INTRODUCTION
An estimated 3.5 million women in the United States are living with breast cancer, with nearly 290,000 new cases expected in 2022.1 During the past several decades, there have been significant strides in breast cancer diagnosis and management. The appreciation for tumor subtypes defined by receptor status has fundamentally changed our understanding of breast cancer and is used to direct treatment strategies. For estrogen receptor-positive (ER+) tumors, treatment with endocrine therapy such as ER modulators or aromatase inhibitors dramatically improves outcomes.2 For those with overexpression or amplification of the human epidermal growth factor receptor-2 (HER-2), targeted treatment with HER-2 antibody-based therapy is now standard.3
For many years, investigators have studied whether these receptors can also be used for imaging breast tumors.4,5 Such targeted molecular imaging has the promise of improved tumor detection, potentially determination of response to therapy, and could guide treatment strategies and improve surgical approaches. The imaging agent 18F-fluoroestradiol (18F-FES) is a PET radiopharmaceutical used for noninvasive imaging of the ER in vivo. In this article, we discuss the history and development of 18F-FES PET, its clinical applications, its potential utility in invasive lobular carcinoma (ILC), and its use with the novel imaging tool, dedicated breast PET (dbPET).
DISCUSSION
History and Development of 18F-Fluoroestradiol
The development of 18F-FES is largely credited to Dr John A. Katzenellenbogen, a chemist from the University of Illinois. His early study began by efforts to obtain gamma-emitting estrogens, specifically using radioiodinated steroidal estrogens with estradiol substituted at the 16α-position. Guided by the study of Dr Richard Hochberg, who found that 16α-[125I]iodoestradiol had better ER-binding affinities in vivo, Katzenellenbogen began experimenting with other radioisotopes substituted at the 16α-position. Eventually, his team identified that 16α-[77Br]bromoestradiol had improved binding over 16α-[125I]iodoestradiol, but translation from rats to humans proved disappointing.6 A change in isotope to fluorine-18 allowed the team to benefit from the timely progress in PET imaging technology. The team prepared a variety of [18F]-labeled steroidal and nonsteroidal estrogens but focusing on the 16α-[18F]-FES in particular, which they named 18F-FES. In 1984, Katzenellenbogen and his team first reported favorable bio-distribution characteristics of 18F-FES in rats, and the first images of ER + breast tumors in human subjects were published in 1988.7 Subsequent years have seen studies evaluating the technical validity, clinical validity, and clinical utility of 18F-FES in the diagnosis and management of breast cancer, with more studies ongoing.8 Approval from the US Food and Drug Administration (FDA) for its use in recurrent or metastatic ER + breast cancers in conjunction with biopsy was received in May 2020.
Clinical Applications of 18F-Fluoroestradiol in Breast Cancer
18F-FES has binding affinity for the ER, ranging from 60% to 100% across reported studies.9-11 As such, when paired with standard imaging procedures such as PET and computed tomography (CT), 18F-FES can serve as a “noninvasive whole-body biopsy” to identify ER+ lesions.9
18F-FES is administered intravenously over 1 to 2 minutes, with PET image acquisition occurring after a 30 to 100-minute uptake period, with imaging at 80 minutes recommended.9,12-15 The agent is metabolized by the liver and excreted through the biliary tract into the small bowel, with additional excretion by the kidneys. Of note, physiologic uptake is more pronounced in liver and small bowel than kidney and bladder.16 Ligand quantities are low enough to avoid physiological effects.17 Because 18F-FES binds to the ER, the use of ER antagonists or degraders results in decreased 18F-FES PET signal.18 The currently recommended washout period before imaging with 18F-FES is 8 weeks for selective ER modulators (SERMs) and 28 weeks for selective ER downregulators/degraders (SERDs). As a result, repeat 18F-FES PET imaging is generally only feasible in patients not on SERMs or SERDs.
