Abstract
Diabetes is now considered as a ‘silent epidemic’ that claims over four million lives every year, and the disease knows no socioeconomic boundaries. Despite extensive efforts by the National and International organizations, and cutting-edge research, about 11% world’s population is expected to suffer from diabetes (and its complications) by year 2045. This life-long disease damages both the microvasculature and the macrovasculature of the body, and affects many metabolic and molecular pathways, altering the expression of many genes. Recent research has shown that external factors, such as environmental factors, lifestyle and pollutants can also regulate gene expression, and contribute in the disease development and progression. Many epigenetic modifications are implicated in the development of micro- and macro- vascular complications including DNA methylation and histone modifications of several genes implicated in their development. Furthermore, several noncoding RNAs, such as micro RNAs and long noncoding RNAs, are also altered, affecting many biochemical pathways. Epigenetic modifications, however, have the advantage that they could be passed to the next generation, or can be erased. They are now being explored as therapeutical target(s) in the cancer field, which opens up the possibility to use them for treating diabetes and preventing/slowing down its complications.
Keywords: Diabetes, Complications, Epigenetics
1. Introduction
The incidence of diabetes is rising across the globe at an alarming rate, and it has now become an epidemic of the twenty first century; over 460 million people with diabetes in 2019 to 700 million people with diabetes in 2045. It is considered as the 7th leading cause of death in the United States, and accounts for 4.2 million deaths worldwide in 2020. As per International Diabetes Federation, “Diabetes is a serious threat to global health that respects neither socioeconomic status nor national boundaries”. It is a chronic disease, and sustained high circulating glucose due to body’s inability to produce sufficient insulin, or effectively use it, damages both the small (micro) and large (macro) blood vessel. This results in a number of long-term complications; in fact, diabetes epidemic is considered as an ‘epidemic of its complications’. Some of the major complications related to the small blood vessels, ‘microvascular complications’, include retinopathy (eye disease), nephropathy (kidney disease) and neuropathy (neural damage). Over 80% of patients develop retinopathy after 15 years of diabetes; about 50% of diabetic patients develop neuropathy and the risk of amputation, and end-stage kidney disease is several folds greater in diabetic patients than people without diabetes [1]. The major ‘macrovascular’ complications (damage to the arteries) of diabetes include accelerated cardiovascular and cerebrovascular diseases; diabetic patients have 70% higher risk of developing cardiovascular disease [2], and are 2–6 times more susceptible to a stroke, compared to nondiabetic individuals [3]. Diabetes and its complications encompass many metabolic, structural, functional and molecular changes, and alter expression of several genes associated with these abnormalities [4–10]. Although high circulating glucose is considered as the main instigator of diabetic complications, other systemic factors, such as hyperlipidemia and hypertension, also contribute to their development [11,12]. Despite extensive research in the field, the molecular mechanism(s) of the development of these complications, however, remains unclear.
2. Epigenetic modifications
Genes have an important role in health and diseases, but external factors, such as behavior and environment, also are critical in health and diseases, and DNA sequence is not the only determinant of clinical phenotype [13,14]. While genetic changes alter which protein is going to be made, epigenetic changes can turn genes “on” and “off”, and epigenetic modifications, without affecting the primary DNA sequence, are considered as key regulators of gene expression in a disease state. Epigenetics addresses how behavior and environment can cause changes that affect the way the genes begin to work, and these modifications have the advantage that they could be imprinted within the genome to be passed to the next generation, or can also be erased [15]. The major epigenetic changes constitute DNA methylation, histone modifications and noncoding RNAs; while DNA and histone modifications close or open the chromatin structure regulating access of the transcription factors, noncoding RNAs control gene expression at the RNA level [16].
2.1. DNA methylation
Addition of a methyl group to the 5′ position of the cytosine pyrimidine ring by a family of DNA methyl transferases (Dnmts) forms 5-methyl cytosine (5mC) and condenses the chromatin, and among the Dnmts, while Dnmt1 is the maintenance enzyme, Dnmt3a and 3b are de novo enzymes [17]. DNA methylation is a dynamic process and 5mC can be rapidly hydroxymethylated to 5-hydroxymethyl cytosine (5hmC) by a cyclic enzymatic cascade, dioxygenases-ten-eleven translocation (Tets). In general, methylation of CpG silences gene expression, and hydroxymethylation activates them [18–20]. Interpretation of the correct methylation marks is mediated by a family of proteins that bind methylated DNA, the methyl-CpG binding domain proteins (MBDs) [21]. In addition to the methylation of the genomic DNA, DNA in the mitochondria (mtDNA) also undergoes methylation, and is considered to play important role in disease processes [9,22].
2.2. Histone modifications
Certain amino acids (e.g., lysine and arginine) in a histone protein are modified by the addition of one, two, or three methyl groups with the help of histone methyltransferases (HMTs), and the balance is maintained by histone demethylases that remove the methyl group. Depending on the site of methylation and number of methyl groups, histone methylation can either increase or decrease transcription of the gene, for example, trimethylation of lysine 4 on histone H3 (H3K4me3) is associated with an active gene expression, but dimethylation (H3K4me2) can result in both inactive and active euchromatic genes, and dimethylation at lysine 9 (H3K9me2) generally results in gene silencing [23,24].
Histones can also be acetylated on lysine residues in the N-terminal tail, and acetylation relaxes the chromatin structure, allowing access to transcription factors for gene transcription. Thus, histone acetylation is generally associated with active gene expression. A balance between histone acetyltransferases (HATs) and histone deacetylases (HDACs) helps maintain steady-state histone acetylation, and aberrant histone acetylation/deacetylation are implicated in many pathologic conditions, including inflammatory and degenerative diseases [25–27].
The histone code is read in part by histone post-translational modifications modules and their associated complexes, leading to chromatin-templated processes, and they have specific readout mechanisms for individual marks, influencing the outcome of the histone modification [28]. Bromodomain containing protein family is one of the epigenetic reader proteins that bind to specific acetylated lysine residues on histone to facilitate the assembly of transcription complexes [29].
Thus, epigenetic modifications (DNA methylation and histone modifications) are introduced by specific enzymes, the “writers”, recognized and interpreted by the specialized domain containing proteins, the “readers”, and can be erased by a dedicated group of enzymes that remove these chemical tags, the “erasers”.
2.3. Noncoding RNAs
The noncoding RNAs, RNAs with no open reading frame for translation, are highly abundant and functionally important RNAs. They include short non-coding RNAs with less than 200 nucleotides (such as microRNAs, miRNAs), RNAs with more than 200 nucleotides (long non-coding RNAs, LncRNAs) and circular RNAs. Human genome project has shown that a large portion of the noncoding RNAs including miRNAs and LncRNAs, play a variety of biological roles in a multitude of cellular processes, such as regulation of DNA replication, transcriptional activity, and translation and stability [30–32]. Although noncoding RNAs are not considered as epigenetic components, they are involved in epigenetic modifications. miRNAs, a class of 21–23 nucleotide single-stranded RNA molecules, is one of the more abundant classes of gene regulatory molecules. miRNAs can influence the output of many protein-coding genes by targeting mRNAs for cleavage or translational repression and control diverse biological processes [33,34], and their dysregulation is associated with several diseases including cancer, diabetes and Alzheimer’s disease [30,31,35]. Contrary to miRNAs, LncRNAs have more than 200 nucleotides, but do not have any protein coding potential, and are either located within the intergenic stretches of the genome or overlap (sense or antisense direction) protein coding genes. RNA-seq data analysis has revealed over 270,000 lncRNA transcripts in humans [36], with their tissue specificity over four fold higher compared to miRNAs [37]. LncRNAs are also involved in a wide range of cellular mechanisms, and they can regulate metabolic processes via several mechanisms including gene expression, binding with proteins and miRNA sponging [38]. Recent research has documented that LncRNAs play crucial roles in the regulation of pathophysiological processes in many chronic diseases including cancer and diabetes & its complications [39–43].
These epigenetics modifications can act independently, or one modification can lead to the other (Fig. 1), ultimately altering the gene expression [20,44]. Although the role of epigenetic modifications in the cancer field is being investigated for few decades, the role of epigenetics in diabetes and its complications is an emerging area of research.
Fig. 1.
