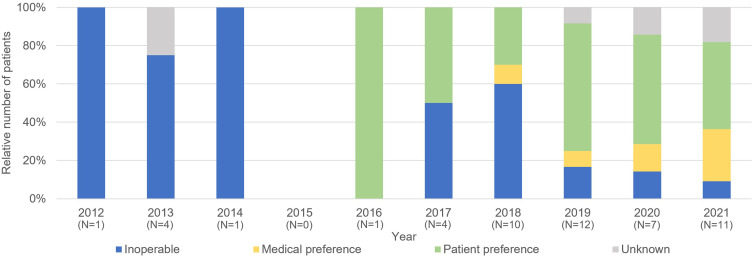
Fifty-one MIBC patients were treated with primary RT, of whom 19 with and 32 without concurrent chemotherapy. Between 2012 and 2016, only 7 patients were referred for primary RT. The majority was referred because of inoperability (5 out of 6 patients with a known reason for RT referral) as shown by Figure 2. All of these patients were also deemed unfit for radiochemotherapy and received therefore RT-alone (25 × 1.8Gy to the bladder with a sequential boost of 5 × 5Gy to the tumor site). After 2017, forty-four patients were referred for primary RT and most patients were referred for RT because of patient or medical preference (29 out of 41 patients with a known reason for RT referral) (Figure 2). Patients fit for radiochemotherapy received 20 × 2.6Gy (2017–2020) or 20 × 2.75Gy (from 2021 on) to the bladder with concurrent gemcitabine. As of 2017, almost all patients deemed unfit for radiochemotherapy received ultra-hypofractionated RT (6 × 6Gy to the bladder, once weekly) (N=19).
Figure 2.
Reason of referral for primary radiotherapy, percentage of patients per reason, per year. The absolute number of patients treated with primary radiotherapy per year (N) is reported under the corresponding year on the x-axis.
Neo-Adjuvant/ Adjuvant/ Peri-Operative RT
Fifty patients received RT in the post-operative setting of whom 84% received adjuvant RT as part of a clinical trial (study enrolment between August 2014–October 2020). All patients were treated with 25 × 2Gy to the pelvic lymph node areas at risk ± the cystectomy bed (in case of a positive surgical margin). Ten patients were treated with peri-operative radiotherapy consisting of low-dose neo-adjuvant RT (3 × 3.5Gy) to the tumor site followed by a partial cystectomy and post-operative brachytherapy (60Gy, pulse dose rate 0.5Gy per pulse, 1 pulse per hour). Apart from this peri-operative treatment, no patients were treated with neoadjuvant RT.
Local Recurrent Disease
Six patients who developed a locoregional recurrence post-cystectomy were treated with radical RT to obtain maximal locoregional control, ie, 25 × 2Gy to the pelvic lymph node areas at risk with a simultaneous integrated boost (65–70Gy) to the recurrence.
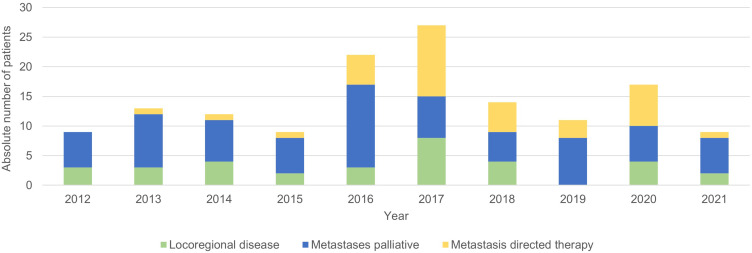
Palliative Setting
Thirty-three patients received palliative RT for pelvic disease, of whom 12 because of locoregional recurrence after their cystectomy. Of the patients treated for pelvic disease 13 and 20 patients received a multi-fraction, ie, 10 × 3Gy or 5 × 4Gy, and single fraction ie, 1 × 7Gy or 1 × 8Gy, RT schedule, respectively. A total of 186 extra-pelvic lesions, divided over 110 patients, were treated with RT. Of these patients, seventy-four patients were treated with a palliative scheme, ie, 1 × 8Gy or 10 × 3Gy or 5 × 4Gy, mostly because of symptomatic disease. Thirty-six patients received MDRT (most used schedules were 3 × 8Gy or 3 × 10Gy), of whom 23 within clinical trials.10,11 The number of MIBC patients treated with palliative RT, per type of treatment, per year is shown in Figure 3.