Detection of estrogen receptor
There have been several studies suggesting a strong correlation between 18F-FES uptake and ER positivity as measured by immunohistochemistry (IHC). Compared with IHC, 18F-FES PET was found to have a pooled sensitivity of 82% and specificity of 95% for ER positivity in a meta-analysis of 9 prospective studies.19 A more recent meta-analysis evaluating the ability of FES to determine ER status of breast and non-breast lesions in patients with metastatic breast cancer found an overall sensitivity of 81% and specificity of 85%.20 Fig. 1 demonstrates a left breast cancer visible on dynamic contrast-enhanced MRI, with no uptake on 18F-FES PET, consistent with biopsy-proven ER-negative status. One study found that 18F-FES had a positive predictive value of 100% and a negative predictive value of 78%, which changed depending on the threshold of the maximum standardized uptake value (SUVmax),12 with the caveat that patients with bone metastases were excluded. In this study, the authors suggest that tumors that are ER + on IHC but negative on 18F-FES PET might reflect the lack of ER functionality as opposed to a false-negative imaging test; more investigation into this hypothesis is needed.
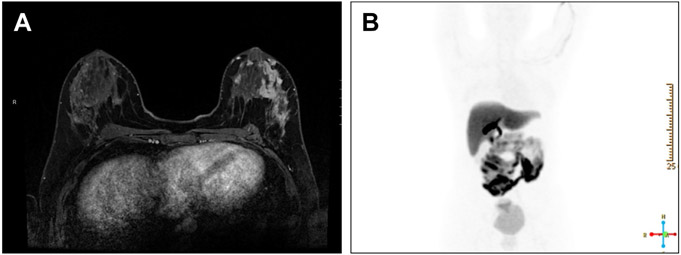
Fig. 1.
Patient with left breast multicentric left breast ER-negative, progesterone receptor-negative, HER 2-positive IDC. (A) shows dynamic contrast-enhanced MRI showing extensive mass and nonmass enhancement in outer left breast. In (B), 18F-FES PET scan shows no uptake in left breast, consistent with ER negativity of known tumor, with expected uptake in liver and gastrointestinal tract.
Although IHC analysis remains the gold standard for determining the presence of ER, there are benefits of 18F-FES over biopsy alone. One potential advantage to 18F-FES is the ability to noninvasively assay the whole tumor, providing a more comprehensive assessment of functional ER status than IHC of a limited tumor sample. Evaluation of 18F-FES uptake within a tumor could reflect intratumoral heterogeneity not elucidated from biopsy alone. Moreover, receptor status may not be uniform across all tumors in a given patient with metastatic disease. Yang and colleagues showed that 37.5% of patients with metastatic breast cancer presented with both ER+ and ER-disease, which may or may not be identified based on biopsy alone, depending on the number of sites biopsied. 18F-FES, however, can help identify metastatic lesions based on the uptake of the tracer in a single test, which has the potential to guide treatment, improve response to therapy, and perhaps even prolong survival.9 Additionally, whole-body 18F-FES PET can be used to evaluate multiple lesions in a noninvasive manner, including sites such as the brain that would be challenging to biopsy. In fact, imaging of brain metastases is of particular clinical interest because PET scanning using fluorodeoxyglucose (FDG-PET) can be limited due to the high FDG avidity of normal cerebral cortex and deep gray nuclei.21 In one study by Ivanidze and colleagues21, 18F-FES brain PET/CT demonstrated increased avidity in a brain lesion suggesting metastatic disease, although also showing decreased avidity in a lesion that was thought to represent posttreatment change.
Systemic therapy selection
One of the proposed clinical applications for 18F-FES is for therapy selection. Some of the initial studies assessing 18F-FES and treatment response were in patients with advanced breast cancer treated with tamoxifen.22-24 Mortimer and colleagues23 postulated that 18F-FES PET could be used to identify hormonally responsive cancers. In their pivotal 2011 study, the authors found that the functional status of ER can be determined using 18F-FES PET and can predict response to tamoxifen. In another study of 51 patients with advanced ER + breast cancer, higher baseline 18F-FES uptake was predictive of response to tamoxifen; additionally, a detectable “metabolic flare” on FDG-PET after estradiol challenge was observed in patients who were more responsive to tamoxifen.25 Indeed, combining characteristics of tumors on both 18F-FES and FDG-PET may allow for further patient stratification.26
In the metastatic setting, disease with low uptake of 18F-FES has been associated with worse response to endocrine treatment, with a cohort study of 47 patients with pretreated metastatic breast cancer identifying a threshold SUV of less than 1.5 being predictive of lack of response.24 Interestingly, van Kruchten and colleagues27 found that although baseline 18F-FES uptake was not associated with disease progression, the persistence of uptake on follow-up 18F-FES PET after SERD initiation was associated with earlier progression, possibly indicating incomplete ER degradation.
