Abstract
Vertical transmission as a route of infection has been well reported in many viral infections. Scrub typhus is a zoonotic disease transmitted by ticks which has had a resurgence in recent times in several tropical countries. It affects all age groups including neonates. Reports of neonates affected with scrub typhus are few, and vertical transmission is rare. We report a case, where a newborn was symptomatic with signs of infection within the first 72 hours of life and Orientia tsutsugamushi, the causative organism was confirmed by PCR in both mother and baby.
Keywords: Infectious diseases, Paediatrics
Background
Sepsis is one of the most common causes of mortality and morbidity in neonates. Early onset infection, in particular, has a significantly higher mortality than late onset sepsis. Though gram negative septicaemia is the most common in neonates worldwide, infection with other pathogens is also common in endemic areas. Scrub typhus is a common infection in tropical countries with a prevalence as high as 25% in cases with undifferentiated febrile illness.1 2 It is important to consider the loco-regional disease burden and epidemiology, when evaluating for infections with atypical presentations in neonates.
Case presentation
A healthy term neonate admitted in the postnatal ward presented with complaints of decreased oral acceptance and irritability at 36 hours of life. She was born to a second gravida mother through an emergency caesarean section in view of meconium-stained liquor and fetal distress with a birth weight of 2.7 kg at 40 weeks gestation. The baby had APGARs of 8 and 9 at 1 min and 5 min of life, respectively. She was initiated on direct breast feeds and was being roomed in with the mother.
At admission to NICU (Neonatal intensive care unit), the baby was febrile (axillary temperature of 38.5°C) and irritable. On examination, she had hepatosplenomegaly (liver 4 cm and spleen 2 cm below the costal margin and liver span of 10 cm) and a diffuse blanchable macular rash over trunk (figure 1). She had a weight loss of 7% on day 2 of life, with tachycardia, tachypnoea and prolonged capillary refill time. After initial stabilisation, blood culture samples were taken and baby was initiated on intravenous antibiotics for suspected sepsis. Presence of rash and fever early in the neonatal period prompted us to consider viral infections like perinatal dengue, chikungunya, COVID-19 and HSV (Herpes simplex virus), so detailed maternal history was elicited. Mother claimed that she had developed a febrile illness with rash for which she was admitted and treated at around 34 weeks of gestation. Her records revealed that PCR for scrub typhus was positive and she was treated with 5 days of oral azithromycin. So, a work-up for tropical and intrauterine infections including dengue, chikungunya, scrub typhus, malaria and TORCH (toxoplasmosis, others (syphilis, hepatitis B), rubella, cytomegalovirus, herpes simplex infections) was also done for the baby.
Figure 1.
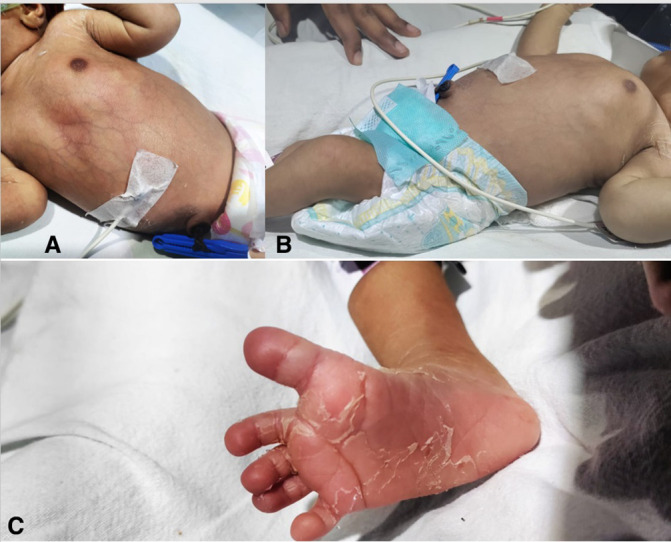
(A) Erythema over the chest and abdomen with visible veins. (B) Resolution of erythema. (C) Peeling of skin over extremities.