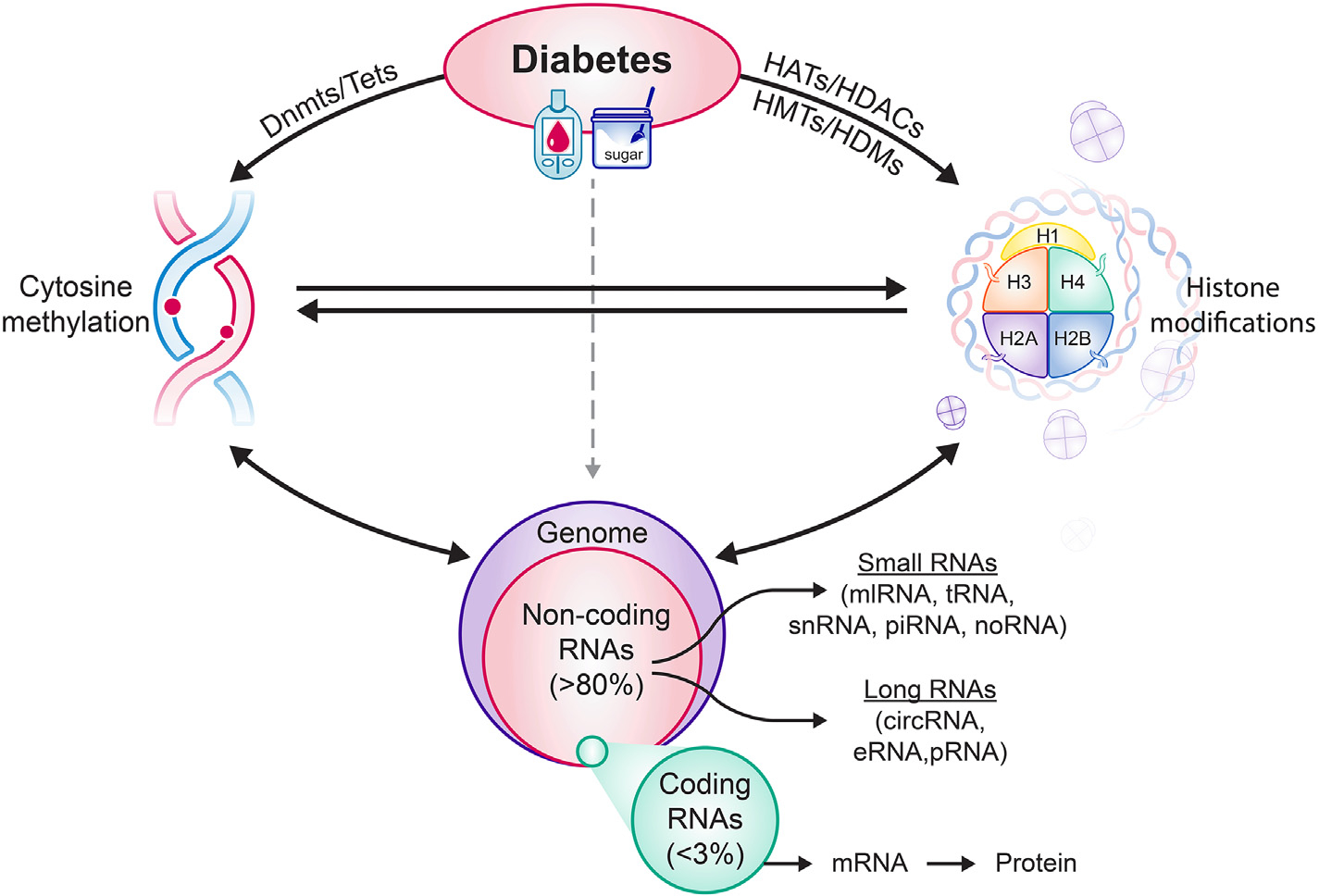
Diabetes alters the activities of the enzymes responsible for maintaing DNA methylation status and histone modifications, and this changes DNA methylation and histone acetylation/methylation status of many genes. Levels of many noncoding RNAs are also altered (up- or down regulated), contributing to suppression or overexpression of many genes. Alterations in DNA methylation affect histone modifcations and noncoding RNAs, and vice-versa. Dnmts = DNA methyl transferases, Tets = dioxygenases-ten-eleven translocases, HATs = Histone acetyltransferases, HDACs = Histone deacetylases, HMTs = histone methyl transferases and HDMs = histone demethylases.
3. Epigenetics and diabetic complications
Nature and nurture interact in a complex manner in the development of diabetes, and epigenetic modifications can modulate the interplay between genes and environment, thus making them as one of the mechanisms by which the environment could be interacting with the genome to modify the risk of diabetes [45]. The role of epigenetics in the development of diabetes is reviewed by many leading investigators [46,47], and is not the focus of this review.
Mounting evidence suggests that DNA methylation, post-translational modifications of histones and long non-coding RNAs play an important role in the initiation, maintenance and progression of both macro- and micro-vascular complications of diabetes [48]. A strong association between DNA methylation and metabolic memory is observed in patients enrolled in The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study [49]. This review will focus on epigenetics and diabetic complications, especially a microvascular complication-diabetic retinopathy.
3.1. Diabetic nephropathy
Diabetic nephropathy, a complex multifactorial disease, is one of the leading causes of chronic kidney disease and end-stage renal disease globally. Kidney disease is prevalent in more than half of the patients with type 2 diabetes and 30% with type 1 diabetes, and a large number of these patients progress to end-stage renal disease [50]. A case control study of 123 type 2 diabetic patients (53 patients with albuminuria and 70 without albuminuria) has shown significantly higher global DNA methylation levels in peripheral blood mononuclear cells of patients with albuminuria compared with those in normal range of albuminuria [51]. Whole blood genomic DNA analysis from diabetic patients with nephropathy have identified differential methylation in a number of genes including Protein Unc-13 Homolog B (UNC13B0, a gene linked with diabetic nephropathy), and analysis of the gene expression omnibus public database has shown 121 genes with hypermethylated sites and 579 genes with hypomethylated sites in the kidney including hypomethylation of Peroxisome proliferator-activated receptor alpha and Glutaminase in tubular cells and hypermethylation of phosphatidylinositol-4-Phosphate 3-Kinase Catalytic Subunit Type 2 Beta,a gene associated with cell proliferation, in glomeruli [52]. Oxidative stress is considered to play a major role in diabetic nephropathy, and Enhancer of zeste homolog 2 (Ezh2), a histone-lysine N-methyltransferase enzyme important in histone methylation-gene suppression, is implicated in the suppression of endogenous antioxidant inhibitor thioredoxin-interacting protein. In podocytes, histone acetyltransferase is shown to regulate a critical sensor of oxidative stress, p66Shc [53]. In addition, many miRNAs and LncRNAs are also implicated in the development of kidney disease; e.g., miR-192 is implicated in podocyte apoptosis, glomerular and tubular hypertrophy and fibrosis and miR-214 and miR-21 in the regulation of inflammatory gene expression and signaling [54–57]. In addition to miRNAs, LncRNA MALAT1 expression is also upregulated in kidneys from diabetic patients with nephropathy [58], and while LncRNA MALAT1 is implicated in inflammation, LncRNA LINC00462 in apoptosis of renal tubular epithelial cells [57]. Thus, epigenetic modifications are intimately associated with the development of diabetic nephropathy via several pathways including inflammation, cell proliferation and apoptosis and autophagy.
3.2. Diabetic neuropathy
Peripheral nerve dysfunction is commonly seen in 20% of patients with type 1 diabetes and 10–15% patients with type 2 diabetes after 20 years of diabetes [59]. As with nephropathy, the pathophysiology of diabetic neuropathy is also not clearly defined. Whole-genome DNA methylation analysis of 186 patients with type 2 diabetes has shown significantly decreased genomic DNA methylation levels in diabetic patients with peripheral neuropathy compared to patients without peripheral neuropathy [60]. A genome-wide study has shown an association between DNA methylation and diabetic peripheral neuropathy in the transcriptional analysis of the mouse models [61]. In diabetic rodents, while miR29c is upregulated in dorsal root ganglions and sciatic nerve, miR146a and 106a are downregulated [62]. Single nucleotide polymorphisms study has shown that while miR-128a variation rs11888095 is significantly correlated to a higher risk of developing diabetic polyneuropathy, miR-146a variation rs2910164 is associated with a lower risk [63]. Furthermore, increased levels of MiR-199a-3p levels are seen in plasma of diabetic patients affected with polyneuropathy [62,64]. Over four hundred differentially expressed LncRNAs, including LncRNA MALAT1 and LncRNA NEAT2, are identified in diabetic patients with peripheral neuropathy [65].
Thus, epigenetic modifications are involved in various aspects of diabetic neuropathy, and targeting them with therapeutic modalities presents a promising opportunity.