18F-FES has also been used to assess potential benefit of other therapeutic agents used in metastatic breast cancer, including cyclin-dependent kinase (CDK) inhibitors. Although adding CDK inhibitors to endocrine treatment has been shown to improve invasive disease-free survival in some patients with metastatic ER + breast cancer, better understanding of ER heterogeneity could potentially improve patient selection for treatment.28 In a prospective analysis of 30 patients with metastatic ER + breast cancer, ER heterogeneity was determined by measuring what proportion of lesions visible on either FDG-PET or CT were avid on 18F-FES PET.29 Those with the highest proportion of 18F-FES-positive disease at baseline had the longest time to progression on combination endocrine therapy with CDK4/6 inhibition. Additionally, those with better response to combination treatment, as measured by reduced lesion metabolic activity on FDG-PET, had higher 18F-FES uptake. These findings suggest that combining 18F-FES imaging with other imaging modalities can be used to differentiate among those with ER-positive disease and identify heterogeneous disease patterns that might benefit from differing treatment strategies.
A novel potential application of 18F-FES imaging includes determining whether resistance to endocrine therapy has been overcome. In a recent study, histone deacetylase inhibition with vorinostat was used with the goal of restoring endocrine therapy sensitivity in 23 patients with metastatic ER + breast cancer.30 Although subsequent 18F-FES PET imaging did not show increased uptake compared with baseline to indicate restored ER ligand binding, higher baseline 18F-FES uptake was again associated with improved progression free survival.30 The authors note, however, that although 18F-FES uptake indicates the ability of the ER to bind ligand, this is not necessarily indicative of endocrine therapy sensitivity, particularly given multiple pathways influencing such sensitivity, and challenges with the definition of sensitivity which may differ by disease site (eg, disease progression in visceral versus bone metastases). However, achieving complete blockade or suppression of ER as measured by lack of 18F-FES uptake on known ER + lesions has been reported for purposes of finding optimal doses for ER-modulating agents.31
Resolving clinical dilemmas
18F-FES PET may be useful in patients with ER + breast cancer who present with clinical dilemmas where conventional workup is inconclusive. For example, a Dutch study included patients with metastatic breast cancer whose staging imaging, including CT chest/abdomen/pelvis, abdominal ultrasound, and bone scan, yielded equivocal findings.32 18F-FES PET was most sensitive for bone metastases and improved diagnostic understanding in 88% of patients, leading to a change in therapy in 48% of those patients. Similar results were presented by Sun and colleagues,33 who found that 18F-FES PET aided the diagnosis and changed treatment plans in approximately half of patients in their study. Fig. 2 demonstrates imaging findings from a patient with biopsy-proven ER + ILC of the left breast with imaging studies identifying an oropharyngeal lesion of unclear cause despite attempted biopsy; this case illustrates the potential additive role of 18F-FES PET for clinical decision-making.
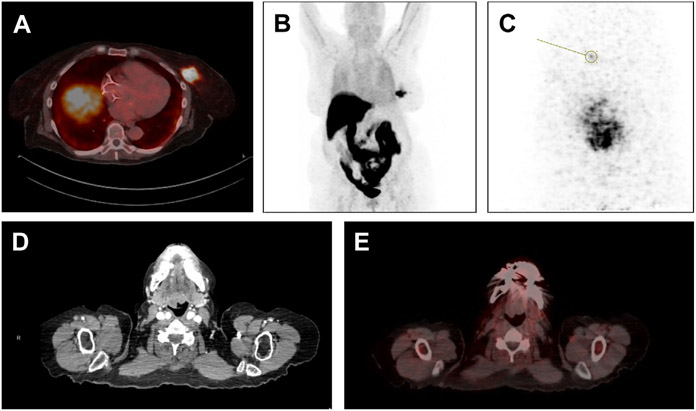
Fig. 2.