The baby was haemodynamically stable at admission but developed shock on the fourth day of life, for which she required non-invasive ventilatory support and inotropes. Meanwhile PCR for scrub typhus returned positive, following which she was started on intravenous doxycycline. Even after 48 hours of doxycycline, baby continued to have fever and encephalopathy; hence, lumbar puncture was done, which was suggestive of meningitis. Haemophagocytic lymphohistiocytosis work-up was negative. Azithromycin was added, after which her encephalopathy improved and organomegaly regressed. The rash changed from diffuse erythema to a pale ash colour followed by resolution with skin peeling (figure 1). After 10 days of azithromycin, baby was discharged home on exclusive breastfeeds.
With a history of scrub typhus in the mother and the infant presenting with symptomatic infection at 48 hours of life, perinatal transmission was suspected, confirmed by positive PCR of both mother and baby. Serology of mother was negative though, suggesting that mother had transmitted the organism to the baby, but not the antibodies as she had not seroconverted.
Investigations
At admission, the baby had thrombocytopaenia (platelets: 100 000/µL) and direct hyperbilirubinaemia and transaminitis (total bilirubin: 6.1 mg/dL, direct: 3.2 mg/dL and mild AST (aspartate transaminase) elevation: 69 IU/L). RT-PCR (Real time polymerase chain reaction) for scrub typhus (56 kDa type specific antigen gene) was positive in blood and negative in cerebrospinal fluid (CSF), though CSF showed 150 cells/high power field with hypoglycorrhachia and elevated protein (sugar: 34 mg/dL and protein: 147 mg/dL).
Differential diagnosis
With the background of symptoms in the neonate at 36 hours initially an early onset infection was considered. A review of maternal history and rash in the newborn prompted us to look for congenital infections and perinatally acquired infections like dengue and chikungunya. Positive scrub typhus PCR report in the mother strongly suggested the possibility of scrub typhus in the baby.
Treatment
Baby received intravenous cefoperazone and amikacin for suspected sepsis which was changed to doxycycline once the PCR for scrub typhus was positive. Azithromycin was added in view of persisting fever spikes, after which the baby improved. Dobutamine was required on fourth day of life for shock.
Outcome and follow-up
The child is currently 5 months old with age-appropriate growth and development with no ongoing problems.
Discussion
About 4.5% of the patients diagnosed as scrub typhus in endemic areas are pregnant women.3 While scrub typhus during pregnancy treated adequately is associated with favourable maternal and fetal outcomes, adverse fetal outcomes such as spontaneous abortion, preterm labour, small for gestational age and stillbirth have also been reported.4 Scrub typhus in neonates is rare, with only 22 cases reported so far.5–15 All affected neonates manifested with fever and hepatosplenomegaly (100%), whereas thrombocytopaenia (21/22), shock (12/21), Disseminated intravascular coagulation or DIC (8/21), Multi-organ dysfunction syndrome or MODS (6/21), respiratory failure (9/22) and eschar (4/22) were the other clinical manifestations. Overall mortality was 18% (4/22). Eschar was absent in most of the neonates, as was the case with ours. Among others, the baby described in our case also had thrombocytopaenia, fever, hepatosplenomegaly and shock. Vertical transmission confirmed by positive serology for scrub typhus was reported in four cases.5–7 15 Among the cases where vertical transmission was suspected, PCR was either not done or negative in three of the cases,5 6 15 and positive in one7 but none of the previously reported studies reported on maternal PCR. PCR was positive in both the neonate and the mother in our case, making the evidence for vertical transmission more robust. Early onset of symptoms (at 36 hours of life) also suggests that the infection was transmitted transplacentally. Variations in host immune response would probably explain why the mother did not pass on protective antibodies to the fetus. Moreover, presence of IgG antibodies against scrub typhus does not appear to be protective,16 which explains why the fetus was affected despite mother recovering from her illness. Most of the affected neonates were treated with doxycycline (11/22). Interestingly, survival was 100% among neonates who received doxycycline, whereas the four neonates who succumbed received other antibiotics (ofloxacin, azithromycin).
Scrub typhus should be considered as a differential diagnosis for neonates presenting with fever, hepatosplenomegaly, thrombocytopaenia and elevated CRP, especially in endemic regions.6 Tropical infections are still a major cause for morbidity and mortality in LMICs (low and middle income countries). Knowledge about commonly occurring endemic infections is important and helps in early identification and treatment of the same.
Patient's perspective.
We understood that our baby had an infection called scrub typhus which was treated with antibiotics and the nature of spread was probably transplacental. She recovered well during treatment and was active and feeding well at discharge.