3.3. Diabetic retinopathy
Retinopathy is the leading cause of blindness among diabetic patients in 20–74 age group. The retina is damaged by continuously being bathed by high glucose, and the damage is observed in its vasculature and neuronal cells components. Diabetes is a complex disease, and like its other complications, retinopathy is also a multifactorial disease, making it difficult to identify the molecular mechanism of its development. Many metabolic abnormalities are implicated in its development including increased polyol and hexosamine pathways and formation of advanced glycation end products, activation of protein kinase C and increase in oxidative stress with increased accumulation of reactive oxygen species (ROS) [66,67]. In addition to dysfunctional mitochondria, hyperglycemia-induced activation of Ras-related C3 botulinum toxin substrate 1 (Rac1)- NADPH oxidase 2 (Nox2), and other metabolic abnormalities including activation of polyol pathway contribute to increased production of ROS. Experimental data have shown that the production of cytosolic ROS by Rac1-Nox2 precedes mitochondrial damage, and cytochrome c escapes from the leaky mitochondrial membranes into the cytosol, inducing apoptosis, a phenomenon which precedes the development of diabetic retinopathy [68].
Retinal mitochondria are dysfunctional and have partial cristolysis and leaky membranes, and mtDNA is damaged in diabetes. Activated cytosolic Nox2 continues to produce ROS, damaging the mitochondrial membranes and fueling into the vicious cycle of free radicals [9,66,69]. Furthermore, increased oxidative stress also activates a protease, matrix metalloproteinase-9 (MMP-9), and heat shock protein-mediated translocation of MMP-9 to the mitochondria breaks down mitochondrial integrity [70]. The situation is further worsened by impairments in the protective machinery including mitochondrial ROS quenching enzyme manganese superoxide dismutase (encoded by Sod2), mtDNA repair enzyme MutLH (Mlh1), fusion protein mitofusin 2 (Mnf2), and a compromised cytosolic antioxidant system including decrease in intracellular antioxidant glutathione (GSH), reduced activity of the nuclear factor erythroid 2-related factor 2 (Nrf2) and inhibition of catalase and copper-zinc superoxide dismutase [9,66,69].
Diabetes also alters the expression of many genes important in maintaining metabolic, functional and structural integrity of the retina. Diabetic patients with similar risk factors and glycemia can still present variable severity of retinopathy, or even no retinopathy, making genetic variant a good marker. However, despite extensive efforts by some of the leading scientists, good genetic associations in the development of diabetic retinopathy is not yet clearly established. Meta-analyses studies have identified variation in the AKR1B1 gene, the gene encoding for the rate limiting enzyme of the polyol pathway [4,71–73], and also Ala allele of the Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-γ2 gene in type 2 diabetic patients [74]. ‘Diabetic Retinopathy Genetics’ study has shown a novel set of genetic variants involved in the angiogenesis and inflammatory pathways contributing to the progression of diabetic retinopathy, and further investigation of variants is in progress [75]. A recent genome-wide association study of diabetic retinopathy with over 5000 participants, however, has shown no genome-wide significant findings, instead the analysis of protein-protein interaction pathways has suggested possible candidate pathways associated with inflammation for proliferative diabetic retinopathy in African Americans and identified genes implicated in inflammation candidate pathways for proliferative diabetic retinopathy [76]. Thus, any association between genetic factors and diabetic retinopathy remains elusive.
As mentioned above, DNA methylation status is maintained by Dnmts-Tets, and diabetes activates both of these enzymes in the retina and its vasculature [77]. In diabetes, the binding of Dnmt1 is increased at the retinal mtDNA, and this results in its hypermethylation. Hypermethylation of mtDNA leads to attenuated transcription of mtDNA-encoded genes that are critical for the functioning of the electron transport chain, and the electron transport chain continues to be compromised [9,66,69,78]. Cytosine and 5mC are, however, not very stable; while cytosine can be deaminated to form uracil, 5mC can be spontaneously converted to thymine, and the mutation rate of 5mC is much higher than that of cytosine [79]. Regulation of DNA methylation is shown to attenuate diabetes-induced increase in base-pair mismatches in the retinal mtDNA, implying the role of DNA methylation in mtDNA damage [78]. Diabetes also hypermethylates promoter of the mismatch repair enzyme Mlh1 and that of DNA polymerase gamma (POLG), the only polymerase found in mitochondria, further contributing to a compromised mtDNA repair system and suboptimal mtDNA copy numbers [80,81]. DNA methylation status of the promoters of mitochondrial fusion and fission proteins Mfn2, and dynamin-related protein 1 (Drp1) are also altered, resulting in transcriptional suppression of Mfn2 and activation of Drp1 [81], and unpublished results). Thus, epigenetics has a major role in disturbing retinal mitochondrial homeostasis in diabetes, and the damaged mitochondria continues to self-propagate the vicious cycle of free radicals.
As stated above, in the pathogenesis of diabetic retinopathy, cytosolic ROS production by Nox2 and activation of MMP-9 precede mitochondrial damage [70]. In diabetes, DNA at the promoter of Rac1 undergoes active methylation-hydroxymethylation, and 5mC formed by Dnmt is rapidly hydroxymethylated to 5hmC by active Tets, resulting in Rac1 transcriptional activation [82,83]. Similarly, dynamic cytosine methylation- hydroxymethylation of DNA at MMP-9 promoter plays a major role in its transcriptional activation in diabetes [77].
Peripheral blood from patients with proliferative diabetic retinopathy have higher methylation of mtDNA compared to diabetic patients without any signs of retinopathy. Furthermore, these patients also have significantly higher methylated DNA at the promoters of MLH1 and Sod2 [84]. Global DNA methylation in blood is also shown to be a predictive biomarker of proliferative diabetic retinopathy including genes associated with inflammation and ischemia [85]. Thus, the role of DNA methylation in the development of diabetic retinopathy is becoming more convincing.
In addition to DNA methylation, many histone modifications have also been implicated in the development of diabetic retinopathy. A Finnish Study has found an association between the polymorphism in the gene encoding histone methyltransferase, suppressor of variegation 39 homolog 2 (SUV39H2), and diabetic microvascular complications, including retinopathy, suggesting the role of histone modifications in the development of diabetic retinopathy [86]. Increased levels of trimethylated H4K20 and histone methyl transferase SUV420H2 binding at the promoter and the enhancer of retinal Sod2 in diabetes is implicated in its gene repression [87], and increased recruitment of lysine demethylase, LSD1, by demethylating H3K4me at Sod2 promoter plays an important factor in the downregulation of Sod2. Decrease in H3K4me3 and H3K4me1 at the promoter of glutamate cysteine ligase-antioxidant response element region 4 is considered to play an important role in regulating the oxidative stress via regulating the production of intracellular antioxidant, glutathione. Increase in H3K4me1 at Keap1, an intracellular inhibitor of Nrf2, by SET domain-containing 7 histonely-sine methyltransferase (SETD7) is implicated in its overexpression in diabetes [88,89], further compromising the antioxidant defense system. In addition, diabetes decreases H3K9me2 at the promoter of MMP-9, and increases the recruitment of histone-lysine N-methyltransferase enzyme, Ezh2 and elevating H3K27me3 levels [90]. The promoter of Rac1 is shown to have increased H3K9me3 and Suv39H1 binding, but decreased levels of H3K9me2 in diabetes [83].
Histone acetylation of many genes is also affected in diabetes; activities of retinal histone acetylases and deacetylases are altered, and global acetylation of histone H3 is reduced [91,92]. Acetyl H3K9 levels are increased at the promoters of retinal Sod2, MMP-9 and p66Shc [93]. Epigenetic modifications of thioredoxin interacting protein, an endogenous inhibitor of antioxidant thioredoxin, are implicated in sustained Cox2 expression seen in the retina in diabetes [94].
Different epigenetic modifications can be interrelated [20], and the same gene can be regulated by DNA and histone methylation and acetylation. In diabetic retinopathy, hypomethylation of H3K9 at MMP-9 by LSD1 frees up the lysine 9 for acetylation, and acetylated H3K9 allows the recruitment of the transcription factor resulting in MMP-9 transcriptional activation, suggesting a crosstalk between histone methylation and histone acetylation [95]. H3K9 methylation can facilitate Dnmt1 recruitment at the promoter CpG sites [96], and in diabetes, activation of Ezh2 trimethylates H3K27 at retinal MMP-9 promoter, which allows the binding of DNA methylation/hydroxymethylation enzymes, resulting in its transcriptional activation [90]. Furthermore, increased Suv39H1 binding at retinal Rac1 promoter is shown to assists in the recruitment of Dnmt1, resulting in an active DNA methylation-hydroxymethylation and transcriptional activation [82]. As it is clear from the above discussion, the role of epigenetic tools, the ‘writers’ and the ‘erasers’, in the pathogenesis of diabetic complications is gaining a great deal of attention, the role of the ‘readers’ remain an understudied area of research, and needs further attention.