18F-FES imaging in patient with left breast ER-positive HER2-negative ILC and oropharyngeal mass for which nondiagnostic biopsy had been performed. (A) Shows left breast mass with 18F-FES uptake on fused PET-CT reflecting ER positivity. (B) Shows 18-F PET highlighting tumor in left breast, with expected uptake of 18F-FES in liver and gastrointestinal tract. In (C), left breast is imaged with dedicated breast PET using 18-F FES, identifying a possible satellite lesion anterior to known tumor. Finally, (D) shows image from CT scan demonstrating irregular oropharyngeal mass, and fused image from 18-F FES PET-CT (E) shows no uptake in mass, suggesting that this mass was unrelated to primary ILC tumor.
The Use of 18F-Fluoroestradiol in Invasive Lobular Carcinoma of the Breast
Although 18F-FES PET may have wide applicability in the diagnosis and management of breast cancer, there are certain subtypes of breast cancer that may benefit even more from this technology. One such subtype is ILC. ILC is the second most common type of breast cancer, accounting for 10% to 15% of all patients with breast cancer. Due to the infiltrative growth pattern of ILC compared with the more common invasive ductal carcinoma (IDC), it is often harder to detect with standard imaging modalities, including FDG-PET. Moreover, nearly 95% of all lobular cancers are ER positive. As such, 18F-FES PET is promising for the evaluation of this breast cancer subtype.
One of the first studies to evaluate the use of 18F-FES PET in ILC was a case series by Venema and colleagues in 2017.34 The authors reported 3 lobular breast cancer cases, where confirmation of metastatic disease was imperative for subsequent treatment, and biopsy was not possible. In these 3 cases, standard imaging modalities such as CT, MRI, and FDG-PET returned equivocal results, whereas 18F-FES PET provided definitive diagnosis of metastatic lesions. The authors concluded that 18F-FES PET may have added value compared with conventional staging mechanisms.
Further studies have compared the use of 18F-FES versus FDG-PET in the diagnosis of metastatic ILC. Ulaner and colleagues35 evaluated results from 7 patients with ILC who underwent both 18F-FES and FDG-PET imaging. The authors found that 18F-FES detected more metastatic lesions in patients with ILC compared to FDG-PET, and no patients presented with only FDG-avid metastases. As such, 18F-FES was considered to compare favorably to FDG for assessing metastases in ILC patients. Fig. 3 illustrates a case of de novo metastatic ER + ILC in which additional lesions were seen on 18F-FES PET compared with FDG-PET.
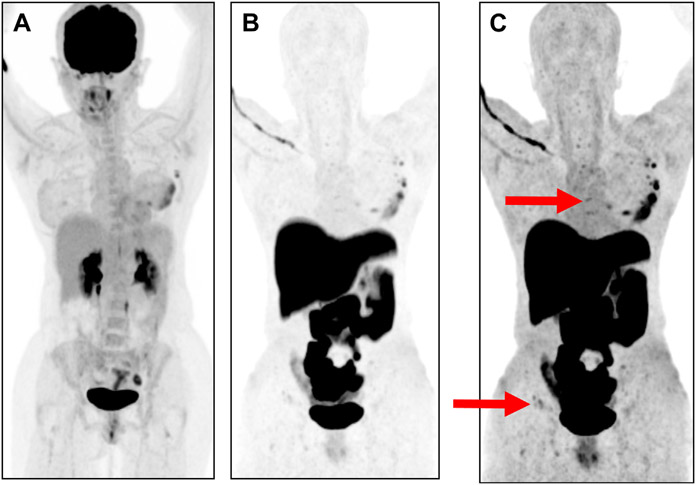
Fig. 3.
Patient with left breast palpable ER-positive HER2-negative ILC with de novo stage IV disease. Panel A shows FDG-PET with uptake at known left breast mass. (B, C) show 18-F FES PET demonstrating foci of low-level avidity on rewindowing images for higher sensitivity, consistent with bone metastases. Bone metastases in sternum and iliac crest denoted by red arrows.