Figure 3.
Overview of the absolute number of MIBC patients treated with radiotherapy (RT) in the palliative setting, per type of treatment, per year.
Discussion
The absolute number of new bladder cancer patients with an RT indication is predicted to increase in the immediate future. In several countries, bladder cancer is expected to settle in the top 5 most frequent cancers according to evidence-based indications.2 Underutilization of RT in the field of bladder cancer has been observed in Belgium and this inevitably leads to denying a potential treatment option to bladder cancer patients.3 An important step in adherence to clinical guidelines is the awareness of the actual guidelines. Agreement between and clarity of guidelines are key for adopting and adhering to guidelines in daily practice.12
In the primary setting, all guidelines recommend radiotherapy in combination with a radiosensitizer, ie, trimodality therapy or multi-modality treatment, as an alternative treatment option for selected MIBC patients, especially in those patients unsuitable for cystectomy or seeking an alternative to cystectomy. Several guidelines still list criteria to select optimal candidates for multi-modality treatment (MMT) (Supplementary Table 2). It is important to emphasize that many of these criteria are known to be prognostic factors and should not be considered as absolute contra-indications. Patients who do not meet these selection criteria will also have a poorer prognosis when treated with cystectomy. At this moment, there is no strong evidence for one MIBC treatment to be superior to the other. This is best translated in the recommendation of the NICE guidelines, stating that a choice between cystectomy and MMT needs to be offered after a full discussion between patient, urologist, radiation-oncologist and clinical nurse specialist. Important adaptations have been made, since our previous review (survey of 2017), in favour of MMT. As indicated in Table 1, modifications in the EAU and ESMO recommendations now indicate that patient preference may influence treatment. A small but notable difference in the phrasing of the EAU recommendation is the change of “highly selected” to “selected” patients. Also, the NCCN guideline changed its recommendation for MMT from category 2A to category 1. At our center, we observed an increase in overall RT use for MIBC patients during the last few years. We attribute this raise in RT use to an increased awareness of optimal use of RT in MIBC patients. One of the measures taken at our center, was the advocacy of MMT, ie, radiochemotherapy, as bladder-sparing alternative for surgery. Consequential, more fit patients, also eligible for radiochemotherapy, were referred to our RT department. Meanwhile, patient preference has become the main reason for primary RT instead of inoperability.
Regarding the use of RT-alone as primary treatment, some discrepancies between guidelines were noted. The AUA/ASCO/ASTRO/SUO and EAU both strongly recommend against offering RT-alone as curative treatment. However, the EAU guideline, as well as the NCCN guideline, mentions that RT-alone could be considered in patients unfit for cystectomy or concurrent chemotherapy. The ESMO and NICE guidelines discuss RT-alone only as a palliative treatment strategy. Undertreatment in older MIBC patients is well documented, and high cancer-specific mortality in this elderly population indicates that omitting active treatment in patients unfit for RC or MMT, is not always justified.13–15 RT-alone used in a hypofractionated scheme, ie, 6 × 6Gy, is safe and seems to provide a better local disease control than a purely palliative treatment.16 Since we implemented this hypofractionated scheme at our center, the use of RT-alone increased. We suspect that this could possibly indicate a more active treatment attitude in this often undertreated group.