Expression of many miRNAs are altered in the retina/vitreous/serum in diabetes e.g., miRs 20a, 20b, miR-206 and miR-381–3 are dysregulated in the retina and serum of diabetic mice, affecting the expressions of many important factors associated with diabetic retinopathy including vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor and cAMP response element-binding protein 1 [97]. Upregulation of miR-21 is associated with downregulation of PPARα, a ligand-activated nuclear receptor important in regulating the expression of many genes associated with lipid metabolism and insulin signaling [98]. Furthermore, in diabetic patients, expression of miR-216a, miR-34c, miR-410 and miR-203a are significantly upregulated and that of miR-212 is downregulated [99]. Serum from patients with proliferative diabetic retinopathy have altered levels of miR-21, miR-181c, and miR-1179 [100]. Aberrant expressions of several miRNAs are also associated with the metabolic abnormalities considered important in the development of diabetic retinopathy including miR-152, miR-423, miR-146, miR 200b, miR 133b and miR365 [101].
Recent research is also implicating many LncRNAs in the pathogenesis of diabetic retinopathy, e.g., LncRNA MALAT1 is significantly upregulated in the retina in diabetes, and in addition to contributing to increase in the inflammatory mediators, it is also implicated in the regulation of cellular antioxidant defense system; it facilitates the binding of the transcription factor at Keap1 promoter, resulting in activation of Keap1 transcription [41,102]. Upregulation of LncRNA ANRIL and LncRNA NEAT1 is implicated in the nuclear factor NF-kB activation and regulation of VEGF and transforming growth factor-β1 [40,103,104]. LncRNA HOTAIR is suggested to play a role in angiogenesis, oxidative damage and mitochondrial aberrations in experimental models of diabetic retinopathy, and regulation of LncRNA HOTTIP in the retina is shown to regulate the inflammatory mediators [105,106]. Furthermore, knockdown of LncRNA MIAT reduces vascular leakage and inflammation by inhibiting tumor necroptosis factor α and intercellular adhesion molecule [32].
Thus, better understanding of the complex epigenetic mechanisms in the pathogenesis of diabetic retinopathy, and better designed studies utilizing prospective samples would open up the field to transition into clinical use.
3.4. Macrovascular complications
Long-term hyperglycemia-induced epigenetic changes are shown to accelerate the development of atherosclerosis by interfering with the physiological activities of macrophages, endothelial cells and smooth muscle cells [107]. NF-kB is the key pro-inflammatory transcription factor integral in regulating genes associated with vascular inflammation and atherosclerosis, and hyperglycemia is shown to induce various histone lysine modifications at the promoter of the RELA gene (encoding the NFkB-p65 subunit); increased H3K4me1 at RELA genes is implicated in the upregulation of the inflammatory genes in peripheral blood mononuclear cells of patients with type 2 diabetes [108]. Epigenetic modifications are also considered to play a major role in poor wound healing in diabetics; in addition to the regulation of macrophage plasticity and keratinocyte and fibroblast function during wound repair, epigenetic modifications also affect both immune and structural cells in wounds, influencing cell phenotypes and the healing process [108]. In addition, many LncRNAs are aberrantly regulated in diabetic cardiac disease including LncRNA MIAT and LncRNA MALAT1 [109], and miR-129 and miR-335, via MMP-9, regulate diabetic wound healing [110].
Thus, it is clear that epigenetic modifications occupy an important place in the development of diabetic microvascular and macrovascular complications (Fig. 2).
Fig. 2.
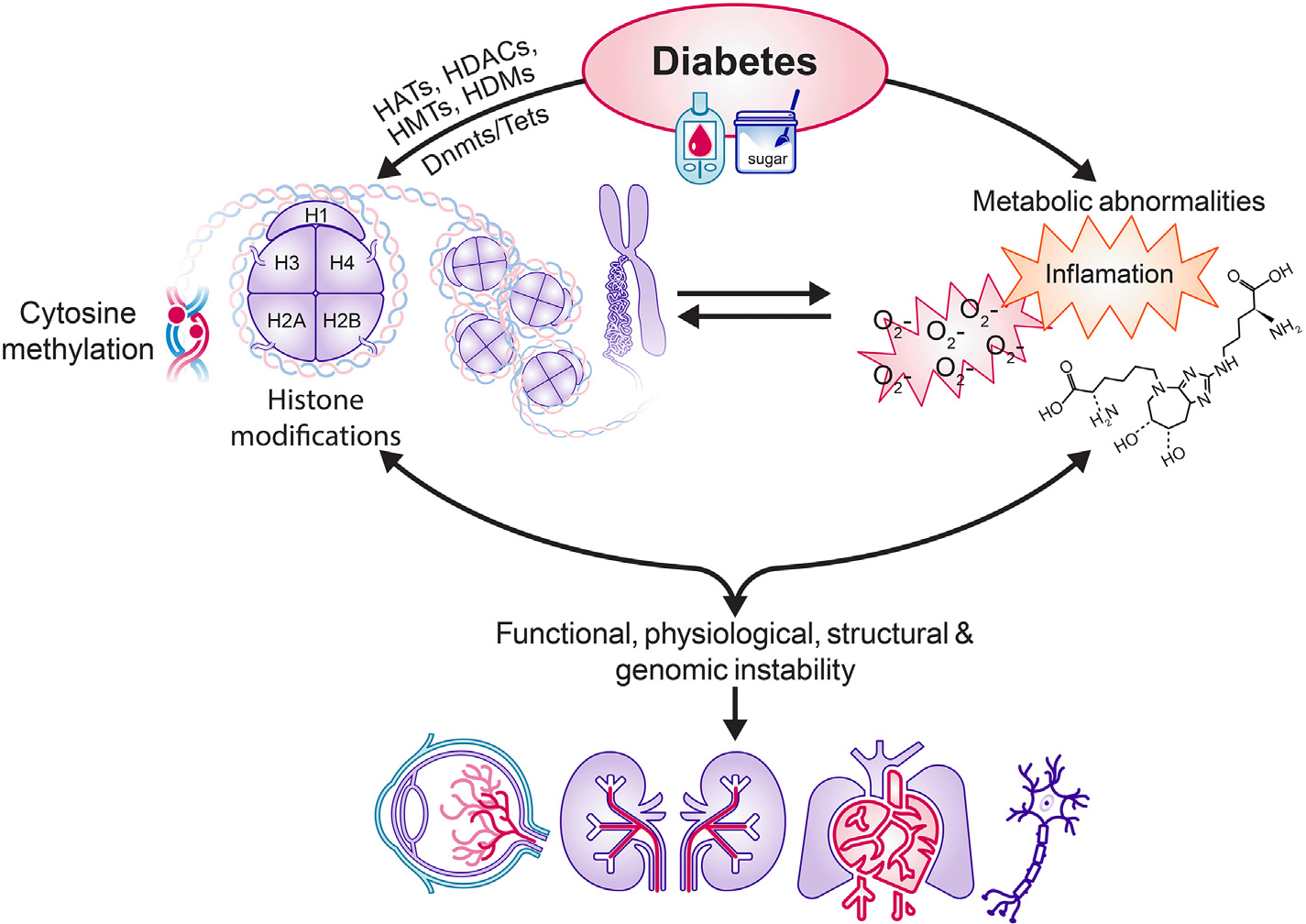
Diabetes facilitates many epigenetic modifications and also results in several metabolic abnormalities including increased oxidative stress, inflammation and formation of advanced glycation end products. Epigentic modifications can result in metabolic abnormalities and vice versa. Ultimately, epigenetic modifications/metabolic abnormalities lead to functional, structural, physiological and genomic instability, resulting in diabetic complications.
However, epigenetic changes detected in biological fluids could play an essential role in the diagnosis of diabetic complications, and targeting them may prevent/slow down further progression of these debilitating complications that a diabetic patients is constantly fearful of facing.
4. Epigenetic drugs and diabetic complications
Epigenetics has a major role in diabetic complications; the dynamic nature of these epigenetic modifications and the ability of the epigenome to be reprogrammed, makes them as attractive targets for therapeutic interventions. Many small molecule compounds that can alter DNA and chromatin structure by modulating the activities of enzymes responsible for maintaining methylation status of DNA and histone modifying enzymes are now being tested in experimental models, and some of them are in ongoing clinical trials. Azacytidine and decitabine, the cytidine analogues that integrate into DNA instead of cytosine forming a covalent bond with Dnmts, are approved by the Food and Drug Administration, and also by the European Medicines Agency for the treatment of acute myeloid leukemia and chronic myelomonocytic leukemia [111–113]. In addition, non-nucleoside analogues, independent of DNA incorporation, including oligonucleotides, natural compounds, S-adenosyl methionine (SAM) competitors, and repurposed drugs, i.e., are also shown to have therapeutic effects incorporation [114]. Curcumin, an active constituent of Curcuma longa is shown to demethylate Nrf2 promoter, increasing its expression [115]. Another phytochemical Sulforaphane, by inhibiting Dnmts, increases the expression of Nrf2 [116]. Flavonoids from tea, soft fruits and soya are potent inhibitors of Dnmts in vitro, and Folates, a group of water-soluble B vitamins found in high concentration in green leafy vegetables, regulate DNA methylation through their ability to generate SAM; people who regularly consume low levels of folate have a significantly increased risk of developing several cancers and cardiovascular disease [117]. This raises a possibility of potential use of Dnmt inhibitors in ameliorating diabetic complications. In fact, experimental models have shown that inhibition of Dnmts in diabetic rodents ameliorate retinal metabolic and functional abnormalities and prevents the development of histopathology characteristic of diabetic retinopathy [83], and the future use of these compounds looks promising.