Given the predilection of ILC for a diffuse growth pattern, further research is needed to assess the use of 18F-FES PET in settings of poorly visualized disease, including peritoneal carcinomatosis, leptomeningeal disease, and pleural effusions.
Challenges in the Implementation of 18F-FES Imaging Studies
One of the primary limitations of 18F-FES PET is the evaluation of liver metastases. As described previously, there is a high level of normal physiologic uptake of 18F-FES in the liver resulting from rapid metabolism of the agent. This issue led one research group to conclude that 18F-FES PET should not be used to evaluate liver metastases.34 However, a recent article by Boers and colleagues sought to evaluate whether 18F-FES could be used to identify ER + liver metastases, confirmed by biopsy, comparing visual and quantitative measures, and evaluating the impact of modifying region of interest. Although quantitative analysis improved sensitivity of detection over visual analysis, specificity was reduced.36 Currently, 18F-FES PET may have limited clinical utility in the detection of liver metastases.
An additional concern about 18F-FES PET is the cost when compared with biopsy alone, assuming that biopsy is feasible. There has been only one cost-effectiveness model that has been published to date about the use of 18F-FES in metastatic breast cancer, which was based on hospitals within the Dutch health-care system.37 Although more metastatic lesions were identified using 18F-FES PET, the diagnostic costs to evaluate receptor status and treatment costs were higher compared with biopsy alone.
As with many PET radiotracers, 18F-FES uptake quantitation can be influenced by body mass index, with higher body mass index being associated with increased uptake; this can be overcome by correcting quantitative measurements for lean body mass.38 Additionally, many ER + lesions have a low tumor to background ratio; the low SUVmax threshold for positivity on 18F-FES PET can pose a sensitivity challenge in FES PET image interpretation.
Dedicated Breast Positron Emission Tomography and 18F-Fluoroestradiol
Although the literature contains many studies evaluating the use of 18F-FES with whole-body PET imaging, dbPET is a promising new technology that may be a complementary tool. Imaging the breast only, dbPET provides higher resolution of breast lesions than whole-body PET, and it may be especially relevant for the evaluation of early stage disease and surgical planning.
Compared with whole-body PET, dbPET uses a lower dose of radiotracer (185 vs 370 MBq) and less radiation, potentially allowing more opportunities for serial imaging.39 Moreover, the positioning of the patient prone rather than supine in dbPET prevents breast compression, thereby allowing full breast volume imaging akin to breast MRI. dbPET has demonstrated higher sensitivity in detecting subcentimeter lesions and may identify response to neoadjuvant chemotherapy earlier than MRI.40 Importantly, however, this high sensitivity comes with the possibility of detecting benign lesions and higher false-positive rates.41 Recently, there has been a push to standardize reporting and descriptors of uptake in dbPET given its increasing use.41
The literature evaluating the use of 18F-FES in dbPET is extremely limited. One feasibility study by Jones and colleagues40 outlined their initial experiences with dbPET using 18F-FES in assessing ER + breast cancer in 6 patients, including 2 with ILC. The results suggest the potential of 18F-FES PET imaging to provide early predictions of neoadjuvant treatment efficacy and thus aid in therapy selection. The authors also noted important limitations to the technology, including variations in 18F-FES uptake in different ER-positive breast cancer subtypes and the exclusion of axillary lymph nodes.40
Future Directions
As of this writing, 18F-FES PET is FDA-approved for imaging ER-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer. However, 18F-FES PET could be used as a beneficial adjunct to FDG-PET and other diagnostic imaging modalities to aid in initial staging.42 In particular, 18F-FES may be able to reduce false-positive FDG-PET results caused by inflammation or improve staging in difficult to detect tumors such as ILC, as described above.42,43
Currently, there is an open clinical trial evaluating the use of 18F-FES for staging and detection of recurrent ER-positive breast cancer compared with standard of care with chest, abdominal, and pelvic CT and bone scan (NCT04883814).44 Other ongoing trials evaluating the clinical utility of 18F-FES include the ECOG-ACRIN EAI 142 trial (NCT02398773), a phase II study of patients with ER + metastatic breast cancer prospectively evaluating 18F-FES PET as a predictor of clinical benefit and progression free survival to first-line endocrine therapy. Similarly, the ongoing ET-FES TRANSCAN trial (EUDRACT 2013–000–287–29) is testing tumoral heterogeneity on 18F-FES PET as a predictor of endocrine therapy response.45 The Imaging Patients for Cancer Drug Selection – Metastatic Breast Cancer study (NCT01957332) tests the clinical utility of 18F-FES PET for reducing biopsies and improving treatment selection. Results from these results may solidify 18F-FES’s place in staging and detection of recurrent breast cancer, and treatment selection for metastatic disease.