Currently, the role of neoadjuvant RT seems to be very limited and is only discussed by the EAU and NCCN guidelines. Based on a meta-analysis showing no overall survival benefit when using neoadjuvant RT, the EAU guideline strongly recommends against offering pre-operative RT for operable patients.17 The NCCN guideline briefly mentions that low-dose pre-operative RT prior to a partial cystectomy can be considered. At our center, neoadjuvant low-dose RT was used a few times in preparation for bladder-sparing surgery followed by brachytherapy. This is in accordance with the Dutch guidelines last updated in 2019.18 These guidelines state that despite favourable reported outcomes, the level of evidence for this treatment strategy is limited and should only be considered in strictly selected MIBC patients (cT1-3aN0M0, solitaire tumor ≤3 cm located outside the trigonum/bladder neck, no carcinoma in situ and/or severe urinary complaints).18
In the adjuvant setting, both EAU and NCCN guidelines state that there are only limited data for the use of radiotherapy following cystectomy. Both guidelines cite a Phase II trial that compared adjuvant chemotherapy vs adjuvant chemoradiation.19 This trial showed an improvement in local relapse-free survival, with low rates of late-grade ≥3 toxicity. Based on these results, the EAU and NCCN guidelines conclude that adjuvant RT can be considered in case of high pathological risk for local recurrence. In contrast, the ESMO guideline mentions that there is no role for adjuvant RT in high-risk patients and further states that these patients have been included in adjuvant immunotherapy trials. Interestingly, patients with a positive resection margin were excluded from the adjuvant immunotherapy trials. A Belgian phase II trial reports that only 3 out of 14 patients with a positive resection margin developed a locoregional failure after adjuvant RT (median follow-up of 18 months).20 This suggests the importance of adjuvant RT in patients with a positive resection margin. Currently, the role of adjuvant RT is further investigated in 2 Phase III trials (GETUG-AFU30 (NCT03333356) and BART trial (NCT02951325)), both including patients with a positive margin.
Patients with a locoregional recurrence have a poor prognosis and often experience debilitating symptoms.21 Patients treated for recurrence showed a better survival than those without treatment (1-year survival: 27% vs 2%).22 Both the EAU and NCCN guidelines recommend RT as a treatment option for recurrent disease. At our center, few patients received RT for recurrent disease. A possible explanation for this is a reduction in the number of locoregional relapses, through the use of adjuvant RT and/or immunotherapy in high-risk MIBC patients. Further, almost all guidelines recommend RT as a means to palliate 1 or more of following local symptoms in patients unsuitable for curative disease: haematuria, lower urinary tract symptoms, pelvic pain. No guideline specifically discusses the role of RT as treatment of symptomatic distant metastases. This, despite the well-established role of RT in the treatment of uncomplicated painful bone metastases.23 There is limited evidence that MDRT can achieve durable disease control in selected patients with minimal metastatic disease.24 Currently, only the EAU guideline briefly mentions a potential role for RT as metastasis-directed therapy. The increased MDRT use at our center was mainly induced by the treatment of patients with MDRT as part of a Phase 1 and Phase 2 trial running between 2016 and 2020 at our hospital.10,11 In the absence of stronger evidence, the role for MDRT outside of clinical trials remains limited. The role of MDRT in oligometastatic bladder cancer is further evaluated in the ongoing EFFORT-MIBC trial.25
There are some limitations to this study. Review of the guidelines was not a systematic review. The pattern of care analysis was a single-center retrospective analysis. Observed changes in treatment frequency are based on absolute numbers of patients. Therefore, the reported changes in treatment practice must be interpreted with caution. However, the observed increase in overall RT use was more pronounced than the increase in newly registered MIBC patients at our hospital.
Conclusion
International guidelines recommend MMT as an alternative to cystectomy, but differ in patient selection. Patient preference is gaining importance in several guidelines. Our single-center experience suggests that increased awareness of MMT as a treatment option can lead to an increase in its use, particularly by including patient preference as a reason for referral. Adjuvant RT for MIBC is currently not well established, and ongoing trials may provide more clarity. RT is recommended for palliating local symptoms in patients ineligible for curative treatment, but guidelines do not discuss its use for symptomatic metastases. The role of MDRT outside of clinical trials remains limited.