HDAC inhibitors are now in use clinically for a wide variety of disorders ranging from hematopoietic malignancies to psychiatric disorders, and some are in clinical trials for other diseases. Therapies for non-oncology indications including HIV infection, muscular dystrophies, inflammatory diseases as well as neurodegenerative diseases such as Alzheimer’s disease, frontotemporal dementia and Friedreich’s ataxia are achieving promising clinical progress [118]. Vorinostat, an inhibitor of all zinc-dependent HDACs (except HDAC IIa) was approved by FDA in 2006 for refractory cutaneous, Belinostat, a novel and potent class I and II Hb-HDACI, is in a phase II trial, for women with ovarian cancer [119].
As detailed above, noncoding RNAs play crucial roles in gene expression, and their aberrant expression can lead to disease development. This has resulted in major efforts to therapeutically target these noncoding RNAs, and antisense oligonucleotides are considered as the most direct way to target them in a selective manner. In fact, many antisense oligonucleotide–based therapies have been tested in phase I clinical trials, and some have reached to phase II/III. For example, fomivirsen and mipomersen have received FDA approval to treat cytomegalovirus retinitis and high blood cholesterol, respectively [35]. CRISPR-Cas technology, with a crucial single guide RNA, and potential to edit DNA directly to generate therapeutic effects has gained a great deal of interest, and is in clinical trials for cancer, blood disorders and Leber congenital amaurosis [120].
However, there are many challenges in the development of drugs targeting epigenetic modifications including dynamic nature of these modifications, tissue and cell specificity and the possibility of different degrees of toxicity due to wide distribution of epigenetic enzymes in different tissues. It is critical to first identify the epigenetic marker to be targeted in a disease and use its class or isoform-specific enzyme inhibitors, and carefully determine the optimal dose and identify how the drug should be transferred prior to their clinical applications in bone tissue engineering. Also, targeting noncoding RNAs with oligonucleotides to the correct tissues of interest remains a challenge.
5. Conclusions
It is clear that the human epigenome contributes to diabetic complications and also interacts with, and responds to, various physiological conditions. The dynamic nature of these modifications, and the potential of specific epigenetic alterations to be modified with therapeutics, have paved the way for the emergence of molecular-based epigenetic therapy (Fig. 3). While glycemic control, which may be difficult for some patients to achieve and maintain life-long, undoubtedly remains the best option to avoid or slow down diabetic complications, better understanding and exploitation of the fine-tuning of epigenetic mechanisms operating in diabetic complications are promising in driving forward an unprecedented advance in precision medicine.
Fig. 3.
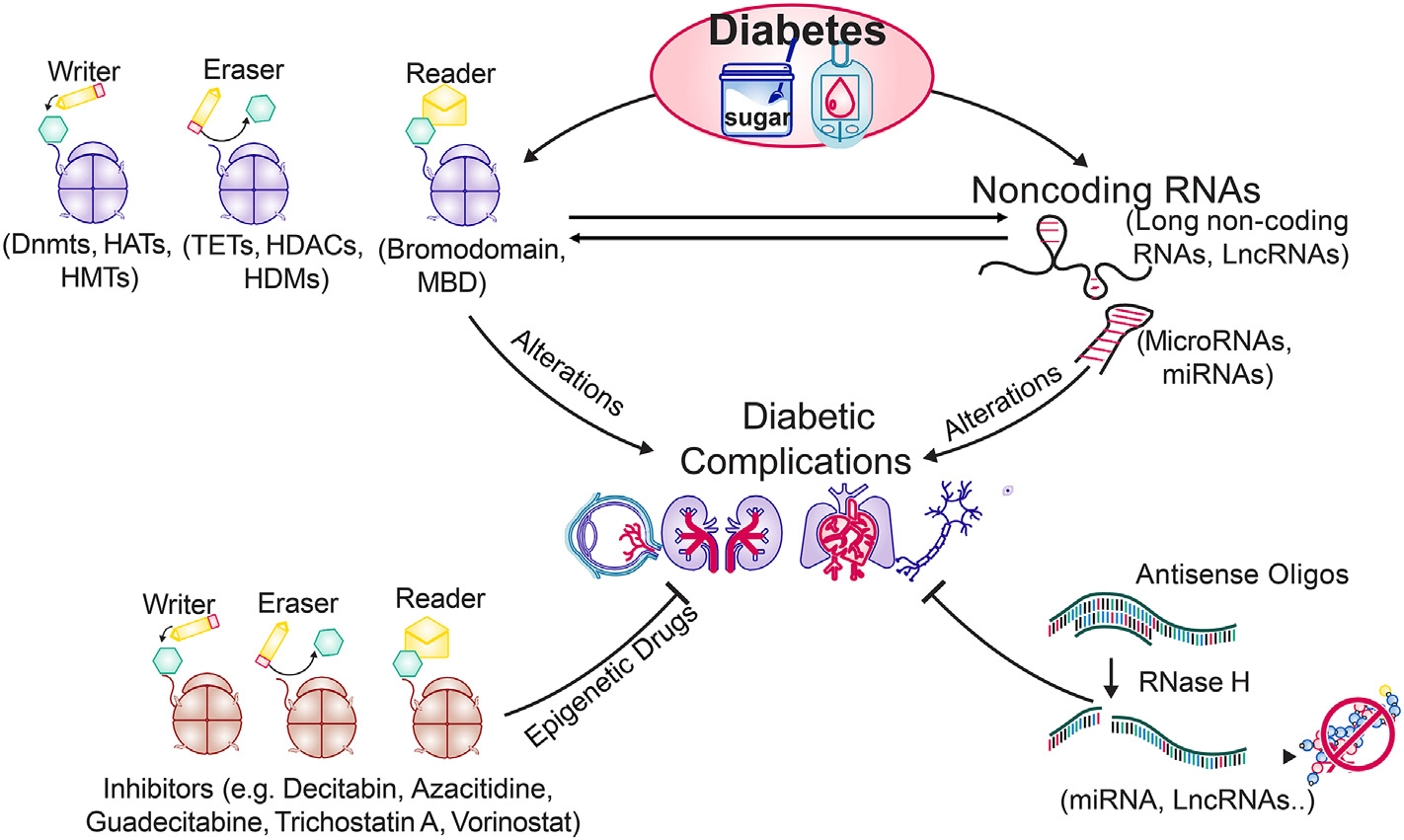
The epigenetic tools, writers/readers/erasers, and noncoding RNAs (miRNA, LncRNA etc.) are altered in diabetes, contributing in the development of diabetic complications. Recent technical advances have identified many small molecule componds that can interfere in the activation/inhibition of these epigenetic tools and target noncoding RNAs. Some of them are in pre-clinical or clinical trials for other chronic diseases (e.g., cancer), and the future for such epigenetic drugs for treating diabetic complications looks promising. (MBD = Methyl-CpG binding domain).
5.1. Expert opinion
The discussion provided in this article clearly suggests that multiple metabolic abnormalities, initiated in a hyperglycemic milieu, are affected by epigenetic modifications, and these modifications play a significant role in modifying the course of the development of diabetic complications. Thus, targeting epigenetic modifications appears a viable option to slow down the development or progression of diabetic complications. Several epigenetic drugs have been approved by US Food and Drug Administration for other diseases, or are in clinical trials (Tables 1 and 2). In addition, novel epigenetic drugs with longer halflife and better safety profile and bioavailability and co-administration of two different epigenetic drugs is now gaining attention. Successful results from other diseases should open up the use of such strategies for the treatment of diabetes and its complications. We recognize that the delivery of the drug to the correct site, especially to the back of the eye (retina) also poses a challenge, however, novel tools in collaboration with biomedical engineering and nanoparticle developmental efforts have shown encouraging outcomes for better tissue targeting. The development of innovative modifications and delivery systems will help in future epigenetic-based therapeutics. Thus, the future of drugs targeting epigenetic modifications, although challenging, poses a great promise for treating diabetes and its complications, and provides hope to a diabetic patient to lead a life with less anxiety of losing vision or kidneys, sensory nerve or heart function.
Table 1.