With increased resolution compared with whole body PET, dbPET may prove useful in accurate assessment of breast tumor size, facilitating surgical planning, and potentially reducing the need for re-excisions. In addition, dbPET may be a useful adjunct to MRI for assessing response to neoadjuvant therapy.
SUMMARY
Recently FDA-approved, 18F-FES is a well-studied radiopharmaceutical with the ability to provide molecular imaging of ER-positive breast cancer. In the setting of whole-body PET scanning, 18F-FES uptake can confirm the presence of ER + metastases and provide insight into tumor heterogeneity. Uptake values may reflect sensitivity to therapy and guide treatment selection. In the setting of ILC, 18F-FES may provide improved disease detection compared with standard FDG-PET. The novel dedicated breast PET technology may provide improved tumor resolution that can be used both for evaluating the response to neoadjuvant treatment and for providing more accurate staging for surgical planning.
KEY POINTS.
18F-Fluoroestradiol (18F-FES) is a radiopharmaceutical for molecular imaging of ER + breast cancers
Baseline 18F-FES uptake may be used to guide treatment strategies
Molecular imaging may improve disease staging
Dedicated breast positron emission tomography scanning with 18F-FES may provide more accurate tumor assessments in early-stage disease, and noninvasive therapy response indicators
Estrogen receptor (ER) modulators and degraders will block 18F-FES binding, and should be held for a minimum of 6 to 8 weeks selective ER modulators or 28 weeks selective ER downregulators/degraders before imaging to avoid false negatives
Funding:
R.A. Mukhtar was supported by the National Cancer Institute Award K08CA256047. E. F. Jones was supported in part by the Department of Defense W81XWH-18-1-0671 and National Institutes of Health R01CA227763.
Footnotes
DISCLOSURE
No disclosures for all authors included in this article.
REFERENCES
- 1.Breast cancer facts and statistics. Available at: https://www.breastcancer.org/facts-statistics. Accessed March 6, 2022.
- 2.Carlson RW, Hudis CA, Pritchard KI. National comprehensive cancer network breast cancer clinical practice guidelines in oncology, american society of clinical oncology technology assessment on the use of aromatase inhibitors, St Gallen international expert consensus on the primary therapy of early breast cancer. Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: evolution of NCCN, ASCO, and St Gallen recommendations. J Natl Compr Cancer Netw 2006;4(10):971–9. [DOI] [PubMed] [Google Scholar]
- 3.Brown-Glaberman U, Dayao Z, Royce M. HER2-targeted therapy for early-stage breast cancer: a comprehensive review. Oncol Williston Park N 2014;28(4):281–9. [PubMed] [Google Scholar]
- 4.Li H, Liu Z, Yuan L, et al. Radionuclide-based imaging of breast cancer: state of the art. Cancers 2021;13(21):5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linden HM, Dehdashti F. Novel methods and tracers for breast cancer imaging. Semin Nucl Med 2013;43(4):324–9. [DOI] [PubMed] [Google Scholar]
- 6.McElvany KD, Katzenellenbogen JA, Shafer KE, et al. 16 alpha-[77Br] bromoestradiol: dosimetry and preliminary clinical studies. J Nucl Med 1982;23(5):425–30. [PubMed] [Google Scholar]
- 7.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology 1988;169(1):45–8. [DOI] [PubMed] [Google Scholar]
- 8.Boers J, de Vries EFJ, Glaudemans AWJM, et al. Application of PET tracers in molecular imaging for breast cancer. Curr Oncol Rep 2020;22(8):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabher BJ. Breast cancer: evaluating tumor estrogen receptor status with molecular imaging to increase response to therapy and improve patient outcomes. J Nucl Med Technol 2020;48(3):191–201. [DOI] [PubMed] [Google Scholar]
- 10.van Kruchten M, de Vries EGE, Brown M, et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol 2013;14(11):e465–75. [DOI] [PubMed] [Google Scholar]