Acknowledgments
Increasing awareness and conducting bladder cancer research would not have been possible without the financial support of Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society. We thank the Cancer Registry for providing us with bladder cancer data of our hospital.
Funding Statement
This work was supported by Kom op tegen Kanker (Stand up to Cancer), the Flemish Cancer Society. The funding body is not involved in the design of the study and collection, management, analysis, and interpretation of data and in writing or submitting of the manuscript.
Abbreviations
ASCO, American Society of Clinical Oncology; ASTRO, American Society for Radiation Oncology; AUA, American Urological Association; EAU, European Association of Urology; ESMO, European Society for Medical Oncology; MDRT, metastasis directed radiotherapy; MIBC, Muscle-invasive bladder cancer; NCCN, National Comprehensive Cancer Network; NICE, National Institute for Health and Care Excellence; RT, Radiotherapy; SBRT, stereotactic body radiation therapy; SUO, Society of Urologic Oncology.
Data Sharing Statement
Raw data were generated at the Ghent University Hospital. Derived data supporting the findings of this study are available from the corresponding author on reasonable request.
Disclosure
The authors report no competing interests in this work.
References
- 1.Compérat E, Amin MB, Cathomas R, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet. 2022;400(10364):1712–1721. doi: 10.1016/s0140-6736(22)01188-6 [DOI] [PubMed] [Google Scholar]
- 2.Borras JM, Lievens Y, Barton M, et al. How many new cancer patients in Europe will require radiotherapy by 2025? An ESTRO-HERO analysis. Radiother Oncol. 2016;119(1):5–11. doi: 10.1016/j.radonc.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 3.Lievens Y, De Schutter H, Stellamans K, Rosskamp M, Van Eycken L. Radiotherapy access in Belgium: how far are we from evidence-based utilisation? Eur J Cancer. 2017;84:102–113. doi: 10.1016/j.ejca.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 4.Fonteyne V, Rammant E, Ost P, et al. Evaluating the Current Place of Radiotherapy as Treatment Option for Patients With Muscle Invasive Bladder Cancer in Belgium. Clin Genitourin Cancer. 2018;16(6):e1159–e1169. doi: 10.1016/j.clgc.2018.07.026 [DOI] [PubMed] [Google Scholar]
- 5.European Association of Urology. EAU guidelines on muscle-invasive and metastatic bladder cancer; 2022. Available from: https://uroweb.org/guidelines/muscle-invasive-and-metastatic-bladder-cancer. Accessed October 1, 2022.
- 6.Powles T, Bellmunt J, Comperat E, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(3):244–258. doi: 10.1016/j.annonc.2021.11.012 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network [homepage on the internet]. NCCN guidelines bladder cancer; 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed October 10, 2022.