Epigenetic drugs approved by US Food and Drug Administration.
| Commercial name | Target | Disease |
|---|---|---|
|
| ||
| Vorinostat (Zolinza) | Histone acetvlation | Lymphoma |
| 5 Azacitidine (Vidaza, Decitabine) | DNA methvlation | Mvelodvsplastic svndrome |
| Romidepsin (Ixodax) | Histone acetvlation | Lymphoma |
| Valproic acid | Histone acetvlation | Anti-depressive Neurologic disorders |
| Belinostat (Belodaq) | Histone acetvlation | Peripheral T Cells Lymphoma |
| Panobinostat | Histone acetvlation | Multiple myeloma |
| Tazemetostat | Histone methvlation | Epithelioid sarcomas |
| Panobinostat + Bortezomib + Dexamethasone (Farvdak) | Histone acetvlation | Multiple myeloma |
| Azacitidine+ decitabine or low-dose cvtarabine (Venclexta) | DNA methvlation | Acute myeloid leukemia |
Table 2.
Epigenetic drugs in on-going clinical trials.
| Commercial name | Target | Additional drug | Disease | Trial identifier | Trial phase |
|---|---|---|---|---|---|
|
| |||||
| Azacitidine | DNA methylation | Lenalidomide | Acute Myeloid Leukemia in Remission | NCT04490707 | III |
| Decitabine | DNA methylation | Carboplatin+ Paclitaxel | Ovarian cancer | NCT02159820 | II & III |
| Decitabine | DNA methylation | Camrelizumab | Hodgkin lymphoma | NCT04510610 | II & III |
| Decitabine | DNA methylation | Carboplatin- Paclitaxel | Malignant neoplasm of ovary | NCT02159820 | II & III |
| Decitabine | DNA methylation | Oxaliplatin | Metastatic renal cell carcinoma | NCT04049344 | II |
| SB939 | Histone acetylation | Castration resistant prostate cancer | NCT01075308 | II | |
| Panobinostat | Histone acetylation | Bicalutamide | Prostate cancer | NCT00878436 | II |
| Chidamide | Histone acetylation | Tislelizumab | Bladder Cancer | NCT04562311 | II |
| Azacitidine | DNA methylation | Pembrolizumab | Pancreas Cancer | NCT03264404 | II |
| Sodium phenylbutyrate | Histone acetylation | Huntington’s disease | NCT00212316 | II | |
| Decitabine | DNA methylation | TQB2450 injection+Anlotinib | Digestive system tumors | NCT04611711 | II |
| Sintilimab/ Chidamide | Histone acetylation | Angioimmunoblastic T-cell lymphoma | NCT04831710 | II | |
| Valproic Acid | Histone acetylation | Levocarnitine | Spinal muscular atrophy | NCT00227266 | II |
| Decitabine | DNA methylation | Ara-C | Myeloid carcinoma | NCT03417427 | II |
| Entinostat | Histone acetylation | Pembrolizumab | Melanoma | NCT03765229 | II |
| Tazemetostat | Histone methylation | B-cell lymphomas and follicular lymphoma | NCT01897571 | I & II | |
| Panobinostat | Histone acetylation | HIV infection | NCT01680094 | I & II | |
| Abexinostat | Histone acetylation | Ibrutinib | B-cell and Mantle cell lymphoma | NCT03939182 | I & II |
| Entinostat | Histone acetylation | Tezolizumab + Bevacizumab | Metastatic cancer | NCT03024437 | I & II |
| Azacitidine | DNA methylation | Docetaxel/prednison | Metastatic castration-resistant prostate cancer | NCT00503984 | I & II |
| Decitabine | DNA methylation | Genistein | Leukemias and solid tumors | NCT02499861 | I & II |
| Vorinostat | Histone acetylation | Olaparib | Metastatic breast cancer | NCT03742245 | I |
| Romidepsin | Histone acetylation | Brentuximab vedotin | Cutaneous lymphoma | NCT02616965 | I |
| Tinostamustine | Histone acetylation | Nivolumab | Malignant melanoma | NCT03903458 | I |
| GSK2816126 | HMT-Ezh2 | B cell lymphoma | NCT02082977 | I | |
| Tranylcypromine | Histone demethylation | Tretinoin | Acute myeloid leukemia | NCT02273102 | I |
| Entinostat | Histone acetylation | Enzalutamide | Prostate adenocarcinoma | NCT03829930 | I |
| Valproic acid | Histone acetylation | Bevacizumab | Advanced cancer | NCT00530907 | I |
| MS-275 | Histone acetylation | Enzalutamide | Castration-resistant prostate cancer | NCT03829930 | I |
| Decitabine | DNA methylation | TQB2450 injection | Digestive system tumors | NCT04611711 | I |
Acknowledgements
Authors thank Mr. Steven Pierce of Bio-Medical Communications at Wayne State University for help with developing figures. The work presented in this review was supported in part by grants from the National Institutes of Health (EY014370, EY017313 and EY022230) and from the Thomas Foundation to RAK, and an unrestricted grant to the Ophthalmology Department from Research to Prevent Blindness.
Footnotes
Declaration of competing interest
RAK and GM do not have any conflict of interest.
CRediT authorship contribution statement
RAK collected the literature and wrote/edited the manuscript, GM researched the data and edited the manuscript. RAK and GM approved the final submission.
References
- [1].Maric-Bilkan C Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin Sci (Lond). 2017;131(9):833–46. [DOI] [PubMed] [Google Scholar]
- [2].Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular complications of diabetes: focus on stroke. Endocrine, Metab Immune Dis Drug Targets. 2012;12(2):148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. [DOI] [PubMed] [Google Scholar]
- [5].Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. [DOI] [PubMed] [Google Scholar]
- [6].Kato M, Natarajan R. Diabetic nephropathy–emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Balakumar P, Maung UK, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharm Res. 2016;113(Pt A):600–9. [DOI] [PubMed] [Google Scholar]
- [8].Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30(1):37–45. [DOI] [PubMed] [Google Scholar]
- [9].Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kowluru RA, Mishra M. Epigenetic regulation of redox signaling in diabetic retinopathy: role of Nrf2. Free Radic Biol Med. 2017;103:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Control Diabetes and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- [12].Frank RN. Diabetic retinopathy and systemic factors. Middle East Afr J Ophthalmol. 2015;22(2):151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fraga MF, Esteller M. Towards the human cancer epigenome: a first draft of histone modifications. Cell Cycle. 2005;4(10):1377–81. [DOI] [PubMed] [Google Scholar]
- [14].Moosavi A, Motevalizadeh Ardekani A. Role of epigenetics in biology and human diseases. Iran Biomed J. 2016;20(5):246–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tammen SA, Friso S, Choi SW. Epigenetics: the link between nature and nurture. Mol Asoects Med. 2013;34(4):753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Handy DE, Castro R, Loscalzo J. Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation. 2011;123(19):2145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bergman Y, Cedar H. DNA methylation dynamics in health and disease. Nat Struct Mol Biol. 2013;20(3):274–81. [DOI] [PubMed] [Google Scholar]
- [18].Zhu JK. Active DNA demethylation mediated by DNA glycosylases. Annu Rev Genet. 2009;43:143–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu H, Zhang Y. Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell. 2014;156(1–2):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Du J, Johnson LM, Jacobsen SE, Patel DJ. DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol. 2015;16(9):519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gigek CO, Chen ES, Smith MA. Methyl-CpG-Binding Protein (MBD) family: epigenomic read-outs functions and roles in tumorigenesis and psychiatric diseases. J Cell Biochem. 2016;117(1):29–38. [DOI] [PubMed] [Google Scholar]
- [22].Kowluru RA. Mitochondrial stability in diabetic retinopathy: lessons learned from epigenetics. Diabetes. 2019;68(2):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6(11):838–49. [DOI] [PubMed] [Google Scholar]
- [24].Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol Cell. 2012;48(4):491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–5. [DOI] [PubMed] [Google Scholar]
- [26].Vaissiere T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008;659(1–2):40–8. [DOI] [PubMed] [Google Scholar]
- [27].Xu YM, Du JY, Lau AT. Posttranslational modifications of human histone H3: an update. Proteomics. 2014;14(17–18):2047–60. [DOI] [PubMed] [Google Scholar]
- [28].Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol. 2007;14(11):1025–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Borck PC, Guo LW, Plutzky J. BET epigenetic reader proteins in cardiovascular transcriptional programs. Circ Res. 2020;126(9):1190–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci. 2009;106(28):11667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Paepe B, Lefever S, Mestdagh P. How long noncoding RNAs enforce their will on mitochondrial activity: regulation of mitochondrial respiration, reactive oxygen species production, apoptosis, and metabolic reprogramming in cancer. Curr Genet. 2018;64(1):163–72. [DOI] [PubMed] [Google Scholar]