- 11.Yoo J, Dence CS, Sharp TL, et al. Synthesis of an estrogen receptor beta-selective radioligand: 5-[18F]fluoro-(2R,3S)-2,3-bis(4-hydroxyphenyl)pentanenitrile and comparison of in vivo distribution with 16alpha-[18F]fluoro-17beta-estradiol. J Med Chem 2005;48(20):6366–78. [DOI] [PubMed] [Google Scholar]
- 12.Chae SY, Ahn SH, Kim SB, et al. Diagnostic accuracy and safety of 16α-[18F]fluoro-17β-oestradiol PET-CT for the assessment of oestrogen receptor status in recurrent or metastatic lesions in patients with breast cancer: a prospective cohort study. Lancet Oncol 2019;20(4):546–55. [DOI] [PubMed] [Google Scholar]
- 13.Chae SY, Kim SB, Ahn SH, et al. A randomized feasibility study of 18F-Fluoroestradiol PET to predict pathologic response to neoadjuvant therapy in estrogen receptor-rich postmenopausal breast cancer. J Nucl Med 2017;58(4):563–8. [DOI] [PubMed] [Google Scholar]
- 14.Gemignani ML, Patil S, Seshan VE, et al. Feasibility and predictability of perioperative PET and estrogen receptor ligand in patients with invasive breast cancer. J Nucl Med 2013;54(10):1697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FDA label cerianna. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212155s000lbl.pdf. Accessed March 10, 2022.
- 16.Mankoff DA, Peterson LM, Tewson TJ, et al. [18F]Fluoroestradiol radiation dosimetry in human PET studies. J Nucl Med 2001;42(4):679. [PubMed] [Google Scholar]
- 17.Mankoff DA, Clark AS. PET oestrogen receptor imaging: ready for the clinic? Lancet Oncol 2019;20(4):467–9. [DOI] [PubMed] [Google Scholar]
- 18.Linden HM, Kurland BF, Peterson LM, et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res 2011;17(14):4799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evangelista L, Dieci MV, Guarneri V, Conte PF. 18F-Fluoroestradiol positron emission tomography in breast cancer patients: systematic review of the literature & meta-analysis. Curr Radiopharm 2016;9(3):244–57. [DOI] [PubMed] [Google Scholar]
- 20.Kurland BF, Wiggins JR, Coche A, et al. Whole-body characterization of estrogen receptor status in metastatic breast cancer with 16α-18F-Fluoro-17β-Estradiol positron emission tomography: meta-analysis and recommendations for integration into clinical applications. Oncologist 2020;25(10):835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanidze J, Subramanian K, Youn T, et al. Utility of [18F]-fluoroestradiol (FES) PET/CT with dedicated brain acquisition in differentiating brain metastases from posttreatment change in estrogen receptor-positive breast cancer. Neurooncol Adv 2021;3(1):vdab178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katzenellenbogen JA. The quest for improving the management of breast cancer by functional imaging: The discovery and development of 16α-[18F]fluoroestradiol (FES), a PET radiotracer for the estrogen receptor, a historical review. Nucl Med Biol 2021;92:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortimer JE, Dehdashti F, Siegel BA, et al. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol 2001;19(11):2797–803. [DOI] [PubMed] [Google Scholar]
- 24.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol 2006;24(18):2793–9. [DOI] [PubMed] [Google Scholar]
- 25.Dehdashti F, Mortimer JE, Trinkaus K, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat 2009;113(3):509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurland BF, Peterson LM, Lee JH, et al. Estrogen receptor binding (18F-FES PET) and glycolytic activity (18F-FDG PET) predict progression-free survival on endocrine therapy in patients with ER+ breast cancer. Clin Cancer Res 2017;23(2):407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kruchten M, de Vries EG, Glaudemans AW, et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov 2015;5(1):72–81. [DOI] [PubMed] [Google Scholar]
- 28.Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 2020. 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boers J, Venema CM, de Vries EFJ, et al. Molecular imaging to identify patients with metastatic breast cancer who benefit from endocrine treatment combined with cyclin-dependent kinase inhibition. Eur J Cancer Oxf Engl 1990 2020;126:11–20. [DOI] [PubMed] [Google Scholar]