Learning points.
Vertical transmission is possible in scrub typhus during pregnancy.
A neonate with suspected sepsis and atypical clinical presentation should always be evaluated for other aetiologies like tropical infections, and metabolic diseases whenever there is a probability of the same.
Fever, hepatosplenomegaly and thrombocytopaenia should arouse suspicion of scrub typhus in endemic regions.
Footnotes
Contributors: RD, NPT, AM and NP were responsible for drafting of the text, sourcing and editing of clinical images, investigation results, drawing original diagrams and algorithms, and critical revision for important intellectual content. RD, NPT, AM and NP gave final approval of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1.Abhilash KPP, Jeevan JA, Mitra S, et al. Acute undifferentiated febrile illness in patients presenting to a tertiary care hospital in South India: clinical spectrum and outcome. J Glob Infect Dis 2016;8:147–54. 10.4103/0974-777X.192966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrispal A, Boorugu H, Gopinath KG, et al. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors - an experience from a tertiary care hospital in South India. Trop Doct 2010;40:230–4. 10.1258/td.2010.100132 [DOI] [PubMed] [Google Scholar]
- 3.Rajan SJ, Sathyendra S, Mathuram AJ. Scrub typhus in pregnancy: maternal and fetal outcomes. Obstet Med 2016;9:164–6. 10.1177/1753495X16638952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poomalar GK, Rekha R. A case series of scrub typhus in Obstetrics. J Clin Diagn Res 2014;8:1–3. 10.7860/JCDR/2014/9718.5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang CL, Yang KD, Cheng SN, et al. Neonatal scrub typhus: a case report. Pediatrics 1992;89(5 Pt 1):965–8. [PubMed] [Google Scholar]
- 6.Suntharasaj T, Janjindamai W, Krisanapan S. Pregnancy with scrub typhus and vertical transmission: a case report. J Obstet Gynaecol Res 1997;23:75–8. 10.1111/j.1447-0756.1997.tb00809.x [DOI] [PubMed] [Google Scholar]
- 7.Aarthi P, Bagyalakshmi R, Mohan KR, et al. First case series of emerging Rickettsial neonatal sepsis identified by polymerase chain reaction-based Deoxyribonucleic acid sequencing. Indian J Med Microbiol 2013;31:343–8. 10.4103/0255-0857.118874 [DOI] [PubMed] [Google Scholar]
- 8.Gothwal S, Dhruw SS, Choudhry R. Neonatal scrub typhus: case report. Pediatr Emerg Care Med Open Access 2016;1:2. [Google Scholar]
- 9.Perumal SK. Primary neonatal scrub typhus-a case report. University Journal of Medicine and Medical Specialities, 2017. [Google Scholar]
- 10.Vajpayee S, Gupta RK, Gupta ML. Scrub typhus causing neonatal hepatitis with acute liver failure-A case series. Indian J Gastroenterol 2017;36:239–42. 10.1007/s12664-017-0761-5 [DOI] [PubMed] [Google Scholar]
- 11.Jajoo M, Kumar D, Manchanda S. Scrub typhus in a new born. J Clin Diagn Res 2017;11:SD05–6. 10.7860/JCDR/2017/26607.10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paramanantham P, Sathyamoorthy DP, Sekar DP. Neonatal scrub typhus: case report. World J Pharm Res 2018;7:836–40. [Google Scholar]
- 13.Samad TEA, Kamalarathnam CN. Clinical profile of scrub typhus in newborns. Indian Pediatr 2020;57:579. [PubMed] [Google Scholar]
- 14.Reddy J, Rajan N, Dinakaran S, et al. Neonatal scrub typhus–A sepsis Mimic. Indian Pediatrics Case Reports 2021;1:173. 10.4103/ipcares.ipcares_138_21 [DOI] [Google Scholar]
- 15.Mehta A, Choudhary S, Bagri DR, et al. Neonatal scrub typhus—A case report. Journal of Neonatology 2022;36:236–9. 10.1177/09732179221100609 [DOI] [Google Scholar]
- 16.Schmidt W-P, Devamani CS, Rose W, et al. Antibody response following scrub typhus infection: clinical cohort study. Trop Med Int Health 2019;24:1455–64. 10.1111/tmi.13322 [DOI] [PubMed] [Google Scholar]