- [32].Zhang L, Dong Y, Wang Y, Gao J, Lv J, Sun J, et al. Long non-coding RNAs in ocular diseases: new and potential therapeutic targets. FEBS J. 2019;286(12):2261–72. [DOI] [PubMed] [Google Scholar]
- [33].Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174(4):1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huang W MicroRNAs: biomarkers, diagnostics, and therapeutics. Methods Mol Biol. 1617;2017:57–67. [DOI] [PubMed] [Google Scholar]
- [35].Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127(3):761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ma L, Cao J, Liu L, Du Q, Li Z, Zou D, et al. LncBook: a curated knowledgebase of human long non-coding RNAs. Nucleic Acids Res. 2019;47(D1):D128–d34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sun L, Lin JD. Function and mechanism of long noncoding RNAs in adipocyte biology. Diabetes. 2019;68(5):887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gordon AD, Biswas S, Feng B, Chakrabarti S. MALAT1: a regulator of inflammatory cytokines in diabetic complications. Endocrinol Diabetes Metab. 2018;1(2):e00010–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen S, Zhong H, Wang Y, Wang Z, Liang X, Li S, et al. The clinical significance of long non-coding RNA ANRIL level in diabetic retinopathy. Acta Diabetol. 2020;57 (4):409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Radhakrishnan R, Kowluru RA. Long noncoding RNA MALAT1 and regulation of the antioxidant defense system in diabetic retinopathy. Diabetes. 2021;70:227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Boon RA, Jaé N, Holdt L, Dimmeler S. Long noncoding RNAs: from clinical genetics to therapeutic targets? J Am Coll Cardiol. 2016;67(10):1214–26. [DOI] [PubMed] [Google Scholar]
- [43].Leung A, Natarajan R. Long noncoding RNAs in diabetes and diabetic complications. Antioxid Redox Signal. 2018;29(11):1064–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Molina-Serrano D, Schiza V, Kirmizis A. Cross-talk among epigenetic modifications: lessons from histone arginine methylation. Biochem Soc Trans. 2013;41(3):751–9. [DOI] [PubMed] [Google Scholar]
- [45].Ling C, Groop L. Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes. 2009;58:2718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bansal A, Pinney SE. DNA methylation and its role in the pathogenesis of diabetes. Pediatr Diabetes. 2017;18(3):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tremblay J, Hamet P. Environmental and genetic contributions to diabetes. Metabolism. 2019;100s:153952. [DOI] [PubMed] [Google Scholar]
- [48].Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen Z, Miao F, Paterson AD, Lachin JM, Zhang L, Schones DE, et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc Acad Natl Sci. 2016;113(21):E3002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kikkawa R, Koya D, Haneda M. Progression of diabetic nephropathy. Am J Kidney Dis. 2003;43:S19–21. [DOI] [PubMed] [Google Scholar]
- [51].Maghbooli Z, Larijani B, Emamgholipour S, Amini M, Keshtkar A, Pasalar P. Aberrant DNA methylation patterns in diabetic nephropathy. J Diabetes Metab Disord. 2014;13:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Xu L, Natarajan R, Chen Z. Epigenetic risk profile of diabetic kidney disease in highrisk populations. Curr Diab Rep. 2019;19(3):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Keating ST, van Diepen JA, Riksen NP, El-Osta A. Epigenetics in diabetic nephropathy, immunity and metabolism. Diabetologia. 2018;61(1):6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Denby L, Ramdas V, McBride MW, Wang J, Robinson H, McClure J, et al. miR-21 and miR-214 are consistently modulated during renal injury in rodent models. The Am J Pathol. 2011;179(2):661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kato M Noncoding RNAs as therapeutic targets in early stage diabetic kidney disease. Kidney Res Clin Pract. 2018;37(3):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15(6):327–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lv J, Wu Y, Mai Y, Bu S. Noncoding RNAs in diabetic nephropathy: pathogenesis, biomarkers, and therapy. J Diabetes Res. 2020;2020 (3960857-). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tanwar VS, Reddy MA, Natarajan R. Emerging role of long non-coding RNAs in diabetic vascular complications. Front Endocrinol (Lausanne). 2021;12 (665811-). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang HH, Han X, Wang M, Hu Q, Li S, Wang M, et al. The association between genomic DNA methylation and diabetic peripheral neuropathy in patients with Type 2 diabetes mellitus. J Diabetes Res. 2019;2019:2494057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hur J, O’Brien PD, Nair V, Hinder LM, McGregor BA, Jagadish HV, et al. Transcriptional networks of murine diabetic peripheral neuropathy and nephropathy: common and distinct gene expression patterns. Diabetologia. 2016;59(6):1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Simeoli R, Fierabracci A. Insights into the role of MicroRNAs in the onset and development of diabetic neuropathy. Int J Mol Sci. 2019;20(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ciccacci C, Morganti R, Di Fusco D, D’Amato C, Cacciotti L, Greco C, et al. Common polymorphisms in MIR146a, MIR128a and MIR27a genes contribute to neuropathy susceptibility in type 2 diabetes. Acta Diabetol. 2014;51(4):663–71. [DOI] [PubMed] [Google Scholar]
- [64].Li YB, Wu Q, Liu J, Fan YZ, Yu KF, Cai Y. miR-199a-3p is involved in the pathogenesis and progression of diabetic neuropathy through downregulation of SerpinE2. Mol Med Rep. 2017;16(3):2417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Luo L, Ji LD, Cai JJ, Feng M, Zhou M, Hu SP, et al. Microarray analysis of long noncoding RNAs in female diabetic peripheral neuropathy patients. Cellular physiology and biochemistry. Int J Exp Cell Physiol. 2018;46(3):1209–17. [DOI] [PubMed] [Google Scholar]
- [66].Kowluru RA, Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Bophys Acta. 2015;1852(11):2474–83. [DOI] [PubMed] [Google Scholar]
- [67].Kowluru RA, Santos JM, Mishra M. Epigenetic modifications and diabetic retinopathy. Biomed Res Int. 2013;2013:635284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sahajpal N, Kowluru A, Kowluru RA. The regulatory role of Rac1, a small molecular weight GTPase, in the development of diabetic retinopathy. J Clin Med. 2019;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kowluru RA, Mishra M. Therapeutic targets for altering mitochondrial dysfunction associated with diabetic retinopathy. Expert Opin Ther Targets. 2018;22(3):233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kowluru RA, Mishra M. Regulation of matrix metalloproteinase in the pathogenesis of diabetic retinopathy. Prog Mol Biol Transl Sci. 2017;148:67–85. [DOI] [PubMed] [Google Scholar]
- [71].Donaghue KC, Margan SH, Chan AK, Holloway B, Silink M, Rangel T, et al. The association of aldose reductase gene (AKR1B1) polymorphisms with diabetic neuropathy in adolescents. Diabet Med. 2005;22:1315–20. [DOI] [PubMed] [Google Scholar]
- [72].Kitada M, Zhang Z, Mima A, King GL. Molecular mechanisms of diabetic vascular complications. J Diabetes Invest. 2010;1:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Santos JM, Mohammad G, Zhong Q, Kowluru RA. Diabetic retinopathy, superoxide damage and antioxidant. Curr Pharm Biotechnol. 2011;12:352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ma J, Li Y, Zhou F, Xu X, Guo G, Qu Y. Meta-analysis of association between the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-γ2 gene and diabetic retinopathy in Caucasians and Asians. Mol Vis. 2012;18:2352–60. [PMC free article] [PubMed] [Google Scholar]
- [75].Cabrera AP, Monickaraj F, Rangasamy S, Hobbs S, McGuire P, Das A. Do genomic factors play a role in diabetic retinopathy? J Clin Med. 2020;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pollack S, Igo RP Jr, Jensen RA, Christiansen M, Li X, Cheng CY, et al. Multiethnic genome-wide association study of diabetic retinopathy using liability threshold modeling of duration of diabetes and glycemic control. Diabetes. 2019;68:441–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kowluru RA, Shan Y, Mishra M. Dynamic DNA methylation of matrix metalloproteinase-9 in the development of diabetic retinopathy. Lab Investig. 2016;96(10):1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mishra M, Kowluru RA. DNA methylation-a potential source of mitochondria DNA base mismatch in the development of diabetic retinopathy. Mol Neurobiol. 2019;56:88–101. [DOI] [PubMed] [Google Scholar]
- [79].Holliday R, Grigg GW. DNA methylation and mutation. Mutat Res. 1993;285(1):61–7. [DOI] [PubMed] [Google Scholar]