- 30.Peterson LM, Kurland BF, Yan F, et al. 18F-Fluoroestradiol PET imaging in a phase II trial of vorinostat to restore endocrine sensitivity in ER+/HER2− metastatic breast cancer. J Nucl Med 2021;62(2):184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Ayres KL, Goldman DA, et al. 18F-Fluoroestradiol PET/CT measurement of estrogen receptor suppression during a phase I trial of the novel estrogen receptor-targeted therapeutic GDC-0810: using an imaging biomarker to guide drug dosage in subsequent trials. Clin Cancer Res 2017;23(12):3053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Kruchten M, Glaudemans AWJM, de Vries EFJ, et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med 2012;53(2):182–90. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Yang Z, Zhang Y, et al. The preliminary study of 16α-[18F]fluoroestradiol PET/CT in assisting the individualized treatment decisions of breast cancer patients. PLoS One 2015;10(1):e0116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venema C, de Vries E, Glaudemans A, et al. 18F-FES PET has added value in staging and therapy decision making in patients with disseminated lobular breast cancer. Clin Nucl Med 2017;42(8):612–4. [DOI] [PubMed] [Google Scholar]
- 35.Ulaner GA, Jhaveri K, Chandarlapaty S, et al. Head-to-head evaluation of 18F-FES and 18F-FDG PET/CT in metastatic invasive lobular breast cancer. J Nucl Med 2021;62(3):326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boers J, Loudini N, de Haas RJ, et al. Analyzing the estrogen receptor status of liver metastases with [18F]-FES-PET in patients with breast cancer. Diagn Basel Switz 2021;11(11). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koleva-Kolarova RG, Greuter MJW, Feenstra TL, et al. Molecular imaging with positron emission tomography and computed tomography (PET/CT) for selecting first-line targeted treatment in metastatic breast cancer: a cost-effectiveness study. Oncotarget 2018;9(28):19836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson LM, Kurland BF, Link JM, et al. Factors influencing the uptake of 18F-fluoroestradiol in patients with estrogen receptor positive breast cancer. Nucl Med Biol 2011;38(7):969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hathi DK, Li W, Seo Y, et al. Evaluation of primary breast cancers using dedicated breast PET and whole-body PET. Sci Rep 2020;10(1):21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones EF, Ray KM, Li W, et al. Initial experience of dedicated breast PET imaging of ER+ breast cancers using [F-18]fluoroestradiol. NPJ Breast Cancer 2019;5(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyake KK, Kataoka M, Ishimori T, et al. A proposed dedicated breast pet lexicon: standardization of description and reporting of radiotracer uptake in the breast. Diagn Basel Switz 2021;11(7):1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao GJ, Clark AS, Schubert EK, et al. 18F-Fluoroestradiol PET: current status and potential future clinical applications. J Nucl Med 2016;57(8):1269–75. [DOI] [PubMed] [Google Scholar]
- 43.van Kruchten M, Glaudemans AWJM, de Vries EFJ, et al. Positron emission tomography of tumour [(18)F]fluoroestradiol uptake in patients with acquired hormone-resistant metastatic breast cancer prior to oestradiol therapy. Eur J Nucl Med Mol Imaging 2015;42(11):1674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulaner G. 18F-Fluoroestradiol (FES) PET/CT Compared to standard-of-care imaging in patients with breast cancer. clinicaltrials.gov. 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT04883814. Accessed March 3, 2022. [Google Scholar]
- 45.Gennari A, Brain E, Nanni O, et al. 114P - Molecular imaging with 18F-fluoroestradiol (18F-FES) to assess intra-patient heterogeneity in metastatic breast cancer (MBC): a European TRANSCAN program. Annals of Oncology 2017;28(suppl_5):v22–v42. doi: 10.1093/annonc/mdx363.030 [DOI] [Google Scholar]