- 8.National Institute for Health and Care Excellence. Bladder cancer: diagnosis and management [NICE Guideline 2]; 2015. Available from: https://www.nice.org.uk/guidance/ng2/evidence/full-guideline-pdf-3744109. Accessed October 10, 2022. [PubMed]
- 9.Chang SS, Bochner BH, Chou R, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol. 2017;198(3):552–559. doi: 10.1016/j.juro.2017.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaas M, Sundahl N, Hulstaert E, et al. Checkpoint inhibition in combination with an immunoboost of external beam radiotherapy in solid tumors (CHEERS): study protocol for a phase 2, open-label, randomized controlled trial. BMC Cancer. 2021;21(1):514. doi: 10.1186/s12885-021-08088-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundahl N, Vandekerkhove G, Decaestecker K, et al. Randomized Phase 1 Trial of Pembrolizumab with Sequential Versus Concomitant Stereotactic Body Radiotherapy in Metastatic Urothelial Carcinoma. Eur Urol. 2019;75(5):707–711. doi: 10.1016/j.eururo.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 12.Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. The case of pediatric vaccine recommendations. Med Care. 1996;34(9):873–889. doi: 10.1097/00005650-199609000-00002 [DOI] [PubMed] [Google Scholar]
- 13.Varughese M, Treece S, Drinkwater K. Radiotherapy management of muscle invasive bladder cancer: evaluation of a national cohort. Clin Oncol. 2019;31(9):637–645. doi: 10.1016/j.clon.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Gray PJ, Fedewa SA, Shipley WU, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol. 2013;63(5):823–829. doi: 10.1016/j.eururo.2012.11.015 [DOI] [PubMed] [Google Scholar]
- 15.Noon AP, Albertsen PC, Thomas F, Rosario DJ, Catto JW. Competing mortality in patients diagnosed with bladder cancer: evidence of undertreatment in the elderly and female patients. Br J Cancer. 2013;108(7):1534–1540. doi: 10.1038/bjc.2013.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huddart R, Hafeez S, Lewis R, et al. Clinical Outcomes of a Randomized Trial of Adaptive Plan-of-The-Day Treatment in Patients Receiving Ultra-hypofractionated Weekly Radiation Therapy for Bladder Cancer. Int J Radiat Oncol Biol Phys. 2021;110(2):412–424. doi: 10.1016/j.ijrobp.2020.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huncharek M, Muscat J, Geschwind JF. Planned preoperative radiation therapy in muscle invasive bladder cancer; results of a meta-analysis. Anticancer Res. 1998;18(3b):1931–1934. [PubMed] [Google Scholar]
- 18.Nederlandse Vereniging voor Urologie. Richtlijnmodule Brachytherapie bij de behandeling van patiënten met een spierinvasief blaascarcinoom; 2016. Available from: https://richtlijnendatabase.nl/richtlijn/blaascarcinoom_-_brachytherapie/startpagina_-_brachytherapie.html., Accessed October 15, 2022.
- 19.Zaghloul MS, Christodouleas JP, Smith A, et al. Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy a randomized phase 2 trial. Article. JAMA Surg. 2018;153(1):654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonteyne V, Dirix P, Van Praet C, et al. Adjuvant Radiotherapy After Radical Cystectomy for Patients with High-risk Muscle-invasive Bladder Cancer: results of a Multicentric Phase II Trial. Eur Urol Focus. 2022;8(5):1238–1245. doi: 10.1016/j.euf.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 21.Baumann BC, Guzzo TJ, He J, et al. Bladder cancer patterns of pelvic failure: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(2):363–369. doi: 10.1016/j.ijrobp.2012.03.061 [DOI] [PubMed] [Google Scholar]
- 22.Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer. 2011;9(1):14–21. doi: 10.1016/j.clgc.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 23.Wu JS, Wong RK, Lloyd NS, Johnston M, Bezjak A, Whelan T. Radiotherapy fractionation for the palliation of uncomplicated painful bone metastases - an evidence-based practice guideline. BMC Cancer. 2004;4:71. doi: 10.1186/1471-2407-4-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longo N, Celentano G, Napolitano L, et al. Metastasis-Directed Radiation Therapy with Consolidative Intent for Oligometastatic Urothelial Carcinoma: a Systematic Review and Meta-Analysis. Cancers. 2022;14:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verghote F, Poppe L, Verbeke S, et al. Evaluating the impact of 18F-FDG-PET-CT on risk stratification and treatment adaptation for patients with muscle-invasive bladder cancer (EFFORT-MIBC): a phase II prospective trial. BMC Cancer. 2021;21(1):1113. doi: 10.1186/s12885-021-08861-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at the Ghent University Hospital. Derived data supporting the findings of this study are available from the corresponding author on reasonable request.