- [80].Tewari S, Zhong Q, Santos JM, Kowluru RA. Mitochondria DNA replication and DNA methylation in the metabolic memory associated with continued progression of diabetic retinopathy. Invest Ophtahlmol Vis Sci. 2012;53(8):4881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kowluru RA, Mohammad G. Epigenetics and mitochondrial stability in the metabolic memory phenomenon associated with continued progression of diabetic retinopathy. Sci Rep. 2020;10(1):6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Duraisamy AJ, Mishra M, Kowluru A, Kowluru RA. Epigenetics and regulation of oxidative stress in diabetic retinopathy. Invest Ophtahlmol Vis Sci. 2018;59(12):4831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kowluru RA, Radhakrishnan R, Mohammad G. Diabetic retinopathy and epigenetic modifications: role of histone methylation and DNA methylation. Sci Rep. 2021;11 (1):14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Duraisamy AJ, Radhakrishnan R, Seyoum B, Abrams GW, Kowluru RA. Epigenetic modifications in peripheral blood as potential noninvasive biomarker of diabetic retinopathy. Transl Vis Sci Technol. 2019;8(6):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Agardh E, Lundstig A, Perfilyev A, Volkov P, Freiburghaus T, Lindholm E, et al. Genome-wide analysis of DNA methylation in subjects with type 1 diabetes identifies epigenetic modifications associated with proliferative diabetic retinopathy. BMC Med. 2015;13:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Syreeni A, El-Osta A, Forsblom C, Sandholm N, Parkkonen M, Tarnow L, et al. Genetic examination of SETD7 and SUV39H1/H2 methyltransferases and the risk of diabetes complications in patients with type 1 diabetes. Diabetes. 2011;60:3073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zhong Q, Kowluru RA. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60:1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mishra M, Zhong Q, Kowluru RA. Epigenetic modifications of Nrf2-mediated glutamate-cysteine ligase: implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression. Free Radic Biol Med. 2014;75:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mishra M, Zhong Q, Kowluru RA. Epigenetic modifications of Keap1 regulate its interaction with the protective factor Nrf2 in the development of diabetic retinopathy. Invest Ophtahlmol Vis Sci. 2014;55(11):7256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Duraisamy AJ, Mishra M, Kowluru RA. Crosstalk between histone and DNA methylation in regulation of retinal matrix metalloproteinase-9 in diabetes. Invest Ophtahlmol Vis Sci. 2017;58(14):6440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhong Q, Kowluru RA. Role of histone acetylation in the development of diabetic retinopathy and the metabolic memory phenomenon. J Cell Biochem. 2010;110 (6):1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kowluru RA, Santos JM, Zhong Q. Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Invest Ophtahlmol Vis Sci. 2014;55:5653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mishra M, Duraisamy AJ, Bhattacharjee S, Kowluru RA. Adaptor protein p66Shc: a link between cytosolic and mitochondrial dysfunction in the development of diabetic retinopathy. Antioxid Redox Signal. 2019;30:1621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Perrone L, Devi TS, Hosoya K, Terasaki T, Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221:262–72. [DOI] [PubMed] [Google Scholar]
- [95].Zhong Q, Kowluru RA. Regulation of matrix metalloproteinase-9 by epigenetic modifications and the development of diabetic retinopathy. Diabetes. 2013;62 (7):2559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Estève PO, Chin HG, Benner J, Feehery GR, Samaranayake M, Horwitz GA, et al. Regulation of DNMT1 stability through SET7-mediated lysine methylation in mammalian cells. Proc Natl Acad Sci. 2009;106:5076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Platania CBM, Maisto R, Trotta MC, D’Amico M, Rossi S, Gesualdo C, et al. Retinal and circulating miRNA expression patterns in diabetic retinopathy: an in silico and in vivo approach. Brit J Pharmacol. 2019;176(13):2179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chen Q, Qiu F, Zhou K, Matlock HG, Takahashi Y, Rajala RVS, et al. Pathogenic role of microRNA-21 in diabetic retinopathy through downregulation of PPARα. Diabetes. 2017;66(6):1671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Shafabakhsh R, Aghadavod E, Mobini M, Heidari-Soureshjani R, Asemi Z. Association between microRNAs expression and signaling pathways of inflammatory markers in diabetic retinopathy. J Cell Physiol. 2019;234(6):7781–7. [DOI] [PubMed] [Google Scholar]
- [100].Qing S, Yuan S, Yun C, Hui H, Mao P, Wen F, et al. Serum miRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell Physiol Biochem. 2014;34 (5):1733–40. [DOI] [PubMed] [Google Scholar]
- [101].Mastropasqua R, Toto L, Cipollone F, Santovito D, Carpineto P, Mastropasqua L. Role of microRNAs in the modulation of diabetic retinopathy. Prog Retin Eye Res. 2014;43C:92–107. [DOI] [PubMed] [Google Scholar]
- [102].Biswas S, Sarabusky M, Chakrabarti S. Diabetic retinopathy, lncRNAs, and inflammation: a dynamic, interconnected network. J Clin Med. 2019;8(7):1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Thomas AA, Feng B, Chakrabarti S. ANRIL: a regulator of VEGF in diabetic retinopathy. Invest Ophtahlmol Vis Sci. 2017;58(1):470–80. [DOI] [PubMed] [Google Scholar]
- [104].Shao K, Xi L, Cang Z, Chen C, Huang S. Knockdown of NEAT1 exerts suppressive effects on diabetic retinopathy progression via inactivating TGF-β1 and VEGF signaling pathways. J Cell Physiol. 2020;235(12):9361–9. [DOI] [PubMed] [Google Scholar]
- [105].Sun Y, Liu YX. LncRNA HOTTIP improves diabetic retinopathy by regulating the p38-MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22(10):2941–8. [DOI] [PubMed] [Google Scholar]
- [106].Biswas S, Feng B, Chen S, Liu J, Aref-Eshghi E, Gonder J, et al. The long non-coding RNA HOTAIR is a critical epigenetic mediator of angiogenesis in diabetic retinopathy. Invest Ophtahlmol Vis Sci. 2021;62(3):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Keating ST, Plutzky J, El-Osta A. Epigenetic changes in diabetes and cardiovascular risk. Circ Res. 2016;118(11):1706–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].den Dekker A, Davis FM, Kunkel SL, Gallagher KA. Targeting epigenetic mechanisms in diabetic wound healing. Transl Res. 2019;204:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Pant T, Dhanasekaran A, Fang J, Bai X, Bosnjak ZJ, Liang M, et al. Current status and strategies of long noncoding RNA research for diabetic cardiomyopathy. BMC Cardiovasc Disord. 2018;18(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wang W, Yang C, Wang XY, Zhou LY, Lao GJ, Liu D, et al. MicroRNA-129 and −335 promote diabetic wound healing by inhibiting Sp1-mediated MMP-9 expression. Diabetes. 2018;67(8):1627–38. [DOI] [PubMed] [Google Scholar]
- [111].Pasculli B, Barbano R, Parrella P. Epigenetics of breast cancer: biology and clinical implication in the era of precision medicine. Semin Cancer Biol. 2018;51:22–35. [DOI] [PubMed] [Google Scholar]
- [112].Bansal A, Pinney SE. DNA methylation and its role in the pathogenesis of diabetes. Pediatr Diabetes. 2017;18(3):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Nunes SP, Henrique R, Jerónimo C, Paramio JM. DNA methylation as a therapeutic target for bladder cancer. Cells. 2020;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Castillo-Aguilera O, Depreux P, Halby L, Arimondo PB, Goossens L. DNA methylation targeting: the DNMT/HMT crosstalk challenge. Biomolecules. 2017;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Khor TO, Huang Y, Wu TY, Shu L, Lee J, Kong AN. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem Pharmacol. 2011;82(9):1073–8. [DOI] [PubMed] [Google Scholar]
- [116].Zhang C, Su ZY, Khor TO, Shu L, Kong AN. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochem Pharmacol. 2013;85(9):1398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Duthie SJ. Epigenetic modifications and human pathologies: cancer and CVD. Proc Nutr Soc. 2011;70(1):47–56. [DOI] [PubMed] [Google Scholar]
- [118].Bondarev AD, Attwood MM, Jonsson J, Chubarev VN, Tarasov VV, Schiöth HB. Recent developments of HDAC inhibitors: emerging indications and novel molecules. Br J Clin Pharmacol. 2021. May 10 (Online ahead of print). [DOI] [PubMed] [Google Scholar]
- [119].Lakshmaiah KC, Jacob LA, Aparna S, Lokanatha D, Saldanha SC. Epigenetic therapy of cancer with histone deacetylase inhibitors. J Cancer Res Ther. 2014;10(3):469–78. [DOI] [PubMed] [Google Scholar]
- [120].Zhou LY, Qin Z, Zhu YH, He ZY, Xu T. Current RNA-based therapeutics in clinical trials. Curr Gene Ther. 2019;19(3):172–96. [DOI] [PubMed] [Google Scholar]


